Abstract
Background and Objectives
Patients who undergo liver resection for metastatic colorectal cancer (mCRC) have reported 5-year survivals ranging from 25-50%. The current study updated long-term survival for patients with resected liver metastases treated with adjuvant hepatic arterial infusion (HAI) and systemic (SYS) chemotherapy.
Methods
Updated survival and recurrence free survival for patients treated on four consecutive adjuvant protocols with HAI and SYS from 1991 to 2009. Patients were divided into two groups: those treated on protocols before 2003 and after 2003. Median follow-up for all patients was 11 years.
Results
All 287 patients enrolled in four prospective protocols after liver resection are included. Patients treated before 2003 had a median follow-up of 15 years, 5 and 10-year survivals of 56% [95% CI: 49%-64%] and 40% [95% CI: 32%-47%], respectively, and median survival of 71 months. Patients treated after 2003 had a median follow-up of 9 years, 5 and 10-year survivals of 78% [95% CI: 70%-84%] and 61% [95%CI: 51%-70%], respectively, and median survival has not been reached.
Conclusions
Survival is improving for patients with mCRC who undergo liver resection. These data support the durability of long-term survival in patients who undergo resection followed by adjuvant HAI and SYS therapy.
Keywords: colorectal cancer, liver metastases, adjuvant therapy, hepatic arterial infusion, survival
Synopsis for Table of Contents
The 5-year survival after liver resection for metastatic colorectal cancer is approximately 25-50%. This report suggests an increase in 5- survival of 78% can be obtained for patients who undergo adjuvant hepatic arterial infusion and systemic chemotherapy after liver resection.
Introduction
In the last two decades there has been a significant improvement in survival for patients with metastatic colorectal cancer, with 5-year survivals increasing from 25-39% to approximately 40-50% [1-3]. This improvement is due to many factors, including the development of new systemic chemotherapy agents, such as irinotecan [4] and oxaliplatin [5], and molecular targeted agents including vascular endothelial growth factor (VEGF) inhibitors [6], and epidermal growth factor receptor (EGFR) inhibitors [7,8], as well as improvement in surgical technique [9,10]. However, most patients will recur after hepatic resection, and the majority of these recurrences will be in the liver [11,12].
Hepatic metastases derive blood supply from the hepatic artery, whereas normal liver cells derive blood supply from the portal vein [13]. Infusion of chemotherapy via hepatic arterial infusion (HAI) directly into the hepatic artery exposes the metastases to high drug concentrations [14]. Treatment with HAI after liver resection increased progression free survival (PFS) and hepatic PFS in three of four randomized studies that compared HAI plus systemic (SYS) to SYS chemotherapy alone or to surgery alone [15-19]. In one study where the end-point was 2-year survival, HAI plus SYS versus SYS alone produced a 2-year survival of 85% versus 69% (p=0.02), and a 2-year hepatic PFS of 85% and 50% (p=0.001), respectively [15,19]. We now report a 20-year follow-up on this study [15] as well as an update on three additional prospective trials [20-22] using adjuvant HAI and SYS therapy. To compare our results with other reports in the literature and to determine whether survival has improved over time, patients were divided into two groups: before and after 2003.
Materials and Methods
After approval by the Institutional Review Board (IRB), a pooled analysis of patients enrolled onto four consecutive prospective adjuvant protocols using HAI and systemic after liver resection was conducted. Patients were enrolled from October 1991 to September 2009 at Memorial Sloan Kettering Cancer Center (MSKCC). Patients who enrolled onto protocols initiated after 2009 were not included because of the limited follow-up time.
All patients signed MSKCC IRB approved informed consent before initiation of protocol treatment. Eligible patients had histologically confirmed CRC with resection or ablation of all liver metastases, a Karnofsky performance status ≥ 60% and must have met the following lab values: WBC ≥ 3,000 cells/μL, platelets > 100,000 cells/μL, serum total bilirubin < 1.5 mg/dl. Exclusion factors included presence of extrahepatic disease, prior regional therapy and prior hepatic radiation. Prior systemic chemotherapy was permitted provided the last dose was given more than three weeks before trial entry. Prior bevacizumab had to be more than six weeks before trial entry.
Contrast enhanced computed tomography (CT) scans of the chest, abdomen, and pelvis were required within six weeks of liver resection and hepatic pump placement. Hepatic arterial supply was studied pre-operatively in all patients. Surgical guidelines for pump placement have been previously reported [21,23]. Patients were evaluated after resection according to clinical risk score: positive margins after hepatic resection, number of liver metastases, presence of positive lymph nodes in the primary tumor, size of liver tumors, and elevated pre-operative CEA value [2,24].
Chemotherapy Administration
Patients commenced chemotherapy four to five weeks after surgery. On Day 1 of a five-week cycle, HAI with floxuridine (FUDR) (Bedford Laboratories, Bedford, OH) was infused at a dose of 0.12 mg or 0.14 mg × kg × pump volume (30 mL), divided by pump flow rate (supplied by the pump manufacturer; Codman and Shurtleff, Raynham, MA) for a 14-day infusion. In the first protocol [15], the dose of FUDR was 0.14 mg × kg × pump volume; all other protocols used FUDR 0.12 mg [20-22]. Dexamethasone (dex) was administered concurrently with FUDR [25]. In all protocols, patients received HAI FUDR/dex on Day 1 of each cycle for 14-day infusion.
On Days 15 and 29 of each cycle, SYS chemotherapy was administered and the pump was refilled with 30 mL heparinized saline. The different SYS chemotherapies used with HAI FUDR/dex on the four protocols have been previously published and are shown in Table 1 [15,20-22]. The first protocol used fluorouracil administered as an intravenous bolus of 325 mg/m2, preceded by an infusion of leucovorin (LV) at 200 mg/m2 for five consecutive days [15]. The second phase I/II protocol used irinotecan (CPT) alone with a starting dose of 60 mg/m2 and escalation to 200 mg/m2 [20]. The third protocol used oxaliplatin 85 mg/m2 to 100 mg/m2 (2-hour infusion) given concurrently with LV 400 mg/m2 via a Y-connection, followed by 5-fluouracil (5-FU) 2,000 mg/m2 delivered via an external pump for a 48-hour infusion [21]. In the fourth protocol, patients who had not had prior oxaliplatin received oxaliplatin 85 mg/m2 (2-hour infusion) concurrently with LV 400 mg/m2 via a Y-connection, followed by 5-FU 2,000 mg/m2 delivered via external pump for a 48-hour infusion [22]. Patients who had prior oxaliplatin received CPT 150 mg/m2 (over 30 minutes) with the same does of 5-FU and LV. Patients were randomized to receive bevacizumab at 5 mg/kg given on the days of SYS chemotherapy.
Table 1. Protocol Treatment.
| Protocol Start Year | HAI Pump | Systemic Chemotherapy |
|---|---|---|
|
| ||
| 1991* | FUDR/Dex | 5FU/LV |
| 1998 | FUDR/Dex | CPT |
| 2003 | FUDR/Dex | FOLFOX |
| 2004 | FUDR/Dex | FOLFIRI or FOLFOX +/- Bevacizumab |
Abbreviations: HAI=hepatic arterial infusion; FUDR= floxuridine; Dex= dexamethasone; 5FU/LV= 5-fluouracil/leucovorin; CPT=irinotecan; FOLFOX=5-fluouracil/leucovorin/oxaliplatin; FOLFIRI=5-fluouracil/leucovorin/irinotecan
This 1991 study compared patients treated with HAI and systemic therapy to systemic therapy alone. In this analysis, we only included patients treated in the HAI and systemic therapy arm.
Follow-up scans were performed every three months for the first two years, then every four months for the next two years, then every six months for the fifth year, and then yearly thereafter. Patient outcomes, toxicities, and accrual goals of recurrence-free survival and overall survival were monitored throughout the study by the MSKCC Data and Safety Monitoring Committee.
KRAS Molecular Genotyping
KRAS testing became the standard of care at MSKCC in 2009, and consequently, we only have KRAS information on 106 patients. Description of technique and microdissection of tumor are described in previous manuscripts [26,27].
Statistical Analysis
Overall survival (OS), recurrence free survival (RFS) and hepatic RFS were calculated from the time of liver resection and pump placement until the time of death (for OS) or until the first recurrence or death, whichever came first. Patients who were alive and did not experience the event of interest were censored at the date of last follow-up. Log-rank test was used to compare survival distributions between treatment era (prior to 2003 vs after 2003) and KRAS status. Patients were divided according to the date they entered protocol: those before 2003 and those after 2003. The year 2003 was chosen as the cutoff date because new drugs became available after 2003 and two protocols started after 2003. OS and RFS were estimated according to clinical characteristics, separately for patients treated before 2003 and for patients treated after 2003, using Kaplan-Meier method and survival distributions between clinical characteristics were compared using log-rank test [28,29]. Chi-square test and Wilcoxon Rank-Sum test were used to examine the clinical/pathological characteristics between patients treated prior to 2003 and those treated post-2003. Multivariate Cox regression model was used to examine the survival outcomes (OS and RFS) between patients treated before or after 2003 adjusting for known risk factors such as age, number of lesions (1 vs 2-4 vs 5-7 vs ≥8), clinical risk score (0-2 vs 3-5), surgical margin (0 vs ≤1 cm vs >1 cm), tumor size (<5 cm vs ≥5 cm), positive lymph nodes, synchronous tumor, and prior chemotherapy. All p values were based on 2-tailed statistical analysis and p-value <0.05 was considered to indicate statistical significance. All analyses were performed with SAS version 9.3 (SAS Institute, Cary, North Carolina).
Results
All 287 patients included were enrolled in one of four consecutive trials using adjuvant HAI and systemic therapy after liver resection from October 1991 to September 2009. Patients were planned to receive six months of adjuvant HAI and SYS on the protocols shown in Table 1.
Patients were divided into two groups: those who entered protocol prior to January 2003 (n=169) and those who entered after January 2003 (n=118). The median follow-up among the survivors for the entire group was 11 years [Range: 5.3-22.7 years]. Median follow-up for patients treated before 2003 and after 2003 was 15 years [Range: 12-22 years] and 9 years [Range: 5.3-11.9 years], respectively. Toxicities for each treatment regimen have been reported in previous manuscripts [15,20-22]. The most serious toxicity was biliary sclerosis requiring biliary stenting, which occurred in 3% of all 287 patients. Characteristics of patients treated on the four protocols are listed in Table 2.
Table 2. Patient Characteristics of Four Adjuvant HAI + SYS Protocols.
| HAI + 5FU/LV | HAI + CPT | HAI + FOLFOX | HAI + SYS +/- Bev | |
|---|---|---|---|---|
| Characteristic | Total % of Patients (N=73) | Total % of Patients (N=103) | Total % of Patients (N=38) | Total % of Patients (N=73) |
| Age | ||||
| 22 – 50 | 32 | 23 | 34 | 43 |
| 51 – 74 | 64 | 71 | 63 | 56 |
| ≥ 75 | 4 | 6 | 3 | 1 |
| Gender | ||||
| Male | 60 | 55 | 68 | 52 |
| Female | 40 | 45 | 32 | 48 |
| # Hepatic Metastases | ||||
| 1 | 36 | 38 | 31 | 23 |
| 2 – 4 | 42 | 48 | 53 | 44 |
| 5 – 7 | 19 | 7 | 8 | 22 |
| ≥ 8 | 3 | 7 | 8 | 11 |
| Clinical Risk Score | ||||
| 0 – 2 | 60 | 55 | 63 | 52 |
| 3 – 5 | 40 | 45 | 37 | 48 |
| Margins | ||||
| > 1 cm | 45 | 44 | 38 | 25 |
| ≤ 1 cm | 47 | 52 | 57 | 67 |
| 0 cm | 8 | 4 | 5 | 8 |
| Liver Tumor Size | ||||
| < 5 cm | 64 | 73 | 79 | 82 |
| ≥ 5 cm | 36 | 27 | 21 | 18 |
| Primary Lymph Node Status | ||||
| Negative | 42 | 37 | 37 | 44 |
| Positive | 58 | 63 | 63 | 56 |
| CEA Post-op | ||||
| < 5 ng/ml | 71 | 64 | 84 | 79 |
| ≥ 5 ng/ml | 29 | 36 | 16 | 21 |
| Synchronous Disease | ||||
| No | 58 | 56 | 53 | 36 |
| Yes | 42 | 44 | 47 | 64 |
| Chemotherapy Naïve | ||||
| Yes | 47 | 25 | 29 | 14 |
Abbreviations: CEA=carcinoembryonic antigen; HAI=hepatic arterial infusion; 5FU=5-Fluorouracil; CPT=Irinotecan; Oxal=Oxaliplatin; Bev= Bevacizumab
Overall Survival
The median OS for the entire cohort (n=287) was 100 months [95% CI: 80-170 months]. The median OS for patients treated before 2003 was 71 months [95% CI: 59-89 months] and has not been reached for those treated after 2003 (p<0.01) (Figure 1). The 5 and 10-year OS for the entire cohort was 66% [95% CI: 60%-71%] and 48% [95% CI: 42%-54%], respectively. The 3, 5, and 10-year OS for patients treated before 2003 was 73% [95% CI: 66%-80%], 56% [95% CI: 49%-64%], and 40% [95% CI: 32%-47%], respectively. The 3, 5, and 10-year OS for patients treated after 2003 was 92% [95% CI: 85%-95%], 78% [95% CI: 70%-84%], and 61% [95%CI: 51%-70%], respectively.
Figure 1. Overall Survival According to Treatment Period.

The influence of various clinical characteristics on OS for patients treated before and after 2003 is shown in Table 3. OS was decreased in patients with known poor prognostic characteristics such as high clinical risk score, positive margins after hepatic resection, number of liver metastases, presence of positive lymph nodes in the primary tumor, and elevated post-operative CEA value [2,24,30].
Table 3. Overall Survival for Patients According to Treatment Era.
| Before 2003 n=169 | After 2003 n=118 | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| % | % 5-year OS | % 10-year OS | p | % | % 5-year OS | p | |
|
| |||||||
| Age | .08 | .02 | |||||
|
| |||||||
| 22 – 50 | 27 | 61 | 48 | 38 | 73 | ||
| ≥ 50 | 73 | 55 | 37 | 62 | 81 | ||
|
| |||||||
| Gender | .28 | .58 | |||||
|
| |||||||
| Male | 57 | 56 | 37 | 58 | 80 | ||
| Female | 43 | 58 | 44 | 42 | 76 | ||
|
| |||||||
| # Hepatic Metastases | <.01 | <.01 | |||||
|
| |||||||
| 1 | 38 | 71 | 57 | 25 | 86 | ||
| 2 – 4 | 46 | 57 | 35 | 47 | 84 | ||
| 5 – 7 | 11 | 26 | 16 | 18 | 67 | ||
| ≥ 8 | 5 | 13 | 0 | 10 | 50 | ||
|
| |||||||
| Clinical Risk Score | <.01 | .09 | |||||
|
| |||||||
| 0 – 2 | 59 | 64 | 47 | 54 | 84 | ||
| 3 – 5 | 41 | 47 | 30 | 46 | 70 | ||
|
| |||||||
| Margins (cm) | .08 | <.01 | |||||
|
| |||||||
| > 1 cm | 44 | 66 | 48 | 31 | 91 | ||
| ≤ 1 cm | 50 | 52 | 37 | 63 | 77 | ||
| 0 cm | 6 | 50 | 10 | 6 | 38 | ||
|
| |||||||
| Margins (mm) | .02 | <.01 | |||||
|
| |||||||
| > 1 mm | 84 | 60 | 42 | 86 | 80 | ||
| ≤ 1 mm | 10 | 30 | 30 | 8 | 88 | ||
| 0 mm | 6 | 50 | 10 | 6 | 38 | ||
|
| |||||||
| Size | .32 | .16 | |||||
|
| |||||||
| < 5 cm | 69 | 58 | 42 | 81 | 80 | ||
| ≥ 5 cm | 31 | 54 | 35 | 19 | 70 | ||
|
| |||||||
| Primary Lymph Nodes | .03 | <.01 | |||||
|
| |||||||
| Negative | 40 | 65 | 52 | 40 | 90 | ||
| Positive | 60 | 52 | 32 | 60 | 71 | ||
|
| |||||||
| CEA Post-operative | .05 | .07 | |||||
|
| |||||||
| < 5 ng/ml | 68 | 64 | 44 | 80 | 82 | ||
| ≥ 5 ng/ml | 32 | 43 | 30 | 20 | 63 | ||
|
| |||||||
| Synchronous Disease | .37 | .38 | |||||
|
| |||||||
| No | 58 | 60 | 40 | 41 | 80 | ||
| Yes | 42 | 52 | 39 | 59 | 77 | ||
|
| |||||||
| Prior Chemotherapy | .80 | .45 | |||||
|
| |||||||
| No | 34 | 60 | 42 | 19 | 87 | ||
| Yes | 66 | 55 | 38 | 81 | 76 | ||
The differences in characteristics of patients treated before and after 2003 are shown in Table 4. When the group treated after 2003 was compared to the group treated before 2003, there was a greater number of patients with ≥ 8 metastases (10% versus 5%, p=0.02), a higher percentage with margins ≤1cm or 0 cm (70% versus 56%, p=0.05), and more patients with synchronous disease (59% versus 42%, p<0.01). A lower percentage of patients treated after 2003 were chemotherapy naïve before resection (20% versus 34%, p<0.01) but this factor was not significantly associated with survival.
Table 4. Patient Characteristics Before and After 2003.
| Before 2003 (n=169) | After 2003 (n=118) | p-value | |
|---|---|---|---|
| Characteristics | |||
| Age | 0.01 | ||
| Median (range) | 58 (22-80) | 54 (27-77) | |
| Gender | 0.77 | ||
| Male | 96 (57%) | 69 (57%) | |
| # Hepatic Metastases | 0.02 | ||
| 1 | 65 (39%) | 29 (25%) | |
| 2-4 | 77 (46%) | 56 (48%) | |
| 5-7 | 19 (11%) | 21 (18%) | |
| ≥ 8 | 8 (5%) | 12 (10%) | |
| Clinical Risk Score | 0.46 | ||
| 0-2 | 99 (59%) | 64 (54%) | |
| 3-5 | 70 (41%) | 54 (46%) | |
| Margins (cm) | 0.05 | ||
| > 1 cm | 73 (44%) | 35 (30%) | |
| ≤ 1 cm | 83 (50%) | 74 (63%) | |
| 0 cm | 10 (6%) | 8 (7%) | |
| Margins (mm) | 0.84 | ||
| > 1 mm | 143 (84%) | 100 (85%) | |
| ≤ 1 mm | 16 (10%) | 9 (8%) | |
| 0 mm | 10 (6%) | 8 (6%) | |
| Liver Tumor Size | 0.03 | ||
| < 5 cm | 117 (69%) | 95 (81%) | |
| ≥ 5 cm | 52 (31%) | 23 (19%) | |
| Primary Lymph Node Status | 0.99 | ||
| Positive | 101 (60%) | 70 (60%) | |
| Synchronous Disease | <0.01 | ||
| Yes | 71 (42%) | 70 (59%) | |
| Chemotherapy naïve | <0.01 | ||
| Yes | 57 (34%) | 23 (20%) |
In multivariate analysis, after adjusting for known risk factors for poor prognosis such as age, number of lesions (1 vs 2-4 vs 5-7 vs ≥8), clinical risk score (0-2 vs 3-5), surgical margins (>1cm vs ≤1 cm vs 0 cm), tumor size (<5 cm vs ≥5 cm), positive lymph nodes, synchronous disease, and prior chemotherapy, patients treated after 2003 still had better OS as compared to patients treated prior to 2003 (HR: 0.4 95%CI: 0.3-0.6, p<0.01).
Recurrence Free Survival
The median RFS for the entire group was 20 months [95% CI: 17-27 months], with a 5-year RFS of 38% [95% CI: 32%-43%] and a 10-year RFS of 34% [95% CI: 29%-40%]. Patients treated before 2003 had a median RFS of 19 months [95% CI: 17-28 months] versus 21 months [95% CI: 16-63 months] for patients treated after 2003 (p=0.20). The 3 and 5-year RFS for patients treated before 2003 was 41% [95% CI: 34%-48%] and 35% [95% CI: 28%-42%], respectively, while patients treated after 2003 had 3 and 5-year RFS of 42% [95% CI: 33%-51%] and 41% [95% CI: 35%-50%], respectively (Figure 2).
Figure 2. Recurrence Free Survival According to Treatment Period.

The influence of various clinical characteristics on RFS for patients treated before and after 2003 is shown in Table 5. The same factors associated with OS were also associated with RFS. For instance, a clinical risk score of 0-2 versus 3-5 produced a 10-year survival of 41% versus 17%, respectively, for patients treated before 2003 (<0.001).
Table 5. Recurrence Free Survival According to Treatment Era.
| Before 2003 n=169 | After 2003 n=118 | ||||||
|---|---|---|---|---|---|---|---|
| % | % 5-year RFS | % 10-year RFS | p | % | % 5-year RFS | p | |
| Age | .19 | .26 | |||||
| 22 – 50 | 27 | 43 | 37 | 38 | 33 | ||
| ≥ 50 | 73 | 33 | 29 | 62 | 47 | ||
| Gender | .84 | .94 | |||||
| Male | 57 | 35 | 37 | 58 | 41 | ||
| Female | 43 | 36 | 44 | 42 | 43 | ||
| # Hepatic Metastases | <.001 | .07 | |||||
| 1 | 38 | 51 | 51 | 25 | 58 | ||
| 2 – 4 | 46 | 34 | 25 | 47 | 43 | ||
| 5 – 7 | 11 | 5.3 | 5.3 | 18 | 24 | ||
| ≥ 8 | 5 | 0 | 0 | 10 | 25 | ||
| Clinical Risk Score | <.001 | .40 | |||||
| 0 – 2 | 59 | 44 | 41 | 54 | 44 | ||
| 3 – 5 | 41 | 23 | 17 | 46 | 39 | ||
| Margins (cm) | .14 | .001 | |||||
| > 1 cm | 44 | 40 | 38 | 31 | 60 | ||
| ≤ 1 cm | 50 | 34 | 30 | 63 | 36 | ||
| 0 cm | 6 | 30 | 0 | 6 | 13 | ||
| Margins (mm) | .06 | .02 | |||||
| > 1 mm | 84 | 37 | 34 | 86 | 42 | ||
| ≤ 1 mm | 10 | 23 | 23 | 8 | 55 | ||
| 0 cm | 6 | 30 | 0 | 6 | 13 | ||
| Size | .95 | .16 | |||||
| < 5 cm | 69 | 36 | 31 | 81 | 44 | ||
| ≥ 5 cm | 31 | 35 | 33 | 19 | 30 | ||
| Primary Lymph Nodes | <.01 | .16 | |||||
| Negative | 40 | 47 | 41 | 40 | 49 | ||
| Positive | 60 | 28 | 25 | 60 | 37 | ||
| CEA Post-operative | .05 | .43 | |||||
| < 5 ng/ml | 68 | 38 | 35 | 80 | 43 | ||
| ≥ 5 ng/ml | 32 | 30 | 25 | 20 | 33 | ||
| Synchronous Disease | .08 | .47 | |||||
| No | 58 | 40 | 35 | 41 | 46 | ||
| Yes | 42 | 30 | 27 | 59 | 39 | ||
| Prior Chemotherapy | .47 | .95 | |||||
| No | 34 | 37 | 33 | 20 | 43 | ||
| Yes | 66 | 33 | 28 | 81 | 41 | ||
In multivariate analysis, after adjusting for known risk factors for poor prognosis such as age, number of lesions (1 vs 2-4 vs 5-7 vs ≥8), clinical risk score (0-2 vs 3-5), surgical margin (>1cm vs ≤1 cm vs 0 cm), tumor size (<5 cm vs ≥5 cm), positive lymph nodes, synchronous disease, and prior chemotherapy, there was no statistical significant association between treatment era and RFS (HR: 0.8 95%CI: 0.5-1.02, p=0.07).
Hepatic Recurrence Free Survival
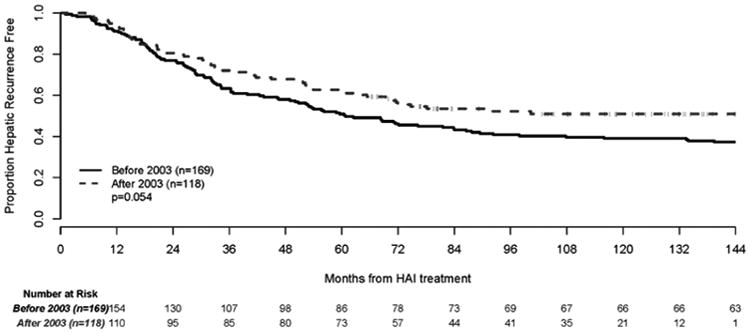
The median hepatic RFS for the entire group was 72 months [95% CI: 60-107 months], and the 3, 5 and 10-year hepatic RFS for the entire group was 66% [95% CI: 61%-72%], 55% [95% CI: 49%-61%] and 43% [95% CI: 38%-50%], respectively. The 3 and 5-year hepatic RFS for the group treated before 2003 was 63% [95% CI: 55%-70%] and 51% [95% CI: 43%-58%], respectively. The 3 and 5-year hepatic RFS for patients treated after 2003 was 72% [95% CI: 63%-79%] and 62% [95% CI: 52%-70%], respectively (p=0.054) (Figure 3).
Figure 3. Hepatic Recurrence Free Survival According to Treatment Period.
KRAS Data
KRAS information was available on 106 patients. The 3 and 5-year OS for KRAS wild-type patients was 96% [95% CI: 89%-98%] and 86% [95% CI: 76%-92%], respectively, while the 3 and 5-year OS for KRAS mutated patients was 89% [95% CI: 70%-96%], and 67% [95% CI: 47%-81%], respectively (p=0.074) (Figure 4). The 3 and 5-year RFS for KRAS wild-type patients were both 49% [95% CI: 37%-59%], whereas the 3 and 5-year RFS for KRAS mutated patients was 32% [95% CI: 16%-50%] and 28% [95% CI: 13%-45%], respectively (p=0.048) (Figure 5).
Figure 4. Overall Survival According to KRAS Status.

Figure 5. Recurrence Free Survival According to KRAS Status.

Discussion
Colorectal cancer is a major cause of cancer death in the United States [31]. Over 50% of patients with colorectal cancer develop liver metastases, which is a major cause of mortality. In the last 50 years the resection of hepatic metastases has become more prevalent in patients with limited hepatic-only disease. Resection of hepatic metastases is associated with prolonged survival and may lead to cure in some patients [3]. The 5-year survival in the older series have been reported between 27-44% [32-34] and in newer series, approximately 50% [35-39]. In a review of literature reporting 10-year survivals for patients after liver resection, 10-year survivals have been reported in the 20-36% range [32-39] (Table 6). Only studies reporting 10-year survival are included in Table 6, with the exception of Nordlinger et al [36] who reported 8-year survival. Some of these reports used systemic chemotherapy after hepatic resection. In this study, all patients received adjuvant HAI plus SYS chemotherapy. With a median follow-up of 11 years, the 5 and 10-year survivals for the entire group was 66% and 48%, respectively. With a median follow-up of 9 years, patients treated after 2003 had 5 and 10-year survivals of 78% and 61%, respectively (Figure 1).
Table 6. Patient Characteristics and Survival after Resection of Colorectal Liver Metastases.
| Patient Characteristics | Overall Survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Year Pts Treated | Pts (n) | Median Follow-up (mos) | Margins < 1 cm (%) | Size > 5cm (%) | Positive LN (%) | > 4 Lesions (%) | Synch disease (%) | % 5-year | %10-year |
| Jamison30 | 1960 – 1987 | 280 | 121 | 44 | 47 | - | 8 | - | 27 | 20 |
| Scheele31 | 1960 – 1992 | 350 | - | 58 | 33* | 62 | 9* | 41 | 39 | 24 |
| Minagawa32 | 1980 – 1997 | 235 | 28 | 50 | 29 | 69 | 28* | 45 | 38 | 26 |
| Hamady33 | 1993 – 2001 | 293 | - | 47** | 32 | 56 | 18 | 50 | 44 | 36 |
| Giuliante34 | 1992 – 2007 | 251 | 37++ | - | 24 | 61 | 13 | 41 | 39 | 24 |
| Nordlinger35 + | 2000 – 2004 | 182 | 36 | - | - | 55 | 0 | 34 | 51 | 30≠ |
| Kemeny | 1991 – 2009 | 287 | 132 | 52 | 23 | 60 | 21 | 49 | 66 | 48 |
| Before 2003 | 1991 – 2002 | 169 | 180 | 47 | 27 | 60 | 16 | 42 | 56 | 40 |
| After 2003 | 2003 – 2009 | 118 | 108 | 61 | 16 | 60 | 28 | 59 | 78 | 61 |
Abbreviations: Pts=patients; LN=lymph nodes; Synch=synchronous; OS=overall survival; – = not reported
only includes 182 patients treated with perioperative chemotherapy (FOLFOX) and not entire group
reported 8-year OS, not 10-year OS
reported as ≥
reported as ≤
reported as mean follow-up
Patients treated after 2003 had a higher 3-year hepatic RFS than patients treated before 2003 (72% versus 63%, respectively), and both groups had a greater hepatic RFS compared to other reports. Regional HAI therapy may protect the liver, which would allow for a greater OS and hepatic RFS. Our favorable survival (78% alive at five years for those treated after 2003) suggests control of hepatic recurrence is important. After complete liver resection, progression in one small lung metastasis can be treated with surgery or radio-frequency ablation (RFA), and may not have an impact on overall survival [40]. Therefore, in evaluating results after liver resection, the location of progression and different treatment interventions may be more important.
Recently, knowledge of molecular changes has been shown to impact survival. Patients with KRAS mutation in both the general colorectal population and in patients who have liver resection tend to have higher recurrence rate and decreased survival [41]. This information was available for patients who entered after 2003. In this report, patients with KRAS wild-type or KRAS mutation had a 5-year OS of 86% versus 67%, and 5-year RFS of 47% versus 28%, respectively.
The longer survival after 2003 is unlikely to be related to better patient characteristics, since the patients treated after 2003 had a higher proportion of adverse prognostic characteristics (Table 4). A potential explanation for this improvement in survival could be more options for systemic chemotherapy and more aggressive treatment at recurrence [5-8,42,43]. After 2003, 37% of KRAS wild-type patients received Cetuximab or Panitumumab after recurrence. Other potential reasons for improvement in survival are better imaging, including intraoperative ultrasound, more extensive salvage surgery [44], and improvement and greater use of ablation techniques [40]. Additionally, recurrences are now treated more aggressively, including second liver resections, lung resections, and even lymph node or abdominal resections.
Conclusions
Historically, the presence of liver metastases from colorectal cancer has been associated with extremely poor survival. Today, in patients who can undergo liver resection followed by adjuvant systemic therapy plus HAI, 5 and 10-year survivals as high as 78% and 61% can be achieved. Our favorable results may reflect selection bias, since these patients had no known extra-hepatic disease and may have had other good characteristics. However, if a comparison is made between patients included in this analysis and patients included in previous studies that report 10-year survival after liver resection (Table 6), the number of patients with poor clinical characteristics is comparable [32-39].
These results suggest that adjuvant HAI therapy in conjunction with modern systemic chemotherapy can improve outcomes. Other centers should be encouraged to design clinical trials using HAI plus systemic therapy after liver resection. Patients with resectable disease should be encouraged to undergo liver resection and to be seen by a multidisciplinary group which includes surgeons, medical oncologists and interventional radiologists.
Acknowledgments
Funding Source: This study was supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
The authors disclose no conflict of interest
Author Contributions: Conception and design: Nancy Kemeny, Michael D'Angelica, Marinela Capanu
Provision of study materials or patients: Michael D'Angelica, William Jarnagin, Peter Kingham, Peter Allen, Ronald DeMatteo
Collection and assembly of data: Marinela Capanu, Joanne Chou, Taryn Boucher
Data analysis and interpretation: Nancy Kemeny, Michael D'Angelica, Peter Allen, Marinela Capanu, Joanne Chou, Taryn Boucher
Manuscript writing: Nancy Kemeny, Taryn Boucher
Final approval of manuscript: All authors
References
- 1.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:4677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–321. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 4.Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 5.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz HI, Fehrenbacher L, Hainsworth JD, et al. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol. 2005;23:3502–3508. doi: 10.1200/JCO.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 8.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 9.Frankel TL, D'Angelica MI. Hepatic resection for colorectal metastases. J Surg Oncol. 2014;109:2–7. doi: 10.1002/jso.23371. [DOI] [PubMed] [Google Scholar]
- 10.Andres A, Majno PE, Morel P, et al. Improved long-term outcome of surgery for advanced colorectal liver metastases: reasons and implications for management on the basis of severity score. Ann Surg Oncol. 2008;15:134–143. doi: 10.1245/s10434-007-9607-1. [DOI] [PubMed] [Google Scholar]
- 11.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 12.de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 13.Ackerman NB. The blood supply of experimental liver metastases. IV. Changes in vascularity with increasing tumor growth. Surgery. 1974;75:589–596. [PubMed] [Google Scholar]
- 14.Ensminger WD, Gyves JW. Clinical pharmacology of hepatic arterial chemotherapy. Semin Oncol. 1983;10:176–182. [PubMed] [Google Scholar]
- 15.Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039–2048. doi: 10.1056/NEJM199912303412702. [DOI] [PubMed] [Google Scholar]
- 16.Kemeny MM, Adak S, Gray B, et al. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy - an intergroup study. J Clin Oncol. 2002;20:1499–1505. doi: 10.1200/JCO.2002.20.6.1499. [DOI] [PubMed] [Google Scholar]
- 17.Lygidakis NJ, Sgourakis G, Vlachos L, et al. Metastatic liver disease of colorectal origin: the value of locoregional immunochemotherapy combined with systemic chemotherapy following liver resection. Results of a prospective randomized study. Hepatogastroenterology. 2001;48:1685–1691. [PubMed] [Google Scholar]
- 18.Lorenz M, Müller HH, Schramm H, et al. Randomized trial of surgery versus surgery followed by adjuvant hepatic arterial infusion with 5-fluorouracil and folinic acid for liver metastases of colorectal cancer. German Cooperative on Liver Metastases (Arbeitsgruppe Lebermetastasen) Ann Surg. 1998;228:756–762. doi: 10.1097/00000658-199812000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemeny NE, Gonen M. Hepatic arterial infusion after liver resection. N Engl J Med. 2005;352:734–735. doi: 10.1056/NEJM200502173520723. [DOI] [PubMed] [Google Scholar]
- 20.Kemeny N, Jarnagin W, Gonen M, et al. Phase I/II study of hepatic arterial therapy with floxuridine and dexamethasone in combination with intravenous irinotecan as adjuvant treatment after resection of hepatic metastases from colorectal cancer. J Clin Oncol. 2003;21:3303–3309. doi: 10.1200/JCO.2003.03.142. [DOI] [PubMed] [Google Scholar]
- 21.Kemeny N, Capanu M, D'Angelica M, et al. Phase I trial of adjuvant hepatic arterial infusion (HAI) with floxuridine (FUDR) and dexamethasone plus systemic oxaliplatin, 5-fluorouracil and leucovorin in patients with resected liver metastases from colorectal cancer. Ann Oncol. 2009;20:1236–1241. doi: 10.1093/annonc/mdn769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kemeny NE, Jarnagin WR, Capanu, et al. Randomized phase II trial of adjuvant hepatic arterial infusion and systemic chemotherapy with or without bevacizumab in patients with resected hepatic metastases from colorectal cancer. J Clin Oncol. 2010;29:884–889. doi: 10.1200/JCO.2010.32.5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kemeny N. Is hepatic infusion of chemotherapy effective treatment for liver metastases? Yes! Important Adv Oncol. 1992:207–227. [PubMed] [Google Scholar]
- 24.Nordlinger B, Guiguet M, Baillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection based on 1568 patients Association Francaise de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 25.Kemeny N, Seiter K, Niedzwiecki D, et al. A randomized trial of intrahepatic infusion of fluorodeoxyuridine with dexamethasone versus fluorodeoxyuridine alone in the treatment of metastatic colorectal cancer. Cancer. 1992;69:327–33. doi: 10.1002/1097-0142(19920115)69:2<327::aid-cncr2820690209>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 26.Yaeger R, Cowell E, Gewirtz A, et al. RAS mutations affect pattern of metastatic spread and increase propensity for brain metastasis in colorectal cancer. Cancer. 2015;121:1195–1203. doi: 10.1002/cncr.29196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janakiraman M, Vakiani E, Zeng Z, et al. Genomic and biological characterization of exon 4 KRAS mutations in human cancer. Cancer Res. 2010;70:5901–5911. doi: 10.1158/0008-5472.CAN-10-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Therneau T, Grambsch P. Modeling Survival Data. New York: Springer; 2000. [Google Scholar]
- 29.Collett D. Modeling Survival Data in Medical Research. 2. London, United Kingdom: Chapman & Hall; 2003. [Google Scholar]
- 30.Are C, Gonen M, Zazzali K, et al. The impact of margins on outcome after hepatic resection for colorectal metastases. Ann Surg. 2007;246:295–300. doi: 10.1097/SLA.0b013e31811ea962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 32.Jamison RL, Donohue JH, Nagorney DM, et al. Hepatic resection for metastatic colorectal cancer results in cure for some patients. Arch Surg. 1997;465:505–510. doi: 10.1001/archsurg.1997.01430290051008. [DOI] [PubMed] [Google Scholar]
- 33.Scheele J, Stang R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 34.Minagawa M, Makucchi M, Torzilli G, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg. 2000;231:487–499. doi: 10.1097/00000658-200004000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamady ZZR, Cameron IC, Wyatt J, et al. Resection margin in patients undergoing hepatectomy for colorectal liver metastases: a critical appraisal of the 1 cm rule. Eur J Surg Oncol. 2006;32:557–563. doi: 10.1016/j.ejso.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Giuliante F, Ardito F, Vellone M, et al. Role of the surgeon as a variable in long-term survival after liver resection for colorectal metastases. J Surg Oncol. 2009;100:538–545. doi: 10.1002/jso.21393. [DOI] [PubMed] [Google Scholar]
- 37.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomized control, phase 3 trial. Lancet Oncol. 2013;14:1208–1215. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 38.Abbas S, Lam V, Hollands M. Ten-year survival after liver resection for colorectal metastases: systematic review and meta-analysis. ISRN Oncol. 2011 doi: 10.5402/2011/763245. Article ID 763245:1-11, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vigano L, Capussotti L, Lapointe Real, et al. Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol. 2014;21:1276–1286. doi: 10.1245/s10434-013-3421-8. [DOI] [PubMed] [Google Scholar]
- 40.Gillams A, Khan Z, Osborn P, et al. Survival after radiofrequency ablation in 122 patients with inoperable colorectal lung metastases. Cardiovasc Intervent Radiol. 2013;36:724–730. doi: 10.1007/s00270-012-0500-3. [DOI] [PubMed] [Google Scholar]
- 41.Diaz-Rubio E, Gomez-Espana A, Massuti B, et al. Role of KRAS status is patients with metastatic colorectal cancer receiving first-line chemotherapy plus bevacizumab: a TTD group cooperative study. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0047345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609–1618. doi: 10.1056/NEJMoa1403108. [DOI] [PubMed] [Google Scholar]
- 43.Douillard JY, Siena S, Cassidy J, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346–1355. doi: 10.1093/annonc/mdu141. [DOI] [PubMed] [Google Scholar]
- 44.Butte JM, Gonen M, Allen PJ, et al. Recurrence after partial hepatectomy for metastatic colorectal cancer: potentially curative role of salvage repeat resection. Ann Surg Oncol. 2015;22:2761–2771. doi: 10.1245/s10434-015-4370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


