Abstract
Objective:
To describe the clinical and genetic characteristics of presynaptic congenital myasthenic syndrome secondary to biallelic variants in SLC18A3.
Methods:
Individuals from 2 families were identified with biallelic variants in SLC18A3, the gene encoding the vesicular acetylcholine transporter (VAChT), through whole-exome sequencing.
Results:
The patients demonstrated features seen in presynaptic congenital myasthenic syndrome, including ptosis, ophthalmoplegia, fatigable weakness, apneic crises, and deterioration of symptoms in cold water for patient 1. Both patients demonstrated moderate clinical improvement on pyridostigmine. Patient 1 had a broader phenotype, including learning difficulties and left ventricular dysfunction. Electrophysiologic studies were typical for a presynaptic defect. Both patients showed profound electrodecrement on low-frequency repetitive stimulation followed by a prolonged period of postactivation exhaustion. In patient 1, this was unmasked only after isometric contraction, a recognized feature of presynaptic disease, emphasizing the importance of activation procedures.
Conclusions:
VAChT is responsible for uptake of acetylcholine into presynaptic vesicles. The clinical and electrographic characteristics of the patients described are consistent with previously reported mouse models of VAChT deficiency. These findings make it very likely that defects in VAChT due to variants in SLC18A3 are a cause of congenital myasthenic syndrome in humans.
The congenital myasthenic syndromes are genetic disorders of neuromuscular junction function that commonly present with ptosis, ophthalmoplegia, and fatigable weakness. The majority of cases are due to defects in postsynaptic proteins, and in >50%, the muscle nicotinic acetylcholine receptor is affected.1 Presynaptic causes identified to date include variants in CHAT (choline acetyltransferase), which facilitates the production of acetylcholine from choline and acetyl coenzyme A, and the recently identified SYT22 and SNAP25 genes.3
SLC18A3 (solute carrier family 18 [vesicular acetylcholine], member 3) is found on chromosome 10q11.23. It comprises a single exon within the first intron of the CHAT gene and encodes the vesicular acetylcholine transporter (VAChT). VAChT is a 532–amino acid protein with 12-transmembrane domains localizing to the membrane of presynaptic secretory vesicles where it transports acetylcholine into vesicles.4 Acetylcholine release from vesicles at cholinergic nerve endings is important for neurotransmission in both the central and peripheral nervous system. The presence of SLC18A3 within the CHAT gene is evolutionarily conserved from Caenorhabditis elegans, a primitive nematode, to humans, suggesting that it has an important regulatory mechanism to ensure appropriate expression of VAChT.4,5
This study describes 2 patients with congenital myasthenic syndrome secondary to pathogenic variants in SLC18A3.
METHODS
Both patients presented with a congenital myasthenic syndrome but remained undiagnosed after testing for known genetic causes. For patient 1, whole-exome sequencing and whole-genome sequencing were performed at the Broad Institute on genomic DNA from the proband and his unaffected mother. Analysis was performed with the XBrowse bioinformatics platform (https://xbrowse.broadinstitute.org) as previously described.6 A custom search was performed for all variants in all known neuromuscular disease genes at the time of investigation. When no candidate gene was identified, the whole exome was searched by inheritance pattern, considering clinical phenotype and population frequency in the Exome Aggregation Consortium (ExAC) database and in silico prediction programs. Comparative genomic hybridization microarray was performed with Agilent SurePrint G3 ISCA Targeted Microarray 8x60K. Sanger sequencing of CHAT was performed as previously described.7 For patient 2, whole-exome sequencing was performed at Beijing Genome Institute Europe (Copenhagen, Denmark) and analyzed with the use of a bioinformatic pipeline at the Radboud University Medical Center, Department of Human Genetics, essentially performed as described.8 A filter for a muscle disease gene panel was applied first (www.genomediagnosticsnijmegen.nl/exome). Because no candidate gene was identified, the whole exome was searched by inheritance pattern, considering clinical phenotype and population frequency in the in-house patient database, ExAC database, and in silico prediction programs.
Standard protocol approvals, registrations, and patient consents.
This research was approved by the Human Research Ethics Committee of the Children’s Hospital at Westmead, Australia (10/CHW/45) and performed with patient consent. For patient 2, the analysis of the whole exome was done after counseling of and informed consent by the parents and approval by the Medical Review Ethics Committee Region Arnhem-Nijmegen (2011/188).
RESULTS
Patients.
Patient 1 was born to unaffected nonconsanguineous Filipino parents (figure, A). He had multiple episodes of cyanosis that did not require assisted ventilation between 2 and 18 months of age. His early developmental milestones were normal. He fatigued easily during his preschool years and was reported to have ptosis from 1 year of age. He was investigated at 6 years of age and again at 14 years of age when he reported reduced exercise tolerance but no diurnal fluctuation in his abilities. He had episodes of more severe weakness triggered by swimming in cold water. On examination at 14 years of age, he had bilateral fatigable ptosis, ophthalmoplegia, and mild facial weakness. Shoulder abduction, hip flexion, and hip extension power were graded 4+/5. Fatigable weakness was present with sustained shoulder abduction. The remaining muscle groups had normal strength. Deep tendon reflexes were present.
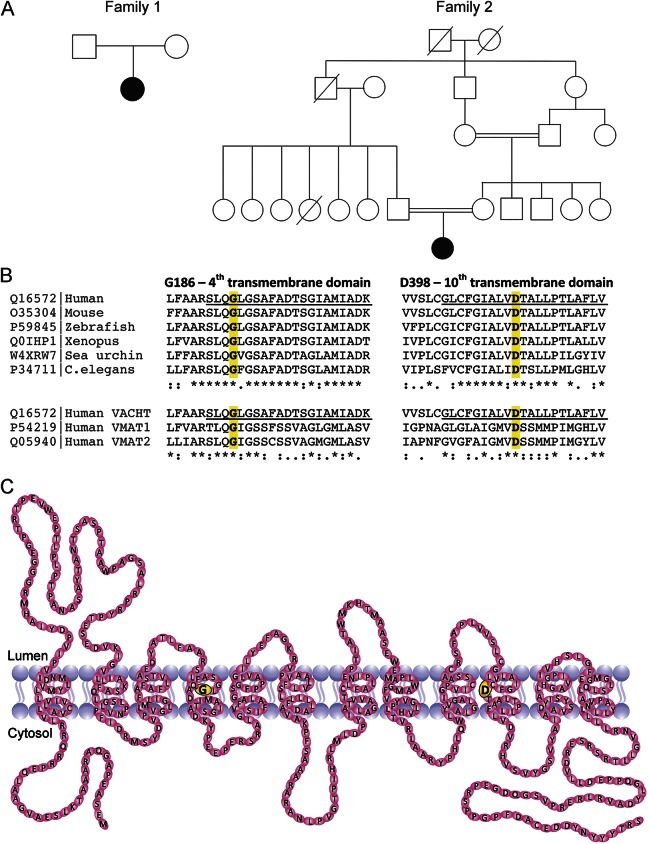
Figure. Family pedigrees, evolutionary conservation of the vesicular acetylcholine transporter (VACHT) receptor, and schematic structure.
(A) Family pedigrees. (B) Evolutionary conservation of G186 and D398 in the 4th and 10th transmembrane domains. Conservation across species is shown on the top; conservation in orthologous genes is shown on the bottom. (C) Schematic diagram showing VACHT. The 12 transmembrane domains are shown. The Gly186Ala variant identified in patient 1 is shown in yellow in the fourth transmembrane domain. The Asp398His variant identified in patient 2 is shown in yellow in the 10th transmembrane domain. VMAT = vesicular monoamine transporter.
Throughout school, he had mild static learning difficulties. A formal psychometric assessment at 16 years of age showed significant deficits in visual perceptual reasoning, attention span, working memory, motor processing speed, and immediate and delayed visual memory. Verbal intellect and verbal learning and memory were within normal limits.
Creatine kinase, thyroid function, lactate, and acetylcholine receptor antibody studies were normal. Repetitive nerve stimulation studies were normal at 9 years of age, including absence of an electrodecrement in the abductor digiti minimi after 10 seconds of isometric exercise. No benefit was observed with an edrophonium test. Electrophysiologic studies repeated at 14 years did not show an electrodecrement in the abductor digiti minimi or abductor pollicis brevis with 5-Hz stimulation. After 10 seconds of isometric exercise, a 5-Hz stimulus resulted in an abnormal decrement of 34% in the abductor pollicis brevis, which persisted when repeated every 30 seconds for 4 minutes.
Pyridostigmine was started at 14 years of age and increased to 60 mg 4 times per day (5.3 mg·kg−1·d−1). He reported reduced fatigue, and his 6-minute walk test improved from 360 to 462 m after 4 weeks of treatment. Sustained arm abduction improved from 45 to 60 seconds and sustained upgaze from 10 to 20 seconds. The dose was increased to 60 mg 5 times per day (7.5 mg·kg−1·d−1), and the clinical improvement was sustained at 16 years of age. Power was graded as 5/5 in all muscle groups; facial weakness was improved; but mild ptosis and ophthalmoplegia persisted.
Transthoracic echocardiogram at 14 years of age, before pyridostigmine treatment, showed mild reduction of left ventricular systolic function (left ventricular end-diastolic dimension 45 mm; end-systolic dimension 33 mm; fractional shortening 26%). After 2 months of treatment with pyridostigmine, a repeat echocardiogram showed normal left ventricular function (fractional shortening 31%). Normal function was maintained 14 months after the start of treatment (fractional shortening 29%).
Patient 2 was the only child of consanguineous East Turkish parents (figure, A). After a complicated labor, she was delivered at 36 + 5 weeks by caesarian section. Apgar scores were 3 at 1 minute, 5 at 5 minutes, and 6 at 10 minutes. She developed meconium ileus after 3 days and apneas after 1 month, which did not require intubation. An MRI of the brain performed because of the complicated delivery showed mild hyperintensity of the white matter and small punctate hemorrhages in the frontal and parietal lobes. At 3 months of age, she was admitted with hypotonia and feeding difficulties with aspiration and required nasogastric feeding. Neurologic examination was typical for a myasthenic syndrome and revealed horizontal nystagmus, horizontal and vertical ophthalmoplegia, bilateral ptosis, and hypotonia, all of which fluctuated during the day. Deep tendon reflexes were normal. Flexure contractures were present at the knees. An edrophonium test was positive, with improvements in tone and head control, a reduction in ptosis, and a louder cry.
Electrophysiologic studies of patient 2 showed normal nerve conduction velocities and amplitudes. Repetitive stimulation at low frequency showed extreme electrodecrement. With a 3-Hz stimulus, an electrodecrement of 47% was recorded in the abductor digiti minimi and 78% in the abductor pollicis brevis. No afterdischarges were recorded. Pyridostigmine was started, and she improved in axial tone, but ptosis, horizontal ophthalmoplegia, and neck extensor weakness persisted. Oral feeding improved, and defecation was no longer dependent on intestinal lavages or enema. 3,4-Diaminopyridine (3,4-DAP; 1 mg·kg−1·24 h−1) was begun at 1 year of age in addition to high-dose pyridostigmine (7 mg·kg−1·24 h−1 as 6 divided doses). At 2 years of age, she had a severe apnea in the night, probably due to a delayed dose of pyridostigmine, and required resuscitation. She had further choking episodes and apneas, and distigmine (2.5 mg) was added with limited benefits. At 3 years of age, ephedrine (0.5 mg/kg as 2 divided doses) was added to pyridostigmine (22 mg·kg−1·24 h−1) and 3,4-DAP (1.7 mg·kg−1·24 h−1) with improvement. Nevertheless, she continued to have apneas during the night, and percutaneous endoscopic gastrostomyfeeds were required. At 4 years of age, she walked for some months, but she lost independent ambulation by age 5. At 6 years of age, patient 2 is able to stand and transfer with assistance when wearing orthoses. She is ambulant over short distances with assistance but has limited exercise tolerance. Knee flexor contractures are developing. Formal neuropyschometric testing has not been done, but she has not demonstrated any learning difficulties in a regular school program. An echocardiogram was normal at 3 months of age, and no evidence of cardiomyopathy was found on continuous ECG monitoring during the intensive care admissions.
Genetic results.
For patient 1, candidate gene sequencing of CHAT, the common N88K RAPSN variant, and a next-generation sequencing neuromuscular gene panel, including all genes known to cause congenital myasthenia in 2014, did not find any pathogenic variants. Whole-exome sequencing identified a homozygous recessive variant in SLC18A3, c.557G>C; p.(Gly186Ala) (NM_003055.2), in the proband that was confirmed by Sanger sequencing. His mother was heterozygous for this variant, and paternal DNA was not available. Chromosomal microarray identified a homozygous 4.83-Mb heterozygous deletion in the proband within chromosome 10q11.22-q11.23, (chr10: 46,949,255–51,780,909, hg19), involving 5 Online Mendelian Inheritance in Man (OMIM)–listed genes, including CHAT and SLC18A3. Thus, the proband carries a deletion of CHAT and SLC18A3 on 1 allele and a missense variant c.557 G>C; p.(Gly186Ala) in SLC18A3 on the second allele, appearing homozygous via Sanger sequencing. Whole-genome sequencing was performed in the proband to exclude a second variant in CHAT, potentially undetected by whole-exome sequencing. No further likely pathogenic variants were identified.
SLC18A3 c.557 G>C; p.(Gly186Ala) is not found in the National Heart, Lung, and Blood Institute Exome Variant Server or the ExAC database. Four in silico prediction software packages predicted the variant to be pathogenic (PolyPhen-2, probably damaging [1.0]; Sorting Intolerant From Tolerant, deleterious [0.000]; Provean, deleterious [−5.62]; Mutation Taster, disease causing1). Gly186 is located in the fourth transmembrane domain of VAChT (Figure, B and C), is invariant among vertebrates, and is conserved in the related vesicular monoamine transporters 1 (SLC18A1) and 2 (SLC18A2), suggesting that this amino acid position is highly important for protein function.
Patient 2 was investigated over many years, including Sanger sequencing and exclusion of known genetic causes of congenital myasthenic syndrome. Whole-exome sequencing studies in patient 2 and her unaffected parents identified a homozygous recessive missense variant, c.1192G>C, p.(Asp398His); (NM_003055.2), in SLC18A3. Both parents were heterozygous for this variant. The variant was confirmed in the trio with Sanger sequencing. SLC18A3 c.1992G>C; p.(Asp398His) is not found in the National Heart, Lung, and Blood Institute Exome Variant Server or the ExAC. p.Asp398Gly and p.Asp398Glu are identified as rare variants in ExAC but with no reported homozygotes. Four in silico prediction software packages predicted the variant to be pathogenic (PolyPhen-2, probably damaging [1.0]; Sorting Intolerant From Tolerant, deleterious [0.000]; Provean, deleterious [−6.46]; Mutation Taster, disease causing [0.999]). Asp398 is located in the 10th transmembrane domain (Figure, B and C) and, like Gly186, is highly conserved in vertebrates and other members of the monoamine transporters.
DISCUSSION
The patients’ clinical presentations were typical for congenital myasthenic syndrome with incomplete improvement after treatment with pyridostigmine (and 3,4-DAP and ephedrine for patient 2). The patients shared features with presynaptic congenital myasthenic syndrome secondary to CHAT deficiency, including apneic crises7,9,10 and deterioration of symptoms in cold water in patient 1.11 The results of repetitive nerve stimulation studies were consistent with a presynaptic defect, with profound electrodecrement on low-frequency repetitive stimulation followed by a prolonged period of postactivation exhaustion. In patient 1, the electrodecrement was unmasked only after sustained isometric contraction, which is recognized as a feature of presynaptic syndromes.12
Two mouse models of reduced SLC18A3 expression, a knockout mouse and a knockdown mouse, have previously been described and provide strong support for SLC18A3 as a genetic cause of presynaptic congenital myasthenic syndrome.13–16 Homozygous SLC18A3 (VAChTdel/del) knockout mice had severe neuromuscular junction dysfunction and died within minutes of birth of respiratory failure. The heterozygous knockout mice (VAChTwt/del) demonstrated a 50% reduction in VAChT protein levels but no clinical phenotype and normal axonal branching of peripheral nerves.14 Homozygous knockdown mice (VAChT KDHOM), with deletion of a regulatory element within the 5′ untranslated region, had a 65% to 70% reduction in VAChT expression. Motor performance and grip strength were impaired, and mice were not able to maintain long periods of physical activity. Grip strength was restored by treatment with a cholinesterase inhibitor.13 These findings support a high safety margin in neuromuscular transmission, with a reduction in protein levels of up to 50% well tolerated.
Postsynaptic myasthenic syndromes affect only peripheral nicotinic acetylcholine receptors, resulting in specific involvement of skeletal muscle. VAChT is crucial for acetylcholine release in the central, autonomic, and peripheral nervous systems. Impairment of VAChT activity thus affects both muscarinic and nicotinic acetylcholine receptors, causing more widespread clinical manifestations.
Cognitive deficits have been described in CHAT-related disease secondary to hypoxic injury after life-threatening apneic episodes.17 Patient 1 demonstrated selective cognitive deficits in the absence of life-threatening apneic crises. These may be secondary to altered cholinergic synaptic transmission mediated by neuronal nicotinic acetylcholine receptors in the brain. Cognitive deficits were not seen in patient 2. Cholinergic tone is considered to be important in learning and memory, and deficits in acetylcholine neurotransmission have been described in several conditions, including Alzheimer disease. The VAChT KDHOM mouse strain performed poorly at memory tasks and in object recognition and social memory.13
Cardiac function was also impaired in VAChTHOM mice secondary to reduced cholinergic neurotransmission. Because cholinergic transmission underpins the parasympathetic nervous system, there is an autonomic imbalance in VAChTHOM mice whereby sympathetic activation of contraction is not appropriately counterbalanced by parasympathetic vasodilation and relaxation. At 3 months of age, mice showed reduced cardiac contractility, which improved significantly after treatment for 2 weeks with a cholinesterase inhibitor.15 Similarly, patient 1 had reduced left ventricular contractile function, which resolved with pyridostigmine therapy, consistent with findings in the mouse model. Patient 2 had a normal echocardiogram at 3 months of age and began pyridostigmine therapy earlier than patient 1. Cardiac dysfunction has not been reported in other cases of congenital myasthenic syndrome, and it remains to be established whether cardiac involvement is a primary manifestation related to SLC18A3 variants and VAChT dysfunction or is an incidental finding in patient 1. Because of the possible cardiac phenotype, we recommend cardiac surveillance in patients with SLC18A3 variants while the clinical spectrum of this new subtype of congenital myasthenic syndrome is being established.
The 4.83-Mb deletion at 10q11.21-q11.23 includes the genes RBP3 (retinitis pigmentosa 66, OMIM 615233), GDF2 (telangiectasia, hereditary hemorrhagic type 5, OMIM 615506), ERCC6 (cerebro-oculo-facio-skeletal syndrome 1, OMIM 214150), and MSMB (prostate cancer, hereditary 13, OMIM 611928), which were not considered relevant to the patient’s phenotype. No second variant in these genes was identified in whole-exome sequencing data. Recurrent deletions and reciprocal duplications involving CHAT and SLC18A3 have been previously reported and range in size from 0.12 to 12 Mb.18,19 The clinical features associated with these changes were variable but included developmental delay and intellectual disability. However, in more than half of the patients, the deletion was present in an unaffected parent, suggesting that it either is not pathogenic when heterozygous or is associated with variable expressivity. Two of 19 patients were reported to have ptosis, but none had other features suggestive of a congenital myasthenic syndrome, strongly suggesting that heterozygous deletion of CHAT and SLC18A3 does not cause congenital myasthenic syndrome.19 Accordingly, heterozygous SLC18A3 mice (VAChTwt/del)13 and heterozygous CHAT mice (chat+/−) showed reduced protein expression but did not have neuromuscular defects, and chat+/− mice exhibited no cognitive deficits.20
Thus, the c.557 G>C; p.(Gly186Ala) missense variant in SLC18A3 identified in patient 1, inherited in trans to the 10q11.21-q11.23 deletion, and the homozygous c.1192G>C, p.(Asp398His) identified in patient 2 are considered the cause of congenital myasthenic syndrome in these patients, likely resulting from defective uptake of acetylcholine into presynaptic vesicles. Both had been extensively investigated to exclude known causes of this disease. In patient 1, whole-genome sequencing was performed to definitively exclude a second causative variant in CHAT.
Congenital myasthenic syndromes are important to diagnose because of the potential for treatment with an acetylcholinesterase inhibitor or, in other subtypes, salbutamol, ephedrine, or 3,4-DAP.1 The early manifestations may be nonspecific, with fatigue and ptosis becoming more evident only later in childhood. Some patients remain undiagnosed until adulthood with slowly progressive limb girdle weakness.21 Failure to demonstrate an electrodecrement is not uncommon, particularly without activation procedures or testing of weak proximal muscles, and given the limitations of performing the study in young children.21 Our findings highlight the importance of repeated nerve conduction studies and consideration of activation procedures. Stimulated single-fiber EMG is recommended as a more sensitive measure of neuromuscular junction dysfunction.22 A trial of acetylcholinesterase inhibitors may be considered, with objective assessment of response and awareness of the risk of deterioration in DOK7–, MUSK–, and LRP4–related disease.1
SLC18A3 is a new genetic cause of presynaptic congenital myasthenic syndrome and should be included in congenital myasthenic syndrome panels. Two well-characterized mouse models of VAChT deficiency strongly support the pathogenesis of SLC18A3 variants in congenital myasthenia.
ACKNOWLEDGMENT
The authors thank the families for their invaluable contribution to this work.
GLOSSARY
- ExAC
Exome Aggregation Consortium
- OMOM
Online Mendelian Inheritance in Man
- 3,4-DAP
3,4 diaminopyridine
- VAChT
vesicular acetylcholine transporter
AUTHOR CONTRIBUTIONS
Gina L. O’Grady: identification of the pathogenic variant in patient 1, data analysis and interpretation, drafting/revising manuscript for content. Corien Verschuuren: clinical care and provision of clinical data for patient 2, data interpretation, drafting/revising manuscript for content. Michaela Yuen: data acquisition, analysis, and interpretation, drafting/revising manuscript for content. Richard Webster: clinical care and provision of clinical data for patient 1, data interpretation, drafting/revising manuscript for content. Manoj Menezes: clinical care and provision of clinical data for patient 1, data interpretation, drafting/revising manuscript for content. Johanna M. Fock: clinical care and provision of clinical data for patient 2, data interpretation, drafting/revising manuscript for content. Natalie Pride: neuropsychologist review of patient 1, data interpretation, drafting/revising manuscript for content. Heather A. Best: data acquisition, data analysis and interpretation, drafting/revising manuscript for content. Tatiana Benavides Damm: data acquisition, analysis, and interpretation, drafting/revising manuscript for content. Christian Turner: data acquisition, data analysis and interpretation, drafting/revising manuscript for content. Monkol Lek: data acquisition, data analysis and interpretation, drafting/revising manuscript for content. Andrew G. Engel: data acquisition, data analysis and interpretation, drafting/revising manuscript for content. Kathryn N. North: data analysis and interpretation, drafting/revising manuscript for content, study design and concept, obtaining funding, study supervision. Nigel F. Clarke: data analysis and interpretation, drafting/revising manuscript for content, study design and concept, obtaining funding, study supervision. Daniel G. MacArthur: data acquisition, analysis and interpretation, drafting/revising manuscript for content, study design and concept, obtaining funding. Erik-Jan Kamsteeg: identification of the pathogenic variant in patient 2, data acquisition, data analysis and interpretation, drafting/revising manuscript for content. Sandra T. Cooper: data analysis and interpretation, drafting/revising manuscript for content, study design and concept, obtaining funding, study supervision.
STUDY FUNDING
This work was supported by the National Health and Medical Research Council of Australia (grant numbers 1022707 to N.F.C. and K.N.N., 1031893 to N.F.C. and K.N.N., 1080587 to D.G.M., K.N.N., N.F.C., and S.T.C., 1056285 to G.L.O., and 1048816 to S.T.C.). Exome sequencing was supported by grants from the National Human Genome Research Institute of the US National Institutes of Health (Medical Sequencing Program grant U54 HG003067 to the Broad Institute principal investigator, Lander). Work done in Dr. Engel’s laboratory was supported by National Institute of Health (grant number NS6277).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Engel AG, Shen X, Selcen D, Sine SM. Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment. Lancet Neurol 2015;14:420–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrmann DN, Horvath R, Sowden JE, et al. Synaptotagmin 2 mutations cause an autosomal-dominant form of Lambert-Eaton myasthenic syndrome and nonprogressive motor neuropathy. Am J Hum Genet 2014;95:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen XM, Selcen D, Brengman J, Engel AG. Mutant SNAP25B causes myasthenia, cortical hyperexcitability, ataxia, and intellectual disability. Neurology 2014;83:2247–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eiden LE, Schafer MK, Weihe E, Schutz B. The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pflugers Arch 2004;44:636–640. [DOI] [PubMed] [Google Scholar]
- 5.Lawal HO, Krantz DE. SLC18: vesicular neurotransmitter transporters for monoamines and acetylcholine. Mol Aspects Med 2013;34:360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menezes MP, Waddell L, Lenk GM, et al. Whole exome sequencing identifies three recessive FIG4 mutations in an apparently dominant pedigree with Charcot-Marie-Tooth disease. Neuromuscul Disord 2014;24:666–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohno K, Tsujino A, Shen XM, et al. Choline acetyltransferase mutations cause myasthenic syndrome associated with episodic apnea in humans. Proc Natl Acad Sci USA 2001;98:2017–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neveling K, Feenstra I, Gilissen C, et al. A post-hoc comparison of the utility of sanger sequencing and exome sequencing for the diagnosis of heterogeneous diseases. Hum Mutat 2013;34:1721–1726. [DOI] [PubMed] [Google Scholar]
- 9.Eymard B, Hanta D, Estournet B. Congenital myasthenic syndromes. In: Dulac O, Lassonde M, Sarnat HB, eds. Handbook of Clinical Neurology (3rd Series), Pediatric Neurology, Part III. Amsterdam, The Netherlands: Elsevier B.V.; 2013;113:1469–1479. [DOI] [PubMed] [Google Scholar]
- 10.Abicht A, Dusl M, Gallenmuller C, et al. Congenital myasthenic syndromes: achievements and limitations of phenotype-guided gene-after-gene sequencing in diagnostic practice: a study of 680 patients. Hum Mutat 2012;33:1474–1484. [DOI] [PubMed] [Google Scholar]
- 11.Maselli RA, Arredondo J, Ferns MJ, Wollmann RL. Synaptic basal lamina-associated congenital myasthenic syndromes. Ann NY Acad Sci 2012;1275:36–48. [DOI] [PubMed] [Google Scholar]
- 12.Juel VC. Evaluation of neuromuscular junction disorders in the electromyography laboratory. Neurol Clin 2012;30:621–639. [DOI] [PubMed] [Google Scholar]
- 13.Prado VF, de Castro BM, Lima RF, et al. Mice deficient for the vesicular acetylcholine transporter are myasthenic and have deficits in object and social recognition. Neuron 2006;51:601–612. [DOI] [PubMed] [Google Scholar]
- 14.De Castro BM, De Jaeger X, Martins-Silva C, et al. The vesicular acetylcholine transporter is required for neuromuscular development and function. Mol Cell Biol 2009;29:5238–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lara A, Damasceno DD, Pires R, et al. Dysautonomia due to reduced cholinergic neurotransmission causes cardiac remodeling and heart failure. Mol Cell Biol 2010;30:1746–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigues HA, Fonseca Mde C, Camargo WL, et al. Reduced expression of the vesicular acetylcholine transporter and neurotransmitter content affects synaptic vesicle distribution and shape in mouse neuromuscular junction. PLoS One 2013;8:e78342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulrike S, Christen H, Durmus H, et al. Long-term follow-up in patients with congenital myasthenic syndrome due to CHAT mutations. Eur J Ped Neurol 2010;14:326–333. [DOI] [PubMed] [Google Scholar]
- 18.DECIPHER Consortium. DECIPHER. http://decipher.sanger.ac.uk. Accessed June 13, 2015.
- 19.Stankiewicz P, Kulkarni S, Dharmadhikari AV, et al. Recurrent deletions and reciprocal duplications of 10q11.21q11.23 including CHAT and SLC18A3 are likely mediated by complex low-copy repeats. Hum Mutat 2011;33:165–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandon EP. Choline transporter 1 maintains cholinergic function in choline acetyltransferase haploinsufficiency. J Neurosci 2004;24:5459–5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nogajski JH, Kiernan MC, Ouvrier RA, Andrews PI. Congenital myasthenic syndromes. J Clin Neurosci 2009;16:1–11. [DOI] [PubMed] [Google Scholar]
- 22.Pitt M. Update in electromyography. Curr Opin Pediatr 2013;25:676–681. [DOI] [PubMed] [Google Scholar]