Abstract
Borealin, a member of the chromosomal passenger complex, plays a key regulatory role at centromeres and the central spindle during mitosis. Loss of Borealin leads to defective cell proliferation and early embryonic lethality. The in vivo functions of Borealin in mammalian postnatal development, tissue homeostasis, and tumorigenesis remain elusive. We specifically analyzed the role of Borealin in regulating postnatal liver development, damage-induced liver regeneration, and liver carcinogenesis using mice carrying conditional Borealin alleles. Perinatal loss of Borealin caused increased genome ploidy and enlarged cell size in hepatocytes, likely due to the impaired function of the chromosomal passenger complex in mitosis. Borealin deletion also showed attenuated expansion of Sox9+HNF4α+ progenitor-like cells in liver regeneration during 3,5-diethoxycarbonyl-1,4-dihydrocollidine diet-induced liver injury. Moreover, ΔN90-β-Catenin and c-Met-induced hepatocarcinogenesis development was largely impeded by Borealin deletion. These findings indicate that Borealin plays a key role in liver development, regeneration, and tumorigenesis and suggests that Borealin could be a potential target for related liver diseases.
Keywords: cell cycle, hepatocellular carcinoma, liver cancer, liver injury, stem cells, Borealin, liver development, liver progenitor-like cell
Introduction
The liver possesses a remarkable regeneration capacity after damage, which is achieved by the proliferation of differentiated hepatocytes or facultative progenitors when the proliferative capacity of hepatocytes is blocked or compromised (1). Recent studies also proposed that transiently formed Sox9+HNF4α+ progenitor-like cells undergo extensive proliferation and replenish liver mass after chronic liver injuries (2–4). Although liver regeneration repairs damaged liver tissues and restores impaired liver function, the fast regeneration process is susceptible to malignant transformation, with the potential for the eventual development of hepatocellular carcinoma (HCC)4 (5–7). HCC is currently the third most deadly and fifth most common cancer worldwide (8). Identifying the key players with important roles in liver regeneration and HCC development will provide potential targets and pave the way for the treatment of these liver diseases.
Chromosomal passenger complex (CPC) contains Survivin, Borealin, INCENP, and Aurora B kinase, of which Aurora B is the core enzyme and the other proteins are regulatory components (9). The CPC complex is essential for genomic stability and works by controlling multiple processes during both nuclear and cytoplasmic division (10). Previous studies found that Survivin deficiency during postnatal liver development impaired hepatocyte mitosis, induced hepatocyte death and oval cell activation, and complete reconstitution of the liver (11, 12). Moreover, Survivin deletion in the liver markedly repressed HCC development (13, 14). Loss of Survivin is associated with impaired Aurora B activation, which implies the CPC complex plays a critical role in liver regeneration and tumorigenesis. However, given that Survivin has additional functions as an inhibitor of apoptosis protein, it remains unclear whether the functions of Survivin in the regulation of postnatal liver development, oval cell reaction, and hepatocarcinogenesis are mediated only through its function in the CPC complex. A careful characterization of other regulatory members in the CPC complex will provide additional insights about the CPC complex in liver regeneration and tumorigenesis.
The function of Borealin (also called cell division cycle associated 8, CDCA8) is confined in the CPC complex and mitosis by facilitating CPC assembling, Aurora B kinase activation, and localization in kinetochores (15). Consistent with its prominent role in mitosis, Borealin is indispensable for ontogenesis. In Drosophila melanogaster, inactivation of Borealin results in polyploidy, delayed mitosis, and abnormal tissue development (16). In mice, Borealin is widely expressed in embryonic tissues and Borealin-deficient mice died in utero due to mitotic defects and apoptosis in blastocyst cells (17). Moreover, Borealin played key roles in tumor onset and recurrence and Borealin was also overexpressed in colorectal and lung cancers and contributed to the proliferation of cancer cells (18, 19). Furthermore, Borealin expression was related to poor prognosis in gastric cancers (20). Suppression of Borealin expression by RNA interference significantly suppressed the growth of cancer cells (18, 19). These findings suggest inhibition of Borealin could be a promising strategy for cancer treatment; however, the in vivo functions of Borealin in postnatal development, tissue homeostasis, and tumor development remain largely undefined.
In this study, which used mouse lines carrying conditional alleles, we found that in neonatal livers, hepatocytes lacking Borealin were blocked in mitosis and displayed increased genome ploidy and enlarged cell size. Moreover, the loss of Borealin dramatically reduced DDC diet-induced Sox9+HNF4α+ progenitor-like cell proliferation and ΔN90-β-Catenin/c-Met-induced HCC development, indicating that Borealin is a potential target for HCC prevention and therapy.
Results
Efficient Deletion of Borealin in Hepatocytes of BorealinΔli Mice
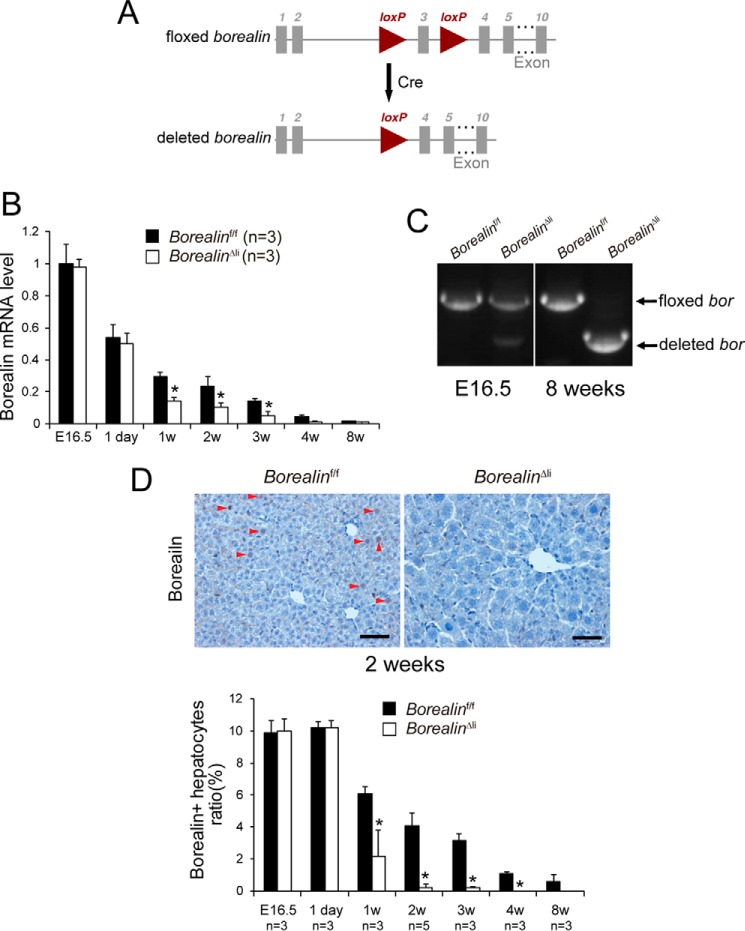
Mice homozygous for floxed Borealin alleles were crossed with mice that carry Cre recombinase under control of the albumin promoter (Alb-Cre) (21) to specifically delete Borealin in hepatocytes perinatally (Fig. 1A). The Alb-Cre, Borealinf/f (BorealinΔli) mice were born indistinguishable from their littermates (Borealinf/f) with the expected Mendelian frequency. To monitor deletion efficiency during postnatal liver development, total liver RNA was isolated from the BorealinΔli livers. Borealin mRNA levels progressively decreased in control livers during development (Fig. 1B), consistent with the role of Borealin in cell proliferation. Expression of Borealin was unchanged on E16.5 and postnatal day 1 in the BorealinΔli livers compared with the control livers (Fig. 1B). Notably, Borealin mRNA levels were reduced by 50% at the age of 1 and 2 weeks, and were further decreased at the age of 3 and 4 weeks, indicating a progressive and efficient deletion of Borealin in the BorealinΔli livers (Fig. 1B). Because the mRNA levels at the age of 8 weeks were almost undetectable in both the wild-type and the BorealinΔli livers, we validated the deletion efficiency of Borealin using liver genomic DNA. Genotyping PCR showed that the floxed allele was only slightly deleted in E16.5 BorealinΔli livers and efficiently deleted in the livers of the 8-week-old BorealinΔli mice (Fig. 1C). Moreover, immunohistochemical (IHC) staining confirmed that the Borealin protein levels were comparable in BorealinΔli and control livers at E16.5 and postnatal day 1 (Fig. 1D). Borealin was already efficiently deleted in the BorealinΔli hepatocytes at 2 weeks of age (Fig. 1D). These results indicated that Borealin was efficiently deleted in the BorealinΔli livers during postnatal liver development.
FIGURE 1.
Efficient deletion of Borealin in hepatocytes of postnatal BorealinΔli mice. A, schematic diagram of the Borealin gene locus and related alleles. The Borealin floxed alleles have two loxP sites (triangles) flanking the third exon (boxes). Mice with Borealin floxed alleles were crossed with a Cre line to generate the deleted Borealin allele. B, Borealin mRNA levels were measured by q-PCR in livers at the indicated ages. C, genotypes were determined by PCR using liver genomic DNA. D, borealin protein levels were characterized by IHC staining in livers. Arrowheads indicate Borealin-positive cells, which have brown-stained nuclei. Scale bars, 50 μm.
Borealin Deficiency Induces Hepatocyte Hypertrophy and Polyploidy
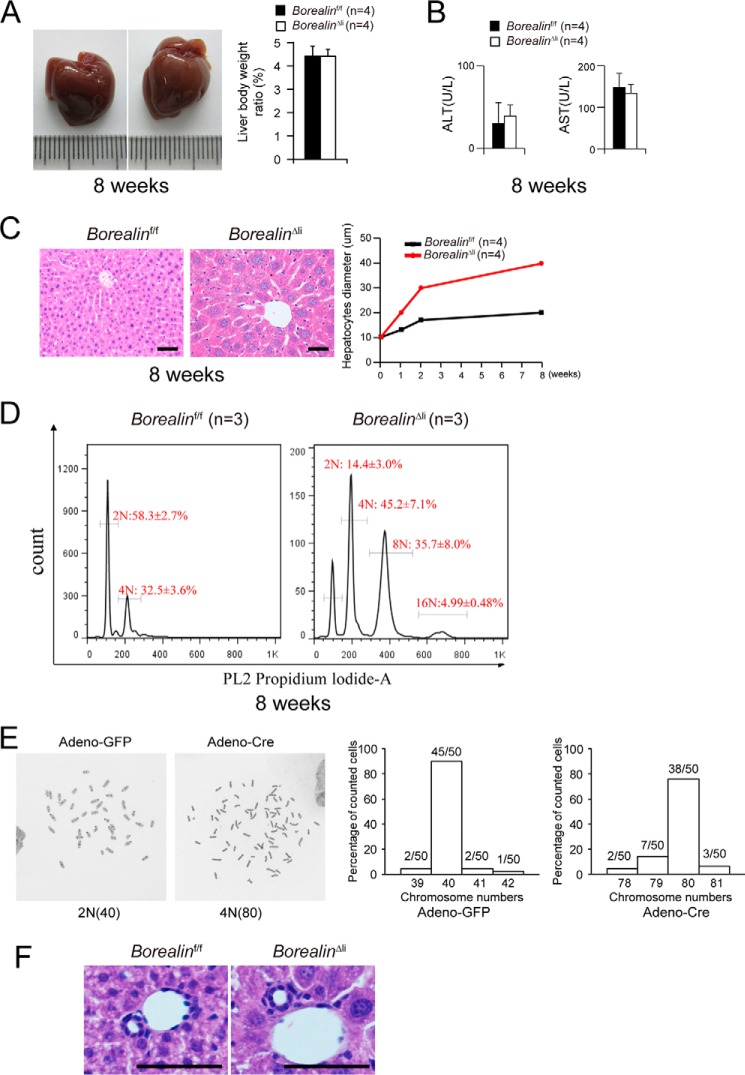
The 8-week-old BorealinΔli mice appeared healthy and their liver morphology and liver/body weight ratios were comparable with controls (Fig. 2A). The serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were also within the normal range (Fig. 2B) suggesting that liver functions of the BorealinΔli mice were apparently normal. Hepatocyte morphology was further analyzed on hematoxylin and eosin (H&E)-stained paraffin sections. Histological inspection revealed that the hepatocyte diameters were comparable in BorealinΔli and control livers in postnatal day 1 (supplemental Fig. S1). Then, the BorealinΔli hepatocytes underwent progressive hypertrophy with a 2-fold increase in diameter compared with the controls (Fig. 2C). Notably, Borealin-deficient hepatocytes showed enlarged nuclei, suggesting that these cells may be polyploid. To further characterize the genome ploidy of Borealin-deficient hepatocytes, single hepatocytes were prepared by collagenase perfusion and the genome ploidy was determined by propidium iodide staining and FACS analysis. We found that Borealin-deficient hepatocytes were highly polyploid (Fig. 2D), suggesting impaired mitosis in their cell cycle. To analyze the overall chromosomal stability after Borealin deletion, we used Borealinf/f tail-tip fibroblasts and deleted Borealin by Adeno-Cre virus. We found that the number of tetraploid cells was dramatically increased after Borealin deletion and tetraploid cells had around 80 chromosomes. When the frequency of aneuploidy cells were calculated, there was no difference between Borealin-deficient cells and control cells (Fig. 2E, 12/50 versus 5/50, p = 0.11, Fisher's exact test). These data suggest that Borealin deletion may not have a dramatic effect on overall karyotype stability. Interestingly, the morphology of liver lobules and bile ducts was unaffected in the BorealinΔli livers (Fig. 2F).
FIGURE 2.
Borealin maintains hepatocyte cell size and genome ploidy. A, the 8-week-old BorealinΔli mice showed normal livers and liver/body weight ratios of BorealinΔli and control Borealinf/f mice were measured. B, serum ALT and AST levels were measured in Borealinf/f and BorealinΔli mice. C, hematoxylin and eosin staining of liver sections of the 8-week-old mice. Hepatocyte diameters were quantified. Scale bars, 50 μm. D, the genome ploidy of hepatocytes was characterized by propidium iodide staining followed by fluorescence-activated cell sorting. E, karyotype analysis showed that Borealin deletion caused tetraploid cells in cultured tail-tip fibroblasts. F, H&E staining of liver sections from the 2-week-old mice. Scale bars: 50 μm.
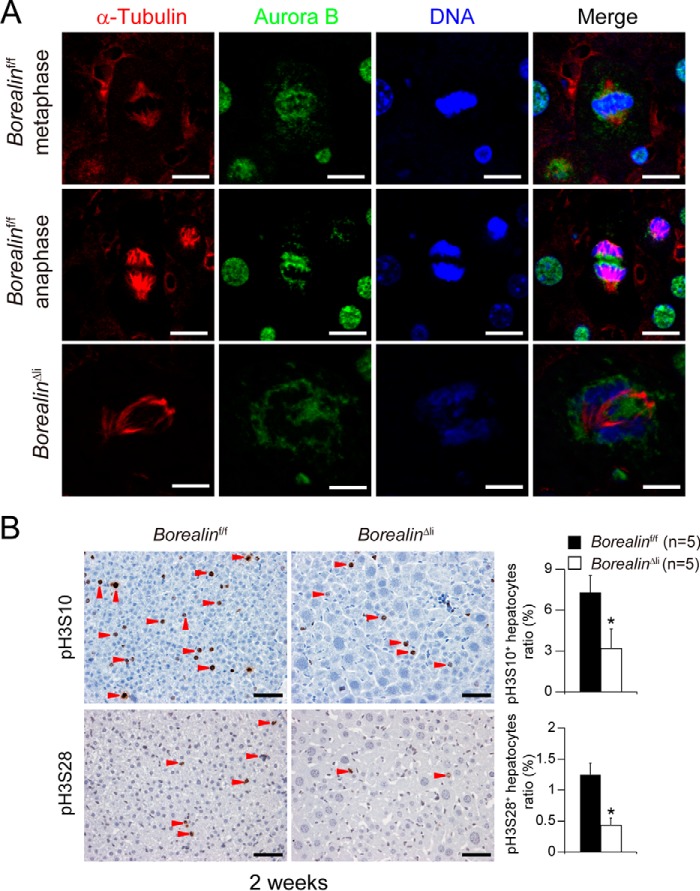
Previous studies have shown that Borealin was highly expressed in the G2/M phase and was required for mitotic transition to control CPC complex assembly and localization (15). The impaired mitosis of Borealin-deficient hepatocytes may be caused by mislocalization of the CPC complex. To verify this hypothesis, we analyzed Aurora B kinase localization during hepatocyte mitosis in the 2-week-old BorealinΔli livers. In the Borealinf/f hepatocytes, Aurora B kinase was located at centromeres and midbodies during metaphase and anaphase, respectively; however, Aurora B kinase diffusely localized on the chromosome arms with no detectable centromere enrichment in the BorealinΔli hepatocytes (Fig. 3A). It is well known that H3S10 is phosphorylated by Aurora B kinase during mitosis. We found the number of phosphorylated histone H3Ser-10 (pH3S10)-positive or pH3Ser-28 (pH3S28)-positive hepatocytes were both dramatically decreased in the 2-week-old BorealinΔli livers (Fig. 3B). Because both H3S10 and H3S28 were directly phosphorylated by Aurora B kinase, these data indicate that Borealin deficiency caused disturbed localization and dysfunction of Aurora B kinase. These data demonstrate that during postnatal liver development, Borealin deficiency induces hepatocyte mitotic defects and leads to genome polyploidy and cell hypertrophy.
FIGURE 3.
Borealin is required for mitotic transition during postnatal liver development. A, co-immunofluorescent staining of α-tubulin and Aurora-B kinase in BorealinΔli livers. Scale bars, 5 μm. B, IHC staining showed that the numbers of pH3S10-positive or pH3S28-positive hepatocytes were reduced in borealinΔli livers. *, p < 0.05. Scale bars, 50 μm.
No Induction of Liver Progenitors in Borealin-deficient Livers
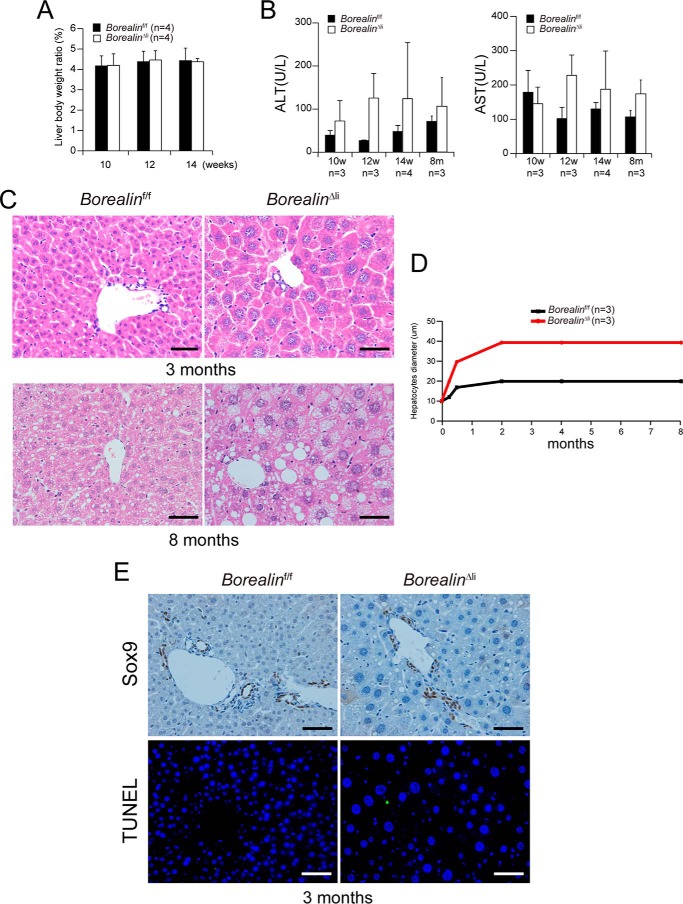
In our previous study, we found that liver-specific deletion of another CPC component, Survivin, caused cell death and liver damage, which triggered extensive oval cell proliferation in the 3-month-old Alb-Cre, Survivinf/f (svvΔli) mice (11). However, in contrast to increased liver/body weight ratio, cell death, and liver damage in the svvΔli mice, the liver/body weight ratio and liver functions of the 3- and 8-month-old BorealinΔli mice were unexpectedly normal (Fig. 4, A and B). Histological inspection revealed that hepatocyte size of 3- and 8-month-old BorealinΔli mice remained enlarged (Fig. 4, C and D). Cell death was undetectable and there was no obvious oval cell expansion in the 3-month-old BorealinΔli mice (Fig. 4E). The distinct phenotypes between Borealin and Survivin liver-specific deletion indicate that Survivin may regulate other signaling pathways, e.g. cell apoptosis, independent of Borealin and CPC (Table 1).
FIGURE 4.
Borealin deletion does not lead to oval cell expansion in 3–8-month-old BorealinΔli mice. A, BorealinΔli mice showed normal liver/body weight ratios. B, serum ALT and AST levels were measured in Borealinf/f and BorealinΔli mice at the indicated ages. C, hematoxylin and eosin staining of liver sections from 3- and 8-month-old mice. Scale bars, 50 μm. D, hepatocyte diameters were quantified. E, IHC staining showed that there was no Sox9+ oval cell expansion. TUNEL staining showed that apoptosis of hepatocytes was undetectable.
TABLE 1.
Phenotype comparison between BorealinΔli and SurvivinΔli mice
| SurvivinΔli | BorealinΔli | |
|---|---|---|
| M phase block | Yes | Yes |
| Polyploidy | Yes | Yes |
| Hepatocyte hypertrophy | Yes | Yes |
| Hepatocyte death | Yes | No |
| Inflammation | Yes | No |
| Ductal reaction/LPC | Yes | No |
| HCC resistance | Yes | Yes |
Borealin Is Essential to DDC-induced Formation of Sox9+HNF4α+ Progenitor-like Cells
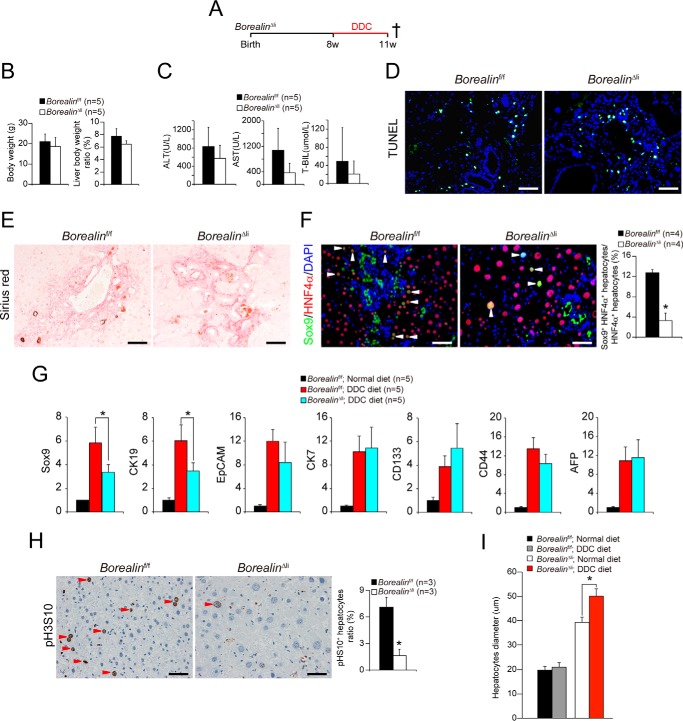
Because Borealin deficiency in liver did not induce hepatocyte death and oval cell reaction, we questioned whether regeneration induced by additional injuries might have also interfered in the BorealinΔli mice. We used the DDC diet to induce liver damage in 2-month-old BorealinΔli mice (Fig. 5A). After 3 weeks of DDC feed, the liver weights of the control mice were significantly increased, consistent with previous studies (11). The liver weight was comparably increased in the BorealinΔli mice (Fig. 5B). The serum ALT and AST levels and periportal cell death in the BorealinΔli mice were comparable with the Borealinf/f livers after DDC treatment (Fig. 5, C and D). Moreover, the DDC diet induced the deposition of collagen in both the BorealinΔli and the Borealinf/f livers (Fig. 5E). All of these results indicated that the liver damage induced by the DDC diet was comparable in the BorealinΔli and Borealinf/f livers.
FIGURE 5.
Borealin deficiency inhibits DDC-induced Sox9+HNF4α+ progenitor-like cell proliferation. A, 8-week-old Borealinf/f and BorealinΔli mice were treated with DDC diet. These mice were killed 3 weeks later. B, liver/body weight ratios of BorealinΔli and control Borealinf/f mice 3 weeks after DDC treatment were measured. C, serum ALT, AST, and T-BIL levels were measured in Borealinf/f and BorealinΔli mice after DDC treatment. D, apoptosis of hepatocytes was confirmed by TUNEL staining. E, Sirius Red staining in DDC-treated BorealinΔli and Borealinf/f livers. F, Sox9 and HNF4α immunofluorescent staining and Sox9+HNF4α+ progenitor-like cells were quantified. G, the mRNA levels of hepatocyte progenitor genes were measured by q-PCR in DDC-treated BorealinΔli and Borealinf/f livers. H, hepatocyte mitosis was determined by pH3S10 IHC staining. Ratio of pH3S10 positive hepatocytes was quantified. I, hepatocyte diameters were quantified.
The DDC treatment induced oval cell reaction in the Borealinf/f livers. Interestingly, the oval cell reaction was reduced in the BorealinΔli livers (Fig. 5F). Recent studies showed that Sox9+HNF4α+ progenitor-like cells, which might be derived from hepatocytes in response to injury, expanded and subsequently contributed to restoration of the liver mass (2, 22). Consistently, we found that DDC treatment induced the expansion of Sox9+HNF4α+ progenitor-like cells in the Borealinf/f livers (Fig. 5F). Notably, the number of Sox9+HNF4α+ progenitor-like cells was markedly decreased in the BorealinΔli livers (Fig. 5, F and G), which may be caused by disabled proliferation capacity of the Borealin-deficient cells. Consistently, the total pH3S10-positive cells were markedly decreased in the BorealinΔli livers after DDC treatment (Fig. 5H). Interestingly, we found the hepatocyte diameter was further increased to about 50 μm in BorealinΔli livers after DDC treatment (Fig. 5I), which indicated that liver regeneration was impaired by the decrease of Sox9+HNF4α+ progenitor-like cells and accomplished by compensatory hypertrophy of hepatocytes. We further analyzed liver injury and hepatocyte death for a prolonged DDC treatment of 8 weeks. We found that hepatocyte death in BorealinΔli livers was comparable with that in the control mice (supplemental Fig. S2, A–D). Our data demonstrate that Borealin deficiency inhibits DDC diet-induced formation of Sox9+HNF4α+ progenitor-like cells and impairs the liver regeneration.
Borealin Deficiency Prevents Oncogene-induced HCC Development
The above data show that Borealin was essential for hepatocyte proliferation both in postnatal liver development and in damage-induced formation of progenitor-like cells. Our previous study showed that Survivin was highly expressed in HCCs and promoted HCC initiation and development (11, 13, 14). Others found that Borealin was highly expressed in gastric, colorectal, and lung cancers, and was essential to cancer cell proliferation (18–20). These data indicated that Borealin might also play an important role in HCC development.
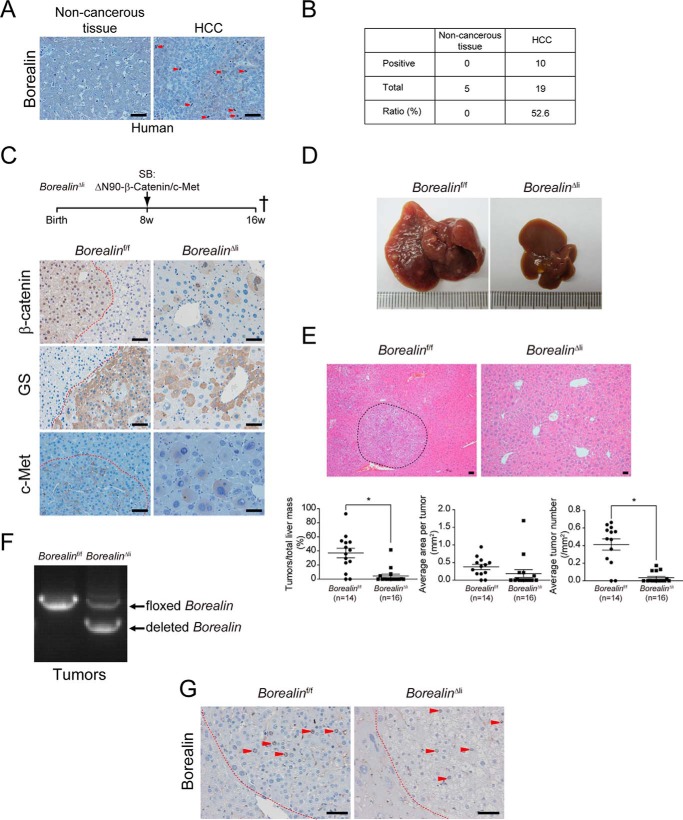
In clinical HCC samples, we found that 52.6% (10/19) of HCCs were Borealin positive and the paired non-cancerous tissues were all Borealin negative (Fig. 6, A and B). These data indicated that Borealin had important functions in HCC development. We analyzed the in vivo function of Borealin in HCC development by Sleeping Beauty transposase (SBT)-mediated human ΔN90-β-Catenin and c-Met overexpression in the liver. In this model, ΔN90-β-Catenin and c-Met oncogenes were inserted into the hepatocyte genome by SBT, and the overexpression of these two oncogenes induced malignant HCCs (23). The 8-week-old BorealinΔli mice were transfected with SBT-ΔN90-β-Catenin/c-Met plasmids through hydrodynamic tail vein injection (24) (Fig. 6C). IHC staining showed that human β-Catenin protein and c-Met were both highly expressed in the BorealinΔli and Borealinf/f livers (Fig. 6C). The target gene of β-Catenin, glutamine synthetase, was also highly induced (Fig. 6C).
FIGURE 6.
Borealin deficiency inhibits ΔN90-β-Catenin/c-Met-induced HCC development. A, borealin protein levels were characterized by IHC staining in human HCC samples. Arrowheads indicate Borealin-positive cells. B, borealin positive ratio in human HCC samples was quantified. C, HCC was induced by the hydrodynamic transfection of plasmids encoding the Sleeping Beauty transposase and transposons with oncogenes of ΔN90-β-Catenin and c-Met in 8-week-old BorealinΔli and control Borealinf/f mice. These mice were sacrificed 8 weeks later to quantify the HCC mass. IHC staining showed that human β-Catenin protein and its downstream target glutamine synthetase (GS) were both highly expressed in the BorealinΔli and Borealinf/f livers 8 weeks after transfection. D, large amounts of HCCs developed in control Borealinf/f mice but not in BorealinΔli livers. E, H&E staining of liver sections from the BorealinΔli and control Borealinf/f mice. Liver cancers were encircled by dashed lines. Total tumor mass, tumor number, and tumor size were quantified. *, p < 0.05; t test. F, genotypes were determined by PCR using HCC genomic DNA. G, IHC staining showed that there were many Borealin-positive cells in HCCs of the BorealinΔli mice. Scale bars, 50 μm.
HCCs were developed in the control Borealinf/f mice 8 weeks after transfection (Fig. 6, D and E). By contrast, HCC development was almost completely inhibited in the BorealinΔli livers (Fig. 6, D and E). Tumor quantification demonstrated that both total tumor mass and average tumor size were reduced in the BorealinΔli mice (Fig. 6E). Interestingly, a few tumors developed in the BorealinΔli mice. We found that the deletion efficiency of Borealin was low and Borealin-positive cells were detected in HCCs of the BorealinΔli mice (Fig. 6, F and G), indicating that Borealin was essential for HCC development and hepatocytes that escaped Cre recombinase-mediated excision of floxed alleles were transformed by ΔN90-β-Catenin/c-Met and developed into HCC.
Discussion
The liver provides the important function of detoxification of chemicals and metabolites, which increases the likelihood that the liver will experience damage and a loss of tissue mass. Although the damage can be repaired by rapid liver regeneration, hepatocyte death is apt to elicit a cyclical inflammatory response that triggers further cell death and compensatory proliferation, followed by eventual development of HCC. Explicating the mechanisms underlining the liver damage-regeneration response and HCC development will provide potential targets for liver diseases.
The CPC plays important roles in cell mitosis and proliferation and is therefore highly expressed in cells with a high mitotic index, such as embryonic and neonatal tissues, adult progenitor cells, and tumor cells. The essential role of the CPC components, Borealin, Survivin, INCENP, and Aurora B kinase for ontogenesis has long been known (16, 17, 25, 26). However, the specific functions of each component in physiological and pathological conditions remain largely uncharacterized. We previously found that hepatic loss of Survivin resulted in hepatocyte mitotic arrest, hypertrophy, genome polyploidy, and chronic cell death during postnatal liver development, which caused oval cell proliferation and reconstitution of the liver mass (11). Moreover, we found that Survivin was essential to HCC initiation and development (13, 14). Because Survivin regulates cell mitosis through CPC and cell apoptosis as an inhibitor of apoptosis protein, analyzing the function of other CPC components during postnatal liver development, liver homeostasis, and HCC development will enhance our understanding of the function of CPC in these physiological and pathological conditions.
In the present study, which used a mouse model of conditional Borealin deletion in the liver, we found that perinatal loss of Borealin impaired hepatocyte mitosis and induced hepatocyte polyploidy and hypertrophy but did not induce oval cell reaction. We found that Survivin or Borealin deletion in the liver resulted in hepatocyte mitotic arrest, hypertrophy, and genome polyploidy. Interestingly, we found that Borealin deficiency caused a milder phenotype and less severe liver damage when compared with those in Survivin-deficient mice. Moreover, Survivin rather than Borealin deletion induced hepatocyte death, oval cell proliferation, and liver mass replenishment during postnatal liver development (11) (Table 1). The oval cells that appeared in the SurvivinΔli livers were Survivin-positive cells that escaped Cre-mediated recombination. However, Borealin-positive oval cells were undetectable in the uninjured BorealinΔli livers. The differences between the phenotypes of the BorealinΔli and the SurvivinΔli mice indicated that Survivin might play other roles besides the one in CPC. It is well known that Survivin is an inhibitor of apoptosis protein and regulates cell apoptosis. Survivin deletion consistently enhanced hepatocyte death and liver damage, which further induces Survivin-positive oval cell proliferation and reconstitution of the liver (11). In contrast, cell death was undetectable in the BorealinΔli mice (Fig. 4E).
Sox9+HNF4α+ progenitor-like cells emerge under injury conditions in the periportal region of the liver (2). We found that the DDC diet induced similar liver injury in the BorealinΔli and control mice; however, Sox9+HNF4α+ progenitor-like cells were dramatically decreased in BorealinΔli mice, which indicated that Borealin deficiency might induce mitosis impairment in Sox9+HNF4α+ progenitor-like cells. Consistently, the pH3S10-positive cells were markedly decreased in the BorealinΔli livers.
Consistent with the essential functions of Borealin in hepatocyte proliferation and injury-induced regeneration, we found that Borealin was highly expressed in human HCC samples and Borealin deficiency suppressed ΔN90-β-Catenin/c-Met-induced HCC development, which was similar to the HCC suppression effect of Survivin deletion. Both Borealin and Survivin deficiency induced disturbed localization and dysfunction of Aurora B kinase, implying that the CPC complex was essential to HCC development and the HCC suppressive effect of Borealin or Survivin deletion was largely caused by the dysfunction of the CPC complex. Our data indicate that Borealin played important roles in HCC development and targeting Borealin appears to be a better strategy for HCC therapy with less adverse effect on liver tissue damage and repair. Screening small compounds that can disturb the interactions between Survivin and Borealin or between Survivin/Borealin and phosphorylated histones, renders prospective value for HCC therapy.
Experimental Procedures
Mice
The BorealinΔli mice were generated by crossing Borealinf/f mice with Alb-Cre mice, with the Cre recombinase gene under the control of the albumin promoter and enhancer (21). Liver samples were collected at the indicated time points for various assays.
Mouse Hepatocyte Isolation
Mouse hepatocytes were isolated following a modified two-step perfusion and collagenase digestion. Briefly, mice were anesthetized with ketamine. Surgery was performed to insert a catheter into the hepatic vein. The livers were then perfused with calcium-free and magnesium-free Earle's balanced salt solution (Hyclone) containing 100 mm EGTA (Sigma) and 10 mm Hepes, pH 7.4 (Sigma), and then 0.025% collagenase type IV (Sigma) in Earle's balanced salt solution with calcium and magnesium (Hyclone). After perfusion, liver cells were suspended in calcium-free and magnesium-free Earle's balanced salt solution containing 90% Percoll (Sigma) and then filtered through a 100-μm cell strainer (BD Bioscience).
Histological Analysis
Liver tissue samples were fixed by paraformaldehyde and embedded in paraffin. Sections were subjected to hematoxylin and eosin (H&E), IHC, immunofluorescent, tyramide signal amplification, and TdT-mediated dUTP nick end labeling (TUNEL) staining.
IHC staining was performed using the Elite ABC kit (Vector Laboratories). For immunohistochemistry, deparaffinized and rehydrated slides were subjected to autoclave antigen retrieval in a 10 mmol/liter of citric acid buffer, pH 6.0, and allowed to cool to room temperature. Slides were blocked with 3% H2O2 for 30 min, washed in phosphate-buffered saline, and then blocked with 5% normal goat serum in PBS. Slides were incubated with diluted primary antibodies overnight at 4 °C. The following primary antibodies were used: rabbit anti-phospho-Histone H3Ser-10 (1:1000 dilution, Upstate Biotechnology), rabbit anti-phospho-Histone H3Ser-28 (1:1000 dilution, Novus Biologicals), mouse anti-Borealin (1:200 dilution, Santa Cruz), rabbit anti-Sox9 (1:1000 dilution, Millipore), mouse anti-β-Catenin (1:500 dilution, BD Biosciences), mouse anti-glutamine synthetase (1:500 dilution, BD Biosciences), and rabbit anti-c-Met (1:200 dilution, Cell Signaling). Biotinylated anti-rabbit (Vector Laboratories) and anti-mouse (Vector Laboratories) antibodies were used as secondary antibodies according to the manufacturer's protocol. Biphenyl-3,3′,4,4′-tetrayltetraammonium tetrachloride (Dako) was used as a chromogen according to the manufacturer's protocol. TUNEL assays were performed using the fluorescein cell death detection kit (Clontech). Images were observed using a Leica BX51 microscope.
For immunofluorescence staining, deparaffinized and rehydrated slides were subjected to autoclave antigen retrieval in a 10 mmol/liter of citric acid buffer, pH 6.0, and allowed to cool to room temperature. Blocking was performed in 1% BSA and 0.1% Triton X-100 for 1 h and then incubated with primary antibodies at room temperature for 2 h or at 4 °C overnight. The following primary antibodies were used: mouse anti-α-tubulin (1:1000 dilution, Sigma), rabbit anti-Aurora-B (1:1000 dilution, Abcam), and goat anti-HNF4α (1:250 dilution, Santa Cruz). After incubation with FITC-conjugated goat anti-rabbit IgG (1:1000 dilution, Molecular Probes), Cy3-conjugated goat anti-mouse IgG (1:1000 dilution; Jackson Laboratories), and FITC-conjugated donkey anti-rat IgG (1:1000 dilution, Molecular Probes) secondary antibodies for 1 h at room temperature and counterstaining with DAPI, fluorescence images were observed using a Leica BX51 microscope.
Serum Contents Measurement
Blood was collected and centrifuged at 12,000 × g for 10 min. Freshly isolated supernatant plasma was used for measuring ALT and AST activity and the total bilirubin level according to the manufacturer's instructions (Shanghai Shensuo UNF Medical Diagnostic Articles Co., Ltd.).
Hydrodynamic Transfection
Procedures were as described previously (24). Between 10 and 50 μg of the plasmids encoding the Sleeping Beauty transposase and transposons with ΔN90-β-Catenin and c-Met oncogenes in a ratio of 2:25 were diluted in 2.5 ml of filtered 0.9% NaCl and then injected into the lateral tail veins of the 8-week-old Borealinf/f and BorealinΔli mice. Ectopic expression of ΔN90-β-catenin and c-Met in the mouse liver can induce HCC efficiently (23).
Quantitative Real-time PCR (q-PCR)
Total RNA was prepared from liver tissue samples using TRIzol (Invitrogen) according to the manufacturer's protocol. Reverse transcriptase PCR was performed using a Moloney murine leukemia virus reverse transcriptase (Promega). Real-time PCR were performed with SYBR Premix Ex Taq (Takara) and 300 nmol/liter of each primer. Amplification was performed according to the manufacturer's protocol of the 7500 Fast Real-time PCR Systems (Applied Biosystems, CA).
Karyotyping
Tail-tip fibroblast cells (TTFs) were isolated from 4-week-old Borealinf/f mice. ∼2 cm length of tail tip was cut and washed with 70% ethanol and PBS. After dermis was peeled away, the remaining part was placed into a 60-mm collagen-coated dish in 5 ml of DMEM (Hyclone) containing 10% FBS (Gibco). After 5 days incubation, fibroblasts that migrated out were trypsinized and expanded to a 10-cm collagen-coated dish.
For karyotypes, TTFs were treated with Adeno-Cre virus or Adeno-GFP virus. After 72 h, TTFs were treated with 50 μg/ml of colchicine (Sigma) for 3 h and harvested by trypsinization. Then, after extensive washing, cells were incubated at 37 °C for 30 min in 75 mm KCl and fixed with methanol:acetic acid (3:1 ratio). Finally, chromosomes from 50 metaphase-arrested TTFs/sample were G-banded with a standard trypsin/Wright's stain protocol. Photographs were taken using CytoVision software from Applied Imaging.
Author Contributions
L. H., D. L., and L. L. designed the experiments and analyzed data. L. L. and D. L. performed most of the experiments. F. T. and Y. J. performed histological analyses. J. C. and X. C. provided technical support and animal care. D. L., L. L., and L. H. wrote the manuscript.
Supplementary Material
This work was supported in part by Ministry of Science and Technology (MOST) of China Grants 2014CB910601 and 2012CB945001, National Science Foundation of China (NSFC) Grants 31225016, 81471948, and 31501111, and Shanghai Science and Technology Committee Grants 12JC1409500 and 14XD1404200. The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Figs. S1 and S2.
- HCC
- hepatocellular carcinoma
- CPC
- chromosomal passenger complex
- IHC
- immunohistochemical
- ALT
- alanine aminotransferase
- AST
- aspartate aminotransferase
- SBT
- Sleeping Beauty transposase
- TTF
- tail-tip fibroblast cell
- q-PCR
- quantitative PCR.
References
- 1. Duncan A. W., Dorrell C., and Grompe M. (2009) Stem cells and liver regeneration. Gastroenterology 137, 466–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tarlow B. D., Pelz C., Naugler W. E., Wakefield L., Wilson E. M., Finegold M. J., and Grompe M. (2014) Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell stem cell 15, 605–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu W. Y., Bird T. G., Boulter L., Tsuchiya A., Cole A. M., Hay T., Guest R. V., Wojtacha D., Man T. Y., Mackinnon A., Ridgway R. A., Kendall T., Williams M. J., Jamieson T., Raven A., et al. (2015) Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat. Cell Biol. 17, 971–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Michalopoulos G. K., and Khan Z. (2015) Liver stem cells: experimental findings and implications for human liver disease. Gastroenterology 149, 876–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamaji S., Zhang M., Zhang J., Endo Y., Bibikova E., Goff S. P., and Cang Y. (2010) Hepatocyte-specific deletion of DDB1 induces liver regeneration and tumorigenesis. Proc. Natl. Acad. Sci. U.S.A. 107, 22237–22242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang E. Y., Yeh S. H., Tsai T. F., Huang H. P., Jeng Y. M., Lin W. H., Chen W. C., Yeh K. H., Chen P. J., and Chen D. S. (2011) Depletion of β-catenin from mature hepatocytes of mice promotes expansion of hepatic progenitor cells and tumor development. Proc. Natl. Acad. Sci. U.S.A. 108, 18384–18389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benhamouche S., Curto M., Saotome I., Gladden A. B., Liu C. H., Giovannini M., and McClatchey A. I. (2010) Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev. 24, 1718–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. El-Serag H. B. (2011) Hepatocellular carcinoma. N. Engl. J. Med. 365, 1118–1127 [DOI] [PubMed] [Google Scholar]
- 9. Jeyaprakash A. A., Klein U. R., Lindner D., Ebert J., Nigg E. A., and Conti E. (2007) Structure of a Survivin-Borealin-INCENP core complex reveals how chromosomal passengers travel together. Cell 131, 271–285 [DOI] [PubMed] [Google Scholar]
- 10. Carmena M., Wheelock M., Funabiki H., and Earnshaw W. C. (2012) The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat. Rev. Mol. Cell Biol. 13, 789–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li D., Cen J., Chen X., Conway E. M., Ji Y., and Hui L. (2013) Hepatic loss of survivin impairs postnatal liver development and promotes expansion of hepatic progenitor cells in mice. Hepatology 58, 2109–2121 [DOI] [PubMed] [Google Scholar]
- 12. Hagemann S., Wohlschlaeger J., Bertram S., Levkau B., Musacchio A., Conway E. M., Moellmann D., Kneiseler G., Pless-Petig G., Lorenz K., Sitek B., and Baba H. A. (2013) Loss of Survivin influences liver regeneration and is associated with impaired Aurora B function. Cell Death Differ. 20, 834–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Min L., Ji Y., Bakiri L., Qiu Z., Cen J., Chen X., Chen L., Scheuch H., Zheng H., Qin L., Zatloukal K., Hui L., and Wagner E. F. (2012) Liver cancer initiation is controlled by AP-1 through SIRT6-dependent inhibition of survivin. Nat. Cell Biol. 14, 1203–1211 [DOI] [PubMed] [Google Scholar]
- 14. Li D., Fu J., Du M., Zhang H., Li L., Cen J., Li W., Chen X., Lin Y., Conway E. M., Pikarsky E., Wang H., Pan G., Ji Y., Wang H. Y., and Hui L. (2016) Hepatocellular carcinoma repression by TNFα-mediated synergistic lethal effect of mitosis defect-induced senescence and cell death sensitization. Hepatology 10.1002/hep.28637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gassmann R., Carvalho A., Henzing A. J., Ruchaud S., Hudson D. F., Honda R., Nigg E. A., Gerloff D. L., and Earnshaw W. C. (2004) Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J. Cell Biol. 166, 179–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hanson K. K., Kelley A. C., and Bienz M. (2005) Loss of Drosophila borealin causes polyploidy, delayed apoptosis and abnormal tissue development. Development 132, 4777–4787 [DOI] [PubMed] [Google Scholar]
- 17. Yamanaka Y., Heike T., Kumada T., Shibata M., Takaoka Y., Kitano A., Shiraishi K., Kato T., Nagato M., Okawa K., Furushima K., Nakao K., Nakamura Y., Taketo M. M., Aizawa S., and Nakahata T. (2008) Loss of Borealin/DasraB leads to defective cell proliferation, p53 accumulation and early embryonic lethality. Mech. Dev. 125, 441–450 [DOI] [PubMed] [Google Scholar]
- 18. Wang Y., Zhao Z., Bao X., Fang Y., Ni P., Chen Q., Zhang W., and Deng A. (2014) Borealin/Dasra B is overexpressed in colorectal cancers and contributes to proliferation of cancer cells. Med. Oncol. 31, 248. [DOI] [PubMed] [Google Scholar]
- 19. Hayama S., Daigo Y., Yamabuki T., Hirata D., Kato T., Miyamoto M., Ito T., Tsuchiya E., Kondo S., and Nakamura Y. (2007) Phosphorylation and activation of cell division cycle associated 8 by Aurora kinase B plays a significant role in human lung carcinogenesis. Cancer Res. 67, 4113–4122 [DOI] [PubMed] [Google Scholar]
- 20. Chang J. L., Chen T. H., Wang C. F., Chiang Y. H., Huang Y. L., Wong F. H., Chou C. K., and Chen C. M. (2006) Borealin/Dasra B is a cell cycle-regulated chromosomal passenger protein and its nuclear accumulation is linked to poor prognosis for human gastric cancer. Exp. Cell Res. 312, 962–973 [DOI] [PubMed] [Google Scholar]
- 21. Postic C., and Magnuson M. A. (2000) DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis 26, 149–150 [DOI] [PubMed] [Google Scholar]
- 22. Font-Burgada J., Shalapour S., Ramaswamy S., Hsueh B., Rossell D., Umemura A., Taniguchi K., Nakagawa H., Valasek M. A., Ye L., Kopp J. L., Sander M., Carter H., Deisseroth K., Verma I. M., and Karin M. (2015) Hybrid periportal hepatocytes regenerate the injured liver without giving rise to cancer. Cell 162, 766–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tward A. D., Jones K. D., Yant S., Cheung S. T., Fan S. T., Chen X., Kay M. A., Wang R., and Bishop J. M. (2007) Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc. Natl. Acad. Sci. U.S.A. 104, 14771–14776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen X., and Calvisi D. F. (2014) Hydrodynamic transfection for generation of novel mouse models for liver cancer research. Am. J. Pathol. 184, 912–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Uren A. G., Wong L., Pakusch M., Fowler K. J., Burrows F. J., Vaux D. L., and Choo K. H. (2000) Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Curr. Biol. 10, 1319–1328 [DOI] [PubMed] [Google Scholar]
- 26. Tatsuka M., Katayama H., Ota T., Tanaka T., Odashima S., Suzuki F., and Terada Y. (1998) Multinuclearity and increased ploidy caused by overexpression of the Aurora- and Ipl1-like midbody-associated protein mitotic kinase in human cancer cells. Cancer Res. 58, 4811–4816 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.