Abstract
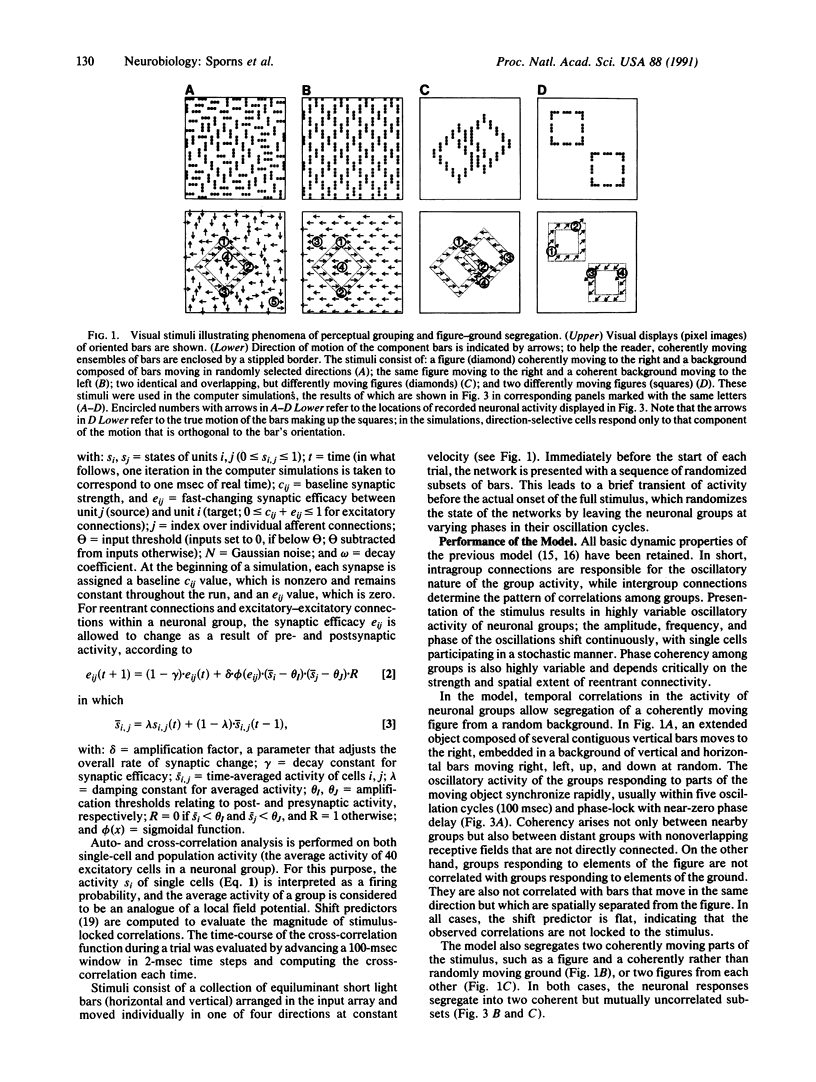
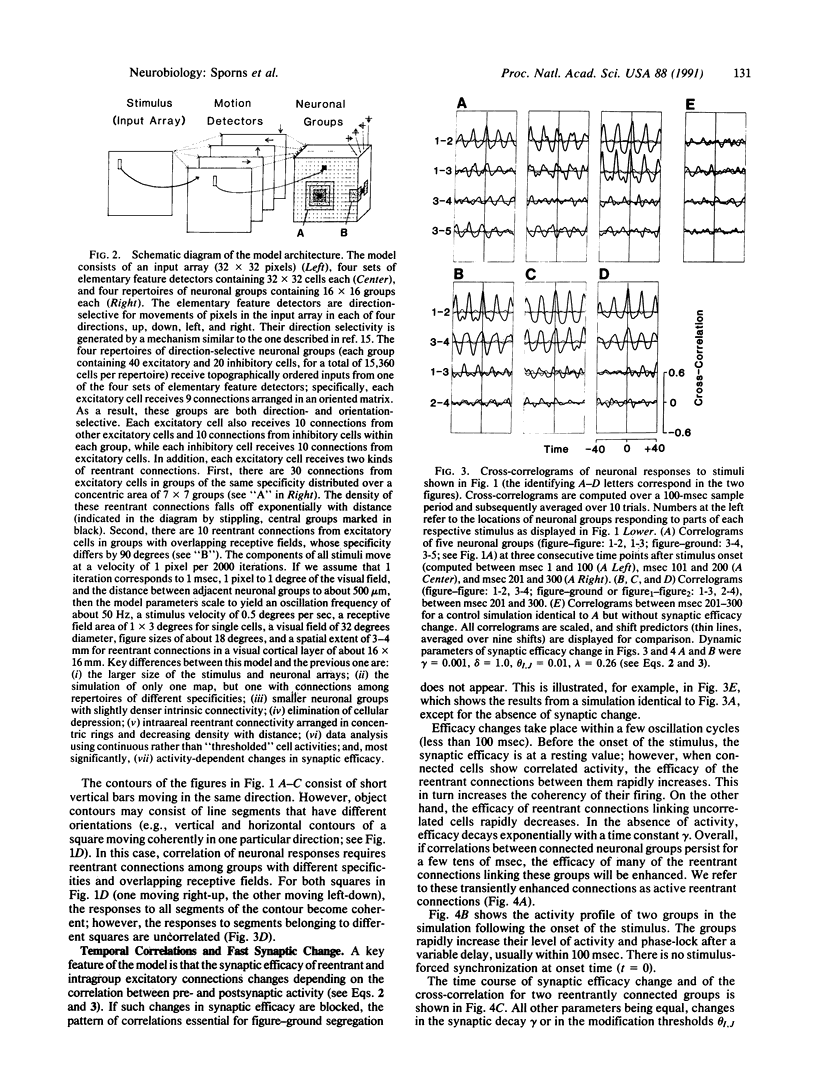
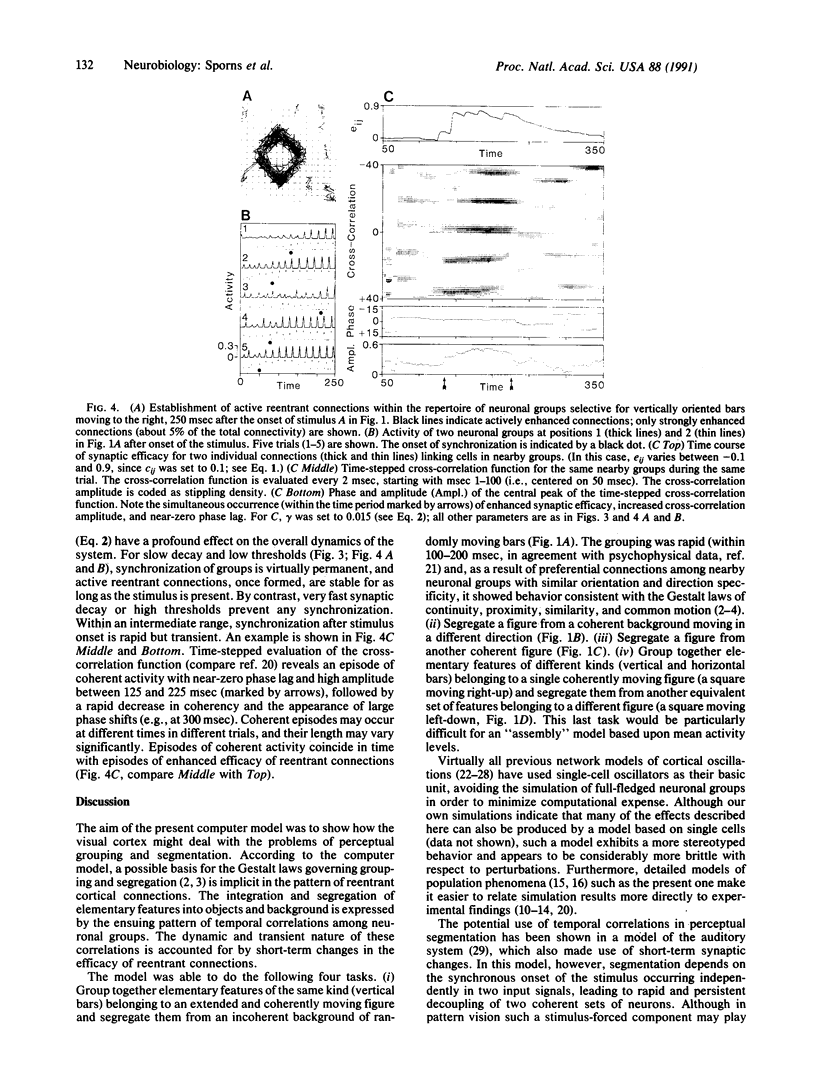
The segmentation of visual scenes is a fundamental process of early vision, but the underlying neural mechanisms are still largely unknown. Theoretical considerations as well as neurophysiological findings point to the importance in such processes of temporal correlations in neuronal activity. In a previous model, we showed that reentrant signaling among rhythmically active neuronal groups can correlate responses along spatially extended contours. We now have modified and extended this model to address the problems of perceptual grouping and figure-ground segregation in vision. A novel feature is that the efficacy of the connections is allowed to change on a fast time scale. This results in active reentrant connections that amplify the correlations among neuronal groups. The responses of the model are able to link the elements corresponding to a coherent figure and to segregate them from the background or from another figure in a way that is consistent with the so-called Gestalt laws.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aertsen A. M., Gerstein G. L., Habib M. K., Palm G. Dynamics of neuronal firing correlation: modulation of "effective connectivity". J Neurophysiol. 1989 May;61(5):900–917. doi: 10.1152/jn.1989.61.5.900. [DOI] [PubMed] [Google Scholar]
- Bergen J. R., Julesz B. Parallel versus serial processing in rapid pattern discrimination. Nature. 1983 Jun 23;303(5919):696–698. doi: 10.1038/303696a0. [DOI] [PubMed] [Google Scholar]
- Eckhorn R., Bauer R., Jordan W., Brosch M., Kruse W., Munk M., Reitboeck H. J. Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol Cybern. 1988;60(2):121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- Engel Andreas K., König Peter, Gray Charles M., Singer Wolf. Stimulus-Dependent Neuronal Oscillations in Cat Visual Cortex: Inter-Columnar Interaction as Determined by Cross-Correlation Analysis. Eur J Neurosci. 1990;2(7):588–606. doi: 10.1111/j.1460-9568.1990.tb00449.x. [DOI] [PubMed] [Google Scholar]
- Fox K., Sato H., Daw N. The location and function of NMDA receptors in cat and kitten visual cortex. J Neurosci. 1989 Jul;9(7):2443–2454. doi: 10.1523/JNEUROSCI.09-07-02443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci. 1989 Jul;9(7):2432–2442. doi: 10.1523/JNEUROSCI.09-07-02432.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gochin P. M., Gerstein G. L., Kaltenbach J. A. Dynamic temporal properties of effective connections in rat dorsal cochlear nucleus. Brain Res. 1990 Mar 5;510(2):195–202. doi: 10.1016/0006-8993(90)91367-p. [DOI] [PubMed] [Google Scholar]
- Gray C. M., König P., Engel A. K., Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989 Mar 23;338(6213):334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Gray C. M., Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray Charles M., Engel Andreas K., König Peter, Singer Wolf. Stimulus-Dependent Neuronal Oscillations in Cat Visual Cortex: Receptive Field Properties and Feature Dependence. Eur J Neurosci. 1990;2(7):607–619. doi: 10.1111/j.1460-9568.1990.tb00450.x. [DOI] [PubMed] [Google Scholar]
- Lester R. A., Clements J. D., Westbrook G. L., Jahr C. E. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990 Aug 9;346(6284):565–567. doi: 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- Luhmann H. J., Singer W., Martínez-Millán L. Horizontal Interactions in Cat Striate Cortex: I. Anatomical Substrate and Postnatal Development. Eur J Neurosci. 1990;2(4):344–357. doi: 10.1111/j.1460-9568.1990.tb00426.x. [DOI] [PubMed] [Google Scholar]
- Miller K. D., Chapman B., Stryker M. P. Visual responses in adult cat visual cortex depend on N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5183–5187. doi: 10.1073/pnas.86.13.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel D. H., Gerstein G. L., Moore G. P. Neuronal spike trains and stochastic point processes. II. Simultaneous spike trains. Biophys J. 1967 Jul;7(4):419–440. doi: 10.1016/S0006-3495(67)86597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky H., Golomb D., Kleinfeld D. Global processing of visual stimuli in a neural network of coupled oscillators. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7200–7204. doi: 10.1073/pnas.87.18.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O., Gally J. A., Reeke G. N., Jr, Edelman G. M. Reentrant signaling among simulated neuronal groups leads to coherency in their oscillatory activity. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7265–7269. doi: 10.1073/pnas.86.18.7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Malsburg C., Schneider W. A neural cocktail-party processor. Biol Cybern. 1986;54(1):29–40. doi: 10.1007/BF00337113. [DOI] [PubMed] [Google Scholar]





