Autophagy is required for hepatocytes to resist injury from high concentrations of LPS. With a genetic decrease in hepatocyte autophagy, increased liver injury occurred in response to LPS from sensitization to TNF-dependent hepatocellular death in association with an impairment in Akt signaling. Human conditions such as aging and hepatic steatosis may worsen the clinical outcome from sepsis as the result of their concomitant decrease in hepatic levels of autophagy.
Keywords: Akt, apoptosis, liver, sepsis, tumor necrosis factor
Abstract
During sepsis, bacterial products, particularly LPS, trigger injury in organs such as the liver. This common condition remains largely untreatable, in part due to a lack of understanding of how high concentrations of LPS cause cellular injury. In the liver, the lysosomal degradative pathway of autophagy performs essential hepatoprotective functions and is induced by LPS. We, therefore, examined whether hepatocyte autophagy protects against liver injury from septic levels of LPS. Mice with an inducible hepatocyte-specific knockout of the critical autophagy gene Atg7 were examined for their sensitivity to high-dose LPS. Increased liver injury occurred in knockout mice, as determined by significantly increased serum alanine aminotransferase levels, histological evidence of liver injury, terminal deoxynucleotide transferase-mediated deoxyuridine triphosphate nick end-labeling, and effector caspase-3 and -7 activation. Hepatic inflammation and proinflammatory cytokine induction were unaffected by the decrease in hepatocyte autophagy. Although knockout mice had normal NF-κB signaling, hepatic levels of Akt1 and Akt2 phosphorylation in response to LPS were decreased. Cultured hepatocytes from knockout mice displayed a generalized defect in Akt signaling in response to multiple stimuli, including LPS, TNF, and IL-1β. Akt activation mediates hepatocyte resistance to TNF cytotoxicity, and anti-TNF antibodies significantly decreased LPS-induced liver injury in knockout mice, indicating that the loss of autophagy sensitized to TNF-dependent liver damage. Hepatocyte autophagy, therefore, protects against LPS-induced liver injury. Conditions such as aging and steatosis that impair hepatic autophagy may predispose to poor outcomes from sepsis through this mechanism.
NEW & NOTEWORTHY
Autophagy is required for hepatocytes to resist injury from high concentrations of LPS. With a genetic decrease in hepatocyte autophagy, increased liver injury occurred in response to LPS from sensitization to TNF-dependent hepatocellular death in association with an impairment in Akt signaling. Human conditions such as aging and hepatic steatosis may worsen the clinical outcome from sepsis as the result of their concomitant decrease in hepatic levels of autophagy.
sepsis represents a systemic response to bacterial products, prominent among which is endotoxin or LPS (13, 25). Sepsis is a major source of morbidity and mortality that yearly affects over 400,000 individuals in the United States and contributes to one-third of all hospital deaths (2, 18, 20). The availability of a wide spectrum of effective antibiotics has failed to reduce mortality from this condition, which remains over 25% (25). Prominent among the sites of end-organ damage from sepsis is the liver, where LPS-induced injury is mediated through the mechanisms of tissue ischemia, immune cell infiltration, and the direct cytotoxicity of cytokines. An enhanced understanding of the pathophysiological basis of the end-organ damage and dysfunction in sepsis is critical to the development of more effective therapies for this condition.
Macroautophagy or autophagy is a lysosomal degradation pathway that performs critical cellular functions in the liver, such as maintaining energy homeostasis and degrading aged or damaged cellular organelles and proteins (7). LPS induces autophagy in the liver (1), suggesting that this pathway may be important in modulating the hepatic effects of LPS. The cell type in which autophagy has been linked to immune responsiveness and inflammation is the macrophage. Autophagy in macrophages modulates the clearance of infectious pathogens and inflammasome-dependent production of the proinflammatory cytokine IL-1β (9). Recently, our laboratory demonstrated that macrophage autophagy regulates hepatic immune responsiveness to low-dose LPS through effects on macrophage polarization and inflammasome activation (14, 17).
Little is known about the function of hepatocyte autophagy in the liver's response to LPS. Our investigations of TNF-dependent liver injury from the hepatotoxin galactosamine have demonstrated that autophagy protects against hepatocyte injury and cell death from TNF (1). Although these studies were conducted in a model in which low-dose LPS injures the liver through sensitization by the toxin galactosamine, these findings suggested that autophagy may modulate hepatocyte injury and death from the high levels of LPS that occur with sepsis. Cross talk between the autophagic and apoptotic pathways has been demonstrated at multiple levels, and, although autophagy can mediate cell death under certain conditions, this pathway is considered largely an antiapoptotic, prosurvival mechanism (16). Hepatocellular toxicity to TNF is regulated by a number of signaling pathways, including NF-κB, Akt, and the MAPK JNK (26). Autophagy can modulate signaling pathways through the degradation of regulatory proteins, such as IκBα, which allows NF-κB activation (6). In addition, decreased Akt signaling in response to insulin has been reported in hepatocyte autophagy-deficient mice in the setting of obesity (37). The realization that autophagy can regulate signal transduction suggested that autophagy may modulate some of these TNF resistance pathways in response to high concentrations of LPS.
In the present study, we examined whether hepatocyte autophagy functions in the liver's response to high concentrations of LPS alone. We demonstrate that mice with a hepatocyte-specific knockout of Atg7-dependent autophagy are sensitized to LPS-induced liver injury and hepatocyte death. Injury was associated with a defect in hepatic Akt signaling, and primary hepatocytes had impaired Akt activation in response to a number of stimuli, including TNF. The decreased Akt activation in response to LPS resulted in TNF-dependent liver injury, as it was abrogated by treatment with anti-TNF antibodies. Hepatocyte autophagy serves a critical function in the resistance to liver injury from LPS.
MATERIALS AND METHODS
Animal model.
Mice were maintained in a pathogen-free facility with 12:12-h light-dark cycles and unlimited access to food and water. All studies were performed in 10- to 14-wk-old male mice. Atg7F/F mice containing floxed alleles for the autophagy gene Atg7 were crossed with ERt-albumin-Cre mice with a tamoxifen-inducible, albumin promoter-driven Cre recombinase to generate ERt-albumin-Cre-Atg7F/F or Atg7Δhep mice with a hepatocyte-specific knockout of autophagy, as previously described (1). Both mouse strains are on a C57BL/6 background. Genotypes were confirmed by PCR with established primers. To activate Cre expression and generate mice with a hepatocyte-specific knockout of Atg7, Atg7Δhep mice were injected intraperitoneally with 0.1 mg of tamoxifen (Sigma, St Louis, MO) daily for 5 consecutive days, as previously described (1). Controls for all experiments with Atg7Δhep mice were littermate Atg7F/F male mice lacking the Cre transgene and identically injected with tamoxifen. Studies on transgenic mice were performed 5 days posttamoxifen treatment. LPS (E. coli 0111:B4; Sigma) was dissolved in sterile PBS and injected intraperitoneally at 7.5 mg/kg. Some mice were pretreated 4 h before LPS administration with a rat/mouse chimeric monoclonal IgG2a against mouse TNF (CNTO5048) or an isotypic IgG control antibody (both the kind gift of Janssen Research and Development, Spring House, PA). All animal studies were approved by the Albert Einstein Institutional Animal Care & Use Committee and followed the NIH guidelines on the care and use of animals.
ALT assay.
Serum alanine aminotransferases (ALTs) were measured using a commercial kit (TECO Diagnostics, Anaheim, CA).
Histology.
Livers were fixed in 10% neutral formalin, stained with hematoxylin and eosin, and graded in a blinded fashion by a single pathologist for the degree of liver injury and inflammation. The percentage of hepatic parenchyma with apoptosis/necrosis or inflammation was semiquantitatively graded on a sliding scale as follows: 0, absent; 0.5, minimal; 1, mild; 1.5, mild to moderate; 2, moderate; 2.5, moderate to marked; and 3, marked.
TUNEL assay.
Terminal deoxynucleotide transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL)-positive cells in liver sections were detected using the commercial kit DeadEnd Colorimetric System (Promega, Madison, WI). Tissue sections were deparaffinized in xylene and gradually rehydrated in decreasing concentrations of ethanol, and the assay was performed according to the manufacturer's instructions. Under light microscopy, the numbers of TUNEL-positive cells in 10 randomly selected fields (×400 magnification) were counted per liver section.
Protein isolation and Western blotting.
Total liver protein was isolated, as previously described (32). Protein concentrations were determined by the Bio-Rad (Hercules, CA) protein assay, and Western blotting was performed as previously described. Membranes were exposed to antibodies that recognized NF-κB p50 (Santa Cruz Biotechnology, Santa Cruz, CA; no. SC-114-G), NF-κB p65 (Santa Cruz Biotechnology; no. SC-109), IκBα (Santa Cruz Biotechnology; no. SC-203), LC3 (Cell Signaling, Beverly, MA; no. 2775), Atg7 (Cell Signaling; no. 2631), caspase-3 (Cell Signaling; no. 9665), caspase-7 (Cell Signaling; no. 9492), tubulin (Cell Signaling; no. 2148), GAPDH (Cell Signaling; no. 2118), Akt (Cell Signaling; no. 9272), P308-Akt (Cell Signaling; no. 9275), P473-Akt (Cell Signaling; no. 9278), P473-Akt1 (Cell Signaling; no. 9018), P473-Akt2 (Cell Signaling; no. 8599), P-GSK-3β (glycogen synthase kinase-3β) (Cell Signaling; no. 9331), cytochrome oxidase (Abcam, Cambridge, MA; no. MS-407), cytochrome c (BD Biosciences, San Jose, CA; no. 556433), β-actin (Sigma Aldrich; no. A5441), NOPP140 (U. Thomas Meier, Albert Einstein College of Medicine, Bronx, NY), and SQSTM1/p62 (p62) (Enzo, Plymouth Meeting, PA; no. BML-PW9860). Western blot signals were quantitated by a FluorChem densitometer (Alpha Innotech, San Leonardo, CA).
Caspase-3 activity.
Mouse liver caspase-3 activity was determined biochemically by commercial kit (R&D Systems, Minneapolis, MN). Activity is expressed as the level relative to that in untreated control mice.
Immunofluorescence.
At death, a piece of liver tissue was coated with OCT, frozen in 2-methylbutane for 15 min, and stored at −80°C until sectioning. Frozen sections (5 μm) were cut with a cryostat, air dried (10 min), fixed in methanol (−20°C, 10 min), rehydrated in PBS (10 min), and then incubated with blocking solution (2% normal donkey serum, 1% BSA, and 0.05% Tween 20 for 1 h). After removal of the blocking solution, sections were incubated overnight at 4°C with rat anti-mouse Ly6G antibody (Biolegend, San Diego, CA; no. 127602) or rat anti-mouse CD68 antibody (Abd Serotec, Raleigh, NC; no. MCA1957GA), diluted 1:200 in the blocking solution. Sections were then washed in PBS twice (10 min) and incubated for 1 h with donkey anti-mouse Cy3 antibody (Jackson ImmunoResearch, West Grove, PA; no. 111–165–152) diluted 1:200. Sections were washed twice in PBS and then air dried and mounted in ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (Life Technologies, Carlsbad, CA). Positive cells were counted from 10 randomly selected high-power fields per section.
Quantitative real-time reverse-transcription PCR.
Total liver RNA was isolated using the commercial kit RNeasy Plus (QIAGEN, Valencia, CA). Reverse transcription was carried out with 1 μg of RNA in an Eppendorf Mastercycler (Hamburg, Germany) using a high-capacity cDNA reverse transcription kit (ABI, Foster City, CA). Annealing of primers was done at 25°C for 10 min, followed by elongation at 37°C for 2 h and inactivation of the enzyme at 85°C for 5 min. Negative controls (no added transcriptase) were performed in parallel. PCR for Tnf, Il-6, Il-1β, Ifnγ, Il-10, Traf2, Birc2, Birc3, and Gapdh was performed in duplicate in a 7500 Fast Real-Time PCR System (ABI). The primer sequences in Table 1 were purchased from Integrated DNA Technologies (Coralville, IA). PCR was carried out using Power SYBR Green Master Mix (ABI). Taq polymerase was activated at 95°C for 10 min. The cycling parameters were denaturation at 95°C for 30 s and extension at 60°C for 1 min (for 40 cycles). Data analysis was performed using the 2−ΔΔCT method for relative quantification. All samples were normalized to Gapdh.
Table 1.
PCR primer sequences
| Gene | Primer Sequence | |
|---|---|---|
| 18S | Forward | 5′-TAGAGGGACAAGTGGCGTTC-3′ |
| Reverse | 5′-CGCTGAGCCAGTCAGTGT-3′ | |
| Birc2 | Forward | 5′-TCAAATCTAAGTATGCAGACACAC-3′ |
| Reverse | 5′-GGACAACAGCTGCTCAAGAA-3′ | |
| Birc3 | Forward | 5′-ACCTGAGCATGCAGACAC-3′ |
| Reverse | 5′-ACGTAGATAATAGCTGCTCAAGT-3′ | |
| Cyt c1 | Forward | 5′-GCCCCAGATATAGCATTCCC-3′ |
| Reverse | 5′-GTTCATCCTGTTCCTGCTCC-3′ | |
| Gapdh | Forward | 5′-AGGTCGGTGTGAACGGATTTG-3′ |
| Reverse | 5′-TGTAGACCATGTAGTTGAGGTCA-3′ | |
| Ifnγ | Forward | 5′-ATGAACGCTACACACTGCATC-3′ |
| Reverse | 5′-CCATCCTTTTGCCAGTTCCTC-3′ | |
| Il-1β | Forward | 5′-GCAACTGTTCCTGAACTCAACT-3′ |
| Reverse | 5′-ATCTTTTGGGGTCCGTCAACT-3′ | |
| Il-6 | Forward | 5′-CACATGTTCTCTGGGAAATCGTGGA-3′ |
| Reverse | 5′-TCTCTCTGAAGGACTCTGGCTTTGT-3′ | |
| Il-10 | Forward | 5′-CGGACTGCCTTCAGCCAGGTG-3′ |
| Reverse | 5′-CACAGGGGAGAAATCGATGACAGC-3′ | |
| Tnf | Forward | 5′-CCCTCACACTCAGATCATCTTCT-3′ |
| Reverse | 5′-GCTACGACGTGGGCTACAG-3′ | |
| Traf2 | Forward | 5′-ATGGATGCACTTGGAAGGGG-3′ |
| Reverse | 5′-ATGGTCCTGAAACGTCTCCC-3′ |
Mitochondrial DNA content.
Total liver DNA was isolated using the commercial kit DNeasy Blood & Tissue (QIAGEN). Real-time PCR for the cytochrome c1 and 18S rRNA genes was performed as described above using the primers in Fig. 1. Mitochondrial DNA content was quantified by normalizing values for the cytochrome c1 gene (mitochondrial DNA) to that for the 18S rRNA gene (nuclear DNA).
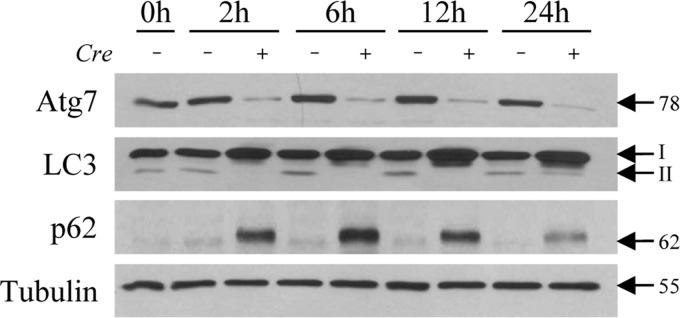
Fig. 1.
Atg7Δhep mice have a decrease in autophagy. Littermate control (Cre −) and Atg7Δhep knockout (Cre +) mice that had all been pretreated with tamoxifen, as detailed in materials and methods, were killed at the indicated times after LPS injection. Total liver protein from individual mice was immunoblotted for Atg7, LC3, p62, and tubulin as a loading control. The protein bands with molecular masses and the LC3-I and LC3-II forms are indicated by arrows.
ATP assay.
Liver and mitochondrial ATP content was measured with a commercial kit (Biovision, Milpitas, CA) and normalized to protein content.
GSH assay.
The 5,5′-dithiobis (2-nitrobenzoic acid)-GSH disulfide recycling assay was used for the determination of total GSH in whole livers and mitochondrial GSH in isolated mitochondria, as previously described (36). Values were normalized to protein content.
Mitochondrial isolation.
Livers were separated into mitochondrial and cytosolic fractions, as previously described (33). Briefly, the liver was dounce homogenized, and the homogenate was centrifuged at slow speed to pellet membranes, nuclei, and other debris. The supernatant was then centrifuged at high speed to pellet mitochondria.
Nuclear separation.
Nuclear and cytosolic fractions were isolated from liver, as previously described (29). Briefly, the liver was dounce homogenized, and the homogenate was centrifuged on a sucrose gradient to separate the nuclear and cytosolic fractions.
Primary hepatocytes.
Primary hepatocytes were obtained by liver perfusion from tamoxifen injected Atg7Δhep mice and Atg7F/F littermate controls, as previously described (11). Only hepatocyte isolations with a viability of ≥90% were used, and cells were subsequently purified on a Percoll gradient. Hepatocytes were plated in serum-free Williams E medium on collagen-coated dishes. After 3 h, cells were supplied with fresh media and cultured overnight. The medium was replaced 2 h before treatment with LPS 100 ng/ml, TNF 15 ng/ml, or IL-1β 17 ng/ml (R&D Systems, Minneapolis, MN) for various times.
Statistical analysis.
Numerical results are reported as means ± SE and are derived from at least three independent experiments, unless otherwise indicated. The unpaired Student's t-test was used to assess significance between control and treated groups. Statistical significance was defined as P < 0.05.
RESULTS
Decreased hepatocyte autophagy sensitizes mice to LPS-induced liver injury.
To determine whether autophagy functions in hepatocyte resistance to liver injury from LPS, Atg7Δhep mice with a hepatocyte-specific knockout of Atg7-dependent autophagy were evaluated for their sensitivity to liver injury from high-dose LPS. Initially, tamoxifen-injected littermate control and knockout mice were examined for the effectiveness of the Atg7 knockout. Atg7Δhep mouse livers had decreased levels of Atg7 and an inhibition of autophagy, as demonstrated by immunoblot findings of decreased Atg7 and LC3-II content with a compensatory increase in LC3-I, and elevated p62 levels (Fig. 1). LPS upregulates autophagy in mouse liver (1), but the induction of autophagy by LPS was blocked in knockout mice.
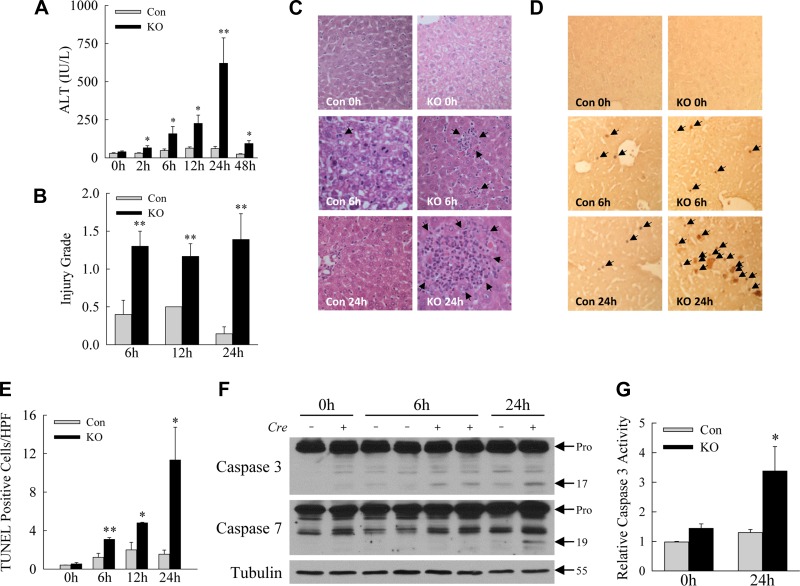
Mild liver injury occurred in response to LPS in littermate control mice, as reflected by increased serum ALT levels that peaked at <60 IU/l within 48 h (Fig. 2A). ALT levels in Atg7Δhep mice peaked within the same time period but were significantly increased compared with control mice at all time points, including a 10-fold increase at 24 h (Fig. 2A). Blinded, semiquantitative assessment of hematoxylin and eosin-stained sections revealed increased levels of hepatic injury in LPS-treated Atg7Δhep mice (Fig. 2B), with increased numbers of apoptotic cells (Fig. 2C). A greater induction of cell death was also demonstrated by findings of significantly higher numbers of TUNEL-positive cells in the livers of knockout mice (Fig. 2, D and E). Finally, increased hepatocyte apoptosis was confirmed by the presence of elevated levels of the active, cleaved protein forms of effector caspase-3 and -7 in Atg7Δhep mouse livers by immunoblotting (Fig. 2F). Knockout mouse livers also had increased levels of caspase-3 biochemical activity (Fig. 2G). Loss of hepatocyte Atg7-dependent autophagy, therefore, sensitized mice to significantly increased liver injury and cell death from LPS.
Fig. 2.
Decreased hepatocyte autophagy sensitizes mice to LPS-induced liver injury. A: serum ALT levels in littermate control (Con) and Atg7Δhep knockout (KO) mice at the indicated hours after LPS treatment. B: semiquantitative histological grading of the degree of liver injury in the same mice. C: representative hematoxylin- and eosin-stained liver sections (arrows indicate areas of injury). D: images of TUNEL-stained livers from Con and KO mice at the times indicated (arrows indicate TUNEL-positive cells). E: quantification of the numbers of TUNEL-positive cells. F: immunoblots of total liver protein from single mice probed for caspase-3 and -7 and tubulin as a loading control. Arrows indicate the procaspases (Pro), cleaved p17 and p19 caspase forms, and tubulin molecular mass. *P < 0.05, **P < 0.01, compared with Con mice at the same time; n = 3–11. G: relative levels of caspase-3 activity in mouse livers at 24 h after LPS administration. *P < 0.04; n = 3–6. Values are means ± SE.
LPS-induced activation of the inflammatory response is unaffected by decreased hepatocyte autophagy.
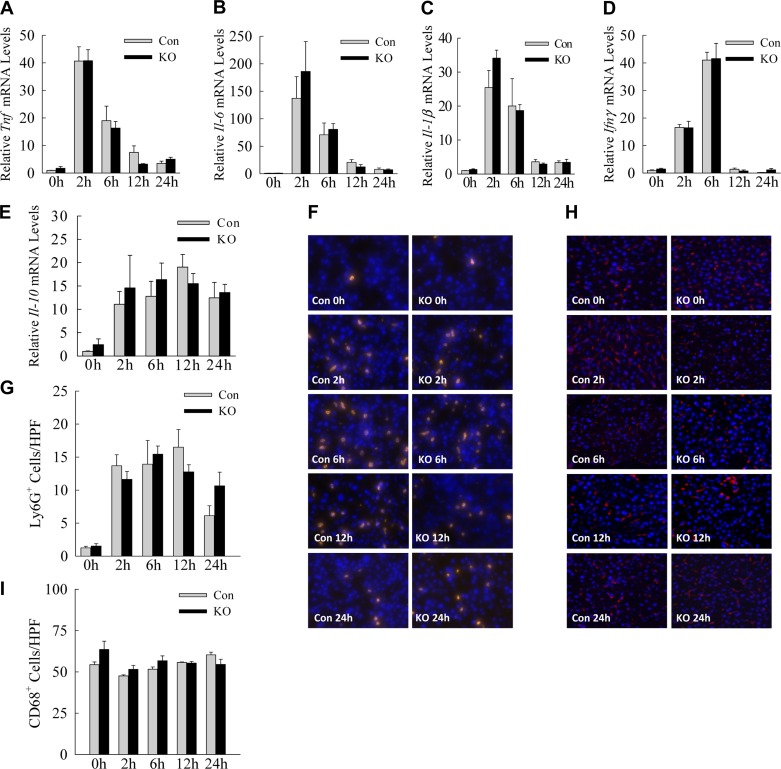
In response to LPS, neutrophils and macrophages infiltrate the liver parenchyma and promote liver injury through the elaboration of proinflammatory cytokines that trigger cellular damage. Although these studies employed hepatocyte-specific autophagy knockout mice, the possibility existed that greater initial injury in knockout hepatocytes led to the release of factors that amplified the innate immune response to LPS, thereby causing increased liver injury. Assessment of the induction of critical LPS-induced cytokines by quantitative real-time reverse-transcription PCR (qRT-PCR) revealed marked increases in hepatic cytokine gene expression with LPS, but no significant differences in the relative mRNA levels of Tnf, Il-6, Il-1β, Ifnγ, or Il-10 between control and knockout mice (Fig. 3, A–E). Immunofluorescence staining with a neutrophil-specific anti-Ly6G antibody demonstrated a marked infiltration of neutrophils with LPS treatment, but equivalent neutrophil numbers in the livers of control and knockout mice (Fig. 3, F and G). Macrophage numbers as measured by CD68 positivity were not altered by LPS treatment in either control or knockout mice (Fig. 3, H and I). These findings indicate that the increase in LPS-induced liver injury in Atg7Δhep mice was not the result of a hyperactive innate immune response to LPS.
Fig. 3.
Inflammatory response to LPS is unaffected by decreased hepatocyte autophagy. A–E: relative mRNA levels for the indicated cytokine genes measured by qRT-PCR in littermate control (Con) and Atg7Δhep knockout (KO) mice (n = 3–8). F: immunofluorescence staining for Ly6G in the livers of Con and KO mice at the indicated times after LPS treatment (×400 magnification). G: quantification of the numbers of Ly6G-positive cells (n = 3–5). H: immunofluorescence staining for CD68 (×200 magnification). I: numbers of CD68-positive cells (n = 3–5). Values are means ± SE. HPF, high-power field.
Increased liver injury is not mediated by impaired mitophagy.
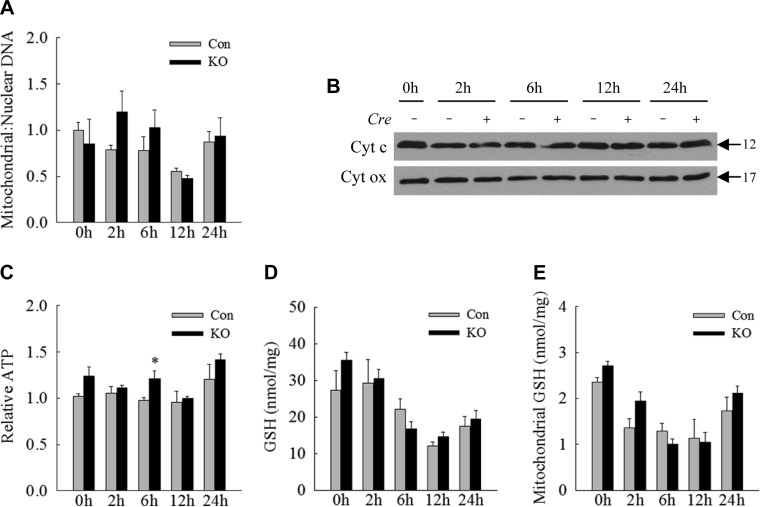
Previous studies have implicated defects in mitophagy, the selective removal of damaged or dysfunctional mitochondria by autophagy, as a mechanism of cellular injury during sepsis (23). We, therefore, assessed whether decreased hepatocyte autophagy led to the retention of damaged and potentially injury-triggering mitochondria or compromised mitochondrial integrity. Measure of mitochondrial number by means of the ratio of mitochondrial to nuclear DNA demonstrated a similar decrease in mitochondrial number at 12 h with a return to baseline levels by 24 h in both control and knockout mouse livers (Fig. 4A). Also indicative of equal mitochondrial numbers in control and Atg7Δhep mouse livers was the finding of equivalent hepatic levels of the mitochondrial proteins cytochrome c and cytochrome oxidase by immunoblotting of total hepatic protein (Fig. 4B). Mitochondrial energy homeostasis was maintained in knockout mice as hepatic ATP levels were essentially unchanged with LPS treatment in both control and Atg7Δhep mice (Fig. 4C). Oxidant stress occurred with LPS treatment, as indicated by decreased total liver (Fig. 4D) and mitochondrial (Fig. 4E) GSH, but decreases were equivalent in control and knockout mice. The failure of the decrease in hepatocyte autophagy to affect hepatic mitochondrial number, liver ATP content, or hepatic and mitochondrial GSH levels in Atg7Δhep mice indicate that the sensitization to LPS-induced injury was not secondary to impaired mitophagy.
Fig. 4.
Mitochondrial accumulation and dysfunction do not occur in knockout (KO) mice. A: ratios of mitochondrial to nuclear DNA in the livers of control (Con) and Atg7Δhep KO mice untreated and LPS-treated for the indicated number of hours. B: immunoblots of total liver protein from individual Con (Cre −) and KO (Cre +) mice probed for the mitochondrial proteins cytochrome c (Cyt c) and cytochrome oxidase (Cyt ox). C: relative levels of ATP in the livers. D: whole liver GSH levels. E: mitochondrial GSH levels. Values are means ± SE; n = 3–6. *P < 0.05, compared with Con mice at the same time point.
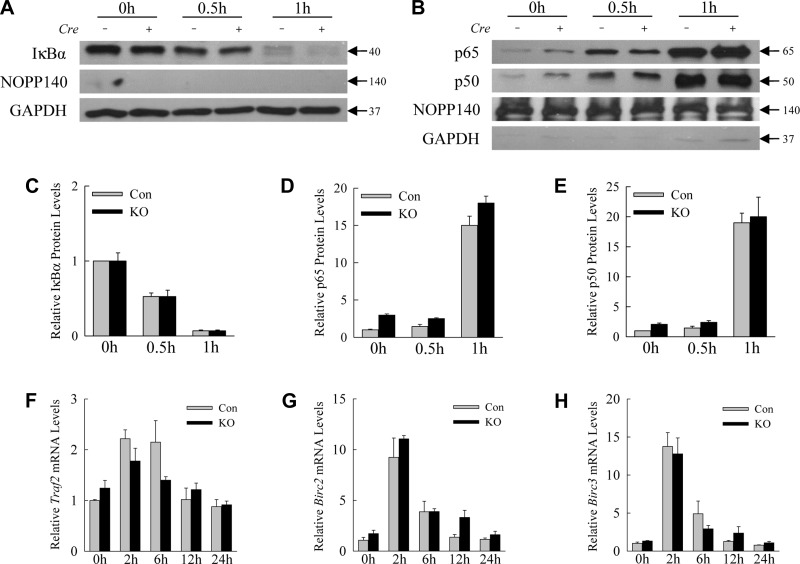
NF-κB activation is unaffected by the inhibition of hepatocyte autophagy.
Activation of the transcription factor NF-κB is critical for hepatocyte resistance to LPS-induced TNF cytotoxicity (27, 35), and autophagy regulates NF-κB signaling (19). To determine whether decreased NF-κB activation mediated the sensitization of knockout mice to hepatotoxicity from LPS, the effect of autophagy inhibition on LPS-induced NF-κB signaling was examined at several levels. Immunoblots for the cytosolic NF-κB inhibitor IκBα, which is degraded during NF-κB activation, revealed equivalent decreases in IκBα from 30 to 60 min following LPS treatment in the livers of control and knockout mice (Fig. 5, A and C). Nuclear translocation of the active p65 (Fig. 5, B and D) and p50 NF-κB subunits in response to LPS was also equivalent in control and knockout livers (Fig. 5, B and E). The purities of the cytosolic and nuclear preps were demonstrated by the restricted presence of GAPDH in the cytoplasmic fractions and NOPP140 in the nuclear isolates (Fig. 5, A and B). Finally, equal induction of the NF-κB-dependent genes Traf2, Birc2, and Birc3 occurred in control and knockout mice, as assessed by qRT-PCR of whole liver (Fig. 5, F–H). Together these findings prove that loss of hepatocyte autophagy did not alter protective NF-κB signaling in response to LPS.
Fig. 5.
NF-κB activation is unaltered by the decrease in hepatocyte autophagy. A: hepatic cytosolic protein fractions from individual control (Con) (Cre −) and knockout (KO) (Cre +) mice untreated or treated with LPS for the indicated hours and probed for IκBα, the nuclear loading control NOPP140, and the cytosolic protein loading control GAPDH. B: nuclear protein fractions from the same mice probed for the p65 and p50 subunits of NF-κB, NOPP140, and GAPDH. C–E: relative protein levels of IκBα, p65, and p50 proteins, respectively, by densitometric scanning of immunoblots (n = 4). F–H: relative Traf2, Birc2, and Birc3 mRNA levels, respectively, determined by qRT-PCR in Con and KO mice untreated, or treated with LPS for hours indicated. Values are means ± SE.
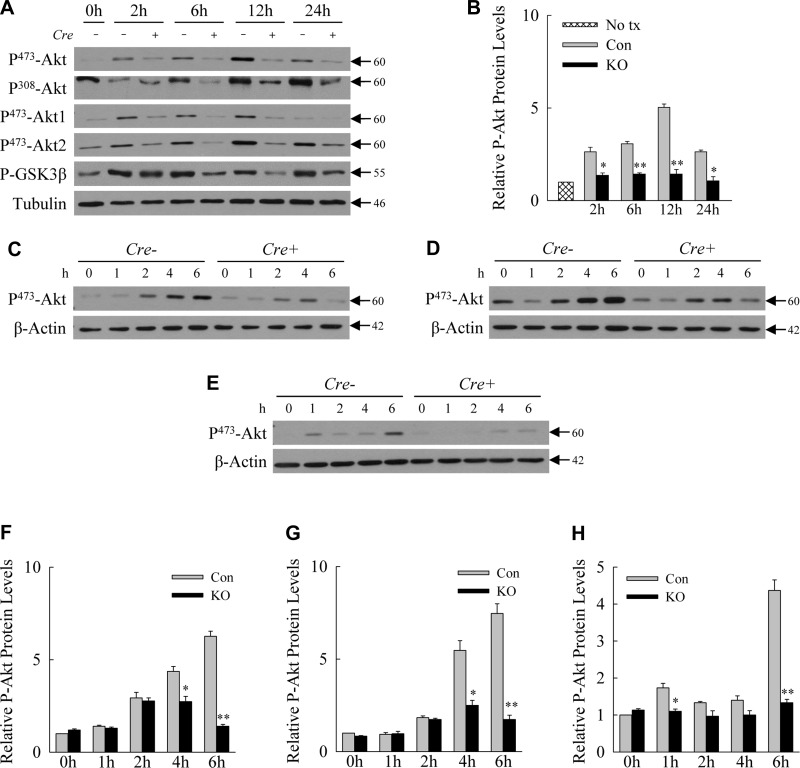
Decreased hepatocyte autophagy impairs Akt signaling.
In addition to NF-κB, Akt signaling serves as a central prosurvival pathway in hepatocytes in response to LPS-induced TNF (12, 22). We, therefore, examined whether decreased hepatocyte autophagy altered hepatic Akt signaling from LPS. Immunoblots of whole liver protein demonstrated a significant increase in Akt phosphorylation following LPS administration that peaked at 12 h in control mice (Fig. 6A). Levels of active, phosphorylated Akt were markedly decreased in the livers of knockout mice (Fig. 6, A and B). Decreased phosphorylation occurred at both Ser473 and Thr308 and in both the Akt1 and Akt2 isomers (Fig. 6A). Akt signaling was functionally impaired as the reduction in Akt phosphorylation was associated with decreased phosphorylation and, therefore, activation of the Akt downstream substrate GSK-3β (Fig. 6A).
Fig. 6.
Loss of hepatocyte autophagy is associated with decreased Akt signaling in vivo and in vitro. A: immunoblots of total liver protein from single control (Con) (Cre −) and knockout (KO) (Cre +) mice untreated or treated with LPS for the indicated hours probed for the phosphorylated (P-) forms of Akt and GSK-3β and tubulin. B: relative levels of P473-Akt in Con and KO hepatocytes by densitometric scanning of immunoblots from 3 independent experiments. *P < 0.01, **P < 0.001 compared with Con mice at the same time point; n = 3. C–E: total protein from primary hepatocytes obtained from littermate Con (Cre −) and Atg7Δhep (Cre +) mice and treated in vitro with LPS, TNF, and IL-1β, respectively. Proteins were probed for phosphorylated Akt and β-actin as a loading control. F–H: relative levels of P473-Akt in Con and knockout hepatocytes treated with LPS, TNF, and IL-1β, respectively. *P < 0.01, **P < 0.001 compared with Con mice at the same time point; n = 3. Values are means ± SE.
The use of a hepatocyte knockout, and the overall decrease of Akt phosphorylation in whole liver, which is composed mainly of hepatocytes, suggested that decreased phosphorylation occurred in hepatocytes. To confirm this fact, Akt activation was examined in primary hepatocytes isolated from the livers of tamoxifen-injected control and knockout livers and treated in vitro, not only with LPS but also with the proinflammatory cytokines TNF and IL-1β. Primary hepatocytes from Atg7Δhep mice had decreased Akt phosphorylation in response to LPS (Fig. 6, C and F), TNF (Fig. 6, D and G), and IL-1β (Fig. 6, E and H). Inhibition of autophagy, therefore, leads to a hepatocyte defect in Akt signaling in response to inflammatory factors.
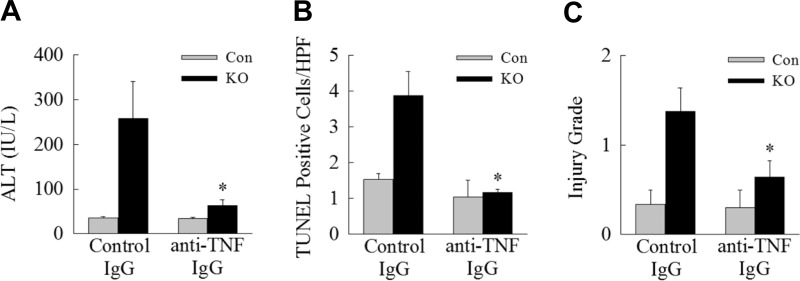
Inhibition of autophagy sensitizes the liver to LPS-induced TNF toxicity.
The reduction in knockout mice of Akt signaling critical for hepatocyte resistance to TNF toxicity suggested that increased liver injury resulted from sensitization to LPS-induced TNF. The administration of anti-TNF antibodies to Atg7Δhep mice before LPS injection almost completely abrogated liver injury compared with mice treated with IgG isotype control antibody, as determined by serum ALT levels (Fig. 7A) and TUNEL staining (Fig. 7B). Anti-TNF treatment also significantly decreased the severity of histological liver injury (Fig. 7C). Together these data demonstrate that, with reduced autophagy, high-dose LPS triggers apoptotic hepatocyte death that is mediated to a large extent by sensitization to TNF cytotoxicity.
Fig. 7.
Inhibition of TNF decreases liver injury in knockout (KO) mice. Control (Con) and KO mice were pretreated 4 h before LPS administration with an isotypic IgG control antibody or a monoclonal IgG2a against mouse TNF and killed at 24 h for analysis of serum ALT levels (*P < 0.03, compared with KO mice treated with control antibody; n = 7–9; A); TUNEL staining (*P < 0.001, compared with KO mice treated with control antibody; n = 4–8; B); and histological grade of liver injury (*P < 0.04, compared with KO mice treated with control antibody; n = 4–8; C). Values are means ± SE.
DISCUSSION
Sepsis is a major clinical problem for which the development of an effective therapy has been hindered by an incomplete understanding of how the bacterial product LPS triggers injury in end organs, such as the liver. LPS induces autophagy in mouse liver and hepatocytes (1, 5), and increased numbers of autophagosomes under electron microscopy have been reported in human livers from septic patients, suggesting that hepatic autophagy is increased by LPS in humans as well (34). The induction of autophagy by LPS, and the known hepatoprotective functions of autophagy (8), suggested a potential role for autophagy in preventing liver injury from high levels of LPS. Studies with nonspecific lysosomal inhibitors or a global knockdown of autophagy worsened liver injury in the cecal ligation and puncture model of sepsis (5, 30). The present study employed a hepatocyte-specific knockout of autophagy and high-dose LPS to demonstrate that hepatocyte autophagy protects the liver from the damaging effects of LPS. These findings establish for the first time that tissue-specific levels of autophagy mediate end-organ damage from septic levels of LPS.
LPS induces a hyperinflammatory state in which cytokines such as TNF and IL-1 are overproduced to the extent that they cause tissue injury. An increase in this inflammatory response was not the mechanism of liver injury in Atg7Δhep mice, as the degree of hepatic macrophage and neutrophil infiltration and the amount of proinflammatory cytokine induction were unaffected by the decrease in autophagy. In the absence of an effect on inflammation, we investigated whether the loss of autophagy led to a decrease in hepatocyte resistance to the cytotoxic effects of LPS-induced cytokines. One of the central mechanisms by which autophagy functions to protect hepatocytes from injury is through the selective removal of damaged mitochondria by mitophagy (15). LPS can induce mitochondrial damage, and previous studies of the effects of LPS on the heart have indicated that mitophagy is a mechanism of cellular resistance to cytotoxicity from LPS (23). Whereas wild-type mice recovered cardiac function after an injection of LPS, mice deficient in mitophagy due to a knockout of PARK2/Parkin developed metabolic mitochondrial dysfunction, together with an impaired recovery of cardiac contractility (23). In the present studies, the sensitization of autophagy-deficient hepatocytes to death from LPS did not occur through a lack of hepatic mitophagy, as the numbers of mitochondria and the levels of mitochondrial ATP and oxidative stress were unaffected by the loss of hepatocyte autophagy. Thus there was no evidence that decreased removal of damaged mitochondria in response to LPS was the mechanism of increased liver injury in the knockout mice. It remains possible that, although mitophagy did not play a role in knockout mouse sensitization to LPS-induced injury, knockout mice still developed increased mitochondrial injury that promoted hepatocyte death. Normal energy and redox status suggest intact mitochondrial function, but do not completely exclude this possibility.
The lack of mechanistic involvement of mitophagy in the sensitization to LPS-induced injury, together with findings that the production of potentially toxic cytokines was unaltered, led to an examination of whether knockout mice had a defect in hepatocyte-protective signaling pathways. NF-κB is critical to protect hepatocytes from LPS-induced TNF, but NF-κB signaling as assessed by IκB degradation, p50/p65 NF-κB nuclear translocation, and the induction of NF-κB-dependent genes was unaltered in knockout mice. When the hepatoprotective Akt signaling pathway was examined, the livers of Atg7Δhep mice had reduced Akt activation, as reflected in decreased phosphorylation of both Akt1 and Akt2 after LPS injection. That the decrease in Akt phosphorylation in whole liver was due at least in part to a hepatocyte effect was demonstrated by the finding that cultured hepatocytes from Atg7Δhep mice had decreased Akt phosphorylation in vitro. Akt phosphorylation was reduced in response to both LPS directly and to the LPS-inducible cytokines TNF and IL-1β. The mechanism by which autophagy promotes Akt phosphorylation is unclear. The decrease in Akt activation was not due to a defect in LPS receptor binding, as NF-κB signaling was intact in the knockout mice. A reduction in autophagy could prolong activation of a signaling pathway by preventing the normal timely degradation of activated pathway proteins (3). The mechanism by which a decrease in autophagy could block activation is less clear.
Akt activation is likely a generalized cellular protective response to LPS toxicity, as Akt protects against cardiac dysfunction, as well through the prevention of apoptosis by an undefined mechanism (10). Our study specifically examined whether liver injury from decreased Akt activation secondary to impaired autophagy was TNF dependent, as Akt signaling is a critical resistance pathway against hepatocyte TNF toxicity (12, 22). Injury was prevented by TNF neutralization, demonstrating the decrease in Akt signaling sensitized the liver to cytotoxicity from LPS-induced TNF. These findings, along with our laboratory's previous study demonstrating the critical function of autophagy in preventing TNF-dependent hepatotoxic liver injury (1), point to the importance of the lysosomal degradative pathway of autophagy in hepatoprotection against inflammatory mediators.
The finding that decreased hepatocyte autophagy sensitizes the liver to increased injury from LPS may explain in part the known susceptibility of certain patient populations to an adverse clinical outcome from sepsis. Over 75% of the deaths from sepsis in the United States occur in individuals over the age of 65 yr (21). Aging is associated with an impairment in autophagic function (24), and the decrease in hepatic autophagy that occurs with aging has been previously implicated in the progression of hepatic steatosis to liver injury and steatohepatitis (11). Decreased hepatocyte autophagy with aging may underlie in part the increased morbidity from sepsis in the elderly. Similarly, we and others have demonstrated decreased autophagic function in the livers of diet-induced and genetically obese mice (28, 37), and this defect in autophagy may play a role in the increased mortality from sepsis in humans with obesity (4, 31). As the decrease in autophagy that occurs in the liver with aging and obesity may contribute to poor outcomes from sepsis, these high-risk groups may benefit from therapeutic agents to augment hepatocyte autophagic function.
GRANTS
This work was supported in part by National Institutes of Health Grants R01-DK-044234, R01-DK-061498, R01-AA-022601 (M. J. Czaja), F32-DK-096791 (K. Liu), and P30-DK-041296, and an American Liver Foundation Postdoctoral Research Fellowship Award (K. Liu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.L., G.I., S.A.M., K.L., E.Z., M.A., Y.L., and K.E.T. performed experiments; G.L., K.E.T., and M.J.C. analyzed data; G.L. and M.J.C. interpreted results of experiments; G.L. and M.J.C. prepared figures; G.L. and M.J.C. edited and revised manuscript; G.L., G.I., K.L., E.Z., M.A., Y.L., K.E.T., and M.J.C. approved final version of manuscript; M.J.C. conception and design of research; M.J.C. drafted manuscript.
ACKNOWLEDGMENTS
The anti-murine TNF and isotypic control antibodies were the kind gifts of Janssen Research & Development, and Thomas Meier kindly provided the NOPP140 antibody.
REFERENCES
- 1.Amir M, Zhao E, Fontana L, Rosenberg H, Tanaka K, Gao G, Czaja MJ. Inhibition of hepatocyte autophagy increases tumor necrosis factor-dependent liver injury by promoting caspase-8 activation. Cell Death Differ 20: 878–887, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Belaid A, Ndiaye PD, Klionsky DJ, Hofman P, Mograbi B. Signalphagy: scheduled signal termination by macroautophagy. Autophagy 9: 1629–1630, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bercault N, Boulain T, Kuteifan K, Wolf M, Runge I, Fleury JC. Obesity-related excess mortality rate in an adult intensive care unit: A risk-adjusted matched cohort study. Crit Care Med 32: 998–1003, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Carchman EH, Rao J, Loughran PA, Rosengart MR, Zuckerbraun BS. Heme oxygenase-1-mediated autophagy protects against hepatocyte cell death and hepatic injury from infection/sepsis in mice. Hepatology 53: 2053–2062, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colleran A, Ryan A, O'Gorman A, Mureau C, Liptrot C, Dockery P, Fearnhead H, Egan LJ. Autophagosomal IκBα degradation plays a role in the long term control of tumor necrosis factor-α-induced nuclear factor-κB (NF-κB) activity. J Biol Chem 286: 22886–22893, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czaja MJ. Functions of autophagy in hepatic and pancreatic physiology and disease. Gastroenterology 140: 1895–1908, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czaja MJ, Ding WX, Donohue TM, Friedman SL, Kim JS, Komatsu M, Lemasters JJ, Lemoine A, Lin JD, Ou JH, Perlmutter DH, Randall G, Ray RB, Tsung A, Yin XM. Functions of autophagy in normal and diseased liver. Autophagy 9: 1131–1158, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol 13: 722–737, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong M, Hu N, Hua Y, Xu X, Kandadi MR, Guo R, Jiang S, Nair S, Hu D, Ren J. Chronic Akt activation attenuated lipopolysaccharide-induced cardiac dysfunction via Akt/GSK3β-dependent inhibition of apoptosis and ER stress. Biochim Biophys Acta 1832: 848–863, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontana L, Zhao E, Amir M, Dong H, Tanaka K, Czaja MJ. Aging promotes the development of diet-induced murine steatohepatitis but not steatosis. Hepatology 57: 995–1004, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatano E, Brenner DA. Akt protects mouse hepatocytes from TNF-α- and Fas-mediated apoptosis through NK-κB activation. Am J Physiol Gastrointest Liver Physiol 281: G1357–G1368, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 348: 138–150, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Ilyas G, Zhao E, Liu K, Lin Y, Tesfa L, Tanaka KE, Czaja MJ. Macrophage autophagy limits acute toxic liver injury in mice through down regulation of interleukin-1β. J Hepatol 64: 118–127, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 462: 245–253, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol 9: 1004–1010, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu K, Zhao E, Ilyas G, Lalazar G, Lin Y, Haseeb M, Tanaka KE, Czaja MJ. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy 11: 271–284, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, Iwashyna TJ. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 312: 90–92, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Luo MX, Wong SH, Chan MT, Yu L, Yu SS, Wu F, Xiao Z, Wang X, Zhang L, Cheng AS, Ng SS, Chan FK, Cho CH, Yu J, Sung JJ, Wu WK. Autophagy mediates HBx-induced nuclear factor-κB activation and release of IL-6, IL-8, and CXCL2 in hepatocytes. J Cell Physiol 230: 2382–2389, 2015. [DOI] [PubMed] [Google Scholar]
- 20.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348: 1546–1554, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med 34: 15–21, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Osawa Y, Uchinami H, Bielawski J, Schwabe RF, Hannun YA, Brenner DA. Roles for C16-ceramide and sphingosine 1-phosphate in regulating hepatocyte apoptosis in response to tumor necrosis factor-α. J Biol Chem 280: 27879–27887, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Piquereau J, Godin R, Deschenes S, Bessi VL, Mofarrahi M, Hussain SN, Burelle Y. Protective role of PARK2/Parkin in sepsis-induced cardiac contractile and mitochondrial dysfunction. Autophagy 9: 1837–1851, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell 146: 682–695, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Russell JA. Management of sepsis. N Engl J Med 355: 1699–1713, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Schwabe RF, Brenner DA. Mechanisms of liver injury. I. TNF-α-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol 290: G583–G589, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Schwabe RF, Uchinami H, Qian T, Bennett BL, Lemasters JJ, Brenner DA. Differential requirement for c-Jun NH2-terminal kinase in TNFα- and Fas-mediated apoptosis in hepatocytes. FASEB J 18: 720–722, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature 458: 1131–1135, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh R, Wang Y, Schattenberg JM, Xiang Y, Czaja MJ. Chronic oxidative stress sensitizes hepatocytes to death from 4-hydroxynonenal by JNK/c-Jun overactivation. Am J Physiol Gastrointest Liver Physiol 297: G907–G917, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi W, Watanabe E, Fujimura L, Watanabe-Takano H, Yoshidome H, Swanson PE, Tokuhisa T, Oda S, Hatano M. Kinetics and protective role of autophagy in a mouse cecal ligation and puncture-induced sepsis. Crit Care 17: R160, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vachharajani V. Influence of obesity on sepsis. Pathophysiology 15: 123–134, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Schattenberg JM, Rigoli RM, Storz P, Czaja MJ. Hepatocyte resistance to oxidative stress is dependent on protein kinase C-mediated down-regulation of c-Jun/AP-1. J Biol Chem 279: 31089–31097, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Singh R, Lefkowitch JH, Rigoli RM, Czaja MJ. Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J Biol Chem 281: 15258–15267, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe E, Muenzer JT, Hawkins WG, Davis CG, Dixon DJ, McDunn JE, Brackett DJ, Lerner MR, Swanson PE, Hotchkiss RS. Sepsis induces extensive autophagic vacuolization in hepatocytes: a clinical and laboratory-based study. Lab Invest 89: 549–561, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Y, Bialik S, Jones BE, Iimuro Y, Kitsis RN, Srinivasan A, Brenner DA, Czaja MJ. NF-κB inactivation converts a hepatocyte cell line TNF-α response from proliferation to apoptosis. Am J Physiol Cell Physiol 275: C1058–C1066, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Xu Y, Jones BE, Neufeld DS, Czaja MJ. Glutathione modulates rat and mouse hepatocyte sensitivity to tumor necrosis factor toxicity. Gastroenterology 115: 1229–1237, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab 11: 467–478, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]