Abstract
Intestinal wounds often occur during inflammatory and ischemic disorders of the gut. To repair damage, intestinal epithelial cells must rapidly spread and migrate to cover exposed lamina propria, events that involve redox signaling. Wounds are subject to extensive redox alterations, particularly resulting from H2O2 produced in the adjacent tissue by both the epithelium and emigrating leukocytes. The mechanisms governing these processes are not fully understood, particularly at the level of protein signaling. Crk-associated substrate, or Cas, is an important signaling protein known to modulate focal adhesion and actin cytoskeletal dynamics, whose association with Crk is regulated by Abl kinase, a ubiquitously expressed tyrosine kinase. We sought to evaluate the role of Abl regulation of Cas at the level of cell spreading and migration during wound closure. As a model, we used intestinal epithelial cells exposed to H2O2 or scratch wounded to assess the Abl-Cas signaling pathway. We characterized the localization of phosphorylated Cas in mouse colonic epithelium under baseline conditions and after biopsy wounding the mucosa. Analysis of actin and focal adhesion dynamics by microscopy or biochemical analysis after manipulating Abl kinase revealed that Abl controls redox-dependent Cas phosphorylation and localization to influence cell spreading and migration. Collectively, our data shed new light on redox-sensitive protein signaling modules controlling intestinal wound healing.
Keywords: restitution, cytoskeleton, focal adhesions, reactive oxygen species
NEW & NOTHEWORTHY
The rapid healing of intestinal wounds is critical to gut barrier function and homeostasis. In this report, we demonstrate a new redox-dependent role of Cas, a key focal adhesion complex docking protein that is controlled by Abl kinase in regulating the reorganization of the epithelium in response to increased leukocyte activity. This finding highlights the sophisticated protein signaling pathways used between cell types during wound healing that are mediated by reactive oxygen species.
the intestinal epithelium forms a protective, selectively permeable barrier that protects the underlying mucosa from luminal contents. Mucosal wounds associated with epithelial injury are often observed in infectious, inflammatory, and ischemic disorders of the gut. In response to mucosal damage, epithelial cells migrate as a sheet to cover denuded surfaces to restore the critical barrier, whereas intestinal stem cells promote renewal of cell numbers and regeneration of crypt architecture in the wound bed (23). Migration of the epithelial sheet requires the coordination of movements between each cell in the monolayer, and within each cell the actin cytoskeleton interfaces with cell junctions and focal adhesions (FAs) to control changes in cell shape, spreading, and migration. Additionally, FAs are integrin-dependent cell contacts with the extracellular matrix that function by dynamic protein-protein complexes (Fig. 1), many of which are regulated by protein phosphorylation and can control the dynamics of the actin cytoskeleton (7, 25). A key component of FAs is Cas, a 130-kDa protein that plays a pivotal scaffolding role in FA assembly (8, 9). To drive FA dynamics, Cas interacts with a distinct set of adaptor proteins, including Crk, in a phosphorylation-dependent manner through Src homology (SH) 2 and SH3 domains (26). However, the role of Cas in promoting intestinal wound healing is not known.
Fig. 1.
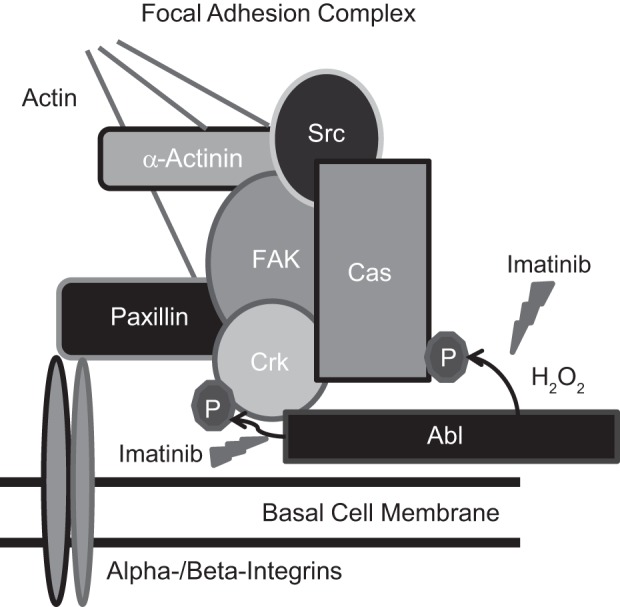
Focal adhesions are subject to redox control. Diagram of focal adhesion complexes including the common proteins is shown. Imatinib is a pharmacological agent that inhibits Abl kinase activity. Abl normally phosphorylates Crk but increases in activity for Cas phosphorylation during H2O2 treatment.
Reactive oxygen species (ROS) are generated at the wound site in the epithelium (19, 20), upon integrin engagement during cell attachment and spreading (6), immediately after contact with endogenous microbiota (30), and by the activity of circulating leukocytes that emigrate into wound sites (22). High levels of ROS, such as that seen in phagocytes, have antibacterial effects, whereas lower levels, such as that observed in epithelia, act as a second messenger in cell signaling, regulating protein function at the level of cysteine oxidation and ultimately promoting cell growth (31) and movement (30). It is both the type and amount of ROS generated, along with its spatiotemporal distribution, that dictates the ROS effect. Although the production of H2O2 in the wound bed has been well established and appears to play a beneficial role (29), the exact mechanisms in facilitating wound healing, particularly the protein signaling events involved, remain largely unknown.
Abl kinase is a ubiquitously expressed enzyme involved in numerous cellular processes that have been linked with redox changes in the cell (28) and cytoskeletal dynamics (12). In fibroblasts, the major substrate of Abl is Crk, which, when phosphorylated, dissociates FA complexes and retards the rate of cell migration (15). However, the contributions of the Abl-Cas signaling module to cell migration in the intestinal epithelia are largely unknown. Importantly, Cas has been shown to be under redox control in bladder cells (11). Taken together, data from previous studies suggest that Abl might also play a redox-dependent role in regulation of Cas to control actin and FA dynamics during wound healing.
Therefore, in an attempt to better define H2O2-dependent mechanisms that enhance or regulate wound healing in the intestine, we investigated the effect of redox stimulation on Cas within mouse colonic epithelium by biopsy wounding or with intestinal epithelial cells in culture and examined the effects of changes in Cas phosphorylation on cell spreading, attachment, and migration, as well as the role Abl plays in these processes. Our analysis has substantiated previous findings demonstrating redox control of Cas (10, 11) and further shows that Abl kinase regulates Cas phosphorylation during H2O2 stimulation in intestinal epithelial cells. With the use of the pharmacological inhibitor of Abl, imatinib, it was determined that the Abl-Cas signaling pathway is important in cytoskeletal dynamics, migration, and cell spreading at the wound edge. Taken together, these facts give new insight to the redox-sensitive mechanisms involving Abl and Cas signaling to control epithelial cell spreading and migration during intestinal wound healing.
MATERIALS AND METHODS
Cell culture reagents.
SKCO15, SKCO15-actin-enhanced green fluorescent protein (eGFP), and Caco-2 model human intestinal epithelial cells were used in these studies and maintained in DMEM (Cellgro) supplemented with 10% fetal bovine serum (Sigma), nonessential amino acids, and l-glutamine, or appropriate antibiotics. Cells were routinely checked for mycoplasma contamination and confirmed negative. Cell monolayers were scratch wounded using a 10-μl micropipette tip attached to a Pasteur pipette with vacuum suction and imaged by light microscopy, and the wound area was quantified by ImageJ. Imatinib mesylate was obtained from BioVision, whereas BSA, N-acetyl cysteine (NAC), arsenite, and H2O2 were obtained from Sigma.
Mouse biopsy wounding.
Colonic biopsy wounding in the distal colon was performed essentially as described previously (20). Briefly, wounds of ∼1 mm2 in area that breached the submucosa were generated with biopsy forceps in the distal colon of mice. At 2 days after wounding, wounds were excised and mounted in optimal cutting temperature compound. Frozen sections were prepared and stained using immunofluorescence. As controls, intact epithelium (unwounded) was also stained using the same procedures. All animals were handled in accordance with approved IACUC protocols.
Antibodies.
Cas pY410 and Crk-L pY207 rabbit antibodies were purchased from Cell Signaling. Mouse anti-Crk was obtained from BD Transduction Laboratories, whereas rabbit Cas antibody was purchased from Santa Cruz Biotechnology. Calnexin and tubulin antibodies were obtained from Sigma. Secondary horseradish peroxidase-conjugated antibodies for Western blotting were purchased from Jackson ImmunoResearch Laboratories.
Microscopy.
Cells were fixed in 4% formaldehyde for 10 min and permeabilized with 0.5% Triton X-100 for 5 min, with PBS washes following each step. Cells were briefly incubated with PBS-BSA solution and then with primary antibody (1/250) for 2 h at room temperature. After several PBS washes, secondary antibodies (1/1,000, Molecular Probes Alexa Fluor 488 or 555) or phalloidin (1/1,000, Molecular Probes Alexa Fluor 555) were added and left for 1 h at room temperature. To finish, coverslips were washed in PBS and mounted using Prolong (Invitrogen) and then visualized by fluorescence microscopy. Images in Figs. 2 and 4A were taken with an Olympus FV1000 confocal microscope. Figures 4B and 5B were acquired on a Zeiss AxioCam Epifluorescense microscope. To measure cell height at the wound edge (Fig. 5C), z-stacks were acquired on a Zeiss 510 confocal microscope from the basal to apical membranes of the monolayer adjacent to membranes protruding into the wounded space to generate an x–z plane and cell height measurement. Migration patterns of SK-CO15 intestinal epithelial cells expressing eGFP-tagged actin were visualized using Nikon A1R laser-scanning confocal microscope equipped with CO2 and temperature control (Fig. 6).
Fig. 2.
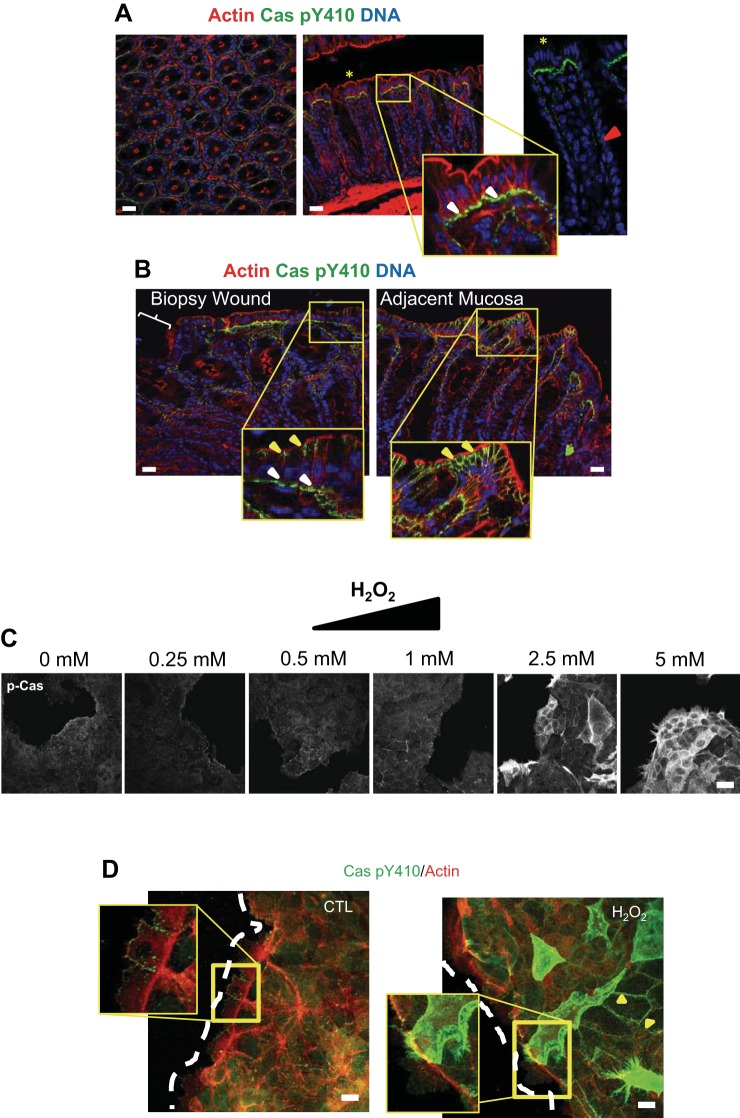
Cas is redox regulated in vivo and in vitro. A and B: immunofluorescence confocal microscopy of cross sections and longitudinal sections from normal (A) and biopsy-wounded mouse intestinal mucosa (B) stained for phospho-Cas (green). The far right inset in A shows the gradient of p-Cas along the transit-amplifying zone (red arrow) and into the apical region of the crypt. In B, the adjacent mucosa (right) is ∼5–6 crypts away from the biopsy wound bed. Actin is shown in red, whereas nuclei are blue. Bars = 100 μm. C: subconfluent SKCO15 cells were treated with a series of H2O2 concentrations ranging from 0.25–5.00 mM, each for 15 min, and then analyzed by immunofluorescence with phospho-Cas antibody. Bars = 100 μm. D: edges of SKCO15 intestinal epithelial cell monolayers 1.5 h after scratch wounding (dashed white line represents wound edge) and then treated for 15 min with 5 mM H2O2. Cells were stained with Cas Y410 phospho-specific antibodies (green) and phalloidin Alexa Fluor 555 (red). Images represent z-stack compressions of wound edge. White arrows point to p-Cas in focal adhesions; yellow arrows point to p-Cas in the lateral cell surface. Bar = 20 μm.
Fig. 4.
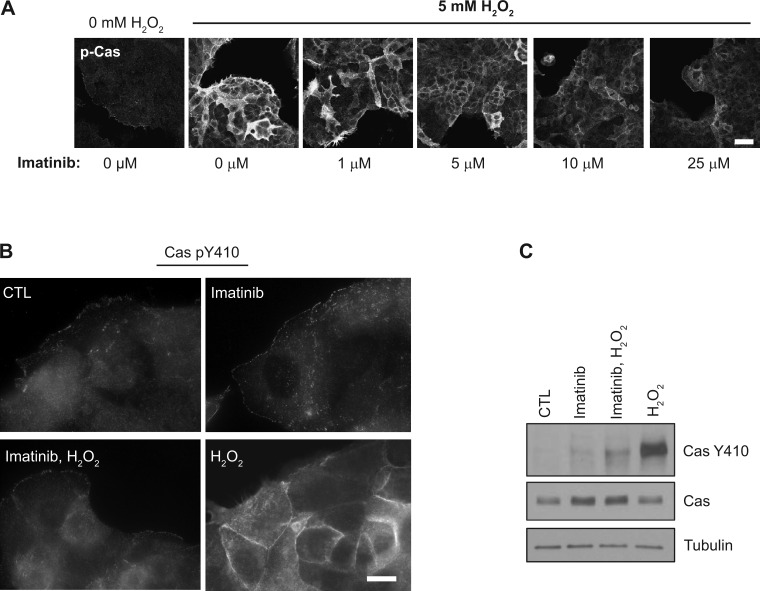
Abl kinase increases Cas pY410 levels during H2O2 treatment. A: subconfluent SKCO15 cells were treated with increasing doses of imatinib ranging from 1–25 μM for 60 min, subsequently treated with 5 mM H2O2 for 15 min, then analyzed by microscopy for phospho-Cas. B and C: SKCO15 intestinal cells were exposed to 20 μM imatinib or control for 1 h before a subsequent exposure to 5 mM H2O2 for 15 min, followed by immunofluorescence microscopy under high magnification (B) or by Western blotting (C) to analyze Cas pY410.
Fig. 5.
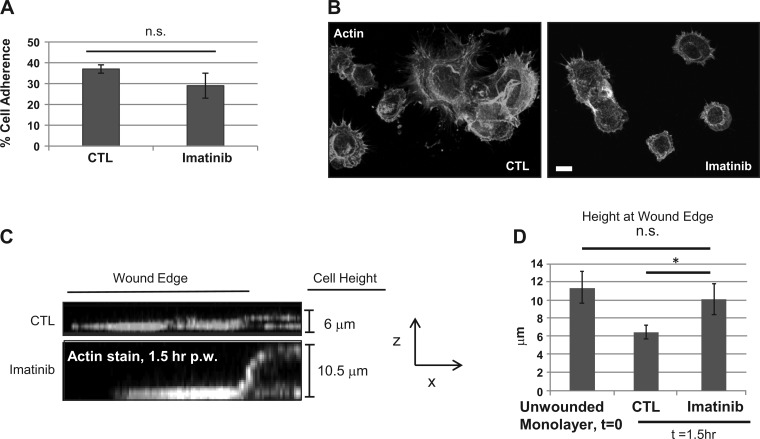
Imatinib affects epithelial cell spreading. Imatinib-treated (20 μM, 1 h) SKCO15 cell suspensions were allowed to attach to collagen-coated coverslips or 96-well plates for 15 min followed by 3 washes in PBS. In A, cells were fixed, permeabilized, and then stained with phalloidin Alexa Fluor 555 and analyzed by fluorescence microscopy. In B, suspended cells were incubated with bis-carboxymethyl-carboxyfluorescein for 1 h before the adhesion assay and fluorescence measured at 488 nm excitation on a plate reader. C: SKCO15 cells were scratch wounded and then allowed to incubate for 1.5 h before fixation, permeabilization, and then staining with phalloidin Alexa Fluor 555. Z-stacks were obtained by confocal microscopy and side images generated by optical slice compression. Corresponding distances between basal and apical surfaces of wound edge were averaged in D and compared with the average height of an unwounded cell monolayer. *P < 0.05.
Fig. 6.
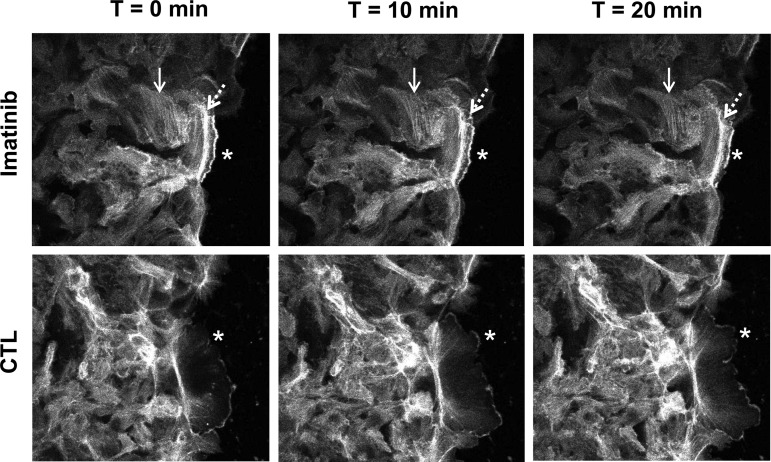
Inhibition of Abl with imatinib decreases actin dynamics. The effects of Abl inhibition on epithelial cell migration using 20 μM imatinib for 1 h were analyzed by fluorescence video or by immunofluorescence microscopy. SKCO15 cells stably expressing actin-green fluorescent protein in the presence of imatinib or under control conditions were analyzed for 30 min by fluorescence video microscopy (shown are images at 0 min, 10 min, and 20 min; 0 min = 1 h treatment, 3 h postwounding). Cell monolayers were scratch wounded, and at 3 h postwounding microscopic analysis was focused on the wound edge. F-actin filaments (solid arrows) and lamellipodia (asterisks) have slower dynamics than controls. A visible band of actin bundle along the longitudinal axis of the forward-moving cell membrane extension was not apparent in control cells (dashed arrows).
Immunoprecipitation and Western blotting.
SKCO15 or Caco-2 grown in T-75 flasks were treated and lysed in HBSS with 1% Triton X-100. Insoluble material was removed by centrifugation, and 1 mg of protein (∼1 ml) was incubated with 1 μg of immunoprecipitating antibody, IgG anti-Crk, or control IgG, for 3–4 h with shaking at 4°C. Afterward, immunocomplexes were captured on protein-G agarose beads (GE Healthcare Life Sciences), and then proteins were resolved by SDS-PAGE for Western blotting using standard protocols.
Adhesion assay.
Attachment of SKCO15 cells was analyzed essentially as described previously (19). Briefly, cell monolayers were lifted, and cells were dispersed into a single cell suspension using Ca++-free and Mg++-free PBS. After a pretreatment with imatinib, ∼50,000 cells/well in a collagen-coated 96-well were allowed to adhere for various time points before several PBS washes and fluorescence detection using standard plate reader settings at 488 nm excitation. Cells were maintained in their respective treatment media during adhesion. To visualize cell spreading after treatment with imatinib, cells were allowed to attach to collagen-coated coverslips for different times and then fixed, permeabilized, and stained with phalloidin Alexa Fluor 555.
Statistical analysis.
Student's t-test was used to calculate statistical differences between each group, with *P < 0.05 statistically significant. Error bars are presented as standard deviation from the mean, with each experiment replicated at least twice and generally performed in duplicate or triplicate.
RESULTS
Cas is under redox control in the intestine.
We first characterized the localization of phosphorylated Cas (at residue Y410) by immunofluorescence staining of the colon from wild-type mice under baseline conditions and found Cas staining to be in a gradient, localized in FAs (white arrow heads, Fig. 2A) from the transit-amplifying zone (Fig. 2A, inset) of the intestinal crypt in low abundance, to the apical end of the crypt, where it was highly abundant. The production of H2O2 in the wound bed has been well established (31). Thus, to assess the effect of this microenvironment on Cas, we introduced biopsy wounds in the distal colon of mice and analyzed them by immunostaining at day 2 after wounding. Interestingly, approximately three to five crypts from the wound bed phospho-Cas were consistently localized in the lateral surfaces of epithelial cells at the apical crypt surface (Fig. 2B, yellow arrows) while also present in FAs (Fig. 2B, white arrows). The extent of lateral phospho-Cas localization was apparent directly around the wound bed, outward ∼20 crypts. Therefore, the increase in H2O2 production in and around the wound bed may contribute to the increase in levels of phospho-Cas in the lateral regions.
We examined a range of H2O2 concentrations on scratch-wounded and subconfluent model intestinal epithelial cells. Figure 2C shows the dose-response effect of phospho-Cas to H2O2 in subconfluent epithelial cells, with effects on phospho-Cas becoming most apparent above 1 mM. Scratch wounding an epithelial cell monolayer alone showed phospho-Cas predominantly in FAs at the wound edge; however, similarly to subconfluent cells, only the addition of H2O2 in the millimolar range caused a lateral accumulation of phospho-Cas (Fig. 2D, yellow arrows), analogous to what we observed in our in vivo biopsy wound experiments. Given the millimolar concentration of H2O2 used, this suggests that exogenous exposure of high levels of ROS in and around the wound bed as a consequence of leukocyte activity may have contributed to the observed changes in phospho-Cas localization. The lack of uniformity in phospho-Cas localization in a subset of cells during H2O2 treatment is dose dependent and is likely due to differences in catalase expression/activity between different cells that create localized differences in H2O2 concentrations. This effect is more obvious in the monolayer where buffering of the H2O2 by catalase appears to be more effective and/or is harder for the H2O2 to gain intracellular access than with subconfluent cells. Nonetheless, phospho-Cas appears to play a redox-sensitive role during wound healing in the intestine in response to localized increases in H2O2 levels.
Cas phosphorylation and Crk binding regulated by H2O2.
Because complex formation between Cas and Crk is known to regulate cell migration (1, 26), we next examined the effect H2O2 has on Cas/Crk binding. In bladder cells, modulation of superoxide dismutase or catalase levels to create different redox environments was shown to cause changes in the formation of Cas/Crk complexes (10, 11), prompting us to measure the effect of H2O2 on Cas pY410 levels and the binding of Cas with Crk. Treating subconfluent cells with H2O2 markedly increased both the phosphorylation of Cas (Fig. 3A) and its binding with Crk (Fig. 3B). The positive control arsenite, which creates oxidative stress through mitochondrial toxicity (32), also modestly increased Cas pY410 levels and Crk binding. The baseline control, along with the antioxidant NAC, did not affect Cas pY410 levels or its association with steady-state Crk. Collectively, these data show that Cas phosphorylation and its ability to form protein complexes known to be important in FA dynamics and cell migration are clearly under redox control in the epithelium and are enhanced by H2O2 exposure.
Fig. 3.
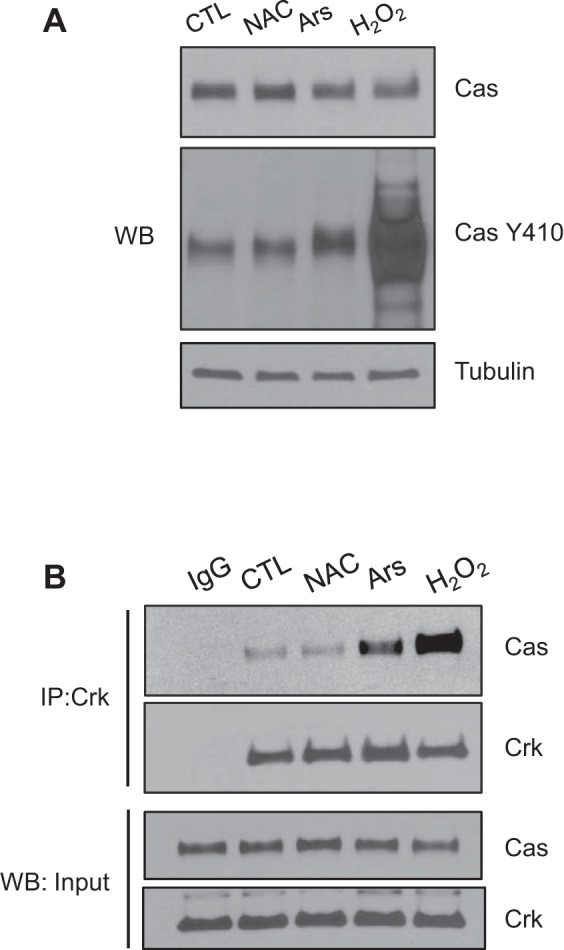
H2O2 regulates Cas Y410 phosphorylation and association with Crk. A: subconfluent Caco-2 intestinal epithelial cells were treated with 10 mM N-acetyl cysteine (NAC) (1 h), 0.5 mM arsenite (Ars) (45 min), 5 mM H2O2 (15 min), or no treatment, and then lysates were collected for Western blotting (WB) with Cas Y410 phospho-specific, total-Cas antibodies and tubulin as a loading control. B: lysates used for immunoprecipitation (IP) with Crk-specific antibodies, followed by Western blotting for Crk and Cas. Bottom: lysate inputs as loading controls.
Abl kinase increases Cas pY410 levels during H2O2 treatment.
Abl kinase regulates the association of Crk with Cas to control migration (16). A series of studies have also demonstrated a redox-regulated role for Abl under a variety of cellular conditions (3, 5, 17, 28, 33). Thus we hypothesized that Abl kinase regulates Cas pY410 levels and its localization in the lateral cell regions during increases in redox stimulation. To test this, we determined whether inhibition of Abl kinase by the small molecule imatinib would affect changes in redox-induced Cas pY410 levels and localization. Dose-response experiments showed that indeed pretreatment of imatinib blocked increases in H2O2-induced phospho-Cas levels and that the effective dose of imatinib on blocking 5 mM H2O2 was between 10 and 25 μM (Fig. 4A). Furthermore, as shown under higher-power magnification in Fig. 4B, subconfluent cells treated for 60 min with 20 μM imatinib alone caused no significant changes in Cas pY410 basolateral relocalization in migrating epithelial cells compared with control cells (Fig. 4B), yet, remarkably, when cells were pretreated with imatinib for 1 h before H2O2 stimulation, the effects of H2O2 on Cas pY410 localization and levels were abolished (Fig. 4, B and C, respectively). This dramatic effect is considerable evidence that Abl kinase regulates both the levels of redox-induced Cas pY410 and its subcellular localization. Given the known functions of Abl and Cas, this redox signaling module in the epithelium likely plays an important role in regulating wound healing in the intestine.
Abl kinase regulates intestinal epithelial cell spreading and migration by influencing FA and actin dynamics.
Considering the role of Abl in regulating Cas phosphorylation and subcellular localization during exposure to H2O2 (Fig. 4), we sought to gain further insight into the function of Abl signaling in the intestinal epithelium. Thus we analyzed the ability of intestinal epithelial cells to attach and spread, a two-step process that requires integrin binding for attachment and actin reorganization for spreading, on collagen-coated surfaces after a 60-min pretreatment with imatinib. Figure 5, A and B, shows that the numbers of cells attached after imatinib treatment are not significantly different; however, control cells showed a greater degree of spreading compared with imatinib-treated cells (Fig. 5B), suggesting that the extracellular substrate binding of integrin was functional but that actin dynamics necessary for normal reorganization of cell shape to flatten were impeded. Additionally, we examined the spreading rate at the wound edge of a freshly scratched epithelial cell monolayer because this requires the reorganization of columnar epithelial cells at the wound edge into a flattened, migrating morphology. Analysis of cell spreading at the wound edge at x–z-axis for 1.5 h after wounding revealed a gradual transition from cells in the monolayer having a columnar morphology and a height of ∼10 μm to becoming flattened with outward membrane extensions with a height of about 6 μm (Fig. 5, C and D). Treatment of scratch-wounded epithelial cell monolayers with imatinib caused a significant delay in the spreading process, with cell flattening along with outward membrane extensions (a process that requires active FA turnover) becoming less apparent in the treated cells than observed for control cells (Fig. 5, A and B).
To more specifically evaluate the effects of Abl kinase activity on the actin cytoskeletal dynamics, we analyzed scratch-wounded SKCO15 cells stably expressing actin-GFP in the presence or absence of imatinib. Using fluorescence time-lapsed microscopy, we observed a dramatic decrease in the fluidity of actin dynamics both within the monolayer and membrane extensions of imatinib-treated cells at the wound edge (Fig. 6). Control cells showed a cyclic accumulation and dispersal of actin within the lamellipodia, along with rapid membrane extension and contraction (Fig. 6, asterisks); however, imatinib-treated cells showed an accumulation of stress fibers (solid arrow) and longitudinal actin bands within cellular membrane extensions (dotted arrow) in cells at the wound edge. The intensity of the actin accumulation in the lamellipodia in imatinib-treated cells also appeared to remain relatively unchanged, further suggesting that actin dynamics, along with FA turnover, had been downregulated, leading to a decrease in the length of the membrane extensions and a dampening of cell migration. Consistent with these observations, the velocity of control cells into the wound bed during these experiments was measured to be 0.75 ± 0.06 μm/min; however, imatinib treatment reduced this rate to 0.5 ± 0.03 μm/min. These data are consistent with Abl kinase ability to control actin remodeling and membrane protrusions (14, 18, 21).
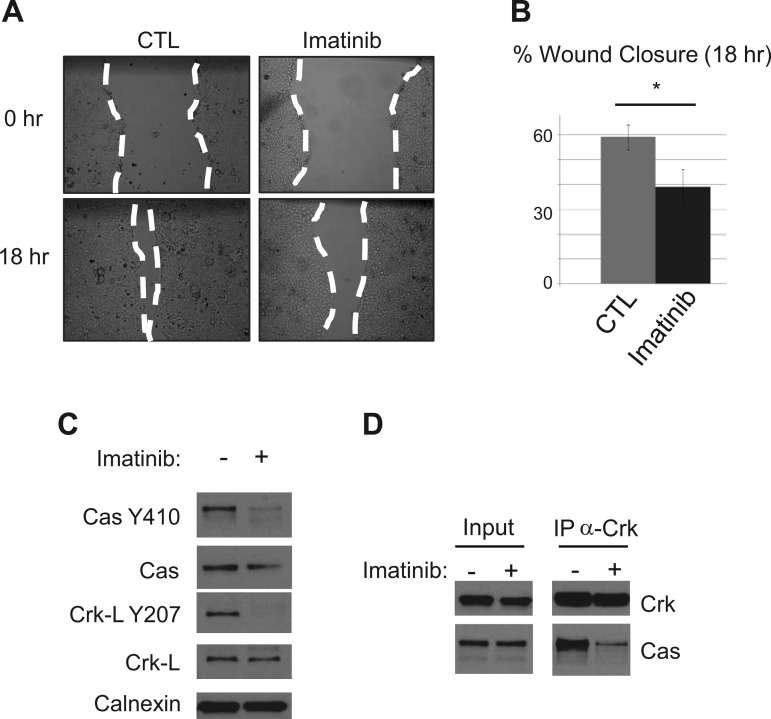
Consistent with our shorter-time experiments, longer-term treatments of cells with imatinib delayed cell migration by disrupting FA dynamics. Scratch-wounded epithelial cell monolayers exposed to imatinib showed a 20% decrease in closure after 18 h (Fig. 7, A and B). By 18 h posttreatment with imatinib, we measured a significant drop in Cas pY410 levels by Western blotting along with an expected drop in Crk-L pY207 levels (Fig. 7C), as this is the primary substrate of Abl. At this time point, we also measured a dramatic decrease in Cas-Crk binding compared with control cell monolayers (Fig. 7D). Thus Abl regulates cell migration in the epithelium through regulation of the cytoskeleton and FA dynamics.
Fig. 7.
Inhibiting Abl decreases in vitro wound closure and focal adhesion dynamics. A: scratch-wounded SKCO15 cells were treated with 20 μM imatinib for 18 h to asses in vitro wound closure. B: closure is represented as a percentage of scratched area covered by migrating cells after 18 h. *P < 0.05. C: after 18 h of imatinib treatment, cells were harvested, and lysates were analyzed for levels of Cas pY410 and Crk-L pY207 by Western blotting. D: coimmunoprecipitation of Cas with Crk using anti-Crk and protein-G agarose followed by Western blotting after 18 h imatinib treatment.
DISCUSSION
The purpose of this study was to investigate the role of Abl and Cas signaling in the intestinal epithelia during wound healing. Cas is known to function as an FA complex scaffolding protein residing in the basal compartments of epithelial cells, where it controls FA dynamics. Our studies showed that phospho-Cas became detectable in the lateral cell surfaces of cells comprising the intestinal epithelium after biopsy wounding the colon or in vitro when exposed to millimolar concentrations of H2O2, which is known to be abundant in wound beds (29, 31). Blocking Abl kinase activity with the pharmacological small molecule imatinib abolished the ROS-dependent increases in Cas phosphorylation and changes in its subcellular localization. Further dissection of the Abl-Cas signaling pathway revealed functions in controlling the rate of cell spreading and migration of the epithelium at the wound edge, an important process in regulating intestinal restitution.
To close a wound, reorganization of the epithelial monolayer at the wound edge requires cross talk between the basolateral and lateral surfaces of the cells. Unlike fibroblasts that can move relatively freely, epithelial cells are firmly connected to one another by junctional protein complexes known as tight and adherens junctions, and thus these cells must negotiate directional movement together. The redistribution of phospho-Cas into the lateral surfaces during ROS exposure (epithelia adjacent to in vivo wound bed or in vitro exposure to H2O2) suggests that under these conditions Cas plays a role in cell adhesion and possible cross talk with lateral cell surfaces and FA. Because Cas plays a known role in proliferation (4) as well as migration, the lateral localization of Cas may contribute to enhancing proliferative signaling through β-catenin, which is known to reside basolaterally with E-cadherin and function in intestinal homeostasis (24). In accordance with previous observations that have shown millimolar concentrations of H2O2 necessary for microbial destruction (2), our experiments revealed that it is likely exogenous production of H2O2 from leukocytes that accounts for the lateral localization of phospho-Cas in the epithelium adjacent to mucosal wounds because our in vitro scratch-wound model that lacked leukocytes only showed Cas reorganization when treated with H2O2 in the millimolar range. Therefore, our findings suggest that, in response to leukocyte activity during mucosal damage, Cas likely plays a redox-dependent role in reorganization of the epithelium through changes in its subcellular localization via phosphorylation to regulate cell migratory and proliferative signaling that ultimately leads to reestablishment of intestinal homeostasis. We show that Cas phosphorylation and lateral localization during H2O2 exposure are regulated by Abl kinase. Although it has been shown that Src kinase is known to regulate Cas phosphorylation in FA, some controversy remains as to the role that other kinases in FA play, such as FA kinase (8, 27). However, evidence suggests that Abl may also play a role (13). By blocking the kinase activity of Abl with imatinib, both the increase in Cas phosphorylation and its localization in the lateral cell surfaces were abolished, providing strong evidence for the role of Abl in regulating this process. It should be noted that PTPN12, a redox-regulated phosphatase, controls levels of Cas phosphorylation through its oxidative inactivation (10), and thus our studies did not rule out whether H2O2-induced phosphorylation of Cas by Abl was due to an increase in Abl activity or by a reduction in PTEN function. Nonetheless, we show a clear link between Abl and Cas function in a redox-dependent manner and further demonstrate that blocking Cas phosphorylation by inhibiting the kinase activity of Abl had detrimental effects on cell spreading and migration at the level of actin dynamics and FA complex assembly.
In conclusion, our findings shed new light on redox regulation of protein signaling in intestinal epithelium during wound healing. The precise mechanisms controlling wound closure are complex, and the role of Abl-Cas signaling in controlling cell spreading and migration elucidated in these studies provides potential new targets in promoting wound healing in the gut. Future studies will be necessary to further define the role of lateral localized Cas within intestinal mucosa and its contribution to promoting mucosal wound repair.
GRANTS
This work was supported by the grant “Role of defective signaling events on epithelial wound healing in IBD,” from the Crohn's and Colitis Foundation of America no. 315781, and the NIH grants DK072564, DK061379, DK079392, and DK055679, DK059888.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.D.M. conception and design of research; J.D.M., R.S., and B.H. performed experiments; J.D.M., R.S., A.N., C.A.P., and A.S.N. analyzed data; J.D.M., R.S., B.H., A.N., C.A.P., and A.S.N. interpreted results of experiments; J.D.M. and R.S. prepared figures; J.D.M. drafted manuscript; J.D.M., A.N., C.A.P., and A.S.N. edited and revised manuscript; J.D.M., R.S., B.H., A.N., C.A.P., and A.S.N. approved final version of manuscript.
REFERENCES
- 1.Abassi YA, Rehn M, Ekman N, Alitalo K, Vuori K. p130Cas couples the tyrosine kinase Bmx/Etk with regulation of the actin cytoskeleton and cell migration. J Biol Chem 278: 35636–35643, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Allaoui A, Botteaux A, Dumont JE, Hoste C, De Deken X. Dual oxidases and hydrogen peroxide in a complex dialogue between host mucosae and bacteria. Trends Mol Med 15: 571–579, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Cabigas EB, Liu J, Boopathy AV, Che PL, Crawford BH, Baroi G, Bhutani S, Shen M, Wagner MB, Davis ME. Dysregulation of catalase activity in newborn myocytes during hypoxia is mediated by c-Abl tyrosine kinase. J Cardiovasc Pharmacol Ther 20: 93–103, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabodi S, Tinnirello A, Di Stefano P, Bisaro B, Ambrosino E, Castellano I, Sapino A, Arisio R, Cavallo F, Forni G, Glukhova M, Silengo L, Altruda F, Turco E, Tarone G, Defilippi P. p130Cas as a new regulator of mammary epithelial cell proliferation, survival, and HER2-neu oncogene-dependent breast tumorigenesis. Cancer Res 66: 4672–4680, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Cao C, Leng Y, Kufe D. Catalase activity is regulated by c-Abl and Arg in the oxidative stress response. J Biol Chem 278: 29667–29675, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Chiarugi P, Pani G, Giannoni E, Taddei L, Colavitti R, Raugei G, Symons M, Borrello S, Galeotti T, Ramponi G. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J Cell Biol 161: 933–944, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark EA, Brugge JS. Integrins and signal transduction pathways: The road taken. Science 268: 233–239, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Fonseca PM, Shin NY, Brabek J, Ryzhova L, Wu J, Hanks SK. Regulation and localization of CAS substrate domain tyrosine phosphorylation. Cell Signal 16: 621–629, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Harte MT, Hildebrand JD, Burnham MR, Bouton AH, Parsons JT. p130Cas, a substrate associated with v-Src and v-Crk, localizes to focal adhesions and binds to focal adhesion kinase. J Biol Chem 271: 13649–13655, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Hempel N, Bartling TR, Mian B, Melendez JA. Acquisition of the metastatic phenotype is accompanied by H2O2-dependent activation of the p130Cas signaling complex. Mol Cancer Res 11: 303–312, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hempel N, Melendez JA. Intracellular redox status controls membrane localization of pro- and anti-migratory signaling molecules. Redox Biol 2: 245–250, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu H, Bliss JM, Wang Y, Colicelli J. RIN1 is an ABL tyrosine kinase activator and a regulator of epithelial-cell adhesion and migration. Curr Biol 15: 815–823, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Jia L, Tang DD. Abl activation regulates the dissociation of CAS from cytoskeletal vimentin by modulating CAS phosphorylation in smooth muscle. Am J Physiol Cell Physiol 299: C630–C637, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin H, Wang JY. Abl tyrosine kinase promotes dorsal ruffles but restrains lamellipodia extension during cell spreading on fibronectin. Mol Biol Cell 18: 4143–4154, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kain KH, Klemke RL. Inhibition of cell migration by Abl family tyrosine kinases through uncoupling of Crk-CAS complexes. J Biol Chem 276: 16185–16192, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol 140: 961–972, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar S, Mishra N, Raina D, Saxena S, Kufe D. Abrogation of the cell death response to oxidative stress by the c-Abl tyrosine kinase inhibitor STI571. Mol Pharmacol 63: 276–282, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Lapetina S, Mader CC, Machida K, Mayer BJ, Koleske AJ. Arg interacts with cortactin to promote adhesion-dependent cell edge protrusion. J Cell Biol 185: 503–519, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leoni G, Alam A, Neumann PA, Lambeth JD, Cheng G, McCoy J, Hilgarth RS, Kundu K, Murthy N, Kusters D, Reutelingsperger C, Perretti M, Parkos CA, Neish AS, Nusrat A. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J Clin Invest 123: 443–454, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leoni G, Neumann PA, Kamaly N, Quiros M, Nishio H, Jones HR, Sumagin R, Hilgarth RS, Alam A, Fredman G, Argyris I, Rijcken E, Kusters D, Reutelingsperger C, Perretti M, Parkos CA, Farokhzad OC, Neish AS, Nusrat A. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest 125: 1215–1227, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller AL, Wang Y, Mooseker MS, Koleske AJ. The Abl-related gene (Arg) requires its F-actin-microtubule cross-linking activity to regulate lamellipodial dynamics during fibroblast adhesion. J Cell Biol 165: 407–419, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20: 1126–1167, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. Wnt5a potentiates TGF-beta signaling to promote colonic crypt regeneration after tissue injury. Science 338: 108–113, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 17: 1709–1713, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubinstein B, Pinto IM. Epithelia migration: A spatiotemporal interplay between contraction and adhesion. Cell Adh Migr 9: 340–344, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakai R, Iwamatsu A, Hirano N, Ogawa S, Tanaka T, Mano H, Yazaki Y, Hirai H. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J 13: 3748–3756, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma A, Mayer BJ. Phosphorylation of p130Cas initiates Rac activation and membrane ruffling. BMC Cell Biol 9: 50, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun X, Majumder P, Shioya H, Wu F, Kumar S, Weichselbaum R, Kharbanda S, Kufe D. Activation of the cytoplasmic c-Abl tyrosine kinase by reactive oxygen species. J Biol Chem 275: 17237–17240, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki N, Mittler R. Reactive oxygen species-dependent wound responses in animals and plants. Free Radic Biol Med 53: 2269–2276, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Swanson PA 2nd, Kumar A, Samarin S, Vijay-Kumar M, Kundu K, Murthy N, Hansen J, Nusrat A, Neish AS. Enteric commensal bacteria potentiate epithelial restitution via reactive oxygen species-mediated inactivation of focal adhesion kinase phosphatases. Proc Natl Acad Sci USA 108: 8803–8808, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Vliet A, Janssen-Heininger YM. Hydrogen peroxide as a damage signal in tissue injury and inflammation: murderer, mediator, or messenger? J Cell Biochem 115: 427–435, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yih LH, Huang HM, Jan KY, Lee TC. Sodium arsenite induces ATP depletion and mitochondrial damage in HeLa cells. Cell Biol Int Rep 15: 253–264, 1991. [DOI] [PubMed] [Google Scholar]
- 33.Zulueta-Coarasa T, Tamada M, Lee EJ, Fernandez-Gonzalez R. Automated multidimensional image analysis reveals a role for Abl in embryonic wound repair. Development 141: 2901–2911, 2014. [DOI] [PubMed] [Google Scholar]