Summary
A novel approach to immunization against glioma tumors is described. Immunization against eleven antigens expressed in malignant brain tumors elicits responses to one or more antigens in a large percentage of patients. This novel approach suggests that it could be extended to a Phase III trial.
In this issue of CLINICAL CANCER RESEARCH, Rampling and colleagues show the data from a first in man immunization of glioma patients with a vaccine containing 10 antigenic epitopes known to be expressed in glioma1. Immunization to treat solid tumors has a long history. Non-specific immunization led to some individual responses, and these early results were then adapted by novel contemporary understandings of immune system function. More recent successes in the treatment of melanoma, small cell lung carcinoma and kidney cancer, as well as others being evaluated, with immunotherapy has reignited hope that similar approaches will work in patients with malignant brain tumors. Inhibitors of co-stimulating molecule pairs CTLA4-CD28 and PD1-PDL1 have shown strong anti-tumoral activities across disease stages, even after failure of standard of care, and various stages of disease progression2,3. This comes after many years of various approaches to systemic immunization providing partially effective results without any definite breakthroughs. Vaccination in cancer has employed potential tumor antigens deduced from tumor proteins, in vitro activation of tumor infiltrating leucocytes, alone, or in combination with suppression of endogenous immune system followed by bone marrow transplantation to potentiate leukocyte engraftment. Results were marginally effective, with only occasional complete responses and long term patient survival. Checkpoint inhibitors have transformed the landscape of solid cancer immunotherapy achieving, in a clinically significant percentage of patients, complete remission, and partial remission in significant numbers4,5.
Highly malignant gliomas (WHO grade IV; glioblastoma multiforme; GBM) remain universally fatal with median survival ~16–18 months. These are some of the most frequently targeted tumors as long term survival has improved marginally during the last 80 years. Surgery, radiation and chemotherapy constitute the standard of care; yet long term survival beyond 2–3 years is rare6. Immunotherapy has been proposed and tested in patients with GBM. Potential tumor antigens derived from potentially immunogenic proteins have been used to stimulate the immune response to recognize these antigens in the patients’ GBM. Alternatively, groups have utilized patients’ own tumor lysates to stimulate the patients’ dendritic cells to be used to as vaccines to elicit immunity against their endogenous tumor antigens. In spite of measurable T cell responses, therapeutic results have been marginal with occasional clinically significant responses5.
Several of these approaches have entered phase III clinical trials. In spite of positive earlier phase clinical trials results, the recent failure of a vaccine against EGFRvIII to extend survival of patients in a phase III trial continues to challenge to approaches that target single antigenic epitopes (http://ir.celldex.com/releasedetail.cfm?ReleaseID=959021)7. Analogously, trials utilizing some of the other vaccination approaches have shown increases in PFS, but limited extension of OS. Why has immunization not shown stronger clinical efficiency in extending life of patient with GBM?
Possible explanations are that immunizations and innate immune adjuvants are not strong enough, that exogenous antigens used are not the same ones as the endogenous ones presented by tumors on HLA, that not enough antigens are retrieved from tumors in approaches attempting to load dendritic cells with tumor antigens, or that tumor downregulation of HLA would sequester antigens from T-cell recognition. Studies of tumor antigen repertoire are cumbersome, and thus the recent description of the neoantigen repertoire in many different tumors is of great importance to tumor immunotherapy. Tumor neoantigen expression is highest in melanoma and lung cancer and lowest in AML, ALL, and in pilocytic astrocytoma. Interest in expression of tumor neoantigens reflects the theory that such antigens would not have been negatively selected during T-cell development. They therefore represent novel targets for the immune system. The correlation between the neoantigen expression repertoire and the clinical efficacy of the immune checkpoint inhibitors supports this hypothesis. It also predicts why vaccines utilizing a low number of antigens would be less likely to succeed.
Taking these practical and theoretical elements into consideration Rampling et al. () present data on a novel vaccination clinical trial for patients with glioblastoma in which vaccines are loaded with endogenous epitopes known to be presented on HLA molecules in GBM. Specifically, Dutoit et al.7 characterized 46000 HLA-bound peptides in GBM, of which 3000 were selectively bound to the most common HLA in humans, i.e., HLA-A*02. They then concentrated on studying 10 GBM-associated antigens based on levels of expression in GBM, and low expression in normal organs. Importantly, and as expected of neoantigens, actual patients did not display any T-cell tolerance to the selected peptides. Equally, patients’ CD8 + T cells induced specific lysis of tumour cells in vitro which expressed the novel antigens. Further, the identification of T-cells specific for the novel antigens in the GBM microenvironment supports the idea that such T-cells could be functionally lytic in vivo as well.
Rampling et al. in this issue (), report the translation of these exciting basic science findings into a first in human phase I trial of a novel multi-peptide therapeutic vaccine in patients recently diagnosed with GBM [FIGURE 1]. The study was performed in two-cohorts of patients treated with standard of care and vaccine immunotherapy. Patients were all HLA-A*02, and were treated with 11 intradermal immunizations. GM-CSF was used as adjuvant and vaccines were administered over 6 months. Patients responded to either single antigens (90%), or to multiple antigens (50%). Importantly pre-treatment levels of Tregs, administration of chemo-radiotherapy, or treatment with steroids did not affect the capacity of the vaccine to stimulate immune responses to GBM antigens. These data together with the previous finding of T-cells specific to the GBM antigens within the GBM microenvironment strongly support the assertion of future combination of the novel type of immunization with checkpoint inhibitors.
FIGURE 1.
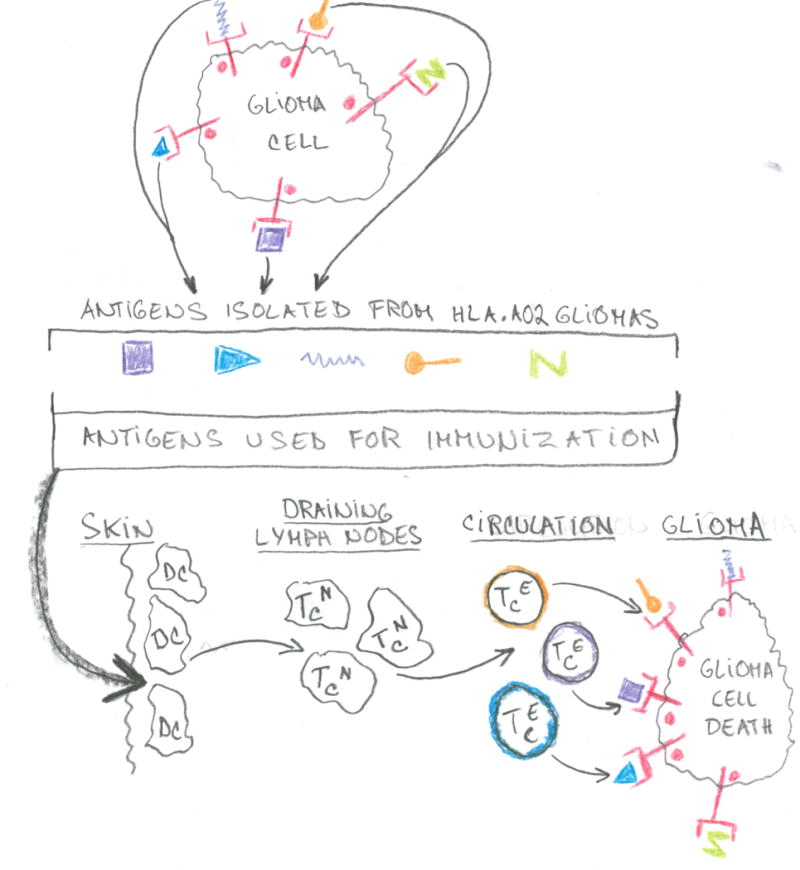
The figure shows schematically the translational aspects of this work. Specifically the top part of the figure describes the work of Dutoit et al. (7) of isolating glioma antigens expressed specifically in glioma tumor cells. The middle part of the figure illustrates how Rampling et al. () utilized these antigens to immunize patients. The lower part of the figure illustrates the mechanism of action whereby the antigens used as immunogens are transported to the draining lymph nodes where they are presented to naïve T-cells. The resulting T-effector cells then migrate to the tumor in the brain where they kill antigen expressing glioma cells.
Overall survival was not increased when patients responding to multiple antigens were compared to those only responding to one antigen. Median survival was comparable to median survival of the general patient population. However, striking difference in survival was detected when patients were evaluated in relation to the presence or absence of an injection site response. Under this comparison, patients in whom an injection site response was evident, displayed twice the survival of those without injection site response. Though statistical analysis provides a significant p value, the authors are careful not to overinterpret these data given that this was a phase I, rather than a double blind randomized phase III trial. The data from this trial thus provide strong support for the continued development of this vaccination approach. Though follow up larger trials of this approach would be predictable, rapid phase I trials combining this vaccination strategy and checkpoint inhibitors could provide stronger responses, and increase the availability of a potentially useful treatment to desperately ill patients.
In context there are currently a number of trials with varying approaches ongoing for GBM. Amongst others, oncolytic viruses are being tested in early phase trials8, as are small molecule inhibitors of enzymes thought to be essential for the survival of glioma cells9, gene and immunotherapy to reengineer the brain immune system (https://clinicaltrials.gov/ct2/show/NCT01811992)10, and novel radiation approaches are all currently ongoing. In view of the profound and urgent need of novel treatments for patients suffering from GBM we hope that all our efforts will significantly contribute to achieving clinical breakthrough results soon.
Footnotes
The authors have no conflicts to declare.
References
- 1.Rampling R, Cruickshank G, Lewis AD, Fitzsimmons SA, Workman P. Direct measurement of pO2 distribution and bioreductive enzymes in human malignant brain tumors. International journal of radiation oncology, biology, physics. 1994 Jun 15;29(3):427–431. doi: 10.1016/0360-3016(94)90432-4. [DOI] [PubMed] [Google Scholar]
- 2.Hughes PE, Caenepeel S, Wu LC. Targeted Therapy and Checkpoint Immunotherapy Combinations for the Treatment of Cancer. Trends Immunol. 2016 Jul;37(7):462–476. doi: 10.1016/j.it.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016 Mar;13(3):143–158. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 4.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015 Jun 10;33(17):1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calinescu AA, Kamran N, Baker G, Mineharu Y, Lowenstein PR, Castro MG. Overview of current immunotherapeutic strategies for glioma. Immunotherapy. 2015;7(10):1073–1104. doi: 10.2217/imt.15.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wirsching HG, Galanis E, Weller M. Glioblastoma. Handb Clin Neurol. 2016;134:381–397. doi: 10.1016/B978-0-12-802997-8.00023-2. [DOI] [PubMed] [Google Scholar]
- 7.Dutoit V, Herold-Mende C, Hilf N, Schoor O, Beckhove P, Bucher J. Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain. 2012 Apr;135(Pt 4):1042–1054. doi: 10.1093/brain/aws042. [DOI] [PubMed] [Google Scholar]
- 8.Cloughesy TF, Landolfi J, Hogan DJ, Bloomfield S, Carter B, Chen CC, et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci Transl Med. 2016 Jun 1;8(341):341ra375. doi: 10.1126/scitranslmed.aad9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gounder MM, Nayak L, Sahebjam S, Muzijansky Am Sanchez AJ, Desideri S, et al. Evaluation of the Safety and Benefit of Phase I Oncology Trials for Patients With Primary CNS Tumors. J Clin Oncol. 2015 Oct 1;33(28):3186–3192. doi: 10.1200/JCO.2015.61.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009 Jan 13;6(1):e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]


