Caloric restriction-induced prevention of nonalcoholic fatty liver disease (NAFLD) is lost after only 4 wk of ad libitum feeding in hyperphagic Otsuka Long-Evans Tokushima fatty rats despite only modest increases in body weight and adiposity. While some beneficial hepatic mitochondrial adaptations were maintained, NAFLD development occurred in conjunction with dramatic increases in hepatic de novo lipogenesis. These findings suggest that prior caloric restriction offers little metabolic protection against future development of NAFLD should healthy eating patterns not persist.
Keywords: caloric restriction, hepatic steatosis, mitochondria, de novo lipogenesis
Abstract
Hyperphagic Otsuka Long-Evans Tokushima fatty (OLETF) rats develop obesity, insulin resistance, and nonalcoholic fatty liver disease (NAFLD), but lifestyle modifications, such as caloric restriction (CR), can prevent these conditions. We sought to determine if prior CR had protective effects on metabolic health and NAFLD development following a 4-wk return to ad libitum (AL) feeding. Four-week-old male OLETF rats (n = 8–10/group) were fed AL for 16 wk (O-AL), CR for 16 wk (O-CR; ∼70% kcal of O-AL), or CR for 12 wk followed by 4 wk of AL feeding (O-AL4wk). CR-induced benefit in prevention of NAFLD, including reduced hepatic steatosis, inflammation, and markers of Kupffer cell activation/number, was largely lost in AL4wk rats. These findings occurred in conjunction with a partial loss of CR-induced beneficial effects on obesity and serum triglycerides in O-AL4wk rats, but in the absence of changes in serum glucose or insulin. CR-induced increases in hepatic mitochondrial respiration remained significantly elevated (P < 0.01) in O-AL4wk compared with O-AL rats, while mitochondrial [1-14C]palmitate oxidation, citrate synthase activity, and β-hydroxyacyl-CoA dehydrogenase activity did not differ among OLETF groups. NAFLD development in O-AL4wk rats was accompanied by increases in the protein content of the de novo lipogenesis markers fatty acid synthase and stearoyl-CoA desaturase-1 and decreases in phosphorylated acetyl-CoA carboxylase (pACC)/ACC compared with O-CR rats (P < 0.05 for each). The beneficial effects of chronic CR on NAFLD development were largely lost with 4 wk of AL feeding in the hyperphagic OLETF rat, highlighting the importance of maintaining energy balance in the prevention of NAFLD.
NEW & NOTEWORTHY
Caloric restriction-induced prevention of nonalcoholic fatty liver disease (NAFLD) is lost after only 4 wk of ad libitum feeding in hyperphagic Otsuka Long-Evans Tokushima fatty rats despite only modest increases in body weight and adiposity. While some beneficial hepatic mitochondrial adaptations were maintained, NAFLD development occurred in conjunction with dramatic increases in hepatic de novo lipogenesis. These findings suggest that prior caloric restriction offers little metabolic protection against future development of NAFLD should healthy eating patterns not persist.
nonalcoholic fatty liver disease (NAFLD) is a chronic, progressive liver disorder that affects ∼30% of the adult population in the United States and ∼75–100% of obese or morbidly obese individuals (2, 3). This condition occurs in the absence of excess alcohol consumption (>20 g/day) and encompasses a spectrum ranging from simple hepatic steatosis [hepatic triglyceride (TG) accumulation ≥5% by weight] to nonalcoholic steatohepatitis (NASH), advanced fibrosis, and cirrhosis (26) and is considered the hepatic manifestation of the metabolic syndrome (12). Although simple steatosis has traditionally been considered to be relatively benign, mounting evidence suggests that it may promote inflammation and contribute to disease progression (18). In fact, ∼20% of obese individuals with NAFLD will develop more advanced liver disease (39), and those with advanced liver disease have increased risk of morbidity and mortality (17). The number of individuals added to the liver transplant list due to NASH has increased by ∼170% since 2004 (38), further demonstrating the growing prevalence of this condition and emphasizing the need to prevent or combat this disease in its early stages.
Caloric restriction (CR) has been shown to prevent NAFLD in animals and humans (11, 19, 31), as well as promote weight management, which is associated with reduced liver fat content and improved glucose control in NAFLD (20, 35). Recently, it was shown that short-term excess energy intake (3 days of high-fat feeding) increased liver TG content in low-capacity runner rats (24), suggesting that acute dietary overconsumption can have negative effects on liver health. Yet it remains unclear if TG accumulation persists with longer periods of caloric overconsumption. Additionally, we recently showed that prior chronic physical activity largely had protective effects on NAFLD development when animals became physically inactive in combination with excess energy intake (21), but it remains unclear if prior chronic CR can provide similar protection from disease onset with a return to overnutrition.
To examine the effectiveness of prior CR on the prevention of NAFLD during energy excess, we utilized the Otsuka Long-Evans Tokushima fatty (OLETF) rat. These animals are selectively bred with a mutated and functionally inoperative cholecystokinin-1 receptor (22, 23), which causes them to become hyperphagic. As a result, they develop obesity, insulin resistance, type 2 diabetes, and NAFLD (29), all of which can be prevented by CR (31) or increased physical activity (27, 28, 30, 31). We previously demonstrated that when hyperphagic OLETF rats are transitioned to a state of physical inactivity for 4 wk, prior physical activity conferred protective effects on NAFLD, including continued suppression of hepatic TG accumulation, increased hepatic palmitate oxidation, and suppression of de novo lipogenesis, compared with chronically sedentary rats (21). However, it remains unclear if prior CR may have similar protective effects on the liver. Here we sought to test our hypothesis that prior habitual moderate CR (∼30% reduction compared with hyperphagic sedentary animals) would have modest protective effects on NAFLD when hyperphagic OLETF rats are allowed AL access to food for 4 wk.
METHODS
Animal protocol.
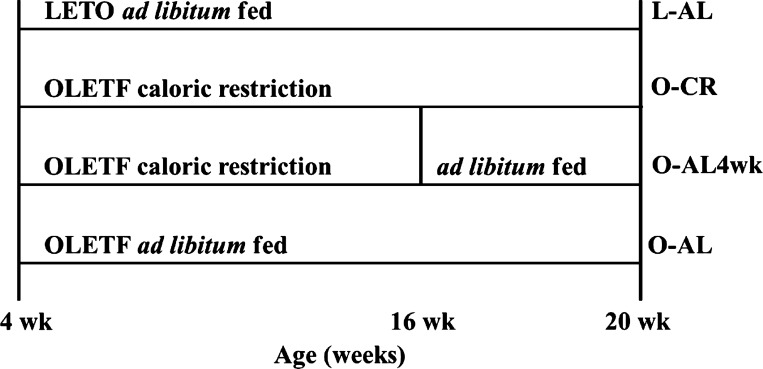
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri. Two cohorts (separated by ∼6 mo) of 4-wk-old male OLETF rats (Tokushima Research Institute, Otsuka Pharmaceutical, Tokushima, Japan) were randomly assigned to the following conditions (n = 4–5 per group per cohort, total 8–10 per group): fed AL for 16 wk (O-AL), CR for 16 wk (O-CR, ∼30% reduction vs. O-AL), or ∼30% CR for 12 wk with AL access to food for 4 wk (O-AL4wk) prior to euthanasia (Fig. 1). Nonhyperphagic Long-Evans Tokushima Otsuka (LETO) rats served as control (L-AL) animals. Animals were individually housed with a 12:12-h light-dark cycle within temperature-controlled animal quarters (21°C) and fed standard rodent chow (Formulab 5008, Purina Mills, St. Louis, MO). Body mass and food intake were assessed weekly. At 20 wk of age, animals underwent a 5-h fast and were anesthetized with pentobarbital sodium (100 mg/kg) and exsanguinated by removal of the heart. Samples for each assay were run in duplicate or triplicate.
Fig. 1.
Experimental design. LETO, Long-Evans Tokushima Otsuka; L, LETO rat; OLETF, Otsuka Long-Evans Tokushima fatty; O, OLETF rat; AL, ad libitum access to food; CR, calorie-restricted.
Serum assays.
Serum glucose (Thermo Scientific, Waltham, MA), TG (Sigma, St. Louis, MO), free fatty acids (FFAs; Wako Chemicals, Richmond, VA), and insulin (EMD Millipore, Billerica, MA) were assessed using commercially available assays.
Tissue collection and preparation procedure.
Livers were quickly removed from anesthetized rats and flash-frozen in liquid nitrogen, placed in 10% formalin, or placed in ice-cold isolation buffer (100 mM KCl, 40 mM Tris·HCl, 10 mM Tris base, 5 mM MgCl2·6H2O, 1 mM EDTA, and 1 mM ATP, pH 7.4). Retroperitoneal, epididymal, and omental adipose tissue fat pads were excised from animals and weighed. Hepatic mitochondria were isolated using centrifugation procedures, as previously described (13, 25). Isolated mitochondria were resuspended in 1,000 μl of SET buffer (250 mM sucrose, 1 mM EDTA, 10 mM Trizma hydrochloride, and 2 mM ATP, pH 7.4) for palmitate oxidation or 1,000 μl of MiP03 buffer (0.5 mM EGTA, 3 mM MgCl2·6H2O, 60 mM K-lactobionate, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, 110 mM sucrose, 1 g/l BSA, 20 mM histidine, 20 μM vitamin E succinate, 3 mM glutathione, 1 μM leupeptin, 2 mM glutamate, 2 mM malate, and 2 mM Mg-ATP) for hepatic mitochondrial respiration.
Fatty acid oxidation.
As previously described (28), radiolabeled [1-14C]palmitate (American Radiochemicals) was used in fatty acid oxidation assays of hepatic mitochondrial preparations. The oxidation rate of [14C]palmitate was measured by collecting the 14CO2 produced (representing complete fatty acid oxidation) and 14C-labeled acid-soluble metabolites (representing incomplete fatty acid oxidation) within a trapping device and counting with a liquid scintillation counter (31).
Hepatic mitochondrial respiration.
Mitochondrial respiration was assessed using high-resolution respirometry (Oxygraph-2k, Oroboros Instruments, Innsbruck, Austria), as previously described (13). Isolated mitochondria (100–150 μg of protein) were loaded into respiration chambers in mitochondrial respiration medium (MiR05: 100 mM sucrose, 60 mM K-lactobionate, 0.5 mM EGTA, 3 mM MgCl2, 20 mM taurine, 10 mM KH2PO4, and 20 mM HEPES, adjusted to pH 7.1 with KOH at 37°C, and 1 g/l fatty acid-free BSA) to assess basal respiration. State 2 respiration was assessed by addition of 5 mM glutamate and 2 mM malate to the chambers in the absence of ADP, and oxygen flux was determined. Oxidative phosphorylation with electron flux through complex I was quantified by titration of 25–125 μM ADP for assessment of state 3 respiration (state 3, complex I). ADP respiration with electron flux through complex I and complex II was assessed by addition of 10 mM succinate (state 3, complex I + II). Finally, the maximal capacity of the electron transport system was assessed with the addition of carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP, 0.25 μM, uncoupled).
Measures of mitochondrial content.
Citrate synthase and β-hydroxyacyl-CoA dehydrogenase (β-HAD) activities were determined using the methods of Srere (34) and Bass et al. (1), respectively, as previously described (28).
Intrahepatic lipid content and morphology.
To examine liver morphology, formalin-fixed, paraffin-embedded livers were sectioned and stained with hematoxylin-eosin. Biochemical intrahepatic TG content was determined as previously described (28). Briefly, ∼30 mg of frozen tissue were placed in 1 ml of lipid extraction solution [1:1 (vol/vol) methanol-chloroform], homogenized, and exposed to gentle agitation overnight at 4°C. One milliliter of 4 mM MgCl was added to each sample, and the samples were centrifuged for 1 h at 1,000 g at 4°C. The organic phase was removed, the remaining liquid was evaporated, and the sample was reconstituted in t-butanol-Triton X-114 [3:2 (vol/vol)]. After reconstitution of the sample, lipid content was measured using a commercially available assay (Sigma).
Western blot analysis.
Western blot analyses were used to determine hepatic protein content for α-smooth muscle actin (α-SMA; Abcam, Cambridge, MA), 5′-AMP-activated protein kinase (AMPK; Cell Signaling Technology, Beverly, MA), AMPK Thr172 phosphorylation-specific (Cell Signaling Technology), interleukin-1β (IL-1β; Abcam), MitoProfile total oxidative phosphorylation (OXPHOS; Abcam), serine/threonine protein kinase unc-51-like autophagy-activating kinase 1 (ULK1; Cell Signaling Technology), ULK1 Ser757 phosphorylation-specific (pULK1; Cell Signaling Technology), Bcl2/adenovirus E1B 19-kDa interacting protein 3 (BNIP3; Cell Signaling Technology), autophagy-related 12 (ATG12; Cell Signaling Technology), fatty acid translocase (FAT)/CD36 (Santa Cruz Biotechnology, Dallas, TX), acetyl coenzyme A carboxylase (ACC; Cell Signaling Technology), ACC Ser79 phosphorylation-specific (pACC; Cell Signaling Technology), fatty acid synthase (FAS; Cell Signaling Technology), and stearoyl-CoA desaturase-1 (SCD-1; Alpha Diagnostics International, San Antonio, TX). Content of phosphorylated proteins (using phosphospecific antibodies) was calculated from the density of the band of the phosphorylated protein divided by the density (content) of the protein (total) using the appropriate antibody.
Quantitative RT-PCR.
Hepatic mRNA expression was quantified using the ABI 7500 Fast sequence detection system and software (Applied Biosystems, Carlsbad, CA), as previously described by our group (25). RT-PCR was conducted to assess mRNA expression for genes associated with inflammation [TCACAAAAAGGCTGCCACTCTT (forward) and CGTAGGGCTTCGTTGCTGTGCTT (reverse) for CD68, CTATGTCTTGCCCGTGGAG (forward) and CACACACTAGCAGGTCGTCA (reverse) for IL-1β], hepatic stellate cell activation [GGAAATCAATGGGATCAGTC (forward) and CTGAAGCAGTAGTTGGTATC (reverse) for TGF-β], and cellular apoptosis [TCCATAAAAGCACTGGAATG (forward) and CTGTGATCTTCCTTAGAAACAC (reverse) for caspase-3 and ATCAACAACGTGAACTTCTG (forward) and GACCATTTTCTTAGCAGTCAG (reverse) for caspase-9] using iTAQ Universal SYBR Green Super Mix (Bio-Rad) and primers obtained from Sigma. Differences in genes were assessed using the comparative threshold (2−ΔΔCT) method, with β-actin serving as the housekeeping gene and L-AL serving as the referent group.
Statistical analyses.
Each outcome measure was examined in 8–10 animals per group. For each outcome measure, a one-way analysis of variance was performed (SPSS/22.0, IBM, Chicago, IL), with significant interactions followed up using Fisher's least significant difference post hoc comparisons. Values are means ± SE, and statistical significance was determined as P < 0.05.
RESULTS
Animal characteristics.
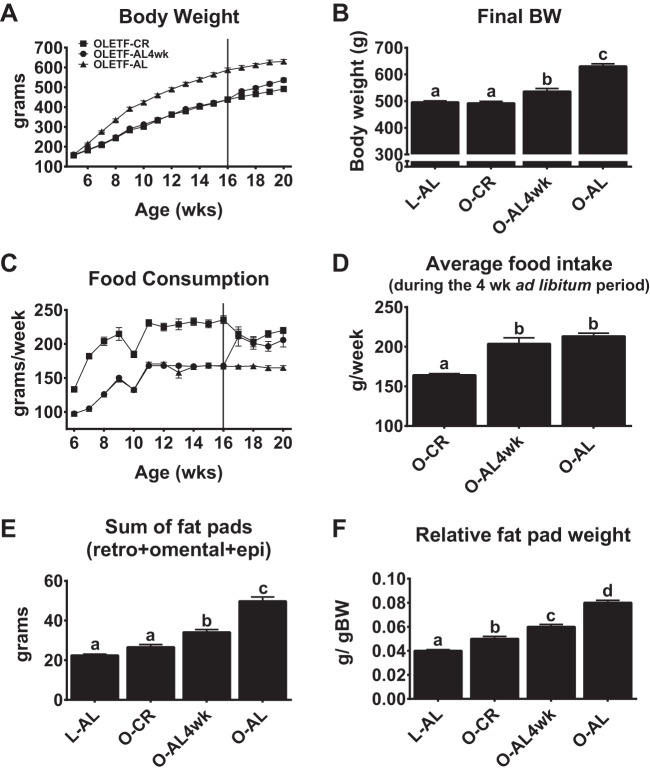
A ∼30% reduction in food intake by OLETF rats resulted in a mean body weight similar to that of the lean L-AL rats (Fig. 2, A and B). Body weight was 9% greater in O-AL4wk than O-CR rats (P < 0.01; Fig. 2, A and B); however, O-AL4wk rats still weighed ∼15% less than O-AL rats (P < 0.001). The increase in body weight in O-AL4wk rats resulted, at least in part, from a return of food intake equal to that in O-AL animals (Fig. 2, C and D) and was accompanied by increased adiposity, as fat pad mass was greater in O-AL4wk than O-CR rats (P < 0.004; Fig. 2E). It is important to note that prior CR provided significant protection against gains in adiposity compared with O-AL rats, with O-AL4wk rats sustaining ∼30% lower fat pad mass than O-AL rats (P < 0.001).
Fig. 2.
Body weight, food consumption, and adiposity. A–D: weekly body weight, final body weight (BW), weekly food consumption, and average food intake with a 4-wk return to AL feeding. E and F: sum of fat pads [retroperitoneal (retro) + omental + epididymal (epi)] and fat pad weight relative to body weight. Values are means ± SE; n = 8–10/group. Values with different superscripts (a, b, c, and d) are significantly different (P < 0.05).
Serum profile.
CR resulted in lower serum TG (P < 0.001), FFA (P < 0.001), glucose (P < 0.05), and insulin (P < 0.001) than in O-AL rats. While most of these parameters were similar between L-AL and O-CR rats, serum FFAs (P < 0.05) and glucose (P < 0.01) were higher in O-CR than L-AL rats. Prior CR had continued beneficial effects on glucose, insulin, and serum lipids, with similar concentrations of these parameters in AL4wk and O-CR animals (Table 1). Although serum glucose and TG concentrations were significantly higher (P = 0.003 and 0.041, respectively) in AL4wk than L-AL rats, systemic TG levels remained 60% lower (P < 0.001) and glucose remained 15% lower (P < 0.05) in AL4wk than O-AL animals (Table 1).
Table 1.
Serum parameters
| L-AL | O-CR | O-AL4wk | O-AL | |
|---|---|---|---|---|
| Triglyceride, mg/dl | 42.3 ± 2.9a | 79.3 ± 14.0a,b | 108.8 ± 20.3b | 278.6 ± 33.6c |
| Free fatty acids, μmol/l | 251.7 ± 12.1a | 183.8 ± 16.3b | 227.2 ± 14.3a,b | 387.7 ± 22.0c |
| Glucose, mg/dl | 179.4 ± 4.5a | 233.9 ± 9.2b | 229.9 ± 18.0b | 271.5 ± 10.9c |
| Insulin, ng/ml | 9.7 ± 0.5a | 9.0 ± 0.7a | 9.7 ± 1.2a | 13.9 ± 0.9b |
Values are means ± SE; n = 8-10/group. L-AL, Long-Evans Tokushima Otsuka rat with ad libitum (AL) access to food; O-CR, calorie-restricted (CR) Otsuka Long-Evans Tokushima (OLETF) fatty rat; O-AL4wk, OLETF rat with AL access to food for 4 wk; O-AL, OLETF rat with AL access to food. Values with different letter superscripts
(a, b, and c) are significantly different.
Hepatic lipid accumulation and liver phenotype.
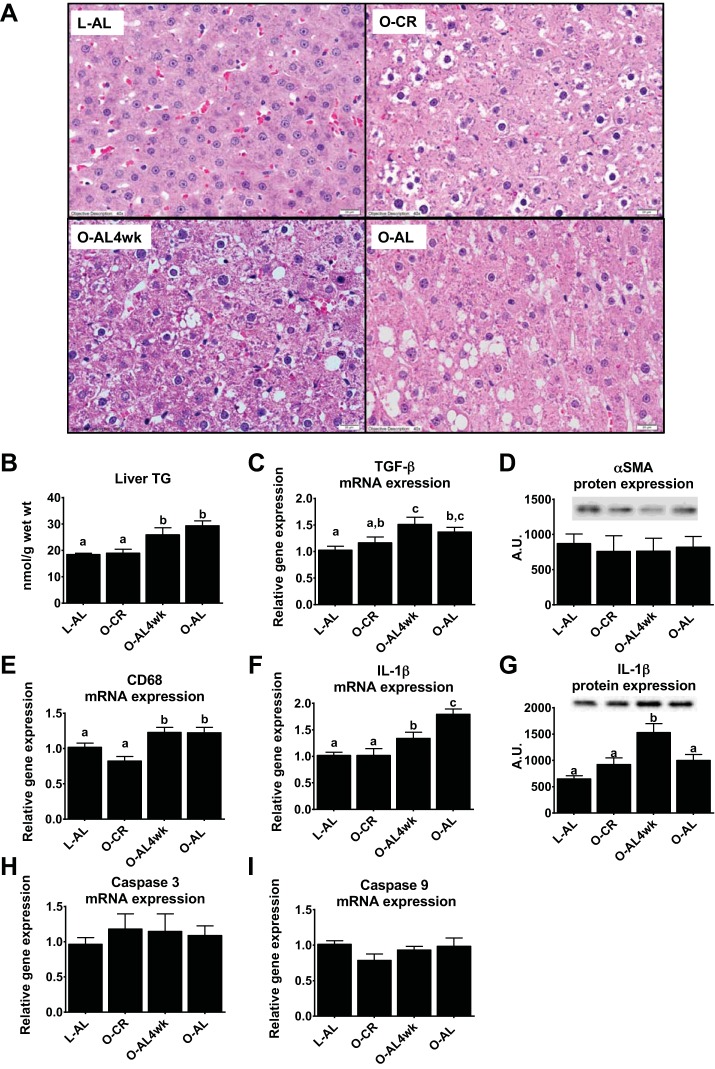
Similar to our earlier findings (31), CR effectively prevented hepatic steatosis in OLETF rats; however, prior CR did not confer protection against return of intrahepatic lipid accumulation when AL access to food was allowed for 4 wk. Note increased lipid vacuolization in the representative hematoxylin-eosin-stained image from a randomly selected section in the AL4wk rat compared with the O-CR rat (Fig. 3A). These histological findings were confirmed with biochemical hepatic TG analyses, which showed hepatic TG content in O-CR rats similar to that in LETO control animals, but the CR-induced benefits were lost after only 4 wk of AL feeding (Fig. 3B). Assessment of hepatic fibrogenesis markers revealed significantly increased hepatic TGF-β mRNA expression in O-AL4wk compared with L-AL (P < 0.01; Fig. 3C) and O-CR (P < 0.05) rats. However, this occurred in the absence of differences in α-SMA protein content (Fig. 3D). Expression of hepatic CD68 and IL-1β, hepatic macrophage/Kupffer cell and inflammatory markers, was also elevated in O-AL4wk compared with O-CR rats (P < 0.01; Fig. 3, E–G). No differences were observed between groups for hepatic mRNA expression of the apoptosis markers caspase-3 (Fig. 3H) and caspase-9 (Fig. 3I).
Fig. 3.
Liver phenotype. A: representative images of hematoxylin-eosin-stained liver sections. Magnification ×40. B–I: quantification of hepatic triglyceride (TG) content, hepatic TGF-β mRNA expression, α-smooth muscle actin (SMA) protein expression, CD68 mRNA expression, IL-1β mRNA and protein expression, caspase-3 mRNA expression, and caspase-9 mRNA expression. Values are means ± SE; n = 8–10/group. Values with different superscripts (a, b, and c) are significantly different (P < 0.05). AU, arbitrary units.
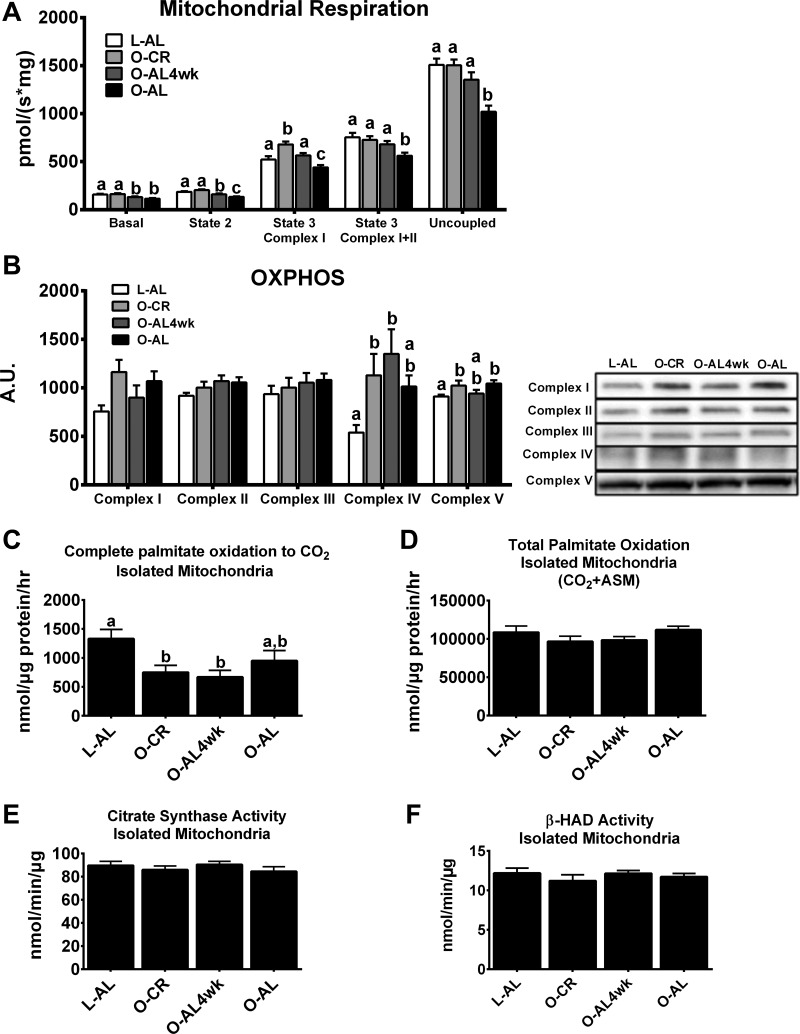
Hepatic mitochondrial function.
Next, we assessed markers of hepatic mitochondrial function, because abnormalities in mitochondrial function are associated with NAFLD (4, 29, 33). Interestingly, state 3 complex I, state 3 complex I + II, and maximal uncoupled respiration were significantly higher in animals subjected to CR for 16 wk than in O-AL animals (P < 0.01 for each; Fig. 4A). However, state 3 complex I respiration was lower in O-AL4wk than O-CR animals (P < 0.05) yet remained elevated compared with O-AL rats (P < 0.01). OXPHOS protein content was assessed to determine if it may have contributed to these changes in mitochondrial respiration (Fig. 4B). Complex IV and V protein contents were significantly higher in O-CR than L-AL rats (P < 0.05 for each); however, no differences were observed among the OLETF groups.
Fig. 4.
Hepatic mitochondrial content and function. A: hepatic mitochondrial respiration. B: oxidative phosphorylation (OXPHOS) complex I–V protein content. C and D: complete and total [1-14C]palmitate oxidation in isolated mitochondria. ASM, acid-soluble metabolites. E: citrate synthase activity in isolated mitochondria. F: β-hydroxyacyl-CoA dehydrogenase (β-HAD) activity in isolated mitochondria. Values are means ± SE; n = 8–10/group. Values with different superscripts (a, b, and c) are significantly different (P < 0.05).
Additional markers of mitochondrial function, including mitochondrial palmitate oxidation, citrate synthase activity, and β-HAD activity, were assessed. Hepatic mitochondrial complete palmitate oxidation was dramatically (∼55%) lower in O-CR and O-AL4wk than L-AL animals (P < 0.01; Fig. 4C), but no significant differences were observed between the OLETF groups. Additionally, no differences were observed in total palmitate oxidation (Fig. 4D), citrate synthase activity (Fig. 4E), or β-HAD activity (Fig. 4F) in the isolated liver mitochondria.
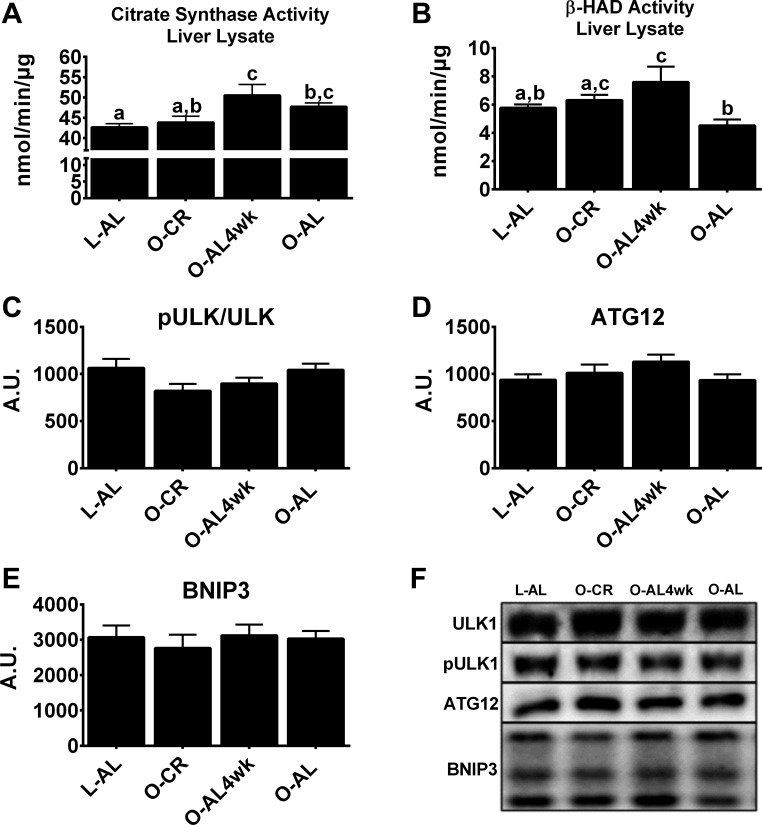
Hepatic markers of mitochondrial content and autophagy/mitophagy.
CR had no effect on whole liver lysate citrate synthase activity, as it did not differ between O-CR and O-AL rats (Fig. 5A), but β-HAD activity was greater in O-CR than O-AL rats (P < 0.05; Fig. 5B). Interestingly, citrate synthase activity was ∼15% higher in O-AL4wk than O-CR rats (P < 0.01), suggesting a potential increase in hepatic mitochondrial content. Markers of autophagy/mitophagy were assessed because of their potential role in the removal of dysfunctional mitochondria, which may account for these increases in markers of liver mitochondrial content (15, 32). No differences were observed between groups for the autophagic/mitophagic protein markers ULK1 (Fig. 5C), ATG12 (Fig. 5D), and BNIP3 (Fig. 5E).
Fig. 5.
Liver lysate mitochondrial content and hepatic autophagy/mitophagy markers. A and B: citrate synthase and β-HAD activity in whole liver homogenate. C–E: phosphorylated unc-51-like autophagy-activating kinase 1 (pULK1)/ULK1, autophagy-related 12 (ATG12), and Bcl2/adenovirus E1B 19-kDa interacting protein 3 (BNIP3) protein content. F: representative Western blots of pULK1/ULK1, ATG12, and BNIP3 protein. Values are means ± SE; n = 8–10/group. Values with different superscripts (a, b, and c) are significantly different (P < 0.05).
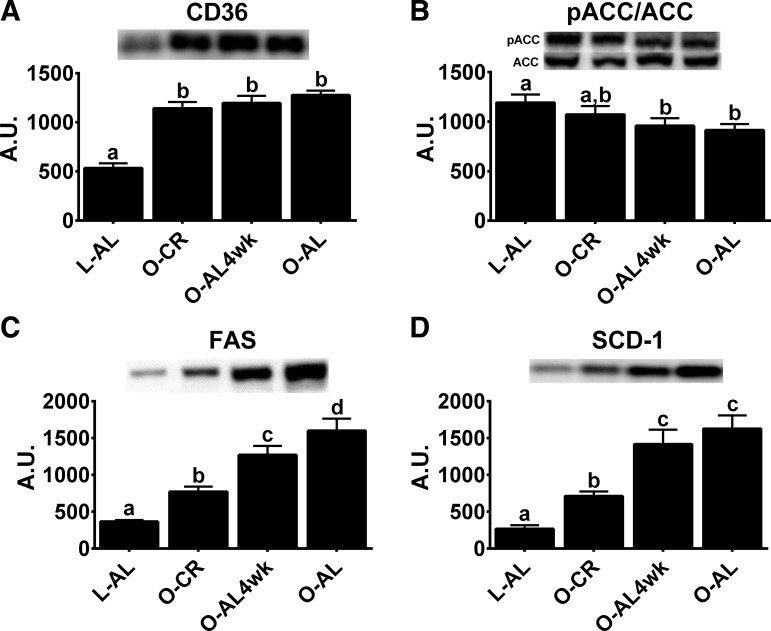
Hepatic fatty acid uptake and de novo lipogenesis markers.
As we have previously shown (20), this modest CR did not alter the phosphorylation status of AMPK (data not shown). However, pACC/ACC, a known target of AMPK and an inhibitor of de novo lipogenesis, was similar between O-CR and L-AL animals (Fig. 6B). CR had no effect on protein expression of FAT/CD36, which aids in fatty acid uptake (Fig. 6A); however, protein content of the de novo lipogenesis markers FAS (Fig. 6C) and SCD-1 (Fig. 6D) was significantly lower in O-CR than O-AL animals (P < 0.05 for each). AL-4wk animals had intermediate protein content of FAS, which remained lower than that in O-AL rats (P < 0.05 vs. O-AL4wk), while pACC/ACC and SCD-1 protein expression returned to levels similar to those in chronically overfed animals (P > 0.05, O-AL4wk vs. O-AL).
Fig. 6.
Hepatic markers of de novo lipogenesis. A–D: hepatic protein expression of CD36, phosphorylated acetyl-CoA carboxylase (pACC)/total ACC, fatty acid synthase (FAS), and stearoyl-CoA desaturase-1 (SCD-1). Values are means ± SE; n = 8–10/group. Values with different superscripts (a, b, c, and d) are significantly different (P < 0.05).
DISCUSSION
We previously reported that when physically active OLETF rats transition to a physically inactive condition for 4 wk, they develop hepatic steatosis, but some degree of protection is conferred, especially in hepatic TG, palmitate oxidation, and de novo lipogenesis (21). Here we expand on these findings by demonstrating that prior CR provided limited protection from NAFLD development when OLETF rats were allowed ad libitum access to food for only 4 wk. The excess energy consumed by O-AL4wk animals contributed to hepatic TG accumulation similar to that in O-AL animals, despite only modest increases in body weight and adiposity and in the absence of changes in serum glucose or insulin. The development of NAFLD in this model was associated not only with increased capacity for de novo lipogenesis, but also higher mRNA expression for markers related to NASH (TGF-β, CD68, and IL-1β). Interestingly, some of the hepatic mitochondrial adaptations incurred with CR, particularly mitochondrial respiration, were maintained following 4 wk of overnutrition.
AL feeding (∼2 wk) following short-term CR (2.5 wk, ∼25% reduction of food intake vs. AL control) and following chronic CR (6 mo, ∼25% reduction vs. AL control) has been shown to result in higher liver weights (9, 14), with the increases likely due, in part, to increased liver glycogenesis (7, 16); however, precursors to NAFLD have also been shown to be upregulated (36), suggesting that hepatic lipid accumulation may occur with a return to AL feeding. In the present report, prior CR conferred little protection against induction of hepatic steatosis and TG accumulation in the O-AL4wk rats, responses that were quite different from our previous findings that physically active OLETF rats were in transition to an inactive state for 4 wk and were largely protected against a return to hepatic TG accumulation (21). Furthermore, while not assessed in our previous report (21), examination of markers of hepatic inflammation and macrophage/Kupffer cell activation revealed that AL feeding for 4 wk was associated with increases in the expression of CD68, IL-1β, and the profibrogenic factor TGF-β. Given that ∼20% of obese individuals develop liver disease that includes inflammation, fibrosis, and/or cirrhosis (39), these findings highlight the possibility that, even in this model of early liver disease, precursors to NASH may be negatively affected by excess energy intake in a relatively short time frame.
Mitochondrial abnormalities are related to and can precede the development of NAFLD (4, 29, 33); however, CR has been shown to partially protect OLETF rats from aberrations in mitochondrial function (20, 31). With more severe CR (∼50% kcal reduction) in normophagic Sprague-Dawley rats, 3 days of reduced energy intake lowered hepatic state 3 respiration ∼30% (10), while 2 wk of CR had no effects on state 3 respiration (5). In contrast, longer-duration (3 mo) CR (∼40% kcal restriction) was shown to increase hepatic state 3 complex I respiration in Wistar rats (37). These results are consistent with our current findings of CR-induced increases in basal, state 2, state 3, and maximal uncoupled hepatic mitochondrial respiration compared with hyperphagic O-AL rats. Interestingly, refeeding following CR has been shown to initially increase hepatic mitochondrial state 3 respiration, but these increases were lost following 2 wk of refeeding (6). We expand on these findings and report that 4 wk of AL feeding following chronic CR lowered basal, state 2, and state 3 complex I mitochondrial respiration compared with chronically CR animals; however, state 3 complex I + II and maximal uncoupled respiration remained elevated at the level of O-CR rats following 4 wk of AL feeding, implying some conferred protection of prior CR on hepatic mitochondrial respiration. In contrast to CR-induced increases in hepatic mitochondrial respiration, CR did not effectively increase isolated mitochondrial complete palmitate oxidation, which is consistent with whole liver lysate palmitate oxidation work from our laboratory (31). These findings collectively suggest that mitochondrial fatty acid oxidation and mitochondrial respiration/utilization of tricarboxylic acid cycle intermediates are influenced differently by CR and AL feeding, a finding that warrants future investigation.
Autophagy is a process that aids in the removal of ubiquitinated proteins or damaged organelles, such as mitochondria, to maintain cellular health, and BNIP3 is an important mediator of mitochondrial autophagy (mitophagy) (32). Because we observed an increase in citrate synthase activity in liver lysates and lowered hepatic mitochondrial respiration in O-AL4wk rats, we hypothesized that a return to AL feeding may perhaps be limiting the autophagy/mitophagy regulatory process and allowing for the accumulation of dysfunctional mitochondria that could contribute to NAFLD. Previous work has shown that hepatic expression of the autophagy marker LC3II/LC3I [the ratio of microtubule-associated protein 1A/1B light chain 3-II (LC3II) to LC3I] is increased with dietary restriction (∼25% kcal reduction) but is lost with 2 wk of AL access to food (14). To our surprise, the protein content of the autophagy/mitophagy-related markers ULK1, ATG12, and BNIP3 did not differ with CR or AL4wk. While this suggests the lack of alterations in autophagy/mitophagy in this model, additional studies with dynamic assessment of autophagic/mitophagic flux are warranted.
Fatty acid uptake and hepatic de novo lipogenesis contribute to NAFLD, with >25% of hepatic TG accumulation resulting from de novo lipogenesis in NAFLD patients (8). We previously demonstrated that chronic CR partially restored markers of de novo lipogenesis in OLETF rats to levels observed in lean control animals at 40 wk of age (31). Additionally, 2 days–1 wk of AL feeding following CR resulted in greater hepatic mRNA expression of FAS and SCD-1 and a doubling of FAS activity in rats (6, 36). The current findings demonstrate a limited ability of prior CR to protect the liver from elevated de novo lipogenesis during prolonged overnutrition, as a return to AL feeding for 4 wk resulted in increased FAS and SCD-1 and lower inhibition of ACC. Together, these data indicate that prior CR may actually prime the de novo lipogenesis pathway and allow for very rapid return of hepatic TG accumulation and is quite distinct from the conferred protection provided by prior exercise training (21).
In summary, we demonstrate that CR-induced prevention of NAFLD is lost with a 4-wk return to AL feeding in the hyperphagic OLETF rat. The development of NAFLD occurred despite only modest increases in body weight and adiposity and with continued protection of other metabolic factors, including fasting serum lipids, glucose, and insulin. NAFLD development also occurred in conjunction with partial losses in certain indexes of hepatic mitochondrial function and increases in hepatic de novo lipogenesis. The lack of a more robust protective effect of prior CR is in stark contrast to the known protective effects of prior exercise training on NAFLD-related outcomes in this model (21). These findings suggest that prior CR offers little metabolic protection against future development of NAFLD should healthy eating patterns not persist and may have important health implications as we design and implement future intervention strategies for NAFLD.
GRANTS
This work was supported with the resources and use of facilities at the Harry S Truman Memorial Veterans Hospital. This work was partially supported by National Institutes of Health Grants T32 AR-048523-07 (J. A. Fletcher), DK-088940 (J. P. Thyfault), and R01 HL-036088 (M. H. Linden), Department of Veterans Affairs Merit Review Award I01 RX000123 (J. P. Thyfault), and Veterans Health Administration Grant CDA2 IK2BX001299 (R. S. Rector).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A.L., J.P.T., M.H.L., and R.S.R. developed the concept and designed the research; M.A.L., J.A.F., G.M.M., and R.S.R. performed the experiments; M.A.L., J.A.F., G.M.M., J.P.T., and R.S.R. analyzed the data; M.A.L., J.A.F., G.M.M., J.P.T., M.H.L., and R.S.R. interpreted the results of the experiments; M.A.L. and R.S.R. prepared the figures; M.A.L. and R.S.R. drafted the manuscript; M.A.L., J.A.F., G.M.M., J.P.T., M.H.L., and R.S.R. edited and revised the manuscript; M.A.L., J.A.F., G.M.M., J.P.T., M.H.L., and R.S.R. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the excellent technical assistance of Kayla Kanosky and Nicholas Shea.
REFERENCES
- 1.Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem 10: 198–206, 1969. [DOI] [PubMed] [Google Scholar]
- 2.Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med 132: 112–117, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 40: 1387–1395, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell SH, Swerdlow RH, Khan EM, Iezzoni JC, Hespenheide EE, Parks JK, Parker WD Jr. Mitochondrial abnormalities in non-alcoholic steatohepatitis. J Hepatol 31: 430–434, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Crescenzo R, Bianco F, Falcone I, Coppola P, Dulloo AG, Liverini G, Iossa S. Mitochondrial energetics in liver and skeletal muscle after energy restriction in young rats. Br J Nutr 108: 655–665, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Crescenzo R, Bianco F, Falcone I, Prisco M, Dulloo AG, Liverini G, Iossa S. Hepatic mitochondrial energetics during catch-up fat after caloric restriction. Metabolism 59: 1221–1230, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Dohm GL, Tapscott EB, Garris DR. The influence of glycogen level on hepatic glucose efflux in the anesthetized rat. Biochem Med 30: 157–161, 1983. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115: 1343–1351, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duarte FO, Sene-Fiorese M, Cheik NC, Maria AS, de Aquino AE Jr, Oishi JC, Rossi EA, Garcia de Oliveira Duarte AC, Damaso AR. Food restriction and refeeding induces changes in lipid pathways and fat deposition in the adipose and hepatic tissues in rats with diet-induced obesity. Exp Physiol 97: 882–894, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Dumas JF, Roussel D, Simard G, Douay O, Foussard F, Malthiery Y, Ritz P. Food restriction affects energy metabolism in rat liver mitochondria. Biochim Biophys Acta 1670: 126–131, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Elias MC, Parise ER, de Carvalho L, Szejnfeld D, Netto JP. Effect of 6-month nutritional intervention on non-alcoholic fatty liver disease. Nutrition 26: 1094–1099, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 43: S99–S112, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher JA, Meers GM, Linden MA, Kearney ML, Morris EM, Thyfault JP, Rector RS. Impact of various exercise modalities on hepatic mitochondrial function. Med Sci Sports Exerc 46: 1089–1097, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giller K, Huebbe P, Hennig S, Dose J, Pallauf K, Doering F, Rimbach G. Beneficial effects of a 6-month dietary restriction are time-dependently abolished within 2 weeks or 6 months of refeeding-genome-wide transcriptome analysis in mouse liver. Free Radic Biol Med 61: 170–178, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Glick D, Zhang W, Beaton M, Marsboom G, Gruber M, Simon MC, Hart J, Dorn GW 2nd, Brady MJ, Macleod KF. BNip3 regulates mitochondrial function and lipid metabolism in the liver. Mol Cell Biol 32: 2570–2584, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holness MJ, MacLennan PA, Palmer TN, Sugden MC. The disposition of carbohydrate between glycogenesis, lipogenesis and oxidation in liver during the starved-to-fed transition. Biochem J 252: 325–330, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hui JM, Kench JG, Chitturi S, Sud A, Farrell GC, Byth K, Hall P, Khan M, George J. Long-term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology 38: 420–427, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Itoh M, Kato H, Suganami T, Konuma K, Marumoto Y, Terai S, Sakugawa H, Kanai S, Hamaguchi M, Fukaishi T, Aoe S, Akiyoshi K, Komohara Y, Takeya M, Sakaida I, Ogawa Y. Hepatic crown-like structure: a unique histological feature in non-alcoholic steatohepatitis in mice and humans. PLos One 8: e82163, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larson-Meyer DE, Newcomer BR, Heilbronn LK, Volaufova J, Smith SR, Alfonso AJ, Lefevre M, Rood JC, Williamson DA, Ravussin E. Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity (Silver Spring) 16: 1355–1362, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linden MA, Lopez KT, Fletcher JA, Morris EM, Meers GM, Siddique S, Laughlin MH, Sowers JR, Thyfault JP, Ibdah JA, Rector RS. Combining metformin therapy with caloric restriction for the management of type 2 diabetes and nonalcoholic fatty liver disease in obese rats. Appl Physiol Nutr Metab 40: 1038–1047, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linden MA, Meers GM, Ruebel ML, Jenkins NT, Booth FW, Laughlin MH, Ibdah JA, Thyfault JP, Rector RS. Hepatic steatosis development with four weeks of physical inactivity in previously active, hyperphagic OLETF rats. Am J Physiol Regul Integr Comp Physiol 304: R763–R771, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moran TH, Bi S. Hyperphagia and obesity in OLETF rats lacking CCK-1 receptors. Philos Trans R Soc Lond B Biol Sci 361: 1211–1218, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moran TH, Bi S. Hyperphagia and obesity of OLETF rats lacking CCK1 receptors: developmental aspects. Dev Psychobiol 48: 360–367, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Morris EM, Jackman MR, Johnson GC, Liu TW, Lopez JL, Kearney ML, Fletcher JA, Meers GM, Koch LG, Britton SL, Rector RS, Ibdah JA, MacLean PS, Thyfault JP. Intrinsic aerobic capacity impacts susceptibility to acute high-fat diet-induced hepatic steatosis. Am J Physiol Endocrinol Metab 307: E355–E364, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris EM, Meers GM, Booth FW, Fritsche KL, Hardin CD, Thyfault JP, Ibdah JA. PGC-1α overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. Am J Physiol Gastrointest Liver Physiol 303: G979–G992, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rector RS, Thyfault JP. Does physical inactivity cause nonalcoholic fatty liver disease? J Appl Physiol 111: 1828–1835, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Rector RS, Thyfault JP, Laye MJ, Morris RT, Borengasser SJ, Uptergrove GM, Chakravarthy MV, Booth FW, Ibdah JA. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. J Physiol 586: 4241–4249, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol 294: G619–G626, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol 52: 727–736, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rector RS, Uptergrove GM, Borengasser SJ, Mikus CR, Morris EM, Naples SP, Laye MJ, Laughlin MH, Booth FW, Ibdah JA, Thyfault JP. Changes in skeletal muscle mitochondria in response to the development of type 2 diabetes or prevention by daily wheel running in hyperphagic OLETF rats. Am J Physiol Endocrinol Metab 298: E1179–E1187, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rector RS, Uptergrove GM, Morris EM, Borengasser SJ, Laughlin MH, Booth FW, Thyfault JP, Ibdah JA. Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. Am J Physiol Gastrointest Liver Physiol 300: G874–G883, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rikka S, Quinsay MN, Thomas RL, Kubli DA, Zhang X, Murphy AN, Gustafsson AB. Bnip3 impairs mitochondrial bioenergetics and stimulates mitochondrial turnover. Cell Death Differ 18: 721–731, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 120: 1183–1192, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Srere PA. Citrate synthase. Methods Enzymol 13: 3–5, 1969. [Google Scholar]
- 35.Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol 56: 255–266, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Turyn J, Stojek M, Swierczynski J. Up-regulation of stearoyl-CoA desaturase 1 and elongase 6 genes expression in rat lipogenic tissues by chronic food restriction and chronic food restriction/refeeding. Mol Cell Biochem 345: 181–188, 2010. [DOI] [PubMed] [Google Scholar]
- 37.Valle A, Guevara R, Garcia-Palmer FJ, Roca P, Oliver J. Sexual dimorphism in liver mitochondrial oxidative capacity is conserved under caloric restriction conditions. Am J Physiol Cell Physiol 293: C1302–C1308, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 148: 547–555, 2015. [DOI] [PubMed] [Google Scholar]
- 39.Younossi ZM, Diehl AM, Ong JP. Nonalcoholic fatty liver disease: an agenda for clinical research. Hepatology 35: 746–752, 2002. [DOI] [PubMed] [Google Scholar]