This is the first study investigating the effect dietary nitrate supplementation on hepatic blood flow and on incretin and C-peptide concentrations in young and older adults. Despite a physiologically relevant elevation in plasma nitrite concentration following an acute dose of 11.9 mmol nitrate, there was no effect on hepatic blood flow, plasma glucose, C-peptide, or incretin concentration. Acute supplementation of nitrate does not appear to alter hepatic diffusion or modulate postprandial glucose homeostasis.
Keywords: nitrate, nitric oxide, C-peptide, glucose, incretin
Abstract
Nitric oxide alters gastric blood flow, improves vascular function, and mediates glucose uptake within the intestines and skeletal muscle. Dietary nitrate, acting as a source of nitric oxide, appears to be a potential low-cost therapy that may help maintain glucose homeostasis. In a randomized, double-blind, placebo-controlled crossover study, 31 young and older adult participants had a standardized breakfast, supplemented with either nitrate-rich beetroot juice (11.91 mmol nitrate) or nitrate-depleted beetroot juice as placebo (0.01 mmol nitrate). MRI was used to assess apparent diffusion coefficient (ADC), portal vein flux, and velocity. Plasma glucose, incretin, and C-peptide concentrations and blood pressure were assessed. Outcome variables were measured at baseline and hourly for 3 h. Compared with a placebo, beetroot juice resulted in a significant elevation in plasma nitrate and plasma nitrite concentration. No differences were seen for the young or older adult cohorts between placebo and beetroot juice for ADC, or portal vein flux. There was an interaction effect in the young adults between visits for portal vein velocity. Nitrate supplementation did not reduce plasma glucose, active GLP-1, total GLP-1, or plasma C-peptide concentrations for the young or older adult cohorts. Despite a significant elevation in plasma nitrite concentration following an acute dose of (11.91 mmol) nitrate, there was no effect on hepatic blood flow, plasma glucose, C-peptide, or incretin concentration in healthy adults.
NEW & NOTEWORTHY
This is the first study investigating the effect dietary nitrate supplementation on hepatic blood flow and on incretin and C-peptide concentrations in young and older adults. Despite a physiologically relevant elevation in plasma nitrite concentration following an acute dose of 11.9 mmol nitrate, there was no effect on hepatic blood flow, plasma glucose, C-peptide, or incretin concentration. Acute supplementation of nitrate does not appear to alter hepatic diffusion or modulate postprandial glucose homeostasis.
nitric oxide is produced within the body via two independent pathways. The first pathway in which nitric oxide is produced is via, nitric oxide synthase enzymes (NOS), acting on the amino acid l-arginine in an O2-dependent reaction (1). The second pathway, known as the enterosalivary pathway, is O2 independent. It involves nitrate from the diet being ingested and absorbed through the stomach wall and proximal small intestine (3, 19) and entering the circulation where it is concentrated in the salivary glands. A reduction of nitrate to nitrite via facultative anaerobic bacteria occurs within the mouth (18). Nitrite is then swallowed and some nitrite is converted to nitric oxide in the acidic environment of the stomach (5) while some of the nitrite is absorbed into the circulation and acts as a storage pool for subsequent conversion to nitric oxide (48). Elevated concentrations of nitric oxide have been shown to increase gastric blood flow (55) while acidified nitrite has been shown to protect against enteric pathogens (23).
Another possible mechanism exists for the conversion of nitrate to nitrite, whereby hepatic xanthine oxidoreductase catalyzes the reduction of nitrate to nitrite (31). This mechanism in conjunction with the uptake of nitrite into the portal circulation may explain why one of the highest concentrations of nitrite in any organ is found within the liver (10, 59). Subsequent increases in the bioavailability of nitric oxide within the liver may be expedited by a number of nitrite reductases such as xanthine oxidoreductase (43, 50), aldehyde oxidase (39, 43), cytoglobin (11, 20, 45), and deoxyhemoglobin (16). The potential increase of nitric oxide within the hepatic vasculature may lead to vasodilation of the parenchyma and lead to greater surface area for glucose uptake to occur. However, with aging there is evidence for both diminished nitric oxide production and impaired vascular responses to nitric oxide (49, 52). These age-related changes may be associated with different responses to nitrate supplementation or other interventions aimed at increasing nitric oxide bioavailability.
Diets rich in vegetables have been shown to have beneficial effects on cardiovascular health (23), morbidity, and mortality (33) and to reduce the risk of developing type 2 diabetes mellitus (T2DM) (13) and are rich in inorganic nitrate. There is growing evidence to suggest nitrate is at least in part responsible for these beneficial effects (28a). Recent reports have described how nitrate supplementation can reduce systolic and diastolic blood pressure (BP) in healthy older adults (30, 34, 35) and in clinical cohorts with elevated cardiovascular risk factors (6, 36, 37, 42). Other reports have shown no effect of nitrate supplementation on systolic and diastolic BP in clinical cohorts (24, 58, 58a), although in an overweight and obese group of individuals nitrate supplementation has been shown to improve postprandial endothelial function following a mixed meal (32). Nitric oxide mediates glucose uptake from the intestines (27) and facilitates its disposal into skeletal muscle in animal models (51) and in individuals with T2DM (38). Increases in the bioavailability of nitric oxide stimulate insulin secretion (54) and increase GLUT4 translocation (46). Supplementation of nitrite in the drinking water of eNOS-deficient mice (for 10 wk) reduces glycated hemoglobin concentrations and lowers baseline and postprandial glucose concentrations (12). A study in young adults supplemented with dietary nitrate has shown a reduction in plasma glucose concentrations postexercise compared with placebo (67). Recently, another study described a reduction in baseline plasma glucose concentrations 2.5 h after supplementation with pharmacological sodium nitrate in individuals with T2DM but there was no effect on postprandial glucose concentrations following an oral glucose tolerance test (14). In contrast, Betteridge et al. (7) showed no change in glucose kinetics during exercise in a group of recreationally active individuals. Moreover, beetroot juice taken in conjunction with a 75-g glucose load did not augment glucose uptake in 16 obese, insulin-resistant men (21). Another potential mechanism for changes in glucose concentrations may be related to incretins and their insulinotropic effects. Incretin hormones are released from the small intestine in response to ingestion of food and are a key component in glucose homeostasis via their insulinotropic effect (28). Incretins mediate the uptake of glucose in the intestines in a nitric oxide-dependent fashion (27) and have been shown to promote the production of nitric oxide within the portal vein (17).
Purpose/hypothesis.
Aging may affect the bioavailability of nitric oxide and thus hepatic diffusion. Therefore we will assess our outcomes in a young adult and an older adult cohort. The aim of this study was to assess whether inorganic nitrate modulates portal vein flux and velocity and hepatic microvascular diffusion and secondly to assess whether supplementation with nitrate alters postprandial plasma glucose, incretin, and C-peptide concentrations and BP. We hypothesized that supplementation of the diet with dietary nitrate would increase blood flow to the liver and vasodilate the microvasculature, causing improved postprandial glucose uptake.
MATERIALS AND METHODS
Volunteers.
Thirty-seven individuals (17 healthy young individuals and 20 healthy older adults) provided written, informed consent to participate in this double-blind, placebo-controlled, crossover design study (see Table 1 for subject characteristics). The healthy young individuals were recruited via word of mouth. The older adults were recruited via the National Institute for Health Research (NIHR) Exeter Clinical Research Facility, Exeter 10,000 cohort. This is a database of individuals who have been prescreened and consented to be contacted as research volunteers. The trial commenced in July 2014 and ended in April 2015. Ethical approval was obtained from the Exeter National Research Ethics Service Committee (14/SW/0092). This trial was registered on the ClinicalTrials.gov website (NCT02195856). Healthy young individuals were recruited if they were aged between 18 and 35 and older adults aged between 50 and 75.
Table 1.
Participant characteristics included in the final analysis
| Young Adults | Older Adults | |
|---|---|---|
| n | 16 | 15 |
| Men, % | 68.8 | 53.3 |
| Age, yr | 26.6 ± 6 | 59.2 ± 6 |
| Height, m | 1.75 ± 01 | 1.69 ± 01 |
| Weight, kg | 76.2 ± 13 | 75.3 ± 12 |
| BMI, kg/m2 | 24.6 ± 3.1 | 26.3 ± 3.8 |
| Baseline SBP, mmHg | 121 ± 12 | 130 ± 12 |
| Baseline DBP, mmHg | 70 ± 9 | 79 ± 9 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Participants were excluded if they were unable to consent; took vasoactive medications; had uncontrolled hypertension (systolic BP > 160 mmHg); received antibiotic therapy within the preceding 2 wk; took regular organic nitrate, thiazolidinidiones, or nicorandil; had severe claustrophobia; were smokers (smoked within past 3 mo); had an estimated glomerular filtration rate <30 ml·min−1·1.73 m−2, had a myocardial infarction or cerebrovascular event within the preceding 3 mo; had previous brain surgery; had a cardiac pacemaker; or had metal fragments in the eye or larger metal objects that would interfere with data collection or analysis. Volunteers who had medical interventions where metal implants were inserted were assessed to determine safety in the scanner.
Experimental overview.
Screening and consent took place at the Diabetes and Vascular Research Centre at the NIHR Exeter Clinical Research Facility. Following screening checks volunteers were randomly assigned (within their respective age-group) to a double-blind crossover experimental design to consume 140 ml of nitrate-rich beetroot juice (beetroot juice; containing 11.91 mmol of nitrate; Beet it, James White Drinks, Ipswich, UK) or nitrate-depleted beetroot juice (placebo; nitrate-depleted beetroot juice containing 0.01 mmol of nitrate; Beet it, James White Drinks). The placebo production has been detailed previously (25); the final product is identical in appearance, odor, taste, color, and texture.
On the day of testing, volunteers fasted overnight (from 10 PM) although water consumption was allowed to ensure they arrived in a hydrated state. Volunteers were asked to refrain from antibacterial mouthwash throughout the study and for at least 7 days prior to experimental visits. Antibacterial mouthwash has been demonstrated to reduce the concentration of oral bacterial anaerobes responsible for the reduction of nitrate in the enterosalivary pathway (26). Volunteers were also asked to avoid caffeine for 12 h, alcohol and strenuous activity for 24 h, and nitrate-rich foods on the day prior to their visits.
Volunteers arrived at the Exeter Magnetic Resonance Research Centre at the University of Exeter. A 30-min acclimatization period was implemented prior to the magnetic resonance imaging (MRI) scans. During this acclimatization period a cannula was inserted to take baseline plasma concentrations for glucose (fluoride and EDTA tubes; Sarstedt, S-Monovette, Nümbrecht, Germany). Plasma nitrate and nitrite were collected (lithium heparin tubes; Sarstedt, S-Monovette) and analysis was performed as previously described (24). Prior to the baseline MRI scan, five resting, seated BP measurements were taken (Schiller Medical, Wissembourg, France) and an average of the final three was recorded.
Following the baseline MRI scans the volunteers were provided with either the nitrate-rich beetroot juice or the placebo with two slices of toast, butter, and jam. The combined quantity of carbohydrate equated to 76 g and is approximately equivalent to that that would be consumed during an oral glucose tolerance test (4). Every hour, for 3 subsequent hours, from the consumption of the beetroot juice, another set of scans were performed. Immediately prior to each scan brachial artery BP and venous blood samples were taken and processed as previously described. A minimum 7-day washout period between the crossover was employed.
MRI scans.
A 1.5-T (Philips, Amsterdam, The Netherlands) MRI scanner was used to examine changes in velocity and flux in the portal vein and microvascular diffusion in the posterior right lobe of the liver.
Initial structural images were obtained to orientate the portal vein and an 8-mm slice was selected perpendicular to the long axis of the vein. To determine flux and velocity, a cardiac-triggered velocity-sensitive phase-encoding imaging sequence (22) was employed that obtained image data at 20 time points throughout the cardiac cycle. Analysis of the portal vein was subsequently undertaken using a package supplied as part of the general scanner software. For each separate measurement the circumference of the vessel was manually drawn and recorded for each of the 20 time points to establish a defined region of interest (ROI). Within this ROI, flow and velocity were automatically calculated to give profiles throughout the cardiac cycle. A mean across the cardiac cycle for flux and velocity was created. Day-to-day repeatability for portal vein velocity and flux was assessed in six individuals studied on two occasions and was 16 and 13%, respectively.
To examine the microvascular diffusion in the posterior right lobe of the liver, a magnetic resonance sequence sensitive to flow was employed via the application of magnetic field gradients in three orthogonal directions. Microvascular diffusion was averaged over all directions within the region of interest and is known as the apparent diffusion coefficient (ADC). Day-to-day repeatability was assessed in six individuals on 2 days for ADC [1 ROI: ADC = 1.15 ± 0.12, coefficient of variation (CV) = 9.77%] and with multiple ROI. One ROI away from any major vessels had greater repeatability than two, three, and six sites (2 ROI: ADC = 1.11 ± 0.21, CV = 15.29%; 3 ROI: ADC = 1.10 ± 0.23, CV = 21.34%; 6 ROI: ADC = 1.09 ± 0.33, CV = 30.32%). To calculate ADC within the posterior left lobe of the liver, a ROI (typically 2,500 mm3) was manually drawn using the scanner software and the signal intensity within determined. For multiple ROI during repeatability testing, different locations within the posterior left lobe of the liver were selected. Each selection was in the same approximate location for each individual. ADC was subsequently calculated based on the ratio of signal intensity from the two images generated from the magnetic resonance sequence employed, one of which was sensitive to flow, whereas the other had a low sensitivity to flow,
where S0 is the signal intensity in the low flow sensitivity image, S1 is the signal intensity in the flow-sensitive image, b0 is the magnetic field gradient used in the low flow sensitivity image = 250 s/mm2, and b1 is the magnetic field gradient used in the flow-sensitive image = 750 s/mm2.
Incretin and C-peptide analysis.
To preserve total and active glucagon-like peptide-1 (GLP-1) for analysis, 10 μl of dipeptidyl peptidase 4 inhibitor (Merck Millipore, Darmstadt, Germany) per 1 ml of whole blood was injected into ice-chilled EDTA tubes (Sarstedt, S-Monovette) prior to adding the blood. Samples were immediately centrifuged at 3,600 rpm for 10 min at 4°C, and plasma was aliquoted and flash frozen with liquid nitrogen. Quantification of total and active GLP-1 were performed using an enzymatic immunoassay (Total and Active GLP-1 kits; MSD, Rockville, MD). The interassay variation for total and active GLP-1 was 9.7 and 11.3%, respectively.
C-peptide quantification was performed using a Roche E170 analyzer (Roche Diagnostics, Mannheim, Germany). The assay utilized a direct electrochemiluminescence immunoassay with mouse monoclonal antibodies that were coupled to the paramagnetic particles. Venous blood samples were taken into ice-chilled gel serum tubes (Sarstedt, S-Monovette). Samples were immediately centrifuged at 3,600 rpm for 10 min at 4°C, aliquoted, and stored at −80°C.
Sample size and randomization.
The ADC was our primary outcome. No study to date has assessed the effect of dietary nitrate supplementation on liver diffusion. Therefore, no data were available to power our outcome. For 90% power with an α-level set at P = 0.05 (two tailed), to detect a 1 SD difference, 13 volunteers were required to compare within group for placebo and active conditions. For 80% power with an α-level set at P = 0.05 (two tailed), to detect a 1.05 SD difference, 16 volunteers were required to compare between groups for placebo and active conditions.
Data and statistical analysis.
All data were tested for normality. Where data were not normally distributed a nonparametric test was performed. Data are presented as means ± SD. Statistical analyses were performed on SPSS software version 21.0 (Chicago, IL). Statistical difference was accepted when P < 0.05. Statistical differences were assessed by repeated-measures ANOVAs. Where baseline differences were present, analyses of covariance (ANCOVAs) were used with baseline as a covariate. For age comparisons, group was used as a covariate. Where statistic differences were present post hoc (Bonferroni-corrected) analysis were performed.
RESULTS
Thirty-seven individuals (17 healthy young individuals and 20 healthy older adults) gave written, informed consent to participate. After screening and consent, six individuals were withdrawn from the trial: one individual had abnormal liver function, one had a metal pin (in an area that would interfere with data collection), and four had previously undiagnosed claustrophobia. Thirty-one individuals (16 healthy young individuals and 15 healthy older adults) were randomized to start in either the nitrate-rich beetroot arm or the placebo arm. No differences between dietary intake or exercise patterns were recorded prior to both study visits. The beetroot juice was well tolerated and no adverse events were reported.
Plasma nitrate concentration.
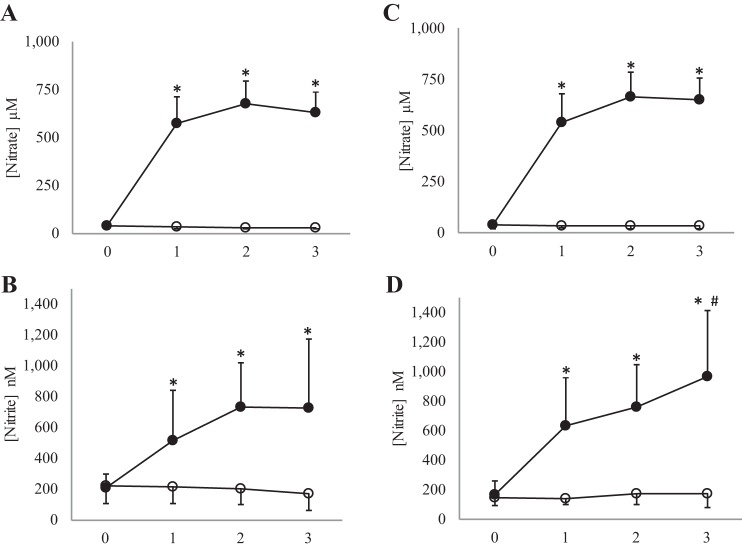
Supplementation with inorganic dietary nitrate caused an increase in plasma nitrate concentration compared with placebo (see Fig. 1). The elevated plasma nitrate concentration was maintained for the entire testing period. Post hoc analysis revealed no significant differences at baseline (prior to any supplementation on the placebo and the nitrate-rich juice arm of the study) for plasma nitrate concentration (young adults: mean difference 2 ± 4.4 μM, P = 0.64, 95% CI −7.4, 11.5; older adults: mean difference 1.5 ± 4.1 μM, P = 0.72, 95% CI −10.4, 7.4). Post hoc analysis revealed a significant increase when beetroot juice was compared with placebo at 1 h postsupplementation (young adult: mean difference 543 ± 37 μM, P < 0.001, 95% CI 463, 624; older adult: mean difference 505 ± 39 μM, P < 0.001, 95% CI 420, 590), 2 h post (young adult: mean difference 645 ± 29 μM, P < 0.001, 95% CI 581, 707; older adult: mean difference 632 ± 35 μM, P < 0.001, 95% CI 556, 710), and 3 h post (young adult: mean difference 598 ± 31 μM, P < 0.001, 95% CI 530, 665; older adult: mean difference 616 ± 26 μM, P < 0.001, 95% CI 559, 673). No statistical difference was present for the young adult group compared with the older adult group for nitrate concentration (F(1,25) = 0.1, P = 0.75).
Fig. 1.
Plasma nitrate and nitrite concentration. Figure depicts changes across time for beetroot and placebo juice. ○, Placebo; ●, beetroot. A and B: young adult cohort. C and D: older adult cohort. *Significant difference when beetroot juice is compared with placebo < 0.001. #Significant difference in the beetroot condition when hour 2 is compared with hour 3.
Plasma nitrite concentration.
Supplementation with inorganic dietary nitrate caused an increase in plasma nitrite concentration compared with placebo (see Fig. 1). This increase was faster in older adults compared with young adults (F(3,75) = 2.93, P = 0.039). The elevated plasma nitrite concentration was maintained for the entire testing period. Post hoc analysis revealed no significant differences at baseline (prior to any supplementation on the placebo and the nitrate-rich juice arm of the study) for plasma nitrite concentration (young adults: mean difference −3.3 ± 74 nM, P = 0.86, 95% CI −43, 36; older adults: mean difference 26.5 ± 78 nM, P = 0.21, 95% CI −17, 69). Post hoc analysis revealed a significant increase when beetroot juice was compared with placebo at 1 h postsupplementation (young adults: mean difference 283 ± 201 nM, P < 0.001, 95% CI 176, 391: older adults: mean difference 471 ± 381 nM, P < 0.001, 95% CI 260, 682), 2 h post (young adults: mean difference 497 ± 259 nM, P < 0.001, 95% CI 353, 640: older adults: mean difference 545 ± 325 nM, P < 0.001, 95% CI 364, 325), and 3 h post (young adults: mean difference 559 ± 201 nM, P < 0.001, 95% CI 442, 675: older adults: mean difference 797 ± 525 nM, P < 0.001, 95% CI 493, 1,100). There was also a significant increase at peak plasma nitrite concentration (hour 3), compared with hour 2 (mean difference 201 ± 344 nM, P = 0.039, 95% CI 11, 392).
ADC.
There was no effect of supplementation (absolute; young adults: F(1,15) = 0.314, P = 0.58; older adults; F(1,14) = 1.65, P = 0.22; change from baseline; young adults: F(1,15) = 0.701, P = 0.42; older adults; F(1,14) = 2.91, P = 0.11) or an interaction effect (time by supplement) when comparing supplementation with inorganic dietary nitrate on hepatic diffusion compared with placebo (absolute; young adults: F(3,45) = 0.25, P = 0.74; older adults; F(3,42) = 1.3, P = 0.28; change from baseline; F(2,30) = 0.13, P = 0.67; older adults; F(2,28) = 0.45, P = 0.64). See Fig. 2 for details.
Fig. 2.
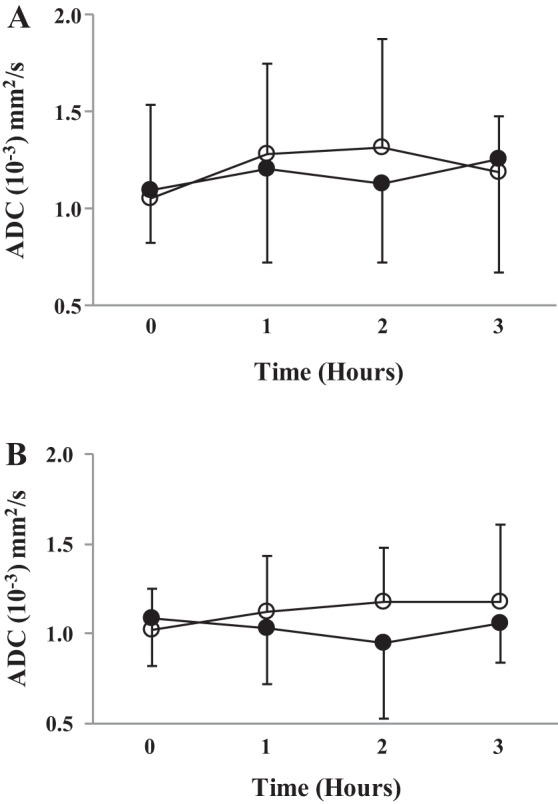
Microvascular diffusion. Figure depicts ADC changes across time for beetroot and placebo juice. A: young adult cohort. B: older adult cohort. ○, Placebo; ●, beetroot.
Portal vein flux.
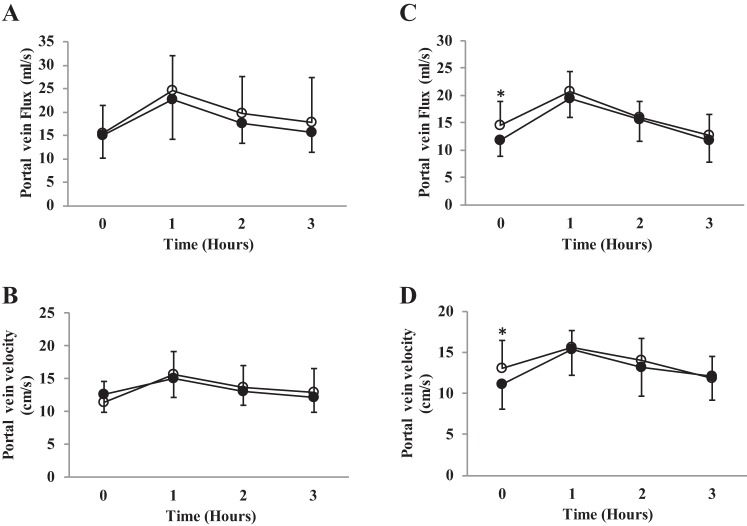
There was a baseline difference for the older adult cohort (older adults: placebo 14.6 ± 4.3 vs. beetroot juice 11.7 ± 2.9 ml/s, P = 0.04, 95% CI −5.67, −0.13) but not in the young adults. See Fig. 3. Therefore, data were analyzed by ANCOVA for the older adult cohort. There was no supplementation (absolute; young adults: F(1,15) = 1.00, P = 0.33; older adults; F(1,12) = 1.28, P = 0.28) or interaction effect (young adults: F(3,45) = 0.34, P = 0.79; older adults: F(2,24) = 0.68, P = 0.52) when comparing supplementation with inorganic dietary nitrate on portal vein flux compared with placebo.
Fig. 3.
Portal vein flux and portal vein velocity. Figure depicts changes across time for beetroot and placebo. A and B: young adult cohort. C and D: older adult cohort. ○, Placebo; ●, beetroot. *Statistically different baseline values between conditions.
Portal vein velocity.
There was a baseline difference for the older adult cohort (older adults: placebo 13 ± 3.4 vs. beetroot 11.1 ± 3 cm/s, P = 0.04, 95% CI −3.7, −1.1) but not in the young adults. See Fig. 3. Therefore, data were analyzed by ANCOVA for the older adult cohort. Portal vein velocity was decreased following beetroot juice, compared with placebo juice for the young adults (F(1,15) = 2.9, P = 0.04); however, no effect was seen in the older adults (F(2,24) = 0.84, P = 0.44).
Plasma glucose concentration.
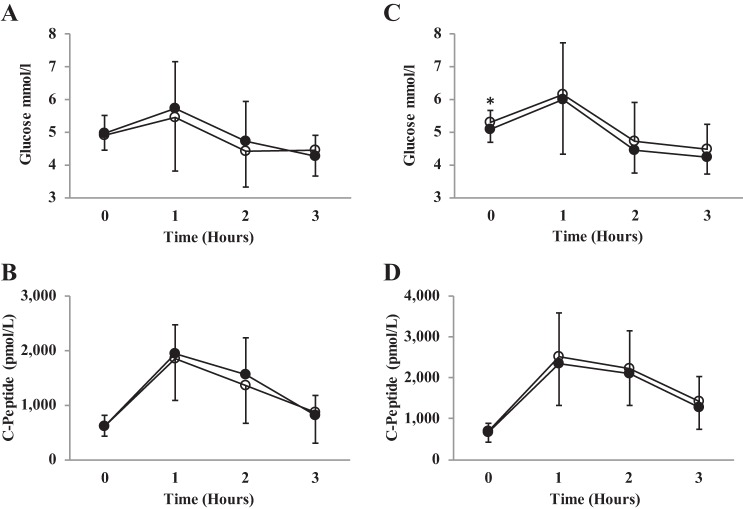
There was a baseline difference for the older adult cohort (older adults: placebo 5.3 ± 0.4 vs. beetroot 5.1 ± 0.4 cm/s, P = 0.02, 95% CI −3.6, −0.04) but not in the young adults. See Fig. 4. Therefore, data were analyzed by ANCOVA for the older adult cohort. No effect of supplementation (young adults: F(1,15) = 0.96, P = 0.35; older adults; F(1,12) = 1.4, P = 0.25) or an interaction effect was present when comparing supplementation with inorganic dietary nitrate on plasma glucose concentrations compared with placebo (young adults: F(3,45) = 0.96, P = 0.42; older adults: F(3,42) = 1.07, P = 0.36). See Fig. 4.
Fig. 4.
Plasma glucose and C-peptide concentration. Figure depicts changes across time for beetroot and placebo juice. A and B: young adult cohort. C and D: older adult cohort. ○, Placebo; ●, beetroot. *Statistically different baseline glucose concentration between conditions.
Effects on incretins and C-peptide.
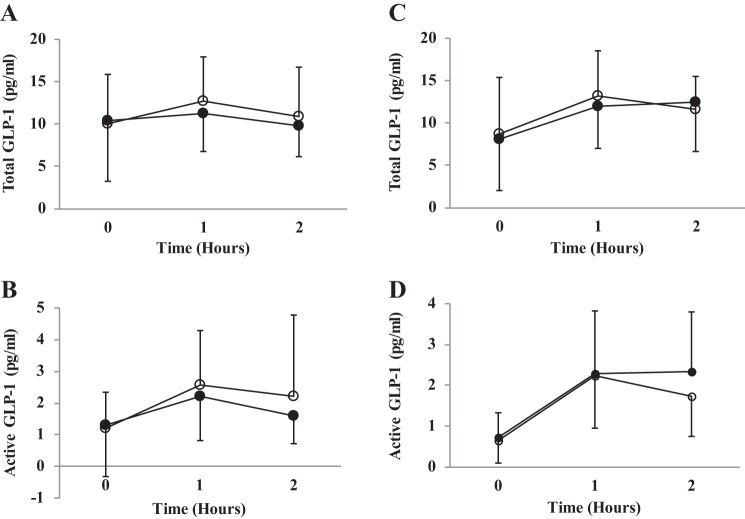
There was no effect of supplementation (young adults: F(1,15) = 0.48, P = 0.49; older adults: F(1,13) = 0.26, P = 0.62) or an interaction effect when comparing supplementation with inorganic dietary nitrate on total GLP-1 concentrations compared with placebo (young adults: F(2,30) = 0.81, P = 0.45; older adults: F(2,26) = 1.63, P = 0.22), active GLP-1 concentrations (young adults: F(2,30) = 0.85, P = 0.43; older adults: F(2,24) = 0.67, P = 0.09) or C-peptide (young adults: F(3,45) = 0.79, P = 0.50; older adults: F(3,42) = 0.39, P = 0.76). See Fig. 5.
Fig. 5.
Total GLP-1 and active GLP-1 concentration. Figure depicts changes across time for beetroot and placebo juice. A and B: young adult cohort. C and D: older adult cohort. ○, Placebo; ●, beetroot.
Effects on resting BP.
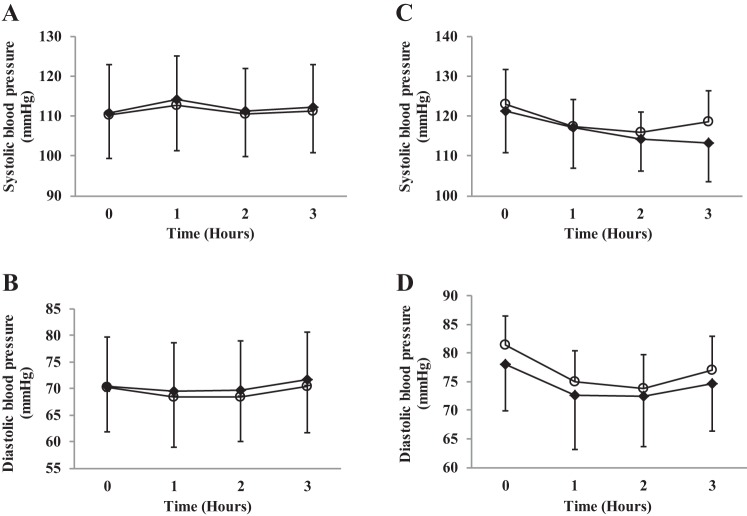
There was no effect of supplementation (young adults: F(1,15) = 1.2, P = 0.28; older adults: F(1,14) = 1.7, P = 0.20) or an interaction effect when comparing supplementation with inorganic dietary nitrate on systolic BP compared with placebo (young adults: F(3,45) = 0.20, P = 0.89; older adults: F(3,42) = 1.7, P = 0.18) or diastolic BP for supplementation (young adults: F(1,15) = 2.6, P = 0.13; older adults: F(1,14) = 4.0, P = 0.06) or an interaction (young adults: F(3,45) = 0.25, P = 0.86; older adults: F(3,42) = 0.45, P = 0.72). See Fig. 6.
Fig. 6.
Systolic blood pressure and diastolic blood pressure. Figure depicts changes across time for beetroot and placebo juice. A and B: young adult cohort. C and D: older adult cohort. ○, Placebo; ●, beetroot.
DISCUSSION
This is the first study to investigate the effects of dietary nitrate supplementation on hepatic blood flow, incretin, and C-peptide concentrations in young and older adults. The primary outcomes were to assess changes in microvascular diffusion (ADC), portal vein flux, and velocity. Nitrate supplementation increased plasma nitrate and nitrite in both the young and older individuals but did not alter portal vein flux or affect ADC. There was, however, an interaction effect in the young adults; however, no effect was present in the older adults between visits for portal vein velocity. Secondary outcomes were to assess plasma glucose, incretin, C-peptide concentrations, and BP changes. Nitrate supplementation did not alter plasma glucose, incretin, or C-peptide concentration and the response was not different in young compared with older individuals. Nitrate supplementation did not lower systolic or diastolic BP in young or older individuals.
Nitrate supplementation and plasma nitrate and plasma nitrite concentration.
Plasma nitrate concentration peaked (∼2,000%) at 2 h following nitrate-rich beetroot juice compared with placebo for both the young and older adult groups. Similarly, plasma nitrite concentration rose following nitrate-rich beetroot juice compared with the placebo and peaked at 2 h for the young group and was highest at 3 h for the older adult group, representing a ∼230 and 460% increase, respectively. The pharmacokinetic response for both plasma nitrate and nitrite concentrations of both cohorts are similar to a previously reported dose-response study in young healthy individuals (66). However, the pharmacokinetic responses of plasma nitrate and nitrite concentrations have not previously been reported in a group of healthy older adults. Kelly et al. (35) recently reported a rise in plasma nitrite concentration of a similar magnitude of 418% in a group of healthy older adults.
Nitrate supplementation and hepatic blood flow.
Despite a statistically significant and physiologically meaningful rise in plasma nitrite concentration, this did not lead to an increase in microvascular diffusion (ADC) or portal vein flux. There was, however, an interaction effect for the young adults for a reduction in portal vein velocity. The portal vein supplies 75% of inflow, with the remainder supplied by the hepatic artery (56), and although the blood it supplies is partially deoxygenated, the portal vein supplies ∼50% of the liver's O2 delivery (see Refs. 29 and 62 for reviews). A higher concentration of deoxyhemoglobin may lead to a faster conversion of nitrite to nitric oxide (16) in the portal vein. It is therefore likely that the highest concentrations of nitric oxide and plasma glucose concentrations would be found in the liver. There was a statistical difference in the older adult cohort for baseline portal vein flux and velocity on the two visits. This study was powered to detect a 1 SD change in our primary outcome, ADC, and as portal vein flux and velocity had poorer reproducibility this may have contributed to the apparent baseline differences. The mean interaction change for portal vein velocity is −0.81 ± 3; in a similarly designed study we would need to recruit 291 individuals to see this effect.
Nitrate supplementation and plasma glucose C-peptide and incretin concentrations.
There was no significant difference between active or placebo juice for plasma glucose concentration. It should be noted that in the older adult cohort there was a statistically significant baseline difference in plasma glucose concentration (mean difference = −0.2 mmol/l or 3.8%) on the placebo and nitrate-rich supplementation day. Given the order of the two arms was randomized this was unlikely to be due to carry over effect from the previous supplementation period. Nitric oxide has been shown to mediate glucose uptake from the intestines and mediate glucose uptake into skeletal muscle (27, 38, 51). Potential mechanisms for this include an elevated nitric oxide bioavailability, which in turn may stimulate insulin secretion (54) and increase GLUT4 translocation (46). Increased levels of GLP-1 have been shown to slow gastric emptying and reduce levels of satiety, which may have significant health benefits by controlling food intake (68). Increasing these incretin concentrations may also lower plasma glucose concentrations via their insulinotropic effects. This would be particularly important in individuals with T2DM as they have an impaired incretin response (53). Although there was no statistical difference for the active or total GLP-1 concentration there was a trend for an increase in plasma concentration of active GLP-1s in the older adult cohort in the active arm (mean difference 0.55 ± 0.86 pg/ml representing a 32.5% increase; see Fig. 5D). If this represents a real difference we would require 41 participants to detect this change. Further analysis is warranted in an older adult population. Despite this, no such trend was evident between the active or placebo juice for C-peptide concentrations. Beetroot juice has been shown to lower the postprandial insulin response in healthy adults; however, it is unclear whether this is due to nitric oxide, polyphenols, or betalains (65). Results from the present study with a true placebo would suggest that nitric oxide is not the active ingredient and further exploration of antioxidants and polyphenols in this area are warranted.
Nitrate supplementation and resting BP.
There was no significant difference in systolic or diastolic BP following nitrate-rich beetroot juice supplementation (at peak plasma nitrite concentration) compared with placebo in the healthy young adult cohort. This is similar to some acute nitrate supplementation studies (15, 57, 64). However, most studies have shown hypotensive effects with acute supplementation regimens (40, 41, 60, 61, 63). There was no significant reduction in systolic BP following nitrate-rich beetroot juice compared with placebo in the healthy older adult cohort. We report a nonsignificant 5 mmHg drop in systolic BP. Kelly et al. (35) report a statistically significant 5 mmHg drop in systolic BP, while another groups reported larger reductions (30). Another study in healthy older adults also reported no effect of nitrate supplementation on systolic BP (8). A detailed pharmacokinetic study in healthy older adults over a prolonged period may identify at what time peak plasma nitrite concentrations occur. If the pharmacokinetic response of plasma nitrite concentrations in the healthy older adult group continued to rise past 3 h, a greater hypotensive effect than measured here may have occurred. However, a dose-response study of young adults showed the elevation in plasma nitrite concentration described above was sufficient to cause a drop in systolic and diastolic BP (66).
Strengths and limitations.
This is the first study to examine the effect of nitrate supplementation on hepatic blood flow. This study has a robust experimental design as a double-blind, placebo-controlled, crossover trial. A limitation to this study is that we did not measure plasma nitrite concentration beyond 3-h supplementation. Future research should aim to elucidate whether plasma nitrite concentration rises beyond 3 h in healthy older adults. The study was powered to detect a change of 1 SD in the outcome measures, changes smaller than this may have occurred but not been noted as significant. Finally, variability in our baseline measurement from the MRI analysis were more than anticipated; however, analysis was performed on absolute and change from baseline, therefore, this should not be a significant impediment.
Conclusion.
This was the first study to examine the hepatic blood flow response to nitrate supplementation. Despite physiologically meaningful elevation in plasma nitrite concentration following an acute dose of 11.91 mmol of nitrate, there was no effect on hepatic blood flow, plasma glucose, incretin, C-peptide concentrations, or systolic and diastolic BP for young or older adults.
GRANTS AND DISCLOSURES
The views and opinions shown within this paper are those of the authors and do not necessarily represent those of the NIHR, National Health Service, or the Department of Health.
N. Benjamin is a director of Heartbeet Ltd. Heartbeet Ltd. has patents granted relevant to this work (production of nitrate-depleted beetroot juice).
The Mason Medical Research Trust funded the GLP-1 analysis in this study. No grant reference number was provided. This grant is held by M. Gilchrist.
J. Fulford's salary was supported via an NIHR grant.
All other authors report no conflict of interest.
AUTHOR CONTRIBUTIONS
A.I.S., D.P.W., P.G.W., N.B., A.C.S., and M.G. conception and design of research; A.I.S., J.F., and M.G. performed experiments; A.I.S., D.P.W., J.F., A.C.S., and M.G. analyzed data; A.I.S., D.P.W., J.F., P.G.W., A.C.S., and M.G. interpreted results of experiments; A.I.S. prepared figures; A.I.S. and M.G. drafted manuscript; A.I.S., D.P.W., J.F., P.G.W., A.C.S., and M.G. edited and revised manuscript; A.I.S., D.P.W., J.F., P.G.W., N.B., A.C.S., and M.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the support of the NIHR Exeter Clinical Research facility. We also thank the research nurses involved in the study and importantly the volunteers.
REFERENCES
- 1.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J 357: 593–615, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartholomew B, Hill M. The pharmacology of dietary nitrate and the origin of urinary nitrate. Food Chem Toxicol 22: 789–795, 1984. [DOI] [PubMed] [Google Scholar]
- 4.Bartoli E, Fra G, Schianca GC. The oral glucose tolerance test (OGTT) revisited. Eur J Intern Med 22: 8–12, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin N, O'Driscoll F, Dougall H, Duncan C, Smith S, Golden M, McKenzie H. Stomach NO synthesis. Nature 368: 502, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Berry MJ, Justus NW, Hauser JI, Case AH, Helms CC, Basu S, Rogers Z, Lewis MT, Miller GD. Dietary nitrate supplementation improves exercise performance and decreases blood pressure in COPD patients. Nitric Oxide 48: 22–30, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betteridge S, Bescós R, Martorell M, Pons A, Garnham AP, Stathis CG, McConell GK. No effect of acute beetroot juice ingestion on oxygen consumption, glucose kinetics or skeletal muscle metabolism during submaximal exercise in males. J Appl Physiol 120: 391–398, 2016. [DOI] [PubMed] [Google Scholar]
- 8.Bondonno CP, Liu AH, Croft KD, Ward NC, Yang X, Considine MJ, Puddey IB, Woodman RJ, Hodgson JM. Short-term effects of nitrate-rich green leafy vegetables on blood pressure and arterial stiffness in individuals with high-normal blood pressure. Free Radic Biol Med 77: 353–362, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, Maloney RE, Bharti A, Rodriguez J, Feelisch M. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol 1: 290–297, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Burmester T, Ebner B, Weich B, Hankeln T. Cytoglobin: a novel globin type ubiquitously expressed invertebrate tissues. Mol Biol Evol 19: 416–421, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Carlström M, Larsen FJ, Nyström T, Hezel M, Borniquel S, Weitzberg E, Lundberg JO. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc Natl Acad Sci USA 107: 17716–17720, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. BMJ 341: c4229, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cermak NM, Hansen D, Kouw IW, van Dijk JW, Blackwell JR, Jones AM, Gibala MJ, van Loon LJ. A single dose of sodium nitrate does not improve oral glucose tolerance in patients with type 2 diabetes mellitus. Nutr Res 35: 674–680, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Coles LT, Clifton PM. Effect of beetroot juice on lowering blood pressure in free-living, disease-free adults: a randomized, placebo-controlled trial. Nutr J 11: 1475–2891, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9: 1498–1505, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Ding KH, Zhong Q, Xu J, Isales CM. Glucose-dependent insulinotropic peptide: differential effects on hepatic artery vs. portal vein endothelial cells. Am J Physiol Endocrinol Metab 286: E773–E779, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Duncan C, Dougall H, Johnston P, Green S, Brogan R, Smith L, Golden M, Benjamin N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med 1: 546–551, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Florin THJ, Neale G, Cummings JH. The effect of dietary nitrate on nitrate and nitrite excretion in man. Br J Nutr 64: 387–397, 1990. [DOI] [PubMed] [Google Scholar]
- 20.Fordel E, Thijs L, Moens L, Dewilde S. Neuroglobin and cytoglobin expression in mice. FEBS J 274: 1312–1317, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs D, Nyakayiru J, Draijer R, Mulder TP, Hopman MT, Eijsvogels TM, Thijssen DH. Impact of flavonoid-rich black tea and beetroot juice on postprandial peripheral vascular resistance and glucose homeostasis in obese, insulin-resistant men: a randomized controlled trial. Nutr Metab (Lond) 13: 34, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gatehouse PD, Keegan J, Crowe LA, Masood S, Mohiaddin RH, Kreitner KF, Firmin DN. Applications of phase-contrast flow and velocity imaging in cardiovascular MRI. Eur Radiol 15: 2172–2184, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Gilchrist M, Winyard P, Benjamin N. Dietary nitrate — good or bad? Nitric Oxide 22: 104–109, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Gilchrist M, Winyard PG, Aizawa K, Anning C, Shore A, Benjamin N. Effect of dietary nitrate on blood pressure, endothelial function, and insulin sensitivity in type 2 diabetes. Free Radic Biol Med 60: 89–97, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Gilchrist M, Winyard PG, Fulford J, Anning C, Shore A, Benjamin N. Dietary nitrate supplementation improves reaction time in type 2 diabetes: development and application of a novel nitrate-depleted beetroot juice placebo. Nitric Oxide 40: 67–74, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Govoni M, Jansson EÅ, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 19: 333–337, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Guan X, Stoll B, Lu X, Tappenden KA, Holst JJ, Hartmann B, Burrin DG. GLP-2-mediated up-regulation of intestinal blood flow and glucose uptake is nitric oxide-dependent in TPN-fed piglets. Gastroenterology 125: 136–147, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 87: 1409–1439, 2007. [DOI] [PubMed] [Google Scholar]
- 28a.Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr 90: 1–10, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Hwang S. Microcirculation of the liver. In: Venous Embolization of the Liver, edited by Madoff DC, Makuuchi M, Mizuno T, Vauthey JN. New York: Springer, 2011, p. 9–13. [Google Scholar]
- 30.Jajja A, Sutyarjoko A, Lara J, Rennie K, Brandt K, Qadir O, Siervo M. Beetroot supplementation lowers daily systolic blood pressure in older, overweight subjects. Nutr Res 34: 868–875, 2014. [DOI] [PubMed] [Google Scholar]
- 31.Jansson EA, Huang L, Malkey R, Govoni M, Nihlen C, Olsson A, Stensdotter M, Petersson J, Holm L, Weitzberg E, Lundberg JO. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol 4: 411–417, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Joris PJ, Mensink RP. Beetroot juice improves in overweight and slightly obese men postprandial endothelial function after consumption of a mixed meal. Atherosclerosis 231: 78–83, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Colditz G, Ascherio A, Rosner B, Spiegelmann D, Willet WC. The effect of fruit and vegetable intake on risk of coronary heart disease. Ann Intern Med 134: 1106–1114, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension 65: 320–327, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly J, Fulford J, Vanhatalo A, Blackwell JR, French O, Bailey SJ, Gilchrist M, Winyard PG, Jones AM. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. Am J Physiol Regul Integr Comp Physiol 304: R73–R83, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Kenjale AA, Ham KL, Stabler T, Robbins JL, Johnson JL, VanBruggen M, Privette G, Yim E, Kraus WE, Allen JD. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol 110: 1582–1591, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerley CP, Cahill K, Bolger K, McGowan A, Burke C, Faul J, Cormican L. Dietary nitrate supplementation in COPD: an acute, double-blind, randomized, placebo-controlled, crossover trial☆. Nitric Oxide 44: 105–111, 2015. [DOI] [PubMed] [Google Scholar]
- 38.Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK. Nitric oxide synthase inhibition reduces glucose uptake during exercise in individuals with type 2 diabetes more than in control subjects. Diabetes 51: 2572–2580, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Kundu TK, Velayutham M, Zweier JL. Aldehyde oxidase functions as a superoxide generating NADH oxidase: an important redox regulated pathway of cellular oxygen radical formation. Biochemistry 51: 2930–2939, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lansley KE, Winyard P, Fulford J, Vanhatalo A, Bailey JB, Blackwell JR, DiMenna FJ, Gilchrist M, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol 110: 591–600, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Lansley KE, Winyard PG, Bailey SJ, Vanhatalo A, Wilkerson DP, Blackwell JR, Gilchrist M, Benjamin N, Jones AM. Acute dietary nitrate supplementation improves cycling time trial performance. Med Sci Sports Exerc 43: 1125–1131, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Leong P, Basham JE, Yong T, Chazan A, Finlay P, Barnes S, Bardin PG, Campbell D. A double blind randomized placebo control crossover trial on the effect of dietary nitrate supplementation on exercise tolerance in stable moderate chronic obstructive pulmonary disease. BMC Pulm Med 15: 52, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H, Cui H, Kundu TK, Alzawahra W, Zweier JL. Nitric oxide production from nitrite occurs primarily in tissues not in the blood critical role of xanthine oxidase and aldehyde oxidase. J Biol Chem 283: 17855–17863, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H, Hemann C, Abdelghany TM, El-Mahdy MA, Zweier JL. Characterization of the mechanism and magnitude of cytoglobin-mediated nitrite reduction and nitric oxide generation under anaerobic conditions. J Biol Chem 287: 36623–36633, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Hu X, Selvakumar P, Russell RR, Cushman SW, Holman GD, Young LH. Role of the nitric oxide pathway in AMPK-mediated glucose uptake and GLUT4 translocation in heart muscle. Am J Physiol Endocrinol Metab 287: E834–E841, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Lundberg J, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7: 156–167, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Lyons D, Roy S, Patel M, Benjamin N, Swift CG. Impaired nitric oxide-mediated vasodilatation and total body nitric oxide production in healthy old age. Clin Sci (Lond) 93: 519–525, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Martin HM, Moore KP, Bosmans E, Davies S, Burroughs AK, Dhillon AP, Tosh D, Harrison R. Xanthine oxidoreductase is present in bile ducts of normal and cirrhotic liver. Free Radic Biol Med 37: 1214–1223, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Merry TL, Steinberg GR, Lynch GS, McConell GK. Skeletal muscle glucose uptake during contraction is regulated by nitric oxide and ROS independently of AMPK. Am J Physiol Endocrinol Metab 298: E577–E585, 2010. [DOI] [PubMed] [Google Scholar]
- 52.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol 93: 1644–1649, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia 54: 10–18, 2011. [DOI] [PubMed] [Google Scholar]
- 54.Nystrom T, Ortsater H, Huang Z, Zhang F, Larsen FJ, Weitzberg E, Lundberg JO, Sjoholm A. Inorganic nitrite stimulates pancreatic islet blood flow and insulin secretion. Free Radic Biol Med 53: 1017–1023, 2012. [DOI] [PubMed] [Google Scholar]
- 55.Petersson J, Carlström M, Schreiber O, Phillipson M, Christoffersson G, Jägare A, Roos S, Jansson EÅ, Persson AEG, Lundberg JO, Holm L. Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radic Biol Med 46: 1068–1075, 2009. [DOI] [PubMed] [Google Scholar]
- 56.Rappaport A. Hepatic blood flow: morphologic aspects and physiologic regulation. Int Rev Physiol 21: 1–63, 1979. [PubMed] [Google Scholar]
- 57.Sandbakk SB, Sandbakk Ø, Peacock O, James P, Welde B, Stokes K, Böhlke N, Tjønna AE. Effects of acute supplementation of l-arginine and nitrate on endurance and sprint performance in elite athletes. Nitric Oxide 48: 10–15, 2014. [DOI] [PubMed] [Google Scholar]
- 58.Shepherd AI, Gilchrist M, Winyard PG, Jones AM, Hallmann E, Kazimierczak R, Rembialkowska E, Benjamin N, Shore AC, Wilkerson DP. Effects of dietary nitrate supplementation on the oxygen cost of exercise and walking performance in individuals with type 2 diabetes: a randomized, double-blind, placebo-controlled crossover trial. Free Radic Biol Med 86: 200–208, 2015. [DOI] [PubMed] [Google Scholar]
- 58a.Shepherd AI, Wilkerson DP, Dobson L, Kelly J, Winyard PG, Jones AM, Benjamin N, Shore AC, Gilchrist M. The effect of dietary nitrate supplementation on the oxygen cost of cycling, walking performance and resting blood pressure in individuals with chronic obstructive pulmonary disease: a double blind placebo controlled, randomised control trial. Nitric Oxide 48: 31–37, 2015. [DOI] [PubMed] [Google Scholar]
- 59.Totzeck M, Hendgen-Cotta UB, Luedike P, Berenbrink M, Klare JP, Steinhoff HJ, Semmler D, Shiva S, Williams D, Kipar A, Gladwin MT, Schrader J, Kelm M, Cossins AR, Rassaf T. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation 126: 325–334, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vanhatalo A, Bailey JB, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard P, Jones AM. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol 299: R1121–R1131, 2010. [DOI] [PubMed] [Google Scholar]
- 61.Vanhatalo A, Fulford J, Bailey SJ, Blackwell JR, Winyard PG, Jones AM. Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. J Physiol 589: 5517–5528, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev 89: 1269–1339, 2009. [DOI] [PubMed] [Google Scholar]
- 63.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 51: 784–790, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilkerson DP, Hayward GM, Bailey SJ, Vanhatalo A, Blackwell JR, Jones AM. Influence of acute dietary nitrate supplementation on 50 mile time trial performance in well-trained cyclists. Eur J Appl Physiol 112: 4127–4134, 2012. [DOI] [PubMed] [Google Scholar]
- 65.Wootton-Beard PC, Brandt K, Fell D, Warner S, Ryan L. Effects of a beetroot juice with high neobetanin content on the early-phase insulin response in healthy volunteers. J Nutr Sci 3: e9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jeukendrup AE, Vanhatalo A, Jones AM. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol 115: 325–336, 2013. [DOI] [PubMed] [Google Scholar]
- 67.Wylie LJ, Mohr M, Krustrup P, Jackman SR, Ermiotadis G, Kelly J, Black MI, Bailey SJ, Vanhatalo A, Jones AM. Dietary nitrate supplementation improves team sport-specific intense intermittent exercise performance. Eur J Appl Physiol 113: 1673–1684, 2013. [DOI] [PubMed] [Google Scholar]
- 68.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 359: 824–830, 2002. [DOI] [PubMed] [Google Scholar]