Abstract
Purpose
The estimation of cosmetic effect in 93 patients with early breast cancer treated with breast conserving surgery (BCS) followed by combined radiotherapy, including HDR brachytherapy (HDR-BT) boost.
Material and methods
After BCS (tumorectomy or quadrantectomy) external beam radiation therapy (EBRT) was used in total dose of 50 Gy for the whole breast. Tumor bed was localized basing on clinical and mammographic preoperative examinations and histopathology evaluation. 10 Gy in one fraction was applied to all patients using HDR-BT. Steel interstitial needles stabilized by plastic templates were used. 192-Ir with 10 Ci nominal activity and HDR-GammaMed 12i unit (Mick Radio-Nuclear Instruments, Inc., Mt. Vernon, NY) and ABACUS software were used. 31 patients received additional chemotherapy. Cosmetic effect was evaluated in 36 month after the end of brachytherapy treatment basing on modified EORTC scale. For statistical analysis the rang of correlation test, contingent test, linear regression test and ProbRough rulet induction test were used.
Results and Conclusions
HDR-BT tolerance was good in most of the cases. Excellent and very good cosmetic effect was observed in 79 patients (85%). Statistically important correlations between following examined prognostic factors and cosmetics outcome were observed: clinical and mammographic tumor estimation, method of breast conserving surgery, type of skin incision, number of interstitial applicators, irradiated reference volume (PTV) and type of optimization method. No correlations with cosmetics effect were found in factors such as: age of patients, location of tumor or additional therapy.
Keywords: breast cancer, BCS, HDR, cosmetic effect
Purpose
Breast cancer is one of the most frequent women malignant neoplasm in Poland. According to Tumor National Registry in 2006 there were 13322 new cases recorded as well as 5212 deaths resulted from a breast cancer. Systematic growth of mortality caused by malignant breast cancer is observed, especially in a group of patients between 60-65 years [1, 2]. Recently, the percentage of early diagnosed breast cancer patients is rising which permits radical treatment without organ removal. The amount of cured patients with good cosmetic effect is growing [2–23]. Complex psychological problems related to danger of possible loss of life due to cancer, fear of aggressive treatment and permanent disability as a result of breast amputation are the subject of numerous analyzes [24–27]. The introduction of BCS in early stage of cancer to clinical practice is compared to results acquired from more radical treatments. The better “picture of own self” and patients sexual functioning after BCS was exposed in a series of investigations and – in spite of early apprehensions – lesser concern of illness relapse, comparing with patients after mastectomy. In Fallowfields, Lasry’s, Schaines and Weitzner’s papers, breast conserving therapy with breast preservation benefits in better picture of “own self”, patients well-being and significant increase of control over own life [24, 26–28]. The consequence of combine treatment dissemination was limitation of various surgical treatments and introducing to clinical practice bcs with tumor, healthy tissues margin and axillary lymph nodes removal (separate incision) [2–11, 13, 17, 19–21, 28–36].
For decades, the value of post-surgery irradiation was the subject of numerous clinical researches. With the progression of new techniques and precision of irradiation, the percentage of its complication is significantly decreasing and – at the same time – the possibility of total experience with prevention of locoregional recurrences is rising [6–9, 13, 29, 37–45]. Additional irradiation of the site of tumor removal (boost) with the use of external beam radiation is applied in the majority of oncologic centers. The purposefulness of boost in cases of negative surgery margin was discussed. Authors compatibility for the necessity of local dose increasing, concerns the cases with doubtful surgical margin, especially with present intraductal component or high degree of histopathologic malignancy [45–48].
Brachytherapy was used as a method of the local dose increase (boost) in combine conservative treatment, as an independent way of treatment and as an emergency proceedings in treatment of local recurrences after initial tumor removal, followed by supplemented radiotherapy [37, 49]. Interstitial brachytherapy allows high dose ionizing irradiation within the tissue area, which results in limitation of post radiation injuries. The turning point in extensive application of interstitial irradiation brought dissemination of the after-loading technique and High Dose Rate (HDR above 12 Gy/hour). In Poland, breast conserving therapy (BCT) was introduced to clinical practice in 1981 in Oncologic Surgery Clinic in Lodz. The work of Prof. Jan Berner and his associates encouraged specialists from other oncologic centres in the country to employ BCT on a larger scale [10, 12, 40, 50, 51]. Since 1985, BCT was used in Warsaw Oncologic Centre. Qualification and treatment protocol was elaborated on the base of results of scientific researches of Dongen, Fisher and Veronesi [2, 9, 21, 31]. Since March 1996 the Brachytherapy Department of Cancer Centre – Institute in Warsaw provides the procedure of local increase of ionizing irradiation (boost) on tumorectomy site with the use of interstitial HDR-BT.
Aim
The estimation of cosmetic effect in 93 patients with early breast cancer treated with breast conserving therapy (BCT), followed by combined radiotherapy including HDR-BT as a boost.
Material
Since the first of March 1996 till 31th December 1999 in Brachytherapy Department of Cancer Centre – Institute in Warsaw, 98 patients with locally non-advanced breast cancer received HDR-BT in order to increase iodizing irradiation dose (according to conserving therapy procedures). During the time of observation, within the primary group of 98 patients, 1 death of non-oncologic cause occurred and 1 case of dissemination of cancer with local recurrence was noted in 26th month from the end of the treatment, which makes 98% of entire surviving and 99% of local control. One patient was not involved in the observation, two patients did not participate in control tests in Oncology Centre. The cosmetic outcome was established for 93 patients and this group was analyzed only. The age of patients ranged 30-74 years with the average age of 50.8. Mean dimension of clinical and mammographic tumor estimation was 13.1 mm and 14.3 mm, respectively. Morphologic composition of surgery material is shown in Table 1.
Table 1.
Histopathology
| N | % | |
|---|---|---|
| Carcinoma ductale invasivum | 57 | 59 |
| Carcinoma lobulare ¡nvasivum | 12 | 12 |
| Others | 29 | 29 |
| Node negative | 74 | 76 |
| Node positive | 24 | 24 |
Methods of treatment
Surgery
BCT consisted of tumorectomy (excision of tumor with 1-2 cm margin) – 92 patients (94%) and quadrantectomy – 6 patients (6%). In analyzed material, two types of surgical incision – arched and radial were applied. Arched skin incision corresponds to so called Langer lines, located concentrically around the nipple areola. In lower parts of breast radial skin incision was perform because of better cosmetics effect. After obtaining histopathology diagnosis, in 15 cases (15%) the procedure was extended to another surgery (quadrantecomy).
Radiotherapy
External Beam Radiation Therapy (EBRT)
Irradiation was performed using Co-60 unit or linear accelerator (4-6 MV). Whole breast was irradiated with 50 Gy of the total dose with the use of isocentric technique with two opposite tangential fields. Reference point was located in the centre of both beam axis. Two fractionation schemas were used: 1 – whole breast irradiation, with pN0 and in case of less or equal 3 of lymphatic node metastases found in histopathology diagnosis; 2 – irradiation of whole breast with locoregional lymph node system (in case of more than 3 lymph nodes metastases found). Two fractionation schemas were used: conventional (fraction dose of 2 Gy) and hyperfractionation (fraction dose of 2.5 Gy).
Brachytherapy
Patients with healthy tissue margin and 1 cm skin distance in microscopic analysis were qualified for treatment of locoregional dose increase (boost) with interstitial HDR-BT. The location of postsurgery site of the organ was an additional condition in technical installation of applicators. Steel interstitial applicators with 2.6 mm diameter (steel needles trocar tip) were placed in postsurgery tumor bed with a position defined on the base of primary lesion location in microscopic analysis of presurgery mammographic examination and introductory clinical inquiry. Applicators were placed in an arrangement of nonflexible plastic templates at 1 cm distance. It allowed to obtain homogeneous irradiation dose in appropriate breast tissues volume. The treatment plan was prepared on the base of Planning System Abacus 1.6. In the first period following the introduction of this method to clinical practice, reference points of dose calculation were defined at 1 cm distance from applicators surface with isodose optimization layout automatically transformed from a computer system. Such way of dose calculation was applied in 21 patients (21.4%). After the initial evaluation of local effects and early postradiation reaction, the location of reference points was changed to 0.5 cm from applicators surface, with number of applicators increased in order to maintain the same tissue margin (1-2 cm) around tumor removed site. The introduction of additional manual way of optimization permitted the elimination of dose increased areas, resulting from automatic standing time elongation in border location of an implant. Manual way of dose optimization was used in 77 patients (79%) with 10 Gy dose application. Irradiation was performed with the use of HDR Gammamed 12i (Isotopen-Technik Dr Sauerwein GMBH, Germany) with Ir-192 source and 10 Ci nominal activity. Radiotherapy data is presented in Table 2.
Table 2.
Radiotherapy description
| Factor | Treated patients | Analyzed patients |
|---|---|---|
| Number of patients | 98 | 93 |
| EBRT (conventional) | 74 (75.5%) | 71 (76%) |
| EBRT (hyperfractionation) | 24 (24.5%) | 22 (24%) |
| BT (manual optimalization) | 77 (78.6%) | 74 (79.5%) |
| BT (automatic optimalization) | 21 (21.4%) | 19 (20.5%) |
| Maximal number of applicators | 17 | 17 |
| Median number of applicators | 9 | 9 |
| Minimal number of applicators | 3 | 3 |
| Maximal reference volume (ml) | 56 | 56 |
| Median reference volume (ml) | 32.3 | 30 |
| Minimal reference volume (ml) | 10 | 10 |
EBRT – external beam radiation therapy, BT – brachytherapy
Systemic treatment
After final histopathology diagnosis it was decided whether to include supplementary systemic treatment. In case of presence of metastases in axillary lymph nodes, radiotherapy was additionally combined with chemo-therapy (CHTCH). 31 patients received CHTCH (31.6% of all patients).
Methods
Retrospective analysis was carried out in a group of 93 women with breast cancer treated by BCT followed by HDR-BT boost in Brachytherapy Department, Cancer Centre – Institute, Warsaw. The estimation of cosmetic outcome was evaluated on the base of modified EORTC scale which criteria are presented in Table 3. During a control visit, patients themselves completed a subjective evaluation using the same scale. Evaluation was done by surgeon and radiotherapist after 36 months from the end of brachytherapy. Median time of observation was 55.4 months (ranged from 36 to 80).
Table 3.
Estimation of cosmetic effect – criteria, modified EORTC score, used in Brachytherapy Department, Cancer Centre – Institute, Warsaw
| Excellent and very good cosmetic effect (9-10 points) | No differences between both breasts |
| Good cosmetic effect (7-8 points) | Small grade breast asymmetry, depigmentation in applicators insertion places, little retraction of scar |
| Sufficient cosmetic effect (5-6 points) | Substantial breast asymmetry, scar deformation, intensive teleangiectasis in applicators insertion places |
| Bad cosmetic effect (< 4 points) | Large breast asymmetry – deformation of treated breast, skin necrosis |
Correlation between cosmetics effect and chosen clinical and therapeutic parameters was investigated. Analyzed group of patients was divided into two subgroups: first group with excellent and very good cosmetic effect (10-7 points, what correspondents to the first and second group of EORTC scale) and second group with sufficient and bad cosmetic result (6 points and below) (Table 3).
Statistical analysis
For analysis of the results, statistical methods were used such as:
Analysis of grade correlation (Spearman Test) in order to investigate the dependence of parameters such as: age, number of interstitial applicators used in brachytherapy, reference volume of irradiated tissues (boost), clinical and mammographic tumor estimation. Specific coefficients were established such as: correlation aspect (R – if the value of this coefficient is close to zero – no statistical relevance of the tested pair of features) and p value with statistical relevance of the dependence of analyzing features.
Analysis of linear regression to describe and graphically presenting the dependence tendency of cosmetic effect including factors such as: age, amount of applicators, reference volume and clinical and mammographic tumor estimation.
Analysis of contingency in order to define the correlation of pairs qualitative traits such as: cosmetic outcome and type of treatment, method of dose optimization and application of chemotherapy. χ2 independence test was used. The force of this correlation was define with the use of Pearson (CC) contingency coefficient. The stronger dependency, the more the value of indicator is closer to a unit. Results of hypothesis testing about the independence of a pair of traits is reflected in so called p value. The p value ≤ 0.05 stands for statistically significant correlation. Values > 0.05 indicate no statis-tical relevance.
Rule induction system ProbRough is used to analyze the possibilities of cosmetic outcome prediction after 36 months on the base of analyzing clinical and therapeutic factors – description of directorial rules [55, 63].
Calculations was completed with the use of Med-Calc, 4.16g version, licensed for HollyCross Cancer Centre in Kielce, Poland.
Results
Overall, the immediate tolerance of brachytherapy was good. The plan of treatment was executed in all 98 patients. No early complications like hematoma or skin damage were observed. Within the first year of the end of treatment the evaluation of cosmetic outcome for all treated patients was performed. During this period of time, no significant influence of interstitial HDR-BT on deterioration of local effects was noted. The final estimation of cosmetic results was conducted after 36 months of the end of the treatment. Besides doctors evaluation, patients were ask for an opinion about cosmetic outcome using criteria of evaluation and 10 point scale. Results are presented in Tables 4–10. In the opinion of medical team, at least good effect was achieved in 79 patients, which stands for 85% of cases in analyzed group. In the opinion of patients the percentage was 82% (76 cases). Correlation between achieved cosmetic outcomes and analyzed clinical and therapeutic parameters presented in Tables 4 and 5 are related to the opinion of medical team and patients.
Table 4.
Estimation of cosmetic effect – done by physicians
| Cosmetic effect | Number of patients | % |
|---|---|---|
| 10 points | 6 | 6.5 |
| 9 points | 32 | 34.4 |
| 8 points | 33 | 35.5 |
| 7 points | 8 | 8.6 |
| 6 points | 9 | 9.7 |
| 5 points | 2 | 2.2 |
| 4 points | 3 | 3.2 |
| Excellent and very good cosmetic effect (9-10 points) | 38 | 40.9 |
| Good cosmetic effect (7-8 points) | 41 | 44.1 |
| Sufficient cosmetic effect (5-6 points) | 11 | 11.8 |
| Bad cosmetic effect (≤ 4 points) | 3 | 3.2 |
Table 10.
Estimation of cosmetic effect – correlation with chemotherapy in combined treatment
| Chemotherapy | 10 points score | Total | |
|---|---|---|---|
| 1-6 pts. | 7-10 pts. | ||
| (-) | 8 (12%) | 57 (88%) | 65 |
| (+) | 6 (21%) | 22 (79%) | 28 |
| Total | 14 | 79 | 93 |
Table 5.
Estimation of cosmetic effect – done by patients
| Cosmetic effect | Number of patients | % |
|---|---|---|
| 10 points | 32 | 34.4 |
| 9 points | 18 | 19.3 |
| 8 points | 21 | 22.6 |
| 7 points | 5 | 5.4 |
| 6 points | 10 | 10.8 |
| 5 points | 7 | 7.5 |
| Excellent and very good cosmetic effect (9-10 points) | 50 | 53.8 |
| Good cosmetic effect (7-8 points) | 26 | 28 |
| Sufficient cosmetic effect (5-6 points) | 17 | 18.2 |
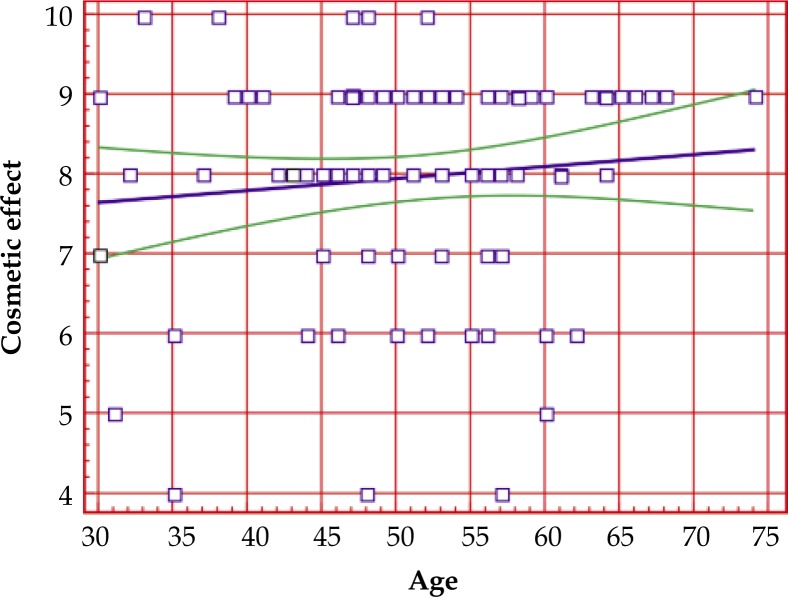
Correlation between cosmetic effect and the age of patients
The correlation between cosmetic effect and the age of patients was analyzed with the use of Spearman Test, and in case of graphic interpretation – analysis of linear regression (Fig. 1). No statistically significant correlation was found between cosmetic result and the age of a patients. The correlation ratio R = 0.0973, coefficient p-value: p = 0.3536.
Fig. 1.
Correlation between cosmetic effect and age
Correlation between cosmetic result and clinical dimension of cancerous lesion
There was statistically significant correlation between cosmetic outcome and clinical dimension of cancerous lesion, confirmed in pretreatment clinical examination. Correlation coefficient: R = –0.2221, p = 0.0324. The dependency show inversely proportional tendency: the larger tumor clinical dimension, the worse cosmetic result estimated according to modified EORTC scale.
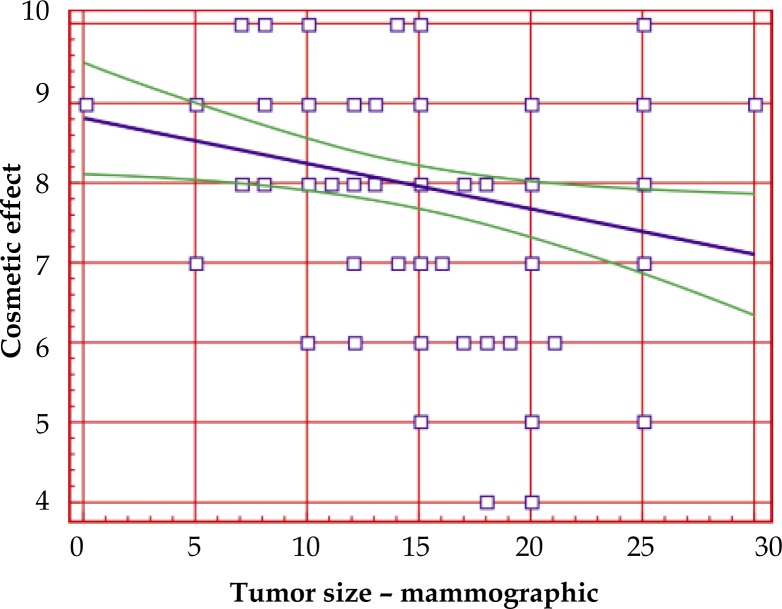
Correlation between cosmetic effect and mammographic dimension of tumor before the treatment
There was statistically significant correlation between cosmetic outcome and mammographic dimension of tumor before treatment. Coefficient R = –0.3333, p = 0.0012. The dependency show inversely proportional tendency: the larger mammographic dimension of tumor, the poorer cosmetic outcome. Graphic presentation of this dependency is presented in Fig. 2. On the x-axis the clinical dimension is presented in millimeters, on y-axis – cosmetic outcome in 10-point scale.
Fig. 2.
Correlation between cosmetic effect and tumor size – mammographic
Correlation between cosmetic effect and original location of the lesion (central localization in internal and external quadrant)
The configuration of evaluation of cosmetic effect in correlation of original location of the lesion is presented in Table 6. Patients were divided into two groups: 10-7 points (excellent, very good and good cosmetic outcome) and second group – 6 and below 6 points (satisfactory or bad cosmetic result). No statistically significant (p = 0.5746) influence of location of the tumor on cosmetic result was noted.
Table 6.
Estimation of cosmetic effect – tumor location
| Location | 10 points score | Total | |
|---|---|---|---|
| 1-6 pts. | 7-10 pts. | ||
| Central location | 1 (25%) | 3 (75%) | 4 |
| Internal quadrants location | 4 (21%) | 15 (79%) | 19 |
| External quadrants location | 9 (13%) | 61 (87%) | 70 |
| Total | 14 (15%) | 79 (85%) | 93 |
Correlation between cosmetic effect and the type of surgical incision in the primary stage of conserving treatment
There was significant correlation between the type of surgical incision and cosmetic outcome. Paerson CC coefficient of contingency was determined with the assistance of contingency analysis used in correlation research within pairs of quality traits. In this case CC = –0.3087 and p = 0.0029. For arched incision the cosmetic result was excellent, very good or good (10-7 points) in 67 cases (90% of patients). For radial incision the cosmetic outcome was excellent, very good or good in 12 patients (63%). Data of increased clinical parameter is presented in Table 7.
Table 7.
Estimation of cosmetic effect – type of skin cutting
| Type of skin cutting | 10 points score | Total | |
|---|---|---|---|
| 1-6 pts. | 7-10 pts. | ||
| Arched | 7 (10%) 50% | 67 (90%) 85% | 74 |
| Radial | 7 (37%) 50% | 12 (63%) 15% | 19 |
| Total | 14 | 79 | 93 |
Correlation between cosmetic effect and the type of surgery
There was significant (CC = 0.4329, p < 0.0001) correlation between cosmetic result and type of surgery. In case of quadrantectomy the cosmetic outcome was satisfactory or bad in 80% of patients (1-6 points), whereas in case of tumorectomy the cosmetic result was excellent, very good or good even in 89% of patients (7-10 points) (Table 8).
Table 8.
Estimation of cosmetic effect – method of breast conserving surgery
| Method | 10 points score | Total | |
|---|---|---|---|
| 1-6 pts. | 7-10 pts. | ||
| Quadrantectomy | 4 (80%) 29% | 1 (20%) 1% | 5 |
| Tumorectomy | 10 (11%) 71% | 78 (89%) 99% | 88 |
| Total | 14 | 79 | 93 |
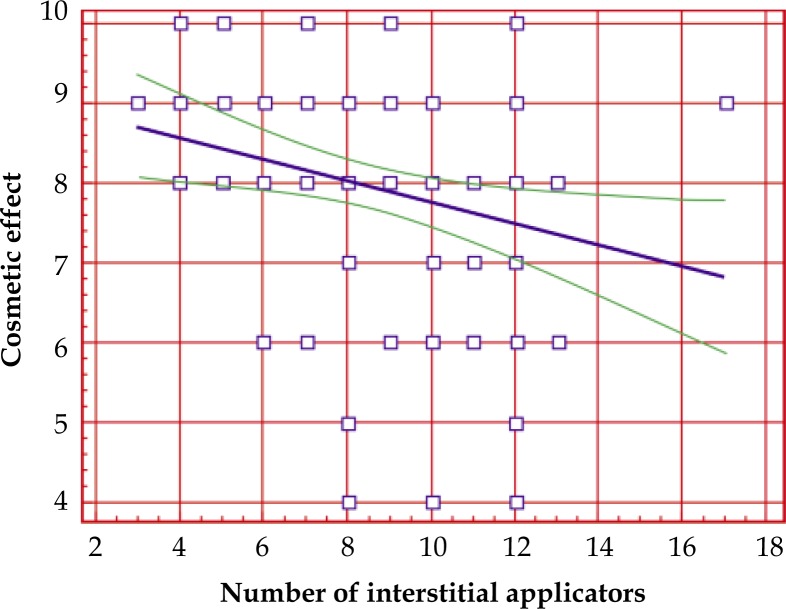
Correlation between cosmetic effect and the number of interstitial applicators
There was statistically significant (R = –0.2727, p = 0.0082) correlation between cosmetic result and the number of interstitial applicators. The bigger number of applicators the worse cosmetic outcome. Graphic interpretation of this correlation is presented in linear regression analysis (Fig. 3).
Fig. 3.
Correlation between cosmetic effect and number of interstitial applicators
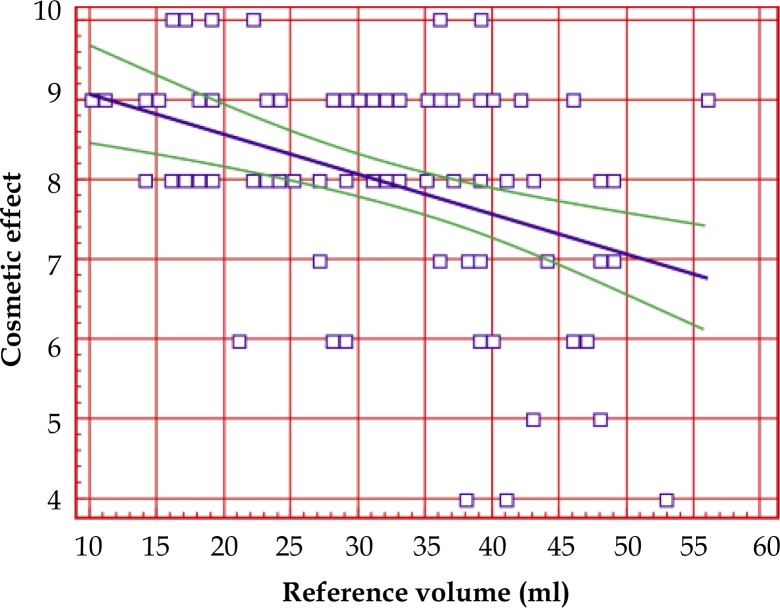
Correlation between cosmetic effect and the reference volume (cm3) of breast tissues during procedure of local increase of dose radiation
There was significant correlation (correlation coefficient R = –0.3726, p = 0.0002) between cosmetic result and the reference volume (cm3). In conjunction with the growth of reference volume, the local effect was poorer (Fig. 4).
Fig. 4.
Correlation between cosmetic effect and reference volume (ml)
Correlation between cosmetic effect and the method of disintegration of dose optimization
There was significant correlation between cosmetic result and the method of disintegration of dose optimization. Coefficient CC = 0.3872, p = 0.0001. In case of automatic system of optimization, the percentage with sufficient or bad (1-6 points) cosmetic outcome and excellent, very good or good cosmetic effect is similar and comes to: 9 patients (43%) and 12 cases (57%), respectively. In case of applying manual method of dose optimization, the percentage of cases with excellent, very good or good effect is bigger and comes to 93% (67 patients). This correlation is presented in Table 9.
Table 9.
Estimation of cosmetic effect – correlation with optimization method
| Optimization method | 10 points score | Total | |
|---|---|---|---|
| 1-6 pts. | 7-10 pts. | ||
| Automatic | 9 (43%) | 12 (57%) | 21 |
| Manual | 5 (7%) | 67 (93%) | 72 |
| Total | 14 | 79 | 93 |
Correlation between cosmetic effect and chemotherapy
Chemotherapy had shown no significant influence on cosmetic outcome. In both compared group of patients (chemotherapy and no chemotherapy group), the percentage of patients with excellent, very good or good cosmetic result was 79% and 88% (CC = 0.1162, p = 0.2592), respectively. The difference is statistically not essential. Individual data of this subgroup of patients is presented in Table 10.
Formulation of regulation rules
In all above analysis, using rules induction system ProbRough, including all clinical and therapeutic factors versus cosmetic results, the strongest decision force is displayed by dimension of mammographic lesion. The conclusions are as follows: if mammographic dimension is >11 mm, the cosmetic effect with great probability would be worse (4-6 points), if mammographic dimension is < 11 mm, the cosmetic outcome with high possibilities would be excellent, very good or good (7-10 points). The above presented formulation of regulation rules system is clinically applicable as the “Knowledge Discovery in Databases”.
Discussion
Clinical researches of breast cancer patients treated with conserving therapy allowed to describe the main factors affecting the final cosmetic result, acquired after combined treatment [6, 7, 12, 14, 52–58]. The unanimity of authors include the influence of: surgical technique (in the first place in relation to the volume of tissue removed and the breast size), the degree of total and fractional dose in supplementary irradiation with the use of external beam, the process of local increase of ionizing irradiation and applying an adjuvant chemotherapy. Each consecutive stage of treatment influence the last effect of therapy. In the beginning, the estimation of cosmetic outcome after conserving therapy was based entirely on a subjective scale. However, in 1976 Harris had introduced 4-point scale of this evaluation, which became a foundation for other scientist. It was used till the end of the 80ties when EORTC Radiotherapy Cooperative Group on the basis of van Limbergen’s work accepted so called objective scale [7, 12, 14, 17, 30, 56, 57, 59–62]. Objective scale is based on measurements of breast retraction and its rotation as well as reaction and late postradiation skin lesion. In Polish literature this kind of evaluation was used by A. Niwińska et al. [14].
In this work the subjective 10-point scale was used in order to separate subgroups of patients where the final cosmetic outcome was influenced by interstitial HDR-BT. Similarly for other authors analyzing the influence of interstitial brachytherapy on the final cosmetic result in conserving therapy, the local effect to decrease the evaluation of the final outcome are: thickening or deformity of postoperative scar or glandular tissue located directly under the scar (within the reference volume during brachytherapy), scar retraction below the skin level, skin depigmentation in places of applicators inserting, different level of teleangiectasia intensification [14, 49, 57, 58, 63–69]. The analyzing group is homogeneous with regard to surgical procedures and EBRT parameters used in treatment, which allowed to estimate the influence of one stage of combine treatment – interstitial brachytherapy to obtain the final cosmetic result.
Clinical investigations of Bentzen et al., van Limbergen, as well as the summary report of EORTC 10.882/22.881, demonstrated directly proportional impact of quantity of absorbed dose and the volume of irradiated tissues resulting in appearance of postradiation fibrosis [49, 50, 55–57, 60, 66, 70, 71]. The initial value of tumor is described by the volume of tissues removed during tumorectomy and reference volume during local increase of ionizing irradiation (boost). Both elements influenced the final cosmetic outcome. The above observations confirmed the choice of surgery procedures – quadrantectomy versus tumorectomy resulting from primary size of tumor. With regard to considerably worse cosmetic results subsequent to expanded treatment (eg. quadrantectomy), there is a tendency to significantly restricted recommendation for this type of treatments in conserving therapy [6, 17, 30, 33, 34]. The dose of ionizing irradiation on the skin surface is the next significant parameter for early and late postradiation reaction, ipso facto cosmetic result. The occurrence of teleangiectasia – small pathologic blood vessels within the area of discolored skin – results from direct clinical outcome of high dose irradiation [16, 50, 55, 61]. Such changes were observed in most of the cases in analyzed material and estimated as good and below good outcomes. It was particularly related to locations of inserting interstitial slides through the skin, however it was not observed within the area of postsurgery scars, neither in opposite areas of applicators insertion. This conforms accuracy to restrict indications of local dose decrease with HDR brachytherapy of lesions localized deeper under the skin (minimum 1 cm margin from upper cut range in postsurgery histopathology investigation).
With regard to therapeutic target and in order to assure homogeneity of a dose, minimum biplane implantations are recommended with additional parameter optimization of source standing time in certain position of active section, which visibly improves dose homogeneity within the target. No complication were found within analyzed group of patients such as postradiation pneumonia or myocardial injury which are observed in some percentage of cases after radiotherapy with EBRT [2, 37, 68, 72–74]. Amongst aspects that are considered to influence the final local effect, age as a factor is not investigated by all authors. In presented material, age is not a factor to significantly influence cosmetic outcome.
In investigated material, statistically significant correlation was found between cosmetic result and clinical and mammographic dimension of the lesion. Similar observation had been noted in a literature analyzing this type of parameters [5, 6, 12, 14, 30, 58]. Direct factor related to size of tumor is the extensiveness of surgical procedure. In case of surgery with minor tissue extension (tumorectomy) the result was better with well define statistical relevance compared to quadrantectomy. Similar results were observed in relation to the increase of irradiated tissue volume during interstitial brachytherapy (number of applicators, reference volume). This results comes in accordance with literature data [70, 75–79]. All parameters that are implicated by tumor size (clinical and mammographic dimension, extent of surgical procedure, volume of irradiated tissues) influence the final cosmetic result in inversely proportional way. In analyzed group of patients statistically significant influence of dose degradation was observed on the final local outcome. The analysis of reasons for overdosage occurrences in site of inserted applicators, demonstrated the dose calculation in reference points localized at a distance of 1 cm from final point of active section of source travel way and recognized the distance as too big. In presented material, the automatic way of dose calculation was applied within one group of patients, however in this group the cosmetic outcome was poorer. The principle parameter that deteriorate cosmetic result is overdosage in breast skin area which leads to skin thickening, intensification of skin decolouration and escalation of teleangiectasia. The tolerance dose of skin blood vessels is relatively low. This problem does not depend directly on the technique of local dose increase (boost), but on the degree of the dose [57, 60, 61, 66, 80, 81]. In case of interstitial HDR-BT this issue concern only sites of interstitial applicators.
In group with automatic dose optimization in order to restrict the number of interstitial applicators, with entire target defined, dose calculation reference points were at 1 cm distance from their surface. As a result, the source standing time in consecutive points of extreme applicators experienced significant elongation where iodize superposition of surrounding slides did not occur. Doses acquired in final points of active section were particularly essential, since they were responsible for the overdosage in sites of applicators inserted through the skin surface, resulting in late postradiation symptoms (increased teleangiectasia within the area). This indication was the most essential factor of cosmetic outcome aggravation and directly associated with brachytherapy. Another issue was the appearance of areas with significant exceed of dose above prescribed amount in relation to the entire therapeutic target. It resulted as a reference points distance from the surface of interstitial applicators with dose heterogeneity in target. Within this group of patients, thickening and postsurgery scar being pulled in was clinically observed.
In case of patients with manual optimization, two main elements were changed in treatment planning: the distance of reference points from 1 to 0.5 cm from slides surface and the final way of dose optimization. Introductory automatic optimization was corrected with source standing time parameter in consecutive points of travel way for elimination of overdosage area. First of all, the dose degree close to the extreme slides in final points of active section was corrected, which lowered the dose degree in slides insertion points on the skin surface.
The localization of breast tumor frequently decides about the choice of the type of surgery incision. The radial incision was applied in tumor lower parts because this type of cutting in this area permits better cosmetic results. Since the tumor localization did not show significant influence on final result, the radial incision was the parameter that statistically deteriorated cosmetic effect.
Conclusions
HDR brachytherapy is an important element in conserving therapy of non advanced breast cancer. Local increase of ionizing dose radiation allows to achieve at least good level of cosmetic outcome in 85% of patients.
There are important clinical factors that influence cosmetic results in this way of treatment such as: tumor dimension evaluated in physical or mammographic examination (the lower value of this parameter, the better cosmetic outcome) or therapeutic investigation: the type of surgical procedure (with better tumorectomy than quadrotectomy results), the type of surgical incision (arched cutting with better results compared to radial incision), the number of interstitial slides and reference volume of irradiated tissues (the lower the value of parameters, the better final result), the method of dose optimization in brachytherapy (better manual method compared to automatic technique).
In the above way of treatment, the most important factors to determine the cosmetic outcome are: type of surgery, amount of interstitial slides and technique of dose optimization.
Factors like: age of patients, tumor localization in individual quadrants and chemotherapy demonstrate no significant influence on final cosmetic result.
The article was written using some parts of doctoral thesis of Anna Kulik, with Prof. Bogdan Gliński MD, PhD as a promotor.
References
- 1.Didkowska J, Wojciechowska U, Zatoński W. Wydawnictwo Krajowego Rejestru Nowotworów przy Centrum Onkologii – Instytutu im. Warszawa: M. Skłodowskiej-Curie; 2008. Nowotwory złośliwe w Polsce w 2006 roku. [in Polish] [Google Scholar]
- 2.Jassem J, editor. Podręcznik dla studentów i lekarzy. Warszawa: Springer PWN; 1998. Rak sutka. [in Polish] [Google Scholar]
- 3.Adair F, Berg J, Joubert L, et al. Long-term follow-up of breast cancer patients: the 30-year report. Cancer. 1974;33:1145–1150. doi: 10.1002/1097-0142(197404)33:4<1145::aid-cncr2820330438>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Arriagada R, Le MG, Rochard F. Conservative treatment versus mastectomy in early breast cancer: patterns of failure with 15 years of follow-up data. J Clin Oncol. 1996;14:1558–1564. doi: 10.1200/JCO.1996.14.5.1558. [DOI] [PubMed] [Google Scholar]
- 5.Calle R, Pilleron JP, Schlienger P, et al. Conservative management of operable breast cancer. Cancer. 1978;42:2045–2053. doi: 10.1002/1097-0142(197810)42:4<2045::aid-cncr2820420455>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Clark RM, Wilkinson RH, Mahoney LJ, et al. Breast cancer: a 21 year experience with conservative surgery and radiation. Int J Radiat Oncol Biol Phys. 1982;8:967–979. doi: 10.1016/0360-3016(82)90163-8. [DOI] [PubMed] [Google Scholar]
- 7.Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. Early Breast Cancer Trialists’ Collaborative Group. N Engl J Med. 1995;333:1444–1455. doi: 10.1056/NEJM199511303332202. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Anderson S, Redmond CK, et al. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1995;333:1456–1461. doi: 10.1056/NEJM199511303332203. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312:665–673. doi: 10.1056/NEJM198503143121101. [DOI] [PubMed] [Google Scholar]
- 10.Jeziorski A, Berner J. Wyniki leczenia oszczędzającego u chorych na raka sutka we wczesnym stopniu zaawansowania. Nowotwory. 1993;43:11–19. [in Polish] [Google Scholar]
- 11.Jeziorski A, Berner J, Wrężel B. Wyniki odległe leczenia oszczędzającego chorych na raka sutka. Pol Przegl Chir. 1997;69:469–482. [in Polish] [Google Scholar]
- 12.Jodkiewicz Z, Malinowski Z, Skowrońska-Gardas A. Ocena wczesnych wyników leczenia i efektu kosmetycznego u chorych napromienianych po oszczędzającym zabiegu chirurgicznym z powodu raka piersi. Nowotwory. 2000;50:141–147. [in Polish] [Google Scholar]
- 13.Korzeniowski S. Leczenie chorych na niezaawansowanego raka sutka z oszczędzeniem piersi. Analiza wyników badań randomizowanych. Nowotwory. 1996;46:459–473. [in Polish] [Google Scholar]
- 14.Niwińska A, Nagadowska M, Tchórzewska H. Ocena efektu kosmetycznego u chorych na raka piersi po leczeniu oszczędzającym. Nowotwory. 1998;48:835–846. [in Polish] [Google Scholar]
- 15.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 16.Pierce SM, Recht A, Lingos TI, et al. Long-term radiation complications following conservative surgery (CS) and radiation therapy (RT) in patients with early stage breast cancer. Int J Radiat Oncol Biol Phys. 1992;23:915–923. doi: 10.1016/0360-3016(92)90895-o. [DOI] [PubMed] [Google Scholar]
- 17.Recht A. Selection of patients with early stage invasive breast cancer for treatment with conservative surgery and radiation therapy. Semin Oncol. 1996;23:19–30. [PubMed] [Google Scholar]
- 18.Recht A, Come SE, Gelman RS. Integration of conservative surgery, radiotherapy and chemotherapy for the treatment of early-stage, node-positive breast cancer: sequencing, timing and outcome. J Clin Oncol. 1991;9:1662–1667. doi: 10.1200/JCO.1991.9.9.1662. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro GG, Magee B, Swindell R, et al. The Christie Hospital breast conservation trial: an update at 8 years from inception. Clin Oncol. 1993;5:278–283. doi: 10.1016/s0936-6555(05)80900-8. [DOI] [PubMed] [Google Scholar]
- 20.Veronesi U, Banfi A, Salvadori B, et al. Breast conservation is the treatment of choice in small breast cancer: long-term results of a randomized trial. Eur J Cancer. 1990;26:668–670. doi: 10.1016/0277-5379(90)90113-8. [DOI] [PubMed] [Google Scholar]
- 21.Veronesi U, Salvadori B, Luini A, et al. Breast conservation is a safe method in patients with small cancer of the breast. Long-term results of three randomised trials on 1,973 patients. Eur J Cancer. 1995;31A:1574–1579. doi: 10.1016/0959-8049(95)00271-j. [DOI] [PubMed] [Google Scholar]
- 22.Vinh-Hung V, Burzykowski T, Van de Steene J, et al. Post-surgery radiation in early breast cancer: survival analysis of registry data. Radiother Oncol. 2002;64:281–290. doi: 10.1016/s0167-8140(02)00105-6. [DOI] [PubMed] [Google Scholar]
- 23.Wise L, Mason AY, Ackerman LV. Local excision and irradiation: an alternative method for the treatment of early mammary cancer. Ann Surg. 1971;174:392–401. doi: 10.1097/00000658-197109000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fallowfield LJ. Psychosocial adjustment after treatment for early breast cancer. Oncology. 1990;4:89–96. [PubMed] [Google Scholar]
- 25.Mika K, Tchórzewska H. Wpływ leczenia i rehabilitacji na jakość życia kobiet chorych na raka piersi. In: Meyza J, editor. Jakość życia w chorobie nowotworowej – wybrane zagadnienia. Warszawie: Centrum Onkologii – Instytut im. M. Skłodowskiej-Curie w; 1997. pp. 261–268. [in Polish] [Google Scholar]
- 26.Schain WS, d’Angelo TM, Lichter AS, et al. Mastectomy versus conservative surgery and radiation therapy. Psychosocial consequences. Cancer. 1994;73:1221–1228. doi: 10.1002/1097-0142(19940215)73:4<1221::aid-cncr2820730416>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 27.Weitzner MA. Funkcjonowanie psychospołeczne i jakość życia chorych na nowotwory gruczołu piersiowego. In: Meyza J, editor. Jakość życia w chorobie nowotworowej – wybrane zagadnienia. Warszawie: Centrum Onkologii – Instytut im. M. Skłodowskiej-Curie w; 1997. pp. 225–247. [in Polish] [Google Scholar]
- 28.Lasry JC, Morgolese RG. Fear of recurrence, breast-conserving surgery and trade-off hypothesis. Cancer. 1992;69:2111–2115. doi: 10.1002/1097-0142(19920415)69:8<2111::aid-cncr2820690817>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 29.Calle R, Vilcoq JR, Zafrani M, et al. Local control and survival of breast cancer treated by limited surgery followed by irradiation. Int J Radiat Oncol Biol Phys. 1986;12:873–878. doi: 10.1016/0360-3016(86)90379-2. [DOI] [PubMed] [Google Scholar]
- 30.Christiaens MR, Cataliotti L, Fentiman I, et al. Comparison of the surgical procedures for breast conserving treatment of early breast cancer in seven EORTC centres. Eur J Cancer. 1996;32A:1866–1875. doi: 10.1016/0959-8049(96)00206-7. [DOI] [PubMed] [Google Scholar]
- 31.Van Dongen JA, Bartelink H, Fentiman IS, et al. Factors influencing local relapse and survival and results of salvage treatment after breast-conserving therapy in operable breast cancer: EORTC trial 10801, breast conservation compared with mastectomy in TNM stage I and II breast cancer. Eur J Cancer. 1992;28A:801–805. doi: 10.1016/0959-8049(92)90118-l. [DOI] [PubMed] [Google Scholar]
- 32.Graves TA, Bland KI. Surgery for early and minimally invasive breast cancer. Curr Opin Oncol. 1996;8:468–477. doi: 10.1097/00001622-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Hayward J. Conservative surgery in the treatment of early breast cancer. Br J Surg. 1974;61:770–771. doi: 10.1002/bjs.1800611006. [DOI] [PubMed] [Google Scholar]
- 34.Kilkenny JW, III, Bland KI. Surgery of breast cancer. Curr Opin Oncol. 1997;9:520–526. doi: 10.1097/00001622-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Korzeniowski S. Uzupełniające leczenie systemowe chorych na niezaawansowanego raka piersi. Wyniki metaanaliz Early Breast Cancer Trialists’ Collaborative Group. Nowotwory. 1999;49:637–647. [in Polish] [Google Scholar]
- 36.de la Rochefordière A, Abner AL, Silver B, et al. Are cosmetic results following conservative surgery and radiation therapy for early breast cancer dependent on technique? Int J Radiat Oncol Biol Phys. 1992;23:925–931. doi: 10.1016/0360-3016(92)90896-p. [DOI] [PubMed] [Google Scholar]
- 37.Gage I, Harris JR. Radiation therapy and breast cancer. Curr Opin Oncol. 1997;7:527–531. doi: 10.1097/00001622-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 38.ICRU (International Commision on Radiation United and Measurments) – Prescribing, recording and reporting photon beam therapy. Bethesda: 1993. ICRU Report 50. [Google Scholar]
- 39.Korzeniowski S. Wartość pooperacyjnej radioterapii w skojarzonym leczeniu chorych na operacyjnego raka piersi. Nowotwory. 1997;47:33–58. [in Polish] [Google Scholar]
- 40.Kułakowski A, Towpik E, editors. Wydawnictwo Polskiej Fundacji Europejskiej Szkoły Onkologii. Warszawa: 1997. Zasady rozpoznawania i leczenia nowotworów zalecane przez Centrum Onkologii w Warszawie. [in Polish] [Google Scholar]
- 41.Peters MV. Wedge resection with or without radiation in early breast cancer. Int J Radiat Oncol Biol Phys. 1977;2:1151–1156. doi: 10.1016/0360-3016(77)90124-9. [DOI] [PubMed] [Google Scholar]
- 42.Pierquin B, Owen R, Maylin C, et al. Radical radiation therapy of breast cancer. Int J Radiat Oncol Biol Phys. 1980;6:17–24. doi: 10.1016/0360-3016(80)90197-2. [DOI] [PubMed] [Google Scholar]
- 43.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–962. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- 44.Sledge GW., Jr Adjuvant therapy for early stage breast cancer. Semin Oncol. 1996;23:51–54. [PubMed] [Google Scholar]
- 45.Wong JS, Recht A, Beard CJ, et al. Treatment outcome after tangential radiation therapy without axillary dissection in patients with early-stage breast cancer and clinically negative axillary nodes. Int J Radiat Oncol Biol Phys. 1997;39:915–920. doi: 10.1016/s0360-3016(97)00456-2. [DOI] [PubMed] [Google Scholar]
- 46.Fijuth J, Nagadowska M. Czy należy dopromienić lożę po tumorectomii u chorych na raka sutka we wczesnych stopniach zaawansowania leczonych oszczędzająco? Nowotwory. 1996;46:553–559. [in Polish] [Google Scholar]
- 47.Pezner RD, Lipsett JA, Desai K, et al. To boost or not to boost: decreasing radiation therapy in conservative breast cancer treatment when “inked” tumor resection margins are pathologically free of cancer. Int J Radiat Oncol Biol Phys. 1988;14:873–877. doi: 10.1016/0360-3016(88)90008-9. [DOI] [PubMed] [Google Scholar]
- 48.Touboul E, Belkacemi Y, Lefranc J-P, et al. Early breast cancer: influence of type of boost (electrons vs iridium-192 implant) on local control and cosmesis after conservative surgery and radiation therapy. Radiother Oncol. 1995;34:105–113. doi: 10.1016/0167-8140(95)01508-e. [DOI] [PubMed] [Google Scholar]
- 49.Pieters BR, Maher M, Gerbaulet AL. Brachytherapy in the management of breast cancer: a review. Cancer Treat Rev. 1995;21:527–539. doi: 10.1016/0305-7372(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 50.Makarewicz R, Kabacińska R. Materiały Regionalnego Centrum Onkologii w Bydgoszczy. Bydgoszcz: 1995. Miejsce i rola brachyterapii w leczeniu raka sutka. IV Jesienna Szkoła Fizyki Medyczne. [in Polish] [Google Scholar]
- 51.Murawa P. W trosce o poprawę standardów w leczeniu raka piersi. Nowotwory. 2000;50:181–185. [in Polish] [Google Scholar]
- 52.Baillet F. Brachytherapy in breast cancer; Sixth International Congress on Anti-Cancer Treatment; 6-9.02.1996; Paris, France. abstract. [Google Scholar]
- 53.Fowble B, Solin LJ, Martz KL, et al. The influence of the type of boost (electrons vs. implant) on local control and cosmesis in patients with stages I and II breast cancer undergoing conservative surgery and radiation. Int J Radiat Oncol Biol Phys. 1986;12(Suppl 1):150. [Google Scholar]
- 54.Gray JR, McCormick B, Cox L, et al. Primary breast irradiation in large-breasted or heavy women: analysis of cosmetic outcome. Int J Radiat Oncol Biol Phys. 1991;21:347–354. doi: 10.1016/0360-3016(91)90781-x. [DOI] [PubMed] [Google Scholar]
- 55.Habibollahi F, Phil M, Mayles HMO, et al. Assessment of skin dose and its relation to cosmesis in the conservative treatment of early breast cancer. Int J Radiat Oncol Biol Phys. 1988;14:291–296. doi: 10.1016/0360-3016(88)90435-x. [DOI] [PubMed] [Google Scholar]
- 56.Harris JR, Levene MB, Svensson G, et al. Analysis of cosmetic results following primary radiation therapy for stages I and II carcinoma of the breast. Int J Radiat Oncol Biol Phys. 1979;5:257–261. doi: 10.1016/0360-3016(79)90729-6. [DOI] [PubMed] [Google Scholar]
- 57.Van Limbergen E. Modern Brachytherapy Techniques. Bratislava: 2001. Radiation dose response and volume relationships for cosmetic damage in breast conservative treatment. Ballistic considerations on the boost technique; pp. 289–311. [Google Scholar]
- 58.Resch A, Potter R, van Limbergen, et al. Long-term results (10 years) of intensive breast conserving therapy including a high-dose and large-volume interstitial brachytherapy boost (LDR/HDR) for T1/T2 breast cancer. Radiother Oncol. 2002;63:47–58. doi: 10.1016/s0167-8140(02)00022-1. [DOI] [PubMed] [Google Scholar]
- 59.Harris JR, Botnick L, Bloomer WD, et al. Primary radiation therapy for early breast cancer: the experience at the Joint Center for Radiation Therapy. Int J Radiat Oncol Biol Phys. 1981;7:1549–1552. doi: 10.1016/0360-3016(81)90087-0. [DOI] [PubMed] [Google Scholar]
- 60.Van Limbergen E. Modern Brachytherapy Techniques. Bratislava: 2001. Where should the booster go (if any) in breast conserving therapy. Dose – response relationship for local control; pp. 275–288. [Google Scholar]
- 61.Van Limbergen E, Briot E, Drijkoningen M. The source-skin distance measuring bridge: a method to avoid radiation teleangiectasia in the skin after interstitial therapy for breast cancer. Int J Radiat Oncol Biol Phys. 1990;18:1239–1244. doi: 10.1016/0360-3016(90)90464-u. [DOI] [PubMed] [Google Scholar]
- 62.Vrieling C, Collette L, Fourquet A, et al. The influence of the boost in breast-conserving therapy on cosmetic outcome in the EORTC “boost versus no boost” trial. EORTC Radiotherapy and Breast Cancer Cooperative Groups. European Organization for Research and Treatment of Cancer. Int J Radiat Oncol Biol Phys. 1999;45:677–685. doi: 10.1016/s0360-3016(99)00211-4. [DOI] [PubMed] [Google Scholar]
- 63.Mansfield CM. Intraoperative Ir-192 Implantation for early breast cancer. Techniques and results. Cancer. 1990;66:1–5. doi: 10.1002/1097-0142(19900701)66:1<1::aid-cncr2820660102>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 64.Mansfield CM, Komarnicky LT, Schwartz GF, et al. Perioperative implantation of Ir-192 as the boost technique for stage I and II breast cancer: results of a 10-year study of 655 patients. Radiology. 1994;192:33–36. doi: 10.1148/radiology.192.1.8208960. [DOI] [PubMed] [Google Scholar]
- 65.McCormick B, Wesson MF, Cox L, et al. Iridium-192 implants for primary breast cancer: experience with placement at the time of wide local excision. Int J Radiat Oncol Biol Phys. 1988;15:745–748. doi: 10.1016/0360-3016(88)90320-3. [DOI] [PubMed] [Google Scholar]
- 66.Nag S, Gupta N. A simple method of obtaining equivalent doses for use in HDR brachyterapy. Int J Radiat Oncol Biol Phys. 2000;46:507–513. doi: 10.1016/s0360-3016(99)00330-2. [DOI] [PubMed] [Google Scholar]
- 67.Perez CA, Taylor ME, Halverson K, et al. Brachytherapy or electron beam boost in conservation therapy of carcinoma of the breast: a nonrandomized comparison. Int J Radiat Oncol Biol Phys. 1996;34:995–1007. doi: 10.1016/0360-3016(95)02378-x. [DOI] [PubMed] [Google Scholar]
- 68.Póti Z, Nemeskéri C, Fekésházy A, et al. Partial breast irradiation with interstitial Co-60 brachytherapy results in frequent grade 3 or 4 toxicity. Evidence based on a 12 year follow-up of 70 patients. Int J Radiat Oncol Biol Phys. 2004;58:1022–1033. doi: 10.1016/j.ijrobp.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 69.Schulz U, Busch M, Bormann U. Interstitial high dose-rate brachytherapy: principle, practice and first clinical experiences with a new remote-controlled afterloading system using Ir-192. Int J Radiat Oncol Biol Phys. 1983;10:915–920. doi: 10.1016/0360-3016(84)90395-x. [DOI] [PubMed] [Google Scholar]
- 70.Major T, Polgar C, Fodor J, et al. Conformity and homogeneity of dose distributions in interstitial implants at idealized target volumes: a comparison between the Paris and dose-point optimized systems. Radioth Oncol. 2002;62:103–111. doi: 10.1016/s0167-8140(01)00447-9. [DOI] [PubMed] [Google Scholar]
- 71.Schmidt-Ullrich RK, Wazer DE, DiPetrillo T, et al. Breast conservation therapy for early stage breast carcinoma with outstanding 10-year locoregional control rates: a case for aggressive therapy to the tumor bearing quadrant. Int J Radiat Oncol Biol Phys. 1993;27:545–552. doi: 10.1016/0360-3016(93)90378-9. [DOI] [PubMed] [Google Scholar]
- 72.Gałecki J, Pieńkowski T, Grudzień-Kowalska M, et al. Powikłania po oszczędzającym leczeniu chorych na raka piersi w I i II stopniu zaawansowania. Nowotwory. 1999;49:145–152. [in Polish] [Google Scholar]
- 73.Hellman S, Harris JR, Levene MB. Radiation therapy of early carcinoma of the breast without mastectomy. Cancer. 1980;46:988–994. doi: 10.1002/1097-0142(19800815)46:4+<988::aid-cncr2820461323>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 74.Lingos TI, Recht A, Vicini F, et al. Radiation pneumonitis in breast cancer patients treated with conservative surgery and radiation therapy. Int J Radiat Oncol Biol Phys. 1991;21:355–360. doi: 10.1016/0360-3016(91)90782-y. [DOI] [PubMed] [Google Scholar]
- 75.DeBiose DA, Horwitz EM, Martinez AA, et al. The use of ultrasonography in the localization of the lumpectomy cavity for interstitial brachytherapy of the breast. Int J Radiat Oncol Biol Phys. 1997;38:755–759. doi: 10.1016/s0360-3016(97)00069-2. [DOI] [PubMed] [Google Scholar]
- 76.Pieters BR, Saarnac AE, Steggerda MJ, et al. A method to improve the dose distribution of interstitial breast implants using geometrically optimized stepping source techniques and dose normalization. Radiother Oncol. 2001;58:63–70. doi: 10.1016/s0167-8140(00)00313-3. [DOI] [PubMed] [Google Scholar]
- 77.Rathmell AJ, Ash DV. Radiotherapy after conservative surgery for breast cancer: selective use of Ir-192 wire boost to tumour bed in high risk patients. Clin Oncol. 1991;3:204–208. doi: 10.1016/s0936-6555(05)80740-x. [DOI] [PubMed] [Google Scholar]
- 78.Sedlmayer F, Rahim HB, Kogelnik HD, et al. Quality assurance in breast cancer brachytherapy: geographic miss in the interstitial boost treatment of the tumor bed. Int J Radiat Oncol Biol Phys. 1996;34:1133–1139. doi: 10.1016/0360-3016(95)02176-0. [DOI] [PubMed] [Google Scholar]
- 79.Vicini FA, Clarke D, Martinez A. Breast. In: Nag S, editor. Principles and Practice of Brachytherapy. New York: Futura Publishing Company; 1997. pp. 351–366. [Google Scholar]
- 80.Fentiman IS, Poole C, Tong D, et al. Inadequacy of iridium implant as sole radiation treatment for operable breast cancer. Eur J Cancer. 1996;32A:608–611. doi: 10.1016/0959-8049(95)00639-7. [DOI] [PubMed] [Google Scholar]
- 81.Kutcher GJ, Smith AR, Fowble BL, et al. Treatment planning for primary breast cancer: a patterns of care study. Int J Radiat Oncol Biol Phys. 1996;36:731–737. doi: 10.1016/s0360-3016(96)00368-9. [DOI] [PubMed] [Google Scholar]