We show that apical exposure of the intestinal epithelium to the serine proteases trypsin and matriptase enhances barrier function in a manner that is dependent upon the mobilization of occludin, but not upon the activation of protease-activated receptor 2. Furthermore, while serine proteases enhance barrier function in intact epithelia, they do not enhance the recovery of barriers disrupted by calcium chelation or cytokines. Proteases may be important regulators of epithelial barrier in the gut.
Keywords: barrier function, tight junction, proteases, inflammation
Abstract
Barrier dysfunction is a characteristic of the inflammatory bowel diseases (IBD), Crohn's disease and ulcerative colitis. Understanding how the tight junction is modified to maintain barrier function may provide avenues for treatment of IBD. We have previously shown that the apical addition of serine proteases to intestinal epithelial cell lines causes a rapid and sustained increase in transepithelial electrical resistance (TER), but the mechanisms are unknown. We hypothesized that serine proteases increase barrier function through trafficking and insertion of tight junction proteins into the membrane, and this could enhance recovery of a disrupted monolayer after calcium switch or cytokine treatment. In the canine epithelial cell line, SCBN, we showed that matriptase, an endogenous serine protease, could potently increase TER. Using detergent solubility-based cell fractionation, we found that neither trypsin nor matriptase treatment changed levels of tight junction proteins at the membrane. In a fast calcium switch assay, serine proteases did not enhance the rate of recovery of the junction. In addition, serine proteases could not reverse barrier disruption induced by IFNγ and TNFα. We knocked down occludin in our cells using siRNA and found this prevented the serine protease-induced increase in TER. Using fluorescence recovery after photobleaching (FRAP), we found serine proteases induce a greater mobile fraction of occludin in the membrane. These data suggest that a functional tight junction is needed for serine proteases to have an effect on TER, and that occludin is a crucial tight junction protein in this mechanism.
NEW & NOTEWORTHY
We show that apical exposure of the intestinal epithelium to the serine proteases trypsin and matriptase enhances barrier function in a manner that is dependent upon the mobilization of occludin, but not upon the activation of protease-activated receptor 2. Furthermore, while serine proteases enhance barrier function in intact epithelia, they do not enhance the recovery of barriers disrupted by calcium chelation or cytokines. Proteases may be important regulators of epithelial barrier in the gut.
barrier function is the ability of the epithelial cells lining the gastrointestinal (GI) tract to prevent harmful components of the lumen, such as pathogenic bacteria, from entering the underlying tissue, while simultaneously maintaining the absorptive functions of the gut. This fine balance is achieved by a variety of cells including goblet, Paneth, and enterocytes through mucus secretion, antimicrobial peptides, and electrolytes and water. Another important component of barrier function is the apical junctional complex (AJC), consisting of adherens and tight junctions (TJs), which form a physical barrier to the passage of material between epithelial cells. The alteration of any one of these components may result in a disruption of gut homeostasis.
The inflammatory bowel diseases (IBD), Crohn's Disease (CD) and ulcerative colitis (UC), are chronic, relapsing diseases of the gastrointestinal tract, and are associated with changes in barrier function. During active inflammation, patients with IBD display increased permeability of the GI tract, and a reduction in barrier function has been shown to predict disease relapse (43, 68). First-degree relatives of patients with CD also display increased intestinal permeability (5, 14, 22, 60), which suggests a genetic component to the changes in barrier function. To complement these observations, recent genomewide association studies (GWAS) have identified several risk alleles that could be associated with UC related to changes in barrier function. These include HNF4A, CDH1 (E-cadherin), LAMB1, and GNA12 (1, 13, 20). However, the link between a change in barrier function and onset of inflammation in susceptible individuals has not been fully elucidated. Any additional information on how intestinal permeability is maintained and modulated may provide further avenues of treatment of diseases characterized by reduced barrier function such as IBDs.
The TJ is not a static structure in the cell, but undergoes constant regulation in response to environmental and internal signals. TJ proteins undergo constant exchange with intramembrane and cytoplasmic pools (64), and TJ protein binding and association are modified through various kinases and phosphatases (15, 40). Cytokines, reactive oxygen species, and long-term calcium depletion induce the removal of TJ proteins from the membrane in vesicles, resulting in a reduction in barrier function (27, 59, 71, 76). During the formation of the junction or as it recovers after injury, proteins are shuttled to the membrane (69, 75). Increased levels of occludin, tricellulin, claudin-1 and claudin-4 at the tight junction are correlated with increases in TER and a reduction in permeability to large molecules (18, 29, 41, 65). Conversely, increased claudin-2, a cation-selective pore-forming claudin, is associated with decreased TER without increasing permeability to large molecules (30). In this study, tight junction protein interactions and their modification in response to stimuli have been determined through various methods including immunoprecipitation, fractionation, and fluorescence recovery after photobleaching (FRAP).
The GI tract is exposed to a variety of serine proteases that aid in digestion, cell turnover, and immune cell functions. During inflammation the GI tract is exposed to increased levels of serine proteases (3), likely released from recruited immune cells such as neutrophils, and circulating proteases from leaky vasculature. It has been shown that transmigrating neutrophils reduce barrier function in epithelia (12). Activation of protease-activated receptor 2 (PAR2) has also been shown to alter barrier function and to induce acute intestinal inflammation in mice (10, 11), and PAR2 knockout mice are protected against models of colitis (24). This evidence points toward a detrimental role of proteases during inflammation.
However, there is also evidence to suggest that proteolytic activity of endogenous serine proteases in epithelial cells is needed to first create, then maintain, the barrier. Matriptase is a type-II membrane-bound serine protease expressed in all epithelial cells and substrates include PAR2, urokinase-type plasminogen activator (uPA), and prostasin (44). Matriptase expression is decreased in patients with IBD (46, 48), and matriptase hypomorphic mice are more susceptible to DSS-induced colitis and recover at a slower rate (46). Intestinal specific knockout of matriptase in mice causes altered tight junction structure, increased intestinal permeability and spontaneous colitis (28, 37). While matriptase has been shown to play important roles in the formation of the tight junction in epithelial cell lines (8), it is not well understood how it is expressed, activated, and regulated. Even though the role of matriptase in the formation of barrier function has been investigated, very few studies have determined whether the exogenous addition of proteases such as matriptase to mature intestinal epithelia can alter barrier function.
Previous work in our lab demonstrated that the apical addition of serine proteases such as trypsin, chymotrypsin, and elastase to a variety of intestinal epithelial cell lines induces a rapid and sustained increase in transepithelial electrical resistance (TER), a measurement of barrier function (66). However, the mechanism behind this response is not well understood. It is also not known whether serine proteases can reverse barrier disruption induced by inflammation. We hypothesized that serine proteases increase barrier function via modulation of the tight junction through trafficking and insertion of tight junction proteins into the membrane, and that serine proteases can reverse barrier disruption induced by inflammatory cytokines.
MATERIALS AND METHODS
Cell culture.
The SCBN canine epithelial cell line was chosen for these studies as they form tight polarized monolayers and, compared with Caco-2 and T84 cells, respond to serine proteases with the greatest TER change (66). Cells were obtained from Dr. André Buret (Univ. of Calgary) (6). Cells between passages 15 and 45 were maintained at 37°C in a humidified 5% CO2 incubator in DMEM High Glucose with 5% FBS, 4 mM l-glutamine, 100 U/ml penicillin, 100 U/ml streptomycin, and 5 μg/ml plasmocin (InvivoGen, San Diego, CA). Cell culture reagents were purchased from Hyclone (Logan, UT) unless otherwise stated. Media were changed on cells every other day. Cells were checked periodically for mycoplasma contamination using the PCR-based Venor GeM Mycoplasma detection kit (Sigma, St. Louis, MO). Cells were passaged using 1X Trypsin-EDTA and plated at 105 cell/well on Snapwell inserts or Transwells (1.12 cm2, 0.4 μm pore size, polycarbonate) (Corning, Corning, NY) unless otherwise stated. Media was changed on plates every other day only in the basolateral compartment, and the ability to completely hold back media from the apical compartment was used as an indicator of tight monolayer formation. Cells were used 5 days post plating.
Ussing chambers and protease treatment.
Cells grown in Snapwells were mounted in modified Ussing chambers (Physiologic Instruments, San Diego, CA). Cells were bathed in pH 7.4 Krebs buffer [115 mM NaCl, 2 mM KH2PO4, 2.4 mM MgCl2·6H2O, 25 mM NaHCO3, 8 mM KCl, 1.25 mM CaCl2 (all from Sigma)] with 10 mM mannitol (apical) or glucose (basolateral), maintained at 37°C, and bubbled with 95% O2-5% CO2. Tissue was voltage clamped to 0 V and TER measured every 20 s with a 5 mV potential difference using Acquire and Analyze software (Physiologic Instruments). Cells were treated apically with porcine pancreatic trypsin (Sigma) or the catalytic subunit of matriptase (generously provided by Dr. Richard Leduc, Université de Sherbrooke). Proteolytic activity of matriptase was determined using a protease activity assay with the fluorogenic trypsin-like substrate glutamine-alanine-arginine (QAR)-AMC (Bachem, Torrance, CA). Activity for matriptase was standardized to trypsin biologically active units (BAU), as provided by the manufacturer. Cell viability was determined at the end of each experiment through measurement of change in current induced by forskolin (Alfa Aesar, Ward Hill, MA). PAR2 was activated on cells using the synthetic peptide agonist 2fLIGRLO (5 mM) (provided by Dr. Morley Hollenberg, synthesized at the Univ. of Calgary peptide facility).
Protein collection and fractionation.
Cells transfected with siRNA or treated with cytokines as described below were lysed with a buffer containing 100 mM NaCl, 20 mM Tris-HCl, 1% Triton X-100, 0.5% SDS, 1 mM EDTA, 2% Protease Inhibitor Cocktail, 1 μM okadaic acid, 1 mM PMSF, 1 mM Na3VO4, and 12.5 mM NaF. Lysates were sonicated and centrifuged at 20,000 g at 4°C and the supernatant collected. To perform the fractionation, SCBN cells grown on transwells were equilibrated in Krebs buffer at 37°C, pH 7.4 for 10 min to mimic Ussing chamber conditions. Cells were then treated apically with 135 BAU/ml trypsin or 1.5 BAU/ml matriptase for 15 min. Fractionation was then carried out as follows, based on methods from Swystun et al. (66) and Singh and Harris (65): Cells were washed with PBS and a lysis buffer containing 10 mM HEPES (Hyclone), 1% Triton X-100, 100 mM NaCl, 2 mM EDTA, 1 mM benzamidine HCl, 1 mM PMSF, 2% protease inhibitor cocktail, 12.5 mM NaF, 1 mM Na3VO4, 1 μM okadaic acid (EMD Millipore, Billerica, MA), 1 μM fostreicin (Santa-Cruz Biotechnology, Dallas, TX), and 55 U/ml soybean trypsin inhibitor (SBTI) was added to the cells and scraped. All components were purchased from Sigma unless otherwise specified. Cells were incubated on ice for 20 min with vortexing every 10 min, and centrifuged at 4°C for 20 min at 10,000 g. The supernatant was removed and saved as the soluble fraction. The pellet was resuspended in a lysis buffer containing all of the components as listed above, except the Triton X-100 was replaced with 1% SDS. Pellet suspensions were sonicated and centrifuged at 4°C for 20 min at 10,000 g. The supernatant was removed and saved as the insoluble fraction.
Western blotting.
Protein concentration of supernatants was determined using the DC Protein Assay (Bio-Rad Laboratories, Mississauga ON). SDS-PAGE was performed using 35 μg of protein per lane in a 12 well Bio-Rad Criterion Precast gradient gel (4–12%), and proteins were transferred to a nitrocellulose membrane. Membranes were blocked with 5% milk in Tween-Tris buffered saline [TTBS-0.1% Tween-20, 50 mM Tris-HCl, 137 mM NaCl, 2.7 mM KCl (Sigma)] for 1 h at room temperature and incubated in primary antibody at 4°C overnight. Fractionated samples were blotted using primary antibodies for ZO-1 [Invitrogen (Carlsbad, CA) 339100], occludin (Invitrogen 331500), claudin-1 (Invitrogen 51–9000), claudin-2 (Invitrogen 51–6100), claudin-4 (Invitrogen 32–9400), tricellulin (Invitrogen 700191), JAM-A (Invitrogen 36–1700), β-actin (Sigma A5441), GAPDH (Santa Cruz sc-59540), caveolin-1 (Cell Signaling Technology, Danvers MA, 3267p) 1:1,000 in 5% milk in TTBS. Apoptosis was assessed using a cleaved caspase-3 (Cell Signaling 9661, 1:500) and PARP (Cell Signaling 9542 1:1,000) antibodies in 5% BSA in TTBS (25). Blots were then washed and incubated in appropriate secondary antibody [either anti-rabbit or anti-mouse HRP-linked (Jackson ImmunoResearch Laboratories, West Grove, PA)] for 1 h at room temperature. Bands were visualized using Clarity Western Enhanced Chemiluminescent Substrate ECL (Bio-Rad) and a Bio-Rad Chemidoc. Band density was analyzed using QuantityOne software (Bio-Rad).
Calcium switch assay in Ussing chambers.
In Ussing chambers, the Krebs buffer bathing SCBN cells was removed and replaced with calcium-free Krebs with either mannitol or glucose on the apical or basolateral sides, respectively, with the addition of 0.4 mM EGTA (Sigma) to chelate excess calcium. Cells were maintained in calcium-free Krebs for 15 min and the Krebs was replaced with standard, calcium-containing Krebs. TER slowly increased following readdition of calcium. To account for fluctuations induced by removal of the buffer, a mock switch was performed, in which the Krebs was removed and replaced with Krebs with calcium. Trypsin (45 BAU/ml) or matriptase (0.5 BAU/ml) was added during various time points during the calcium switch, either under calcium-free conditions, as soon as the cells were returned to normal calcium, or 10, 30, or 60 min after return to normal calcium. Fluorescein isothiocyanate (FITC)-dextran (3–5 kDa) (Sigma) 25 μM was added to the apical side of the cells at each switch of the Krebs and samples were taken from the basolateral side every 2 min (under calcium-free Krebs) or 5, 10, 30, 60, and 90 min post switch (under normal Krebs with calcium).
Cytokine treatment.
SCBN cells were treated basolaterally on day 3 post plating with 2.5, 10, or 25 ng/ml canine recombinant IFNγ and/or TNFα (R&D Systems, Minneapolis, MN) every 24 h for 48 h. Concentrations and treatment times were based on work by Wang et al. (71, 72). In some experiments cells were simultaneously treated with 50 μM ZVAD-FMK (Santa Cruz) (4) to inhibit apoptosis. Positive control for apoptosis was SCBN cells treated for 16 h with staurosporine (1 μM) (Sigma). Treated cells were mounted in Ussing chambers and baseline TER, change in TER in response to 45 BAU/ml trypsin or 0.5 BAU/ml matriptase, permeability to FITC-dextran, and change in FITC-dextran flux were assessed.
Immunofluorescence.
Cells grown in transwells and treated with cytokines were fixed with ice-cold methanol for 30 min at 4°C. Cells were blocked with 10% bovine serum albumin (BSA) in 0.1% Triton X-100 in PBS for 2 h at room temperature, then incubated overnight in primary antibodies (1:200 in 2% BSA in 0.1% TX in PBS: occludin, ZO-1, or claudin-4, the same antibodies used in Western blotting). Cells were washed and incubated in goat anti-mouse Alexa488 secondary antibody (Invitrogen A11029, 1:200 in 5% BSA in 0.1% TX in PBS) for 2 h at room temperature. Finally, cells were washed and incubated in DAPI (Life Technologies, Carlsbad CA) for 30 min at room temperature to visualize nuclei. Transwell membranes were removed from their supports and mounted on slides using Fluorosave (EMD Millipore, Billerica MA). Images were taken on an Olympus FV1000 confocal microscope (Olympus Canada, Richmond Hill, ON) using a 40x/N.A. 1.3 objective at zoom 2. Image z-stacks were recorded at 0.5-μm intervals, and the resulting image is a maximum intensity projection of the z-stack.
RNA interference.
SCBN cells were plated on transwells at 104 cells/well and on day 3 post-plating were transfected with 50–300 nM of Stealth RNAi siRNA (Invitrogen), that were designed against canine occludin mRNA (NM_001003195.1) (sequence: UAC AAA UAC UGA UCC AUG UAG AGU C), or on day 2 post-plating were transfected with 300 nM Stealth RNAi siRNA against canine PAR2 mRNA (NM_001145991.1) (sequence: GAU CCC UUC GUC UAU UAC UUU GUU U). Cells were transfected using RNAiMAX Lipofectamine (Invitrogen) in media without antibiotics. Controls included an untransfected, RNAiMAX-only and scrambled control (AllStars Negative Control, Qiagen, Valencia, CA). Cells were mounted on Ussing chambers 48 h later (occludin) or 72 h later (PAR2) on day 5, and baseline TER and change in TER in response to trypsin (45 BAU/ml) or matriptase (0.5 BAU/ml) were determined. To assess occludin knockdown, Western blotting was performed. To determine PAR2 knockdown, transfected cells were mounted on Ussing chambers and change in short-circuit (Isc) was measured in response to basolateral treatment of 5 mM 2fLIGRLO.
Fluorescence recovery after photobleaching.
SCBN cells were transfected using jetPRIME (Polyplus Transfection) with pEGFP-C1-Occludin (62, 64) and selected using G418 (Sigma) for at least 2 wk. Cells were plated at 3 × 105 cells per well on clear Corning Transwells (3450) and grown for 5 days. Media were changed every other day. Cells were treated with sodium butyrate (3 mM, Sigma) overnight to enhance expression of EGFP-occludin. The transwell was removed from its supports and placed in a Live Cell Instruments Chamlide CF flow chamber (Seoul, Korea). Krebs buffer with 10 mM glucose was placed on the basolateral side of the transwell and the apical side was continuously perfused at 0.5 ml/min with preheated CO2-equilibrated Krebs with 10 mM mannitol using a syringe pump. The chamber was mounted on the stage of an FV1000 confocal microscope that was equipped with a heated enclosure to maintain the cell monolayers at 37°C. Images were taken with a 60 × objective (NA 1.42) at zoom 2 with an ROI of 150 × 150 pixels and a bleach area of 15 × 15 pixels (corresponding to an area of 3.1 μm2). Images were acquired with a 488 laser at 4 μs/pixel with 3 s in between each frame, for 10 min. The confocal pinhole was opened to 500 μm to increase the signal to noise ratio of these measurements. Using a separate light path, a bleach pulse was applied at 1 min after the initiation of the image acquisition time lapse. Bleaching was performed using a 405 laser at 100% power for 3 ms at 10 μs/pixel. Triplicate baseline (control) FRAP runs were conducted along the junction between two epithelial cells strongly expressing EGFP-occludin. Cells were then perfused apically with 45 BAU/ml trypsin for 15 min, and then follow-up triplicate FRAP experiments were conducted. The FRAP image series were analyzed using EasyFRAP Software (55).
Statistics.
Data in bar graphs represent means ± SE. All statistical analyses were performed using GraphPad Prism (version 6.0e, GraphPad Software, La Jolla, CA). Details are provided in accompanying figure legends. An associated probability (P) value of <0.05 was considered significant.
RESULTS
Matriptase increases transepithelial electrical resistance in SCBN cells and is more potent than trypsin.
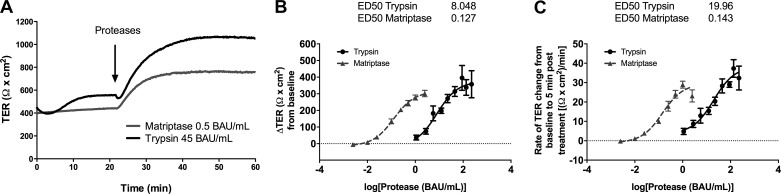
We first determined if exogenous matriptase could increase TER in SCBN cells. The activity of matriptase was determined and the units standardized to porcine pancreatic trypsin. We found that the catalytically active form of matriptase could also increase TER when applied to the apical side of SCBN cells (Fig. 1A). There are two parameters by which this serine protease response can be measured, either the total change in TER, or the rate at which the TER increases (slope). Activity-response curves in these parameters for both trypsin and matriptase were determined. We found that the increase in TER for both proteases is activity-dependent, and that matriptase is much more potent than trypsin, both when measuring total TER change [effective dose 50 (ED50) for trypsin 8.048 BAU/ml and matriptase 0.127 BAU/ml] (Fig. 1B) and rate of change (ED50 trypsin 19.96 BAU/ml, matriptase 0.143 BAU/ml) (Fig. 1C). We chose a dose of 0.5 BAU/ml matriptase, which is at the higher end of the activity-response curve, for further experiments to ensure a robust response.
Fig. 1.
Serine proteases induce an activity-dependent increase in transepithelial electrical resistance (TER). A: SCBN cells were mounted in Ussing chambers and treated apically with trypsin or matriptase and representative tracings shown. B and C: activity-response curves of SCBN cells treated with trypsin (n = 3–7) or matriptase (n = 4–6) analyzed as either the change in TER from baseline 15 min posttreatment (B) or rate of TER change (slope) from treatment time to 5 min posttreatment (C). ED50 values were determined using least-squares nonlinear regression in Prism and presented above each panel (units in BAU/ml).
The serine protease-induced increase in TER is not dependent on PAR2.
Both trypsin and matriptase activate PAR2. Previously, we showed that the apical addition of the PAR2 activating peptide SLIGRLO does not induce an increase in TER (66), which suggests trypsin does not induce a change in TER through canonical activation of the receptor. Serine proteases could have indirect effects on the receptor, however, such as by cleaving a molecule or complex from the apical cell surface that can activate PAR2, activating another receptor to induce transactivation of PAR2, or by inducing an increase in TER through a unique combination of receptor activation cause biased agonist signaling (21, 23).
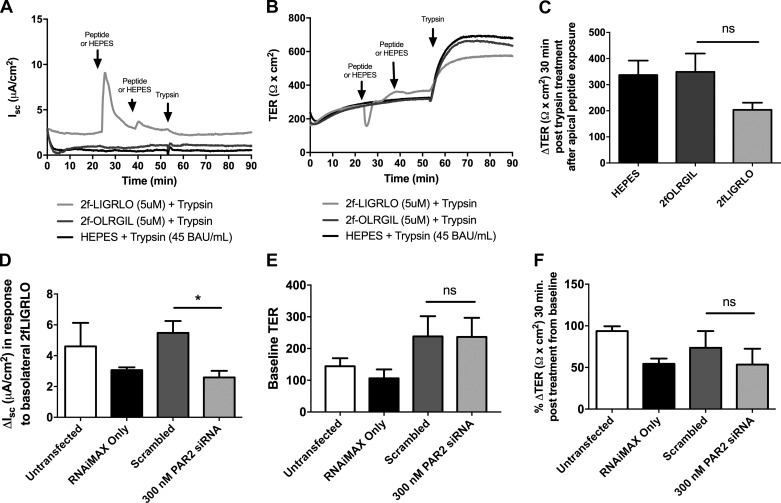
To determine the role of PAR2 in the serine protease-induced increase in TER, we removed PAR2 from SCBN cells using two methods. First, in Ussing chambers, we treated SCBN cells apically with the PAR2 activating peptide 2FLI (5 mM), waited 15 min, and treated again with 2FLI for 15 min. After PAR2 is activated it is internalized and degraded (53, 54). In our studies, addition of 2FLI to the apical side caused a small change in short-circuit current, and this was significantly reduced or zero upon the second treatment, indicating the receptor had been internalized (Fig. 2A). We then treated the cells apically with 45 BAU/ml trypsin and measured the change in TER 30 min later (Fig. 2B). We found a small but insignificant decrease in the ability of trypsin to induce a change in TER after PAR2 internalization (Fig. 2C). Second, we knocked down PAR2 using siRNA. To determine knockdown we assessed the change in Isc in response to basolateral 2FLI and compared PAR2 siRNA treated cells against the scrambled control (Fig. 2D). Knockdown of PAR2 did not alter baseline TER, and did not affect the increase in TER in response to apical trypsin treatment (Fig. 2F).
Fig. 2.
PAR2 is not involved in the trypsin-induced increase in TER in SCBNs. A and B: SCBN cells in Ussing chambers were treated apically with 2fLIGRLO (2FLI, 5 mM) to activate and internalize PAR2 in two 15-min intervals. Controls include reverse peptide, 2fOLRGIL (5 mM) and HEPES (vehicle). Cells were then treated with 45 BAU/ml trypsin apically. Representative tracings are shown for short-circuit current (Isc) and TER, n = 4. C: changes in TER 30 min post trypsin treatment were determined. By t-test, pretreatment with 2FLI did not significantly alter the response to trypsin (compared with reverse peptide), although it is trending toward a decrease (P = 0.1). D: SCBN cells were plated and transfected with 300 nM PAR2 siRNA on day 2 post plating and grown for 3 days (72 h treatment with siRNA). Cells were mounted on Ussing chambers and treated basolaterally with 2fLIGRLO (5 mM) and the change in Isc determined. Treatment with PAR2 siRNA significantly reduced the response to 2FLI treatment, indicative of knockdown of PAR2 (n = 4, *P < 0.05 by t-test comparing siRNA treated to scrambled control). Baseline TER (E) and percent change in TER 30 min post trypsin (45 BAU/ml) treatment from baseline (F) were determined. Percent change was determined instead of total change in TER due to variability in baseline TER. No significant differences in TER change were determined as assessed by t-test between scrambled control and PAR2 siRNA-treated cells.
Treatment with serine proteases does not induce trafficking of tight junction proteins into the membrane as assessed by fractionation.
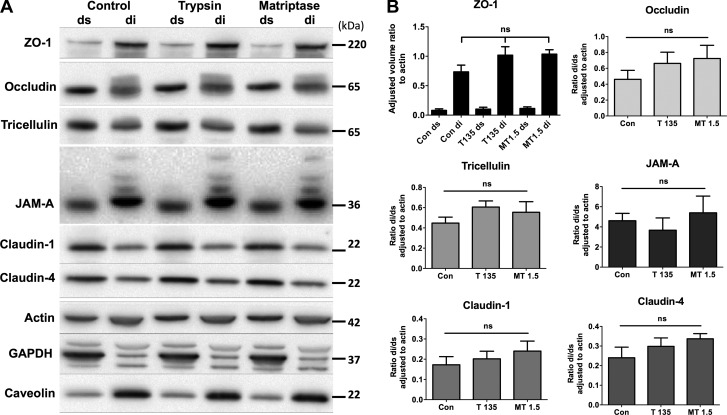
It was hypothesized that serine proteases induce an increase in TER through increased trafficking of TJ proteins to the membrane. Tight junctions are present in a specific membrane environment called detergent-insoluble glycolipid rafts (DIGs) (2, 47). These lipid rafts are insoluble in Triton X-100, and thus can be separated from the rest of the cell using fractionation with this detergent (32). We combined methods from two studies, Swystun et al. (66) and Singh and Harris (65), which allowed for additional phosphatase inhibitors to prevent dephosphorylation of tight junction proteins after serine protease treatment is removed. Fractionation was verified through observation that the majority of GAPDH was present in the detergent-soluble (ds) fraction, caveolin-1 was present in the detergent-insoluble (di) fraction. ZO-1 was almost exclusively present in the di fraction, and a heavier occludin band was seen in the di fraction, indicative of TJ-localized phosphorylated occludin (58). A representative Western blot can be seen in Fig. 3A. Apical treatment with 135 BAU/ml trypsin or 1.5 BAU/ml matriptase did not cause an increase or decrease in any junction protein (ZO-1, occludin, tricellulin, JAM-A, claudin-1, or claudin-4) in either fraction (Fig. 3B). Claudin-2 is not expressed in SCBN cells as assessed by Western blotting and RT-PCR (data not shown).
Fig. 3.
Serine proteases do not induce trafficking to the tight junction as assessed by fractionation. SCBN cells grown for 5 days on transwells were treated with trypsin (T; 135 BAU/ml) or matriptase (MT; 1.5 BAU/ml) apically for 15 min in Krebs buffer. Cell compartments were separated into Triton X-100 detergent-soluble (ds) or -insoluble (di) fractions and run on SDS-PAGE followed by Western blotting. A: representative blot shown of n = 4–7. Controls for the fractionation include actin, GAPDH (middle band, only in soluble fraction) and caveolin-1 (only in insoluble fraction). No increases or decreases in either fraction are seen with serine protease treatment compared with control. B: adjusted volume ratios of each protein to actin were determined and then taken as a ratio of the detergent-insoluble to the detergent-soluble fraction for occludin, tricellulin, JAM-A, claudin-1, and claudin-4. Due to low levels of ZO-1 in the detergent-soluble fraction, a ratio was not taken and the detergent-soluble and -insoluble fraction are shown separately. Analysis by ANOVA shows no significant difference between the treated and control (con) groups for any protein assessed.
Serine proteases do not accelerate tight junction reassembly during calcium switch.
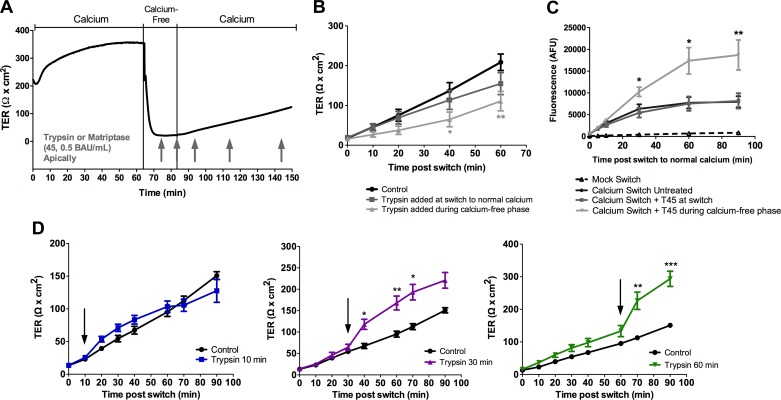
The fact that changes in protein trafficking were not observed following apical exposure to protease may mean that our fractionation methods are not sensitive enough to detect small changes in TJ protein trafficking, or that changes in trafficking do not account for the increased TER induced by protease exposure. To address the former hypothesis, we sought to determine if prior disruption of the barrier would reveal an enhancement of recovery of barrier function which could be indicative of an effect on tight junction protein trafficking. The calcium switch assay was employed to induce a reversible disruption of the barrier (Fig. 4A).
Fig. 4.
Serine proteases prevent the recovery of the barrier after calcium switch and can only increase TER when the tight junction has been sufficiently reformed. A: a calcium switch assay was performed on SCBN cells mounted in Ussing chambers. Krebs buffer bathing the cells was replaced with calcium-free Krebs with 0.4 mM EGTA for 15 min, and the Krebs was then switched to normal calcium containing Krebs. Trypsin or matriptase was added at various points during the assay, either during the calcium-free phase, as soon as the calcium was replaced back into the system (at switch) or 10, 30, or 60 min postswitch (arrows). B: TER measurements after the switch to normal calcium when trypsin is added before or at the switch to normal calcium (n = 8–11). *P < 0.05, **P < 0.01 as assessed by ANOVA with Dunnett's post hoc test comparing each protease-treated group to untreated control. C: FITC-dextran was added to the apical chamber after the switch to normal calcium, and samples were taken at various time points from the basolateral compartment. Trypsin (45 BAU/ml, T45) was added either at the switch to normal calcium or during the calcium-free phase before the switch. Trypsin added when the junction is disrupted during the calcium-free phase inhibited recovery of the junction. n = 3–7. *P < 0.05, **P < 0.01 as assessed by ANOVA with Dunnett's post hoc test comparing each protease-treated group to untreated control. D: TER measurements after the switch to normal calcium where trypsin (45 BAU/ml) is added at 10, 30, or 60 min (arrow). n = 3–5. *P < 0.05, **P < 0.01, ***P < 0.001 as assessed by ANOVA with Dunnett's post hoc test comparing each protease treated group to untreated control (which is the same in each panel).
It was found that the addition of trypsin (45 BAU/ml) under calcium-free conditions causes a significant delay in the recovery of TER of the monolayer, and no difference in recovery was observed when serine proteases are added at the time of the switch to normal calcium (Fig. 4B). This was paralleled in FITC-dextran flux experiments (Fig. 4C), in which trypsin added during calcium-free conditions resulted in a significant increase in the level of fluorescence on the basolateral side of cells compared with untreated cells, indicating a delay in recovery. However, when serine proteases were added 30 or 60 min after the return to normal calcium conditions there was a response (increase in TER) by the cells (Fig. 4D). Similar results were observed with matriptase (0.5 BAU/ml) treatment (data not shown). The longer the cells were able to recover after calcium readdition, the greater the effect of serine proteases. This suggests that a functional tight junction needs to be in place for the increase in TER to be induced, and that serine proteases do not affect the recovery of the tight junction after a brief period of calcium depletion. In mock switch experiments, TER always returned to baseline after a small decrease, caused by removal and replacement of buffer (data not shown) and no changes in FITC-dextran flux were observed (Fig. 4C).
The inflammatory cytokines IFNγ and TNFα induce a disruption of tight junction proteins claudin-4, occludin, and ZO-1.
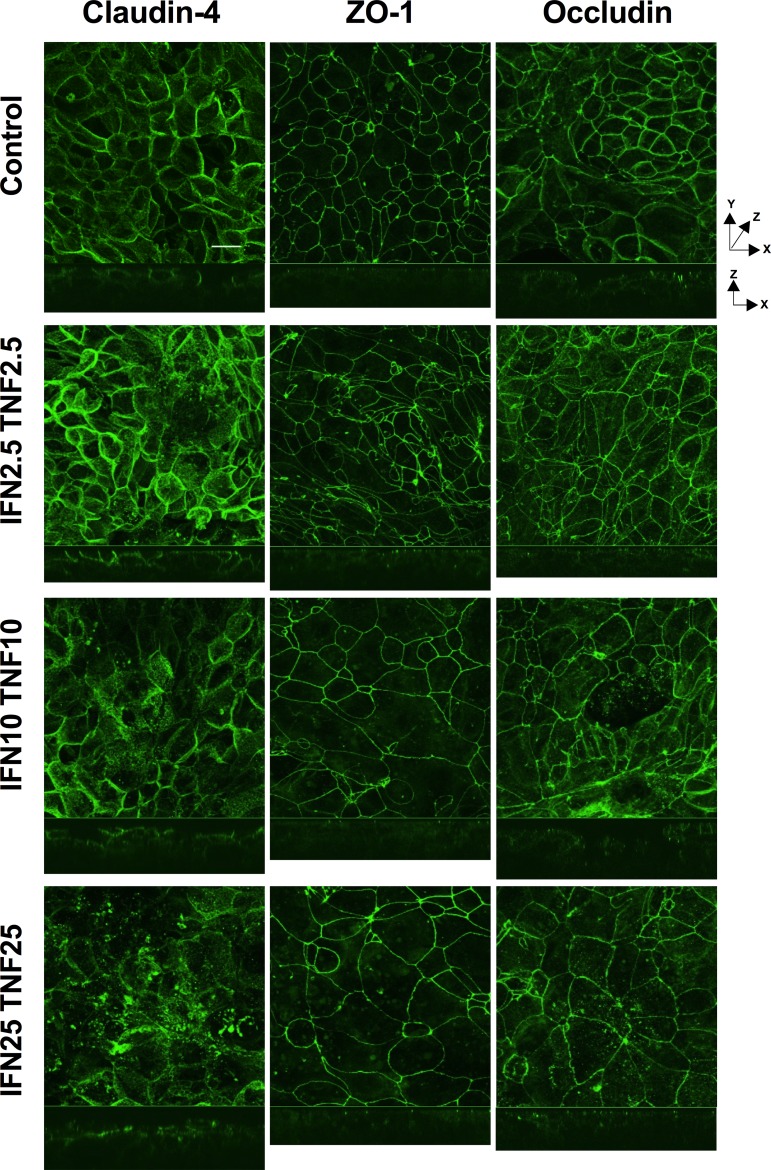
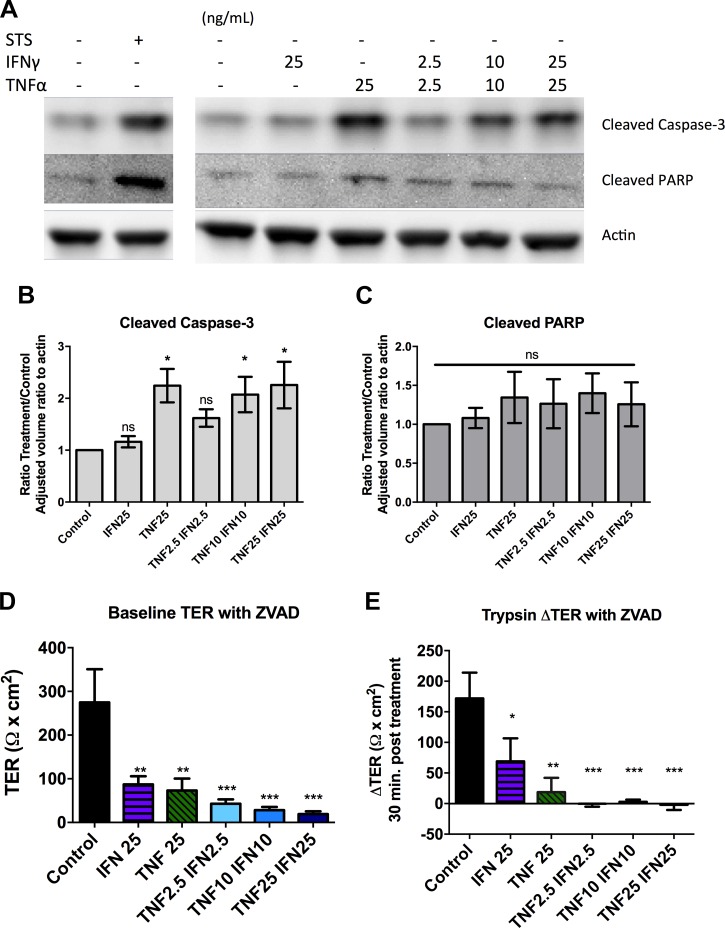
As the calcium switch assay is an artificial method to disrupt barrier that removes the junction in its entirety, we also wanted to cause barrier disruption through a more physiologically relevant method. Cytokines induce barrier disruption independently of apoptosis through selective removal of tight junction proteins from the membrane (4, 70) in combination with alteration of the actin cytoskeleton (9). SCBN cells were treated basolaterally with varying combined concentrations of IFNγ and TNFα for 48 h. Cytokine treatment induced a disruption of barrier function as assessed by immunofluorescence and confocal microscopy (Fig. 5). Cytokines induced an internalization of claudin-4 and occludin into the cytoplasm, as well as discontinuities of ZO-1 junctional staining. Cross sections of the z-stacks show redistribution of claudin-4 and occludin from the lateral and apical membranes to the basolateral side and some internalization. The membrane distribution of ZO-1, however, does not seem to change, and remains close to the apical membrane between cells even with cytokine treatment. At higher concentrations of cytokines, some apoptosis could be observed in the cell monolayer with reduced cell number and evidence of cells being removed through purse-string extraction by adjacent cells (39). We assessed whether the cytokine treatment was inducing apoptosis through assessment of cleaved caspase-3 and cleaved PARP by Western blotting (see Fig. 7). Cells treated with 25 ng/ml TNFα or 10 ng/ml TNFα with 10 ng/ml IFNγ showed significantly increased cleaved caspase-3 but no changes in cleaved PARP compared with untreated controls.
Fig. 5.
Cytokines induce a disruption of the tight junction. SCBN cells were plated on transwells and grown for 3 days before treating with 2.5, 10, or 25 ng/ml IFNγ and/or TNFα, basolaterally every 24 h for 48 h. Cells were fixed in ice-cold methanol and stained for claudin-4, ZO-1, occludin, and/or DAPI (nuclei, not shown). Images were taken on an Olympus IX81 Fluoview (FV1000) laser scanning confocal microscope with a 40× oil objective (NA 1.3) at 2× zoom. Images represent superimposed images of the full thickness of tissue with slices every 0.5 μm. Z-stacks shown below are from the center slice of each image. Images with combined cytokines only are shown; IFNγ 25 ng/ml was similar to control, and TNFα 25 ng/ml was similar to IFN25 TNF25. Scale bar = 20 μm. Images are representative of 4 separate experiments.
Fig. 7.
Barrier disruption induced by inflammatory cytokines is independent of apoptosis. SCBN cells were plated on transwells or Snapwells and treated with 2.5, 10, or 25 ng/ml IFNγ and/or TNFα basolaterally every 24 h for 48 h. A: apoptosis was assessed using Western blotting for cleaved caspase-3 or cleaved PARP. Representative blot shown of n = 9. The positive control is 1 μM staurosporine (STS) treatment of SCBN cells for 16 h. B and C: densitometry of Western blots for cleaved caspase-3 and PARP. Adjusted volume ratio determined and adjusted to actin, taken as a ratio to control cells. *P < 0.05 compared with control as assessed by ANOVA with Dunnett's post hoc test. ns = not significant. D and E: SCBN cells were treated with cytokines and 50 μM ZVAD-FMK for 48 h, and baseline TER and change in TER 30 min post treatment with 45 BAU/ml trypsin assessed as in Fig. 5. n = 4. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control as assessed by ANOVA with Dunnett's post hoc test.
Serine proteases cannot reverse cytokine-induced barrier disruption.
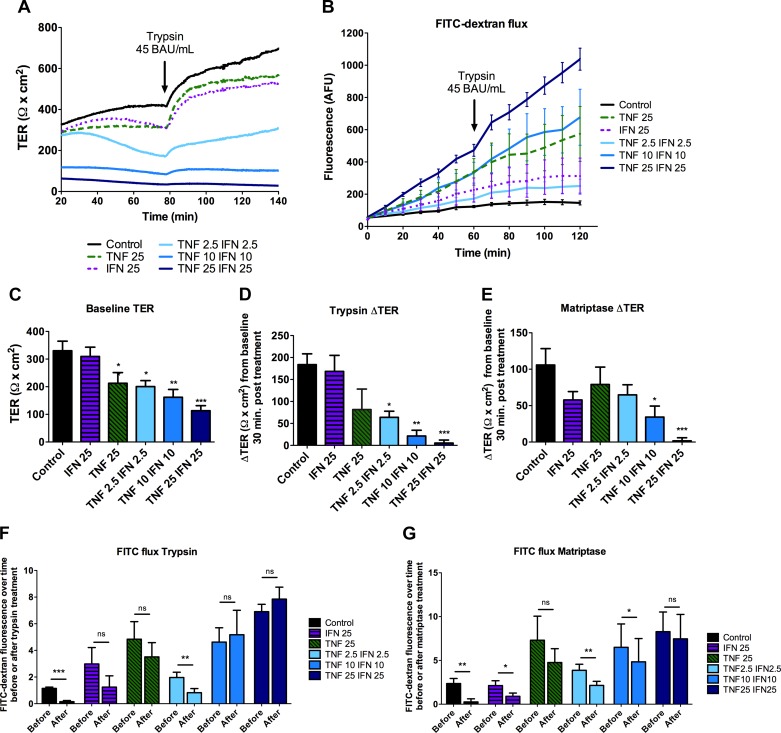
SCBN cells treated with cytokines were placed on Ussing chambers, and FITC-dextran flux and response to trypsin and matriptase were assessed. The higher the concentrations of cytokines, the greater the barrier disruption, as indicated by lower baseline TER and increased FITC-dextran flux at baseline (Fig. 6, A–C). Cells treated with IFNγ or TNFα separately had little change in TER compared with the two cytokines in combination, as expected based on previous literature (71). However, the cells treated with TNFα at 25 ng/ml had greater FITC-dextran flux and more structural changes compared with lower concentrations of the combined cytokines (Figs. 5 and 6B). The greater the dose of cytokines, the less the serine proteases trypsin and matriptase were able to induce an increase in TER (Fig. 6, D and E). These results are paralleled in FITC-dextran flux experiments, as in control cells, proteases significantly decreased the rate of FITC-flux, but this effect was lost when cells were pretreated with cytokines (Fig. 6, F and G). Cytokines were also able to induce significantly decreased baseline TER in the presence of ZVAD-FMK (ZVAD). ZVAD treatment by itself did not affect baseline TER or change in TER 30 min post treatment with 45 BAU/ml trypsin. Interestingly, in the presence of ZVAD, cytokines were able to induce greater baseline TER loss and a greater FITC-dextran flux (data not shown). For example, 25 ng/ml IFNγ does not induce a change in TER, but in the presence of ZVAD it did (Figs. 6 and 7). As in the experiments without it, in the presence of ZVAD, cytokine treatment prevents the change in TER in response to trypsin (Fig. 7E). Thus, similar to the calcium switch experiments, serine proteases do not induce significant increases in barrier function when the junction has already been disrupted.
Fig. 6.
Cytokine-induced barrier disruption of the barrier prevents the serine protease-induced increase in TER. SCBN cells were plated on transwells and grown for 3 days before treating with 2.5, 10, or 25 ng/ml IFNγ and/or TNFα basolaterally every 24 h for 48 h. Cells were mounted in Ussing chambers to assess barrier function. Representative tracing (A) and FITC-dextran flux (B) of cells treated with 45 BAU/ml trypsin (n = 7). Tracings were analyzed for TER before addition of serine proteases (baseline) (n = 13–14) (C), change in TER in response to trypsin 30 min post treatment (n = 5–6) (D), and change in TER in response to 0.5 BAU/ml matriptase (n = 7–8) (E). *P < 0.05, **P < 0.01, ***P < 0.001 compared with untreated control as assessed by ANOVA with Dunnett's post hoc test. To assess change in flux, slopes between 0–60 min and 70–120 min were taken [before or after trypsin (n = 6) (F) or matriptase (n = 4) (G) treatment] and a paired t-test performed within each group. *P < 0.05, **P < 0.01, ***P < 0.001.
The serine protease-mediated increase in TER is dependent on occludin.
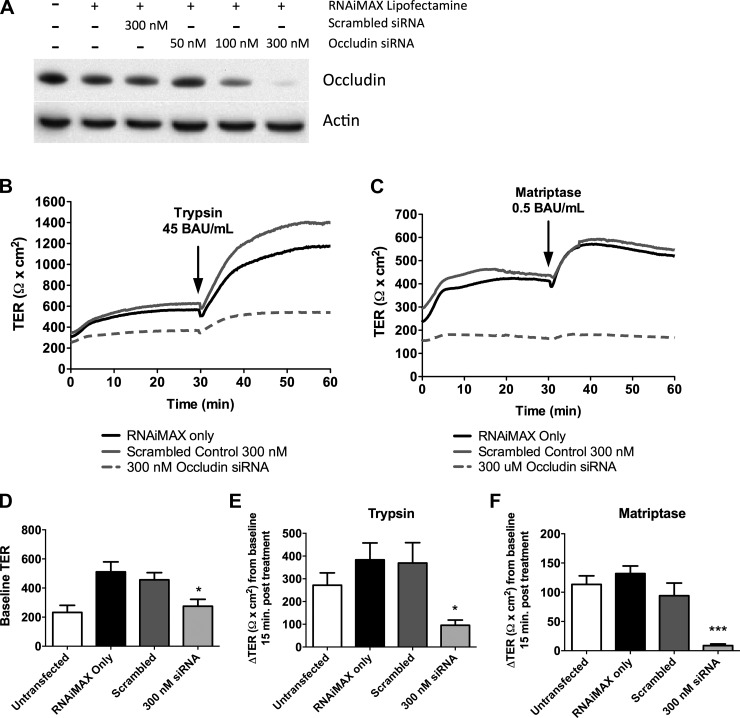
Data indicate that while TJ proteins may not traffic to the membrane to increase TER, a functional tight junction needs to be in place for the effect to occur. Previous literature has suggested that occludin is an important TJ protein in the serine protease response (66). We wanted to confirm this observation by knocking down occludin in our cells and assessing its effect on the serine protease response. Using siRNA we were able to achieve an almost complete knockdown of occludin (Fig. 8A). We then determined the response of these cells to serine protease treatment in Ussing chambers (Fig. 8, B and C). Knockdown resulted in a decreased baseline TER (Fig. 8D) compared with scrambled control transfected cells. Knockdown of occludin significantly reduced the ability of trypsin and matriptase to induce an increase in TER (Fig. 8, E and F).
Fig. 8.
Occludin knockdown attenuates serine protease-induced increase in TER. A: SCBN cells were treated with 50, 100, or 300 nM occludin siRNA for 48 h and knockdown assessed by Western blotting. B and C: representative tracings of SCBN cells transfected with 300 nM occludin siRNA for 48 h, and treated with trypsin (B, n = 6–9) or matriptase (C, n = 4). Controls include untransfected cells, and RNAiMAX Lipofectamine with or without scrambled siRNA. Baseline TER (n = 10–14) (D) and change in TER 15 min post serine protease treatment (E and F) were assessed. Occludin siRNA induced a significant reduction in baseline TER and attenuated the trypsin- and matriptase-induced increase in TER. *P < 0.05, **P < 0.01 compared with scrambled control.
Serine proteases increase the membrane mobility of occludin.
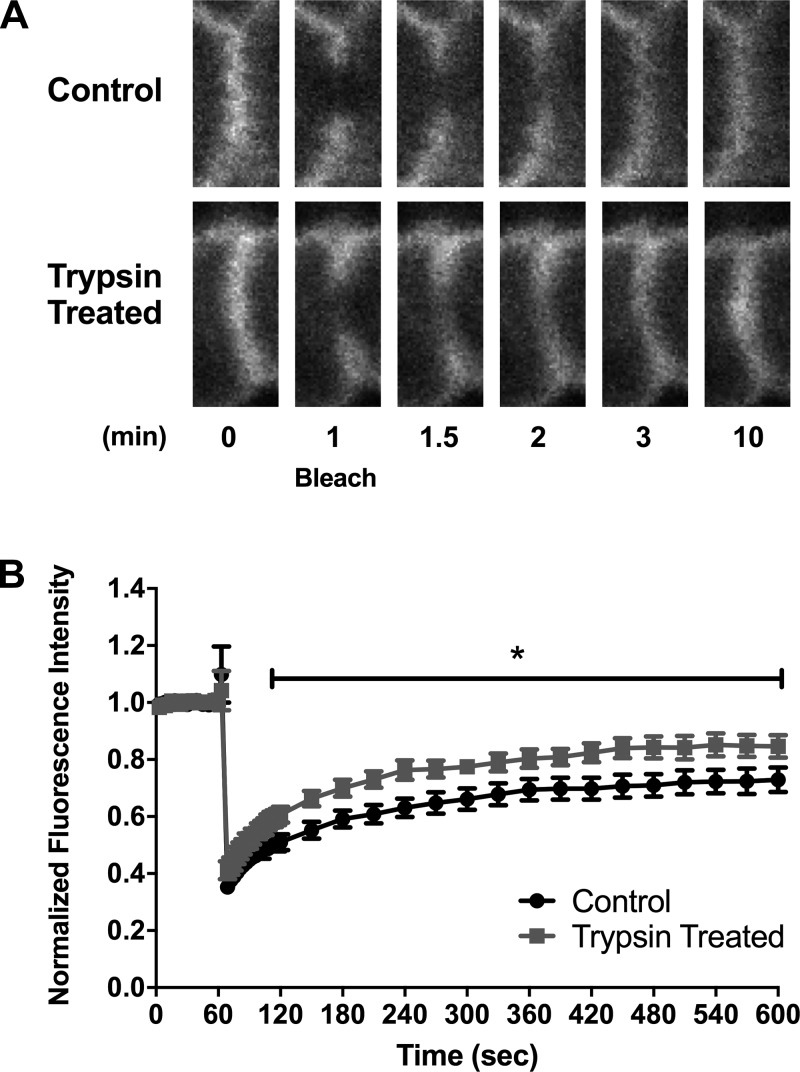
We found that occludin is an important tight junction protein in the serine protease-induced increase in TER (Fig. 8) but could not see changes in trafficking to or from the membrane using fractionation followed by Western blotting (Fig. 3). However, it may be that serine proteases are inducing a change in the complexity of the junction through modifications of protein-protein binding (52). FRAP measures the ability of a GFP-tagged protein to move in the membrane, or its mobility. Mobility is an indirect indicator of how tightly bound a particular protein is to others. It was hypothesized that serine protease treatment would induce a decrease in mobility of occludin, which would suggest it is bound more tightly to other proteins and cannot move as freely. However, using FRAP with EGFP-C1-Occludin stably expressed by SCBN cells, we found that trypsin induced an increase in occludin mobility (Fig. 9).
Fig. 9.
Trypsin induces a change in mobility of occludin in the membrane as assessed by fluorescence recovery after photobleaching (FRAP). Briefly, SCBN cells expressing EGFP-occludin grown on transwells for 5 days were mounted on a flow chamber and placed in a heated FV1000 Olympus Confocal and mobility of occludin assessed by FRAP. A: representative bleaching and recovery images during FRAP at time 0 (baseline), and 1, 1.5, 2, 3, and 10 min into the experiment. Bleaching was performed at 1 min. B: trace of the fluorescence intensity of the photobleached area over time of the junction during FRAP experiments, normalized to background intensity and initial intensity (set to 1). Bleaching depth was not significantly different between the two groups. N = 6–7.
DISCUSSION
Epithelial cells express a wide range of exogenous serine proteases such as matriptase and prostasin that are thought to play roles in many cellular processes such as matrix remodeling and barrier function (67). Patients with IBD exhibit decreased levels of matriptase in their colonic epithelium (46). The relationship between matriptase and tight junction dysregulation, and its potential role in the recovery of tissue after inflammation, warrants further investigation in disease states characterized by reduced barrier function such as IBD.
Matriptase and prostasin, a GPI-anchored serine protease, work in concert to activate each other (7, 17), and loss of either one in mice results in the same phenotype: alteration of epidermal barrier function resulting in fatal water loss after birth (33, 36). In Caco-2 cells, matriptase is expressed on the basolateral side, and prostasin on the apical side of cells (16). In our cells, the increase in barrier function is only observed when serine proteases are added to the apical side of cells. We have previously shown that when applied to the basolateral side, serine proteases activate PAR2, leading to a transient increase in chloride secretion and decreased TER (42). Thus it may be that by adding exogenous serine proteases, we are activating signaling pathways controlled by prostasin, but that are probably activated by endogenous matriptase. Both trypsin and matriptase affect both the pore and leak pathway (63), changing permeability to ions (TER) and larger molecules (FITC-dextran flux) (Fig. 6, F and G).
The activity-response curves for both trypsin and matriptase indicate that the more enzymatic activity present, the faster and greater the response of the cells in terms of enhanced TER. This relationship also suggests some involvement of target receptors or proteins on the cell surface that are eventually saturated or removed by proteolytic cleavage. Matriptase is more potent than trypsin, but trypsin has a greater maximum response. This suggests some difference in their substrate specificity or mechanism of action; however, the mechanism may be difficult to pinpoint due to the number of proteins that serine proteases could potentially cleave on the cell surface. As tight junctions control barrier function, it was thought that serine proteases might have an effect on the complexity of TJ proteins, through increased trafficking into the membrane.
The tight junction can be modulated in a variety of ways in response to stimuli. TJ proteins can be trafficked into or out of the membrane, depending on vesicle trafficking and phosphorylation events, or through a change in actin cytoskeletal dynamics. We first investigated the role of protein trafficking in the change in TER by serine proteases through fractionation and Western blotting (65, 66). This technique separates the tight junction from the rest of the cell based on detergent solubility. However, we saw no change in the location of any tight junction protein. This technique may be limited in sensitivity, however, as small changes in protein movement are difficult to visualize using Western blotting. Fractionation may also not separate the lipid-insoluble raft from vesicles containing TJ proteins, which would not differentiate TJ proteins in the membrane from those being trafficked there.
Under homeostatic conditions, the rate of trafficking may be at its maximum, and thus to further elucidate whether or not TJ proteins were shuttled to the membrane in response to serine proteases, we sought to first disrupt the barrier. We also wanted to determine if serine proteases could reverse barrier disruption. Two methods were used to disrupt the barrier: the calcium switch assay and treatment with cytokines. Calcium is required for the interaction of E-cadherin between adjacent cells, and when this is lost, it induces the retraction of the actin cytoskeleton via phosphorylation of myosin regulatory light chain (MLC2) and dissolution of the adherens junction. This then disrupts TJ structure, ultimately resulting in protein internalization into the cytoplasm (38, 45). We performed the calcium switch in a short time frame. This “fast” assay causes disruption of the adherens and tight junction, but the proteins are not removed into the cytoplasm (31, 49, 57). Inhibitors or compounds can be added at various stages of the calcium switch to determine if they change the rate of recovery of the junction. While the fast calcium switch assay may not give us an indicator of TJ protein trafficking in endosomes, it can indicate whether or not serine proteases can enhance the reformation of the junction. For example, butyrate, a compound known to enhance barrier function, has been shown to increase the speed at which the TJ forms in a calcium switch assay (50). Thus it was hypothesized that serine proteases could also increase the speed at which the barrier recovers.
We found that when trypsin or matriptase was added to the monolayer under calcium-free conditions, the rate at which the barrier recovered when calcium was added back into the system was impeded. This may be because the proteases now have access to the lateral and basolateral membranes and can cleave junction proteins, or activate signaling pathways that activate ion secretion, which would mask increases in TER. There is also no change in the rate of recovery when serine proteases are added as soon as calcium is added back into the system (at switch). The junction needs 30 min of recovery before application of proteases has an effect on TER. If serine proteases are activating receptors and inducing a signaling cascade to the junction, it would be expected that these are occurring even when the junction is not present. It would be expected that when the junction has then recovered sufficiently, the signaling would still be in place to allow an increase in TER. However, this seems to not be the case. This suggests that the serine proteases need a functional tight junction to induce an increase in TER and they cannot induce the formation of a tight junction.
While the calcium switch assay is a good model for the formation of a tight junction, it does not represent the changes in barrier function seen during inflammation. The fast switch also does not cause internalization of TJ proteins, and thus cannot be used to measure trafficking. Thus we decided to induce barrier disruption using inflammatory cytokines, IFNγ and TNFα, which have previously been shown to induce TJ protein internalization and modification of actin cytoskeletal structure (34, 61, 71) to induce changes in barrier function. We first confirmed that our cell system also responded in a similar way. Using the inflammatory cytokines IFNγ and TNFα, we induced a dose-dependent decrease in TER and increase in FITC-dextran flux in our cells. This barrier disruption correlates with an internalization of claudin-4, and a disruption of occludin and ZO-1 from the junction to the cytoplasm, as observed in previous literature (26, 51, 71, 73). Treatment with cytokine induces some apoptosis in our cells, as observed in immunofluorescence images of cells being extruded from the monolayer, and by an increase in cleaved caspase-3. However, we did not see a change in cleaved PARP after cytokine treatment. As PARP is downstream of cleaved caspase-3, this may suggest that the cells were beginning to undergo apoptosis, but PARP had not been activated. To rule out that the barrier dysfunction was independent of apoptosis, we treated cells with ZVAD-FMK at the same time as our cytokines. ZVAD itself had no impact on baseline TER or response to trypsin. Interestingly, ZVAD treatment resulted in a further drop in baseline TER with cytokines. We hypothesize that this is due to the inability of the cells to respond appropriately to the stressful stimulus and remove dying cells from the monolayer in a controlled manner (39). We found that the serine protease-induced increase in TER is significantly impaired by pretreatment with cytokines. Like the calcium switch assay, these experiments demonstrate that a functional tight junction needs to be in place before exogenous trypsin or matriptase can have a barrier-enhancing effect. The removal of specific TJ proteins from the membrane may be the reason why this is lost.
To mimic the changes in TJ structure we observe when the junction is disrupted by cytokines, and to determine which tight junction protein may be the most important in the serine protease response, we depleted occludin in our cells using siRNA. Depletion of occludin did not prevent SCBN cells from forming an electrically tight monolayer, but did reduce baseline TER. The loss of occludin prevented trypsin and matriptase from inducing an increase in TER. Thus occludin is a key tight junction protein in the response to serine proteases. However, from our fractionation experiments, its trafficking to the membrane may not be increased. If trafficking is not altered, serine proteases may be instead changing the arrangement of proteins already present in the membrane through phosphorylation events. To address this, we performed FRAP on SCBN cells transfected with an EGFP-C1-Occludin. We used a chamber on an inverted confocal in which we could perform FRAP on cells in transwells while having perfusion on the apical side for treatment with serine proteases. We found that trypsin induced an increase in the mobile fraction of occludin. This suggests there may be some regulation of tight junction complexity by these proteases through occludin, but the exact mechanism will have to be determined in subsequent experiments. Interestingly, SCBN cells do not express claudin-2, a pore-forming claudin whose expression is associated with decreased barrier function (18, 56, 74). Thus the serine protease-mediated increase in TER is independent of claudin-2.
Relevant to our studies, others have shown that endogenous serine proteases such as matriptase play a role in the development and maintenance of barrier function (35). Previous work investigating the role of matriptase and prostasin in barrier function has used animal models or cell lines where expression of these endogenous proteases had been reduced. Only two other studies have treated cell lines with exogenous serine proteases, one which shows the apical addition of recombinant prostasin can enhance barrier function in Caco-2 cells (7). A second study has corroborated our findings and shows that the addition of matriptase or other serine proteases to epithelial cells induces an increase in transepithelial electrical resistance (19). Here we provide additional evidence that the exogenous apical application of the catalytic subunit of matriptase can also enhance barrier function in polarized intestinal epithelia and have further characterized its addition in disrupted monolayers.
Overall, our results suggest that apical serine proteases activate a pathway that reduces permeability, but that activation of this pathway during conditions where the epithelial barrier has been disrupted, such as in IBD, is not likely to enhance barrier function. However, once the barrier has been reestablished, as in our calcium switch experiments, the protease-mediated pathway can further enhance TER. Targeting this pathway to increase barrier function could perhaps be utilized in patients with IBD under remission to prevent the recurrence of a flare of active disease. Indeed, this pathway may be targeted in other intestinal disorders that are characterized by increased permeability, including celiac disease, diarrhea-predominant IBS, and enteric infection. Manipulating the barrier-enhancing activities of endogenous epithelial proteases like matriptase and prostasin is also a potential therapeutic approach that deserves further study.
GRANTS
This work was supported by grants from the Canadian Institutes of Health Research (MOP-119490, W. K. MacNaughton) and the National Institutes of Health (R01-DK-68271, J. R. Turner). N. J. Ronaghan was supported by a studentship from the Natural Sciences and Engineering Research Council of Canada.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.J.R., V.I., R.Z., P.C., and W.K.M. conception and design of research; N.J.R. and J.S. performed experiments; N.J.R. and W.K.M. analyzed data; N.J.R., P.C., J.R.T., and W.K.M. interpreted results of experiments; N.J.R. prepared figures; N.J.R. drafted manuscript; N.J.R., J.S., P.C., J.R.T., and W.K.M. edited and revised manuscript; N.J.R., J.S., V.I., R.Z., P.C., J.R.T., and W.K.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Live Cell Imaging Facility in the Snyder Institute for Chronic Diseases for expert assistance with the FRAP imaging and analysis.
REFERENCES
- 1.Anderson CA, Boucher G, Lees CW, Franke A, D'Amato M, Taylor KD, Lee JC, Goyette P, Imielinski M, Latiano A, Lagacé C, Scott R, Amininejad L, Bumpstead S, Baidoo L, Baldassano RN, Barclay M, Bayless TM, Brand S, Büning C, Colombel JF, Denson LA, De Vos M, Dubinsky M, Edwards C, Ellinghaus D, Fehrmann RSN, Floyd JAB, Florin T, Franchimont D, Franke L, Georges M, Glas J, Glazer NL, Guthery SL, Haritunians T, Hayward NK, Hugot JP, Jobin G, Laukens D, Lawrance I, Lémann M, Levine A, Libioulle C, Louis E, McGovern DP, Milla M, Montgomery GW, Morley KI, Mowat C, Ng A, Newman W, Ophoff RA, Papi L, Palmieri O, Peyrin-Biroulet L, Panés J, Phillips A, Prescott NJ, Proctor DD, Roberts R, Russell R, Rutgeerts P, Sanderson J, Sans M, Schumm P, Seibold F, Sharma Y, Simms LA, Seielstad M, Steinhart AH, Targan SR, van den Berg LH, Vatn M, Verspaget H, Walters T, Wijmenga C, Wilson DC, Westra HJ, Xavier RJ, Zhao ZZ, Ponsioen CY, Andersen V, Torkvist L, Gazouli M, Anagnou NP, Karlsen TH, Kupcinskas L, Sventoraityte J, Mansfield JC, Kugathasan S, Silverberg MS, Halfvarson J, Rotter JI, Mathew CG, Griffiths AM, Gearry R, Ahmad T, Brant SR, Chamaillard M, Satsangi J, Cho JH, Schreiber S, Daly MJ, Barrett JC, Parkes M, Annese V, Hakonarson H, Radford-Smith G, Duerr RH, Vermeire S, Weersma RK, Rioux JD. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet 43: 246–252, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson JM, Itallie Van CM. Caveolin binds independently to claudin-2 and occludin. Ann NY Acad Sci 1257: 103–107, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Bohe M. Pancreatic and granulocytic endoproteases in faecal extracts from patients with active ulcerative colitis. Scand J Gastroenterol 22: 59–64, 1987. [DOI] [PubMed] [Google Scholar]
- 4.Bruewer M, Nusrat A, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol 171: 6164–6172, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Buhner S, Buning C, Genschel J, Kling K, Herrmann D, Dignass A, Kuechler I, Krueger S, Schmidt HHJ, Lochs H. Genetic basis for increased intestinal permeability in families with Crohn's disease: role of CARD15 3020insC mutation? Gut 55: 342–347, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buret A, Lin YC. Genotypic characterization of an epithelial cell line for the study of parasite-epithelial interactions. J Parasitol 94: 545–548, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Buzza MS, Martin EW, Driesbaugh KH, Desilets A, Leduc R, Antalis TM. Prostasin is required for matriptase activation in intestinal epithelial cells to regulate closure of the paracellular pathway. J Biol Chem 288: 10328–10337, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buzza MS, Netzel-Arnett S, Shea-Donohue T, Zhao A, Lin CY, List K, Szabo R, Fasano A, Bugge TH, Antalis TM. Membrane-anchored serine protease matriptase regulates epithelial barrier formation and permeability in the intestine. Proc Natl Acad Sci USA 107: 4200–4205, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta 1788: 864–871, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cenac N, Chin AC, Garcia-Villar R, Salvador-Cartier C, Ferrier L, Vergnolle N, Buret AG, Fioramonti J, Bueno L. PAR2 activation alters colonic paracellular permeability in mice via IFN-gamma-dependent and -independent pathways. J Physiol 558: 913–925, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cenac N, Coelho AM, Nguyen C, Compton S, Andrade-Gordon P, MacNaughton WK, Wallace JL, Hollenberg MD, Bunnett NW, Garcia-Villar R, Bueno L, Vergnolle N. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am J Pathol 161: 1903–1915, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chin AC, Lee WY, Nusrat A, Vergnolle N, Parkos CA. Neutrophil-mediated activation of epithelial protease-activated receptors-1 and -2 regulates barrier function and transepithelial migration. J Immunol 181: 5702–5710, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Consortium UK IBD Genetics, Barrett JC, Lee JC, Lees CW, Prescott NJ, Anderson CA, Phillips A, Wesley E, Parnell K, Zhang H, Drummond H, Nimmo ER, Massey D, Blaszczyk K, Elliott T, Cotterill L, Dallal H, Lobo AJ, Mowat C, Sanderson JD, Jewell DP, Newman WG, Edwards C, Ahmad T, Mansfield JC, Satsangi J, Parkes M, Mathew CG, Wellcome Trust Case Control Consortium 2 , Donnelly P, Peltonen L, Blackwell JM, Bramon E, Brown MA, Casas JP, Corvin A, Craddock N, Deloukas P, Duncanson A, Jankowski J, Markus HS, McCarthy MI, Palmer CNA, Plomin R, Rautanen A, Sawcer SJ, Samani N, Trembath RC, Viswanathan AC, Wood N, Spencer CCA, Bellenguez C, Davison D, Freeman C, Strange A, Langford C, Hunt SE, Edkins S, Gwilliam R, Blackburn H, Bumpstead SJ, Dronov S, Gillman M, Gray E, Hammond N, Jayakumar A, McCann OT, Liddle J, Perez ML, Potter SC, Ravindrarajah R, Ricketts M, Waller M, Weston P, Widaa S, Whittaker P, Attwood AP, Stephens J, Sambrook J, Ouwehand WH, McArdle WL, Ring SM, Strachan DP. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet 41: 1330–1334, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Inca R, Annese V, Di Leo V, Latiano A, Quaino V, Abazia C, Vettorato MG, Sturniolo GC. Increased intestinal permeability and NOD2 variants in familial and sporadic Crohn's disease. Aliment Pharmacol Ther 23: 1455–1461, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Dörfel MJ, Huber O. Modulation of tight junction structure and function by kinases and phosphatases targeting occludin. J Biomed Biotechnol 2012: 1–14, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friis S, Godiksen S, Bornholdt J, Selzer-Plon J, Rasmussen HB, Bugge TH, Lin CY, Vogel LK. Transport via the transcytotic pathway makes prostasin available as a substrate for matriptase. J Biol Chem 286: 5793–5802, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friis S, Uzzun Sales K, Godiksen S, Peters DE, Lin CY, Vogel LK, Bugge TH. A matriptase-prostasin reciprocal zymogen activation complex with unique features: prostasin as a non-enzymatic co-factor for matriptase activation. J Biol Chem 288: 19028–19039, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol 153: 263–272, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Gall SM, Szabo R, Lee M, Kirchhofer D, Craik CS, Bugge TH, Camerer E. Matriptase activation connects tissue factor-dependent coagulation initiation to epithelial proteolysis and signaling. Blood 127: 3260–3269, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev 13: 3–10, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Hirota CL, Moreau F, Iablokov V, Dicay M, Renaux B, Hollenberg MD, MacNaughton WK. Epidermal growth factor receptor transactivation is required for proteinase-activated receptor-2-induced COX-2 expression in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 303: G111–G119, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med 105: 883–885, 1986. [DOI] [PubMed] [Google Scholar]
- 23.Hollenberg MD, Mihara K, Polley D, Suen JY, Han A, Fairlie DP, Ramachandran R. Biased signalling and proteinase-activated receptors (PARs): targeting inflammatory disease. Br J Pharmacol 171: 1180–1194, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyun E, Andrade-Gordon P, Steinhoff M, Vergnolle N. Protease-activated receptor-2 activation: a major actor in intestinal inflammation. Gut 57: 1222–1229, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Iablokov V, Hirota CL, Peplowski MA, Ramachandran R, Mihara K, Hollenberg MD, MacNaughton WK. Proteinase-activated receptor 2 (PAR2) decreases apoptosis in colonic epithelial cells. J Biol Chem 289: 34366–34377, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Itallie CM, Fanning AS, Holmes J, Anderson JM. Occludin is required for cytokine-induced regulation of tight junction barriers. J Cell Sci 123: 2844–2852, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell 15: 176–188, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosa P, Szabo R, Molinolo AA, Bugge TH. Suppression of Tumorigenicity-14, encoding matriptase, is a critical suppressor of colitis and colitis-associated colon carcinogenesis. Oncogene 31: 3679–3695, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krug SM, Amasheh S, Richter JF, Milatz S, Günzel D, Westphal JK, Huber O, Schulzke JD, Fromm M. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell 20: 3713–3724, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krug SM, Günzel D, Conrad MP, Lee IFM, Amasheh S, Fromm M, Yu ASL. Charge-selective claudin channels. Ann NY Acad Sci 1257: 20–28, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Lacaz-Vieira F, Jaeger MM, Farshori P, Kachar B. Small synthetic peptides homologous to segments of the first external loop of occludin impair tight junction resealing. J Membr Biol 168: 289–297, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Lambert D, O'Neill CA, Padfield PJ. Depletion of Caco-2 cell cholesterol disrupts barrier function by altering the detergent solubility and distribution of specific tight-junction proteins. Biochem J 387: 553–560, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leyvraz C, Charles RP, Rubera I, Guitard M, Rotman S, Breiden B, Sandhoff K, Hummler E. The epidermal barrier function is dependent on the serine protease CAP1/Prss8. J Cell Biol 170: 487–496, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Zhang Q, Wang M, Zhao S, Ma J, Luo N, Li N, Li Y, Xu G, Li J. Interferon-γ and tumor necrosis factor-α disrupt epithelial barrier function by altering lipid composition in membrane microdomains of tight junction. Clin Immunol 126: 67–80, 2008. [DOI] [PubMed] [Google Scholar]
- 35.List K, Bugge TH, Szabo R. Matriptase: potent proteolysis on the cell surface. Mol Med 12: 1–7, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.List K, Haudenschild CC, Szabo R, Chen W, Wahl SM, Swaim W, Engelholm LH, Behrendt N, Bugge TH. Matriptase/MT-SP1 is required for postnatal survival, epidermal barrier function, hair follicle development, and thymic homeostasis. Oncogene 21: 3765–3779, 2002. [DOI] [PubMed] [Google Scholar]
- 37.List K, Kosa P, Szabo R, Bey AL, Wang CB, Molinolo A, Bugge TH. Epithelial integrity is maintained by a matriptase-dependent proteolytic pathway. Am J Pathol 175: 1453–1463, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma TY, Tran D, Hoa N, Nguyen D, Merryfield M, Tarnawski A. Mechanism of extracellular calcium regulation of intestinal epithelial tight junction permeability: role of cytoskeletal involvement. Microsc Res Tech 51: 156–168, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Marchiando AM, Shen L, Graham WV, Edelblum KL, Duckworth CA, Guan Y, Montrose MH, Turner JR, Watson AJM. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterology 140: 1208–1218, e2, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol 4: 225–237, 2003. [DOI] [PubMed] [Google Scholar]
- 41.McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci 109: 2287–2298, 1996. [DOI] [PubMed] [Google Scholar]
- 42.van der Merwe JQ, Hollenberg MD, MacNaughton WK. EGF receptor transactivation and MAP kinase mediate proteinase-activated receptor-2-induced chloride secretion in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 294: G441–G451, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Miele E, Pascarella F, Quaglietta L, Giannetti E, Greco L, Troncone R, Staiano A. Altered intestinal permeability is predictive of early relapse in children with steroid-responsive ulcerative colitis. Aliment Pharmacol Ther 25: 933–939, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Miller GS, List K. The matriptase-prostasin proteolytic cascade in epithelial development and pathology. Cell Tissue Res 351: 245–253, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Nagar B, Overduin M, Ikura M, Rini JM. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature 380: 360–364, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Netzel-Arnett S, Buzza MS, Shea-Donohue T, Désilets A, Leduc R, Fasano A, Bugge TH, Antalis TM. Matriptase protects against experimental colitis and promotes intestinal barrier recovery. Inflamm Bowel Dis 18: 1303–1314, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, Innis-Whitehouse W, Eastburn KK, Madara JL. Tight junctions are membrane microdomains. J Cell Sci 113: 1771–1781, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Oberst MD, Singh B, Ozdemirli M, Dickson RB, Johnson MD, Lin CY. Characterization of matriptase expression in normal human tissues. J Histochem Cytochem 51: 1017–1025, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Parkos CA, Colgan SP, Bacarra AE, Nusrat A, Delp-Archer C, Carlson S, Su DH, Madara JL. Intestinal epithelia (T84) possess basolateral ligands for CD11b/CD18-mediated neutrophil adherence. Am J Physiol Cell Physiol 268: C472–C479, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr 139: 1619–1625, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest 85: 1139–1162, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Raleigh DR, Boe DM, Yu D, Weber CR, Marchiando AM, Bradford EM, Wang Y, Wu L, Schneeberger EE, Shen L, Turner JR. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol 193: 565–582, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramachandran R, Mihara K, Mathur M, Rochdi MD, Bouvier M, DeFea K, Hollenberg MD. Agonist-biased signaling via proteinase activated receptor-2: differential activation of calcium and mitogen-activated protein kinase pathways. Mol Pharmacol 76: 791–801, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramachandran R, Noorbakhsh F, DeFea K, Hollenberg MD. Targeting proteinase-activated receptors: therapeutic potential and challenges. Nat Rev Drug Discov 11: 69–86, 2012. [DOI] [PubMed] [Google Scholar]
- 55.Rapsomaniki MA, Kotsantis P, Symeonidou IE, Giakoumakis NN, Taraviras S, Lygerou Z. easyFRAP: an interactive, easy-to-use tool for qualitative and quantitative analysis of FRAP data. Bioinformatics 28: 1800–1801, 2012. [DOI] [PubMed] [Google Scholar]
- 56.Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, Gunzel D, Fromm M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci 123: 1913–1921, 2010. [DOI] [PubMed] [Google Scholar]
- 57.Rothen-Rutishauser B, Riesen FK, Braun A, Günthert M, Wunderli-Allenspach H. Dynamics of tight and adherens junctions under EGTA treatment. J Membr Biol 188: 151–162, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita S. Possible involvement of phosphorylation of occludin in tight junction formation. J Cell Biol 137: 1393–1401, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwarz BT, Wang F, Shen L, Clayburgh DR, Su L, Wang Y, Fu YX, Turner JR. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology 132: 2383–2394, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Secondulfo M, de Magistris L, Fiandra R, Caserta L, Belletta M, Tartaglione MT, Riegler G, Biagi F, Corazza GR, Carratù R. Intestinal permeability in Crohn's disease patients and their first degree relatives. Dig Liver Dis 33: 680–685, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Shen L, Black ED, Witkowski ED, Lencer WI, Guerriero V, Schneeberger EE, Turner JR. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci 119: 2095–2106, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell 16: 3919–3936, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol 73: 283–309, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol 181: 683–695, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh AB, Harris RC. Epidermal growth factor receptor activation differentially regulates claudin expression and enhances transepithelial resistance in Madin-Darby canine kidney cells. J Biol Chem 279: 3543–3552, 2004. [DOI] [PubMed] [Google Scholar]
- 66.Swystun VA, Renaux B, Moreau F, Wen S, Peplowski MA, Hollenberg MD, MacNaughton WK. Serine proteases decrease intestinal epithelial ion permeability by activation of protein kinase C. Am J Physiol Gastrointest Liver Physiol 297: G60–G70, 2009. [DOI] [PubMed] [Google Scholar]
- 67.Szabo R, Bugge TH. Membrane-anchored serine proteases in vertebrate cell and developmental biology. Annu Rev Cell Dev Biol 27: 213–235, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology 119: 15–22, 2000. [DOI] [PubMed] [Google Scholar]
- 69.Vega-Salas DE, Salas PJ, Rodriguez-Boulan E. Exocytosis of vacuolar apical compartment (VAC): a cell-cell contact controlled mechanism for the establishment of the apical plasma membrane domain in epithelial cells. J Cell Biol 107: 1717–1728, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van De Walle J, Hendrickx A, Romier B, Larondelle Y, Schneider YJ. Inflammatory parameters in Caco-2 cells: Effect of stimuli nature, concentration, combination and cell differentiation. Toxicol Vitr 24: 1441–1449, 2010. [DOI] [PubMed] [Google Scholar]
- 71.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol 166: 409–419, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang F, Schwarz BT, Graham WV, Wang Y, Su L, Clayburgh DR, Abraham C, Turner JR. IFN-γ-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology 131: 1153–1163, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol 290: G496–G504, 2006. [DOI] [PubMed] [Google Scholar]
- 74.Yu ASL, Cheng MH, Angelow S, Gunzel D, Kanzawa SA, Schneeberger EE, Fromm M, Coalson RD. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J Gen Physiol 133: 111–127, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu D, Turner JR. Stimulus-induced reorganization of tight junction structure: the role of membrane traffic. Biochim Biophys Acta 1778: 709–716, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zwanziger D, Staat C, Andjelkovic AV, Blasig IE. Claudin-derived peptides are internalized via specific endocytosis pathways. Ann NY Acad Sci 1257: 29–37, 2012. [DOI] [PubMed] [Google Scholar]