Summary
The root meristem has a centrally located group of quiescent cells, to which current models assign a stem cell organizer function. However, evidence is emerging for decentralized control of stem cell activity, where self-renewing behavior emerges from the lack of cell displacement at the border of opposing differentiation gradients. We term this a “stagnation” model due to its reliance on passive mechanics. The position of stem cells is established by two opposing axes that reciprocally control each other’s differentiation. Such broad tissue organization programs would allow plants, like some animal systems, to rapidly reconstitute stem cells from non-stem cell tissues.
eTOC
In root meristems, current models assign stem cell organizer function to a centrally located group of quiescent cells. Rahni et al. discuss emerging evidence for decentralized control of stem cell activity, whereby self-renewing behavior emerges from passive mechanics and reciprocal control of differentiation by opposing axes establishes stem cell position.
Introduction
Plants grow indeterminately from their meristems, regions of cell division and organ formation (Sinnott, 1960). Within the meristem, a small group of cells, termed initials, remains and generates specialized tissues (Evert et al., 2006). These properties – self-renewal and tissue generation – are the two core, defining features of stem cells, and initials serve an analogous function to animal stem cells (Barlow, 1978). While there has been some discrepancy in application of the term “plant stem cells” – referring at times to all cells in the meristem – the defining attributes of self-renewal and tissue generation limit stem cells to those positions that remain in place to produce long-term lineages (Scheres et al., 1994). Self-renewal requires that one daughter of a stem cell division be maintained in a state of low differentiation. Current models indicate this maintenance is mediated by a central organizer that signals to the surrounding stem cells.
Here, we review the evidence for the control of growth and differentiation in the root meristem as it relates to stem cell behavior. We consider an alternative to a dominant model of centralized control of stem cells by examining the potential of non-local and passive signals to mediate the defining properties of stem cells. The model presented here is an extreme counterpart to a central organizer, wherein it is recognized that future work could support elements of both a central and dispersed organizer model. The question relates to what properties of the meristem maintain its long-term growth.
Tissue-generative properties are widely dispersed
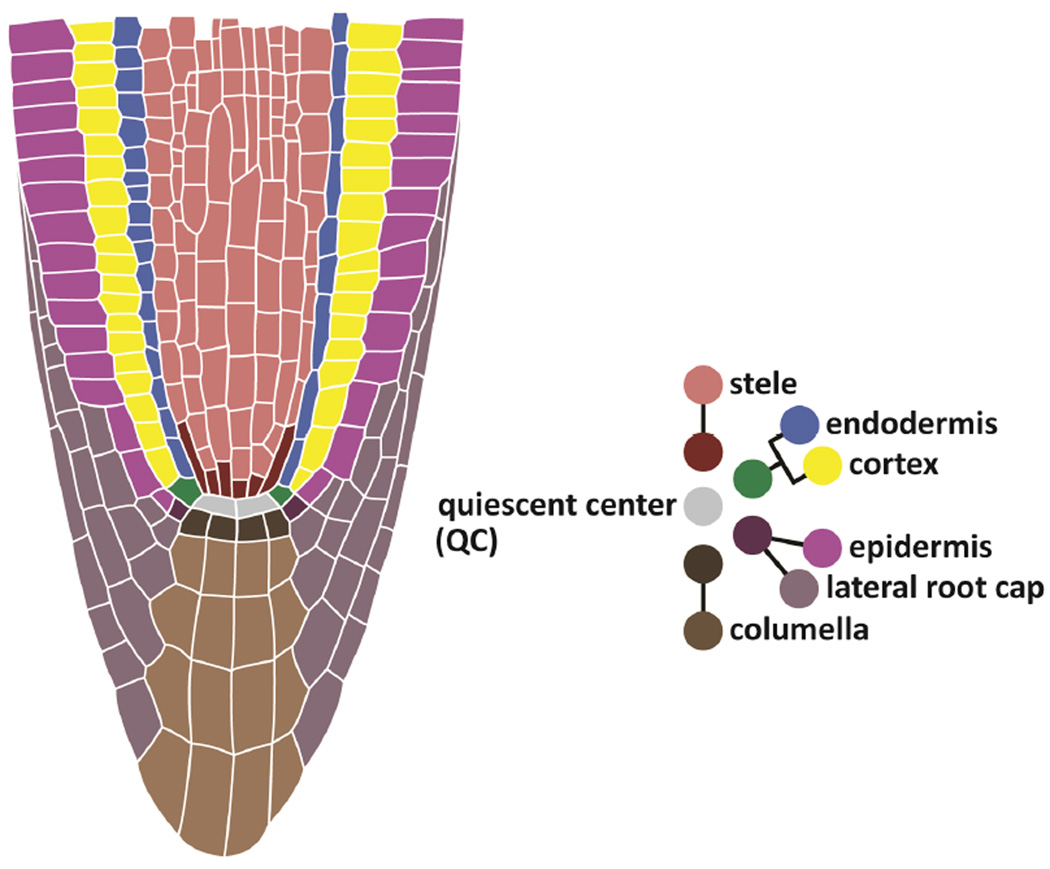
In the Arabidopsis root, stem cells are arranged around the quiescent center (QC), a group of less mitotically active cells (Dolan et al., 1993; Figure 1). Stem cell daughters undergo several transit-amplifying divisions before rapid elongation and differentiation to generate orderly, specialized tissue files (Breuer et al., 2010; Petricka et al., 2012). In animals, stem cells are maintained in niches, where signals from neighboring cells prevent their differentiation (Scadden, 2014). In Arabidopsis, laser ablation of the centrally localized QC led to morphological differentiation of distal neighbors (van den Berg et al., 1997). The result suggested that the QC may serve as a central signaling center, or organizer, that maintains and positions the stem cells, and together the QC and surrounding initials have widely been considered the stem cell niche (Scheres, 2007).
Figure 1. Anatomy of the root meristem and stem cell niche.
The diagram on the right shows the stereotypical contributions of stem cells, or initials, to specialized cell types, which form cell files in the root. The initials surround and directly contact the QC.
Still, a remarkable property of the root meristem is that the stem cells and many surrounding daughters can be excised and the root will rapidly repair itself to restore organization and growth (Feldman, 1976; Prantl, 1874). Recent lineage-tracing experiments showed that, when most of the meristem is excised (including all stem cells), many of the remaining cells within the meristem can give rise to new stem cells, often forming new tissue types distinct from their original identity (Efroni et al., 2016). This showed that almost any meristematic cell has a wide potential to adopt stem cell behavior without any external chemical treatments. Similarly, in the tomato shoot meristem, specific ablation of the stem cells caused other meristematic cells to rapidly regenerate a new, functional meristem, including all stem cells (Reinhardt et al., 2003). Although the origin of these new stem cells was not determined, the potency of adult meristems and the ability to re-establish stem cells appears widespread. These experiments definitively show that stem cells can emerge de novo from a self-organizing tissue assembly in the adult.
Moreover, these results make clear that many cells have the latent capacity for self-renewal and the generation of new tissues when put in the right signaling environment. Indeed, an earlier generation of plant anatomists, examining a wide variety of plant species, noted that plant initials did not have any inherent characteristics of their own. Rather, their permanence in the meristem emerged from larger growth patterns around them, paired with their relative position within the meristem (Esau, 1965; Foster, 1941). Meristematic initials resulted from continuing division behaviors, rather than continuing identity (Newman, 1965). In this view, most cells in the meristem possess a “stem-like” character, allowing them to rapidly adapt to new cellular environments and stresses, but a distinct mechanism is still needed to explain their self-renewing behavior.
Interestingly, the ability of an adult structure to reform its stem cells is not limited to highly plastic plant cells. For example, in the adult mouse, ablated hair follicle stem cells can be “reconstituted” from epithelial cells that do not normally participate in hair generation (Rompolas et al., 2013). Similarly, in the mouse gut, secretory cells can gain stem cell behaviors after radiation damage kills resident stem cells (van Es et al., 2012). These systems are similar to plants in the sense that stem cells need not immediately give rise to specialized tissues, but rather, specialized tissues can give rise to stem cells (Clevers, 2015; Ivanov, 2007). These examples place emphasis on the importance of the integrity of the patterning system rather the stem cell’s memory of its special status. They highlight one aspect of a conceptual framework in the animal field, where the control of stem cell properties can arise from a range of intrinsic to highly contextual inputs (Laplane, 2016). Thus, plants may simply exist on the extreme of such context-dependent stemness.
The self-organizational ability of stem cells does not rule out a scenario wherein patterning re-establishment occurs first and subsequently creates a central organizer that controls aspects of stem cell behavior. The question remains, however, as to what kind of organizational role a potential central organizer plays. The early establishment of broader tissue organization relative to the appearance of stem cells at least opens the possibility that broadly assembled domains could, in concert, control stem cell behavior.
The QC as one end of a distal pole, rather than a stem cell organizer
Measured relative to the QC, cells in the growing root are displaced distally (rootward) in one axis to form the central cap (columella and portions of the lateral root cap), and proximally (shootward) to form virtually all other cell types (Figure 1). Some daughters of the epidermal/lateral root cap initials are first displaced laterally before their daughters undergo distal or proximal displacement. In plants with closed meristems, such as Arabidopsis, this creates a morphology in which all cell files in both axes converge upon the QC (Dolan et al., 1993).
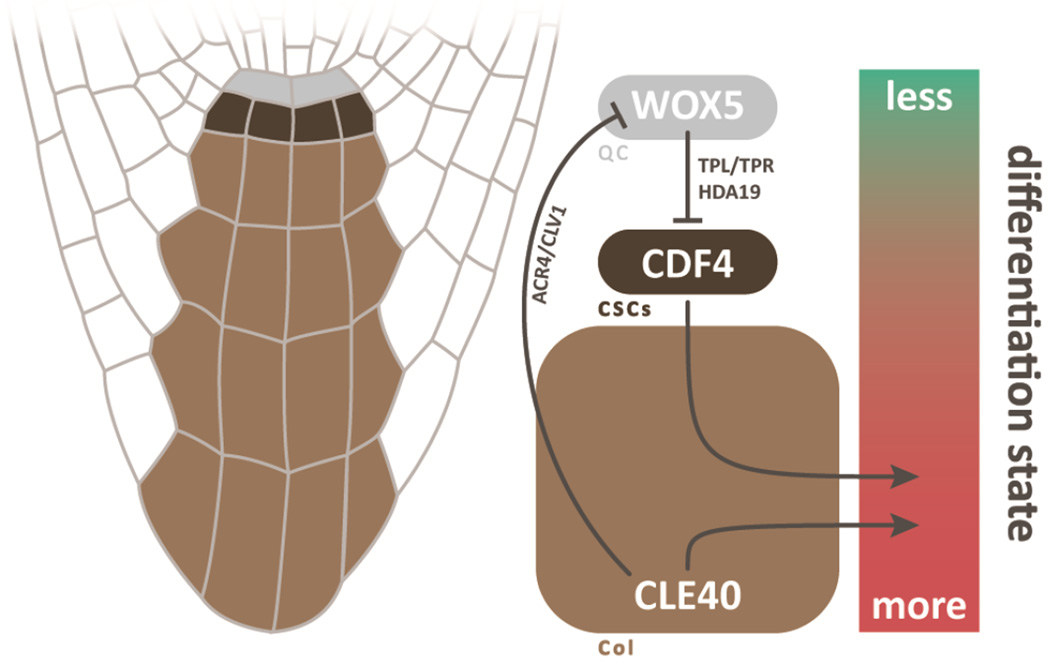
In keeping with its central position, the QC has long been purported to be an organizer. And, there is strong evidence that the QC signals to its distal neighbors to inhibit differentiation. In brief, mutations in the largely QC-localized transcription factor WUSCHEL-RELATED HOMEOBOX5 (WOX5) mimic the effect of QC ablation, causing the columella stem cells (CSCs) to differentiate (Sarkar et al., 2007). It was shown that the WOX5 protein moves from the QC into its distal CSC neighbors, where it acts to silence the differentiation-promoting transcription factor CYCLING DOF FACTOR 4 (CDF4) by directly binding its promoter (Pi et al., 2015). Thus, the QC-specific WOX5 represses differentiation in its neighbor, giving it an important role in regulating CSC behavior. At the other end of this distal axis, there is also regulation of QC and CSCs from differentiated columella daughters. For instance, it was shown that the secreted peptide CLAVATA3/ESR-RELATED 40 (CLE40), which is expressed in differentiated columella cells, non-autonomously represses WOX5 activity through receptor kinases (Stahl et al., 2013; Stahl et al., 2009; Figure 2).
Figure 2. The dual poles controlling differentiation within the distal cells.
At the upper position, WOX5 exerts influence from the QC by moving toward the tip and inhibiting differentiation in the CSCs. There, WOX5 recruits co-repressors TOPLESS/TOPLESS-RELATED (TPL/TPR) and HISTONE DEACETYLASE 19 (HDA19) to repress the differentiation-promoting factor, CDF4. At the tipward position of columella cells, CLE40 inhibits WOX5 expression and promotes differentiation through receptor kinases CLAVATA1 (CLV1) and ARABIDOPSIS CRINKLY4 (ACR4).
However, the position of stem cells in the root tip appears to be more of a balance between two gradients than specific local signaling from QC. For instance, it was shown that the gradient of differentiation from QC to columella cells can be shifted proximally by application of excess CLE40, perturbation of WOX5 function, or both (Stahl et al., 2009). In addition, characteristics of the undifferentiated CSCs can be rescued in wox5 plants with offsetting mutations in differentiation-promoting factors, showing that, while WOX5 opposes differentiation, it is not necessary to maintain the undifferentiated state of the stem cell (Bennett et al., 2014). In addition, mutations in WOX5 or the root cap-specific transcription factor FEZ can severely reduce CSC divisions, but they result in more rapid division of QC cells to instead replenish the columella, indicating that this stem cell behavior can be shifted to another cell type (Bennett et al., 2014).
Indeed, no single entity appears to regulate the undifferentiated state of the CSCs. The self-renewing behavior of distal stem cells appears to fluctuate between QC and the undifferentiated cells below it, as it was shown that, under normal growth, the QC’s occasional divisions gave rise exclusively to columella lineages (Cruz-Ramirez et al., 2013). Furthermore, mutants impaired in QC identity and lacking canonical CSCs can still regenerate functional, gravity-sensing columella cells when their root tips are excised (Sena et al., 2009), suggesting that differentiated cells can be specified in the absence of a functional QC or even the originating stem cells. Supporting this observation, during regeneration of the stem cell niche, stem cell-like behavior is established by ~24h after injury, while an organized, central, WOX5-expressing QC is not fully formed until ~48h, well after the establishment of root morphology (Efroni et al., 2016; Sena et al., 2009).
One distinctive property of the QC is its relative mitotic quiescence, and several recent reports have identified mechanisms mediating division in the QC (Forzani et al., 2014; Heyman et al., 2013). However, the QC’s relative quiescence does not appear to have a function in stem cell maintenance per se, as one recent study showed that loss of quiescence using a QC-targeted knockdown of RETINOBLASTOMA-RELATED (RBR) protein had no effect on meristem growth under normal conditions, although QC cells were more resistant to DNA damaging agents and could replace cells killed by such treatment (Cruz-Ramirez et al., 2013). This shows that one of the unique attributes of the QC – its relative quiescence – is not necessarily needed for stem cell behavior, although it does not rule out that other QC properties could control stem cells.
At least one signal that inhibits differentiation – WOX5 – originates from the QC, so it could be considered an organizer in some sense. However, as will be detailed below, the QC is not unique as a source of signals that inhibit differentiation. An alternative interpretation of the QC’s role in Arabidopsis is to serve as a part of a distal signaling domain, within which it represents one of two poles that send antagonistic signals to generate a differentiation gradient from QC to columella (Figure 2). The columella promotes differentiation from the tip of the root upward, in part through CLE40, while WOX5 and other factors inhibit differentiation from the QC downward (Richards et al., 2015). Beyond semantics, this interpretation puts the emphasis on cell-cell communication among the complex tissues that comprise the plant meristem rather than the QC as a privileged communication center for “stemness.”
Communication between the two axes of the meristem
While the WOX5-CLE40 signaling system helps explain the control of differentiation in the distal axis, it provides no explanation for stem cell behavior in the proximal axis. Indeed, unlike the distal stem cells, short-range signals from the QC to proximal stem cells have yet to be identified. However, the QC is a unique source of long-range regulation of the entire proximal axis. For example, mutation of the endodermis- and QC- expressed transcription factor SCARECROW (SCR) affects the entire meristem, but restoration of its activity specifically in the QC is sufficient to rescue much of the meristematic growth defects (Sabatini et al., 2003). Subsequently, it was shown that SCR mediates local cytokinin signaling in the QC to affect long-distance control of meristem length via regulation of auxin transport (Moubayidin et al., 2013). Thus, the QC does appear to have roles in controlling cell division in the proximal meristem.
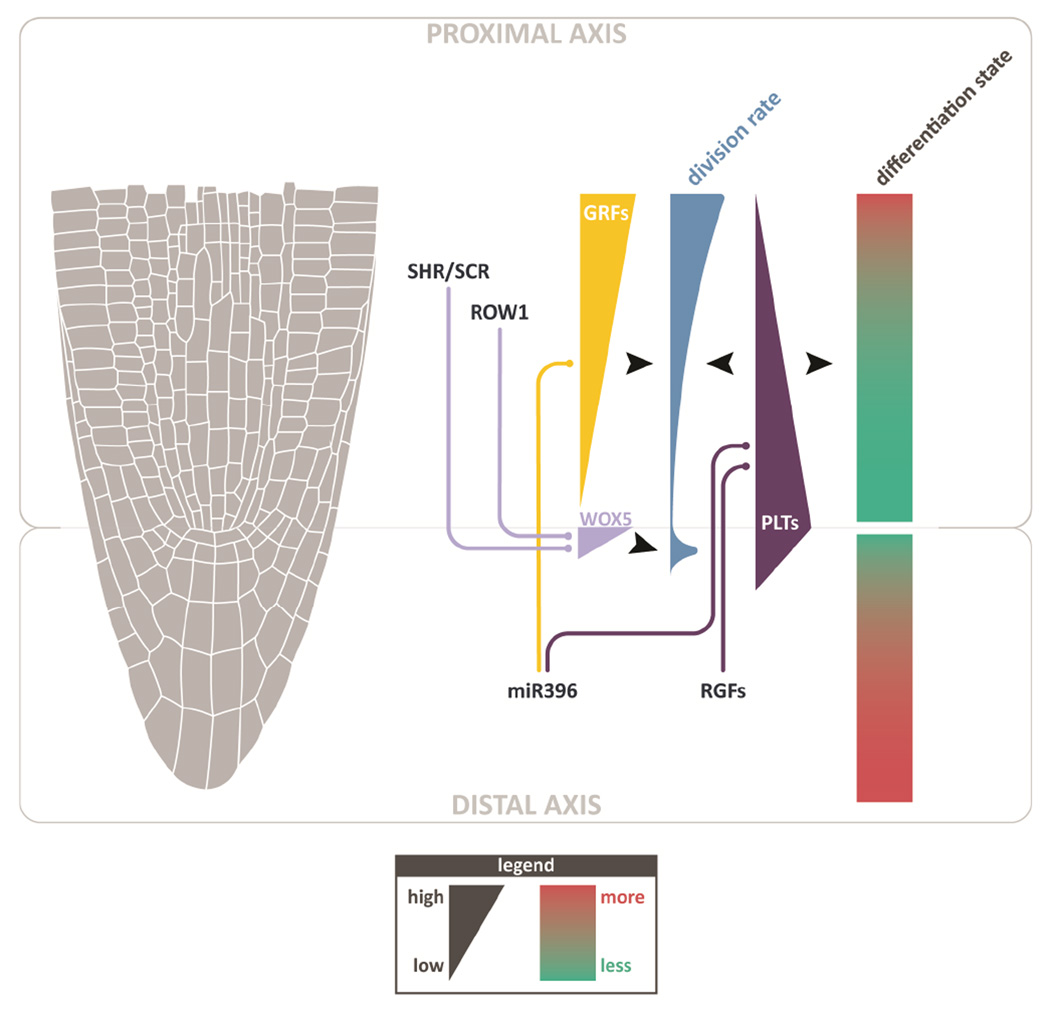
However, far from being the sole critical inputs, these functions exist within the context of a larger signaling domain in controlling proximal meristem growth and differentiation. Other work has demonstrated that, in addition to the QC, columella cells also comprise an important source of long-distance signaling to the proximal meristem (Figure 3). For example, a small family of ROOT GROWTH FACTORS (RGFs), which are expressed in the QC and columella, have been shown to diffuse into the proximal meristem and positively regulate growth (Matsuzaki et al., 2010; Zhou et al., 2010). In addition, it was shown recently that microRNA396, which is transcribed in the QC and columella, non-cell autonomously represses redundant GROWTH REGULATING FACTORs (GRFs), which are localized exclusively in the proximal meristem and were shown to have a role in locally promoting division rates (Rodriguez et al., 2015; Wang et al., 2011). Both factors have been implicated in the regulation of the PLETHORA (PLT) gene family (Matsuzaki et al., 2010; Wang et al., 2011), which regulates division rates and maturation state throughout the meristem (Aida et al., 2004; Galinha et al., 2007). In addition, the QC and columella are the root’s primary sink for auxin (Sabatini et al., 1999), which also displays a complex gradient that is mediated, in part, by the PLTs (Brunoud et al., 2012; Grieneisen et al., 2007; Mahonen et al., 2014). Indeed, the entire cap plays a role in proximal meristem differentiation and division, as the lateral root cap (LRC) also has been shown to non-cell autonomously regulate ROS levels that control cell division in the proximal meristem (Tsukagoshi et al., 2010). Thus, these studies reveal a general theme in which the entire distal axis (QC, columella, and LRC) sends long-range signals to non-autonomously regulate division patterns and differentiation in the opposing, proximal meristem (Figure 3).
Figure 3. Mechanisms that mediate long-distance control of growth and differentiation from two opposing signaling domains.
The distal axis (QC, columella, and LRC) regulates both cell division and differentiation gradients by long-distance influence (e.g., RGFs and miR396). The proximal axis (radial cell files) has top-down influence, setting the position of the QC, the upper-most cell of the distal axis, through SCR/SHR and ROW1. These factors influence distal axis division and differentiation through QC factors and potentially other long distance mechanisms to affect columella identity (not shown).
In the other direction, well-established work has shown that the proximal patterning elements SHR and SCR are important inputs to QC identity (Figure 3, (Sabatini et al., 2003)). In addition, recent work showed that REPRESSOR OF WUSCHEL1 (ROW1) – which binds histone H3 lysine 4 trimethylation marks and is expressed throughout the proximal but not the distal axis – is necessary to limit QC identity from spreading into the proximal meristem; accordingly, row1 mutants display ectopic, proximal expression of WOX5 (Zhang et al., 2015). It is not clear if other mechanisms in the proximal meristem independently control columella and cap identities or whether the primary influence of these proximal factors on distal axis organization is to simply set the coordinates of the QC. Nonetheless, these mechanisms highlight a complementary set of controls from the proximal axis that regulate the distal axis.
Taken together, these results suggest that the distal axis can be viewed as one component of the meristem that regulates the differentiation of the proximal axis. In turn, the proximal axis exerts control on the differentiation of the distal domain (Figure 4).
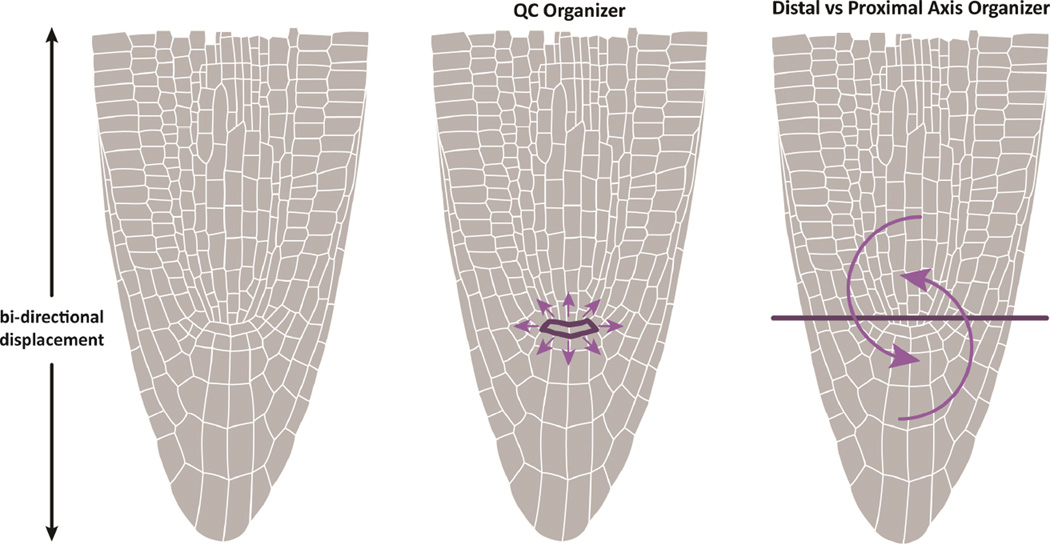
Figure 4. Alternative models of stem cell regulation.
In a central organizer model, signals from QC regulate stem cell behavior in all cells that contact it by means of a short range signal. In the dual axis model, opposing growth axes, which regulate each other’s division and differentiation gradients, displace their cells in opposite directions. Cells at the border of the opposing growth axes are not displaced by their neighbors whose daughters are pushed out of place in the opposite direction. These border cells behave as stem cells simply because they are not pushed away, a passive mechanism. Such a mechanism can result in bi-directional and radiating growth without a central organizer of division patterns.
Several mechanisms that influence division planes around the meristem are beginning to illustrate how two orthogonal axes can be modified to generate a radiating pattern where the distal and proximal axes meet. For example, it was shown recently that a SCR/RBR protein interaction can fluctuate over time via feedback inputs to regulate a lateral (periclinal) division in the stem cell daughter (Cruz-Ramirez et al., 2012). In fez mutants, the lateral (periclinal) epidermal/LRC initial divisions (that give rise to LRC) are reduced, without altering the epidermis-generating divisions of the same stem cells (Willemsen et al., 2008). These two mechanisms illustrate how a bidirectional displacement of daughter cells can be modified to form a pattern of cell files radiating from the QC.
Opposing displacement and distributed regulation of localized stem cells
Perhaps the mechanisms above could already reveal a distributed system that explains the undifferentiated state of stem cells without the need for a stem cell-specific organizer. The QC, columella and LRC establish a distal signaling axis that exerts long-range influence of growth and differentiation in the proximal domain. Conversely, the proximal axis, including all radial cell files, exerts influence back on the distal domain by determining its most proximal/shootward position (Figure 3, 4). While the overall growth of the root is mediated by the proximal stem cells, the distal stem cells create a temporary opposing axis before their daughters are sloughed off. This results in two gradients of differentiation, with a long/shallow gradient on the proximal side, and a short/steep gradient of on the distal side. Positions nearest the opposing axis are kept in the most undifferentiated state, with correlated control of division rates (e.g., miR396 to RGF). Within a given axis, differentiated tissue can exert influence on less differentiated positions; for example, QC and columella cells at the tip set up opposing gradients that regulate differentiation within their own region (Figure 3).
Tissue-generative potential is widely dispersed, but the second attribute of stem cell behavior – self-renewal – requires a division pattern that does not displace stem cells from the niche. This could be generated passively by local differences in the rate of cell division. Considering the original definitions of the initial cell, any cell that retains one daughter in the meristem over successive divisions can exhibit relatively stable self-renewal. If the juxtaposed distal and proximal axes that generate a differentiation gradient also set up a gradient of division, they can create a stagnation zone by slowing the rate of cell division where they meet. An implication is that self-renewing behavior should emerge at the low point of division, as more rapidly dividing cells on either side of the stagnation zone face an immobile substrate on one side while their daughters are displaced in opposite directions. The coupling of low division rates and low differentiation by mechanisms like miR396 ensures a coordination of self-renewing behavior and relatively youthful cell states. By simple mechanics, cells at the border remain in a state of low differentiation by the lack of being pushed away from the youthful domain by neighboring cells. Combined with the wide tissue generative capacity of cells in the meristem, reciprocal influence of proximal and distal domains would offer a unifying model of the control of self-renewal and low differentiation among stem cells at the root tip.
It could be argued here that the QC’s quiescence establishes such a low-point in the meristem, and as such directly promotes self-renewal. However, stagnation as described here is a passive mechanism, distinct from any active role in promoting self-renewal that an organizer implies. Indeed, any set of sufficiently slow-dividing cells could fill this role, so long as division rates increase in opposite directions on either side.
While the interaction between the two axes results in stem cell behavior in this model, the border between the two need not itself be a source of signals that supports self-renewal. Indeed, one of the predictions of this model is that signals from the QC may not be needed for proximal, or even broadly defined, distal stem cell behavior. If division orientation and daughter displacement were controlled broadly – for example, by mechanical effects or genetic and molecular mechanisms that control the differentiation of each axis – self-renewal would be another property of stem cells controlled in a distributed manner.
Variations in meristem organization
Many long-lived meristems in plants with internal stem cells exhibit bi- or even multi-directional cell displacement, such as the apical meristem, lateral meristem, and the cork cambium (Esau, 1965). Similar to the role of WOX5 in the root, one of the seminal discoveries in the shoot was the WUSCHEL-CLAVATA (CLV) pathway demonstrating short-range feedback signaling between supporting cells and stem cells (Gaillochet et al., 2015). There is also evidence of long-range signals that control differentiation from different growth zones in the shoot. For example, CLAVATA3, which is located in the stem cells of the central zone, was shown to non-autonomously control division patterns in the peripheral zone (Reddy and Meyerowitz, 2005). In early-stage cambial meristem development, the mobile peptide CLAVATA3/ESR-RELATED 41 (CLE41) is expressed in the differentiating phloem that lies external to the initials of the procambium, while its receptor, PHLOEM INTERCALATED WITH XYLEM (PXY), is expressed within the cambial initials. While ectopic expression of CLE41 could non-autonomously induce divisions from many nearby locations, its expression in phloem was specifically needed for the proper orientation of divisions in the cambium initials (Etchells and Turner, 2010). It is not clear if bidirectional cell displacement could emerge passively from the setting up of adjacent domains, but mechanisms like that of CLE41 are interesting because they could establish division orientations that help generate a local vector of cell displacement. Local mechanical forces may also be good candidates to mediate regional cellular displacement, such as the ability of outer layers to drive the growth of internal tissues (Savaldi-Goldstein and Chory, 2008; Savaldi-Goldstein et al., 2007).
In addition, while the general patterning of vascular plant meristems is well conserved (Gifford et al., 1989), division patterns within and around the QC can differ dramatically among different species. For example, open meristems show less cellular organization in the vicinity of stem cells, with an ill-defined boundary between root cap and proximal meristem (Esau, 1977). Thus, another prediction of a decentralized model is that the position of stem cells may vary within meristems that exhibit less stable tissue borders. In fact, in some open meristems, the QC shows a gradient of division rates and often contributes to both the proximal and distal axes (Clowes, 1981). In addition, the contributions of QC to these different axes even appeared to fluctuate over time (Clowes, 1981).
Finally, evolutionary trends argue against separate origins of open and closed meristems as the two growth forms appear scattered among angiosperm families in the plant phylogenetic tree (Heimsch and Seago, 2008). Thus, within many related plant root meristems, it is not clear that stem cell behavior can be regularly ascribed to a particular cell, making an explicit, local signaling center a less parsimonious model for the regulation of stem cells.
Perspective
The emphasis in this review has been to point out the possibility of alternative interpretations for the mechanisms that underlie stem cell function in the root meristem. For instance, the disappearance of CSCs below the QC in mutants such as wox5 could be due to the loss of a stem cell, as frequently cited, or, alternatively, due to the shift of a complex gradient that alters the coordinates of youthful to differentiated states. In the latter view, the CSCs represent a position within a differentiation gradient rather than an inherent stem cell state.
Still, the lack of evidence for signals from the QC to the proximal stem cells does not mean they do not exist. While no universal markers for root stem cells have been found, signaling systems can clearly distinguish and regulate precise positions around the QC (Sozzani et al., 2010). This underscores the point that genes expressed in a lineage-specific manner could regulate the stem cell position in each cell file around the QC independently. And, of course, a universal marker for strict-sense stem cells in plants may yet exist but simply be awaiting discovery. Unique transcriptional markers may not even be needed to specify a unique cell state, as distinctive properties of stem cells could be specified by combinations of protein-protein interactions, specific splice forms, or post-transcriptional modifications.
Furthermore, the original QC ablation studies did show an effect on proximal cells in the form of a change in division plane orientation of the cortex/endodermal initial (van den Berg et al., 1997). This result is consistent with a direct influence of QC on a set of proximal stem cells. However, in retrospect, another explanation is that the ablation could cause an alteration mechanical forces that influence cell division planes or in auxin flux, as occurs in the shoot meristem due to damage or mechanical disruption of the tissue (Heisler et al., 2010). It was found subsequently that auxin is indeed one input in the SCR-RBR circuit that transiently altered the orientation of cell division in the endodermal file around the QC (Cruz-Ramirez et al., 2012).
The search for mechanisms that control the behavior of proximal stem cells will be a critical part of understanding long-term growth in the meristem. However, a correlation of division patterns in the whole meristem seems like a critical facet of describing phenotypes in existing meristem mutants. The transparency of the root makes it particularly amenable to live imaging using light sheet and other real-time microscopy techniques (Sozzani et al., 2014). To explore the mechanisms that lead to stem cell behavior, tracking division patterns around the meristem in wild type and growth-perturbed mutants could provide a better correlation of growth patterns to stem cell behavior (e.g. Reddy et al., 2004; Reddy and Meyerowitz, 2005). The examination of growth-regulating mutants in an open meristem model or in species other than Arabidopsis would also help parse out which signaling mechanisms serve common roles across different anatomies.
In this alternative model of stem cell behavior, no local stem cell organizer is needed. Rather, a confluence of broadly acting forces generates a highly localized structure, analogous to the way the eye of a hurricane emerges from wider pressure patterns created by a tropical storm. In the case of the plant, many different signals across the meristem may coordinate their activity to give rise to distinct stem cell properties like division behavior and undifferentiated states. It is recognized here that some combination of local organization along with long-range signals may ultimately prove a better model. This review encourages a broader consideration of models when interpreting growth phenotypes in the meristem, as the control of long-term growth in the meristem need not necessarily be centrally controlled.
Acknowledgments
Funding was provided by the National Institutes of Health (R01 GM078279 to KDB), and the European Molecular Biology Organization (LTF185-2010 for IE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- A M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh Y-S, Amasino R, Scheres B. The PLETHORA Genes Mediate Patterning of the Arabidopsis Root Stem Cell Niche. Cell. 2004;119:109–120. doi: 10.1016/j.cell.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Barlow PW. The concept of the stem cell in the context of plant growth and development. In: Lord BI, Potten CS, Cole RJ, editors. Stem cells and tissue homeostasis In Stem Cells and Tissue Homeostasis. Cambridge: Cambridge University Press; 1978. [Google Scholar]
- Bennett T, van den Toorn A, Willemsen V, Scheres B. Precise control of plant stem cell activity through parallel regulatory inputs. Development. 2014;141:4055–4064. doi: 10.1242/dev.110148. [DOI] [PubMed] [Google Scholar]
- Breuer C, Ishida T, Sugimoto K. Developmental control of endocycles and cell growth in plants. Curr Opin Plant Biol. 2010;13:654–660. doi: 10.1016/j.pbi.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Brunoud G, Wells DM, Oliva M, Larrieu A, Mirabet V, Burrow AH, Beeckman T, Kepinski S, Traas J, Bennett MJ, et al. A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature. 2012;482:103–106. doi: 10.1038/nature10791. [DOI] [PubMed] [Google Scholar]
- Clevers H. What is an adult stem cell? Science. 2015;350:1319–1320. doi: 10.1126/science.aad7016. [DOI] [PubMed] [Google Scholar]
- Clowes FAL. The Difference Between Open and Closed Meristems. Annals of Botany. 1981;46:761–767. [Google Scholar]
- Cruz-Ramirez A, Diaz-Trivino S, Blilou I, Grieneisen VA, Sozzani R, Zamioudis C, Miskolczi P, Nieuwland J, Benjamins R, Dhonukshe P, et al. A bistable circuit involving SCARECROW-RETINOBLASTOMA integrates cues to inform asymmetric stem cell division. Cell. 2012;150:1002–1015. doi: 10.1016/j.cell.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Ramirez A, Diaz-Trivino S, Wachsman G, Du Y, Arteaga-Vazquez M, Zhang H, Benjamins R, Blilou I, Neef AB, Chandler V, et al. A SCARECROW-RETINOBLASTOMA protein network controls protective quiescence in the Arabidopsis root stem cell organizer. PLoS Biol. 2013;11:e1001724. doi: 10.1371/journal.pbio.1001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. Cellular organisation of the Arabidopsis thaliana root. Development. 1993;119:71–84. doi: 10.1242/dev.119.1.71. [DOI] [PubMed] [Google Scholar]
- Efroni I, Mello A, Nawy T, Ip PL, Rahni R, DelRose N, Powers A, Satija R, Birnbaum KD. Root Regeneration Triggers an Embryo-like Sequence Guided by Hormonal Interactions. Cell. 2016 doi: 10.1016/j.cell.2016.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau K. Plant anatomy. 2d. New York: Wiley; 1965. [Google Scholar]
- Esau K. Anatomy of seed plants. 2d. New York: Wiley; 1977. [Google Scholar]
- Etchells JP, Turner SR. The PXY-CLE41 receptor ligand pair defines a multifunctional pathway that controls the rate and orientation of vascular cell division. Development. 2010;137:767–774. doi: 10.1242/dev.044941. [DOI] [PubMed] [Google Scholar]
- Evert RF, Esau K, Esau K. Esau's Plant anatomy : meristems, cells, and tissues of the plant body : their structure, function, and development. 3rd. Hoboken, N.J.: Wiley-Interscience; 2006. [Google Scholar]
- Feldman LJ. The de Novo Origin of the Quiescent Center Regenerating Root Apices of Zea mays. Planta. 1976;128:207–212. doi: 10.1007/BF00393230. [DOI] [PubMed] [Google Scholar]
- Forzani C, Aichinger E, Sornay E, Willemsen V, Laux T, Dewitte W, Murray JA. WOX5 suppresses CYCLIN D activity to establish quiescence at the center of the root stem cell niche. Curr Biol. 2014;24:1939–1944. doi: 10.1016/j.cub.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster AS. Studies on the Structure of the Shoot Apex in Seed Plants. Bulletin of the Torrey Botanical Club. 1941;68:339–350. [Google Scholar]
- Gaillochet C, Daum G, Lohmann JU. O cell, where art thou? The mechanisms of shoot meristem patterning. Curr Opin Plant Biol. 2015;23:91–97. doi: 10.1016/j.pbi.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Galinha C, Hofhuis H, Luijten M, Willemsen V, Blilou I, Heidstra R, Scheres B. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature. 2007;449:1053–1057. doi: 10.1038/nature06206. [DOI] [PubMed] [Google Scholar]
- Gifford EM, Foster AS, Foster AS. Morphology and evolution of vascular plants. 3rd. New York: W.H. Freeman and Co.; 1989. [Google Scholar]
- Grieneisen VA, Xu J, Maree AF, Hogeweg P, Scheres B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature. 2007;449:1008–1013. doi: 10.1038/nature06215. [DOI] [PubMed] [Google Scholar]
- Heimsch C, Seago JL., Jr Organization of the root apical meristem in angiosperms. Am J Bot. 2008;95:1–21. doi: 10.3732/ajb.95.1.1. [DOI] [PubMed] [Google Scholar]
- Heisler MG, Hamant O, Krupinski P, Uyttewaal M, Ohno C, Jonsson H, Traas J, Meyerowitz EM. Alignment between PIN1 polarity and microtubule orientation in the shoot apical meristem reveals a tight coupling between morphogenesis and auxin transport. PLoS Biol. 2010;8:e1000516. doi: 10.1371/journal.pbio.1000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman J, Cools T, Vandenbussche F, Heyndrickx KS, Van Leene J, Vercauteren I, Vanderauwera S, Vandepoele K, De Jaeger G, Van Der Straeten D, et al. ERF115 controls root quiescent center cell division and stem cell replenishment. Science. 2013;342:860–863. doi: 10.1126/science.1240667. [DOI] [PubMed] [Google Scholar]
- Ivanov VB. Stem cells in the root and the problem of stem cells in plants. Russian Journal of Developmental Biology. 2007;38:338–349. [Google Scholar]
- Laplane L. Cancer stem cells : philosophy and therapies. Cambridge, Massachusetts: Harvard University Press; 2016. [Google Scholar]
- Mahonen AP, ten Tusscher K, Siligato R, Smetana O, Diaz-Trivino S, Salojarvi J, Wachsman G, Prasad K, Heidstra R, Scheres B. PLETHORA gradient formation mechanism separates auxin responses. Nature. 2014;515:125–129. doi: 10.1038/nature13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki Y, Ogawa-Ohnishi M, Mori A, Matsubayashi Y. Secreted peptide signals required for maintenance of root stem cell niche in Arabidopsis. Science. 2010;329:1065–1067. doi: 10.1126/science.1191132. [DOI] [PubMed] [Google Scholar]
- Moubayidin L, Di Mambro R, Sozzani R, Pacifici E, Salvi E, Terpstra I, Bao D, van Dijken A, Dello Ioio R, Perilli S, et al. Spatial coordination between stem cell activity and cell differentiation in the root meristem. Dev Cell. 2013;26:405–415. doi: 10.1016/j.devcel.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman IV. Pattern in the meristems of vascular plants: 111. Pursuing the patterns in the apical meristem where no cell is a permanent cell*. J Linn Soc. 1965;59:185. [Google Scholar]
- Petricka JJ, Winter CM, Benfey PN. Control of Arabidopsis root development. Annu Rev Plant Biol. 2012;63:563–590. doi: 10.1146/annurev-arplant-042811-105501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi L, Aichinger E, van der Graaff E, Llavata-Peris CI, Weijers D, Hennig L, Groot E, Laux T. Organizer-Derived WOX5 Signal Maintains Root Columella Stem Cells through Chromatin-Mediated Repression of CDF4 Expression. Dev Cell. 2015;33:576–588. doi: 10.1016/j.devcel.2015.04.024. [DOI] [PubMed] [Google Scholar]
- Prantl K. Untersuchungen uber die Regeneration des Vegetationspunktes an Agiospermenwurzeln. Arb Bot Inst Wurzburg. 1874;4:546–562. [Google Scholar]
- Reddy GV, Heisler MG, Ehrhardt DW, Meyerowitz EM. Real-time lineage analysis reveals oriented cell divisions associated with morphogenesis at the shoot apex of Arabidopsis thaliana. Development. 2004;131:4225–4237. doi: 10.1242/dev.01261. [DOI] [PubMed] [Google Scholar]
- Reddy GV, Meyerowitz EM. Stem-cell homeostasis and growth dynamics can be uncoupled in the Arabidopsis shoot apex. Science. 2005;310:663–667. doi: 10.1126/science.1116261. [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Frenz M, Mandel T, Kuhlemeier C. Microsurgical and laser ablation analysis of interactions between the zones and layers of the tomato shoot apical meristem. Development. 2003;130:4073–4083. doi: 10.1242/dev.00596. [DOI] [PubMed] [Google Scholar]
- Richards S, Wink RH, Simon R. Mathematical modelling of WOX5- and CLE40-mediated columella stem cell homeostasis in Arabidopsis. J Exp Bot. 2015;66:5375–5384. doi: 10.1093/jxb/erv257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez RE, Ercoli MF, Debernardi JM, Breakfield NW, Mecchia MA, Sabatini M, Cools T, De Veylder L, Benfey PN, Palatnik JF. MicroRNA miR396 Regulates the Switch between Stem Cells and Transit-Amplifying Cells in Arabidopsis Roots. Plant Cell. 2015;27:3354–3366. doi: 10.1105/tpc.15.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502:513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;99:463–472. doi: 10.1016/s0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- Sabatini S, Heidstra R, Wildwater M, Scheres B. SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes Dev. 2003;17:354–358. doi: 10.1101/gad.252503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, Scheres B, Heidstra R, Laux T. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature. 2007;446:811–814. doi: 10.1038/nature05703. [DOI] [PubMed] [Google Scholar]
- Savaldi-Goldstein S, Chory J. Growth coordination and the shoot epidermis. Curr Opin Plant Biol. 2008;11:42–48. doi: 10.1016/j.pbi.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaldi-Goldstein S, Peto C, Chory J. The epidermis both drives and restricts plant shoot growth. Nature. 2007;446:199–202. doi: 10.1038/nature05618. [DOI] [PubMed] [Google Scholar]
- Scadden DT. Nice neighborhood: emerging concepts of the stem cell niche. Cell. 2014;157:41–50. doi: 10.1016/j.cell.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres B. Stem-cell niches: nursery rhymes across kingdoms. Nat Rev Mol Cell Biol. 2007;8:345–354. doi: 10.1038/nrm2164. [DOI] [PubMed] [Google Scholar]
- Scheres B, Wolkenfelt H, Willemsen V, Terlouw M, Lawson E, Dean C, Weisbeek P. Embryonic origin of the Arabidopsis primary root and root meristem initials. Development. 1994;120:2475–2487. [Google Scholar]
- Sena G, Wang X, Liu HY, Hofhuis H, Birnbaum KD. Organ regeneration does not require a functional stem cell niche in plants. Nature. 2009 doi: 10.1038/nature07597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott EW. Plant Morphogenesis. 1960:244. [Google Scholar]
- Sozzani R, Busch W, Spalding EP, Benfey PN. Advanced imaging techniques for the study of plant growth and development. Trends Plant Sci. 2014;19:304–310. doi: 10.1016/j.tplants.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozzani R, Cui H, Moreno-Risueno MA, Busch W, Van Norman JM, Vernoux T, Brady SM, Dewitte W, Murray JA, Benfey PN. Spatiotemporal regulation of cell-cycle genes by SHORTROOT links patterning and growth. Nature. 2010;466:128–132. doi: 10.1038/nature09143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl Y, Grabowski S, Bleckmann A, Kuhnemuth R, Weidtkamp-Peters S, Pinto KG, Kirschner GK, Schmid JB, Wink RH, Hulsewede A, et al. Moderation of Arabidopsis root stemness by CLAVATA1 and ARABIDOPSIS CRINKLY4 receptor kinase complexes. Curr Biol. 2013;23:362–371. doi: 10.1016/j.cub.2013.01.045. [DOI] [PubMed] [Google Scholar]
- Stahl Y, Wink RH, Ingram GC, Simon R. A signaling module controlling the stem cell niche in Arabidopsis root meristems. Curr Biol. 2009;19:909–914. doi: 10.1016/j.cub.2009.03.060. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi H, Busch W, Benfey PN. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell. 2010;143:606–616. doi: 10.1016/j.cell.2010.10.020. [DOI] [PubMed] [Google Scholar]
- van den Berg C, Willemsen V, Hendriks G, Weisbeek P, Scheres B. Short-range control of cell differentiation in the Arabidopsis root meristem. Nature. 1997;390:287–289. doi: 10.1038/36856. [DOI] [PubMed] [Google Scholar]
- van Es JH, Sato T, van de Wetering M, Lyubimova A, Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Gu X, Xu D, Wang W, Wang H, Zeng M, Chang Z, Huang H, Cui X. miR396-targeted AtGRF transcription factors are required for coordination of cell division and differentiation during leaf development in Arabidopsis. Journal of experimental botany. 2011;62:761–773. doi: 10.1093/jxb/erq307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen V, Bauch M, Bennett T, Campilho A, Wolkenfelt H, Xu J, Haseloff J, Scheres B. The NAC domain transcription factors FEZ and SOMBRERO control the orientation of cell division plane in Arabidopsis root stem cells. Dev Cell. 2008;15:913–922. doi: 10.1016/j.devcel.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jiao Y, Liu Z, Zhu YX. ROW1 maintains quiescent centre identity by confining WOX5 expression to specific cells. Nat Commun. 2015;6:6003. doi: 10.1038/ncomms7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Wei L, Xu J, Zhai Q, Jiang H, Chen R, Chen Q, Sun J, Chu J, Zhu L, et al. Arabidopsis Tyrosylprotein sulfotransferase acts in the auxin/PLETHORA pathway in regulating postembryonic maintenance of the root stem cell niche. Plant Cell. 2010;22:3692–3709. doi: 10.1105/tpc.110.075721. [DOI] [PMC free article] [PubMed] [Google Scholar]