Abstract
Cranberry phenolic compounds have been linked to many health benefits. A recent report suggested that cranberry bioactives inhibit adipogenesis in 3T3-L1 adipocytes. Thus, we investigated the effects and mechanisms of the cranberry product (CP) on lipid metabolism using the Caenorhabditis elegans (C. elegans) model. CP (0.016% and 0.08%) dose-dependently reduced overall fat accumulation in C. elegans (N2, wild type) by 43% and 74%, respectively, without affecting its pumping rates or locomotive activities. CP decreased fat accumulation in aak-2 (an ortholog of AMP-activated kinase α) and tub-1 (an ortholog of TUBBY) mutants significantly, but only minimal effects were observed in sbp-1 (an ortholog of sterol response element-binding protein-1) and nhr-49 (an ortholog of peroxisome proliferator-activated receptor-α) mutant strains. We further confirmed that CP downregulated sbp-1, cebp, and hosl-1 (an ortholog of hormone-sensitive lipase homolog) expression, while increasing the expression of nhr-49 in wild-type C. elegans. These results suggest that CP could effectively reduce fat accumulation in C. elegans dependent on sbp-1, cebp, and nhr-49, but not aak-2 and tub-1.
Key Words: : C. elegans, cranberry, fat accumulation, nhr-49, sbp-1
Introduction
Obesity has become one of the leading contributors to a number of chronic illnesses all over the world, such as diabetes, cardiovascular diseases, and hypertension.1 It is well known that excessive intake of food and decreased physical activity are important factors contributing to obesity; however, there are many other factors that contribute to the development of obesity.2 Treatment of obesity using drugs is possible, but rather limited, thus using a food-based approach to control obesity is more desirable.
Cranberries are a well-known food with high phytochemical content of natural antioxidants and have many known health benefits, including prevention of urinary tract infection and chronic diseases, such as diabetes mellitus, cardiovascular diseases, and cancers.3–5 Cranberry products (CPs) are very popular in the United States. More than 8 million barrels of cranberries, with a value of 385.5 million, were produced in the United States in 2012.6 Recently, there were reports of cranberries inhibiting adipogenesis in 3T3-L1 cells.7 However, no reports have investigated the mechanisms of cranberries modulating fat accumulation in animal models.
As a model system to test the effects of food components on obesity, we have used Caenorhabditis elegans (C. elegans). C. elegans has been used intensively in biological and medical studies due to their short life span of 30 days and rapid reproduction cycles.8 Moreover, various mutants of C. elegans are available at minimum cost, which makes it a great in vivo model for cellular, genetic, or behavior studies. It is also known that C. elegans's lipid metabolism is conserved in mammalian cells.9 Therefore, studying antiobesity effects using C. elegans is not only efficient and easier to handle but also provides an ideal model to study the genes involved in the process. In fact, Martorell et al.10 suggested that C. elegans could be a useful in vivo model to investigate as well as screen antiobesity drugs or food bioactives. Thus, we chose C. elegans as an in vivo model to investigate the effect and mechanisms of the CPs on fat accumulation in the current study.
Materials and Methods
Materials
A water-soluble cranberry extract powder standardized to 4% proanthocyanidins (HI-PAC 4.0) was provided by Decas botanical Synergies (Carver, MA, USA). N2, bristal (wild-type); CE541, sbp-1 (ep79); RB754, aak-2 (ok524); DG2179, tub-1 (nr2044); GR1307, daf-16 (mgDf50); RB1716, nhr-49 (ok2165); CE548, sbp-1 (ep79) III; epEx141; and Escherichia coli OP50 were obtained from the Caenorhabditis Genetics Center (CGC, University of Minnesota, Minneapolis, MN, USA). The amounts of triglyceride (TG) and protein were quantified using kits from Thermo Scientific (Middletown, VA, USA) and Bio-Rad Co. (Hercules, CA, USA), respectively. TaqMan gene expression assays used for sbp-1, cebp, hosl-1, atgl-1, nhr-49, and daf-16 were purchased from Applied Biosystems (Carlsbad, CA, USA). Fluorodeoxyuridine (FUDR) and carbenicillin were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Fluoromount-G was from Southern Biotechnology Associates (Birmingham, AL, USA). TRIzol was from Thermo Scientific (Rockford, IL, USA), and other chemicals were purchased from Fisher Scientific (Pittsburgh, PA, USA).
Preparation of cranberry in S-complete solution
The CP was previously reported to contain ∼6.6% phenolic acids, 2.2% flavonoids, and 0.6% anthocyanidins.11 The CP was dissolved in S-complete and filtered through a 0.45-μm membrane to make 24 mg of the CP/mL stock solution (2.4%). Previously, it was reported that 0.2% (2 mg/mL) CP has beneficial effects on C. elegans.12,13 Our preliminary tests showed that in the range of 0.016% (0.16 mg/mL) to 0.2% (2 mg/mL) CP could reduce triacylglyceride in wild-type C. elegans dose-dependently (data not shown). Thus, we chose 0.016% and 0.08% CPs to study the mechanisms underlying the fat reduction effect induced by the CP in the current study.
C. elegans culture
M9 buffer, S-basal, S-complete, and nematode growth media agar used in C. elegans culture were prepared as previously described.14 A synchronous worm culture was obtained using the previously described method.14 All C. elegans strains were raised at 20°C in S-complete media supplemented with the CP in 12-well plates. Treatments started from the L4 stage (3 days old) and treatment periods were 2–4 days with FUDR treatment (DNA synthesis inhibitor15) to prevent eggs from hatching during the treatment period. For experiments with green fluorescent protein (GFP) detection and quantitative reverse transcriptase–polymerase chain reaction, we treated nematodes with CP, which were 1 day old, for 3 days (before eggs were produced). This was done because FUDR treatment during the egg-producing period might influence gene expression levels.16 We further confirmed that effects of CP on TG accumulation (treated when either 1 day old or 3 days old for 2–4 days) were comparable (data not shown).
Triacylglyceride quantification
At the end of the treatment, C. elegans were collected and washed twice with M9 buffer to remove bacteria and S-complete media. C. elegans samples were dissolved in 0.05% Tween 20 solution. After sonication, C. elegans samples were used for the TG and protein measurements. The TG assay was conducted with a commercial assay kit (Infinity™ Triglyceride Reagent; Thermo Scientific) and the protein content was measured with the Bio-Rad DC protein assay kit according to the manufacturer's instructions. TG content was normalized with protein concentration.
Pharyngeal pumping rate and locomotion assay
Food intake was measured by counting the rate of pharyngeal muscle contraction from C. elegans under an optical microscope (Olympus Corporation, Tokyo, Japan).17 Locomotion activity was measured by using the Wormlab tracking system (Allied Vision Technologies, Stadtroda, Germany, and Wormlab Software; MBF Bioscience, Williston, VT, USA) as previously reported with minor modifications.18 Each video used for tracking analysis lasted for 1 min. Data for average moving speed [(forward distance + reverse distance)/time] and the width and length of wild-type C. elegans were collected from the tracking system. Body size and locomotive activity of C. elegans were measured after treatment with CP for both 2 and 4 days.
Detection of GFP-labeled sbp-1 expression
The CE548 strain was washed twice with S-basal solution at 1,000 g for 20 s. Then, C. elegans samples were fixed with 4% paraformaldehyde for 2 h at 4°C. Fixed C. elegans samples were washed thrice with phosphate-buffered saline. The fluoromount-G was put onto a glass slide, followed by addition of the fixed C. elegans. Pictures were taken under confocal microscopy (Nikon microscope D-Eclipse C1 80i; Nikon Corporation, Melville, NY, USA).
mRNA expression analysis
Total RNA was extracted from C. elegans using TRIzol® reagent under RNase-free conditions. Total RNA was reverse transcribed to cDNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Real-time PCR was performed on a StepOne Plus real-time PCR system (Applied Biosystems). Integrated sequences for TaqMan gene expression assays used for sbp-1, cebp, hosl-1, atgl-1, nhr-49, and daf-16 were NM_067071.5, NM_182035.3, NM_001047763.3, NM_171167.4, NM_181998.4, and NM_001264561.1. Threshold values were analyzed using the comparative CT method. The RNA polymerase II large subunit ama-1 gene (NM_068122.6) was used as an internal standard.
Statistical analyses
Data are expressed as means ± standard errors and analyzed with the Statistical Analysis System (SAS Institute, Cary, NC, USA). Differences between groups were assessed with one-way analysis of variance, followed by Tukey's multiple range test. Significance of differences was defined at the P < .05 level.
Results
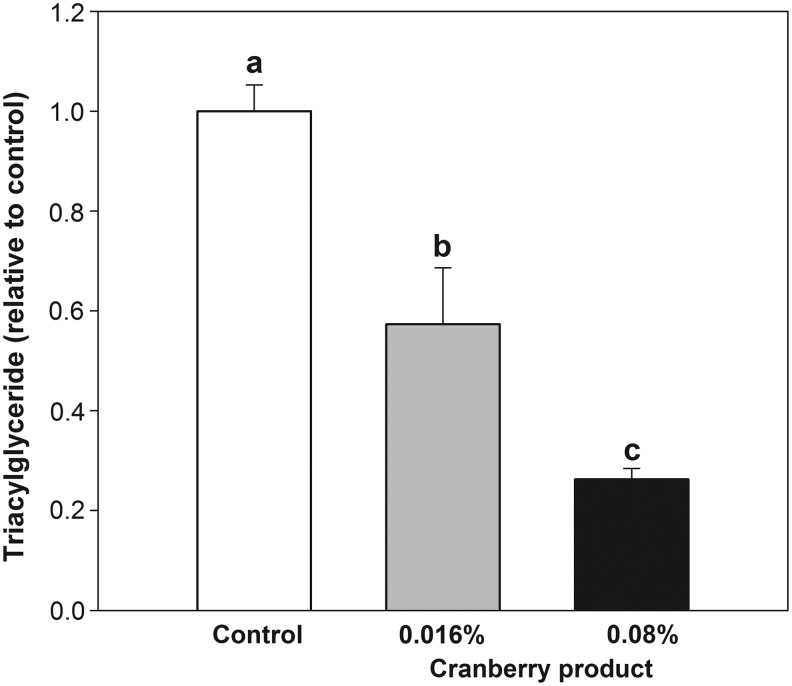
Figure 1 shows the effects of CP on the TG content of wild-type C. elegans. The results showed that the TG content was reduced by treatment with CP in a dose-dependent manner. This is consistent with a previous report that cranberry treatments reduce fat accumulation in 3T3-L1 adipocytes.7
FIG. 1.
Effects of the cranberry product on triacylglyceride accumulation in wild-type (N2) Caenorhabditis elegans. Cranberry treatment of C. elegans started from the L4 stage and treated C. elegans samples were harvested for analysis after a 4-day treatment. Data are expressed as means ± standard errors (n = 3). Means with different letters are significantly different at P < .05.
As it is known that either increased energy intake or decreased energy expenditure can result in increased fat accumulation, we further determined pumping rates (food intake)19 and locomotive activity (energy expenditure) from these nematodes after treatments with the CP (Table 1). Genetic and environmental factors, including food, are also known to affect the body size of C. elegans, which can also influence overall fat accumulation.20 Thus, body sizes after treatment with CP were also measured (Table 1). No significant difference was observed for pumping rates among control and cranberry-treated groups. Cranberry also did not affect the locomotive activity of C. elegans (Table 1). No significant differences were observed for body size between control and CP-treated groups (Table 1). These results suggest that CPs have no influence on food intake, energy expenditure, or body size of C. elegans, suggesting that another mechanism may be responsible for its effect on fat accumulation, such as altering lipid metabolism.
Table 1.
Effects of the Cranberry Product on Pumping Rates, Locomotion Activity, and Body Size of Caenorhabditis elegans
| Body size | ||||
|---|---|---|---|---|
| Pumping rate (times/min) | Speed (μm/s) | Length (μm) | Width (μm) | |
| 2-day treatment | ||||
| Control | 222.6 ± 5.6 | 39.6 ± 2.8 | 542.2 ± 11.5 | 56.0 ± 3.8 |
| Cranberry product | ||||
| 0.016% | 247.8 ± 8.1 | 38.0 ± 2.0 | 522.5 ± 10.3 | 51.9 ± 1.3 |
| 0.08% | 219.3 ± 12.0 | 39.3 ± 2.5 | 513.9 ± 23.1 | 57.1 ± 5.4 |
| 4-day treatment | ||||
| Control | 221.6 ± 7.9 | 31.2 ± 2.0 | 538.9 ± 14.2 | 52.7 ± 1.5 |
| Cranberry product | ||||
| 0.016% | 245.8 ± 14.9 | 31.2 ± 2.2 | 528.2 ± 24.5 | 51.1 ± 2.8 |
| 0.08% | 218.5 ± 14.7 | 34.2 ± 2.0 | 565.8 ± 18.7 | 54.0 ± 1.9 |
Numbers are means ± standard errors (n = 10–15 for pumping rates and n = 15–35 for speed and body size measurements). There are no significant differences between control and the cranberry product treatment group.
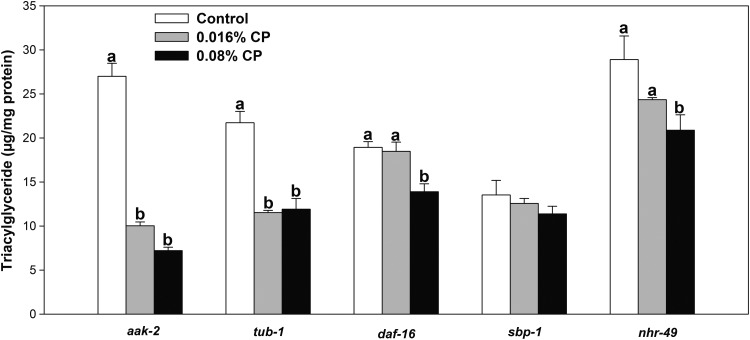
Next, we completed genetic epistasis assays to determine if cranberry compounds influence key genes in lipid metabolism using various available mutants, particularly genes known to be associated with lipid metabolism, such as aak-2, tub-1, sbp-1, nhr-49, or daf-16. The AMP-activated kinase (AMPK) is an important cellular fuel gauge, which responds to the cellular AMP:ATP ratio as well as upstream kinase cascades.21,22 The activation of AMPK promotes energy-generating pathways and inhibits energy-consumptive pathways. AMPK is conserved in C. elegans, and aak-2 is a homolog of α subunit of AMPK in mammals.23 To understand whether aak-2 is involved in the fat accumulation attenuation effect of cranberry in C. elegans, the aak-2 deficiency mutant was studied. These mutants had greater amounts of fat accumulation compared with wild-type animals, suggesting that this is one of the important genes involved in fat metabolism (Fig. 2). CPs (0.016% and 0.08%) significantly reduced fat accumulation in aak-2 mutants (62% and 73% reduction compared with control, respectively), which were not different to the triglyceride reduction trend in wild-type C. elegans (43% and 74%, respectively, compared with control). This suggests that the fat reduction by CP in C. elegans might be independent of aak-2.
FIG. 2.
Effects of the CP on triacylglyceride accumulation in various mutants. CP treatment of C. elegans started from the L4 stage and C. elegans samples were harvested for analysis after a 4-day treatment. Control (white bars) and CPs (gray bars for 0.016% and black bars for 0.08%). Numbers are means ± standard errors (n = 3). Means with different letters are significantly different at P < .05 at each gene. CP, cranberry product.
Tubby is broadly expressed in the central nervous system; mutations in rodent tubby cause adult-onset obesity with insulin resistance.24 Similarly, loss of function in tub-1 in C. elegans (ortholog of Tubby) caused fat accumulation as seen in Figure 2 and previously.25 CPs (0.016% and 0.08%) significantly reduced fat accumulation in the tub-1 deficiency mutant (Fig. 2), suggesting that the fat reduction effect of cranberry in C. elegans might be independent of tub-1.
DAF-16 is known to be involved in lipid metabolism along with other functions.26 DAF-16 is known to be the main target of DAF-2 in C. elegans, which is a homolog to insulin-like receptors in mammals.26 It is reported that daf-2 inhibits DAF-16 (Forkhead family of transcription factors), resulting in shortened life span in C. elegans,25,27 although responses to insulin between mammals and C. elegans are somewhat inconsistent.28,29 To determine whether daf-16 is involved in CP's effect on reduced fat accumulation in C. elegans, we studied the effect of CP on fat accumulation in daf-16 mutant. The TG content of daf-16 mutant is no different from wild type, which might suggest that daf-16 is not critical in regulating overall TG content in C. elegans. We observed that CP decreased TG content in daf-16 mutant at 0.08% only (Fig. 2), and the percentage of fat reduction induced by CP in this mutant was less than that seen in wild-type C. elegans. These results indicate that reduction of TG by CP may not mediate daf-16 in C. elegans.
Next, we tested CP on the sterol response element-binding protein (SREBP) deficiency mutant. SREBP is a key transcriptional regulator of fat and sterol synthesis pathways in mammals30,31 as C. elegans SREBP homolog sbp-1 deletion mutants exhibit significant reductions in fat content (Fig. 2).32 No significant differences between the triglyceride content of the control and cranberry treatment groups in sbp-1 mutants were detected (Fig. 2). This suggests that sbp-1 is an important gene involved in reduced TG accumulation due to cranberry treatment.
The peroxisome proliferator-activated receptor (PPAR)33 family is also known to be a key regulator of fat, cholesterol, and glucose homeostasis. In C. elegans, nhr-49 has similar functions to the PPAR family, particularly subtype α, and increased fatty acid β-oxidation.25,34,35 As expected, there were greater total fat contents in nhr-49 mutant compared with wild type (Fig. 2). When cranberry compounds were used for treatment in nhr-49 mutant, no significant difference was observed between the cranberry treatment groups and the control group in nhr-49 mutant (Fig. 2). These data suggest that cranberry could act on nhr-49 to mediate fat accumulation in C. elegans.
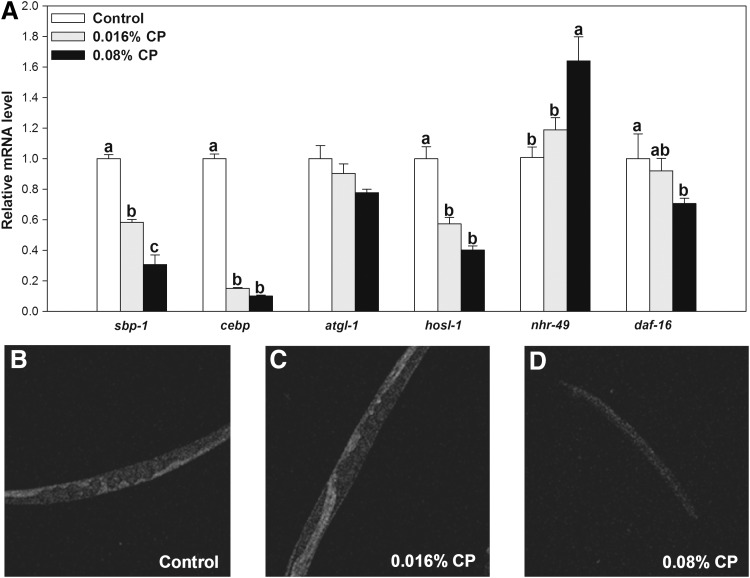
The above data suggest that sbp-1 and nhr-49 genes, but not aak-2, tub-1, and daf-16, may mediate the effects of CP on fat reduction in C. elegans. To further support this conclusion, we measured expression of these genes in wild-type C. elegans (Fig. 3A). CP treatment significantly reduced the expression of sbp-1 and daf-16, while increased nhr-49 gene expression. Reduced sbp-1 was further shown from fluorescence detection of GFP-labeled sbp-1 in CE548 C. elegans (Fig. 3B–D). However, the fluorescence of GFP-labeled sbp-1 expression in the 0.016% CP group (Fig. 3C) apparently was similar to that of control CE548 (Fig. 3B). This is different from the results of sbp-1 expression, which were significantly reduced in wild-type C. elegans after 0.016% CP treatment (Fig. 3A). Since we have not quantified the total fluorescence from these strains, this may not represent the overall effects. Alternatively, this discrepancy might be due to the fact that the CE548 strain is less sensitive to CP treatment than the wild-type strain or the GFP expression method may not be as sensitive as the reverse transcriptase–polymerase chain reaction method.
FIG. 3.
Effects of the CP on sbp-1, cebp, atgl-1, hosl-1, nhr-49, and daf-16 gene expression levels in N2 C. elegans (A). Green fluorescent protein-labeled sbp-1 expression level in CE548 strain: (B) control; (C) 0.016% CP; and (D) 0.08% CP. These images were magnified by 200 times. Cranberry treatment started from the L1 stage and C. elegans samples were harvested for analysis after a 3-day treatment. Numbers are means ± standard errors (n = 3–4). Means with different letters are significantly different at P < .05.
We then determined other genes involved in lipogenesis, cebp (ortholog of C/EBP, required for fat storage36), hosl-1, and atgl-1 (orthologs of hormone-sensitive lipase and adipose triglyceride lipase, respectively, key genes for lipolysis), since mutants for cebp, hosl-1, or atgl-1 are not available currently. CP treatment had significantly inhibited the expression of cebp and hosl-1, but not atgl-1 (Fig. 3A). These results also suggest that CP may inhibit adipogenesis in C. elegans.
Discussion
In this study, we observed that CP dose-dependently reduced triacylglyceride content in wild-type C. elegans without affecting food intake, body size, or locomotive activity. The current results are consistent with others suggesting that CP reduces fat accumulation without adversely influencing other physiological functions.37
The fat reduction by cranberry treatment was completely attenuated by sbp-1 deficiency, while in nhr-49 mutant, the fat reduction effect of cranberry was partly attenuated. Thus, the sbp-1 gene might play a more significant role in the CP's effect on fat reduction compared with nhr-49. Moreover, CP may inhibit fat accumulation by inhibiting cebp. These results are consistent with Kowalska et al.7 where significant effects of CPs on C/EBPα (cebp ortholog) and SREBP (sbp-1 ortholog) were reported in a 3T3-L1 adipocyte model. The current results further suggest that sbp-1 may play a greater role compared with nhr-49 on CP's effect on overall fat accumulation.
In addition to being involved in life span regulation, daf-16 is reported to regulate fat accumulation in C. elegans.38 However, no differences of triglyceride contents between wild-type and daf-16 mutants were observed in the current study. Although we did not determine overall fat contents of wild-type and daf-16 mutants at the same study, we observed the relatively small difference on overall fat contents between experiments (5% of difference between studies). This discrepancy might be, in part, due to different quantification methods used (Triglyceride kit measurement in the current study versus Nile Red O staining in previous). Alternatively, the current results suggest that daf-16 may not contribute to overall TG accumulation. It is also necessary to point out that CP decreased gene expression of daf-16 in the current study, which is different from a previous publication (0.2% CP in water).37 Based on these observations, we can infer that daf-16 is unlikely to contribute to CP's effect on fat accumulation; however, daf-16 may play an important role in other functions of CP, such as its effect on life span. Therefore, additional studies are needed to clarify the role of daf-16 in C. elegans.
Unlike Kowalska et al.7 who reported increased lipolysis by CP in 3T3-L1 adipocytes, the current results with decreased expression of hosl-1, but not atgl-1, are inconsistent effects of CP on lipolysis. It is possible that there might be other mechanisms, such as post-translational regulation of these genes39,40 or sirt (SIRT1 ortholog), responsible for the CP's fat reduction effect, which we have not explored in the current study.41
The CP (HI-PAC 4.0) used was reported to contain ∼6.6% phenolic acids, 2.2% flavonoids, 0.6% anthocyanidins, 38% sugars, and 4.1% dietary fiber.11,42 Several components in CPs were previously reported to have antiobesity functions. First, quercetin, one flavonoid found in CP, was found to inhibit adipogenesis by activation of AMPK genes and downregulating the expression of C/EBPα and SREBP-1.34,43,44 The current results suggest that cranberry bioactive may influence C/EBPα and SREBP-1, but not AMPK.45,46 This inconsistency may derive from differences of CPs used (pure quercetin vs. mixture of compounds in CP), doses of CPs used, or models used (such as potential different sensitivity to AMPKα between C. elegans and mammals).
Next, anthocyanins, another flavonoid found in CP, were also reported to have potential antiadipogenic effects.47 Anthocyanins from purple corn reduced high-fat diet-induced weight gain, but also decreased white and brown adipose tissue weights significantly in mice.47 These effects were further supported by the finding that purple corn reduced the mRNA levels of enzymes involved in fatty acid and triacylglycerol synthesis, as well as the SREBP-1 mRNA level in white adipose tissue.47
Pterostilbene, a stilbenoid found in cranberry, was reported to activate PPAR-α, an nhr-49 homolog. NHR-49 serves a similar function to mammalian PPARα, which modulates pathways controlling the increased fatty acid β-oxidation and decreased triglyceride content in the liver and lowers plasma lipid levels when fed to hamsters.48 Consistently, it has also been reported that deletion mutation of nhr-49 causes a higher fat content in C. elegans.35 It is possible that pterostilbene in CP may have contributed to the current results of CP and nhr-49, which needs to be further confirmed.
Dietary fiber, which exists in the CP, is also reported to reduce fat accumulation in C. elegans.8 Since the CP used contained ∼4.1% fiber, the final concentrations of fiber in current experiments were 0.0007% and 0.003%, respectively. Thus, we speculate that fiber in the CP used has a minimal effect in C. elegans on fat accumulation; however, we cannot rule out the possibility that fiber present in the CP used played a role in the overall effects of CP in the current study. In addition to fiber, there are about 38% sugars, mainly fructose and glucose (per Naturex; South Hackensack, NJ, USA), in the CP, resulting in final sugar concentrations of 0.006% and 0.03% in the treatment used, respectively. Previously, we determined the role of glucose on fat accumulation in C. elegans and found that 0.5% glucose supplemented with C. elegans had no effect on its triacylglyceride content, while 1% glucose increased triacylglyceride content in C. elegans (data not shown). Thus, it is unlikely that sugars in the CP used have a significant effect on C. elegans fat accumulation.
Compared with rodent models, C. elegans have unique advantages. The short life span, rapid reproduction cycles, and large brood sizes, as well as various available mutants, allow for a variety of cellular, molecular, genetic, and behavioral analyses. Core fat metabolic pathways are conserved in C. elegans as many fat regulatory pathways found in C. elegans play similar roles in mammals.50 Thus, C. elegans is a great in vivo model for obesity research.
In conclusion, the current results conclude that the reduction of fat accumulation by CP is dependent on sbp-1 and nhr-49, while independent of aak-2 and tub-1. CP might also regulate adipogenesis in C. elegans, as shown by the decreased cebp gene expression after CP treatment. The current findings may provide evidence to promote the application of cranberries as natural products in the prevention and treatment of obesity.
Acknowledgments
This material is based upon work supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, the Massachusetts Agricultural Experiment Station, and the Department of Food Science, the University of Massachusetts Amherst, under project number MAS00450. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the USDA or NIFA. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). The China Scholarship Council supported Mr. Quancai Sun and Ms. Peiyi Shen. The authors thank Dr. Jolene Zheng (Department of Food Science, Louisiana State University Agricultural Center, Baton Rouge, LA, USA) and Dr. Dan Chase (University of Massachusetts) for help on some experiments. The authors thank Ms. Jayne M. Storkson for help in preparing the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Mark DH: Deaths attributable to obesity. JAMA 2005;293:1918–1919 [DOI] [PubMed] [Google Scholar]
- 2.Kopelman PG: Obesity as a medical problem. Nature 2000;404:635–643 [DOI] [PubMed] [Google Scholar]
- 3.Zafra-Stone S, Yasmin T, Bagchi M, et al. : Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res 2007;51:675–683 [DOI] [PubMed] [Google Scholar]
- 4.Reed J: Cranberry flavonoids, atherosclerosis and cardiovascular health. Crit Rev Food Sci Nutr 2002;42:301–316 [DOI] [PubMed] [Google Scholar]
- 5.Neto CC, Amoroso JW, Liberty AM: Anticancer activities of cranberry phytochemicals: An update. Mol Nutr Food Res 2008;52 Suppl 1:S18–S27 [DOI] [PubMed] [Google Scholar]
- 6.Geisler M: Cranberries Profile. http://agmrc.org/commodities__products/fruits/cranberries-profile/ (accessed October2015)
- 7.Kowalska K, Olejnik A, Rychlik J, Grajek W: Cranberries (Oxycoccus quadripetalus) inhibit adipogenesis and lipogenesis in 3T3-L1 cells. Food Chem 2014;148:246–252 [DOI] [PubMed] [Google Scholar]
- 8.Zheng J, Enright F, Keenan M, et al. : Resistant starch, fermented resistant starch, and short-chain fatty acids reduce intestinal fat deposition in Caenorhabditis elegans. J Agric Food Chem 2010;58:4744–4748 [DOI] [PubMed] [Google Scholar]
- 9.Spiegelman BM, Flier JS: Obesity and the regulation of energy balance. Cell 2001;104:531–543 [DOI] [PubMed] [Google Scholar]
- 10.Martorell P, Llopis S, Gonzalez N, et al. : Caenorhabditis elegans as a model to study the effectiveness and metabolic targets of dietary supplements used for obesity treatment: The specific case of a conjugated linoleic acid mixture (Tonalin). J Agric Food Chem 2012;60:11071–11079 [DOI] [PubMed] [Google Scholar]
- 11.Palikova I, Vostalova J, Zdarilova A, et al. : Long-term effects of three commercial cranberry products on the antioxidative status in rats: A pilot study. J Agric Food Chem 2010;58:1672–1678 [DOI] [PubMed] [Google Scholar]
- 12.Guha S, Natarajan O, Murbach CG, et al. : Supplement timing of cranberry extract plays a key role in promoting Caenorhabditis elegans healthspan. Nutrients 2014;6:911–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinh J, Angeloni JT, Pederson DB, et al. : Cranberry extract standardized for proanthocyanidins promotes the immune response of Caenorhabditis elegans to Vibrio cholerae through the p38 MAPK pathway and HSF-1. PLoS One 2014;9:e103290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solis GM, Petrascheck M: Measuring Caenorhabditis elegans life span in 96 well microtiter plates. J Vis Exp 2011;(49):pii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angeli S, Klang I, Sivapatham R, et al. : A DNA synthesis inhibitor is protective against proteotoxic stressors via modulation of fertility pathways in Caenorhabditis elegans. Aging 2013;5:759–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uchikubo Y, Hasegawa T, Mitani S, Kim HS, Wataya Y: Mechanisms of cell death induced by 5-fluoro-2′-deoxyuridine (FUdR)—necrosis or apoptosis after treated with FUdR. Nucleic Acids Res Suppl 2002:245–246 [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson DS, Taylor RC, Dillin A: Analysis of aging in Caenorhabditis elegans. Methods Cell Biol 2012;107:353–381 [DOI] [PubMed] [Google Scholar]
- 18.Omura DT, Clark DA, Samuel ADT, Horvitz HR: Dopamine signaling is essential for precise rates of locomotion by C. elegans. PLoS One 2012;7:e38649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez-Amaro RL, Valentine ER, Carretero M, et al. : Measuring food intake and nutrient absorption in Caenorhabditis elegans. Genetics 2015;200:443–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.So S, Miyahara K, Ohshima Y: Control of body size in C. elegans dependent on food and insulin/IGF-1 signal. Genes Cells 2011;16:639–651 [DOI] [PubMed] [Google Scholar]
- 21.Kahn BB, Alquier T, Carling D, Hardie DG: AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005;1:15–25 [DOI] [PubMed] [Google Scholar]
- 22.Lindsley JE, Rutter J: Nutrient sensing and metabolic decisions. Comp Biochem Physiol B Biochem Mol Biol 2004;139:543–559 [DOI] [PubMed] [Google Scholar]
- 23.Curtis R, O'Connor G, DiStefano PS: Aging networks in Caenorhabditis elegans: AMP-activated protein kinase (aak-2) links multiple aging and metabolism pathways. Aging Cell 2006;5:119–126 [DOI] [PubMed] [Google Scholar]
- 24.Carroll K, Gomez C, Shapiro L: Tubby proteins: The plot thickens. Nat Rev Mol Cell Biol 2004;5:55–63 [DOI] [PubMed] [Google Scholar]
- 25.Ashrafi K, Chang FY, Watts JL, et al. : Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 2003;421:268–272 [DOI] [PubMed] [Google Scholar]
- 26.Hansen M, Flatt T, Aguilaniu H: Reproduction, fat metabolism, and life span: What is the connection? Cell Metab 2013;17:10–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin K, Hsin H, Libina N, Kenyon C: Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet 2001;28:139–145 [DOI] [PubMed] [Google Scholar]
- 28.Lee RY, Hench J, Ruvkun G: Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr Biol 2001;11:1950–1957 [DOI] [PubMed] [Google Scholar]
- 29.Pierce SB, Costa M, Wisotzkey R, et al. : Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev 2001;15:672–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rawson RB: The SREBP pathway—insights from insigs and insects.(Report). Nat Rev Mol Cell Bio 2003;4:631–640 [DOI] [PubMed] [Google Scholar]
- 31.Eberle D, Hegarty B, Bossard P, Ferre P, Foufelle F: SREBP transcription factors: Master regulators of lipid homeostasis. Biochimie 2004;86:839–848 [DOI] [PubMed] [Google Scholar]
- 32.Yang F, Vought BW, Satterlee JS, et al. : An ARC/mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature 2006;442:700–704 [DOI] [PubMed] [Google Scholar]
- 33.Bonmatin JM, Giorio C, Girolami V, et al. : Environmental fate and exposure; neonicotinoids and fipronil. Environ Sci Pollut Res 2015;22:35–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atherton HJ, Jones OA, Malik S, Miska EA, Griffin JL: A comparative metabolomic study of NHR-49 in Caenorhabditis elegans and PPAR-alpha in the mouse. FEBS Lett 2008;582:1661–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Gilst MR, Hadjivassiliou H, Jolly A, Yamamoto KR: Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol 2005;3:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKay RM, McKay JP, Avery L, Graff JM: C. elegans: A model for exploring the genetics of fat storage. Dev Cell 2003;4:131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guha S, Cao M, Kane RM, et al. : The longevity effect of cranberry extract in Caenorhabditis elegans is modulated by daf-16 and osr-1. Age 2013;35:1559–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashrafi K: Obesity and the regulation of fat metabolism. WormBook 2007:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krintel C, Morgelin M, Logan DT, Holm C: Phosphorylation of hormone-sensitive lipase by protein kinase A in vitro promotes an increase in its hydrophobic surface area. FEBS J 2009;276:4752–4762 [DOI] [PubMed] [Google Scholar]
- 40.Xie X, Langlais P, Zhang X, et al. : Identification of a novel phosphorylation site in adipose triglyceride lipase as a regulator of lipid droplet localization. Am J Physiol Endocrinol Metab 2014;306:E1449–E1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerhart-Hines Z, Rodgers JT, Bare O, et al. : Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J 2007;26:1913–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Synergies DB: HI-PAC 4.0 Nutritional Analysis. http://decascranberry.com/sites/default/files/Hi-PAC%204%200%20Nutritional%20Analysis%206175_0.pdf (accessed October2015)
- 43.Yang JY, Della-Fera MA, Rayalam S, et al. : Enhanced inhibition of adipogenesis and induction of apoptosis in 3T3-L1 adipocytes with combinations of resveratrol and quercetin. Life Sci 2008;82:1032–1039 [DOI] [PubMed] [Google Scholar]
- 44.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM: Transcriptional regulation of adipogenesis. Genes Dev 2000;14:1293–1307 [PubMed] [Google Scholar]
- 45.Ahn J, Lee H, Kim S, Park J, Ha T: The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem Biophys Res Commun 2008;373:545–549 [DOI] [PubMed] [Google Scholar]
- 46.Lee YK, Lee WS, Kim GS, Park OJ: Anthocyanins are novel AMPKalpha1 stimulators that suppress tumor growth by inhibiting mTOR phosphorylation. Oncol Rep 2010;24:1471–1477 [DOI] [PubMed] [Google Scholar]
- 47.Tsuda T, Horio F, Uchida K, Aoki H, Osawa T: Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J Nutr 2003;133:2125–2130 [DOI] [PubMed] [Google Scholar]
- 48.Rimando AM, Nagmani R, Feller DR, Yokoyama W: Pterostilbene, a new agonist for the peroxisome proliferator-activated receptor α-isoform, lowers plasma lipoproteins and cholesterol in hypercholesterolemic hamsters. J Agric Food Chem 2005;53:3403–3407 [DOI] [PubMed] [Google Scholar]
- 49.Smith U: Dietary fibre, diabetes and obesity. Int J Obes 1987;11 Suppl 1:27–31 [PubMed] [Google Scholar]
- 50.Mullaney BC, Ashrafi K: C. elegans fat storage and metabolic regulation. Biochim Biophys Acta 2009;1791:474–478 [DOI] [PMC free article] [PubMed] [Google Scholar]