This study presents new findings on the role of the intestinal biotin transporter (sodium-dependent multivitamin transporter) in the maintenance of normal intestinal mucosal integrity and health. This effect appears to be mediated, at least in part, via providing cells of the gut mucosa (like immune cells) with the essential micronutrient biotin, whose role in immune function has been reported with increased frequency in recent years.
Keywords: sodium-dependent multivitamin transporter knockout, biotin, mucosal integrity, mucosal inflammation, tight junction proteins
Abstract
Utilizing a conditional (intestinal-specific) knockout (cKO) mouse model, we have recently shown that the sodium-dependent multivitamin transporter (SMVT) (SLC5A6) is the only biotin uptake system that operates in the gut and that its deletion leads to biotin deficiency. Unexpectedly, we also observed that all SMVT-cKO mice develop chronic active inflammation, especially in the cecum. Our aim here was to examine the role of SMVT in the maintenance of intestinal mucosal integrity [permeability and expression of tight junction (TJ) proteins]. Our results showed that knocking out the mouse intestinal SMVT is associated with a significant increase in gut permeability and with changes in the level of expression of TJ proteins. To determine whether these changes are related to the state of biotin deficiency that develops in SMVT-cKO mice, we induced (by dietary means) biotin deficiency in wild-type mice and examined its effect on the above-mentioned parameters. The results showed that dietary-induced biotin deficiency leads to a similar development of chronic active inflammation in the cecum with an increase in the level of expression of proinflammatory cytokines, as well as an increase in intestinal permeability and changes in the level of expression of TJ proteins. We also examined the effect of chronic biotin deficiency on permeability and expression of TJ proteins in confluent intestinal epithelial Caco-2 monolayers but observed no changes in these parameters. These results show that the intestinal SMVT plays an important role in the maintenance of normal mucosal integrity, most likely via its role in providing biotin to different cells of the gut mucosa.
NEW & NOTEWORTHY
This study presents new findings on the role of the intestinal biotin transporter (sodium-dependent multivitamin transporter) in the maintenance of normal intestinal mucosal integrity and health. This effect appears to be mediated, at least in part, via providing cells of the gut mucosa (like immune cells) with the essential micronutrient biotin, whose role in immune function has been reported with increased frequency in recent years.
biotin (vitamin B7), a member of the water-soluble family of vitamins, is an indispensable micronutrient for normal human health because of its roles in cellular metabolism, proliferation, and survival. The vitamin acts as a cofactor for five carboxylases that are critical for fatty acid, glucose, and amino acid metabolism (reviewed in Refs. 24, 25, 38). Recent studies have uncovered important new roles for biotin in cellular energy metabolism (ATP production) and in the regulation of the cellular level of reactive oxygen species (26), in the regulation of gene expression (>2,000 human genes appear to be affected by biotin status; 36, 47, 48), and in the function of adaptive and innate immune cells (2, 3, 10, 17–20, 30). In reference to the latter, the vitamin has been shown to be important for activity, generation, maturation, and responsiveness of immune cells (2, 3, 17–19, 30), and its deficiency leads to an increase in the level of proinflammatory cytokines (19, 20). Finally, a role for biotin in the infection and invasiveness of enteropathogenic bacteria has also been recently described (54). Thus it is not surprising that deficiency/suboptimal levels of this vitamin lead to disturbances in the normal function of many systems and thus overall human health (reviewed in Refs. 25, 38, 49). Such deficiency/suboptimal levels occur in a variety of conditions, including inflammatory bowel diseases (IBD) (1, 14).
Because of their inability to synthesize biotin endogenously, humans and other mammals obtain the vitamin from exogenous sources via absorption in the gut. The gut is exposed to two sources of biotin: a dietary source (which is processed and absorbed in the small intestine) and a bacterial source [in reference to the considerable amount of biotin that is generated by gut microbiota (4, 31, 50); this biotin can be absorbed in the large intestine (39)]. Absorption of biotin in both the small and large intestine occurs via an Na+-dependent, carrier-mediated process that involves the sodium-dependent multivitamin transporter (SMVT), a system that also transports pantothenic acid and lipoate (reviewed in Refs. 38, 40, 41). Molecular identity of the SMVT system has been determined by cloning, and the carrier protein was shown to be the product of the SLC5A6 gene (11, 33, 51). Studies utilizing confocal imaging, immunological probing, and functional assays from our laboratory and others have established that the SMVT system is exclusively expressed at the apical membrane domain of polarized epithelia (42–44). The relative contribution of the SMVT system toward intestinal carrier-mediated biotin uptake has also been investigated in our laboratory utilizing an in vitro gene-specific silencing (siRNA) approach and human-derived intestinal epithelial Caco-2 cells (5) and more recently an in vivo conditional (intestine-specific) SMVT knockout (cKO) mouse model generated utilizing Cre/lox technology (15). Results of both approaches have established that intestinal absorption of biotin occurs exclusively via the function of the SMVT system. However, during the course of our investigations of the SMVT-cKO mouse model, we unexpectedly came across an interesting observation that all the SMVT-cKO animals (in addition to becoming biotin deficient) also develop a spontaneous chronic active inflammation, especially in the cecum (15). This inflammation is reminiscent of that seen in IBD (15). The latter finding suggests that the SMVT system plays a role in the maintenance of normal intestinal mucosal integrity and immunity. We are interested in addressing both of these issues, and in this study we report on the results of our investigation into the role of the SMVT system in the maintenance of intestinal mucosal integrity. Three models were used in this investigation: 1) the SMVT-cKO mouse model (15), 2) a dietary-induced biotin-deficient mouse model, and 3) in vitro cultured intestinal epithelial Caco-2 monolayers maintained under a chronic biotin-deficient condition. The results showed that ablating the intestinal SMVT system was associated with a significant increase in intestinal permeability and with changes in the level of expression of important tight junction (TJ) proteins. Similar changes were observed in wild-type (WT) mice made biotin deficient via dietary manipulation but not in cultured intestinal epithelial Caco-2 monolayers maintained in vitro under a chronic biotin-deficient condition. These findings suggest that the SMVT system plays an important role in the maintenance of normal intestinal mucosal integrity and that this is most likely mediated via its function in ensuring normal biotin availability and the role that this vitamin plays in the function of different (e.g., immune) mucosal cells.
MATERIALS AND METHODS
Materials.
All chemicals and reagents used in this study were purchased from commercial vendors and were of analytical/molecular biology grade. Anti-claudin-1 (catalog no. 374900) and -2 (catalog no. 325600), anti-zonula occludens (ZO)-1 (catalog no. sc-8146), and anti-myosin light chain kinase (MLCK) (catalog no. M7905) antibodies were obtained from Life Technologies, Santa Cruz Biotechnology, and Sigma-Aldrich, respectively. Animal studies described in this paper were approved by the Animal Care and Use Committee of the VAMC, Long Beach, CA.
Breeding of the conditional (intestinal-specific) SMVT-cKO mice.
The SMVT-cKO mouse line was generated in our laboratory previously using the Cre/lox technology (15). The animals were genotyped as described previously (15). In this study, we used 6–8-wk-old SMVT-cKO mice and their sex-matched littermates as controls.
Induction of dietary biotin deficiency in WT mice.
Dietary biotin deficiency was induced in animals as described previously (3, 6, 45). Briefly, weight-matched 4-wk-old male C57BL/6J WT mice (Jackson Laboratory) were divided into two groups. The first group was allowed free access to a biotin-deficient diet (containing 30% egg white; Dyets), whereas the other (control) group was pair fed an identical amount of diet supplemented with biotin (20 mg biotin/kg food; Dyets). After 16 wk of pair feeding, the biotin-deficient group developed classic symptoms of biotin deficiency (which includes alopecia, dermatitis around the mouth, and decreased growth rate), whereas mice of the control group were all normal and healthy.
Maintenance of confluent intestinal epithelial Caco-2 monolayers under biotin-deficient condition.
Human-derived intestinal epithelial Caco-2 cells [passage 20, American Type Culture Collection (ATCC), Manassas, VA] were grown in EMEM media supplemented with 10% (vol/vol) FBS, penicillin (100,000 U/l), and streptomycin (10 mg/l) in 75-cm2 plastic flasks at 37°C in a 5% CO2-95% air atmosphere. Maintenance of confluent intestinal epithelial Caco-2 monolayers under biotin-deficient and -sufficient conditions was performed as described previously (37). Briefly, biotin-deficient growth medium (DMEM from GIBCO-BRL) was prepared using 2.5% dialyzed FBS (Gemini Bio-Products) treated with avidin-agarose (Sigma; briefly, 2 ml suspension of avidin-agarose containing 1.5 mg avidin was mixed with 50 ml of FBS for 1 h followed by removal of the biotin-avidin-agarose complex by centrifugation). The biotin-sufficient growth medium was the same medium but with added biotin (10 μM). Caco-2 monolayers were maintained under the biotin-deficient and -sufficient conditions for 14 days with fresh changes of the respective growth medium every other day.
FITC-dextran assay for intestinal permeability in vivo.
Intestinal permeability was determined in vivo by measuring the appearance of FITC-dextran [molecular weight 4 kDa (FD4), Sigma-Aldrich] in the blood, as previously described (7). Briefly, mice were gavaged with 4-kDa FITC-dextran (40 mg/100 g body wt) and then killed after 4 h. Whole blood was collected by cardiac puncture, and FITC-dextran measurements were performed using 100 μl of whole blood by fluorimeter at 488 nm (BMG NOVOstar microplate fluorimeter). The concentration of FITC-dextran in the blood was determined by comparing the sample absorption to the standard curve generated using different dilutions of FITC-dextran in PBS.
Determination of transepithelial resistance in Caco-2 monolayers.
Transepithelial resistance (TER) of Caco-2 monolayers [grown on collagen-coated Transwell filters (Corning Costar) and maintained in biotin-deficient and -sufficient media for 14 days] was determined (as a measure of intestinal permeability) as described previously (52). TER was measured using an EVOM2 Epithelial Voltmeter as described previously (52).
Quantitative real-time PCR.
Total RNA was extracted from mouse tissues and from confluent Caco-2 monolayers using Trizol reagent (Invitrogen) following the manufacturer's protocol. The cDNA was prepared from DNaseI-treated RNA samples using the i-Script kit (Bio-Rad). Quantitative real-time PCR analysis was performed using the CFX96 real-time PCR system (Bio-Rad) according to manufacturer's instructions using the gene-specific primers for mouse or human TNF-α, IFN-γ, ZO-1, claudin-1 and -2, MLCK, JAM-A, occludin, and β-actin (as internal control) (see Table 1 for the list of all primers). Relative gene expression was quantified by normalizing Ct values with the corresponding β-actin.
Table 1.
List of mouse and human primers used in the real-time PCR
| Gene Name | Forward and Reverse Primer Sequences (5′-3′) |
|---|---|
| mClaudin-1 | TGTGGATGGCTGTCATTG; TGGCCAAATTCATACCTG |
| mClaudin-2 | TTAGCCCTGACCGAGAAAGA; AAAGGACCTCTCTGGTGCTG |
| mZO-1 | TTCAAAGTCTGCAGAGACAATAGC; TCACATTGCTTAGTCCAGTTCC |
| mMLCK | ACATGCTACTGAGTGGCCTCTCT; GGCAGACAGGACATTGTTTAAGG |
| mJAM-A | GGTCAGCATCCACCTCACTGT; AGGTCAGCACTGCCCTG |
| mOccludin | TCTCTCAGCCAGCGTACT; AGCCTCTGTCCCAAGCA |
| mTNF-α | CATCTTCTCAAAATTCGAGTGACAA; TCGGAGTAGACAAGGTACAACCC |
| mIFN-γ | TCAAGTGGCATAGATGTGGAAGAA; TGGCTCTGCAGGATTTTCATG |
| mβ-actin | GGCTGTATTCCCCTCCATCG; CCAGTTGGTAACAATGCCATGT |
| hClaudin-1 | CGATGAGGTGCAGAAGATGA; CCAGTGAAGAGAGCCTGACC |
| hClaudin-2 | TCGAACCTCATTGTCAGCAG; ACGCTGAGGAAGTTCTCCAA |
| hZO-1 | AACCCAGCATCATCAACCTC; ATCTACATGCGACAATGATG |
| hMLCK | CCCGTGCTAGGAACTGAGAG; TTCTCGCTGTTCTCCACCTT |
| hJAM-A | GTGAAGTTGTCCTGTGCCTACTC; ACCAGTTGGCAAGAAGGTCACC |
| hOccludin | TCCAATGGCAAAGTGAATGA; AGTCCTCCTCCAGCTCATCA |
| hTNF-α | GGAGAAGGGTGACCGACTCA; CTGCCCAGACTCGGCAA |
| hIFN-γ | CCAACGCAAAGCAATACATGA; CCTTTTTCGCTTCCCTGTTTTA |
| hβ-actin | CATCCTGCGTCTGGACCT; TAATGTCACGCACGATTTCC |
Western blot analysis.
For Western blot analysis, tissues/cells were homogenized in RIPA buffer (Sigma) containing complete protease inhibitor cocktail (Roche). Total protein homogenates were cleared by centrifugation at 8,000 g for 10 min, and an equal amount (35 μg) of the total proteins was loaded on a 4–12% mini gel (Invitrogen). The proteins were then transferred to a PVDF membrane and probed simultaneously with mouse claudin-1 and -2, ZO-1, and MLCK antibodies (raised in mouse or rabbit) and monoclonal β-actin antibody (raised in mouse). The blots were then incubated with anti-rabbit/anti-mouse IR 800 dye and anti-mouse IR 680 dye (LI-COR) secondary antibodies (1:25,000) for 1 h at room temperature. Relative expression was quantified by comparing the fluorescence intensities in an Odyssey Infrared imaging system (LI-COR) using Odyssey application software (version 3.0) with respect to corresponding β-actin.
Estimation of biotin status.
Biotin status in mice fed biotin-deficient diet and their pair-fed controls was estimated as described previously (8, 15, 21) by measuring the total level of biotinylated proteins in the liver of these animals using Western blot analysis. In these investigations, the membrane was incubated first with anti-mouse anti-β-actin antibodies. This is followed by labeling the anti-β-actin primary antibodies with anti-mouse IR 680 dye (LI-COR) and the biotinylated proteins with avidin-IR 800 dye (LI-COR).
Histopathology analysis.
The cecum of biotin-deficient mice and their pair-fed controls was collected immediately after euthanasia and fixed in 10% formalin overnight. Sections of the cecal wall were paraffin embedded, and hematoxylin and eosin-stained slides were prepared using the Long Beach VAMC Histology Laboratory, as described before (15). A board-certified anatomic pathologist then performed the microscopic evaluations of the blinded slides.
Statistical analysis.
Data presented in this study are means ± SE of at least three separate experiments. Significance (calculated using the Student's t-test) was set at P < 0.05.
RESULTS
Effect of SMVT-cKO on intestinal permeability and on the level of expression of TJ proteins.
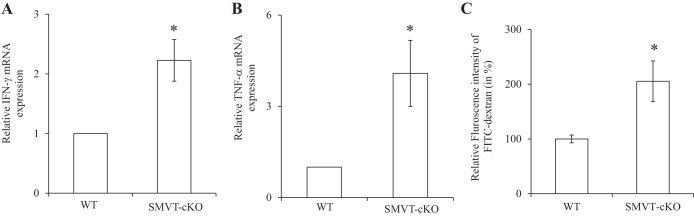
Our previous studies have shown that specific deletion of the SMVT system from mouse intestine leads to the development of chronic active inflammation, especially in the cecum. The latter was further confirmed by the findings in the present study of a significant induction (P < 0.05) in the level of expression of the proinflammatory cytokines IFN-γ (Fig. 1A) and TNF-α (Fig. 1B) in the cecal mucosa of the SMVT-cKO mice compared with WT littermates. Collectively, these findings point to the possible role for the SMVT system in the maintenance of normal intestinal integrity and immunology. Focusing on the role of SMVT in intestinal integrity in the present investigation, we examined possible changes in intestinal permeability in the SMVT-cKO mice using the FITC-dextran approach (see materials and methods). The results showed a significant increase (P < 0.05) in gut permeability in the SMVT-cKO mice compared with their sex-matched WT littermates (Fig. 1C).
Fig. 1.
Effect of intestinal sodium-dependent multivitamin transporter conditional knockout (SMVT-cKO) on the level of mRNA expression of IFN-γ (A) and TNF-α (B) in cecal mucosa. Data are means ± SE of at least 3 sets of mice. (*P < 0.05). Effect of intestinal SMVT-cKO on gut permeability is shown. Intestinal permeability was determined using the 4-kDa FITC-dextran (C) as described in materials and methods. Data are means ± SE of at least 3 determinations (*P < 0.05).
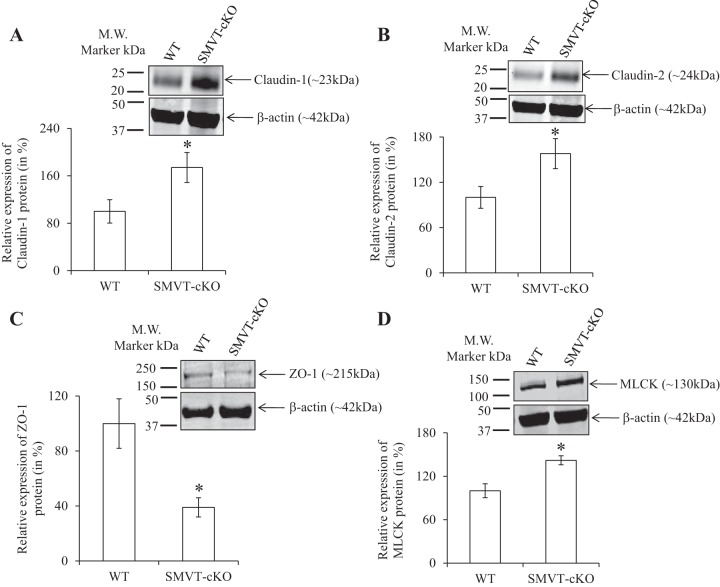
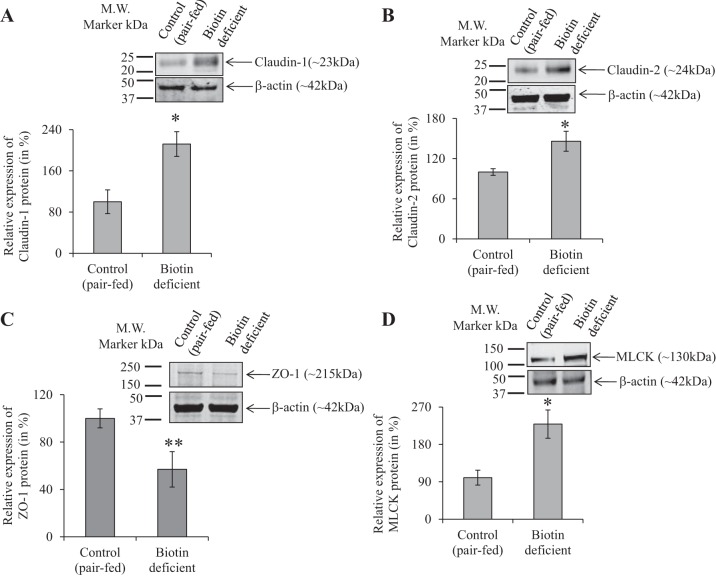
To examine whether the above-described change in intestinal permeability is associated with changes in the level of expression of TJ proteins, we examined (by means of real-time PCR) the level of mRNA expression of important TJ proteins in the intestine of SMVT-cKO mice and compared the findings to their levels in WT littermates. We focused on the cecum of the SMVT-cKO mice because it is the site of the intestinal tract that showed the greatest abnormal intestinal pathology and inflammation (15). The results showed a significant increase in the level of expression of claudin-1 (P < 0.01) and claudin-2 (P < 0.01) and a decrease (P < 0.01) in the level of expression of ZO-1 in the cecum of the SMVT-cKO mice compared with WT littermates (Fig. 2, A–C); the level of the TJ regulator MLCK (12, 27) was also increased significantly (P < 0.05) in the cecum of the SMVT-cKO mice (Fig. 2D) (we also examined the level of expression of occludin and JAM-A but observed no changes between the 2 animal groups; data not shown). Focusing on TJ proteins whose mRNA level showed changes in the SMVT-cKO mice, we also determined whether similar changes are reflected at the protein level. This was done by means of Western blotting (see materials and methods) with the results showing a significant increase in the level of protein expression of claudin-1 (P < 0.05, Fig. 3A), claudin-2 (P < 0.05, Fig. 3B), and MLCK (P < 0.05, Fig. 3D), whereas the protein level of ZO-1 was significantly (P < 0.05) decreased (Fig. 3C).
Fig. 2.
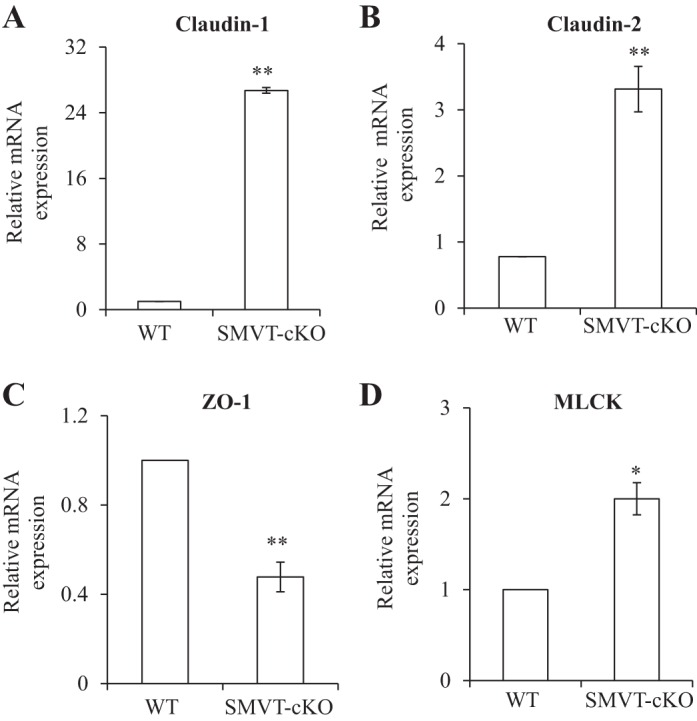
A–D: effect of intestinal SMVT-cKO on the level of mRNA expression of tight junction (TJ) proteins in the cecum. mRNA levels were determined by means of RT-PCR, and data were normalized relative to β-actin (see materials and methods). Data are means ± SE of at least 3 separate sets of mice (*P < 0.05; **P < 0.01). WT, wild-type; ZO, zonula occludens; MLCK, myosin light chain kinase.
Fig. 3.
Effect of intestinal SMVT-cKO on the level of protein expression (Western blotting) of TJ proteins in the cecum. Cecal tissue lysates were used for Western blot, and the blots were probed with anti-claudin-1 and -2, ZO-1, and MLCK antibodies. The expression of TJ proteins was normalized relative to β-actin as described in materials and methods. The graphs show relative protein expression of claudin-1 (A), claudin-2 (B), ZO-1 (C), and MLCK (D) in the cecum of cKO mice and their sex-matched littermates. Data are means ± SE of at least 3 sets of mice (*P < 0.05).
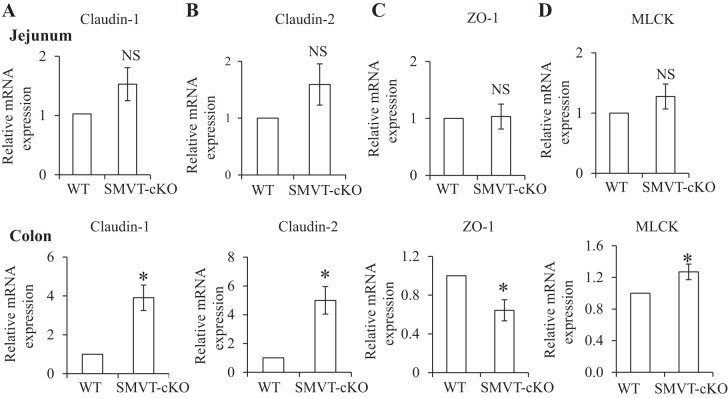
In a related study, we examined possible changes in the level of expression of the TJ proteins in other areas of the gut, namely the jejunum (Fig. 4, top) and the colon (Fig. 4, bottom). We focused on those TJ proteins whose gene expression showed clear changes in the cecum of the SMVT-cKO mice, i.e., claudin-1 and -2, ZO-1, and MLCK. The results showed no significant changes in the level of expression of these TJ proteins in the jejunum of the SMVT-cKO mice compared with WT littermates (Fig. 4, top). On the other hand, changes in the level of expression of claudin-1 and -2, ZO-1, and MLCK that are similar to those seen in the cecum of the SMVT-cKO mice were observed in the colon (Fig. 4, bottom).
Fig. 4.
Effect of intestinal SMVT-cKO on the level of mRNA expression of TJ proteins in the jejunum and colon. The levels of claudin-1 (A), claudin-2 (B), ZO-1 (C), and MLCK mRNA expression (D) in jejunum (top) and colon (bottom) of SMVT-cKO mice and their sex-matched littermates are shown. Data are means ± SE of at least 3 sets of mice. (*P < 0.05; NS, not significant).
Effect of dietary-induced biotin deficiency on normal intestinal homeostasis.
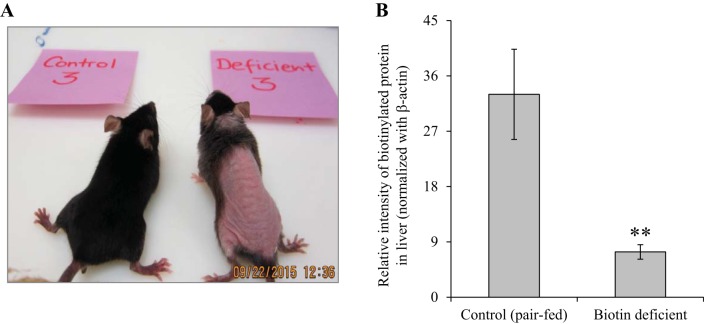
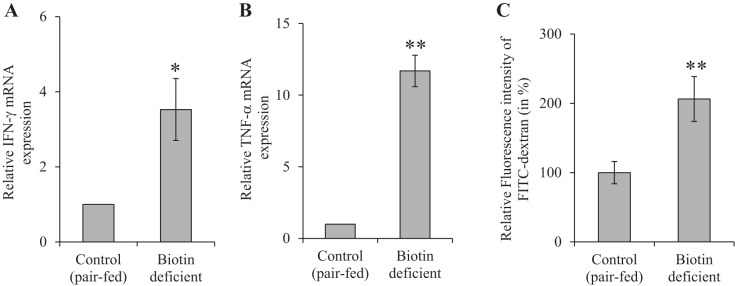
Because the SMVT system transports, not only biotin, but also pantothenic acid and lipoate (reviewed in Refs. 40, 41), and because biotin is known to play an important role in normal immune function (2, 3, 10, 17–20, 30), we sought in these experiments to determine whether the observed changes in intestinal homeostasis in the SMVT-cKO mice are related to the state of biotin deficiency that develops in all these animals. For this, we developed biotin deficiency in WT mice by feeding them a biotin-deficient diet (see materials and methods) (control mice were pair fed the same diet but with added biotin); biotin deficiency was verified as described before (8, 15, 21) (Fig. 5). The results showed that biotin deficiency per se leads to the development of chronic active inflammation with focal cryptitis in the cecal mucosa with neutrophilic infiltration into the lamina propria and focally the submucosa (Fig. 6). We also tested the level of expression of the proinflammatory cytokines IFN-γ (Fig. 7A) and TNF-α (Fig. 7B) in the cecal mucosa of the biotin-deficient mice and observed significant (P < 0.05 and 0.01, respectively) induction compared with their level in the cecal mucosa of pair-fed controls.
Fig. 5.
Effect of dietary-induced biotin deficiency in mice on phenotype and on biotin level. A: representative image of a biotin-deficient mouse (right) and its pair-fed control (left). B: level of total biotinylated proteins in the liver of biotin-deficient mice and their sex-matched pair-fed controls (determinations were done as described in materials and methods). Data are means ± SE of at least 3 separate sets of mice (**P < 0.01).
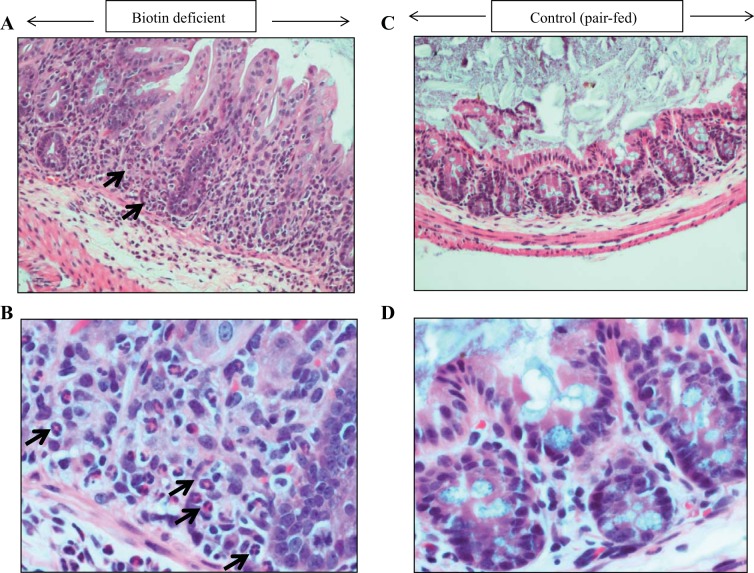
Fig. 6.
Histology of the cecum (A–D) of the biotin-deficient mice and their pair-fed controls. A representative section of cecum of a biotin-deficient mouse (A and B) and its pair-fed control (C and D) is shown with hematoxylin and eosin stain, at ×40 (A and C) and ×200 (B and D). C and D: normal morphology of the pair-fed control cecum. A and B: significant cryptitis and neutrophils within epithelial crypt (arrows).
Fig. 7.
Effect of dietary-induced biotin deficiency on the level of mRNA expression of IFN-γ (A) and TNF-α (B) in large intestinal mucosa. Data are means ± SE of at least 3 sets of mice. (*P < 0.05; **P < 0.01). Effect of dietary-induced biotin deficiency on mouse intestinal permeability is shown. Intestinal permeability was examined using 4-kDa FITC-dextran (C) as described in materials and methods. Data are means ± SE of 3 pairs of biotin-deficient mice and their pair-fed controls (**P < 0.01).
In other studies, we examined possible changes in gut permeability of the mice made biotin deficient via dietary manipulation. This was again performed using the FITC-dextran method with the results showing a significant increase (P < 0.01) in gut permeability in the biotin-deficient mice compared with their pair-fed controls (Fig. 7C).
The effect of dietary-induced biotin deficiency on the level of expression of TJ proteins was also examined in the cecum of the biotin-deficient mice, and the results were compared with their level in pair-fed controls. The results showed a similar pattern of changes in expression of TJ proteins to that seen in the cecum of the SMVT-cKO mice in which a significant increase in the level of expression of claudin-1 (P < 0.01, Fig. 8A), claudin-2 (P < 0.01, Fig. 8B), and MLCK (P < 0.01, Fig. 8D) and a significant (P < 0.01) decrease in the level of expression of ZO-1 were observed (Fig. 8C) (again there was no change in the level of expression of occludin and JAM-A between the 2 animal groups; data not shown). Changes in the levels of expression of claudin-1 (P < 0.05), claudin-2 (P < 0.05), ZO-1 (P < 0.01), and MLCK (P < 0.05) in the biotin-deficient animals were also confirmed at the protein level by Western blotting (Fig. 9, A–D).
Fig. 8.
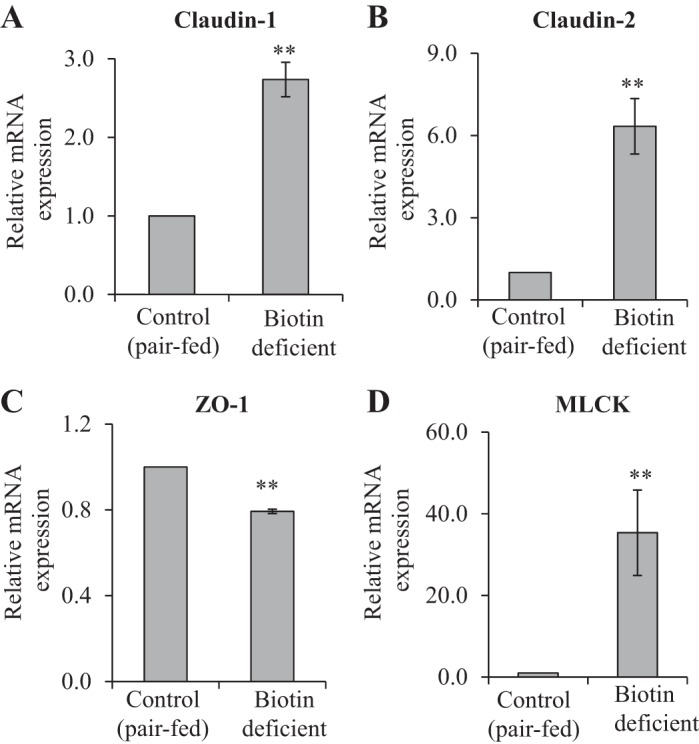
Effect of dietary-induced biotin deficiency in mice on the level of mRNA expression of TJ proteins in the cecum. The mRNA levels were determined by RT-PCR, and data were normalized to β-actin (see materials and methods). Data are means ± SE of at least 3 separate pairs of biotin-deficient mice and their pair-fed controls (**P < 0.01).
Fig. 9.
Effect of dietary-induced biotin deficiency in mice on the level of protein expression (Western blotting) of TJ proteins in the cecum. Legend is similar to that of Fig. 3. The graphs show the relative expression of Western blot analysis of claudin-1 (A), claudin-2 (B), ZO-1 (C), and MLCK (D) proteins in the cecum of biotin-deficient mice and their pair-fed controls. Data are means ± SE of at least 3 separate pairs of biotin-deficient mice and their pair-fed controls (*P < 0.05; **P < 0.01).
Effect of chronic biotin deficiency on permeability and on the level of expression of TJ proteins of cultured intestinal epithelial Caco-2 monolayers in vitro.
In these investigations, we sought to determine whether the changes in intestinal permeability and in the level of expression of TJ proteins observed in the SMVT-cKO mice (all of which develop biotin deficiency) and in mice made biotin deficient via dietary manipulation are due to a direct effect of the biotin deficiency state on intestinal epithelial cells. This was performed using cultured confluent intestinal epithelial Caco-2 monolayers grown on collagen-coated Transwell filters and maintained under a chronic biotin-deficient condition (for 14 days); findings were compared with monolayers maintained under the control (biotin-sufficient) condition. The results showed no changes in permeability (Fig. 10A) or in the level of expression of TJ proteins (claudin-1 and -2, ZO-1, and MLCK; Fig. 10, B–E) in Caco-2 monolayers maintained under biotin-deficient conditions compared with those maintained under biotin-sufficient conditions [we also did not observe any induction in the level of expression of proinflammatory cytokines (e.g., TNF-α and IFN-γ) in Caco-2 monolayers maintained under these two conditions; data not shown].
Fig. 10.
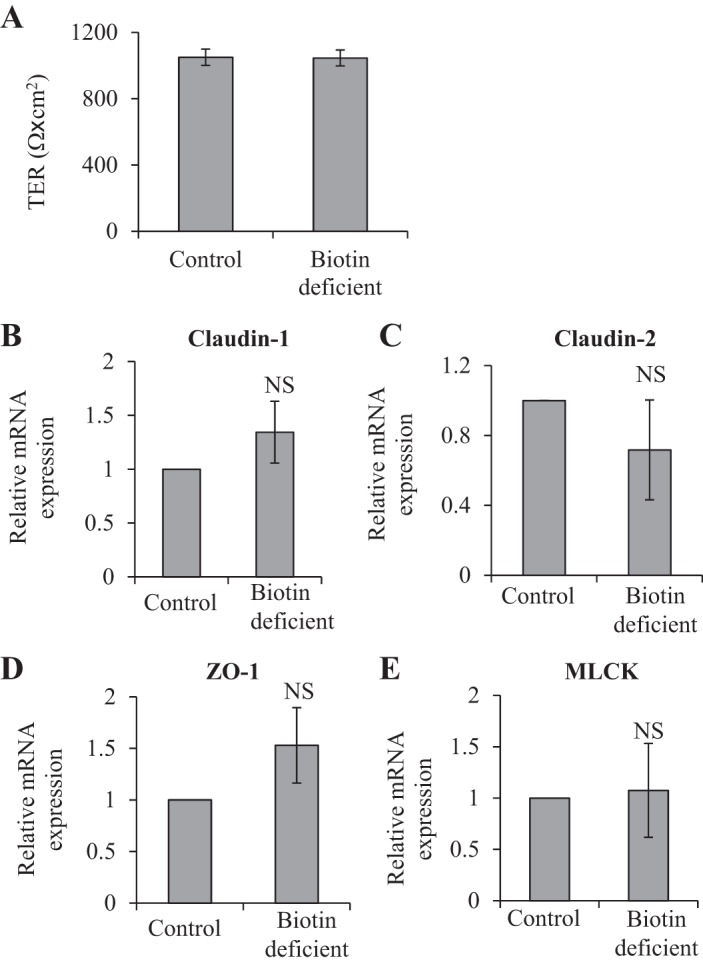
A: transepithelial electrical resistance (TER) of confluent intestinal epithelial Caco-2 monolayers maintained under biotin-deficient and control conditions. Cells were grown on collagen-coated Transwell filters (Corning Costar) and maintained in biotin-deficient and -sufficient media for 14 days. Data are means ± SE of at least 3 separate sets of experiments. B–E: effect of maintaining confluent Caco-2 monolayers under biotin-deficient condition (14 days) on the level of mRNA expression of TJ proteins. mRNA levels were determined by RT-PCR; data were normalized relative to β-actin (see materials and methods). Data are means ± SE of at least 3 separate sets of experiments.
DISCUSSION
Recent studies from our laboratory have shown that ablating the mouse intestinal SMVT system leads to a drastic inhibition in intestinal biotin absorption and to the development of biotin deficiency in the KO animals (15). Unexpectedly, we also observed that all the SMVT-cKO animals develop chronic active inflammation in the cecum reminiscent of that seen in human IBDs (mucosal infiltration with neutrophils and focal cryptitis; 15). These findings suggested a possible role for the SMVT system in the maintenance of normal intestinal integrity and immunity. Our aim in this study was to further understand the role of the intestinal biotin transporter (SMVT) in the maintenance of gut mucosal integrity (focusing on permeability and the level of expression of TJ proteins), and for that we used three models: 1) the intestinal SMVT-cKO mouse model, 2) a dietary-induced biotin-deficient mouse model, and 3) an in vitro model of confluent intestinal epithelial Caco-2 monolayers maintained under a biotin-deficient condition.
Further studies with the SMVT-cKO model confirmed what we know about these animals of gut inflammation by showing a marked increase in the level of expression of proinflammatory cytokines (TNF-α and IFN-γ) in the cecal mucosa. These KO animals showed a significant increase in gut permeability, which was associated with marked changes in the level of expression of important TJ proteins in the cecum. In reference to the latter, there was a significant increase in mRNA and the protein level of expression of the “leaky” TJ proteins claudin-1 and -2 and a decrease in the level of expression of “tight” TJ protein ZO-1 (16, 22); there was also a significant induction in the level of expression of MLCK, the modulator of TJ barrier function (12, 27). Such changes in TJ proteins/MLCK have been observed in cases associated with increased gut leakiness (9, 34, 35, 53). Similar changes in expression of TJ proteins were observed in the colon (but not the jejunum) of the SMVT-cKO mice. Because the large intestine harbors the highest bacterial load in the intestinal tract, it is reasonable to speculate here that luminal bacteria may contribute toward the observed changes in mucosal integrity in the SMVT-cKO mice. Further studies are needed to test this possibility (29, 32, 46).
To determine whether the observed changes in intestinal inflammation and mucosal integrity observed in the SMVT-cKO mice are due (at least in part) to the biotin deficiency state that develops in the gut mucosa in these animals, we generated a biotin deficiency state in WT animals (via dietary manipulation) and examined the effect of that condition on intestinal pathology/inflammation and mucosal integrity. The results showed that dietary-induced biotin deficiency also leads to the development of chronic active cecal inflammation and focal cryptitis similar to that seen in the SMVT-cKO model, together with an increase in the level of expression of proinflammatory cytokines (TNF-α and IFN-γ). In addition, significant increases in intestinal permeability associated with marked changes in the level of expression of cecal TJ proteins similar to those seen with the SMVT-cKO mice were observed. These findings suggest that biotin deficiency per se affects intestinal mucosal integrity/immunity and thus may be (at least in part) responsible for the observed changes in intestinal integrity/immunity in the SMVT-cKO mice.
Our studies with the third model of in vitro cultured confluent intestinal epithelial Caco-2 monolayers maintained under chronic biotin deficiency showed no changes in epithelial permeability or in the level of expression of TJ proteins. The latter findings suggest that the effect of biotin deficiency on intestinal mucosal integrity observed in the above-described mouse models is indirect in nature and most likely mediated via an effect of biotin deficiency on other cell types in the intestinal mucosa, like immune cells. The latter is reasonable in light of the important role that biotin plays in the function of a variety of immune cell types (2, 3, 10, 17–20, 30). Under this scenario, proinflammatory cytokines that are induced in the mucosa of biotin-deficient animals may exert a negative effect on TJ structure and permeability (13, 23, 27, 28). This could lead to penetration of luminal antigens causing inflammation, especially in areas of the gut with high luminal bacterial load. Further studies are needed to test this hypothesis and to gain better understanding of the molecular role of biotin in immune cell functions.
In summary, our findings show for the first time that the intestinal SMVT system is important for the maintenance of normal intestinal mucosal integrity and that this is at least in part mediated via the role it plays in providing biotin to the gut mucosa and its associated immune cells.
GRANTS
This work was supported by grants from the Department of Veterans Affairs and the National Institutes of Health (DK58057 and DK56061).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S. and H.M.S. conception and design of research; S.S., J.A.B., R.K., K.C., A.G., and N.W.G.L. performed experiments; S.S., J.A.B., K.C., A.G., N.W.G.L., and H.M.S. analyzed data; S.S., J.A.B., A.G., N.W.G.L., and H.M.S. interpreted results of experiments; S.S., J.A.B., R.K., K.C., A.G., N.W.G.L., and H.M.S. prepared figures; S.S. and H.M.S. drafted manuscript; S.S., N.W.G.L., and H.M.S. edited and revised manuscript; S.S., J.A.B., R.K., K.C., A.G., N.W.G.L., and H.M.S. approved final version of manuscript.
REFERENCES
- 1.Abad-Lacruz A, Fernandez-Banares F, Cabre E, Gil A, Esteve M, Gonzalez-Huix F, Xiol X, Gassull MA. The effect of total enteral tube feeding on the vitamin status of malnourished patients with inflammatory bowel disease. Int J Vitam Nutr Res 58: 428–435, 1988. [PubMed] [Google Scholar]
- 2.Agrawal S, Agrawal A, Said HM. Biotin deficiency enhances the inflammatory response of human dendritic cells. Am J Physiol Cell Physiol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baez-Saldana A, Diaz G, Espinoza B, Ortega E. Biotin deficiency induces changes in subpopulations of spleen lymphocytes in mice. Am J Clin Nutr 67: 431–437, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Burkholder PR, McVeigh I. Synthesis of vitamins by intestinal bacteria. Proc Natl Acad Sci USA 28: 285–289, 1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balamurugan K, Ortiz A, Said HM. Biotin uptake by human intestinal and liver epithelial cells: Role of the SMVT system. Am J Physiol Gastrointest Liver Physiol 285: G73–G77, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Báez-Saldaña A, Gutiérrez-Ospina G, Chimal-Monroy J, Fernandez-Mejia C, Saavedra R. Biotin deficiency in mice is associated with decreased serum availability of insulin-like growth factor-I. Eur J Nutr 48: 137–144, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Brandl K, Rutschmann S, Li X, Du X, Xiao N, Schnabl B, Brenner DA, Beutler B. Enhanced sensitivity to DSS colitis caused by a hypomorphic Mbtps1 mutation disrupting the ATF6-driven unfolded protein response. Proc Natl Acad Sci USA 106: 3300–3305, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogusiewicz A, Stratton SL, Ellison DA, Mock DM. Biotin accounts for less than half of all biotin and biotin metabolites in the cerebrospinal fluid of children. Am J Clin Nutr 88: 1291–1296, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blair SA, Kane SV, Clayburgh DR, Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest 86: 191–201, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Cowan MJ, Wara DW, Packman S, Ammann AJ, Yoshino M, Sweetman L, Nyhan W. Multiple biotin-dependent carboxylase deficiencies associated with defects in T-cell and B-cell immunity. Lancet 21: 115–118, 1979. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee NS, Kumar CK, Ortiz A, Rubin SA, Said HM. Molecular mechanisms of the intestinal biotin transport process. Am J Physiol Cell Physiol 277: C605–C613, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham KE, Turner JR. Myosin light chain kinase: Pulling the strings of epithelial tight junction function. Ann NY Acad Sci 1258: 34–42, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capaldo CT, Farkas AE, Hilgarth RS, Krug SM, Wolf MF, Benedik JK, Fromm M, Koval M, Parkos C, Nusrat A. Proinflammatory cytokine-induced tight junction remodeling through dynamic self-assembly of claudins. Mol Biol Cell 25: 2710–2719, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Banares F, Abad-Lacruz A, Xiol X, Gine JJ, Dolz C, Cabre E, Esteve M, Gonzalez-Huix F, Gassull MA. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol 84: 744–748, 1989. [PubMed] [Google Scholar]
- 15.Ghosal A, Lambrecht N, Subramanya SB, Kapadia R, Said HM. Conditional knockout of the Slc5a6 gene in mouse intestine impairs biotin absorption. Am J Physiol Gastrointest Liver Physiol 304: G64–G71, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Günzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev 93: 525–569, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuroishi T. Regulation of immunological and inflammatory functions by biotin. Can J Physiol Pharmacol 93: 1091–1096, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Kung JT, Mackenzie CG, Talmage DW. The requirement for biotin and fatty acids in the cytotoxic T-cell response. Cell Immunol 48: 100–110, 1979. [DOI] [PubMed] [Google Scholar]
- 19.Kuroishi T, Endo Y, Muramoto K, Sugawara S. Biotin deficiency up-regulates TNF-α production in murine macrophages. J Leukoc Biol 83: 912–920, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Kuroishi T, Kinbara M, Sato N, Tanaka Y, Nagai Y, Iwakura Y, Endo Y, Sugawara S. Biotin status affects nickel allergy via regulation of interleukin-1β production in mice. J Nutr 139: 1031–1036, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Lewis B, Rathman S, McMahon R. Dietary biotin intake modulates the pool of free and protein-bound biotin in rat liver. J Nutr 131: 2310–2315, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Luettig J, Rosenthal R, Barmeyer C, Schulzke JD. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers 3: e977176, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Q, Zhang Q, Wang M, Zhao S, Ma J, Luo N, Li N, Li Y, Xu G, Li J. Interferon-gamma and tumor necrosis factor-alpha disrupt epithelial barrier function by altering lipid composition in membrane microdomains of tight junction. Clin Immunol 126: 67–80, 2008. [DOI] [PubMed] [Google Scholar]
- 24.McMahon RJ. Biotin in metabolism and molecular biology. Annu Rev Nutr 22: 221–239, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Mock DM. Biotin. In: Handbook of Vitamins, edited by Zempleni J, McCormick DB, Suttie JW. New York, NY: CRC, 2006, p. 361–377. [Google Scholar]
- 26.Madsen CT, Sylvestersen KB, Young C, Larsen SC, Poulsen JW, Andersen MA, Palmqvist EA, Hey-Mogensen M, Jensen PB, Treebak JT, Lisby M, Nielsen ML. Biotin starvation causes mitochondrial protein hyper-acetylation and partial rescue by the SIRT3-like deacetylase Hst4p. Nat Commun 6: 7726, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-α modulation of Caco-2 intestinal epithelial tight junction barrier: Role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol 288: G422–G430, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-α-induced increase in intestinal epithelial tight junction permeability requires NF-κB activation. Am J Physiol Gastrointest Liver Physiol 286: G367–G376, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol 14: 667–685, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Okabe MD, Urabe K, Fujita K, Yamamoto T, Yao T, Doi S. Biotin effects in Crohn's disease. Dig Dis Sci 33: 1495–1501, 1988. [DOI] [PubMed] [Google Scholar]
- 31.O'Keefe SJ, Ou J, Aufreiter S, O'Connor D, Sharma S, Sepulveda J, Fukuwatari T, Shibata K, Mawhinney T. Products of the colonic microbiota mediate the effects of diet on colon cancer risk. J Nutr 139: 2044–2048, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep 7: 688–693, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prasad PD, Wang H, Huang W, Fei YJ, Leibach FH, Devoe LD, Ganapathy V. Molecular and functional characterization of the intestinal Na+-dependent multivitamin transporter. Arch Biochem Biophys 366: 95–106, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Poritz LS, Harris LR, Kelly AA, Koltun WA. Increase in the tight junction protein claudin-1 in intestinal inflammation. Dig Dis Sci 56: 2802–2809, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res 140: 12–19, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Melendez R, Zempleni J. Regulation of gene expression by biotin. J Nutr Biochem 14: 680–690, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Reidling JC, Nabokina SM, Said HM. Molecular mechanisms involved in the adaptive regulation of human intestinal biotin uptake: A study of the hSMVT system. Am J Physiol Gastrointest Liver Physiol 292: G275–G281, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Said HM. Biotin: Biochemical, physiological and clinical aspects. Subcell Biochem 56: 1–19, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Said HM, Ortiz A, McCloud E, Dyer D, Moyer MP, Rubin S. Biotin uptake by human colonic epithelial NCM460 cells: A carrier-mediated process shared with pantothenic acid. Am J Physiol Cell Physiol 275: C1365–C1371, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Said HM. Intestinal absorption of water-soluble vitamins in health and disease. Biochem J 437: 357–372, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Said HM, Trebble T. Intestinal digestion and absorption of micronutrients. In: Slesenger and Fordtran Gastrointestinal and Liver Disease, edited by Feldman M, Friedman LS, and Brandt LJ. New York, NY: Elsevier, 2015, p. 1765–1788. [Google Scholar]
- 42.Said HM, Redha R, Nylander W. A carrier-mediated, Na+ gradient-dependent transport system for biotin in human intestinal brush border membrane vesicles. Am J Physiol Gastrointest Liver Physiol 253: G631–G636, 1987. [DOI] [PubMed] [Google Scholar]
- 43.Said HM, Redha R. Biotin transport in basolateral membrane vesicles of human intestine. Gastroenterology 94: 1157–1163, 1988. [DOI] [PubMed] [Google Scholar]
- 44.Subramanian VS, Marchant JS, Boulware MJ, Ma TY, Said HM. Membrane targeting and intracellular trafficking of the human sodium-dependent multivitamin transporter in polarized epithelial cells. Am J Physiol Cell Physiol 296: C663–C671, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Said HM, Mock DM, Collins JC. Regulation of intestinal biotin transport in the rat: Effect of biotin deficiency and supplementation. Am J Physiol Gastrointest Liver Physiol 256: G306–G311, 1989. [DOI] [PubMed] [Google Scholar]
- 46.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Velazquez-Arellano A. From an inborn error patient to a search for regulatory meaning: a Biotin conducted voyage. Mol Genet Metab 87: 194–197, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Wiedmann S, Rodriguez-Melendez R, Ortega-Cuellar D, Zempleni J. Clusters of biotin-responsive genes in human peripheral blood mononuclear cells. J Nutr Biochem 15: 433–439, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Wolf B. Disorders of biotin metabolism. In: The Metabolic and Molecular Bases of Inherited Disease, edited by Scriver CR, Beaudet AL, Aly WS, Valle D, Childs B, Kinzler KW, Vogelstein B. New York, NY: McGraw-Hill Medical Publishing Division, 2001, p. 3935–3962. [Google Scholar]
- 50.Wrong OM, Edmonds CJ, Chadwick VS. Vitamins. In: The Large Intestine: Its Role in Mammalian Nutrition and Homeostasis. New York, NY: Wiley, 1981, p. 157–166. [Google Scholar]
- 51.Wang H, Huang W, Fei YJ, Xia H, Yang-Feng TL, Leibach FH, Devoe LD, Ganapathy V, Prasad PD. Human placental Na+-dependent multivitamin transporter. J Biol Chem 274: 14875–14883, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Weerachayaphorn J, Pajor AM. Identification of transport pathways for citric acid cycle intermediates in the human colon carcinoma cell line, Caco-2. Biochim Biophys Acta 1778: 1051–1059, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest 88: 1110–1120, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang B, Feng L, Wang F, Wang L. Enterohemorrhagic Escherichia coli senses low biotin status in the large intestine for colonization and infection. Nat Commun 6: 6592, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]