Abstract
Cancers figure among the leading causes of morbidity and mortality worldwide. The number of new cases is expected to rise by about 70% over the next 2 decades. Development of novel therapeutic agents is urgently needed for clinical cancer therapy. Alphavirus M1 is a Getah-like virus isolated from China with a genome of positive single-strand RNA. We have previously identified that alphavirus M1 is a naturally existing oncolytic virus with significant anticancer activity against different kinds of cancer (e.g., liver cancer, bladder cancer, and colon cancer). To support the incoming clinical trial of intravenous administration of alphavirus M1 to cancer patients, we assessed the safety of M1 in adult nonhuman primates. We previously presented the genome sequencing data of the cynomolgus macaques (Macaca fascicularis), which was demonstrated as an ideal animal species for virus infection study. Therefore, we chose cynomolgus macaques of either sex for the present safety study of oncolytic virus M1. In the first round of administration, five experimental macaques were intravenously injected with six times of oncolytic virus M1 (1 × 109 pfu/dose) in 1 week, compared with five vehicle-injected control animals. The last two rounds of injections were further completed in the following months in the same way as the first round. Body weight, temperature, complete blood count, clinical biochemistries, cytokine profiles, lymphocytes subsets, neutralizing antibody, and clinical symptoms were closely monitored at different time points. Magnetic resonance imaging was also performed to assess the possibility of encephalitis or arthritis. As a result, no clinical, biochemical, immunological, or medical imaging or other pathological evidence of toxicity was found during the whole process of the study. Our results in cynomolgus macaques suggested the safety of intravenous administration of oncolytic virus M1 in cancer patients in the future.
Introduction
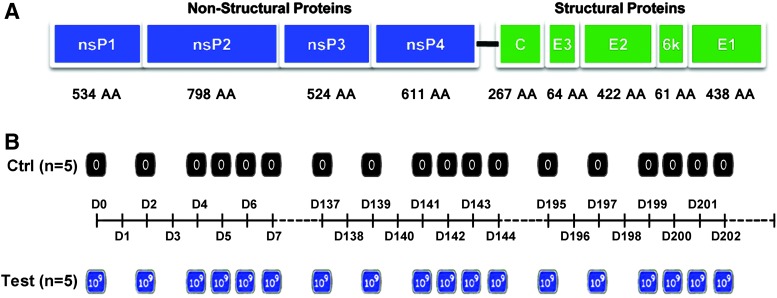
M1 is a strain of Getah-like alphavirus that was isolated from Hainan province in China. As shown in Fig. 1A, its genome is a positive single-strand RNA, which encodes several structural and nonstructural proteins.1 In the previous study, we identified and characterized alphavirus M1 as a naturally existing oncolytic virus for the reason that M1 virus can selectively replicate in cancer cells and destroy them without causing excessive damage to normal tissues.2,3 Most oncolytic viruses are derived from genetically engineered pathogenic viruses. Despite an impressive number of preclinical studies and valuable clinical trials confirming the general safety of the oncolytic viral therapy, the oncolytic virus still retained the capability to reproduce. Therefore, research on the safety of oncolytic viruses are extremely important.
Figure 1.
Safety study of oncolytic virus M1. (A) Genome of alphavirus M1. (B) Injection interval for the safety study of alphavirus M1. Time is depicted in days. Test, five cynomolgus macaques injected with three rounds of intravenous injections with oncolytic virus M1 (109 pfu in 20 ml); control, five cynomolgus macaques injected with the same volume of vehicle in the same schedule.
In the previous study, we used in vivo mouse modelsa to confirm the oncolytic efficacy and safety of oncolytic virus M1. Our data indicated that after intravenous (i.v.) injection, oncolytic virus M1 can target tumor cells such as liver cancer and melanoma for effective and safe lysis, and confirmed the feasibility of i.v. dosing delivery route for further study. Further, this kind of tumor targeting seems likely to be related to the malignant feature of cancer cell, but not the type of tissues from which the cancer derived. For all the kinds of normal tissue, on which there is no significant tropism of the oncolytic virus M1 that can be found, compared with cancer cells the kind of normal tissue does not matter. These results from mice suggested that the application of oncolytic virus M1 would be promising. Nevertheless, the rodent's anatomy, physiology, and pathology are far from our humankind's after all. To better guide clinical trials in the future, we decided to carry out safety experiments in cynomolgus macaques.
Additionally, we have already sequenced the genome of cynomolgus macaques (Macaca fascicularis), which is used extensively in medical experiments, in particular those connected with viral disease.4 Because of their close physiology, they can share some infections with humans. Many groups have reported the use of cynomolgus macaques as infection models in the study of alphaviruses5,6 or oncolytic viruses.7–9 However, using cynomolgus macaques as an animal model in safety study of naturally existing oncolytic virus, especially oncolytic alphavirus, has not been reported.
By comparing the test group and control group of cynomolgus macaques, we conducted various kinds of experiments, including basic biological indicators (body weight, temperature), observation of clinical symptoms, blood routine examination, peripheral blood lymphocytes classification, serum cytokine examination, blood biochemical examination, magnetic resonance imaging (MRI), and titration of virus or neutralizing antibody, to evaluate the potential adverse effects of the multiple rounds of repeated i.v. oncolytic virus M1 injections on nonhuman primates.
Materials and Methods
Ethics statement
The experimental protocol was reviewed and approved by the animal experimentation ethics review committee of Sun Yat-sen University.
Animals
Ten captive-bred 4–6-year-old male- or female-specific pathogen-free cynomolgus macaques (M. fascicularis) weighing between 3.5 and 6 kg were obtained from Kangda Experiment Animal Technology Company before the beginning of the study. Animals were housed and maintained at the animal facility of the Kangyuan Experiment Animal Technology Co. according to regulations and guidelines set forth by the animal experimentation ethics review committee of Sun Yat-sen University. Cynomolgus macaques were examined by attending veterinarians during the 6-week institutional quarantine and were found to be healthy and seronegative for B virus, SIV, STLV, SRV, dengue, and alphavirus M1. Five randomly selected macaques (with different animal IDs as 092604C, 092614C, 092649C, 102737C, and 092725C) were treated with oncolytic virus M1; the left five ones (with different animal IDs as 102706C, 102902C, 093052C, 102705C, and 093135C) were subjected to the control group–receiving vehicle after the same schedule as the virus injections in the test group. All animals were bled 2 days before viral injection to confirm the absence of neutralizing antibodies.
Cell lines and viruses
Oncolytic virus M1 was grown in Vero cells. Virus titer was determined by TCID50 assay using BHK-21 cells and converted to pfu as described previously.10 The variant of M1 in this study was described previously.11 African green monkey kidney (Vero) and baby hamster kidney (BHK-21) cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics, and were incubated in a humidified environment with 5% CO2 at 37°C. After propagation in Vero cells, oncolytic virus M1 was stored at −70°C. Immediately before administration, the virus was thawed. Injections for the test or control group were kept on wet ice throughout. Syringes were loaded with enough viruses (test) or vehicle (control) for a single dose before injection. Excess active viral dilutions were titrated to be 109 pfu in BHK-21 cells after dosing for confirmation of the dose administered. All medium and supplements were purchased from Gibco (Life Technologies).
Clinical signs
Macaques were monitored daily by facility veterinary technicians and animal caretakers for potentially clinical signs of alphavirus disease, including fever, pain as evidenced by reduced mobility, joint swelling, nose/gum bleeding, paresis, paralysis, and other neurological disorders, and routine examinations included monitoring body weight and taking rectal temperature. In addition, trained study personnel assessed macaques daily for activity level, diet status, posture, facial symmetry, gait, behavior, motor function, level of consciousness, and so on.
Samples collection and processing
All blood draws and sample collections were performed under sedation, using ketamine (15 mg/kg) and xylazine (0.5–8.0 mg/kg), administered intramuscularly. Peripheral blood samples were collected daily by venipuncture. Whole-blood samples were collected with ethylenediaminetetraacetic acid (EDTA)-impregnated blood tubes for blood cell counts using an automated cell counter (Life technologies) or for flow cytometry examination. Serum samples were spun down from blood samples containing no anticoagulant, and frozen at −80°C until assayed for virus titration, quantitative real-time polymerase chain reaction (qRT-PCR), neutralization assay, cytokine profiles, and blood biochemistry studies of various indexes, including creatine kinase-MB (CK-MB), aspartate aminotransferase (AST), alanine transaminase (ALT), albumin (ALB), globulin (GLB), alkaline phosphatase (ALP), cholesterol (CHOL), total bilirubin (TBIL), blood urea nitrogen (BUN), and creatinine (Cr).
Hematology
White blood cell and differential cell counts, including lymphocytes, neutrophils, eosinophils, and monocytes, were measured from whole-blood samples using an automated hematology analyzer (Sysmex).
Quantitative real-time polymerase chain reaction
Total RNA was extracted using TRIzol (Life Technologies), and reverse transcription was performed from 3 μg total RNA using gene-specific primer and RevertAid Reverse Transcriptase (Thermo Scientific) according to the supplier's instructions. Quantitative PCR was performed with SuperReal PreMix SYBR Green (Tiangen) using an Applied Biosystems 7500 Fast Real-Time PCR System (Life Technologies). Relative cDNA level was calculated by comparative CT (cycle threshold) method. PCR primers for the M1 genome sequence in the gene of NS1 are 5′GTTCCAACAGGCGTCACCATC3′ (sense) and 5′ACACATTCTTGTCTAGCACAGTCC3′ (antisense).
Serum cytokine levels
Serum samples collected at indicated time points were examined for cytokine profiles. The concentrations of cytokines in serum were determined with human enzyme-linked immunoassay (ELISA) kits as per the instructions of the manufacturer (Beijing North Institute of Biological Technology).
Flow cytometry
Blood samples (2 ml) collected from cynomolgus macaques were centrifuged, and cells were resuspended in 100 μl phosphate-buffered saline. Resuspended cells (50 μl) were then incubated at room temperature for 15 min with antimacaque CD3-APC, CD4-FITC, CD19-PE, CD56-PE, FoxP3-PE, and CD8-PE-Cy7 monoclonal antibodies before running on the FACScan (BD Biosciences) using CellQuest software to estimate lymphocyte proportion. All the antibodies were purchased from BD Biosciences and used according to the instruction from the manufacturer.
Neutralization assay
Macaque sera were diluted with PBS in a volume of 50 μl in serial two-fold dilutions in triplicate in 96-well plates. A total of 50 μl of oncolytic virus M1 (approximately 300 TCID50) in Opti-MEM I reduced-serum medium was added to the diluted serum in each well. The 96-well plates containing serum and virus were incubated at 37°C for 1 hr. Approximately 4000 Vero cells in 50 μl in Opti-MEM were then added to each well. The plates were incubated at 37°C with 5% CO2 for 2 hr. An amount of 50 μl of DMEM supplemented with 10% FBS was added to each well. The plates were incubated at 37°C with 5% CO2 for 2–3 days. Neutralizing titers are given as the reciprocal of the highest dilutions that neutralized the virus activity by 50%.
Magnetic resonance imaging
Before the cease of study, MRIs of the head and knee were performed on all cynomolgus macaques. Subjects were sedated with ketamine (15 mg/kg) and xylazine (0.5–8.0 mg/kg), administered intramuscularly. Atropine (0.5 mg/kg) was administered intravenously, and the animals were intubated, catheterized, and placed on a heat pad. Macaques were maintained on 1–3% isoflurane anesthesia during the procedure. A 1.5T Signa MRI whole-body MRI scanner (GE Healthcare) with an extremity coil was used for image acquisition. Sagittal images were performed with a slice thickness of 4 mm, and axial images were performed with slice thickness of 3 mm. All subject images were analyzed digitally.
Statistical analysis
All statistical analyses were done using SPSS 19.0 software. Most of the data were analyzed by Student's t-test. Repeated measures ANOVA was generally not used because of the relatively large variation among macaques during the time course and limit of detection, which precluded reliable statistical analyses. All error bars indicate SD. The difference was considered significant when the p value was less than 0.05.
Results
The schedule of administration and examination
In keeping with previous studies in vivo in mice and the incoming studies in clinical trials, we used i.v. injection as a unique route of administration in the present study. We administrated oncolytic virus in a mutiple times manner. Based on our previous work on mice, we adjusted the highest effective dosage according to the different body weight of these two kinds of animals. We decided to use 109 pfu in each injection for each individual macaque. As the administration schedule shown in Fig. 1, there are three rounds of repeated injections in this study, each round consisting with six times of independent injections. Sample collections were performed at indicated time points started 1 hr before the first injection in the first round and ended 9 months thereafter. The schedule for the various kinds of examination during the first 105 days after the first injection was shown in Table 1.
Table 1.
Data collection schedule for the safety study of alphavirus M1
| Study day | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Procedure | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 13 | 21 | 28 | 32 | 39 | 46 | 53 | 60 | 67 | 74 | 81 | 88 | 95 | 105 |
| Body weight | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | |||||||
| Temperature | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
| Clinical observation | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
| Hematology | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | ||||
| Blood biochemistry | × | × | × | × | × | × | × | × | × | × | × | × | × | × | ||||||||
| Cytokine | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | ||||
| Urine | × | × | × | × | × | × | × | × | × | × | × | × | × | |||||||||
| M1 titration (blood) | × | × | × | × | ||||||||||||||||||
| qRT-PCR (blood) | × | × | × | × | ||||||||||||||||||
| Neutralization assay | × | × | × | × | ||||||||||||||||||
M1, oncolyticvirus M1; qRT-PCR, quantitative real-time polymerase chain reaction.
General health status
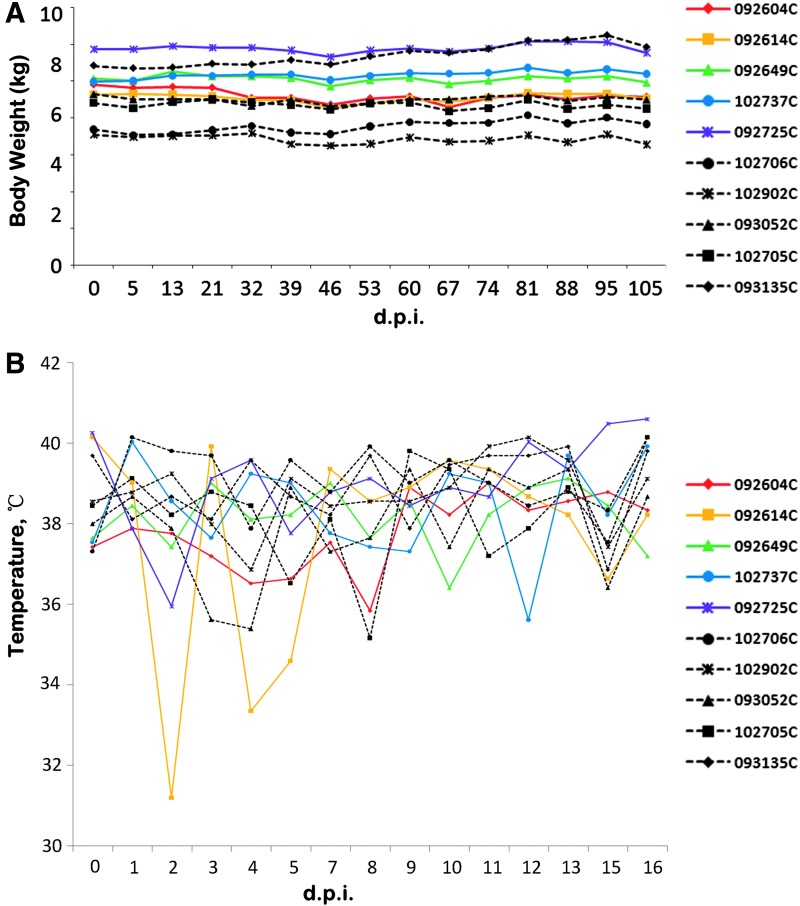
We measured the body weight of experimental macaques (n = 5) and control macaques (n = 5) weekly. As Fig. 2A shows, test and control macaques maintained and gained weight throughout the study, and no significant difference between the two groups was found in body weight level.
Figure 2.
Body weight and body temperature. Data were collected from cynomolgus macaques injected with 109 pfu oncolytic virus M1 (test, i.v. application of oncolytic virus M1) or equal volume of vehicle (control, i.v. application of same volume of vehicle). (A) Profiles of body weight of cynomolgus macaques at indicated days. (B) Kinetics of body temperatures of cynomolgus macaques after i.v. treatment. An overview of the individual cynomolgus macaques of the experiment and their animal codes is shown (right). Data from different animals are marked with different shapes of symbols; the ID number consists of six digits plus a capital letter. Data from control animals are shown in dashed lines versus solid lines for data from test animals. d.p.i., days post the first injection; i.v., intravenous.
Within 2 weeks after the first injection of oncolytic virus M1 or vehicle, the body temperature of cynomolgus macaques was measured daily. As Fig. 2B shows, compared with the control macaques, the temperature levels of all experimental macaques have not been found with any significant difference, except for a temporary temperature fluctuation in one experimental macaque (animal ID, 092604C).
In the whole process of this study, we observed the clinical manifestations of each animal at least twice daily. As Table 2 shows, during the 9-month study of safety, only one macaque (animal ID, 092604C) was found to have diarrhea and diarrhea-associated anorexia among the experimental macaques in the second month after the first injection. Overall, the 10 macaques remained normal in their activity level, posture, facial symmetry, gait, behavior, motor function, and level of consciousness throughout the study.
Table 2.
External clinical symptoms of macaques in the safety study of intravenous injection of oncolytic virus M1
| Animal No. | Anorexia | Emesis | Diarrhea | Aggression | Vocalization | Circling | Hunched | Paddling | Recumbent |
|---|---|---|---|---|---|---|---|---|---|
| M1 injected | |||||||||
| 092604C | + | − | + | − | − | − | − | − | − |
| 092614C | − | − | − | − | − | − | − | − | − |
| 092649C | − | − | − | − | − | − | − | − | − |
| 102737C | − | − | − | − | − | − | − | − | − |
| 092725C | − | − | − | − | − | − | − | − | − |
| Control | |||||||||
| 102706C | + | − | + | − | − | − | − | − | − |
| 102902C | + | − | − | − | − | − | − | − | − |
| 093052C | + | − | − | − | − | − | − | − | − |
| 102705C | − | − | − | − | − | − | − | − | − |
| 093135C | − | − | − | − | − | − | − | − | − |
It was suggested that there was no negative impact of the i.v. injection of oncolytic virus M1 on the health status of the cynomolgus macaques. With the aim to verify it, we also have carried out a series of laboratory examinations as follows.
Serum biochemical tests
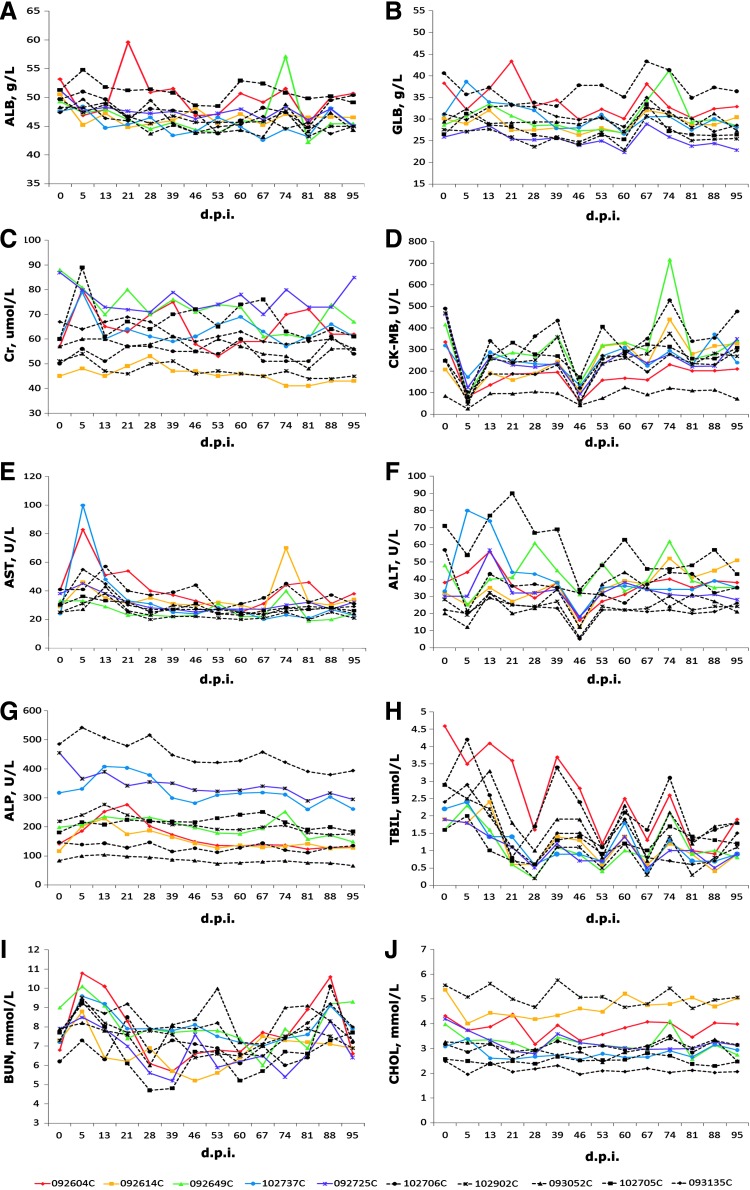
Every week, we routinely collected blood, separated serum, and completed a comprehensive examination of blood biochemistry, including albumin (ALB), globulin (GLB), creatinine (Cr), creatine kinase-MB (CK-MB), aspartate aminotransferase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), total bilirubin (TBIL), blood urea nitrogen (BUN), and cholesterol (CHOL). After careful statistical analysis, the data from each macaque were shown in the charts in Fig. 3. Overall, compared with the control, we found no significant difference in any indicator detected between the two groups. It reduces or eliminates the possibility of any damage on some vital tissues/organs (e.g., liver, kidney, skeletal muscle) caused by acute infection, and further supports the conclusion that the i.v. injection of oncolytic virus M1 does not affect normal biochemical metabolism and physiological status in the experimental macaques.
Figure 3.
Blood biochemistry examinations of the serum samples of macaques. Samples taken at indicated time points were assayed using an automated blood biochemistry analyzer (Piccolo Xpress) to obtain a basic blood biochemistry profile. Different parameters, including albumin (A), globulin (B), creatinine (C), creatine kinase-MB (D), aspartate aminotransferase (E), alanine transaminase (F), alkaline phosphatase (G), total bilirubin (H), blood urea nitrogen (I), and cholesterol (J), of individual macaques were shown in charts. An overview of the individual cynomolgus macaques of the experiment and their animal codes is shown (bottom). Data from different animals are marked with different shapes of symbols; the ID number consists of six digits plus a capital letter. Data from control animals are shown in dashed lines versus solid lines for data from test animals.
Blood cytology examination
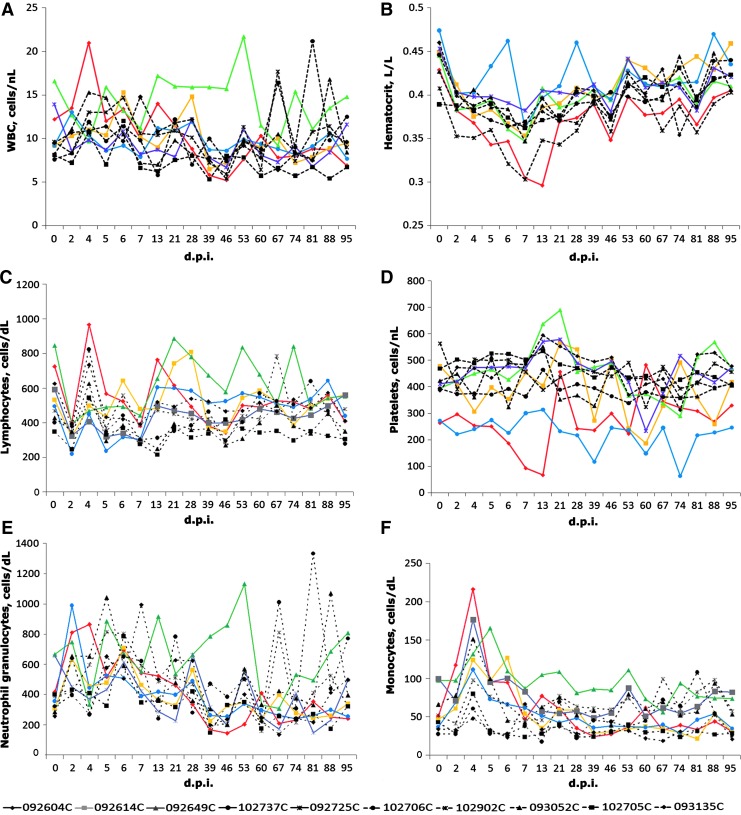
Blood cytology is also an important clinical examination for evaluation of the physiological condition at the level of the entire body. With different virus infections, blood cells often display different patterns of changes. We have collected anticoagulated blood samples of all cynomolgus macaques, and conducted a complete blood count (CBC) analysis, including white blood cell (WBC), neutrophilic granulocyte (NG), lymphocyte (LY), monocyte (MONO), platelet (PLT), and hematocrit (HCT). As shown in Fig. 4F, we found that the proportion of monocyte in the blood of the experimental group was significantly increased after M1 virus injection, reached the peak on the fourth day postinjection, and returned to normal level 2 weeks after. After statistical analysis, we did not find significant difference in the count of the whole white blood cell or any fraction of it compared with correspondent data of the control (Fig. 4A–E).
Figure 4.
Hematological examinations of whole blood samples of macaques. Peripheral blood samples from macaques injected with six doses of oncolytic virus M1 (109 pfu/dose) or with vehicle were used to perform complete blood counts (CBC) analysis using an automated counter (Sysmex pocH-100iV). Charts show the numbers of white blood cells (A), platelet cells (D), and hematocrits (B) and the percentages of lymphocytes (C), neutrophilic granulocytes (E), and monocytes (F) for individual macaques at indicated time points. An overview of the individual cynomolgus macaques of the experiment and their animal codes is shown (bottom). Data from different animals are marked with different shapes of symbols; the ID number consists of six digits plus a capital letter. Data from control animals are shown in dashed lines versus solid lines for data from test animals.
Cellular and molecular immunology detection
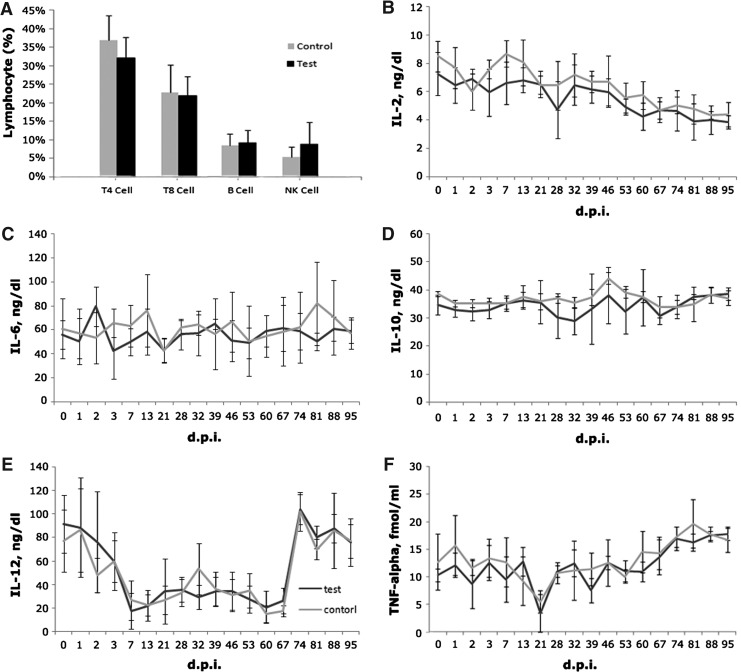
To understand the impact of intravenously injected oncolytic virus M1 on immune cells in the peripheral blood, we take whole blood samples from both groups of cynomolgus macaques on the 45th day after the second round of injections, and checked the lymphocyte subsets and NK cells by flow cytometry. As shown in Fig. 5A, there is no significant difference in the proportion of each cell subset in the peripheral blood between the groups of cynomolgus macaques examined, including B cells, killer T-cells, memory T-cells, regulatory T-cells, and natural killer cells. These results indicated that the oncolytic virus M1 cannot cause proliferation activation or inhibition of these cell subsets in host macaques.
Figure 5.
The effects of intravenous injection of oncolytic virus M1 on the cytokines and the lymphocyte subsets in the peripheral blood of cynomolgus macaques. Protein levels of cytokines, including TNF-α (A), IL-2 (B), IL-6 (C), IL-10 (D), and IL-12 (E), at indicated time points were detected using ELISA. Data from test animals shown in black lines versus data from control animals shown in gray lines are both in ng/dl with the exception of TNF-α (in fmol/ml). Comparative studies on lymphocyte classification (F) in the peripheral blood between the test group and the control group were performed immediately after whole blood collection using immunocytometric methods. Data are shown as mean ± SD, bars indicate standard deviations, and significant results are indicated if the p value is less than 0.05.
Immune cells often modulate each other via the release and action of cytokines. Therefore, detection of cytokines level in the serum help us understand the systemic immune status. We began to collect samples from 24 hr after the first injection, and the protein levels of various cytokines were detected by ELISA. After statistical analysis, we found that all the five kinds of checked cytokines were not significantly changed after the injection of oncolytic virus M1 (Fig. 5B–F). These data, combined with the result of lymphocyte subsets detection, indicated that M1 cannot break the balance between immune cells by altering the levels of cytokines.
Specific humoral immunity is an important factor for the body to clear different kinds of viruses. We collected serum samples after injection of oncolytic virus M1. Using end-point dilutions method, we performed neutralization assay to test the levels of neutralizing antibody in both groups of cynomolgus macaques. As shown in Table 3, neutralizing activity in different macaques increased beginning from the second week after the first i.v. injection of M1 with various speeds, which suggested the different immunity levels of different individual macaques.
Table 3.
Titers of antioncolytic virus M1 antibodies in macaque serum as determined by infection reduction neutralization assay
| Virus-neutralizing antibody titera | ||||||
|---|---|---|---|---|---|---|
| Macaque ID | Day 0 | Day 3 | Day 9 | Day 22 | Day 68 | Day 96 |
| M1 injected | ||||||
| 092604C | 0 | 0 | 0 | 10.75 | 39.81 | 107.34 |
| 092614C | 0 | 0 | 0 | 9.32 | 9.53 | 125.89 |
| 092649C | 0 | 0 | 0 | 25.43 | 11.66 | 16.19 |
| 102737C | 0 | 0 | 125.89 | 177.83 | 105.25 | >256 |
| 092725C | 0 | 0 | >256 | >256 | 180.52 | 75.76 |
| Control | ||||||
| 102706C | 0 | 0 | 0 | 0 | 0 | 0 |
| 102902C | 0 | 0 | 0 | 0 | 0 | 0 |
| 093052C | 0 | 0 | 0 | 0 | 0 | 0 |
| 102705C | 0 | 0 | 0 | 0 | 0 | 0 |
| 093135C | 0 | 0 | 0 | 0 | 0 | 0 |
Defined as the reciprocal of serum dilution that neutralized the virus activity by 50%.
Diagnostic MRI on arthritis and encephalitis
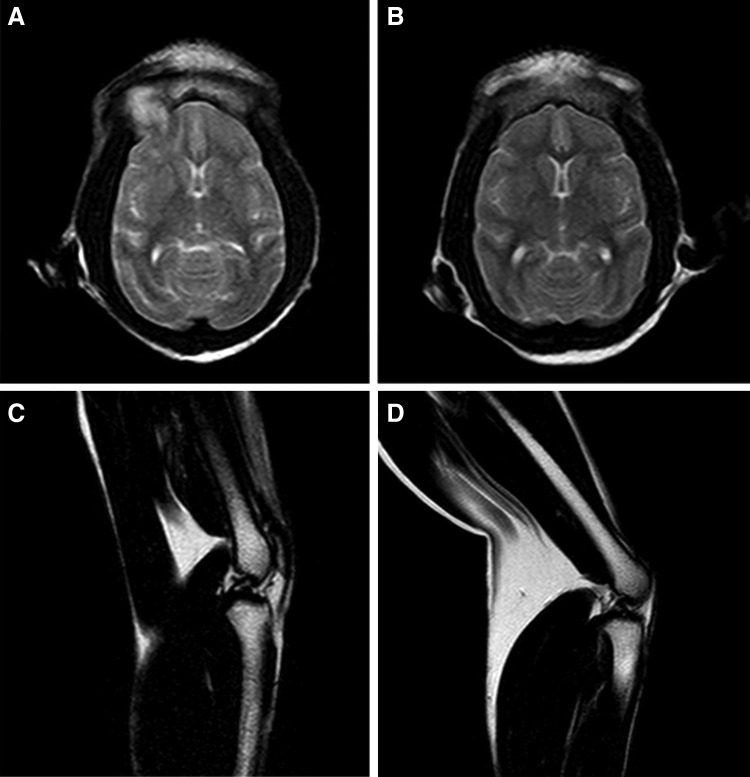
As far as we know, there is scarcely any report of human disease caused by Getah virus, the closest alphavirus of M1. However, there are many reports about some disease generated by other alphavirus, for example, chikungunya,12,13 Eastern equine encephalitis,7,14 and Venezuelan equine encephalitis,5 which can be referred to determine the possible disease types of alphavirus M1. In short, encephalitis and arthritis are more common diseases caused by alphavirus.15 Nearing the end point of the study, we used brain MRI and knee joint MRI to find the potential occurrence of encephalitis or arthritis. Similar to the control macaques, no encephalitis- or arthritis-related imaging characteristics were found in the experimental macaques. MRIs of one experimental macaque (animal ID, 092604C) compared with a control macaque (animal ID, 102902C) are shown in Fig. 6 as the representative results.
Figure 6.
Magnetic resonance imaging findings. T2-weighted images of the brain (axial section; A, B) and knee joint (sagittal section; C, D) were scanned and compared between the test group and the control group macaques using the TSE sequence. The presentative images of two animals, a control macaque with the ID of 102902C (A, C) and a test macaque with the ID of 092604C (B, D), are shown. There is no evidence of encephalitis, arthritis-associated joint destruction, or other abnormality in either animal.
Long-term impacts of multiple rounds of i.v. injection
Considering the possibility of using repeated i.v. injection after several months' interval for the patients in the clinical trial, we continue to complete the two rounds of i.v. administration of oncolytic virus M1 after the first round. All the examinations mentioned above were performed to evaluate the potential comprehensive long-term effects of multiple rounds of injections on macaques. As shown in Table 4, no significant difference can be found in the test group compared with the control, except for the increased percentage of lymphocytes in the white blood cells, which is a common phenomenon during virus infection.
Table 4.
Comparison of main outcome measures between two groups of macaques at the end of the safety study of oncolytic virus M1
| Diagnosis | Control, n = 5 | Test, n = 5 | p ≥ 0.05a |
|---|---|---|---|
| Body weight | 5.26 ± 1.39 | 5.81 ± 0.8 | Yes |
| Anorexia | 0 (0) | 0 (0) | |
| Body temperature | 38.42 ± 0.48 | 38.22 ± 0.52 | Yes |
| Clinical symptomsb | 0 (0) | 0 (0) | |
| WBC, cells/nl | 11.12 ± 4.31 | 9.54 ± 4.01 | Yes |
| PLT, cells/nl | 432.8 ± 51.09 | 350 ± 109.60 | Yes |
| NG, % | 57.46 ± 11.34 | 40.22 ± 12.31 | Yes |
| LY, % | 34.72 ± 9.62 | 51.16 ± 12.14 | 0.0450 |
| MONO, % | 4.86 ± 2.32 | 5.2 ± 3.17 | Yes |
| HCT, % | 40.82 ± 3.35 | 42.64 ± 2.69 | Yes |
| CK-MB, U/liter | 255.6 ± 117.10 | 194.2 ± 39.24 | Yes |
| AST, U/liter | 28 ± 5.24 | 28.2 ± 4.55 | Yes |
| ALT, U/liter | 32.4 ± 16.77 | 26.4 ± 3.78 | Yes |
| ALB, g/liter | 44.66 ± 2.79 | 46.22 ± 1.81 | Yes |
| GLB, g/liter | 32 ± 3.50 | 30.26 ± 4.11 | Yes |
| ALP, U/liter | 175 ± 76 | 181.8 ± 70.48 | Yes |
| CHOL, mM | 3.09 ± 0.94 | 3.60 ± 0.78 | Yes |
| TBIL, μM | 1.3 ± 0.56 | 1.14 ± 0.62 | Yes |
| BUN, mM | 6.44 ± 1.18 | 6.14 ± 1.10 | Yes |
| Cr, μM | 64.8 ± 13.37 | 67 ± 12.86 | Yes |
| Viremia | 0 (0) | 0 (0) | |
| IL-2, ng/dl | 2.75 ± 0.33 | 3.33 ± 0.53 | Yes |
| IL-6, ng/dl | 93.57 ± 6.85 | 95.75 ± 18.17 | Yes |
| IL-10, ng/dl | 28.43 ± 7.33 | 22.85 ± 4.36 | Yes |
| IL-12, ng/dl | 211.33 ± 39.32 | 168.01 ± 91.57 | Yes |
| TNF-alpha, fmol/ml | 21.93 ± 2.05 | 20.1 ± 42.19 | Yes |
| Abnormal MRI | 0 (0) | 0 (0) |
p < 0.05, statistically significant differences between the control group and the test group.
Clinical symptoms including emesis, diarrhea, circling, aggression, vocalization, hunched posture, paddling, and recumbent.
ALB, albumin; ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CHOL, cholesterol; CK-MB, creatine kinase-MB; Cr, creatinine; GLB, globulin; HCT, hematocrit; LY, lymphocyte; MONO, monocyte; NG, neutrophilic granulocyte; PLT, platelet; TBIL, total bilirubin; WBC, white blood cell.
Discussion
As a kind of self-replicating virus specifically targeting tumor tissue, oncolytic viruses are often genetically modified to be a weak virulence. After 9 months of experimental study, our present study shows that a naturally existing oncolytic virus, alphavirus M1, did not cause significant harm to the health of cynomolgus macaques after three rounds of repeated i.v. injections (totally including 18 doses, each dose up to 1 × 109 pfu), except for the temporarily increased monocyte in the peripheral blood of the experimental group after M1 virus injection.
The increment of monocyte in peripheral blood of cynomolgus macaques had been reported in Western equine encephalitis virus study by other researchers.16 In fact, it was also reported that alphavirus can infect monocyte and spread in human body via monocytes.17,18 Although recruitment of CCR2(+) monocytes can contribute to inflammation,19 monocytes also appear to be critical for preventing excessive pathology and resolving inflammation after alphavirus infection.20 In contrast with the negative results in control group macaques, the increased induction of monocytes at least indicated that oncolytic virus M1 was successfully intravenously injected.
In the first week, we collected serum from each macaque daily after the first injection, used qRT-PCR to quantify the viral genomic RNA of oncolytic virus M1, and determined the virus titer by TCID50 assay using BHK-21 cells. To our surprise, both assays generated negative results, and there was not any detectable virus in the serum (data not shown), which cannot attribute to the clearance of neutralizing antibodies considering the late occurrence of these antibodies after injection. A reasonable explanation is that the virus quickly reached and adsorbed to the tissues throughout the body after injection and then did not remain up to a detectable level in the serum. In support of this, our previous biodistribution experiments using qRT-PCR methods in mice found that within 24 hr postinfection the oncolytic virus M1 has completed its enrichment in cancer and other tissues.4
Oncolytic virus M1 is a kind of Getah-like alphavirus whose related disease was just reported in horse. Therefore, that no neutralizing antibody was found in control macaques yet suggests that there remains a naive immune status in the uninfected nonhuman, avoiding a reduced therapeutic efficacy resulted from neutralizing antibodies.21 Metastasis refers to the spread of cancer to different parts of the body, which leads to secondary cancer, and is a main cause of death of cancer patients. Intravenous virus administration can help patients to eradicate metastasized cancer cells and kill the seeds of secondary cancer at the first step. At least, our present findings about the emerging process of neutralizing antibody suggested that there would be a time window shorter than 9 days for the i.v. administration of oncolytic virus M1 in the patients without the obstacle of neutralizing antibody, if there is no possible virus M1 infection during the early time of a cancer patient's life. Further, even though we use different animals, our primate experiments do find an endurable dose equivalent to the possible effective dose according to the data in mice experiments, which will lead us to proceed. Nonetheless, we should go on to find the maximum endurable dose and the minimum effective dose in the preclinical study and clinical trial in the future.
Even though our results in the cynomolgus macaques study supported the preclinical safety of oncolytic virus M1, we cannot completely rule out the potential risk of clinical use of this replication-competent virus for the following reasons: First, for ethics consideration in animal experiments, we minimized the number of macaques. Second, because very few macaques showed severe clinical pathological manifestations, we did not perform an autopsy, complying with corresponding animal ethics. Last but not least, because of its insect-borne characteristics of alphavirus transmission, it was needed to setup an effective surveillance and preventive system before the clinical trial of the oncolytic virus.
In summary, the present results in the adult cynomolgus macaques demonstrated the safety of the multiple rounds of repeated i.v. injections of high titers of oncolytic virus M1 in nonhuman primates, the closest animal relatives to humankind. It prompts the clinical safety of oncolytic virus M1 and can guide future clinical dose and route of administration.
Acknowledgments
This work was supported by National Natural Science Foundation of China (81273531 and 81573447), the Guangdong Provincial Science and Technology project (No. 2015B020211003), the Guangdong Provincial Natural Science Foundation Grant (2015A030313081), and the Fundamental Research Funds for the Central Universities (13ykpy07).
Author Disclosure
The authors have declared that no conflicts of interest exist.
References
- 1.Wen JS, Zhao WZ, Liu JW, et al. . Genomic analysis of a Chinese isolate of Getah-like virus and its phylogenetic relationship with other Alphaviruses. Virus Genes 2007;35:597–603 [DOI] [PubMed] [Google Scholar]
- 2.Lin Y, Zhang H, Liang J, et al. . Identification and characterization of alphavirus M1 as a selective oncolytic virus targeting ZAP-defective human cancers. Proc Natl Acad Sci U S A 2014;111:E4504–4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li K, Zhang H, Qiu J, et al. . Activation of cyclic adenosine monophosphate pathway increases the sensitivity of cancer cells to the oncolytic virus M1. Mol Ther 2016;24:156–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan G, Zhang G, Fang X, et al. . Genome sequencing and comparison of two nonhuman primate animal models, the cynomolgus and Chinese rhesus macaques. Nat Biotechnol 2011;29:1019–1023 [DOI] [PubMed] [Google Scholar]
- 5.Reed DS, Lackemeyer MG, Garza NL, et al. . Severe encephalitis in cynomolgus macaques exposed to aerosolized Eastern equine encephalitis virus. J Infect Dis 2007;196:441–450 [DOI] [PubMed] [Google Scholar]
- 6.Labadie K, Larcher T, Joubert C, et al. . Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest 2010;120:894–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugiyama T, Yoneda M, Kuraishi T, et al. . Measles virus selectively blind to signaling lymphocyte activation molecule as a novel oncolytic virus for breast cancer treatment. Gene Ther 2013;20:338–347 [DOI] [PubMed] [Google Scholar]
- 8.Buijs PR, van Amerongen G, van Nieuwkoop S, et al. . Intravenously injected Newcastle disease virus in non-human primates is safe to use for oncolytic virotherapy. Cancer Gene Ther 2014;21:463–471 [DOI] [PubMed] [Google Scholar]
- 9.Su C, Cao H, Tan S, et al. . Toxicology profiles of a novel p53-armed replication-competent oncolytic adenovirus in rodents, felids, and nonhuman primates. Toxicol Sci 2008;106:242–250 [DOI] [PubMed] [Google Scholar]
- 10.Hadac EM, Peng KW, Nakamura T, et al. . Reengineering paramyxovirus tropism. Virology 2004;329:217–225 [DOI] [PubMed] [Google Scholar]
- 11.Hu J, Cai XF, Yan G. Alphavirus M1 induces apoptosis of malignant glioma cells via downregulation and nucleolar translocation of p21WAF1/CIP1 protein. Cell Cycle (Georgetown, TX) 2009;8:3328–3339 [DOI] [PubMed] [Google Scholar]
- 12.Horwood PF, Buchy P. Chikungunya. Rev Sci Tech 2015;34:479–489 [DOI] [PubMed] [Google Scholar]
- 13.Mathew AJ, Ravindran V. Infections and arthritis. Best Pract Res Clin Rheumatol 2014;28:935–959 [DOI] [PubMed] [Google Scholar]
- 14.Mitchell CJ, Niebylski ML, Smith GC, et al. . Isolation of eastern equine encephalitis virus from Aedes albopictus in Florida. Science (New York, NY) 1992;257:526–527 [DOI] [PubMed] [Google Scholar]
- 15.Taylor A, Herrero LJ, Rudd PA, et al. . Mouse models of alphavirus-induced inflammatory disease. J Gen Virol 2015;96:221–238 [DOI] [PubMed] [Google Scholar]
- 16.Reed DS, Larsen T, Sullivan LJ, et al. . Aerosol exposure to western equine encephalitis virus causes fever and encephalitis in cynomolgus macaques. J Infect Dis 2005;192:1173–1182 [DOI] [PubMed] [Google Scholar]
- 17.Levitt NH, Miller HV, Edelman R. Interaction of alphaviruses with human peripheral leukocytes: In vitro replication of Venezuelan equine encephalomyelitis virus in monocyte cultures. Infect Immun 1979;24:642–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long KM, Ferris MT, Whitmore AC, et al. . Gammadelta T cells play a protective role in chikungunya virus-induced disease. J Virol 2015;90:433–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rulli NE, Rolph MS, Srikiatkhachorn A, et al. . Protection from arthritis and myositis in a mouse model of acute chikungunya virus disease by bindarit, an inhibitor of monocyte chemotactic protein-1 synthesis. J Infect Dis 2011;204:1026–1030 [DOI] [PubMed] [Google Scholar]
- 20.Poo YS, Nakaya H, Gardner J, et al. . CCR2 deficiency promotes exacerbated chronic erosive neutrophil-dominated chikungunya virus arthritis. J Virol 2014;88:6862–6872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willmon C, Harrington K, Kottke T, et al. . Cell carriers for oncolytic viruses: Fed Ex for cancer therapy. Mol Ther 2009;17:1667–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]