Abstract
Background
One of the most common nonsteroidal anti-inflammatory drugs (NSAIDs) used worldwide, diclofenac (DIC), has been linked to increased risk of cardiovascular disease (CVD). The molecular mechanism(s) by which DIC causes CVD is unknown.
Methods
Proteasome activities were studied in hearts, livers, and kidneys from male Swiss Webster mice treated with either 100 mg/kg DIC for 18 h (acute treatment) or 10 mg/kg DIC for 28 days (chronic treatment). Cultured H9c2 cells and neonatal cardiomyocytes were also treated with different concentrations of DIC and proteasome function, cell death and ROS generation studied. Isolated mouse heart mitochondria were utilized to determine the effect of DIC on various electron transport chain complex activities.
Results
DIC significantly inhibited the chymotrypsin-like proteasome activity in rat cardiac H9c2 cells, murine neonatal cardiomyocytes, and mouse hearts, but did not affect proteasome subunit expression levels. Proteasome activity was also affected in liver and kidney tissues from DIC treated animals. The levels of polyubiquitinated proteins increased in hearts from DIC treated mice. Importantly, the levels of oxidized proteins increased while the β5i immunoproteasome activity decreased in hearts from DIC treated mice. DIC increased ROS production and cell death in H9c2 cells and neonatal cardiomyocytes while the cardioprotective NSAID, aspirin, had no effect on ROS levels or cell viability. DIC inhibited mitochondrial Complex III, a major source of ROS, and impaired mitochondrial membrane potential suggesting that mitochondria are the major sites of ROS generation.
Conclusion
These results suggest that DIC induces cardiotoxicity by a ROS dependent mechanism involving mitochondrial and proteasome dysfunction.
Keywords: Cardiac cells, Cell death, Diclofenac, Mitochondria, Proteasome, Reactive Oxygen Species
INTRODUCTION
Nonsteroidal Anti Inflammatory Drugs (NSAIDs) are among the most important therapeutic interventions for the treatment of a variety of painful conditions including arthritis, osteoarthritis, rheumatoid arthritis, and headaches [1]. Their efficacy in reducing fever and inflammation is well established [1]. Despite the effective analgesic, anti-inflammatory, and antipyretic effects of these compounds, most NSAIDs are associated with various side effects including increased risk of heart attack, stroke, as well as gastrointestinal, renal and liver problems [2]. Although people taking NSAIDs without any history of cardiovascular diseases (CVD) show increased risk of cardiovascular incidences, this risk is even higher in people with a history of CVD [3]. While various meta-analysis studies and clinical trials have shown the propensity of NSAIDs to increase the risk of CVD and stroke, no mechanism is currently known to be associated with the side effects of the NSAIDs directly on the heart. One possible mechanism that has been related to the side effects of NSAIDs is the inhibition of the cyclooxygenase pathway in activated platelets or endothelial cells leading to a disruption of the homeostasis between the biosynthesis of eicosanoids like thromboxane and prostacyclin [1]. Both non-selective NSAIDs like diclofenac (DIC), ibuprofen, and indomethacin, as well as the cyclooxygenase 2 selective NSAIDs like celecoxib and rofecoxib, lead to decreased prostacyclin levels and increased levels of thromboxane (which promotes thrombosis) in infarcted hearts, which may cause further progression of the disease [4].
DIC is one of the most commonly used NSAIDs [5]. The risk for development of CVD among DIC users is similar to the risk of using rofecoxib, with DIC increasing the risk of heart attack and stroke by approximately 40% [5]. Although DIC is available over the counter at lower doses than normally prescribed (in certain countries such as Indonesia and Denmark), these lower doses still show a 22% increase in the risk of CVD [5]. Apart from increased CVD risk, NSAIDs have also been reported to induce toxicity in the livers and kidneys [6, 7]. Hepatotoxicity resulting from DIC use (150 mg/day) within 4–6 months has been associated with elevated levels of aminotransferase, an indicator of liver damage [8]. Hepatotoxicity induced by NSAIDs, such as DIC and mefenamic acid, has been suggested to cause hepatocyte injury by uncoupling mitochondrial oxidative phosphorylation, resulting in decreased ATP synthesis [9].
Some studies suggest that inhibition of COX-2 in cardiomyocytes may contribute to heart failure in patients receiving NSAIDs [10, 11] because selective deletion of COX-2 in cardiomyocytes depressed cardiac output and enhanced susceptibility to induced arrhythmogenesis [10]. Recent studies have indicated a COX-independent mechanism of NSAIDs action in different cells like fibroblasts, human gastric adenocarcinoma cells, and endothelial cells [12]. Several studies have reported the generation of reactive oxygen species (ROS) by NSAIDs in different tissues leading to increased cell death [13–15]. However, some NSAIDs like aspirin have been suggested to decrease ROS levels in human endothelial cells [16]. The effect of DIC on ROS levels in cardiac cells, on cardiac myocytes, and the heart has not been previously investigated. Aspirin has been shown to indirectly affect the proteasome [17], and proteasome dysfunction has been associated with various diseases including CVD [18], neurodegenerative diseases [19], and different forms of cancer [20]. Proteasomes are large protein complexes which are part of the ubiquitin proteasome system (UPS). The UPS is responsible for the degradation of normal, damaged, misfolded and oxidized proteins [21]. In the heart, normal cardiac function is maintained by efficient protein degradation of normal, damaged, misfolded, and oxidized proteins to maintain intracellular protein homeostasis. Several studies have revealed the association between altered proteasome function and various pathological conditions of the heart including myocardial ischemia [22], cardiac hypertrophy [23], and atherosclerosis [24].
In our in vitro and in vivo experiments, DIC was found to be associated with cardiac dysfunction. DIC was a potent inducer of ROS, in contrast to aspirin, which did not induce ROS in cardiomyocytes. DIC was also an effective inhibitor of cardiac mitochondrial complex III, reduced mitochondrial membrane potential, increased oxidized protein levels, and decreased proteasome activity. These adverse changes in protein homeostasis and mitochondrial function likely lead to the observed time and dose dependent DIC induced cardiac cell death.
MATERIALS AND METHODS
Animal studies
Male Swiss Webster mice obtained from Charles River Laboratory and weighing 30–35 g were used for the study. Animal study protocol adheres to the guideline adopted and promulgated by U.S. National Institute of Health. The study protocol was approved by Institutional Animal Care and Use Committee (IACUC) of University of California, Davis. Mice were housed in vivarium and maintained at controlled temperature and humidity. The animals had free access to food and water. Mice were either treated with drinking water or drinking water containing diclofenac. The dose of diclofenac was 10 mg/kg/day, and it was administered for 28 days (chronic treatment group; n=4). In a separate study, the mice were orally administered a single dose of diclofenac at 100 mg/kg (acute treatment group; n=4). At the end of the study the animals were sacrificed using isoflurane anesthesia and hearts, livers and kidneys excised from the mice treated with or without DIC after 18h and 28 days of treatment. Briefly, the mice were sacrificed and hearts, livers and kidneys were isolated and quickly washed in ice-cold phosphate buffered saline (PBS) twice to get rid of excess blood. The tissues were then frozen and pulverized in liquid nitrogen and care was taken to ensure that all steps were carried out at a very low temperature. The pulverized tissues were then collected in clean microcentrifuge tubes and stored at −80°C until needed.
Determination of DIC levels in plasma of treated animals
DIC levels in plasma of treated animals were determined using liquid chromatography-tandem mass spectrometry (LC-MS/MS) as previously described and reported [25].
Cell Culture
Rat cardiac H9c2 cells (H9c2(2-1), CRL-1446, a subclone of the original clonal cell line derived from embryonic BD1X rat heart tissue) was obtained from American Type Culture Collection (ATCC), Manassas, Virginia) and grown in 75 ml ventilated cell culture flasks (BioLite, Thermo Scientific) in high glucose Dulbecco’s modified Eagle’s medium (DMEM, Hyclone) with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 50 units/ml penicillin, and 50 µg/ml streptomycin (growth medium, GM), at 37°C and 5% CO2. The cells were passaged by trypsinization (0.25% trypsin-EDTA, Gibco BRL) and dilution into culture dishes after confluency of 70–80% was reached.
Preparation of Murine Neonatal Cardiomyocytes
Neonatal cardiomyocytes were isolated from 1–3 day old mice (B6SJLF1 mice from Jackson Laboratories, Sacramento, CA) using the Pierce Primary Cardiomyocyte Isolation Kit (Thermo Scientific). Briefly, hearts from new born mice were collected in ice cold Hanks’ Balanced salt Solution (HBSS). After mincing the hearts with a sterile razor blade, the minced tissues were incubated with the isolation enzymes at 37°C for 30 min. Following washing with HBSS, the samples were re-suspended in complete media (DMEM containing 10% FBS and 1% Pen-Strep) and plated onto cell culture plates.
Treatment of cells with DIC
DIC was prepared in DMSO at the required concentrations and frozen in aliquots at −80°C and used within a week of its preparation. H9c2 and neonatal cardiac cells were treated with DIC at the desired concentrations for different incubation periods (n=3). Care was taken to keep the volume of DMSO below 0.1% in DMEM containing cells to reduce the effects of solvent on the cells. After the desired treatment duration, the cells were harvested in ice-cold 26S buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 5 mM MgCl2, 0.5 mM DTT, pH 7.5 [26] and sonicated. Centrifugation was carried out at 15,000 × g for 20 minutes and the supernatant containing proteasomes was collected. Protein concentration of the cell lysate was measured using a nanodrop (2000C, Thermo Scientific) and each sample was diluted to 1µg/µl to be used for proteasome assays and western blot analysis.
26S Proteasome Activity Assays of H9c2 Cells, Neonatal Cells and Tissue Lysates
The samples (1µg/µl) prepared as described under the section “Treatment of cells with DIC” were used for studying either β5 chymotrypsin-like activity, β1 caspase-like activity, or β2 trypsin-like activity. Each assay was carried out using 100µM ATP with or without a proteasome specific inhibitor: 10 µM bortezomib for β5 activity and 100 µM bortezomib for β1 and β2 activity. The hearts, livers, and kidneys from mice treated with DIC were similarly prepared in 26S buffer. Approximately 20mg of each pulverized tissue was weighed out and homogenized using a hand-held homogenizer in 26S buffer and centrifuged at 15,000 × g for 20 mins at 4°C. Protein concentration of the supernatant containing the proteasomes was determined and 20 µg of the sample was used for measuring 26S β1, β2 and β5 activity. Specific substrates for each proteasomal β subunits were used for the initiation of the reaction [27–30]. β substrates were purchased from Enzo Life Sciences, NY, USA: β1 substrate Z-LLE-AMC is cleaved after glutamic acid (E) by the β1 catalytic subunit via caspase-like activity. Similarly, β2 substrate Boc-LSTR-AMC is cleaved after arginine (R) via β2 trypsin-like activity and β5 substrate Suc-LLVY-AMC after tyrosine (Y) via β5 chymotrypsin-like activity.
Immunoproteasome β5i activity
10–20 µg of heart samples from DIC treated animals were used to determine β5i activity. Samples were incubated with immunoproteasome buffer containing 50 mM Tris, 5 mM MgCl2, 20 mM KCl, pH 7.4 and freshly added 2 mM DTT. β5i selective inhibitor, ONX 0914 (20 µM) (Cayman Chemical, MI) was utilized to determine the selectivity of the assay. The reaction was initiated by adding the fluorogenic chymotrypsin-like β5i substrate, ANW-R110 (AAT Bioquest, Inc, CA) and the readings were recorded in a Tecan Infinite M1000 over a duration of 2 h at an excitation of 498 nm and an emission of 520 nm at 37°C
Labeling of proteasome active sites by MV-151
10µg of rat heart lysate was utilized for proteasome labeling in MV151 homogenization buffer (50mM Tris, 5mM MgCl2, 250mM sucrose, 2mM ATP and 1mM DTT, pH 7.5). DIC at different concentrations was added to each tube (n=3) and 20µM of MG132, a proteasome inhibitor, or no probe (negative controls) were also used. After subsequent mixing, the tubes were incubated for 30 minutes at 30°C, and 0.1µM MV151 added to each tube, and the tubes incubated for 1h at 30°C in dark. After the addition of 2× Laemmli sample buffer and subsequent heating, the proteins were separated on a 4–20% stain free gel (Bio-Rad) and the fluorescent signals emerging from the MV151 labeled proteins were visualized using a ChemiDoc MP (Bio-Rad).
Characterization of proteasomes
Characterization of proteasomes was done by native gel electrophoresis (3.5%) as described [31]. Briefly, DIC treated H9c2 cells (n=3), were homogenized in lysis buffer containing 50 mM Tris-HCl, 5 mM MgCl2, 0.5 mM EDTA and 1 mM ATP, pH 7.4 followed by centrifugation at 15,000 × g for 30 min. Electrophoresis was carried out in TBE buffer for 3h, 100V at 4°C. The relative amounts of proteasome subunits were determined by transferring the proteins in a nitrocellulose membrane and subsequently blocking and probing the blot with PSMA6 (1:10,000, Abcam # ab109377) antibody and anti-rabbit HRP secondary antibody (1:25000, Cat# A0545, Sigma, St. Louis, MO).
Western Blot analysis
Equal amounts of protein (in 2× Laemmli buffer) prepared from heart, liver and kidney lysates of DIC treated animals were subjected to electrophoresis on 4–20% Criterion polyacrylamide gels (Bio-Rad) under reducing conditions and transferred to Nitrocellulose (Bio-Rad). Total protein for normalization of Western blots was obtained by either staining nitrocellulose membranes with Ponceau S or by imaging in stain-free gels (Bio-Rad) [32]. Blocking of the membrane was performed for 1h with 3% nonfat dry milk (NFM) in TBS, pH 7.4 containing 0.05% (w/v) Tween 20 (TTBS). The membranes were washed three times in TTBS and probed overnight at 4°C in 1% NFM with primary antibodies: mouse anti-Rpt6 (1:1000, Enzo), and anti-PSMA6 (1:10,000, Abcam, Cambridge, MA, # ab109377), and anti-ubiquitin (1:2500, Life Sensors, VU-101). The membranes were then incubated for 1h at room temperature with horseradish peroxidase-conjugated rabbit-anti-mouse or goat anti-rabbit IgG in 1% NFM (1:40000, Sigma, St. Louis). Detection of the tagged secondary antibody was done using ECL detection kit (Westar Supernova, Cyanagen). Images were obtained by the ChemiDoc MP (Bio-Rad) controlled by Image Lab 5.0 (Bio-Rad). The quantitation of blots was performed using Image Lab 5.0.
Oxidized protein level determination
Heart, liver and kidney lysates prepared from mice treated with DIC were heated with 2× Laemmli buffer at 96°C for 5 minutes. The samples were subjected to SDS gel electrophoresis and the proteins transferred to nitrocellulose membranes. Equilibration of the membrane was done in TBS containing 20% methanol and then washed with 2N HCl. The oxidized proteins in the samples were determined by pre-derivatization of the carbonyl group of the protein with dinitrophenylhydrazine (DNPH) in 2N HCl. The blot was probed with rabbit anti-DNP antibody (1:250 Millipore) for 4h at room temperature and then washed and incubated with secondary antibody, anti-rabbit HRP (1:25,000, Sigma). Oxidized protein levels were determined by using the substrate Westar Supernova (Cyanagen). Imaging of the blot was performed in ChemiDoc MP (Bio-Rad) and the quantitation done using Image Lab 5.0.
Alamar Blue Measurement of Cell Proliferation
Alamar Blue (Invitrogen) was utilized according to the manufacturer’s protocol to determine H9c2 and neonatal cardiac cell viability after DIC treatment. Briefly, DIC was added at varying concentration to H9c2 cells and neonatal cardiomyocytes in GM for different incubation periods (n=4–9). In parallel experiments, the cells were treated with H2O2 for positive control experiments. After the desired incubation time, fresh GM, without DIC or H2O2, was added along with 10µl of Alamar Blue into each well. Incubation of the plate was carried out for 1–6 h at 37°C and 5% CO2 and the absorbance of the dye (550 nm) was measured using a Bio-Rad Model 680 microplate reader.
Detection of intracellular reactive oxygen species formation
The cell permeant reagent 2’,7’ – dichlorodihydrofluorescein diacetate (H2DCFDA), a fluorogenic dye (Life Technologies) was utilized to study ROS generation in H9c2 and neonatal cardiac cells. Briefly, cells were plated onto a 96 black well plate and allowed to reach a confluency of ~80%, washed twice with PBS, and then treated with 10µM of H2DCFDA in phenol red free media for 30 min at 37°C (n=4–9). Following incubation, the wells were again washed with PBS and the cells were treated with different concentrations of DIC and H2O2 in 5% FBS in phenol free media. ROS generation was determined immediately by measuring the formation of fluorescent DCF, using an Infinite M1000 PRO-Tecan, at an Ex-490nm and Em-520nm. Measurements were done every 10 minutes for 3 hours.
Fluorescence microscopy was also employed to detect the intensity of fluorescence in these cells in the presence of DIC and rotenone. Rotenone was used at a lower concentration (20 µM) than what was previously found to increase ROS production in another cell type [33]. Briefly, cells were plated in 8 well slides (EZ slides, Millipore) and incubated with H2DCFDA for 30 min. Thereafter, the cells were treated with the compounds for 1.5 h and the fluorescence intensity was measured immediately with a Zeiss Axio Imager M1. Three or more independent experiments were performed for each group.
Measurement of Caspase 3 activity
Hearts, livers and kidneys from mice treated with DIC were used for determination of Caspase 3 activity. Briefly, samples were prepared as described under the section, “26S Proteasome Activity Assays of H9c2 Cells, Neonatal Cells and Tissue Lysates” and incubated in a 96 black well plate with Caspase 3 buffer (50 mM HEPES, 0.1% CHAPS, 100 mM NaCl, 1 mM EDTA, 10% Glycerol and 10 mM DTT; pH 7.4) in the presence or absence of 10 µM caspase-3 inhibitor, Ac-DEVD-CHO (Biomol). 100 µM caspase-3 substrate, Ac-DEVD-AMC (Biomol) was added to the reaction mixture to initiate the reaction. Caspase-3 activity was measured by the release of free AMC which was recorded every 15 min at an excitation wavelength of 390 nm and an emission wavelength of 460 nm for up to 120 min in a Fluoroskan Ascent fluorometer.
Isolation of mitochondria
Heart mitochondria were isolated from 2.5–3 month old mice as described with slight modifications [34]. The isolation process was carried out at 4°C. Briefly, hearts were collected in ice-cold PBS supplemented with 10 mM EDTA and minced. Following incubation with 0.05% trypsin for 30 minutes, samples were centrifugated at 200 × g for 5 min. This was followed by homogenization in buffer containing 67 mM sucrose, 50 mM Tris-HCl, 50 mM KCl, 1 mM EDTA, 0.2% BSA, pH 7.4 and the homogenate was centrifuged at 700 × g for 15 min. The supernatant was again centrifuged at 7,000 × g for 15 min and the pellet resuspended in 250 mM sucrose, 3 mM EGTA and 10 mM Tris-HCl, pH 7.4. The solution containing the mitochondria was centrifuged at 7,000 × g for 15 min and the pellet containing mitochondria was resuspended in minimum amount of buffer. A Bradford assay was performed to determine the mitochondria concentration [34]. To determine if the mitochondria were functional, enzymatic activity assays (complex I, II or III) were carried out immediately with the respective inhibitors. Flash frozen mitochondria were also used for this study; the frozen mitochondria showed similar enzymatic activity when compared to activity of freshly isolated mitochondria [35].
Mitochondria Complex Activities
The activities of the respiratory chain complexes, Complex I (C-I), II (C-II) and III (C-III) were studied in isolated heart mitochondria in the presence of DIC (n=4–6).
C-I activity measurement
Mitochondrial C-I activity was measured by ubiquinone-stimulated NADH oxidation [35]. Purified mitochondria were added to buffer containing 25 mM potassium phosphate buffer (pH 7.5), 2 mM NaN3 (sodium azide) 0.8% C24H39NaO5 (sodium cholate), and 100 µM NADH (nicotinamide-adenine-dinucleotide). 10 µM rotenone (C-I inhibitor) and 5–10 µM DIC were added to the mitochondria in parallel experiments and incubation carried out for 2–5 min. 0.3 mM ubiquinone1 was added to initiate the reaction and the decrease in absorbance was measured at 340 nm for 2–3 min in a Tecan Infinite M1000.
C-II activity measurement
For measuring C-II activity, incubations of purified mitochondria (~0.2 µg) were carried out with 25 mM potassium phosphate buffer (pH 7.5), 1 mg/ml fatty acid-free BSA, 300 µM KCN (potassium cyanide), 20 mM succinate and 0.002% DCPIP (2,6-dichlorophenolindophenol) at 37°C for 5–10 min. In parallel experiments, mitochondria were incubated with C-II selective inhibitor, 500 µM thenoyltrifluoroacetone (TTFA), and 5 µM DIC. Thereafter, the baseline value was read at 595 nm at 37°C in a Bio-Rad Model 680 microplate reader for 2 min. A decrease in absorbance was observed after the addition of 150 µM of decylubiquinone (dUb) at at 595 nm for 2–3 min.
C-III activity measurement
Isolated mitochondria at concentration of ~0.1 µg was added to buffer containing 25mM potassium phosphate buffer (pH 7.5), 75 µM of oxidized cytochrome c, 500 µM KCN, 200 µM EDTA, 0.025% Tween-20. Antimycin A (0.01 mg/ml) and 5 µM DIC was added in parallel experiments under similar conditions. Baseline activity was read at 550 nm for 2 min in a Bio-Rad Model 680 microplate reader at 37°C. To initiate the reaction, 100 µM reduced decylubiquinone (DBH2) was added and an increase in absorbance was immediately recorded at 550 nm for 2–3 min at 37°C.
Determination of mitochondrial membrane potential
The mitochondria selective carbocyanine dye JC-1(5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbo-cyanine iodide) (Enzo Life Sciences) was utilized to study the effect of DIC on mitochondrial membrane potential in H9c2 cells. Briefly, H9c2 cells were plated on a 96 black well plate and incubated with DIC for 24 h (n=4–6). In parallel experiments, H9c2 cells were incubated with 200 µM 2,4-dinitrophenylhydrazine (DNPH [36] and 30µM rotenone [33, 37], used as positive control experiments). The next day the cells were incubated with 5 µg/ml JC-1 dye for 15 min. Mitochondrial membrane potential was measured in a Tecan Infinite M1000 using an excitation of 490 nm and emission of 590 nm.
Statistics
Results are expressed as mean ± SD from at least three independent experiments. Student’s t-test or one way ANOVA was applied to determine the statistical significance between the different samples. Values of P < 0.05 were defined as statistically significant.
Results
Proteasome activity of H9c2 cells and neonatal cardiomyocytes is impaired by DIC treatment
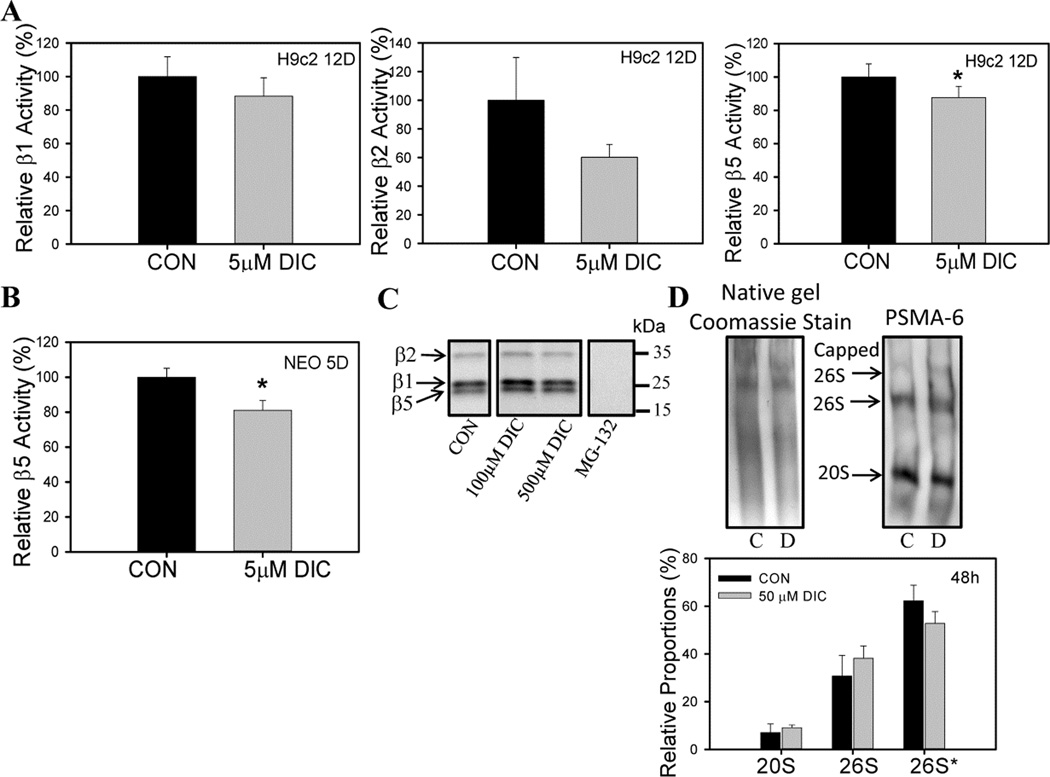
The average plasma concentrations of DIC in rats (treated with 5mg/kg DIC as a single dose) was 14.47 µM [38] and 1.8–8.7 µM in humans treated with a single dose of 50mg DIC [39]. Rat cardiac H9c2 cells treated with 5 µM DIC for 12 days showed decreased β5 (chymotrypsin-like activity) relative to vehicle treated cells (Fig. 1A). The DIC treated cells also showed a trend towards reduced β2 (trypsin-like activity) activity but several repeats showed no statistically significant difference when compared to control samples. The β1 (caspase-like activity) was not affected by DIC under the conditions investigated (Fig. 1A). To determine if DIC also affected proteasome function in freshly isolated murine neonatal cardiomyocytes, cardiomyocytes were incubated with 5 µM DIC for 5 days. Consistent with the results obtained for H9c2 cells, neonatal cardiomyocytes also showed a significant decrease in the proteasome β5 activity (Fig. 1B).
Figure 1. Diclofenac reduces proteasome function in H9c2 and neonatal cardiomyocytes.
A, Effect of 5 µM diclofenac (DIC) treatment for 12 days on β1, β2 and β5 proteasome activity in rat cardiac H9c2 cells. B, Effect of 5 µM DIC treatment for 5 days on β5 proteasome activity in murine neonatal cardiomyocytes. C, Interaction between DIC and active proteasome subunits. D, Amounts of proteasome complexes in diclofenac treated H9c2 cell lysates. Proteasome complexes were separated by native gel electrophoresis. C – control; D – diclofenac. Results are expressed as mean ± SD (n=3), * P < 0.05.
DIC does not directly bind to the proteasome active sites
Since DIC inhibited the proteasome, the fluorescently labeled proteasome inhibitor, MV151 which reversibly binds to the proteasome active sites and inhibits proteasome function, was utilized to determine if DIC directly interacted with the proteolytic proteasome subunits. DIC at concentrations of 100 µM and 500 µM failed to displace the bound inhibitor from the proteasome active sites of rat heart lysates (Fig. 1C). As a negative control, MG-132 (proteasome inhibitor) was found to compete with MV151 and bind to the active site. This result suggests that DIC does not impair proteasome function by any direct interaction with the proteasome active sites.
Impaired proteasome function has been reported to be associated with increased dissociation of the 20S core particle from the 26S proteasome complex [40]. Because in vitro studies in H9c2 cells have suggested decreased β5 activity induced by DIC (Fig. 1A), further studies were carried out to determine the relative proportions of the proteasome in the DIC treated cells. Although DIC significantly reduced β5 activity, no significant changes were observed in the relative proportions of either doubly capped 26S proteasome (19S-20S-19S), singly capped 26S proteasome (19S-20S) or 20S proteasome core particle (Fig. 1D). Our group recently showed that another NSAID, meclofenamate sodium, increased dissociation of the 20S from the 26S complex [41]. These results indicate a possible involvement of an alternate mechanism or some post translational modification of the proteasome through which DIC causes proteasome dysfunction and does not directly affect the relative proportions of the 26S or 20S proteasome levels.
DIC treated mouse hearts exhibit altered proteasome activity
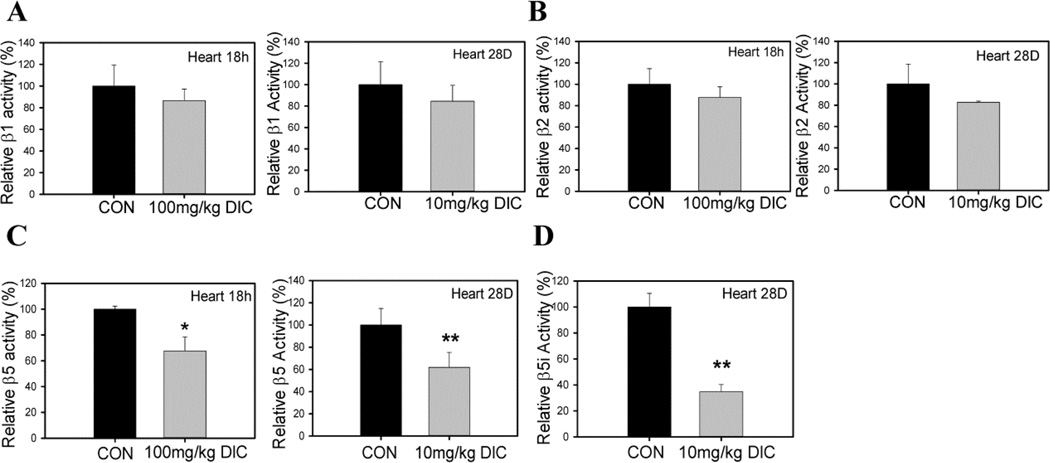
The amounts of diclofenac used in these animal studies resulted in plasma concentrations which were similar to concentrations used in previous studies [42]. Acute treatment of mice with 100 mg/kg of DIC (by gavage) for 18 h or chronic treatment with 10 mg/kg for 28 days (in drinking water) showed decreased proteasome function (Fig. 2C). In hearts, both acute and chronic DIC treatments resulted in decreased β5 activity (Fig. 2C) without significantly affecting the β1 or β2 proteasome activities (Fig. 2A and 2B). These results suggest that DIC preferentially affects the cardiac β5 activity. The β5 activity has been previously suggested to be the most important of the three proteasome activities [43]. The β5i immunoproteasome activity, which is important for degrading oxidized proteins during stress [30], was significantly reduced in hearts after chronic treatment suggesting that DIC may increase the levels of oxidized proteins in cardiomyocytes (Fig. 2D). The extent of the reduction (>60%) in β5i immunoproteasome activity may be physiologically important. Studies related to chronic DIC treatment were considered to be physiologically relevant because the amount of diclofenac in plasma (12 ± 3 ng/ml) was in the pharmacological range found in animals (Supplemental Table 1). The average plasma concentration of DIC in the acute treatment group was 330.8 ± 110.3 ng/ml (Supplemental Table 1).
Figure 2. Diclofenac impairs proteasome function in hearts of diclofenac treated mice.
Proteasome function was determined in both acute (100mg/kg DIC for 18h) and chronic treatment group (10mg/kg DIC for 28 days). A, heart β1 proteasome activity; B, heart β2 proteasome activity; C, heart β5 proteasome activity. D, β5i immunoproteasome activity in hearts from chronic treatment group. Data are expressed as mean ± SD (n=4), * P < 0.05, ** P < 0.001.
Proteasome function is reduced in liver and kidney of mice treated with DIC
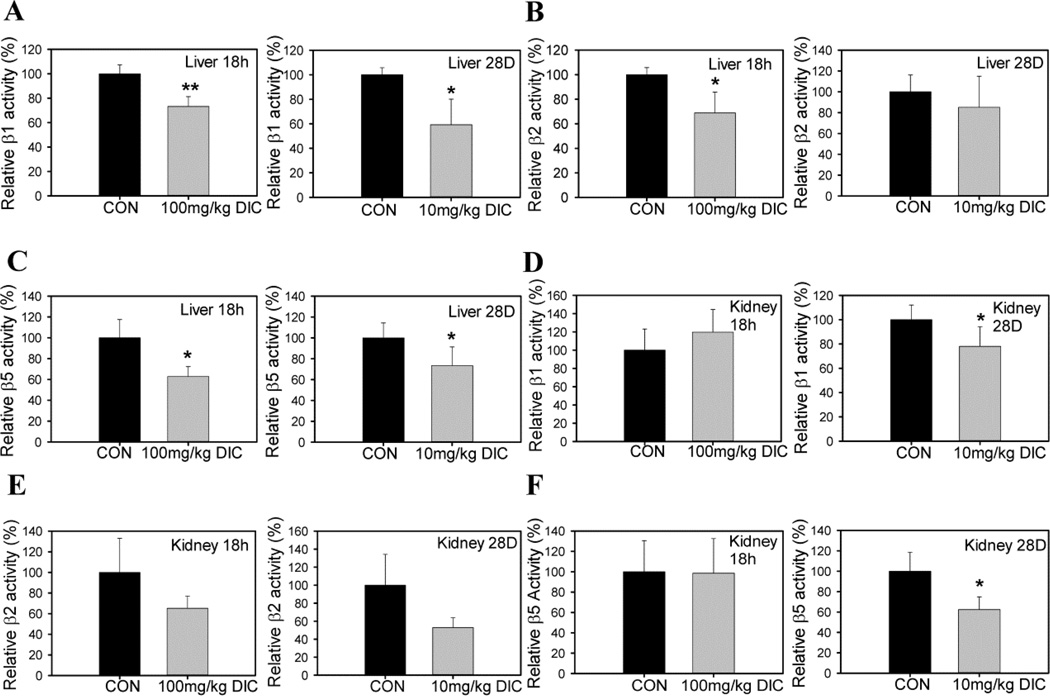
Since NSAIDs have been reported to have potential adverse effects in the hepatic and renal system, we also investigated the role of DIC on the proteasome function of murine liver and kidney treated with DIC. Similar to what was observed in heart tissue, β5 activity was decreased in both liver and kidney samples from the chronic treatment group (Figs. 3C and 3F). Kidney samples from the acute treatment group showed similar β5 activity to control kidneys but liver samples showed decreased β5 activity relative to control livers (Figs. 3C and 3F). Unlike heart, which showed similar β1 and β2 proteasome activities in DIC treated animals, β1 activity was decreased in liver and kidneys in chronically treated animals (Fig. 3A and 3D). The β1 activity was also significantly decreased in liver of the animals from the acute treatment group (Fig. 3A). The β2 activity of the liver proteasome of the acutely treated mice with DIC was significantly reduced (Fig. 3B). On the other hand, the chronic treatment group showed no changes in β2 activity (Fig. 3B). The kidneys from the acute treatment group did not show any significant alteration in either β1 or β2 activities of the proteasome (Figs. 3D and 3E). Although not statistically significant, there was a strong trend towards decrease in the β2 activity in both the acute treatment group (~40 %) and the chronic treatment group (~50 %) (Fig. 3E).
Figure 3. Diclofenac impairs proteasome function in livers and kidneys of diclofenac treated mice.
Proteasome activity in livers of acute (100mg/kg DIC for 18h) and chronic treatment animals (10mg/kg DIC for 28 days): A, liver β1 proteasome activity; B, liver β2 proteasome activity; C, liver β5 proteasome activity. Proteasome activity in kidneys of acute and chronic treatment animals: D, kidney β1 proteasome activity; E, kidney β2 proteasome activity; F, kidney β5 proteasome activity. Data are expressed as mean ± SD (n=4), * P < 0.05, ** P < 0.001.
DIC increases polyubiquitination levels in heart without altering proteasome subunit expression
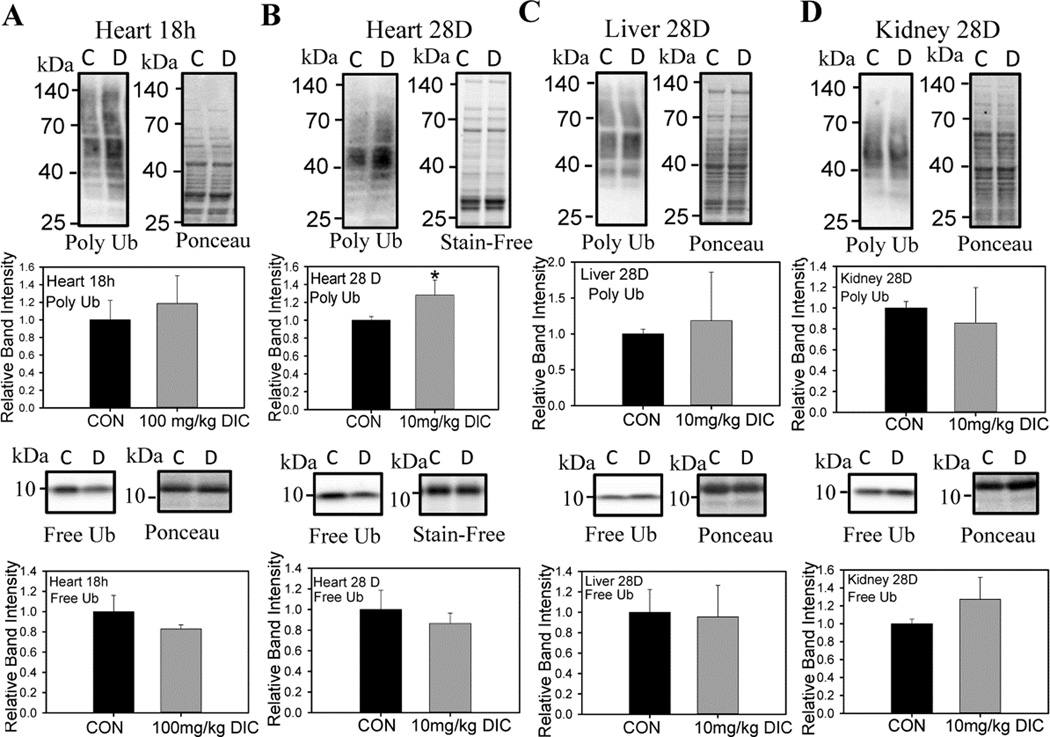
When proteasome function is significantly affected, increased levels of polyubiquitination is often observed [44]. Polyubiquitination levels were increased in hearts of the chronic treatment group (Fig. 4B), but not in the livers, or kidneys from these animals (Fig. 4C and D). The levels of free ubiquitin were not affected by DIC treatment in any of the tissues investigated (Fig. 5). Although not statistically significant, there was a trend towards decrease in the free ubiquitin levels in the hearts of the mice treated with DIC (Fig. 4A and B). These results suggest that compared to livers or kidneys, the heart UPS is more susceptible to the toxic effect of DIC.
Figure 4. Diclofenac increases the levels of polyubiquitinated proteins in hearts from diclofenac treated mice.
A, Western blotting was used to determine the effect of DIC on polyubiquinated proteins and free ubiquitin expression levels in the hearts of the acute treatment group. B, Effect of DIC on heart polyubiquinated proteins and free ubiquitin expression levels in the chronic treatment group. C, Effect of DIC on liver polyubiquinated proteins and free ubiquitin expression levels in the chronic treatment group. D, Effect of DIC on kidney polyubiquinated proteins and free ubiquitin expression levels in the chronic treatment group. C - Control, vehicle treated hearts. D - Diclofenac treated hearts. Results are expressed as mean ± SD (n=4),* P < 0.05.
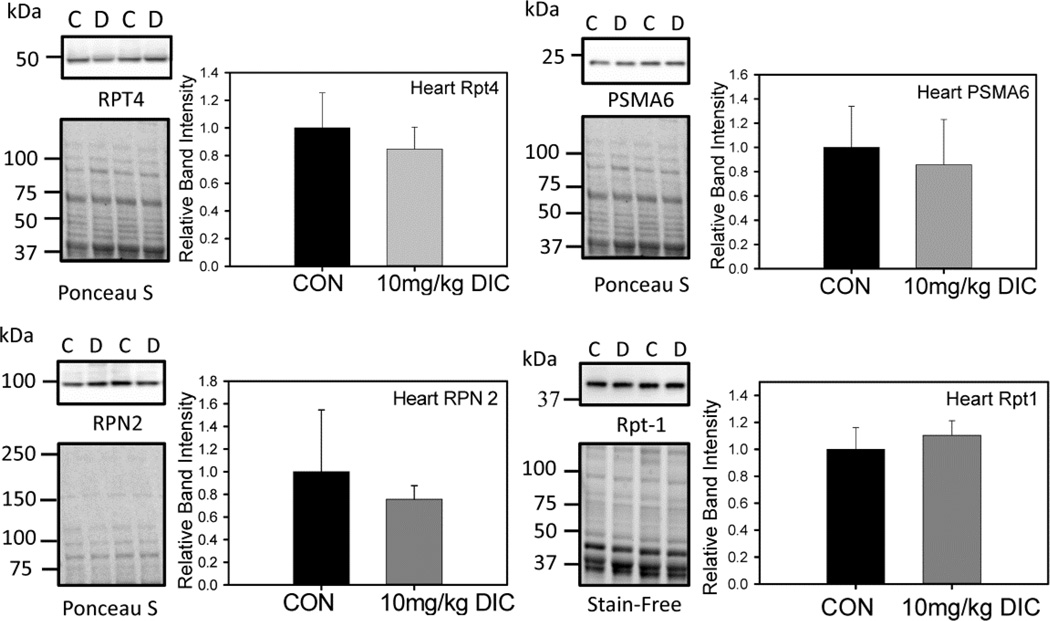
Figure 5. Levels of proteasome subunit expression in hearts are not affected by diclofenac treatment.
Proteasome subunit expression levels in the chronic treatment group were determined by Western blotting. Four proteasome subunits, Rpt4, Rpn2, Rpt1 and PSMA6 were investigated. C - Control, vehicle treated hearts. D - Diclofenac treated hearts. Quantification is expressed as mean ± SD (n=4).
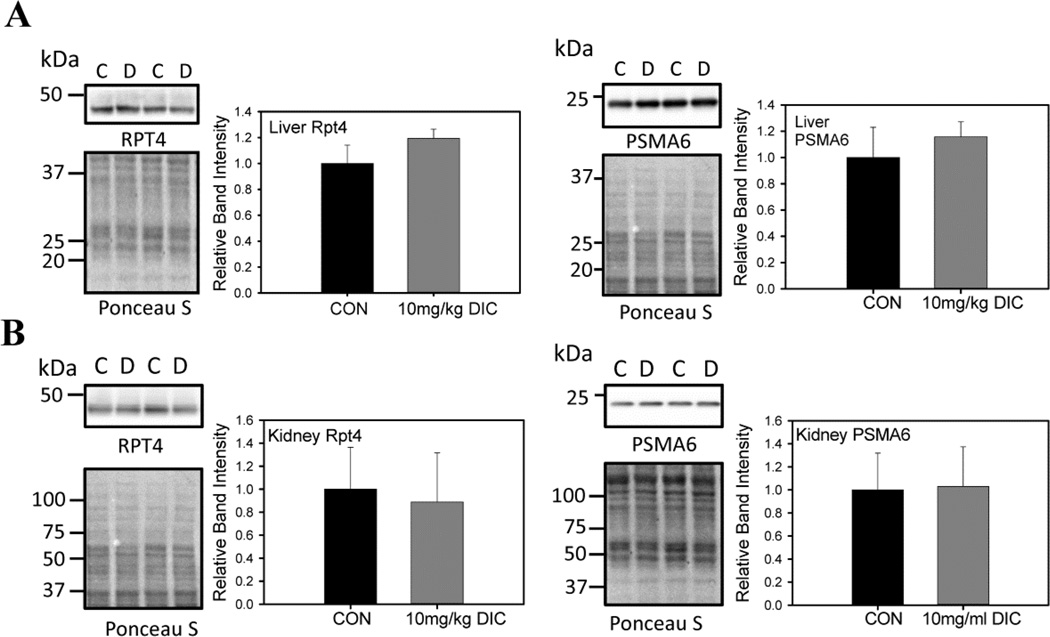
To further evaluate the adverse effects of DIC on proteasome function, the levels of proteasome subunit expression were quantified by Western blot analysis. The expression levels of various proteasome subunits, Rpt4, Rpn2, Rpt1 (19S regulatory subunit) and PSMA6 (20S catalytic core subunit), in the hearts of the mice from the chronic treatment group did not exhibit any significant changes in expression levels (Fig. 5). Similarly the liver (Fig. 6A) and kidneys (Fig. 6B) from the animals of the chronic treatment group did not show any changes in the levels of the proteasome subunits Rpt4 and PSMA 6.
Figure 6. Levels of proteasome subunit expression in livers and kidneys are not affected by diclofenac treatment.
A, The proteasome subunit levels of Rpt4 (19S proteasome subunit) and PSMA6 (20S proteasome subunit) were measured in livers of the chronically treated animals with DIC. B, The proteasome subunit levels of Rpt4 and PSMA6 were measured in kidneys from the acute treatment group. C - control, vehicle treated hearts. D - DIC treated hearts. Values are expressed as mean ± SD (n=4), * P < 0.01, ** p < 0.001.
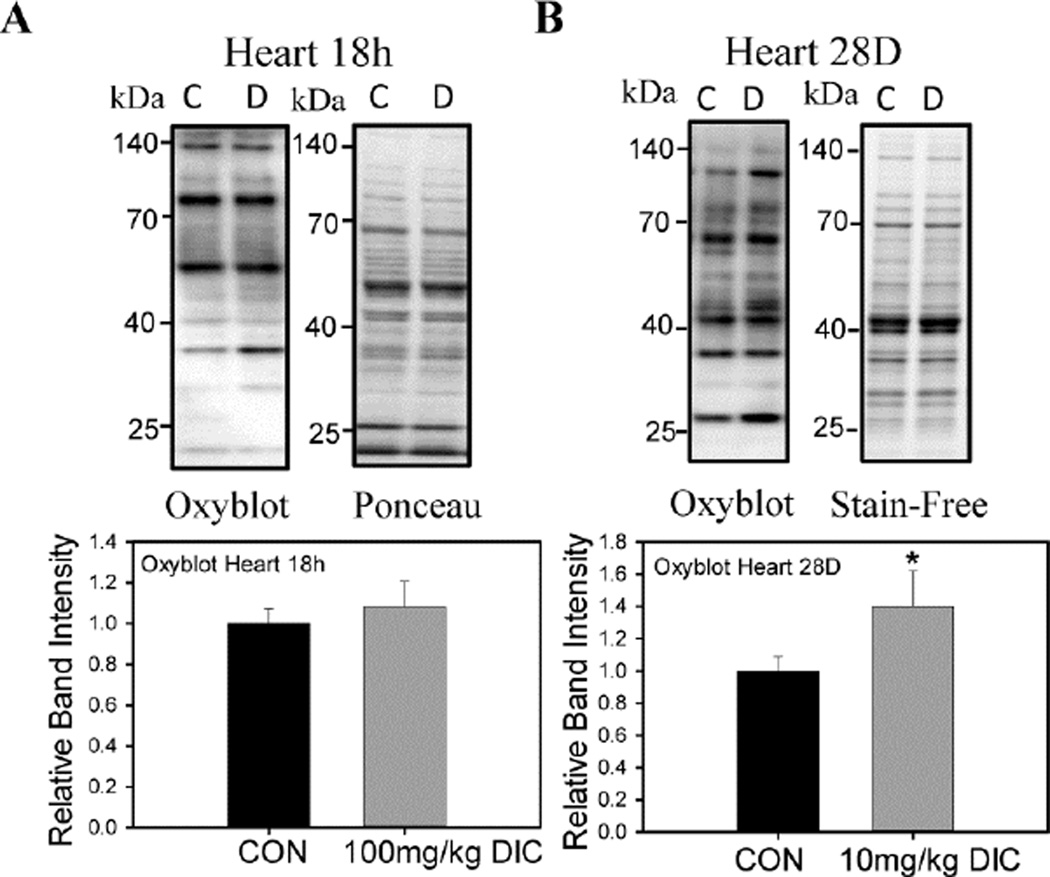
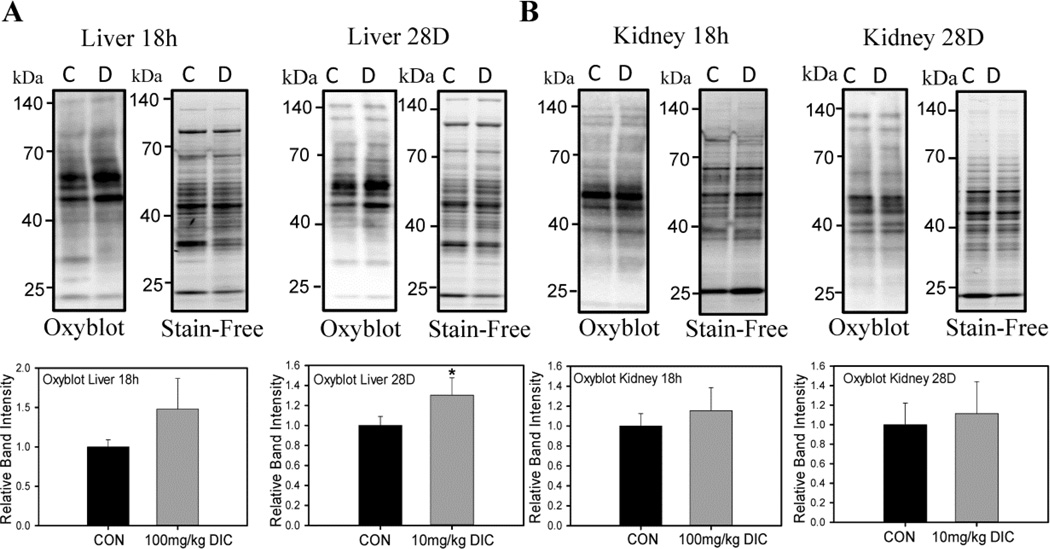
DIC increases oxidized protein levels in heart and liver
Proteasome dysfunction is associated with increased intracellular stress as the proteasome is critical for proteostasis (protein homeostasis). The levels of oxidized proteins, as measured using the oxyblot method, increased in hearts (Fig. 7B) and livers (Fig. 8A), but not in kidneys (Fig. 8B), in the chronic treatment group. The acute treatment group did not show any change in protein oxidation in hearts (Fig. 7A) or kidneys (Fig. 8B). The liver on the other hand, showed a marked increase (~50%) in the levels of oxidized proteins although not statistically significant (Fig. 8A). These results suggest that a chronic low dose of DIC may be more potent at increasing oxidative stress in mice compared to a shorter duration of high dose.
Figure 7. Effect of acute and chronic diclofenac treatment on levels of oxidized proteins in hearts.
Oxyblot analysis was performed to determine the levels of the oxidized proteins in hearts, livers and kidneys of animals treated with DIC. A, Oxidized proteins levels of mouse hearts in the acute treatment group. B, Oxidized proteins in mouse hearts in the chronic treatment group. C - Control, vehicle treated hearts. D - Diclofenac treated hearts. Quantification of the blots are expressed as mean ± SD (n=4), * P < 0.05.
Figure 8. Effect of acute and chronic diclofenac treatment on levels of oxidized proteins in livers or kidneys.
A, Levels of oxidized proteins detected by Western Blotting in the livers of the animals from either the acute treatment group or the chronic treatment group. B, Oxidized proteins in mouse kidneys in the either the acute treatment group or the chronic treatment group. Results are expressed as mean ± SD (n=4), * P < 0.01, ** p < 0.001.
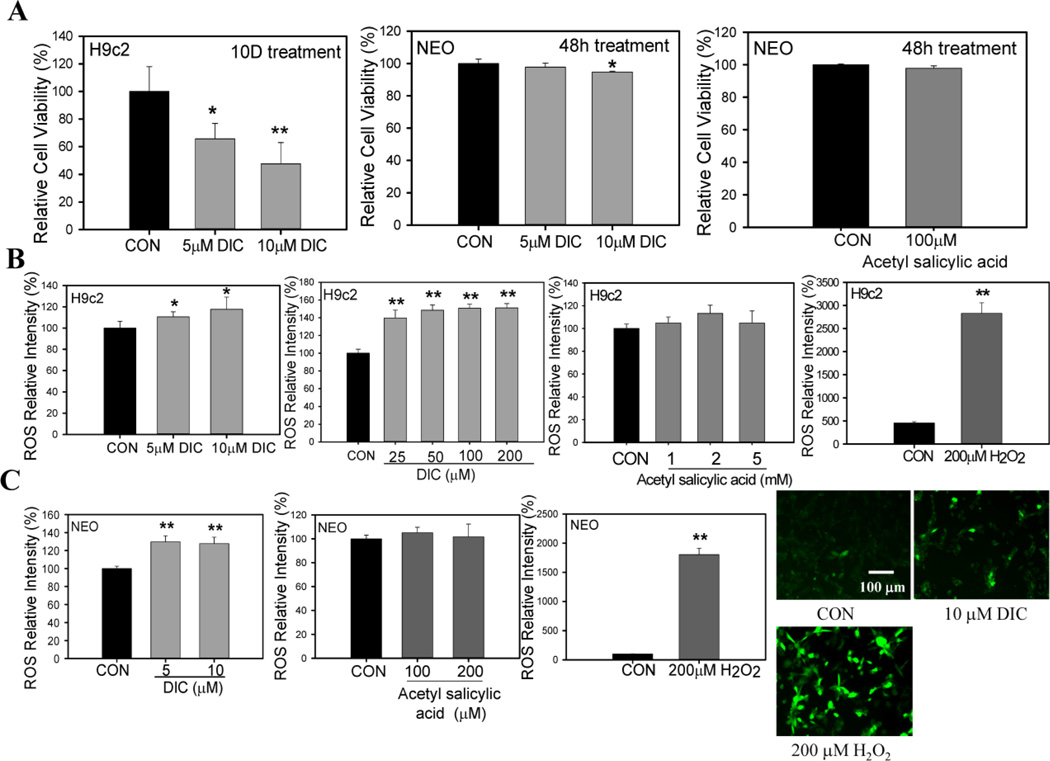
Cell Viability of H9c2 cells and neonatal cardiac cells is decreased by DIC
Since increased oxidized and polybiquinated proteins suggest proteostasis impairment, the effect of DIC on cardiac cell death was investigated. H9c2 cells treated with either 5 µM DIC or 10 µM DIC for 10 days showed a significant decrease in cell viability compared to control cells (Fig. 9A). Similarly, neonatal cardiomyocytes showed a significant reduction in cell viability when treated with 10 µM DIC for 48 h (Fig. 9A).
Figure 9. Diclofenac reduces cardiac cell viability and increases ROS production in H9c2 and neonatal cardiomyocytes.
A, H9c2 cardiac cells treated with vehicle or 5 and 10 µM diclofenac (DIC) for 10 days. Neonatal murine cardiomyocytes were treated with 10 µM DIC or vehicle for 2 days. Cell viability of H9c2 cells and neonatal cardiomyocytes were measured by Alamar Blue. Additionally acetylsalicylic acid (aspirin, 100 µM) was used to measure cell viability in neonatal murine cardiomyocytes. B, ROS levels in H9c2 cardiac cells treated with different concentrations of DIC (25 µM–200 µM) and acetyl salicylic acid (1mM–5mM). 200 µM Hydrogen peroxide (H2O2) was used as a positive control for ROS production. C, Effect of 5 µM DIC, 10 µM DIC, 100 µM acetylsalicylic acid or 200 µM acetylsalicylic acid on ROS levels in neonatal cardiomyocytes. Fluorescent images represent ROS generation in the presence of DIC and H2O2. The images are a representative of 3 or more independent experiments. Results are expressed as mean ± SD (n=4–9), * P < 0.05, ** P < 0.001.
ROS generation in H9c2 cells and neonatal cells is increased by DIC
To find out if cell death induced by DIC may be due to excessive ROS generation, ROS formation was measured in both H9c2 cells and neonatal cardiac cells by treating the cells with different concentrations of DIC (5 µM – 200 µM DIC). In cardiac H9c2 cells, ROS generation was observed at pharmacological concentrations of 5 µM with ROS levels increasing at higher concentrations (Fig. 9B). Consistent with the above results, neonatal cardiomyocytes exhibited significant amounts of ROS formation at concentrations of 5 µM and 10 µM DIC (Fig. 9B). These results suggest that DIC induced cell death may be associated with increased ROS generation. In separate experiments, the effect of acetyl salicylic acid (aspirin) on ROS generation in cardiac cells was studied. Aspirin is a well-established cardioprotective compound compared to all other NSAIDs [45]. H9c2 cells and neonatal cardiomyocytes were similarly incubated with DCFDA for 30 min after which ROS generation was measured in the presence of different concentrations of acetylsalicylic acid. No ROS generation was detected either in the H9c2 cells or neonatal cardiac cells in the presence of this compound (Fig. 9B and 9C). Hydrogen peroxide (H2O2) used as a positive control showed significant increases in ROS generation (Fig. 9B and C). These results were further validated by fluorescence microscopy studies which suggested a significant increase in H9c2 ROS generation in the presence of 10 µM DIC (Fig. 9C).
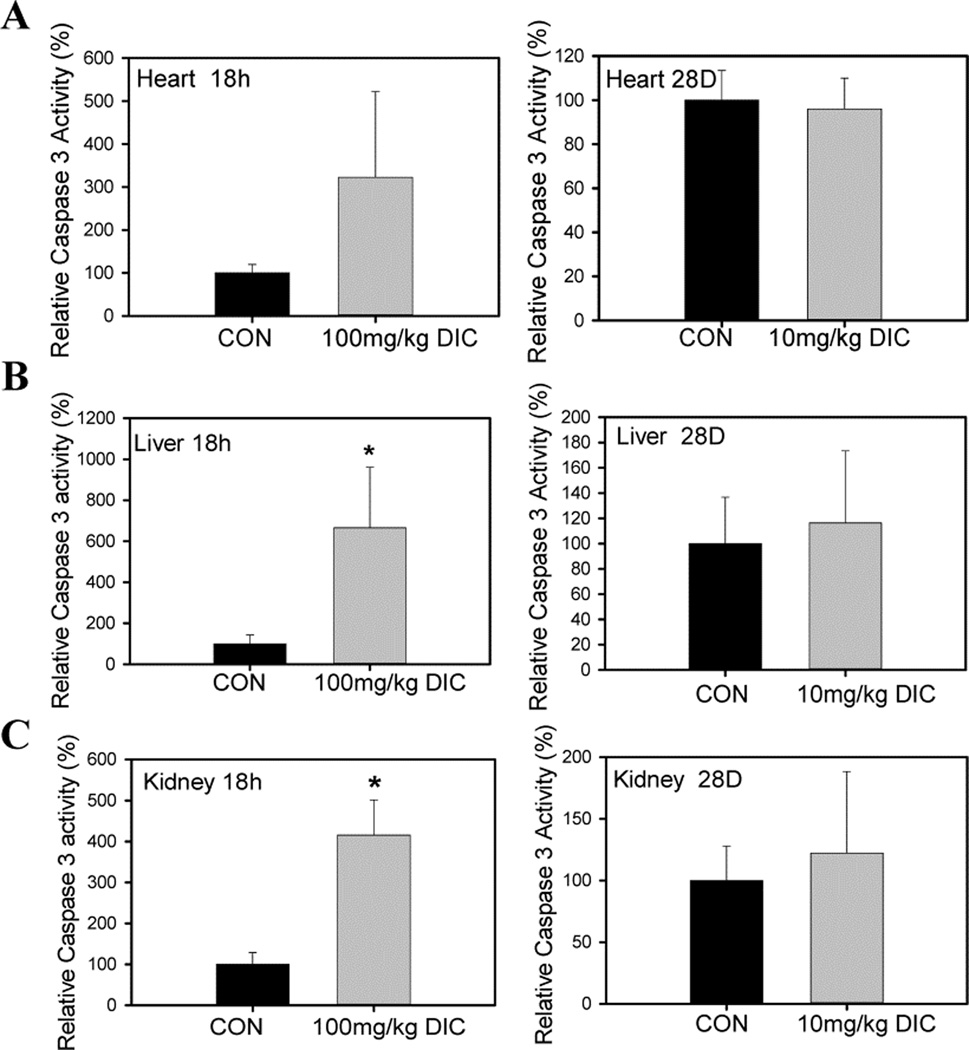
To determine the mechanism of DIC induced cell death, caspase 3 activity was determined in heart, kidney and liver tissues to determine if apoptosis was induced (Fig. 10). After acute DIC treatment, both liver and kidney showed significant (>4 fold) increases in caspase 3 activity (Fig. 10B and 10C). Although not statistically significant due to the high standard deviation, the heart exhibited a 3 fold higher mean caspase 3 activity in the DIC treated mice compared to the vehicle treated mice (Fig. 10 A), suggesting that acute DIC treatment may increase caspase 3 activity in most or all tissues. However, after chronic DIC treatment, heart, liver and kidney did not show any significant changes in the activity (Figs. 10A, B, and C).
Figure 10. Diclofenac increases caspase 3 activity in tissues in the acute treatment group but not in the chronically treated animals.
A, Caspase 3 activity in hearts, B, Caspase 3 activity in livers, C, Caspase 3 activity in kidneys of mice from either the acute treatment group or chronically treated animals. Values are expressed as mean ± SD (n=4), * P < 0.05.
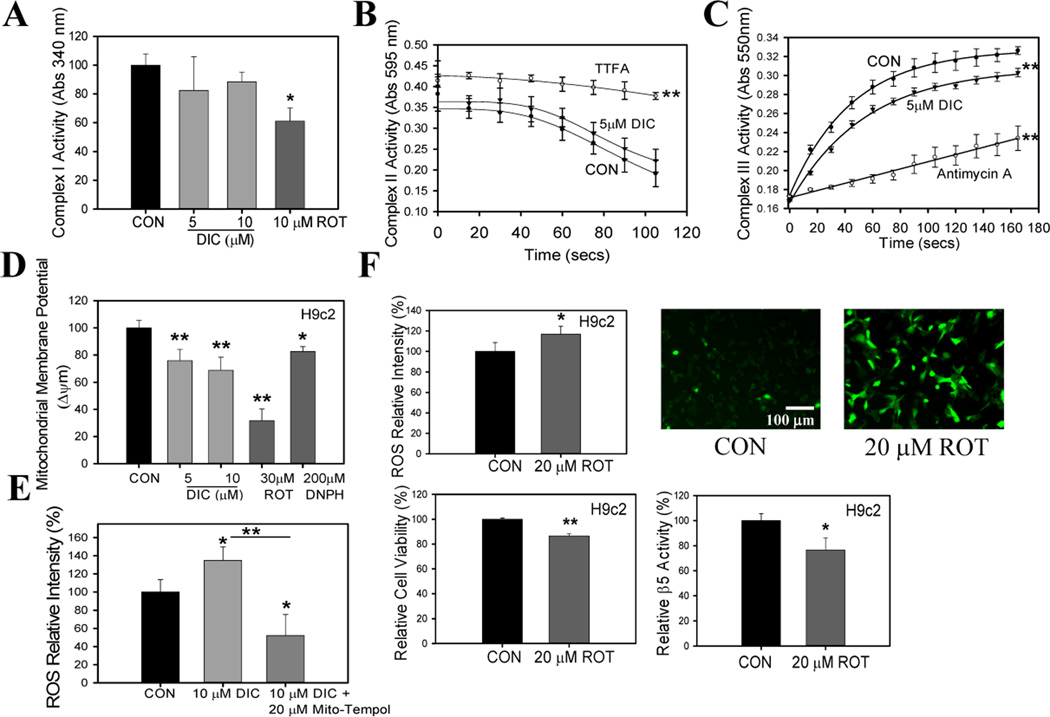
Mitochondria function is impaired by DIC
One of the major sources of ROS generation in the cell system is the mitochondria [46]. To determine if mitochondria are involved in ROS generation in DIC treated cells, measurement of complex activity was carried out in isolated heart mitochondria in the presence and absence of DIC. DIC (5 and 10 µM) did not affect the C-I activity (Fig. 11A). Rotenone (a complex one inhibitor) was used as a positive control and it significantly decreased C-I activity (Fig. 11A). 5 µM DIC did not significantly affect C-II activity (Fig. 11B). However, 5 µM DIC significantly impaired mitochondrial III function (Fig. 11C). Antimycin A, an inhibitor of C-III was used as a positive control. Inhibitions of either C-I, C-II, or C-III have been associated with mitochondrial ROS production [47, 48].
Figure 11. Diclofenac induces mitochondrial dysfunction and mitochondrial complex I inhibitor induces ROS generation and cell death and decreases proteasome activity of H9c2 cells.
A, Effect of DIC on complex I activity in mitochondria isolated from mouse hearts. Rotenone (ROT), a known inhibitor of complex I, was used as a control. B, Effect of DIC on complex II activity in mitochondria isolated from mouse hearts. Thenoyltrifluoroacetone (TTFA), a complex II inhibitor, was used as a control. C, Effect of DIC on complex III activity in mitochondria isolated from mouse hearts. The complex III inhibitor, Antimycin A, was used as a control. D, Effect of DIC on mitochondrial membrane potential measured in H9c2 cells. Rotenone (ROT) and 2,4-dinitrophenylhydrazine (DNPH) were used as controls. E, ROS levels were determined in the presence of DIC and DIC + Mito-Tempol, a mitochondrial ROS scavenger. F, ROS generation, cell viability and proteasome β5 activity of H9c2 cells in the presence of rotenone. H9c2 cells were plated onto 96 plates at 4000 cells/well and H2DCFDA and Alamar blue was utilized to determine ROS generation and cell viability as described in the Methods. ROS generation in H9c2 cells in the presence of rotenone was determined both by fluorimetry and fluorescent microscopy studies. The fluorescent images are a representative of 3 or more independent experiments. Effect of rotenone on proteasome β5 activity was also measured. ROT-rotenone. Results are mean ± SD (n=4–6)* P < 0.05, ** P < 0.001.
To determine if the mitochondrial membrane potential, an important aspect of mitochondria function, was affected by DIC, the mitochondrial membrane potential was measured in H9c2 cells exposed to DIC. DIC (5 and 10 µM) decreased the mitochondrial membrane potential suggesting that this NSAID would cause mitochondrial dysfunction and increase mitochondrial derived ROS levels (Fig. 11D). Rotenone, which has been shown to impair mitochondrial membrane potential, was used as a positive control. Rotenone was used at 30 µM to ensure significant inhibition of the mitochondrial membrane potential because a previous report demonstrated that in another cell type (HepG2 cells) 25 µM rotenone only reduced mitochondrial membrane potential by less than 20% [33].
To determine if the increased ROS levels due to DIC was mainly of mitochondrial origin, H9c2 cells were incubated with the mitochondrial selective antioxidant, mito-Tempol (Fig. 11E). The increase in ROS produced by DIC was prevented by mito-Tempol suggesting that most ROS produced by DIC was from mitochondria. In the presence of 20 µM mito-Tempol DIC treated cells showed lower ROS levels than control cells suggesting that some of the endogenous ROS produced by H9c2 cells was scavenged by mito-Tempol. This is consistent with the mitochondria being considered as major contributors to BASAL levels of endogenous ROS production in healthy cells.
Mitochondrial complex I inhibitor induces ROS generation, decreases cell viability and proteasome activity
Recent studies have reported a close association between mitochondrial function and proteasome activity [49]. However, whether these two pathways regulate each other in cardiac cells is not known. H9c2 cells were treated with 20 µM of rotenone and ROS generation determined [50]. It was found that rotenone itself was a significant inducer of ROS generation in these cells as suggested by fluorimetry analysis as well as microscopy studies (Fig. 11F). This is consistent with previous reports which suggest that reduced C-I activity results in disruption in the flow of electrons in the respiratory chain complex resulting in premature leaking of the superoxide ion at C-I [46]. This led to further studies on the effect of rotenone on H9c2 cell viability. Rotenone at 20 µM significantly reduced cell viability. To further investigate the association between mitochondrial function and proteasome activity, H9c2 cells were treated with 20 µM rotenone for 24 h and proteasome β5 activity was measured. Proteasome activity was significantly impaired in the rotenone treated cells compared to the vehicle treated group (Fig. 11F). These studies indicate that the mitochondria and the proteasome system of cardiac cells may regulate each other and impairment of any one of the pathway may lead to the dysfunction of the other.
Discussion
During the past decade, various clinical trials have reported increased cardiovascular incidences among NSAID users [3, 5]. NSAIDs and highly selective COX-2 inhibitors (Coxibs) are immensely valuable drugs and are used worldwide. However, a variety of side effects are associated with their use specifically extending beyond the ability of these drugs to inhibit eicosanoid syntheses. However the underlying cellular and molecular mechanisms associated with the development of CVD due to NSAIDs is not known. In the present study investigations were made to delineate the possible underlying mechanisms through which DIC may exert its potential adverse effect leading to the development of CVD by studying its effect on cardiac myocytes and whole hearts.
Although very little is known about DIC effects on the heart, DIC at certain concentrations is known to be toxic to liver tissues of animals [51]. Renal failure due to DIC products in vulture tissues has been suggested to cause death in vultures [51]. While DIC plasma concentrations were less than concentrations reported to be toxic, the breakdown products of DIC in vultures show DIC exposure and toxicity of liver tissue [52]. The vulture population in the Indian subcontinent started to decline rapidly in the early 1990s. Since 1991, some vulture species have declined by more than 97% [53]. Livestock, especially cows and goats, were commonly treated with DIC, which was still present in the carcasses of dead animals scavenged by vultures [54]. Significant proteasome dysfunction in cardiomyocytes and hepatocytes could result in toxicity to these cell types. Proteasome dysfunction was observed in the hearts, liver and kidneys of the mice administered with DIC, but the extent of proteasome dysfunction varied widely among these organs. Many drugs are not distributed equally throughout the system, and they preferentially accumulate in specific organs or tissues to exert their clinically desirable effects or to induce toxicity in those tissues [55]. Many NSAIDs including DIC have high affinity for binding to the plasma proteins resulting in an uneven distribution in the physiological system and often exhibit higher concentrations in the liver, kidneys and inflamed cells [55]. Our results indicate that proteasome dysfunction was significantly impaired in the hearts, livers and kidneys of the DIC treated animals. The present study revealed that DIC does not support direct interaction with the proteolytic sites on the proteasome (as demonstrated by the MV151 labelling of the proteasome active sites). This suggests that a different mechanism may be involved leading to the dysfunction of proteasomes.
A significant accumulation of polyubiquitinated protein (an indicator of proteasome dysfunction) was only observed in the hearts of the DIC treated animals compared to wild-type hearts or livers or kidneys from the same animals. We have previously shown that proteasomes purified from heart tissues were more susceptible to inhibition by proteasome inhibitors than proteasomes purified from liver tissues [29]. These results strongly suggest that the UPS system in the heart is more susceptible to DIC than in livers or kidneys.
Additionally, a significant reduction in heart immunoproteasome β5i activity further supported the adverse effect of DIC on proteasome function. Immunoproteasomes are inducible subunits of the proteasome system that replace the standard proteasome subunits under conditions of oxidative stress and exert a protective effect in the cell system [56]. The impaired β5i activity induced by 10 mg/kg DIC (chronic treatment group) probably resulted in the increased levels of oxidized proteins observed in the hearts. Hearts and livers, but not kidneys, from the chronic treatment group also showed increased levels of oxidized proteins suggesting that DIC affected proteostasis in both hearts and livers. The more pronounced oxidative stress in the chronic treatment group compared to the acutely treated animals may be because the acute effects produced by a single dose are sometimes reversible and tend to improve over time.
In some of our experiments there was a marked difference between the treated and control groups, yet the data was not statistically significant (eg. the caspase 3 activity in the hearts of the acute treatment group were around 200% higher than the control group, but the data were not statistically significant (Fig. 10A)). This may be due to different animals responding differently to the DIC causing large standard deviations resulting in insignificant data. Large variations in effects between animals treated with the same chemical have been previously reported [57]. Use of larger number of animals may have reduced to standard error associated with these measurements. Hence, it is likely that the caspase 3 activity is also upregulated in the hearts from the DIC acute treatment group (Fig. 10A).
The amounts of diclofenac used in these animal studies were similar to concentrations used in previous studies (for example, diclofenac at 10 and 25 mg/kg, administered intraperitoneally to mice, significantly delayed glioma growth [42]). In the present study, chronic treatment of mice was capable of inducing cardiotoxic effect in mice indicating the adverse effect of the compound in the heart. Different concentrations (10–1000 µM) of DIC have been used to study various parameters in different kinds of cells [58–60]. In the present study, pharmacological levels of DIC (5 µM – 12 µM) caused cell death in H9c2 cells and neonatal cardiomyocytes. Primary neonatal cardiac cells are commonly used to study the toxic effect of drugs because of the ability of these cells to mimic in vivo results [61]. The ability of DIC in inducing cardiac cell death was found to be correlated with decreased mitochondrial and proteasome function.
The main mechanism by which DIC causes cell death may be due to increased ROS production. In cultured cardiac cells, DIC was found to be a potent inducer of ROS generation. This increased ROS generation caused by DIC was reduced by Mito-Tempol suggesting that ROS generated by DIC treatment may have originated from mitochondria. However, since Mito-Tempol reduced ROS production compared to the control group, it is possible that Mito-Tempol reduces mainly the ROS production normally produced by mitochondria. DIC decreased C-III activity of isolated heart mitochondria further suggesting that mitochondria are main sources of ROS generation in the cardiomyocytes. C-III is known to be a major contributor of ROS generation in mitochondria [62], with reduced C-III activity associated with increased ROS production (possibly due to increased premature leaking of the superoxide ion at C-III [46]. C-III activity was also recently shown to be inhibited by the NSAID meclofenamate sodium [41]. DIC treated cardiac cells also showed lower mitochondrial membrane potential suggesting that significant mitochondrial dysfunction is occurring.
It has been reported that mitochondrial dysfunction is closely associated with impaired proteasome activity [49]. In the present study, mitochondrial complex I inhibitor, rotenone, was found to significantly decrease proteasome activity of H9c2 cells which was also associated with an increase in ROS generation and decreased cell viability. Isolated liver mitochondria incubated with DIC caused mitochondrial swelling which lead to disruption of the mitochondrial membrane potential [9]. DIC induced renal toxicity have also been reported where DIC caused nephrotoxicity in mouse kidneys leading to the generation of reactive oxygen species (ROS) causing cell death due to apoptosis and DNA fragmentation [63]. However, the proteasome dysfunction and oxidative stress observed in our studies was not associated with apoptosis as caspase 3 activity was not affected in the chronic treatment group. No previous reports have looked at how the UPS is affected by DIC.
Overall, our results suggest that DIC at pharmacological levels caused cardiac cell death in neonatal cardiomyocytes and cardiac dysfunction in hearts of DIC treated mice. The results also suggest that DIC may exert its effect on cardiac cells by inhibiting mitochondrial C-III activity which leads to excessive ROS generation, followed by decreased proteasome function and impaired mitochondrial function which lead to cell death.
Study Limitations
The present study has certain limitations. Our findings suggest that pharmacological levels of DIC (5–10µM) was potent in inducing ROS and cell death due to mitochondrial dysfunction and impaired proteasome function. However the specific fundamental mechanism(s) by which DIC has such adverse effects on cardiac function remains to be identified. A mechanistic understanding of NSAID action could provide both diagnostic and predictive capability regarding NSAID side effects, and possibly may lead to reduction or prevention of these side effects. What we present in our manuscript is the consequence of DIC treatment in the animal and cell culture models and not the cause of such effects. In a recent publication by our group another NSAID, meclofenamate sodium, increased 20S proteasome levels and decreased 26S proteasome and increased oxidation of 19S regulatory subunits in cardiac cells. In the present study no significant changes in the relative proportions of 20S and 26S proteasomes was observed in the cells treated with DIC. Although we suggest a crosstalk between mitochondria and proteasome function, further in vitro analyses are required to determine the specific mechanism (post translational modification of the proteasomes like phosphorylation or oxidation of proteasome subunits), if any, through which DIC may lead to decreased proteasome activity in cardiac cells. Furthermore, studies related to determining the levels of antioxidants in the cells treated with DIC would further suggest that DIC induces cell death through the generation of ROS. Finally, investigating the role of antioxidants in counteracting the effect of DIC in cardiomyocytes would give new directions to possibly improve cardiac outcomes in patients treated with DIC for prolonged period of time.
Supplementary Material
Acknowledgments
This work was partly funded by University of California funds, a Hellman Fellowship (AVG), and an American Heart Association grant 6GRNT31350040. Partial support was provided by NIEHS R01 ES002710 and P42 ES004699.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Rajeshwary Ghosh takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Sumanta K. Goswami was responsible for treating the animals with diclofenac.
Luis Felipe B.B. Feitoza carried out experiments on the hearts, livers and kidneys procured from the diclofenac treated animals.
Bruce Hammock was responsible for providing the animals and diclofenac.
Aldrin V. Gomes takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Conflict of Interest: The authors report no relationships that could be construed as a conflict of interest.
References
- 1.Ong CK, Lirk P, Tan CH, Seymour RA. An evidence-based update on nonsteroidal anti-inflammatory drugs. Clinical medicine & research. 2007;5:19–34. doi: 10.3121/cmr.2007.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taubert KA. Cardiology patient pages. Can patients with cardiovascular disease take nonsteroidal antiinflammatory drugs? Circulation. 2008;117:e322–e324. doi: 10.1161/CIRCULATIONAHA.107.749135. [DOI] [PubMed] [Google Scholar]
- 3.Howes LG. Selective COX-2 inhibitors, NSAIDs and cardiovascular events - is celecoxib the safest choice? Therapeutics and clinical risk management. 2007;3:831–845. [PMC free article] [PubMed] [Google Scholar]
- 4.Bing RJ, Lomnicka M. Why do cyclo-oxygenase-2 inhibitors cause cardiovascular events? Journal of the American College of Cardiology. 2002;39:521–522. doi: 10.1016/s0735-1097(01)01749-1. [DOI] [PubMed] [Google Scholar]
- 5.McGettigan P, Henry D. Use of non-steroidal anti-inflammatory drugs that elevate cardiovascular risk: an examination of sales and essential medicines lists in low-, middle-, and high-income countries. PLoS medicine. 2013;10:e1001388. doi: 10.1371/journal.pmed.1001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Connor N, Dargan PI, Jones AL. Hepatocellular damage from non-steroidal anti-inflammatory drugs. QJM : monthly journal of the Association of Physicians. 2003;96:787–791. doi: 10.1093/qjmed/hcg138. [DOI] [PubMed] [Google Scholar]
- 7.Murray MD, Brater DC. Renal toxicity of the nonsteroidal anti-inflammatory drugs. Annual review of pharmacology and toxicology. 1993;33:435–465. doi: 10.1146/annurev.pa.33.040193.002251. [DOI] [PubMed] [Google Scholar]
- 8.Laine L, Goldkind L, Curtis SP, Connors LG, Yanqiong Z, Cannon CP. How common is diclofenac-associated liver injury? Analysis of 17,289 arthritis patients in a long-term prospective clinical trial. The American journal of gastroenterology. 2009;104:356–362. doi: 10.1038/ajg.2008.149. [DOI] [PubMed] [Google Scholar]
- 9.Masubuchi Y, Yamada S, Horie T. Possible mechanism of hepatocyte injury induced by diphenylamine and its structurally related nonsteroidal anti-inflammatory drugs. The Journal of pharmacology and experimental therapeutics. 2000;292:982–987. [PubMed] [Google Scholar]
- 10.Wang D, Patel VV, Ricciotti E, Zhou R, Levin MD, Gao E, et al. Cardiomyocyte cyclooxygenase-2 influences cardiac rhythm and function. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7548–7552. doi: 10.1073/pnas.0805806106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Y, Ricciotti E, Scalia R, Tang SY, Grant G, Yu Z, et al. Vascular COX-2 modulates blood pressure and thrombosis in mice. Science translational medicine. 2012;4:132ra54. doi: 10.1126/scitranslmed.3003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz-Gonzalez F, Sanchez-Madrid F. NSAIDs: learning new tricks from old drugs. European journal of immunology. 2015;45:679–686. doi: 10.1002/eji.201445222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Leeuwen JS, Unlu B, Vermeulen NP, Vos JC. Differential involvement of mitochondrial dysfunction, cytochrome P450 activity, and active transport in the toxicity of structurally related NSAIDs. Toxicology in vitro : an international journal published in association with BIBRA. 2012;26:197–205. doi: 10.1016/j.tiv.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Hortmann M, Daiber A, Oelze M, Ostad MA, Schwarz PM, et al. Cyclooxygenase 2-selective and nonselective nonsteroidal anti-inflammatory drugs induce oxidative stress by up-regulating vascular NADPH oxidases. The Journal of pharmacology and experimental therapeutics. 2008;326:745–753. doi: 10.1124/jpet.108.139030. [DOI] [PubMed] [Google Scholar]
- 15.Vazquez-Meza H, de Pina MZ, Pardo JP, Riveros-Rosas H, Villalobos-Molina R, Pina E. Non-steroidal anti-inflammatory drugs activate NADPH oxidase in adipocytes and raise the H2O2 pool to prevent cAMP-stimulated protein kinase a activation and inhibit lipolysis. BMC biochemistry. 2013;14:13. doi: 10.1186/1471-2091-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dragomir E, Manduteanu I, Voinea M, Costache G, Manea A, Simionescu M. Aspirin rectifies calcium homeostasis, decreases reactive oxygen species, and increases NO production in high glucose-exposed human endothelial cells. Journal of diabetes and its complications. 2004;18:289–299. doi: 10.1016/j.jdiacomp.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Dikshit P, Chatterjee M, Goswami A, Mishra A, Jana NR. Aspirin induces apoptosis through the inhibition of proteasome function. The Journal of biological chemistry. 2006;281:29228–29235. doi: 10.1074/jbc.M602629200. [DOI] [PubMed] [Google Scholar]
- 18.Li YF, Wang X. The role of the proteasome in heart disease. Biochimica et biophysica acta. 2011;1809:141–149. doi: 10.1016/j.bbagrm.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Catalgol B, Grune T. Proteasome and neurodegenerative diseases. Progress in molecular biology and translational science. 2012;109:397–414. doi: 10.1016/B978-0-12-397863-9.00011-0. [DOI] [PubMed] [Google Scholar]
- 20.Crawford LJ, Walker B, Irvine AE. Proteasome inhibitors in cancer therapy. Journal of cell communication and signaling. 2011;5:101–110. doi: 10.1007/s12079-011-0121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. Journal of the American Society of Nephrology : JASN. 2006;17:1807–1819. doi: 10.1681/ASN.2006010083. [DOI] [PubMed] [Google Scholar]
- 22.Calise J, Powell SR. The ubiquitin proteasome system and myocardial ischemia. American journal of physiology Heart and circulatory physiology. 2013;304:H337–H349. doi: 10.1152/ajpheart.00604.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cacciapuoti F. Role of ubiquitin-proteasome system (UPS) in left ventricular hypertrophy (LVH) American journal of cardiovascular disease. 2014;4:1–5. [PMC free article] [PubMed] [Google Scholar]
- 24.Wang F, Lerman A, Herrmann J. Dysfunction of the ubiquitin-proteasome system in atherosclerotic cardiovascular disease. American journal of cardiovascular disease. 2015;5:83–100. [PMC free article] [PubMed] [Google Scholar]
- 25.Goswami SK, Wan D, Yang J, Trindade da Silva CA, Morisseau C, Kodani SD, et al. Antiulcer efficacy of soluble epoxide hydrolase inhibitor TPPU on diclofenac sodium induced intestinal ulcers. The Journal of pharmacology and experimental therapeutics. 2016 doi: 10.1124/jpet.116.232108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomes AV, Zong C, Edmondson RD, Li X, Stefani E, Zhang J, et al. Mapping the murine cardiac 26S proteasome complexes. Circulation research. 2006;99:362–371. doi: 10.1161/01.RES.0000237386.98506.f7. [DOI] [PubMed] [Google Scholar]
- 27.Cui Z, Gilda JE, Gomes AV. Crude and purified proteasome activity assays are affected by type of microplate. Analytical biochemistry. 2014;446:44–52. doi: 10.1016/j.ab.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomes AV, Waddell DS, Siu R, Stein M, Dewey S, Furlow JD, et al. Upregulation of proteasome activity in muscle RING finger 1-null mice following denervation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:2986–2999. doi: 10.1096/fj.12-204495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomes AV, Young GW, Wang Y, Zong C, Eghbali M, Drews O, et al. Contrasting proteome biology and functional heterogeneity of the 20 S proteasome complexes in mammalian tissues. Molecular & cellular proteomics: MCP. 2009;8:302–315. doi: 10.1074/mcp.M800058-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui Z, Hwang SM, Gomes AV. Identification of the immunoproteasome as a novel regulator of skeletal muscle differentiation. Molecular and cellular biology. 2014;34:96–109. doi: 10.1128/MCB.00622-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elsasser S, Schmidt M, Finley D. Characterization of the proteasome using native gel electrophoresis. Methods in enzymology. 2005;398:353–363. doi: 10.1016/S0076-6879(05)98029-4. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh R, Gilda JE, Gomes AV. The necessity of and strategies for improving confidence in the accuracy of western blots. Expert review of proteomics. 2014;11:549–560. doi: 10.1586/14789450.2014.939635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siddiqui MA, Ahmad J, Farshori NN, Saquib Q, Jahan S, Kashyap MP, et al. Rotenone-induced oxidative stress and apoptosis in human liver HepG2 cells. Molecular and cellular biochemistry. 2013;384:59–69. doi: 10.1007/s11010-013-1781-9. [DOI] [PubMed] [Google Scholar]
- 34.Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nature protocols. 2007;2:287–295. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- 35.Spinazzi M, Casarin A, Pertegato V, Salviati L, Angelini C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nature protocols. 2012;7:1235–1246. doi: 10.1038/nprot.2012.058. [DOI] [PubMed] [Google Scholar]
- 36.Buckler KJ, Vaughan-Jones RD. Effects of mitochondrial uncouplers on intracellular calcium, pH and membrane potential in rat carotid body type I cells. The Journal of physiology. 1998;513(Pt 3):819–833. doi: 10.1111/j.1469-7793.1998.819ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chalmers S, McCarron JG. The mitochondrial membrane potential and Ca2+ oscillations in smooth muscle. Journal of cell science. 2008;121:75–85. doi: 10.1242/jcs.014522. [DOI] [PubMed] [Google Scholar]
- 38.Kim YC, Oh EY, Kim SH, Lee MG. Pharmacokinetics of diclofenac in rat model of diabetes mellitus induced by alloxan or steptozotocin. Biopharmaceutics & drug disposition. 2006;27:85–92. doi: 10.1002/bdd.484. [DOI] [PubMed] [Google Scholar]
- 39.Juhlin T, Bjorkman S, Gunnarsson B, Fyge A, Roth B, Hoglund P. Acute administration of diclofenac, but possibly not long term low dose aspirin, causes detrimental renal effects in heart failure patients treated with ACE-inhibitors. European journal of heart failure. 2004;6:909–916. doi: 10.1016/j.ejheart.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Livnat-Levanon N, Kevei E, Kleifeld O, Krutauz D, Segref A, Rinaldi T, et al. Reversible 26S proteasome disassembly upon mitochondrial stress. Cell reports. 2014;7:1371–1380. doi: 10.1016/j.celrep.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh R, Hwang SM, Cui Z, Gilda JE, Gomes AV. Different effects of the nonsteroidal anti-inflammatory drugs meclofenamate sodium and naproxen sodium on proteasome activity in cardiac cells. Journal of molecular and cellular cardiology. 2016;94:131–144. doi: 10.1016/j.yjmcc.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 42.Chirasani SR, Leukel P, Gottfried E, Hochrein J, Stadler K, Neumann B, et al. Diclofenac inhibits lactate formation and efficiently counteracts local immune suppression in a murine glioma model. International journal of cancer Journal international du cancer. 2013;132:843–853. doi: 10.1002/ijc.27712. [DOI] [PubMed] [Google Scholar]
- 43.Kisselev AF, Goldberg AL. Monitoring activity and inhibition of 26S proteasomes with fluorogenic peptide substrates. Methods in enzymology. 2005;398:364–378. doi: 10.1016/S0076-6879(05)98030-0. [DOI] [PubMed] [Google Scholar]
- 44.Mearini G, Schlossarek S, Willis MS, Carrier L. The ubiquitin-proteasome system in cardiac dysfunction. Biochimica et biophysica acta. 2008;1782:749–763. doi: 10.1016/j.bbadis.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Patrono C. Low-dose aspirin in primary prevention: cardioprotection, chemoprevention, both, or neither? European heart journal. 2013;34:3403–3411. doi: 10.1093/eurheartj/eht058. [DOI] [PubMed] [Google Scholar]
- 46.Stowe DF, Camara AK. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxidants & redox signaling. 2009;11:1373–1414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quinlan CL, Orr AL, Perevoshchikova IV, Treberg JR, Ackrell BA, Brand MD. Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. The Journal of biological chemistry. 2012;287:27255–27264. doi: 10.1074/jbc.M112.374629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirst J, King MS, Pryde KR. The production of reactive oxygen species by complex I. Biochemical Society transactions. 2008;36:976–980. doi: 10.1042/BST0360976. [DOI] [PubMed] [Google Scholar]
- 49.Ross JM, Olson L, Coppotelli G. Mitochondrial and Ubiquitin Proteasome System Dysfunction in Ageing and Disease: Two Sides of the Same Coin? International journal of molecular sciences. 2015;16:19458–19476. doi: 10.3390/ijms160819458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiong N, Xiong J, Jia M, Liu L, Zhang X, Chen Z, et al. The role of autophagy in Parkinson's disease: rotenone-based modeling. Behavioral and brain functions: BBF. 2013;9:13. doi: 10.1186/1744-9081-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oaks JL, Gilbert M, Virani MZ, Watson RT, Meteyer CU, Rideout BA, et al. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature. 2004;427:630–633. doi: 10.1038/nature02317. [DOI] [PubMed] [Google Scholar]
- 52.Muralidharan S, Dhananjayan V. Diclofenac residues in blood plasma and tissues of vultures collected from Ahmedabad, India. Bulletin of environmental contamination and toxicology. 2010;85:377–380. doi: 10.1007/s00128-010-0109-7. [DOI] [PubMed] [Google Scholar]
- 53.Shultz S, Baral HS, Charman S, Cunningham AA, Das D, Ghalsasi GR, et al. Diclofenac poisoning is widespread in declining vulture populations across the Indian subcontinent. Proceedings Biological sciences / The Royal Society. 2004;271(Suppl 6):S458–S460. doi: 10.1098/rsbl.2004.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taggart MA, Cuthbert R, Das D, Sashikumar C, Pain DJ, Green RE, et al. Diclofenac disposition in Indian cow and goat with reference to Gyps vulture population declines. Environ Pollut. 2007;147:60–65. doi: 10.1016/j.envpol.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 55.Brune K, Furst DE. Combining enzyme specificity and tissue selectivity of cyclooxygenase inhibitors: towards better tolerability? Rheumatology (Oxford) 2007;46:911–919. doi: 10.1093/rheumatology/kem070. [DOI] [PubMed] [Google Scholar]
- 56.Seifert U, Bialy LP, Ebstein F, Bech-Otschir D, Voigt A, Schroter F, et al. Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell. 2010;142:613–624. doi: 10.1016/j.cell.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 57.Turner PV, Brabb T, Pekow C, Vasbinder MA. Administration of substances to laboratory animals: routes of administration and factors to consider. Journal of the American Association for Laboratory Animal Science : JAALAS. 2011;50:600–613. [PMC free article] [PubMed] [Google Scholar]
- 58.Kudo C, Kori M, Matsuzaki K, Yamai K, Nakajima A, Shibuya A, et al. Diclofenac inhibits proliferation and differentiation of neural stem cells. Biochemical pharmacology. 2003;66:289–295. doi: 10.1016/s0006-2952(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 59.Inoue T, Anai S, Onishi S, Miyake M, Tanaka N, Hirayama A, et al. Inhibition of COX-2 expression by topical diclofenac enhanced radiation sensitivity via enhancement of TRAIL in human prostate adenocarcinoma xenograft model. BMC urology. 2013;13:1. doi: 10.1186/1471-2490-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bort R, Ponsoda X, Jover R, Gomez-Lechon MJ, Castell JV. Diclofenac toxicity to hepatocytes: a role for drug metabolism in cell toxicity. The Journal of pharmacology and experimental therapeutics. 1999;288:65–72. [PubMed] [Google Scholar]
- 61.Chlopcikova S, Psotova J, Miketova P. Neonatal rat cardiomyocytes--a model for the study of morphological, biochemical and electrophysiological characteristics of the heart. Biomedical papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia. 2001;145:49–55. [PubMed] [Google Scholar]
- 62.Bleier L, Drose S. Superoxide generation by complex III: from mechanistic rationales to functional consequences. Biochimica et biophysica acta. 2013;1827:1320–1331. doi: 10.1016/j.bbabio.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 63.Hickey EJ, Raje RR, Reid VE, Gross SM, Ray SD. Diclofenac induced in vivo nephrotoxicity may involve oxidative stress-mediated massive genomic DNA fragmentation and apoptotic cell death. Free radical biology & medicine. 2001;31:139–152. doi: 10.1016/s0891-5849(01)00560-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.