Abstract
Context:
Greater anterior knee laxity (AKL) has been identified as an anterior cruciate ligament (ACL) injury risk factor. The structural factors that contribute to greater AKL are not fully understood but may include the ACL and bone geometry.
Objective:
To determine the relationship of ACL width and femoral notch angle to AKL.
Design:
Cross-sectional study.
Setting:
Controlled laboratory.
Patients or Other Participants:
Twenty recreationally active females (age = 21.2 ± 3.1 years, height = 1.66.1 ± 7.3 cm, mass = 66.5 ± 12.0 kg).
Main Outcome Measure(s):
Anterior cruciate ligament width and femoral notch angle were obtained with magnetic resonance imaging of the knee and AKL was assessed. Anterior cruciate ligament width was measured as the width of a line that transected the ACL and was drawn perpendicular to the Blumensaat line. Femoral notch angle was formed by the intersection of the line parallel to the posterior cortex of the femur and the Blumensaat line. Anterior knee laxity was the anterior displacement of the tibia relative to the femur (mm) at 130 N of an applied force. Ten participants' magnetic resonance imaging data were assessed on 2 occasions to establish intratester reliability and precision. Using stepwise backward linear regression, we examined the extent to which ACL width, femoral notch angle, and weight were associated with AKL.
Results:
Strong measurement consistency and precision (intraclass correlation coefficient [2,1] ± SEM) were established for ACL width (0.98 ± 0.3 mm) and femoral notch angle (0.97° ± 1.1°). The regression demonstrated that ACL width (5.9 ± 1.4 mm) was negatively associated with AKL (7.2 ± 2.0 mm; R2 = 0.22, P = .04). Femoral notch angle and weight were not retained in the final model.
Conclusions:
A narrower ACL was associated with greater AKL. This finding may inform the development of ACL injury-prevention programs that include components designed to increase ACL size or strength (or both). Future authors should establish which other factors contribute to greater AKL in order to best inform injury-prevention efforts.
Key Words: lower extremity, femoral notch angle, injury prevention
Key Points
Females with smaller anterior cruciate ligament widths had greater anterior knee laxity.
Femoral notch angle was not related to anterior knee laxity.
Multiple intrinsic factors related to passive joint structures have been shown to predict anterior cruciate ligament (ACL) injury.1−4 Anterior knee laxity (AKL) is the most common clinical measure of ACL function, as the ACL provides about 85% of the total resisting force to anterior tibial translation.5 Anterior knee laxity has been identified in both prospective2,3 and retrospective6 studies as a risk factor for ACL injury. Specifically, greater side-to-side AKL differences have been associated with increased ACL injury risk in athletic populations,2 and a combination of narrower femoral notch width, higher body mass index, greater generalized joint laxity, and greater AKL predicted ACL injury risk in active military personnel.3 Although greater AKL is associated with noncontact ACL injury risk, little is understood about the structural factors that may contribute to an individual having this condition; more information may help target future injury-prevention efforts.
Research7,8 suggests that the structural properties of the ACL and surrounding bony geometry may contribute to the magnitude of AKL. It is well established in the orthopaedic biomechanics literature that increased cross-sectional area of connective tissue is generally associated with greater resistance to deformation.9 This suggests that greater cross-sectional area, and thus width, of the ligament leads to less deformation at fixed amounts of load. Specific to the ACL, this would mean that greater ACL width would be associated with less ligamentous deformation under the same load and, thus, less AKL. Although this theory is supported by the authors7 of animal studies who reported that total anterior-posterior translation of the knee was associated with ligamentous cross-sectional area after ligament reconstruction, the relationship between ACL width and AKL in healthy humans is relatively unknown.
Bony geometry may also affect joint laxity, as a steeper femoral notch angle has been associated with increased impingement of the ACL against the femoral condyle.8 This impingement could lead to a weakened ACL,10 which may, in turn, contribute to greater knee laxity.8 Such ligamentous impingement associated with a steeper notch angle has been associated with complaints of effusions, anterior knee instability, and anterior knee pain.10 Because the magnitude of ligamentous deformation under a uniform load force has been associated with ligamentous collagen fiber direction,11 a steeper femoral notch angle could result in the ACL collagen fibers being oriented more parallel to the tibia, which could increase knee laxity. Collectively, these factors suggest that femoral notch angle could influence ACL fiber orientation and associated ligamentous impingement, which may thereby affect AKL,12 but whether there is a relationship between bony geometry and ACL function is unknown.
Based on these unknown factors, the purpose of our study was to investigate the contribution of ACL width and femoral notch angle on AKL. A better understanding of the relationships among ACL width, femoral notch angle, and AKL may advance our identification of the structural factors associated with AKL and the corresponding risk of ACL injury. In turn, understanding the factors associated with greater AKL may help inform future injury-prevention efforts.
METHODS
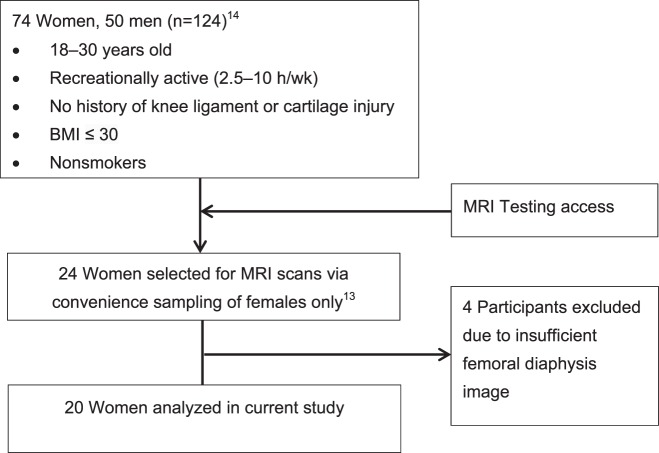
These data represent secondary analyses from a previous larger study that included magnetic resonance imaging (MRI) scans.13 The participants in the larger study were 18 to 30 years old, recreationally active (2.5−10 h/wk), nonsmokers with no history of knee ligament or cartilage injury, and body mass index ≤30.13,14 From this larger study, we selected 24 participants via convenience sampling for MRI examination when the device was available.13 Due to insufficient femoral diaphysis imaging, 4 participants were excluded. Thus, 20 recreationally active women were included along with their corresponding knee-laxity measures from a previous study.13,14 The participant-inclusion process is detailed in Figure 1. All recruits gave informed consent to participate in the study, which was approved by the university's institutional review board. All measurements were obtained on the dominant-stance leg (defined as the preferred-stance leg when kicking a ball).
Figure 1. .
Flow diagram of screened and included participants. Abbreviations: BMI, body mass index; MRI, magnetic resonance imaging.
The T1-weighted, multiplanar MRI scans (1.5 T; voxel size = 0.55 × 0.55 × 0.5 mm) were acquired for imaging of the dominant knee.15 All MRI measures were performed offline by a single investigator (nonradiologist) who was blinded to the individual laxity measures. Anterior cruciate ligament width was measured using Medical Image Processing, Analysis and Visualization software (http://mipav.cit.nih.gov; The National Institutes of Health, Bethesda, MD) per the methods described by Anderson et al.16 First, the sagittal-plane image that provided the clearest image of the Blumensaat line was selected. The Blumensaat line is the landmark that corresponds to the roof of the femoral intercondylar notch as drawn on the sagittal image of the knee joint.17 On this image, the Blumensaat line was drawn through the roof of the intercondylar notch. Anterior cruciate ligament width was determined by a line drawn perpendicular to the Blumensaat line that measured the distance across the ACL (Figure 2).
Figure 2. .
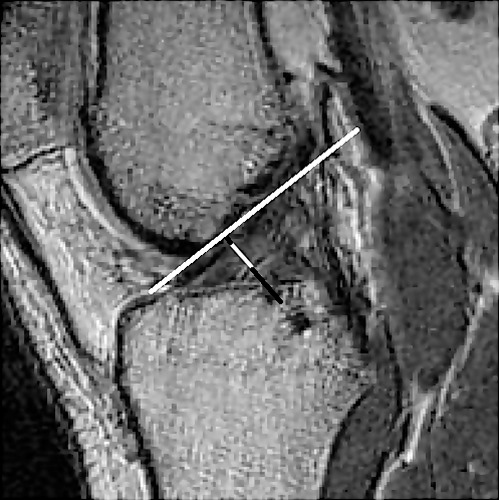
Anterior cruciate ligament width measurement = the distance across the anterior cruciate ligament (white) on a line perpendicular to the Blumensaat line.
The femoral notch angle was obtained from the same T1-weighted, multiplanar MRI scans as described by Jagodzinski et al.8 Using Medical Image Processing, Analysis and Visualization software, the same sagittal-plane image used for the clearest view of the Blumensaat line was also used to determine ACL width. From this image, 2 parallel lines perpendicular to the femoral cortex were drawn at distances of 50 and 70 mm proximal to the joint line. A line drawn through the centers of the parallel lines defined the long axis of the femur. The angle of this long axis line with respect to the Blumensaat line defined the notch angle (Figure 3). To determine the intratester consistency and precision of the measurement, the first 10 MRI scans were remeasured for ACL width and notch angle at least 1 week later. During all MRI measurements, the investigator was blinded to knee-laxity values.
Figure 3. .
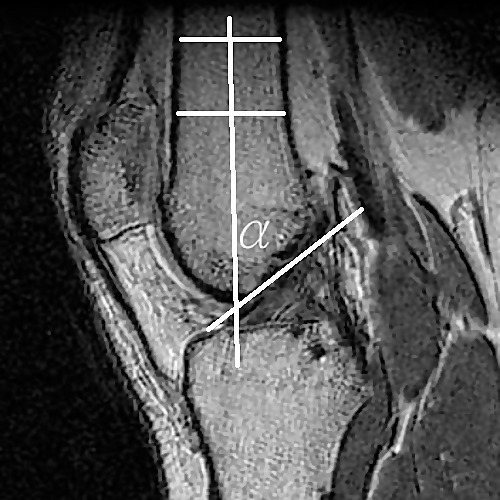
Femoral notch angle measurement α represents the angle of the intersection between the line parallel to the long axis of the femur and the Blumensaat line.
In the previous study,13,14 AKL was measured via a KT-2000 Knee Arthrometer (Medmetric Corporation, San Diego, CA) by a single investigator who had no knowledge of the MRI measures. The participant was placed in a supine position with the knee flexed 25° ± 5° over a thigh bolster. To avoid lower extremity rotation when testing, we used a hook-and-loop strap to fix the participant's thighs while the feet rested in the foot cradles. The examiner first applied a 90-N posteriorly directed force and then a 130-N anteriorly directed force to the tibia, where the tibial displacement (mm) with respect to the femur was recorded via the compuKT V1.6 software (Medmetric Corporation). Anterior knee laxity was defined as the anterior displacement of the tibia relative to the femur from 0 to 130 N of anterior tibial loading. The last 2 measures were obtained and averaged for analyses. The single examiner demonstrated strong interday reliability and precision of the measure (intraclass correlation coefficient [ICC] [SEM] = 0.96 [0.3 mm]).18 Participants were tested during the first 6 days of their menstrual cycle (based on self-report of the first day of menstrual bleeding) to control for cyclic hormonal effects on laxity.14 The AKL data in the current study were originally reported as part of the larger study.14
For ACL width and femoral notch angle measures, we used ICC [2,1] and SEM to assess intratester reliability and precision, respectively. Stepwise backward, multiple linear regression examined the extent to which ACL width, femoral notch angle, and weight were associated with AKL. Anterior cruciate ligament width, femoral notch angle, and weight were entered in the first step, and then the variables that did not improve the overall model were removed in the following steps. Weight was initially entered to account for body size. The α level for significance was set a priori at P ≤ .05. All calculations were performed using PASW (version 18.0; SPSS Inc, Chicago, IL).
RESULTS
Descriptive data for the 20 participants' demographics, ACL width, femoral notch angle, and AKL are shown in Table 1. Of the 10 participants who were measured on 2 occasions, strong intratester measurement consistency and precision were noted for ACL width (ICC [2,1] ± SEM = 0.98 ± 0.3 mm) and femoral notch angle (ICC [2,1] ± SEM = 0.97° ± 1.1°) measures.
Table 1. .
Descriptive Statistics for All Participants (N = 20)
| Measure |
Mean ± SD |
| Age, y | 21.2 ± 3.1 |
| Height, cm | 166.1 ± 7.3 |
| Mass, kg | 66.5 ± 12.0 |
| Anterior cruciate ligament width, mm | 5.9 ± 1.4 |
| Notch angle, ° | 38.0 ± 6.3 |
| Anterior knee laxity, mm14 | 7.2 ± 2.0 |
On the initial step of the regression analyses, ACL width, femoral notch angle, and body weight had no association with AKL (R2 = 0.25, P = .20). The initial regression model is found in Table 2. The second step removed femoral notch angle from the model; ACL width and body weight were still not associated with AKL (R2 = 0.24, P = .10). Body weight was removed in the final step, which resulted in ACL width being negatively associated with AKL (R2 = 0.22, P = .04). The final regression equation was AKL = (−0.67) × ACL width + 11.20, which suggests, on average that for every 1-mm increase in ACL width, AKL decreased 0.67 mm. The scatterplot of the relationship between ACL width and AKL is depicted in Figure 4.
Table 2. .
Initial Regression Coefficients Model
| Coefficient |
β |
Significance |
95% Confidence Interval |
| (Constant) | 11.286 | .015 | 2.555, 20.017 |
| Weight | .024 | .529 | −0.056, 0.104 |
| Anterior cruciate ligament width | −.757 | .037 | −1.462, −0.052 |
| Femoral notch angle | −.032 | .660 | −0.181, 0.118 |
Figure 4. .
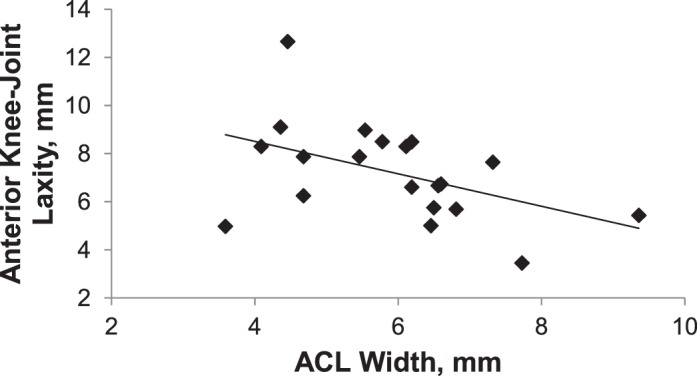
Scatter plot of anterior knee laxity and anterior cruciate ligament (ACL) width.
DISCUSSION
Greater AKL has been prospectively associated with higher ACL injury rates.2,3 However, the structural factors of the ligament that contribute to greater AKL are relatively unknown. In the current study, we focused on potential structural factors that have been implicated in human and animal studies.7,16 Our primary finding was that in a female population, a smaller ACL width was associated with a greater magnitude of AKL in an intact human knee. Although the authors of a previous animal study7 reported that greater total knee anterior-posterior translation was associated with a smaller ligamentous cross-sectional area, this is the first study, to our knowledge, to show a similar association in the intact human knee. In earlier research,19 investigators used a multivariate regression model to predict the structural properties of the ACL and observed that a smaller ACL volume was a contributor to greater ligamentous elongation at failure. Furthermore, smaller graft volumes of male goats' ACLs, as assessed via MRI measures, were associated with lower failure loads.20 Such findings indicate that ACL size is associated with ligamentous biomechanics, which could affect AKL. Additionally, female cadaver ACLs were smaller than those of male cadavers and had weaker mechanical properties, such as lower strain energy density and stress at failure.21 Furthermore, retrospective measurement of the contralateral limb in ACL-injured patients revealed smaller ACL volumes than in control participants.22 Mechanically, decreased cross-sectional area results in greater deformation9 (ie, greater laxity), which could potentially increase ACL injury risk. Collectively, these data suggest that a smaller ligament is associated with greater laxity.
Our hypothesis that greater laxity would be associated with a steeper femoral notch angle was not supported. Our findings differ from those of a previous group8 who reported a positive relationship between a steeper femoral notch angle and greater AKL. The conflicting findings may be because those authors analyzed data on a sample of both women (n = 9) and men (n = 6),8 whereas we examined only women. Females have been noted to have both smaller intercondylar notch morphology (ACL notch index and notch width index)23 and greater laxity15 than males, so including both males and females in the same analysis without first controlling for sex differences may have demonstrated a relationship due to sex differences rather than a direct association between AKL and notch angle. Females' smaller intercondylar notch morphology indicated that the potential occurrence of ACL impingement was higher in females than in males.23 The increased ACL impingement was also associated with greater knee laxity.8 In light of the different bony morphology by sex, which could further affect the ACL impingement risk, including males in the previous study could explain why the results differ compared with our findings and previous work. Additionally, a steeper femoral notch angle was related to greater impingement of the ligament,8 which could cause ligamentous dysfunction.10 It is also important to consider that we recruited healthy participants in whom the range of femoral notch angles may not have reached a critical level to affect ACL function.
We demonstrated the ability to perform consistent and precise (ICC ± SEM) measures of ACL width (0.98 ± 0.3 mm) and femoral notch angle (0.97° ± 1.1°). Although the investigator performing ACL width and femoral notch angle measures was not a radiologist, face validity was established, as the current values compare favorably with those of researchers who previously used such measures. We are unaware of any sex comparison of ACL width, but given that ACL volume is smaller in females than in males,24 within-sex comparisons with the previous literature are likely most appropriate. Specifically, the mean ACL width in female basketball athletes was 7.6 mm,16 and the average ACL width calculated in the current study was 5.9 mm. Height (169.2 versus 166.1 cm) and mass (64.5 versus 66.5 kg) of the participants were similar across studies, yet our recruited population was recreationally active females, whereas the previous study involved basketball athletes. Authors of an earlier cross-sectional investigation25 reported that ACL size in weightlifters was larger than that in an age-, height-, and weight-matched control group, which suggests that training may contribute to increased ACL size. Hence, differences in activity level may explain why the ACL width in our study was approximately 25% smaller than in female basketball athletes from the previous study. Our mean notch angle (38.0° ± 6.3°) is very similar to that of previous research (37.7° ± 4.8°).8 It should be noted that the previous study included both males and females (rather than all female participants as in the current study). However, we are unaware of any work comparing femoral notch angles between sexes. Collectively, our study demonstrated consistent and precise measures of ACL width and notch angle that are similar in magnitude to previously reported values.
Anterior knee laxity has previously been reported as a risk factor for ACL injury,2,3 and we found that lesser ACL width correlated with greater AKL. Therefore, greater AKL may represent a structurally weaker ligament and an increased risk of ACL failure. As a result, focusing on ligamentous size or strength may advance injury-prevention efforts. Previous evidence is quite limited in regard to the potential for modifying ligamentous size or strength. In a human cross-sectional study,25 weightlifters had larger ACL cross-sectional areas than controls matched by age, height, and weight. Furthermore, an animal training study26 showed that medial collateral ligament cross-sectional area increased after 9 weeks of remobilization following 9 weeks of immobilization. Additionally, authors of other animal training studies27−29 have reported that the structural composition of ligaments, such as collagen density and diameter, could be altered postexercise, resulting in higher failure loads. Such findings lend a degree of support to the notion that ACL width may be modified. With limited evidence to support ACL alterations through training in the healthy population, prospective studies are needed to determine if exercise interventions can increase ACL size. Given that only 22% of the variance in knee laxity we observed was explained by ligament width, future investigators should address how other intrinsic structural ligamentous components (eg, collagen density, collagen orientation, water content) may also contribute to less knee laxity. Such knowledge will provide a scientific basis for developing effective injury-prevention protocols that focus on enhancing ACL integrity.
A limitation of the present study is that we used ACL width rather than ACL volume to characterize ACL size. Whereas the use of ACL volume requires sectioning of the ACL in multiple sagittal slices,21,25 we measured ACL width from a single sagittal image that provided the clearest view of the Blumensaat line. Additionally, although 1 previous group15 used T1-weighted scans to measure ACL volume, authors of other MRI-based studies16,20 have commonly used T2-weighted scans to assess ACL size. Because these data were obtained from a previous study in which MRI scans used T1 sequences (as it was focused on bony geometry), we chose to measure ACL width, as the T1-weighted images sometimes failed to overtly delineate the proximal and distal ends of the ACL. However, in pilot testing, we demonstrated a strong correlation of ACL volume and ACL width using the T1 images (r = 0.82, R2 = 0.67, P < .01). Furthermore, because ACL width would provide less information (and thus potentially more error), the relationships might actually have been stronger if a more precise measurement had been obtained. As such, the present findings represent a first step in examining these relationships, and future researchers should confirm the findings with the MRI scan sequences best equipped to assess ACL volume.
The second limitation is the lack of an intercondylar notch area measurement. We did not assess this due to inadequate statistical power, but a smaller intercondylar notch area has been previously associated with a smaller ACL cross-sectional area.23 Additionally, female athletes were reported to have both greater AKL than30 and a smaller ACL cross-sectional area than males.16 This indicates that a smaller intercondylar notch area may be associated with increased AKL. Although intercondylar notch geometry may affect AKL, the distinct mechanisms by which intercondylar notch area affects ACL size and its relationship to the AKL are relatively unknown. Hence, further investigation of which bony structures may play an important role in influencing ACL size and AKL is needed. Also, although the single nonradiologist rater in this study demonstrated reliable and precise measurements, intertester reliability with a radiologist should be established to strengthen the validity of the measures.
In summary, a smaller ACL width was associated with greater AKL in females. Given the relationship of size to failure load,20 this suggests that a more lax ligament would fail under a lesser load. This finding enhances our knowledge regarding the use of AKL as a screening tool. Furthermore, although purely speculative at this time, increasing ACL size might result in lesser AKL and, thus, potentially lower the risk of an ACL injury. Studies are needed to determine the extent to which chronic loading may influence ACL size and other structural compositional factors associated with knee laxity. Should ACL size, structural composition, and laxity be modifiable through training, this may pave the way for the development and refinement of future ACL injury-prevention efforts.
ACKNOWLEDGMENTS
This study was supported in part by the National Institutes of Health-National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant No. R01 AR053172 (S.J.S.).
REFERENCES
- 1. Terauchi M, Hatayama K, Yanagisawa S, Saito K, Takagishi K. Sagittal alignment of the knee and its relationship to noncontact anterior cruciate ligament injuries. Am J Sports Med. 2011; 39 5: 1090– 1094. [DOI] [PubMed] [Google Scholar]
- 2. Myer GD, Ford KR, Paterno MV, Nick TG, Hewett TE. The effects of generalized joint laxity on risk of anterior cruciate ligament injury in young female athletes. Am J Sports Med. 2008; 36 6: 1073– 1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uhorchak JM, Scoville CR, Williams GN, Arciero RA, St Pierre P, Taylor DC. Risk factors associated with noncontact injury of the anterior cruciate ligament: a prospective four-year evaluation of 859 West Point cadets. Am J Sports Med. 2003; 31 6: 831– 842. [DOI] [PubMed] [Google Scholar]
- 4. Branch TP, Browne JE, Campbell JD, et al. Rotational laxity greater in patients with contralateral anterior cruciate ligament injury than healthy volunteers. Knee Surg Sports Traumatol Arthrosc. 2010; 18 10: 1379– 1384. [DOI] [PubMed] [Google Scholar]
- 5. Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee: a biomechanical study. J Bone Joint Surg Am. 1980; 62 2: 259– 270. [PubMed] [Google Scholar]
- 6. Woodford-Rogers B, Cyphert L, Denegar CR. Risk factors for anterior cruciate ligament injury in high school and college athletes. J Athl Train. 1994; 29 4: 343– 346. [PMC free article] [PubMed] [Google Scholar]
- 7. Grood ES, Walz-Hasselfeld KA, Holden JP, et al. The correlation between anterior-posterior translation and cross-sectional area of anterior cruciate ligament reconstructions. J Orthop Res. 1992; 10 6: 878– 885. [DOI] [PubMed] [Google Scholar]
- 8. Jagodzinski M, Richter GM, Pässler HH. Biomechanical analysis of knee hyperextension and of the impingement of the anterior cruciate ligament: a cinematographic MRI study with impact on tibial tunnel positioning in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2000; 8 1: 11– 19. [DOI] [PubMed] [Google Scholar]
- 9. Nordin M, Frankel VH. Basic Biomechanics of the Musculoskeletal System. 2nd ed. Philadelphia, PA: Lea & Febiger; 1989. [Google Scholar]
- 10. Watanabe BM, Howell SM. Arthroscopic findings associated with roof impingement of an anterior cruciate ligament graft. Am J Sports Med. 1995; 23 5: 616– 625. [DOI] [PubMed] [Google Scholar]
- 11. Quapp KM, Weiss JA. Material characterization of human medial collateral ligament. J Biomech Eng. 1998; 120 6: 757– 763. [DOI] [PubMed] [Google Scholar]
- 12. Howell SM, Barad SJ. Knee extension and its relationship to the slope of the intercondylar roof: implications for positioning the tibial tunnel in anterior cruciate ligament reconstructions. Am J Sports Med. 1995; 23(3)288−294. [DOI] [PubMed] [Google Scholar]
- 13. Shultz SJ, Schmitz RJ. Tibial plateau geometry influences lower extremity biomechanics during landing. Am J Sports Med. 2012; 40 9: 2029– 2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shultz SJ, Schmitz RJ, Kong Y, et al. Cyclic variations in multiplanar knee laxity influence landing biomechanics. Med Sci Sports Exerc. 2012; 44 5: 900– 909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beynnon BD, Vacek PM, Newell MK, et al. The effects of level of competition, sport, and sex on the incidence of first-time noncontact anterior cruciate ligament injury. Am J Sports Med. 2014; 42 8: 1806– 1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anderson AF, Dome DC, Gautam S, Awh MH, Rennirt GW. Correlation of anthropometric measurements, strength, anterior cruciate ligament size, and intercondylar notch characteristics to sex differences in anterior cruciate ligament tear rates. Am J Sports Med. 2001; 29 1: 58– 66. [DOI] [PubMed] [Google Scholar]
- 17. Seyahi A, Atalar AC, Koyuncu LO, Cinar BM, Demirhan M. [Blumensaat line and patellar height]. Acta Orthop Traumatol Turc. 2006; 40 3: 240– 247. [PubMed] [Google Scholar]
- 18. Shultz SJ, Schmitz RJ, Nguyen AD, Levine BJ. Joint laxity is related to lower extremity energetics during a drop jump landing. Med Sci Sports Exerc. 2010; 42 4: 771– 780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hashemi J, Mansouri H, Chandrashekar N, Slauterbeck JR, Hardy DM, Beynnon BD. Age, sex, body anthropometry, and ACL size predict the structural properties of the human anterior cruciate ligament. J Orthop Res. 2011; 29 7: 993– 1001. [DOI] [PubMed] [Google Scholar]
- 20. Fleming BC, Vajapeyam S, Connolly SA, Magarian EM, Murray MM. The use of magnetic resonance imaging to predict ACL graft structural properties. J Biomech. 2011; 44 16: 2843– 2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chandrashekar N, Mansouri H, Slauterbeck J, Hashemi J. Sex-based differences in the tensile properties of the human anterior cruciate ligament. J Biomech. 2006; 39 16: 2943– 2950. [DOI] [PubMed] [Google Scholar]
- 22. Chaudhari AM, Zelman EA, Flanigan DC, Kaeding CC, Nagaraja HN. Anterior cruciate ligament-injured subjects have smaller anterior cruciate ligaments than matched controls: a magnetic resonance imaging study. Am J Sports Med. 2009; 37 7: 1282– 1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dienst M, Schneider G, Altmeyer K, et al. Correlation of intercondylar notch cross sections to the ACL size: a high resolution MR tomographic in vivo analysis. Arch Orthop Trauma Surg. 2007; 127 4: 253– 260. [DOI] [PubMed] [Google Scholar]
- 24. Chandrashekar N, Slauterbeck J, Hashemi J. Sex-based differences in the anthropometric characteristics of the anterior cruciate ligament and its relation to intercondylar notch geometry: a cadaveric study. Am J Sports Med. 2005; 33 10: 1492– 1498. [DOI] [PubMed] [Google Scholar]
- 25. Grzelak P, Podgorski M, Stefanczyk L, Krochmalski M, Domzalski M. Hypertrophied cruciate ligament in high performance weightlifters observed in magnetic resonance imaging. Int Orthop. 2012; 36 8: 1715– 1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Woo SL, Gomez MA, Sites TJ, Newton PO, Orlando CA, Akeson WH. The biomechanical and morphological changes in the medial collateral ligament of the rabbit after immobilization and remobilization. J Bone Joint Surg Am. 1987; 69 8: 1200– 1211. [PubMed] [Google Scholar]
- 27. Adams A. Effect of exercise upon ligament strength. Res Q. 1966; 37 2: 163– 167. [PubMed] [Google Scholar]
- 28. Tipton CM, James SL, Mergner W, Tcheng TK. Influence of exercise on strength of medial collateral knee ligaments of dogs. Am J Physiol. 1970; 218 3: 894– 902. [DOI] [PubMed] [Google Scholar]
- 29. Cabaud HE, Chatty A, Gildengorin V, Feltman RJ. Exercise effects on the strength of the rat anterior cruciate ligament. Am J Sports Med. 1980; 8 2: 79– 86. [DOI] [PubMed] [Google Scholar]
- 30. Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am J Sports Med. 1996; 24 4: 427– 436. [DOI] [PubMed] [Google Scholar]