Abstract
Vitamin B12 is a water-soluble molecule composed of a tetrapyrrolic complex with a cobalt atom at its centre. It is an essential regulatory element, synthesized only by bacteria; for this reason it is present only in food of animal origin and the daily requirement for humans is about 1 to 2 mg. Since milk and dairy products provide a significant dietary cobalamin intake, an ultra performance liquid chromatographytandem mass spectrometry method was applied to samples collected at different stages along the process of cheese making in order to evaluate the distribution of this molecule. In particular, samples of milk, rennet, whey, ricotta cheese, curd, mozzarella cheese and caciotta cheese were analysed. Results showed a level of vitamin B12 about 10 times higher in whey and ricotta cheese with respect to the milk they are derived from. These data would confirm the tendency of cobalamine to concentrate in the proteic fractions along the cheese production process.
Key words: Vitamin B12, Cobalamine, UPLC-MS/MS, Dairy products
Introduction
Vitamin B12, or cobalamine, is a water-soluble molecule composed of a tetrapyrrolic complex with a cobalt atom at its centre. It is an essential regulatory element, very important to human physiology since it is involved in the red blood cells formation and nervous system functioning, and its deficiency may lead to anaemia and nerve degenerations. Differently from other vitamins belonging to the B class, vitamin B12 is synthesized only by bacteria; for this reason it is present only in food bacterially fermented or obtained from animals that got this cobalamin from their gastrointestinal microflora or diet (Girard et al., 2009).
Food of animal origin is the main source of this vitamin for humans and daily requirement is about 1 to 2 µg per day (Chassigne and Lobinski, 1998). In foodstuff, vitamin B12 is present in its coenzyme forms: hydroxylcobalamin, 5’-deoxyadenosylcobalamin, methylcobalamin, and cyanocobalamin, which occurs only in small amounts naturally (Kelly et al., 2005). Cyanocobalamin is more stable than the other forms which are light sensitive (Kumar et al., 2010). Cobalamins are naturally protected from degradation by protein-binding; in bovine milk, naturally occurring vitamin B12 is associated with the protein fraction, with high affinity to whey protein (Campos-Giménez et al., 2008).
Since milk and dairy products provide a significant dietary cobalamin intake, an ultra performance liquid chromatography-tandem mass spectrometry method (Zironi et al., 2013) was applied to samples collected at several stages along the process of cheese making in order to evaluate the distribution of this molecule in different dairy products.
Materials and Methods
Sample preparation
Samples of milk, rennet, whey, ricotta cheese, curd, mozzarella cheese and caciotta cheese were collected twice along the production processes, which were repeated four times on different days. Routine control on serum reported a pH of 4.9 and a protein content of 1.14%.
Mozzarella was produced using 50 L of unpasteurized milk and natural whey culture as starter. Briefly, the raw milk was heated to 37-38°C, then the whey and rennet were added and left to ripen at 37-38°C for about 4 h. Afterwards, the curd was extracted from the whey, stretched with hot water (90°C) for about 3 min and then molded into the traditional round shape. Each mozzarella weighed about 250 g (Serraino et al., 2013).
The extraction procedure was conducted on 5 g of the various matrices adding sodium acetate buffer and potassium cyanide; samples were submitted to heat treatment that, in presence of cyanide, converts all the less stable cobalamins in cyanocobalamin (Zironi et al., 2013). For solid matrices it has been necessary to introduce a centrifugation step before solid phase extraction (SPE) clean-up, in order to obtain a better separation between the liquid phase and the solid residue. Samples were then purified by a single SPE step and analysed using reverse phase liquid chromatography coupled with tandem mass spectrometry in positive electrospray ionization (ESI+). Methotrexate was used as internal standard for quantification (Lu et al., 2008).
Analytical conditions
The separation was performed by an acquity ultra-performance liquid chromatographic system consisting of a binary pump, solvent degasser, autosampler and column heater fitted with a Waters HSS T3 column (1.7 µm, 2.1×50 mm) equipped with a guard column with the same packing (Waters Corporation, Milford, MA, USA). Flow rate was 0.3 mL/min and the column temperature was kept at 45°C. Separation was carried out in programmed conditions with mobile phase consisting of water (A) and acetonitrile containing 0.1% formic acid (B), for a total run time of 5 min. The gradient was T0 min: 90% A, 10% B; T2 min: 20% A, 80% B; T3 min to the end: 90% A, 10% B.
The mass spectrometer was a Quattro Premiere XE, a triple quadrupole instrument equipped with an ESCI™ Multi-Mode Ionization Source (Waters Corporation).
The whole analysis was conducted in ESI+ mode using multiple reaction monitoring (MRM). The monitored transitions were m/z 678.36→147.10 and 678.36→359.30 for vitamin B12, and m/z 455.22→175.13 and 455.22→308.22 for methotrexate (IS).
Results and Discussion
The complexity of vitamin B12 quantification in food matrices has been investigated by several Authors but very few works on milk, and in particular on dairy products, made use of mass spectrometry. Vitamin B12 concentrations found in milk were similar to those described in the literature (Arkbåge et al., 2003), although it was not possible to find data comparable to our cheese making process.
Results showed a level of vitamin B12 about 10 times higher in whey and ricotta cheese with respect to the milk they are derived from; at the same time, it is interesting to notice a decrease of cobalamine concentration in curd, caciotta cheese and mozzarella cheese (Figure 1). Vitamin B12 affinity for proteins is well known and several kinds of carrier proteins, called transcobalamines, have been identified; these molecules bind cobalamin to protect it from degradation and some analogues are present in bovine milk as well (Fedosov et al., 1995, 1996; Fox and Kelly, 2003; Le et al., 2011).
Figure 1.
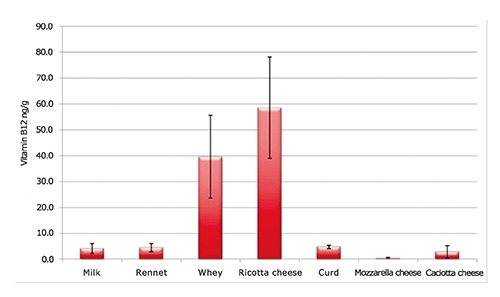
Vitamin B12 concentrations in different samples (n=8, mean value±standard deviation).
Conclusions
These data would confirm the tendency of cobalamine to concentrate in the proteic fraction of whey along the cheese production process. The lower value for mozzarella cheese compared to curd is probably due to the process of production, which implies curd draining and a dilution effect related to the water addition in the stretching phase.
References
- Arkbåge K, Witthöft C, Fondén R, Jägerstad M,. 2003, Retention of vitamin B12 during manufacture of six fermented dairy products using a validated radio protein-binding assay. Int Dairy J 13:101-9. [Google Scholar]
- Campos-Giménez E, Fontannaz P, Trisconi MJ, Kilinç T, Gimenez C, Andrieux P, 2008. Determination of vitamin B12 in food products by liquid chromatography/UV detection with immunoaffinity extraction: single-laboratory validation. J AOAC Int 91:786-93. [PubMed] [Google Scholar]
- Chassigne H, Lobinski R, 1998. Direct species-selective determination of cobalamins by ionspray mass spectrometry and ionspray tandem mass spectrometry. Analyst 123:131-7. [Google Scholar]
- Fedosov SN, Petersen TE, Nexo E, 1995. Binding of cobalamin and cobinamide to transcobalamin from bovine milk. Biochemestry 34:16082-7. [DOI] [PubMed] [Google Scholar]
- Fedosov SN, Petersen TE, Nexo E, 1996. Transcobalamin from milk: isolation and physico-chemical properties. Biochim Biophys Acta 1292:113-9. [DOI] [PubMed] [Google Scholar]
- Fox PF, Kelly AL, 2003. Developments in the chemistry and technology of milk proteins. Food Aust 55:231-4. [Google Scholar]
- Girard CL, Santschi DE, Stabler S P, Allen RH, 2009. Apparent ruminal synthesis and intestinal disappearance of vitamin B12 and its analogs in dairy cows. J Dairy Sci 92:4524-9. [DOI] [PubMed] [Google Scholar]
- Kelly RJ, Gruner TM, Sykes AR, 2005. Development of a method for the separation of corrinoids in ovine tissues by HPLC. Biomed Chromatogr 19:329-33. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Chouhan RS, Thakur MS, 2010. Trends in analysis of vitamin B12. Anal Biochem 398:139-49. [DOI] [PubMed] [Google Scholar]
- Le A, Barton LD, Sanders JT, Zhang Q, 2011. Exploration of bovine milk proteome in colostral and mature whey using an ionexchange approach. J Proteome Res 10: 692-704. [DOI] [PubMed] [Google Scholar]
- Lu B, Ren Y, Huang B, Liao W, Cai Z, Tie X, 2008. Simultaneous determination of four water-soluble vitamins in fortified infant foods by ultra-performance liquid chromatography coupled with triple quadrupole mass spectrometry. J Chromatogr Sci 46:225-32. [DOI] [PubMed] [Google Scholar]
- Serraino A, Giacometti F, Daminelli P, Losio MN, Finazzi G, Marchetti G, Zambrini AV, Rosmini R, 2013. Survival of Arcobacter butzleri during production and storage of artisan water buffalo mozzarella cheese. Foodborne Pathog Dis 10:820-4. [DOI] [PubMed] [Google Scholar]
- Zironi E, Gazzotti T, Barbarossa A, Devicienti C, Scardilli M, Pagliuca G, 2013. Technical note: development and validation of a method using ultra performance liquid chromatography coupled with tandem mass spectrometry for determination of vitamin B-12 concentrations in milk and dairy products. J Dairy Res 96:2832-6. [DOI] [PubMed] [Google Scholar]


