Abstract
Background
Determining risk factors for opioid abuse or dependence will help clinicians practice informed prescribing and may help mitigate opioid abuse or dependence. The purpose of this study is to identify variables predicting opioid abuse or dependence.
Methods
A retrospective cohort study using de-identified integrated pharmacy and medical claims between October 2009 and September 2013. Patients with at least one opioid prescription claim during the index period (index claim) were identified. We ascertained risk factors using data from 12 months prior to index claim (pre-period) and captured abuse or dependency diagnosis using data from 12 months post index claim (post-period). We included continuously eligible (pre- and post-period) commercially insured patients aged 18 or older. We excluded patients with cancer, residence in a long-term care facility, or previous diagnosis of opioid abuse or dependence (identified by International Classification of Diseases-9th revision (ICD-9) code or buprenorphine/naloxone claim in the pre-period). The outcome was a diagnosis of opioid abuse (ICD9 code 304.0×) or dependence (305.5).
Results
The final sample consisted of 694,851 patients. Opioid abuse or dependence was observed in 2,067 patients (0.3%). Several factors predicted opioid abuse or dependence: younger age [per decade (older) odds ratio (OR) 0.68], being a chronic opioid user [OR 4.39], history of mental illness [OR 3.45], non-opioid substance abuse [OR 2.82], alcohol abuse [OR 2.37], high morphine equivalent dose per day user [OR 1.98], tobacco use [OR 1.80], obtaining opioids from multiple prescribers [OR 1.71], residing in the South [OR 1.65], West [OR 1.49], or Midwest [OR 1.24], using multiple pharmacies [OR 1.59], male gender [OR 1.43], and increased 30-day adjusted opioid prescriptions [OR 1.05].
Conclusions
Readily available demographic, clinical, behavioral, pharmacy and geographic information can be used to predict likelihood of opioid abuse or dependence.
Keywords: Opioid abuse, opioid dependence, predictive model, demographic factors, pharmacy claims based factors, prescription drug monitoring program
Introduction
The United States has seen a dramatic rise in opioid prescriptions in the past decade with a concomitant increase in abuse of opioid medications.1 There has been a tripling in the rate of opioid related overdose deaths from 2000 to 2014, with over 28,000 deaths in 2014.2 This epidemic creates a dilemma for prescribers who seek to provide adequate pain relief while minimizing risks of abuse and dependence. Abuse is defined as the intentional self-administration of a medication for a non-medical reason3 while dependence is a maladaptive pattern of substance use.4, 5
Guidelines exist for using opioids in non-cancer pain,6 but prescribers face challenging situations when prescribing opioids and need tools to aid their decisions. Prescription Drug Monitoring Programs can help reveal aberrant behavior. Forty-nine states have enacted these programs; however monitoring alone does not prevent abuse.7–10 Currently, there are limited tools that help predict which patients may develop opioid abuse or dependence. The Opioid Risk Tool (ORT) identifies at risk patients based on past medical, family and social history.11 However, the ORT does not combine patient and prescription drug monitoring program information to assess risk. Clinicians need to know how risk factors ascertained at the time of prescribing opioids predict subsequent abuse or dependence.
The objective of this study is to identify demographic characteristics, clinical, behavioral factors obtained from prescription drug monitoring programs, pharmacy and geographic factors that quantify the risk of developing opioid abuse or dependence. These factors are immediately available to a prescriber by patient interview and by accessing a prescription drug monitoring program and could help assess risk of prescribing opioids. Once at-risk patients are identified, additional screening tests could be employed by the prescriber12, 13 and treatment of abuse and dependence could be pursued.
METHODS
We used de-identified (in accordance with Health Insurance Portability and Accountability Act (HIPAA) requirements) pharmacy and medical claims data from a pharmacy benefit manager (Express Scripts) from October 1, 2009, to September 30, 2013. These data include health insurance claims (inpatient/outpatient medical, and outpatient pharmacy) and enrollment data from large employers and health plans across the United States. This study included patients 18 years old or older as of the index opioid claim date.
International Classification of Diseases, Ninth Revision (ICD–9) codes were used to identify medical diagnoses. First Data Bank ‘Smart Key’ classifications were used to identify opioids based on pharmacy claims.14 Smart Key Specific Therapeutic Class designations (4-digit codes describing therapeutic drug classes) and Generic Code Numbers (5-digit numbers that group equivalent products based on active ingredients) were used to classify pharmacy claims (Appendix 1). Dosage strengths for Specific Therapeutic Class were used in calculating daily morphine equivalent dosing and to classify immediate vs. extended release opioids.
Exclusion criteria included patients with a cancer diagnosis (Appendix 2), claims for chemotherapy or antiemetics (Appendix 3), residence in long-term care facilities (residence code of 03 from the National Council of Prescription Drug Programs 384-4× classification), in convalescence following chemotherapy (ICD-9 V66.2) or in hospice/palliative/end-of-life care (ICD-9 V66.7). Patients with a prior opioid dependency diagnosis (within 365 days prior to the index claim) or were on buprenorphine/naloxone (typically used to treat opioid dependence) also were excluded (Appendix 4).
To predict the likelihood of opioid abuse or dependency, we conducted a retrospective claims analysis. Derivation and validation models were developed. For the derivation model (Figure 1), we identified patients based on ≥1 claim for opioids in the index period (October 1, 2011 to September 30, 2012), the index claim was a randomly selected opioid claim. For the validation model, we identified patients based on ≥1 claim for opioids in the index period (October 1, 2010 to September 30, 2011), again randomly selected. For both models, we ascertained risk factors using data from 12 months prior to index claim (pre-period) and captured abuse or dependency diagnosis by ICD-9 code using data from 12 months post index claim (post-period). All patients were continuously eligible during pre- and post-periods.
Figure 1.
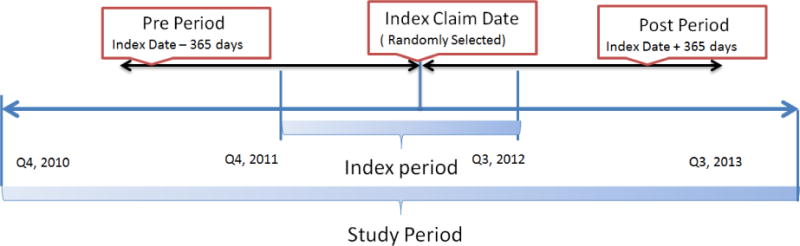
Study Timeline for the Derivation Model
Note: The index period for the out-of-time validation model was Q4, 2010 to Q3 2011.
The primary outcome measure was an ICD-9 diagnosis of nondependent opioid abuse (304.0×) or dependence (305.5×) in the post-period. Patient characteristics based on pharmacy and medical claims were included as independent variables, including demographic, clinical, behavioral, pharmacy claims and geographical factors. All factors were measured prior to the index date.
Variables Included age15 (calculated at the index claim) and the chronic use of opioids16 (defined as claims for more than 90 days of opioids in the 6 months prior to and including the index date). Clinical variables of history of mental illness,17,18 non-opioid substance abuse,17 and nondependent alcohol abuse17,18, 19 were identified by ICD-9 codes (Appendix 5). We identified high morphine equivalent dose users (≥120 mg morphine equivalent dosing daily),20–22 using pharmacy claims. The other clinical variable, tobacco use disorder,18, was identified by ICD-9 code as well.
Prescriber shopping was hypothesized to be a risk factor15, 24, 25 and patients were identified as prescriber shoppers if they received opioid prescriptions from ≥2 prescribers15 within 60 days prior to and inclusive of the index date. Geographic region is associated with opioid abuse or dependence.17 Patients were classified into geographic regions (Northeast, South, West, or Midwest as defined by US Census Bureau26) based on their index claim state of residence.
Pharmacy shopping was also considered as a risk factor15,24,27 and patients were considered pharmacy shoppers if they filled opioid prescriptions at ≥3 pharmacies15, 24 within 60 days prior to and inclusive of the index date. Prior research indicates that men are more likely to be opioid abusers than women.17, 28 We hypothesized that the same relationship would be observed for opioid abuse or dependence.
Pharmacy claims were used to determine the number opioid prescriptions in the pre-period.17 To capture prior use of opioids, the number of 30-day adjusted opioid prescriptions in the pre-period was used. Days’ supply of all opioid prescriptions was divided by 30.4 days/month to convert to months and address the days’ supply differential between dispensing channels. Pharmacy claims also identified long-term use of immediate release opioids. For patients who were on opioids for at least 6 months of the pre-period, we computed a ratio of immediate release to total opioids being taken. Patients were considered chronic immediate release users if this ratio exceeded 0.5.
We computed a binary distance variable of less than and greater than 50 miles from patient to index opioid prescriber based on centroids of the respective zip codes. We hypothesized that potential opioid abusers or dependents would travel farther to receive an opioid prescription.
From a population of approximately 1.4 million patients with at least 1 opioid claim during the index period for the derivation model, 694,851 patients constituted the final analytical sample (Table 1). Datasets were created and statistical analyses were conducted using SAS version 9.3 and SAS Enterprise Guide version 5.1 (SAS Inc., Cary, NC). Descriptive statistics included comparison of bivariate differences in risk factors between opioid abusers or dependents and non-abusers or non-dependents using analysis of variance for continuous variables and chi-square tests for categorical variables. All comparisons were 2-tailed and a p<0.05 was considered statistically significant. Variance inflation factor was analyzed to ascertain multicollinearity among independent variables.
Table 1.
Sample Selection Methodology and Description of Sample Size
| Study Selection Criteria | Derivation Model N |
Validation Model N |
|---|---|---|
| Total patients having opioid prescription claims during index period* | 1,428,137 | 1,453,996 |
| No cancer diagnosis or medication | 1,348,793 | 1,376,236 |
| Not in long term care facilities | 1,345,908 | 1,376,210 |
| Not in hospice care facilities | 1,345,720 | 1,376,052 |
| No diagnosis for prior drug dependency | 1,339,418 | 1,370,631 |
| Continuously eligible during pre- and post-period | 751,937 | 689,519 |
| 18 years of age or older as of the index date | 696,922 | 636,620 |
| No missing values for key covariates | 694,851 | 634,588 |
Derivation Model: Index period Q4 2011–Q3 2012; Validation Model: Index period Q4 2010–Q3 2011
Multivariate logistic regression analyses were performed to predict the likelihood of opioid abuse or dependence. To address potential bias in the estimated coefficient and to test the robustness of the findings, two sensitivity analyses were conducted (Appendix 6).
Validation was conducted to assess performance of the predictive model in an independent sample. The validation model design was identical to the derivation model with the exception of index period. The index period for the validation model was one year earlier, from October 1, 2010 to September 30, 2011, which resulted in a cohort of 634,588 patients.
This research was exempt from Institutional Review Board approval based on the Code of Federal Regulation, §46.101b, from the United States Department of Health and Human Resources,29 and exempted from the Washington University IRB.
Results
The derivation cohort included 694,851 patients, of which 2,067 patients (0.3%) were opioid abusers/dependents. They were significantly younger (Table 2). There were more chronic opioid users (55.8% vs 10.4%) in the group who developed abuse or dependence.
Table 2.
Baseline Characteristicsa
| Derivation Model | Validation Model | |||
|---|---|---|---|---|
| Measure | Dependent | Non-Dependent | Dependent | Non-Dependent |
| N | 2,067 | 692,784 | 1,580 | 633,008 |
| Age [Mean (Std. Dev)] | 44.1 (15.5) | 48.8 (15.6) | 43.6 (15.1) | 49.1 (15.6) |
| Chronic users [N (%)] | 1,154 (55.8) | 72,072 (10.4) | 869 (55.0) | 62,597 (9.9) |
| Mental illness [N (%)] | 1,076 (52.1) | 103,398 (14.9) | 781 (49.4) | 86,546 (13.7) |
| Non-opioid substance abuse [N (%)] | 84 (4.1) | 1,696 (0.2) | 59 (3.7) | 1,280 (0.2) |
| Non-dependent alcohol abuse [N (%)] | 82 (4.0) | 3,191 (0.5) | 55 (3.5) | 2,623 (0.4) |
| Daily MED ≥ 120 mg/day [N (%)] | 398 (19.3) | 13,075 (1.9) | 301 (19.1) | 15,663 (2.5) |
| Tobacco use disorder [N (%)] | 401 (19.4) | 30,584 (4.4) | 290 (18.4) | 23,663 (3.7) |
| Prescriber shoppers [N (%)] | 735 (35.6) | 80,354 (11.6) | 575 (36.4) | 72,373 (11.4) |
| Region [N (%)] | ||||
| Northeast | 386 (18.7) | 218,291 (31.5) | 313 (19.8) | 206,560 (32.6) |
| South | 772 (37.4) | 198,229 (28.6) | 489 (31.0) | 172,457 (27.2) |
| West | 409 (19.8) | 111,168 (16.1) | 368 (23.3) | 104,301 (16.5) |
| Midwest | 500 (24.2) | 165,096 (23.8) | 410 (26.0) | 149,690 (23.7) |
| Pharmacy shoppers [N (%)] | 141 (6.8) | 3,855 (0.6) | 125 (7.9) | 3,406 (0.5) |
| Percent male [N (%)] | 994 (48.1) | 298,126 (43.0) | 780 (49.4) | 271,038 (42.8) |
| Prior opioid 30-day adjusted prescriptions [Mean (Std. Dev)] | 9.3 (9.3) | 1.8 (4.1) | 9.1 (9.2) | 1.7 (4.0) |
| Chronic immediate release users [N (%)] | 665 (32.2) | 46,839 (6.8) | 536 (33.9) | 40,069 (6.3) |
| Distance from patient to prescriber [N (%)] | ||||
| ≤ 50 miles | 1,747 (84.5) | 600,035 (86.6) | 1,343 (85.0)b | 539,518 (85.2)b |
| > 50 miles | 320 (15.5) | 92,749 (13.4) | 237 (15.0)b | 93,490 (14.8)b |
All data were significantly different at P<0.05 between opioid dependents and non-dependents, except for cells marked with a note indicating otherwise
Not significantly different between opioid dependents and non-dependents at P<0.05 MED: Morphine equivalent dose
Clinical factors significantly varied between the 2 groups of patients. Opioid abusers/dependents had a higher proportion of mental illness (52.1% vs. 14.9%). non-opioid substance abuse (4.1% vs 0.2%), and non-dependent alcohol abuse (4.0% vs. 0.5%) compared to non-abusers/non-dependents. Furthermore, opioid abuse/dependence were associated with high morphine equivalent dose users (19.3% vs 1.9%) and tobacco use disorder (19.4% vs 4.4%).
Opioid abuser/dependents were more likely to be prescriber shoppers (35.6% vs 11.6%). There were also significant regional differences among the 2 groups with, South, West and Midwest having a higher percentage of abusers or dependents as compared to the Northeast.
Pharmacy shopping differed between the two groups. There was a higher percentage of pharmacy shoppers (6.8% vs 0.6%) in the opioid abuse/dependence group than the non-opioid abusers/dependents. There was a higher proportion of males in the abuse/dependence group than the non-abusers or non-dependents. Patients who developed abuse or dependency averaged higher numbers of 30-day adjusted opioid prescriptions in the pre-period (9.3 vs. 1.8) and more chronic immediate release users (32.2% vs 6.8%).
The derivation and validation data set found similar effects for all variables, except that a long (>50 miles) distance between patient and index opioid prescriber was significantly more common among opioid abusers/dependents in the derivation dataset but not in the validation data.
As indicated by a variance inflation factor of less than 10 for all variables, independent variables in the model did not have a high level of collinearity. Thus, all variables were retained in the model. The c-statistic for the derivation model was 0.852 and for the validation model was 0.847, indicating that the two models (Table 3) successfully discriminate between opioid abusers/dependents vs. non-abusers or non-dependent patients.
Table 3.
Multivariate Adjusted Odds Ratio for Opioid Dependency Models
| Reference | Derivation Modelc | Validation Modelc | |||
|---|---|---|---|---|---|
| ORa | 95% CI | ORa | 95% CI | ||
| Age (per decade of life) | NA | 0.68 | [0.65,0.70] | 0.65 | [0.63,0.68] |
| Chronic users | Absent | 4.39 | [3.71,5.19] | 4.29 | [3.53,5.22] |
| Mental illness | Absent | 3.45 | [3.13,3.79] | 3.37 | [3.02,3.76] |
| Non-opioid substance abuse | Absent | 2.82 | [2.18,3.64] | 2.87 | [2.11,3.89] |
| Non-dependent alcohol abuse | Absent | 2.37 | [1.84,3.05] | 2.10 | [1.55,2.85] |
| Daily MED ≥ 120 mg/day | Absent | 1.98 | [1.68,2.34] | 1.93 | [1.61,2.32] |
| Tobacco use disorder | Absent | 1.80 | [1.60,2.04] | 2.09 | [1.81,2.40] |
| Prescriber shoppers | Absent | 1.71 | [1.55,1.89] | 1.74 | [1.55,1.95] |
| South Region | Northeast | 1.65 | [1.45,1.87] | 1.44 | [1.25,1.67] |
| West Region | Northeast | 1.49 | [1.29,1.72] | 1.70 | [1.46,1.99] |
| Midwest Region | Northeast | 1.24 | [1.08,1.42] | 1.31 | [1.13,1.53] |
| Pharmacy shoppers | Absent | 1.59 | [1.31,1.92] | 1.98 | [1.61,2.43] |
| Male | Female | 1.43 | [1.31,1.57] | 1.52 | [1.37,1.68] |
| Prior opioid 30-day adjusted prescriptions | NA | 1.05 | [1.04,1.06] | 1.04 | [1.03,1.05] |
| Chronic, immediate release user | Absent | 1.07b | [0.93,1.22] | 1.27 | [1.09,1.48] |
| Distance from patient to prescriber | ≤50 miles | 1.12b | [0.99,1.27] | 0.95b | [0.83,1.10] |
All data were significant at P<0.05 unless marked with a note indicating otherwise
Not significant at P<0.05
The c-statistics were 0.852 for the derivation model and 0.847 for the validation model.
NA: Not applicable for continuous variables
MED: Morphine equivalent dose
Younger age [OR 0.68 per decade older, 95% confidence interval (CI) 0.65–0.70] significantly predicated opioid abuse or dependence. Chronic use of opioids [OR 4.39, 95% CI 3.71–5.19] and history of mental illness [OR 3.45, 95% CI 3.13–3.79] were strong predictors of developing opioid abuse/dependence. Histories of other substance abuse [OR 2.82 95% CI 2.18–3.64] and alcohol abuse [2.37 95% CI 1.84–3.05], and doses of opioids ≥ 120 mg of morphine equivalents per day [OR 1.98 95% CI 1.68–2.34] elevated the risk of developing opioid abuse or dependence. Tobacco use [OR 1.80, 95% CI 1.60–2.04], prescriber shoppers [OR1.71, 95% CI 1.55–1.89], residing in the South [OR 1.65, 95% CI 1.45–1.87], West [OR 1.49 95% CI 1.29–1.72], and Midwest [OR 1.24, 95% CI 1.08–1.42] compared to the northeast were also significant predictors of developing opioid abuse/dependence.
Finally, pharmacy shoppers [OR 1.59 95% CI 1.31–1.92], male gender [OR 1.43, 95% CI 1.31–1.57], and each additional 30-day adjusted opioid prescription [OR 1.05, 95% CI 1.04–1.06] were predictive of developing opioid abuse/dependence.
Distance from patient to prescriber was not statistically significant in either model. Being a chronic immediate release user was insignificant in the derivation model.
With only one exception (chronic immediate-release opioid) each of the predictors of opioid abuse or dependence that were significant in the derivation model, also were significant in the validation model. Additional sensitivity analyses (Appendix 6) corroborated the associations in the derivation and validation models.
Discussion
This study identified 12 patient characteristics that predict increased risk of de novo abuse or dependence in opioid users. The strongest predictors were chronic use, mental illness, non-opioid substance use, alcohol abuse, high morphine equivalent dose dose per day, younger age, and male gender. These effects were in the direction as hypothesized. In this study, the relationships between the distance from patient to prescriber and being a chronic immediate release user to the odds of developing opioid abuse or dependence were not consistently significant. All identified risk factors are available through patient history or a prescription drug monitoring program. Thus, our study provides useful risk factors for prescribers to be able to determine a patient’s risk of developing opioid abuse or dependence in the next 12 months. These factors can help prescribers weigh the risks and benefits of prescribing opioids.
Our findings are consistent with prior research. Dufour and colleagues developed a predictive model using data from one commercial insurer with 3,500 cases of opioid abuse or dependence.30 They also found lower risk with advanced age and high risks among men.
Our results also are consistent with Edlund et al., who evaluated 46,000 Arkansas patients.31 They reported that opioid abuse or dependence was associated with mental health disorders, prior opioid abuse, younger age (18–30 years old), prior non-opioid substance abuse, and higher morphine equivalent dose per day. White et al. developed an abuse prediction model based on 116,382 patients in Maine who used opioids.27 One of their main findings was that ≥4 opioid prescriptions (OR 7.34) and early refills (OR 3.39) predicted abuse. Dose escalation was also a significant risk factor (OR1.88). We did not assess early refills or dose escalation as they cannot be assessed at the time of first prescription. As compared to these seminal studies, our study is larger and more representative of the US population.
Rice et al. also studied a large, representative dataset.17 Their findings of non-opioid drug abuse (OR 9.89) and a history of mental illness (OR 2.45) increasing the risk for opioid abuse support our findings. Our study differentiates from prior studies in that we quantified how readily available demographic, clinical, behavioral, pharmacy and geographic information predict opioid abuse or dependence. Prescribers will be able to use these variables in real-time to make a more accurate risk assessment of developing opioid abuse or dependence.
Limitations
Our study has a few limitations. First, the model is not implementable in states without a prescription drug monitoring program, but 49 states have a program in place or pending. Second, our study did not include Medicare, Medicaid, or Veterans Administration patients and awaits validation in these populations. However, most of the total US population is covered by private insurance.32 Third, because we used one-year of ICD-9 codes after the index claim for identifying opioid abuse or dependence, we likely failed to capture some episodes of abuse or dependence. Future studies could include longer follow up. Finally, the relationship between receiving ≥50% of the total dose in the immediate release form and being diagnosed with opioid abuse or dependence was significant in the validation model, but not in the derivation model.
Conclusions
In light of the opioid abuse epidemic, the findings of this study warrant updating tools that estimate the risk for abuse or dependence. We recommend incorporating factors found in a prescription drug monitoring program into a patient’s risk analysis. We found that risk factors for a patient being diagnosed with opioid abuse or dependence are younger age, being a chronic opioid user, histories of mental illness, non-opioid substance abuse, alcohol abuse, being a high morphine equivalent dose user, a history of tobacco use, using multiple prescribers, residing in the South, West or Midwest, using multiple pharmacies, male gender and an increasing number of opioid prescriptions. Our study quantifies risk factors that are available to prescribers who are considering prescribing opioids. These insights highlight the importance of utilizing readily available demographic, clinical, pharmacy, and geographic information to estimate the risk for opioid abuse or dependence.
Clinical Significance.
Readily available variables can help quantify the risk of developing opioid abuse
Chronic opioid use and history of mental illness are strongest predictors of abuse
Acknowledgments
Thomas Ciesielski receives support from an unrestricted grant from the Foundation for Barnes-Jewish Hospital. Amit Bothra, and Reethi Iyengar receive salary support from Express Scripts, an independent pharmacy benefits manager. Dave Tomala also received salary support from Express Scripts at the time the study was conducted. Brian Gage receives support from Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the NIH. HM Dinesh, MS, Genpact, contributed to data collection. From Express Scripts, Craig Reno, BS MBA, provided clinical expertise on the analysis, Ria Westergaard, PharmD, provided clinical expertise on the analysis. In additional to the authors, Ruth Martinez, RPh, contributed to writing and editing the manuscript.
Appendix 1: Specific Therapeutic Class (STC) and Associated Description for Opioid Claims
| STC | STC Description |
|---|---|
| 0268 | Analgesics, narcotics |
| 6122 | Narcotic antitussive-1st generation antihistamine-decongestant combination |
| 7740 | Analgesics narcotic, anesthetic adjunct agents |
| 8483 | Narcotic antitussive-anticholinergic combination |
| 8485 | Narcotic antitussive-1st generation antihistamine |
| 8502 | Narcotic antitussive-decongestant combinations |
| 8514 | Narcotic antitussive-decongestant-expectorant combination |
| 8518 | Narcotic antitussive-expectorant combination |
| 8769 | Analgesic narcotic agonist- NSAID combination |
| B902 | Analgesic narcotics-dietary supplement combination |
| B947 | Narcotic- non-salicylate analgesic-barbiturate-xanthine combination |
| B955 | Narcotic-salicylate analgesics-barbiturate-xanthine combination |
| B974 | Narcotic analgesic-non-salicylate analgesic combination |
| C423 | Narcotic antitussive-decongestant-analgesic-expectorant combination |
| C431 | Narcotic antitussive-1st generation antihistamine-analgesic, non-salicylate combination |
| C618 | Narcotic- salicylate analgesic combination |
Appendix 2: ICD-9 Cancer Diagnosis Codes for Patient Exclusion Criteria
ICD-9 codes 140–165, 170–176, 179–209 excluding benign neoplasms under 209.× (209.4*, 209.5* and 209.6*) were used to identify patients with cancer.
Notes: *denotes all combination codes under the ICD-9
Appendix 3: GCN’s Associated with Cancer Drug Markers for Patient Exclusion Criteria
| STC Description | GCN Used |
|---|---|
| ANP – Selective Retinoid Receptor Agonists (RXR) | 92373 |
| Antibiotic Antineoplastics | 29203, 34241,34242,34247,34248,35080, 38581,38590,38591,38592,38593,38594, 38600,38601,38602,38610,38613,38622, 38623,38630, 47340,47343, 94175,96679,97242,97271,97272,97277, 97278,97282,99510,99835 |
| Anti-CD20 (B Lymphocyte) Monoclonal Antibody | 27827, 30137 |
| Antiemetic/Antivertigo Agents | 16007,16008,17256,17258, 20011,20228,23756,29247, 33531, 60548, 99260,99267,99335,99862 |
| Antileprotics | 19321, 28301, 95392,98220 |
| Antineoplast HUM VEGF Inhibitor Recomb MC Antibody | 21427 |
| Antineoplast, Histone Deacetylase (HDAC) Inhibitors | 28397, 97345 |
| Antineoplast – Alkylating Agents | 6939, 7182,7196, 9217, 12014,14401,17724, 24699,24701, 34221,34310,38232,38340,38350,38351, 38352,38353,38357,38360,38361,38370, 38380,38390,38410,38420,38422,38431, 38432,38433,38440,38450,38451,38910, 38911,38912,38920, 48862, 60901, 72722,72730,72731,72732, 72733,72734, 92893,92903,92913,92933,97957,98310, 98311,98709,98710,98813 |
| Antineoplast – Antidrogenic Agents | 450, 22642,22645,25740,29886, 33183 |
| Antineoplast – Antimetabolites | 880, 10290,12473,19901, 21179,21473, 21485,21501,21503,22663, 23432,23439,24037,25932,27027,27365, 27663,27664, 30776,30777,30778,31611,31612,32981, 34230,34231,38490,38500,38520,38530, 38531,38532,38540,38541,38542,38543, 93472,97455,97456,97457,97458,97825, 99268 |
| Antineoplast – Aromatase Inhibitors | 17300 |
| Antineoplast – Epothilones & Analogs | 98998,98999 |
| Antineoplast – Halichondrin B Analogs | 29249 |
| Antineoplast – Hedgehog Pathway Inhibitor | 31307 |
| Antineoplast – Janus Kinase (JAK) Inhibitors | 30892,30893,30894,30895,30896 |
| Antineoplast – MTOR Kinase Inhibitors | 20784,20844,28783, 31396,34589,34590,34592, 98597 |
| Antineoplast – Topoisomerase I Inhibitors | 14254,14256, 22661,29519, 97955,97956,99056,99790 |
| Antineoplast – VEGF-A,B & PLGF Inhibitors | 32988,32989 |
| Antineoplast – Vinca Alkaloids | 38560,38572,38580,38820,38970, 97327,97630 |
| Antineoplast Antibody/Radioactive-drug Complexes | 20159,20160 |
| Antineoplast EGF Receptor Blocker Mclon Antibody | 13632,13638,13639,15979,15983, 28471, 32343 |
| Antineoplast Immunomodulator Agents | 26314,26315,27276,27277,29809,29811, 29812, 31911,34147,34148,34149,34150,34743 |
| Antineoplast LHRH (GNRH) Agnonist, Pituitary Suppr. | 13133,15338,15344,16945,16946,17377, 18155,19219, 21004,23768,24301,28506,28507,29894, 30083, 84590,84591,84592,84593,84594,84596, 84597,84598,84601,84602, 99763,99764 |
| Antineoplast Systemic Engyme Inhibitors | 13369,19586,19656,19907,19908, 23793,23794,23795,26263,26452,26453, 26454,27257,27258,27259,27829,28737, 29405, 29406,29817,29818, 30332,30457,30458,31294,31295,32722, 33199,33202,33363,33873,33874,33903, 33904,33905,34723,34724,34726,34727, 98140,99070,99867 |
| Antineoplast Antibody/Antibody-drug Complexes | 14171,18373,18374, 20158,21050,24507, 30404,34234,34235 |
| Antineoplast – Miscellaneous | 7480,7481,7544,7550,7552,7560, 14103, 24094,24231,28663,28762,29066,29591, 29662,29663,29664, 30918,33734,38710,38730,38731,38732, 38740,38750,39000,39150,39152,39153, 39154, 47410,48480,48481,48590, 85410,85602,85602, 93610 |
| Chemotherapy Rescue/Antidote Agents | 1330, 27236, 31194,36901,38950,38953,38955, 87552,87553,87554,87555,87556,87557, 87558,87559,87562,87563,89655 |
| CXCR4 Chemokine Receptor Antogonist | 16124 |
| Cytotoxic T-Lymphocyte Antigen (CTLA-4) RM Antibody | 29688,29689 |
| Immunomodulators | 26405, 46471,46472,47511,47512,47513,47520, 47521,47522,47523,47524,47525,47526, 47527,47528,47529,47530,47600,47601, 47602,47603,47604,47605,47661,47662, 47663,48891,48931,48941,49031, 90823,90833 |
| Keratinocyte Growth Factor (KGF) | 23928 |
| Leukocyte (WBC) Stimulants | 13206,13308,13309,15666, 26001,26220,26221,26222 |
| LHRH (GNRH) Agonist Analog Pituitary Suppressants | 23768, 80254,84350 |
| Selective Estrogen Receptor Modulators (SERMS) | 17307,17308, 38720,38721, 50377 |
| Steroid Antineoplastics | 38640,38661,38700 |
| Tissue Protective TX of Chemotherapy Extravasation | 30562 |
| Topical Antineoplastic & Premalignant Lesion Agents | 89921 |
Appendix 4: GCN’s Associated with Suboxone for Patient Exclusion Criteria
| STC Description | GCN Used |
|---|---|
| Narcotic Withdrawal Therapy Agents | 18973, 18974, 28958, 28959, 33741, 33744, 34904, 34905, 36677, 36678, 36679 |
Appendix 5: ICD-9 Codes associated with independent variables
| Description | ICD-9 Code |
|---|---|
| Non-opioid substance abuse | 304.1 to 304.9 and 305.2 – 305.9, excluding 305.5× |
| Tobacco use disorder | 305.1 |
| Nondependent alcohol abuse | 303.9 and 305.0× |
| Mental illness | 290 to 302 and 306 to 316 |
Appendix 6: Sensitivity Analyses to Test the Robustness of the Findings
The first sensitivity analysis was ‘Firth’s bias-adjusted estimation’ which maximizes a penalized likelihood function and provides finite parameter estimates. Second was ‘oversampling’ to increase the target rate by ten times. This helps address the bias resulting from the margin of sampling error being related to the outcome sample size.
Sensitivity Analysis using Firth Bias-Adjusted Estimation Method and Over Sampling Method
| Reference | Firth’s Bias-Adjusted Estimation Modelc | Over Sampling Method Modelc | |||
|---|---|---|---|---|---|
| ORa | 95% CI | ORa | 95% CI | ||
| Age | NA | 0.96 | [0.96,0.97] | 0.96 | [0.96,0.96] |
| Chronic users | Absent | 4.39 | [3.71,5.19] | 3.98 | [3.28,4.83] |
| History of mental illness | Absent | 3.45 | [3.13,3.79] | 3.63 | [3.28,4.03] |
| History of non-opioid substance abuse | Absent | 2.83 | [2.18,3.63] | 3.93 | [2.84,5.44] |
| History of non-dependent alcohol abuse | Absent | 2.38 | [1.84,3.04] | 2.64 | [1.93,3.60] |
| Daily MED ≥ 120 mg/day | Absent | 1.98 | [1.67,2.34] | 1.83 | [1.50,2.24] |
| History of tobacco use disorder | Absent | 1.80 | [1.59,2.04] | 2.04 | [1.77,2.34] |
| Prescriber shoppers | Absent | 1.71 | [1.55,1.89] | 1.71 | [1.53,1.91] |
| Region | |||||
| South | Northeast | 1.65 | [1.45,1.87] | 1.79 | [1.56,2.05] |
| West | Northeast | 1.49 | [1.29,1.72] | 1.52 | [1.30,1.78] |
| Midwest | Northeast | 1.24 | [1.08,1.42] | 1.29 | [1.12,1.50] |
| Pharmacy shoppers | No | 1.59 | [1.31,1.92] | 2.03 | [1.58,2.61] |
| Male | Female | 1.43 | [1.31,1.57] | 1.52 | [1.37,1.68] |
| Prior opioid 30-day adjusted prescriptions | NA | 1.05 | [1.04,1.06] | 1.06 | [1.05,1.07] |
| Chronic immediate release users | Absent | 1.06b | [0.93,1.22] | 1.03b | [0.88,1.21] |
| Distance from patient to prescriber | ≤50 miles | 1.12b | [0.99,1.27] | 1.12b | [0.98,1.28] |
All data were significant at P<0.05 unless marked with a note indicating otherwise
Not significant at P<0.05
The c-statistics for the Firth’s bias-adjusted estimation model was 0.853 and over sampling method model was 0.876
NA: Not applicable for continuous variables
MED: Morphine equivalent dosing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: All authors – ‘None’
Contributor Information
Thomas Ciesielski, Email: tciesiel@dom.wustl.edu, Instructor in Medicine, Washington University School of Medicine, Department of Internal Medicine, Division of Medical Education, 660 S Euclid Ave, Campus Box 8121, St. Louis, MO 63110, P: +1-314-362-8065, F: 314-747-1080.
Reethi Iyengar, Email: rniyengar@express-scripts.com, Senior Manager, Research, Express Scripts, 4600 N Hanley Road, St Louis, MO, Ph: +1-314-522-5854.
Amit Bothra, Email: akbothra@express-scripts.com, Senior Manager, Advanced Analytics, Express Scripts, 4600 N Hanley Road, St Louis, MO, Ph: +1-314-522-5903.
Dave Tomala, Email: datomala@express-scripts.com, Senior Director, Advanced Analytics, Express Scripts, 4600 N Hanley Road, St Louis, MO, Ph: +1-314-684-6461.
Geoffrey Cislo, Email: gcislo@dom.wustl.edu, Assistant Professor of Medicine, Washington University School of Medicine, Department of Internal Medicine, Division of Medical Education, 660 S Euclid Ave, Campus Box 8121, St. Louis, MO 63110, Phone: +1-314-362-8065.
Brian F. Gage, Email: bgage@dom.wustl.edu, Professor of Medicine, Washington University School of Medicine, Department of Internal Medicine, Division of General Medical Sciences, 660 S Euclid Ave, Campus Box 8005, St. Louis, MO 63110, Phone: P: +1-314-362-8065.
References
- 1.Dart RC, Surrat HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. NEJM. 2015;372(3):241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- 2.Rudd R, Aleshire N, Zibbell JE, Gladden M. Increases in Drug and Opioid Overdose Deaths — United States, 2000–2014. MMWR. 2016;64(50):1378–82. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 3.Katz NP, Adams EH, Chilcoat H, et al. Challenges in the development of prescription opioid abuse-deterrent formulations. Clin J Pain. 2007;23(8):648–660. doi: 10.1097/AJP.0b013e318125c5e8. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 5.Savage SR, Joranson DE, Covington EC, Schnoll SH, Heit HA, Gilson AM. Definitions related to the medical use of opioids: Evolution towards universal agreement. J Pain and Symptom Management. 2003;26(1):655–667. doi: 10.1016/s0885-3924(03)00219-7. [DOI] [PubMed] [Google Scholar]
- 6.Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. The Journal of Pain. 2009;10(2):113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paulozzi LJ, Jones CM, Mack KA, Rudd RA. Vital signs: overdoses of prescription opioid pain relievers United States, 1999–2008. MMWR. 2011;60(43):1487–1492. [PubMed] [Google Scholar]
- 8.Katz N, Panas L, Kim M, et al. Usefulness of prescription monitoring programs for surveillance–analysis of Schedule II opioid prescription data in Massachusetts, 1996–2006. Pharmacoepidemiol Drug Saf. 2010;19(2):115–23. doi: 10.1002/pds.1878. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Christo PJ. The influence of prescription monitoring programs on chronic pain management. Pain Physician. 2009;12(3):507–515. [PubMed] [Google Scholar]
- 10.Centers for Medicare and Medicaid Services. The role of a prescription drug monitoring program in reducing prescription drug diversion, abuse, and misuse. 2014 Jun; http://www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/Medicaid-Integrity-Education/Downloads/prescriptiondrug-monitoring-factsheet.pdf. Accessed May 4, 2015.
- 11.Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the opioid risk tool. Pain Med. 2005;6(6):432–442. doi: 10.1111/j.1526-4637.2005.00072.x. [DOI] [PubMed] [Google Scholar]
- 12.McNeely J, Strauss SM, Saitz R, et al. A Brief Patient Self-administered Substance Use Screening Tool for Primary Care: Two-site Validation Study of the Substance Use Brief Screen (SUBS) The American Journal of Medicine. 2015;128(7):784.e9–784.e19. doi: 10.1016/j.amjmed.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowman S, Eiserman J, Beletsky L, Stancliff S, Bruce RD. Reducing the Health Consequences of Opioid Addiction in Primary Care. The American Journal of Medicine. 2013;126(7):565–571. doi: 10.1016/j.amjmed.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 14.First Databank. http://www.fdbhealth.com/ Accessed April. 24, 2015.
- 15.Cepeda MS, Fife D, Chow W, Mastrogiovanni G, Henderson SC. Opioid shopping behavior: how often, how soon, which drugs, and what payment method. J Clin Pharmacol. 2013;53(1):112–117. doi: 10.1177/0091270012436561. [DOI] [PubMed] [Google Scholar]
- 16.Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24(6):521–527. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rice JB, White AG, Birnbaum HG, Schiller M, Brown DA, Roland CL. A model to identify patients at risk for prescription opioid abuse, dependence, and misuse. Pain Med. 2012;13(9):1162–1173. doi: 10.1111/j.1526-4637.2012.01450.x. [DOI] [PubMed] [Google Scholar]
- 18.Skala K, Reichl L, Ilias W, et al. Can we predict addiction to opioid analgesics? A possible tool to estimate the risk of opioid addiction in patients with pain. Pain Physician. 2013;16(6):593–601. [PubMed] [Google Scholar]
- 19.McCabe SE, Cranford JA, Boyd CJ. The relationship between past-year drinking behaviors and nonmedical use of prescription drugs: prevalence of co-occurrence in a national sample. Drug Alcohol Depend. 2006;84(3):281–288. doi: 10.1016/j.drugalcdep.2006.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manchikanti L, Helm S, II, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15(3 Suppl):ES9–ES38. [PubMed] [Google Scholar]
- 21.Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2 –guidance. Pain Physician. 2012;15(3 Suppl):S67–S116. [PubMed] [Google Scholar]
- 22.Sehgal N, Manchikanti L, Smith HS. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Physician. 2012;15(3 Suppl):ES67–ES92. [PubMed] [Google Scholar]
- 23.Cheatle MD, O’Brien CP, Mathai K, Hansen M, Grasso M, Yi P. Aberrant behaviors in a primary care-based cohort of patients with chronic pain identified as misusing prescription opioids. J Opioid Manag. 2013;9(5):315–324. doi: 10.5055/jom.2013.0174. [DOI] [PubMed] [Google Scholar]
- 24.Parente ST, Kim SS, Finch MD, et al. Identifying controlled substance patterns of utilization requiring evaluation using administrative claims data. Am J Manag Care. 2004;10(11 Pt 1):783–790. [PubMed] [Google Scholar]
- 25.Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613–2620. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 26.United States Census Bureau. Geographic terms and concepts: census divisions and census regions. https://www.census.gov/geo/reference/gtc/gtc_census_divreg.html. Accessed Feb 9, 2015.
- 27.White AG, Birnbaum HG, Schiller M, Tang J, Katz MP. Analytic models to identify patients at risk for prescription opioid abuse. Am J Manag Care. 2009;15(12):897–906. [PubMed] [Google Scholar]
- 28.Roland CL, Joshi AV, Mardekian J, Walden SC, Harnett J. Prevalence and cost of diagnosed opioid abuse in a privately insured population in the United States. J Opioid Manag. 2013;9(3):161–175. doi: 10.5055/jom.2013.0158. [DOI] [PubMed] [Google Scholar]
- 29.Basic Health and Human Services policy for protection of human research subject. 2009 45 C.F.R. § 46. [Google Scholar]
- 30.Dufour R, Markekian J, Pasquale MK, Schaaf D, Andrews G, Patel N. Understanding predcitors of opioid abuse: predictive model development and validation. Am J Pharm Benefits. 2014;6(5):208–216. [Google Scholar]
- 31.Edlund MJ, Martin BC, Fan MY, Devries A, Braden JB, Sullivan MD. Risks for opioid abuse and dependence among recipients of chronic opioid therapy: results from the TROUP study. Drug Alcohol Depend. 2010;112(1–2):90–98. doi: 10.1016/j.drugalcdep.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaiser Family Foundation. Health insurance coverage of the total population. 2015 http://kff.org/other/state-indicator/total-population/. Accessed March 24, 2015.


