Abstract
The aim of the study was to evaluate the biogenic amine (BA) content of Ciauscolo salami made with and without the use of a selected started culture. Two batches of salami were made following the guidelines of the Protected Geographical Indications: with and without adding a commercial starter culture made of Lactobacillus plantarum and Staphylococcus xylosus. Six samples of salami per batch were collected at different ripening times (0, 15, 30, 45 and 60 days) for physical, chemical and microbiological analyses and for the determination of BA content. No differences were recorded for physical, chemical and microbiological analyses except for Staphylococcus spp. count at the time of casing (T0) and total volatile basic nitrogen (TVBN) from 30 days (T2) to the end of the ripening time (60 days, T4). After 60 days of ripening, the use of selected starter culture significantly affected the amount of putrescine (195.15 vs 164.43 mg/100 g in salami without and with starters, respectively), cadaverine (96.95 vs 104.40 mg/100 g in salami without and with starters, respectively), histamine (81.94 vs 69.89 mg/100 g in salami without and with starters, respectively), and spermine (36.88 vs 33.57 mg/100 g in salami without and with starters, respectively). Despite significantly higher values of TVBN, the use of selected starter culture determined no significant effects on the BA content of the products.
Key words: Raw fermented meat products, Biogenic amines, Staphylococcus xilosus, Lactobacillus plantarum, Starter culture
Introduction
The cultural heritage and the connection to local production are two important intrinsic product attributes involved in consumer choice of high quality foods (Martinez et al., 2010). Among Italian local products, Ciauscolo salami is a fermented meat product with an ancient history, produced in a small geographic area in Central Italy and appreciated by consumers for its peculiar taste and traits (Rea et al., 2003). The product is spreadable, slightly smoke flavoured and, due to its peculiar characteristics and geographical localization, in 2009 it was awarded with the Protected Geographical Indication (PGI) certification from the European Union (Reg. EC No 729/2009; European Commission, 2009). The product is traditionally marketed after about 4 weeks of ripening but according to the production guidelines (www.politicheagricole.it) not less than 20 days after casing (15 days of ripening). However, sometimes the product may undergo a more prolonged ripening (2-3 months) based on market requests (Rea et al., 2005a). In reality, the real typical homemade Ciauscolo originally underwent a longer ripening because it was used as a break from farm fieldwork which restarted late in the spring after the winter rest (Rea et al., 2003). Despite the generally artisanal production of PGI Ciauscolo salami, there is an industrial interest in the standardization of the product. The use of selected starter bacteria is probably the most common method for achieving a standard dry fermented product (Leroy et al., 2006). The salami making technique, especially the starter culture used, could cause differences in the production of biogenic amines (BAs) (Martuscelli et al., 2000; Rea et al., 2005a). BAs are low molecular weight basic nitrogenous compounds occurring in many foods and are mainly due to amino acid decarboxylation performed by some microorganisms. Amino acid decarboxylation may play an important energy role for bacteria in nutritionally poor environments. It can generate a charge translocation through the cellular membrane influencing its potential (Martuscelli et al., 2000; Suzzi and Gardini, 2003). In particular, the possibility that histidine decarboxylation and the following histamine (HIS) formation may represent an alternative pathway for lactic acid bacteria (LAB) to produce energy when insufficient amounts of fermentable sugars are present has been reported (Rea et al., 2005a). The amino acid decarboxylation is not the only enzymatic system to form BAs. Some of them, such as putrescine (PUT) which is formed mainly from arginine decarboxylation, can be obtained through different pathways that produce intermediate compounds (ornithine and agmatine in particular) by both Gram negative and Gram positive bacteria following generally more complex reactions in the latter (Wunderlichova et al., 2014). Moreover, some of the BAs present in food, especially PUT, cadaverine (CAD), spermine (SPM) and spermidine (SPD), are also produced by the animal organism itself for physiological purposes (Suzzi and Gardini, 2003). Polyamines are present in many types of body cells and their presence in food is often due to their endogenous origin, although in the human body the largest part of PUT comes from food intake (Wunderlichova et al., 2014).
The presence of BAs can be considered from different points of view according to the meaning given to them (Silla Santos, 1996). Besides their real or potential action as toxic substances, BAs play several important biological roles. Most of these roles are still unclear: their contribution to the development of food aroma (Mascaro et al., 2010); their role as potential precursors of carcinogenic compounds such as nitrosamines (Yamamoto et al., 1982; Stratton et al., 1991; Shalaby, 1996; Wunderlichova et al., 2014); their role as possible indicators of freshness or spoilage levels of food (Rea et al., 2008); their use in the evaluation of hygienic characteristics of raw matter used for food manufacturing, especially in fishery products (Veciana-Nogues et al., 1997; Muscarella et al., 2005; Visciano et al., 2012; Wunderlichova et al., 2014) but also in different meat products (Cantoni, 1995; Hernandez-Jover et al., 1996; Rea et al., 2008).
Many studies have been carried out regarding BAs as toxic compounds and the aspects related to their toxicity have been largely reviewed (Stratton et al., 1991; Shalaby, 1996; Silla Santos, 1996; Wunderlichova et al., 2014), even specifically in dry fermented sausages (Suzzi and Gardini, 2003). Their biological roles have also been reported by several authors: polyamines act as modulators of cellular function, as reviewed in detail by (Igarashi and Kashiwagi (2010); are indispensable components of living cells, especially in rapidly growing tissues and the intestine in particular; and are essential to maintain the metabolic activity of the immunological system of the gut (Silla Santos, 1996). Some BAs, such as histamine (HIS) and catecholamines, fulfill important metabolic functions in the nervous system, control blood pressure, and mediation of allergic responses (HIS), as reported by Silla Santos (1996). Furthermore, polyamines may act as radical scavengers and inhibit the oxidation of polyunsaturated fatty acids. Putrescine is also a precursor in the synthesis of other polyamines such as SPM and SPD (Wunderlichova et al., 2014). Many of the functions described are related to the polycationic nature of polyamines which allows them to interact with many important negatively charged molecules (DNA, RNA, proteins, phospholipids) (Igarashi and Kashiwagi, 2010; Wunderlichova et al., 2014).
Different papers have recently considered Ciauscolo salami from different perspectives (Aquilanti et al., 2007; Trani et al., 2011; Ranucci et al., 2013; Federici et al., 2014), and a preliminary evaluation of BA content in this product has been reported by Rea et al. (2005b). No data is available on the relationships between the addition of a mixed starter culture of Lactobacillus plantarum and Staphylococcus xylosus and BA content in Ciauscolo salami and the aim of this work is to evaluate the effect of the use of selected starter culture on different characteristics and BA content in such a product.
Materials and Methods
Two batches of Ciauscolo salami were made from the same raw materials and ingredients (pork meat/fat, salt, pepper, garlic) in a local processing plant of central Italy with and without the use of starter culture. Products made without starters were considered as control. Commercially available starter cultures (Lyocarni SHA-24; Sacco srl., Cadorago, Italy) containing Lactobacillus plantarum and Staphylococcus xylosus were added to the minced meat, according to manufacturer’s guidelines, followed by the other ingredients and additives (ascorbic acid – E300, and potassium nitrate – E252), as dictated by regulation. Formulation was in accordance with the PGI guidelines for Ciauscolo salami.
Six samples from each batch were collected from each group after casing (T0) and every 15 days until 60 days of ripening (T1=15 days; T2=30 days, T3=45 days, T4=60 days) were reached. Samples were sent under refrigeration to the laboratory for physical, chemical and microbiological analyses and for BA determination.
Some physical and chemical analyses were performed on each sample and at each sampling time. In particular: pH was determined according to Bendall (1977) using a pHmeter (MP 230; MettlerToledo, Schwerzenbach, Switzerland) equipped with a routine electrode; water activity (aw) was determined by HygroLab 3 hygrometer (Rotronic, Huntington, NY, USA); and total volatile basic nitrogen (TVBN) was determined according to Pearson (1991). Only after 60 days of ripening the proximate chemical composition and salt (NaCl) content were determined according to AOAC methods (1990).
For microbiological analyses, 25 g of each sample were collected after aseptic removal of the casing and were placed in a sterile bag with buffered peptone water (Oxoid, Basingstoke, UK). Samples were homogenized (Stomaker 400 circulator; PBI International, Milan, Italy) for 2 min at room temperature and serial decimal dilutions in buffered peptone water were then prepared. Lactobacillus spp. (on MRS agar, Oxoid, incubated at 37°C for 72 h under anaerobic conditions), Enterobacteriaceae (according to ISO 21528-2:2004; ISO, 2004), Enterococcus spp. (according to ISO 7899-2:2003; ISO, 2003) and Staphylococcus spp. [on Baird Parker agar with Egg Yolk Tellurite Emulsion (Oxoid) incubated at 37°C for 48 h] counts were determined. BA contents were determined according to Rea et al. (2005b).
An unpaired T test (Statview, SAS Institute Inc., Cary, NC, USA) was used to compare the differences in proximate chemical composition between samples with and without starter cultures. For the other parameters, an ANOVA model (Statview) with time (T0, T1, T2, T3 and T4) and groups (with and without starters) as fixed factors, followed by Tukey’s test, was used. Significance level was set at P<0.05.
Results
The proximate chemical composition and salt content of Ciauscolo salami, at the end of a 60-day ripening period with starter culture added or not added, is reported in Figure 1. No differences were recorded in all the parameters considered in the two groups. The evolution of pH and aw of the samples throughout the ripening is reported in Figure 2. The pH dropped to a value below 5.6 after 15 days of ripening (T1) with a significantly (P<0.05) lower value in the products made with the addition of starter culture than in samples without starters (5.39 and 5.54, respectively). No differences were recorded in the pH values registered at T2, T3 and T4. The results of physical and chemical analyses showed the total compliance with the production guidelines: pH≥4.8, proteins not less than 15%, fat between 32 and 42%, moisture/protein ratio ≤3.1, fat/protein ratio ≤2.8. The only exception was fat/protein ratio that was slightly lower than 32% at T1 and T2, excluding T0, when the values were not to be applied.
Figure 1.
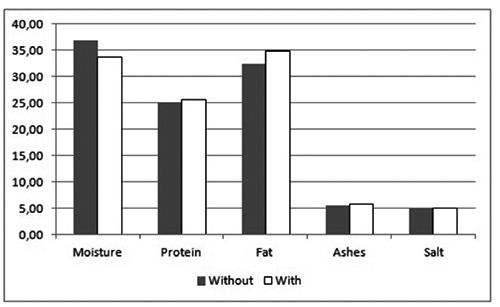
Chemical composition (%) of Protected Geographical Indication Ciauscolo salami made with and without starter cultures (Lactobacillus plantarum and Staphylococcus xylosus) at 60 days of ripening.
Figure 2.
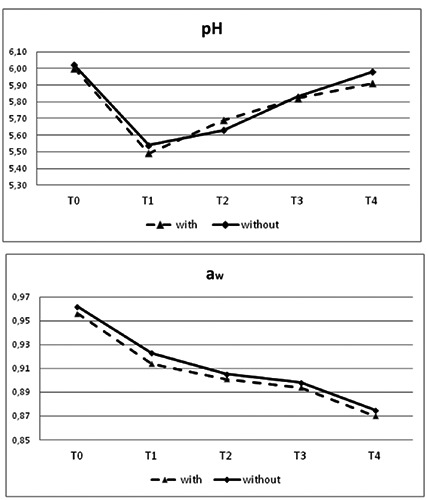
pH and water activity evolution in Protected Geographical Indication Ciauscolo salami with and without starter cultures (Lactobacillus plantarum and Staphylococcus xylosus) at different times of ripening. T0=after casing; T1=15 days; T2=30 days; T3=45 days; T4=60 days.
Aw value also dropped throughout the ripening from expected values of raw salted minced meat (over 0.95) to values below 0.87 recorded at T4 with no differences between the samples of the two groups.
The TVBN values obtained are reported in Figure 3. Differences were registered from T2 to T4 with significantly higher values in Ciauscolo salami made with the addition of starter culture than those made without starters: 52.98 vs 42.84 mg/100 g at T2 (P<0.05), 66.41 vs 55.71 mg/100 g at T3 (P<0.001); 76.37 vs 69.30 mg/100 g at T4 (P>0.001), respectively.
Figure 3.
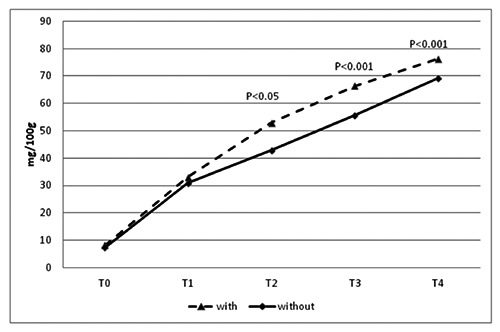
Total volatile basic nitrogen evolution in Protected Geographical Indication Ciauscolo salami with and without starter cultures (Lactobacillus plantarum and Staphylococcus xylosus) at different times of ripening. T0=after casing; T1=15 days; T2=30 days; T3=45 days; T4=60 days.
The results of microbial analyses are reported in Figure 4. No significant differences were recorded between the two groups of samples for all the microbial parameters considered with the exception of Staphylococcus spp. count at T0 when values of 4.18 and 3.08 Log CFU/g were detected for salami made with and without starters, respectively (P<0.05). Also, the count of mesophilic aerobic and lactic bacteria followed the guidelines provided for PGI Ciauscolo salami that fix a lower limit of 7 log CFU/g.
Figure 4.
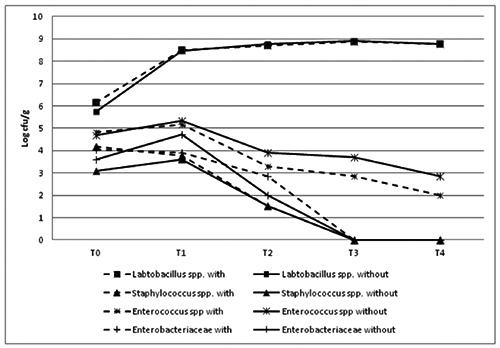
Microbial growth in Protected Geographical Indication Ciauscolo salami made with and without starter cultures (Lactobacillus plantarum and Staphylococcus xylosus) at different times of ripening. T0=after casing; T1=15 days; T2=30 days; T3=45 days; T4=60 days.
The BA contents determined in the two groups of salami are reported in Table 1 and in Figures 5 and 6. The differences detected were significant for all BAs when the ripening factor was considered (P<0.001). Considering the starter addition as a fixed factor, significance was observed for PUT (P=0.006), CAD (P<0.001) and SPM (P=0.003). Considering both time and presence of starters, the differences were only significant (P=0.049) for HIS.
Table 1.
Biogenic amine content (ppm, mean±standard deviation) in Ciauscolo salami made with and without starter cultures.
| Time | Sample | B-Phenilethylamine | Putrescine | Cadaverine | Histamine | Tyramine | Spermidine | Spermine | Total |
|---|---|---|---|---|---|---|---|---|---|
| T0 | With | 0 | 0 | 17.09±0.01a | 0 | 0 | 0 | 28.10±0.01a | 45.19±0.01a |
| Without | 0 | 0 | 9.60±1.07a | 0 | 0 | 0 | 30.81±6.30a | 40.41±7.37a | |
| T1 | With | 0a | 40.24±1.25a | 54.62±2.55b | 15.35±1.41a | 113.86±0.43a | 1.51±2.06a | 31.52±2.24a | 257.11±4.77b |
| Without | 7.16±9.08a | 69.85±7.97b | 56.69±1.17b | 22.59±3.06a | 117.53±2.11a | 2.64±2.93a | 33.28±0.68a | 309.75±3.93b | |
| T2 | With | 26.83±2.94b | 91.35±12.20c | 75.12±9.83c | 30.24±5.09b | 162.73±23.66b | 0a | 35.31±1.02a | 421.59±49.14c |
| Without | 24.15±12.53b | 101.58±30.86c | 70.01±6.52c | 35.17±15.38b | 163.33±24.16b | 3.64±3.10a | 35.39±1.15a | 433.27±85.76c | |
| T3 | With | 30.91±1.24bc | 125.76±17.48d | 91.48±4.52e | 36.88±5.73bc | 208.18±1.08c | 5.40±0.52b | 35.75±1.95b | 534.37±12.32d |
| Without | 30.61±2.86bc | 141.51±30.99d | 82.20±7.13d | 46.31±6.45c | 195.01±26.66c | 4.71±2.33b | 37.48±1.41b | 537.83±72.05d | |
| T4 | With | 37.71±2.41c | 164.43±14.20e | 104.40±4.05f | 69.89±5.14d | 230.16±5.06d | 5.55±0.88b | 33.57±1.88a | 645.69±19.50e |
| Without | 34.37±6.53c | 195.15±36.12f | 96.95±4.94e | 81.94±17.29e | 238.05±5.06d | 5.84±0.66b | 36.88±2.90b | 689.69±73.69e | |
| P value | Time | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Starter | 0.921 | 0.006 | <0.001 | 0.241 | 0.964 | 0.094 | 0.003 | 0.216 | |
| Time×Starter | 0.245 | 0.495 | 0.140 | 0.049 | 0.539 | 0.055 | 0.456 | 0.426 |
T0, after casing; T1, 15 days; T2, 30 days; T3, 45 days; T4, 60 days.
a-fDifferent letters in the same column show significant differences (P<0.05).
Figure 5.
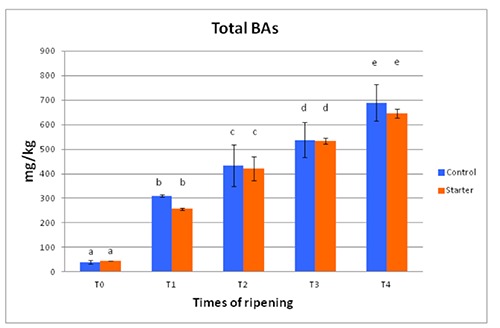
Total amounts of biogenic amines in Protected Geographical Indication Ciauscolo salami made with (starter) and without (control) starter cultures (Lactobacillus plantarum and Staphylococcus xylosus) at different times of ripening. T0=after casing; T1=15 days; T2=30 days; T3=45 days; T4=60 days. Different letters in the same column show significant differences.
Figure 6.
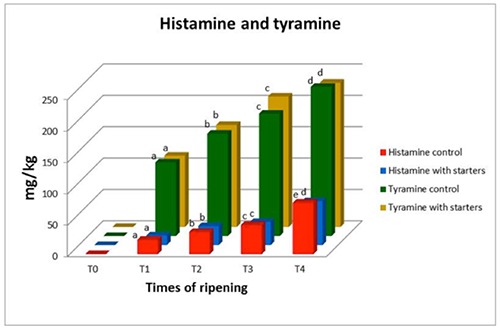
Amounts of histamine and tyramine in Protected Geographical Indication Ciauscolo salami made with and without (control) starter cultures (Lactobacillus plantarum and Staphylococcus xylosus) at different times of ripening. T0=after casing; T1=15 days; T2=30 days; T3=45 days; T4=60 days. Different letters in the same column show significant differences.
The total amount of BAs throughout the study increased progressively showing significant differences among the different sampling times as expected. With the exception of samples at T0, control products (made without starters) showed slightly higher amounts of BAs than samples with starters added at each sampling time but these differences were never significant. The concentration of total BAs at T0 in both groups of products was mostly due to the presence of SPM but PUT was also present. The most evident increase of total BA concentration was observed between T0 and T1, which corresponded to the lowest pH values observed. This was mainly due to TYR, which was the most represented BA at each sampling time [as observed by Rea et al. (2005b) in other kinds of traditional Italian salami] and to PUT and CAD to a lesser extent. In any case, this increase was due to BAs considered as generic and without a relevant increase of HIS, which is the most important BA from a toxicological point of view. This significant progressive increase between T0 and T1, such as the increase observed throughout the study, was real and not related to the progressive decrease of moisture because the values referred to dry matter (data not reported) showed an identical trend.
The concentration of single BAs were almost similar in control samples than in those with added starters. Higher values of PUT, HIS, TYR, SPD and SPM were constantly observed in control samples than in samples with starters added, even at very low extent, with the exception of TYR and SPD at T3 when the values were lower in control samples. However, the differences between the two groups were significant only for PUT at T1 and T4 and for HIS and SPM at T4. Lower values in control samples compared to starter-added samples were constantly observed for B-PHE and CAD with the exception of both BAs at T1, but the differences were significant only for CAD at T3 and T4.
Discussion
The pH values recorded in both starter-added and control Ciauscolo salami were slightly higher than those found by other authors (Rea et al., 2005b; Trani et al., 2010; Ranucci et al., 2013) in the same products even though the LAB count increased from T0 to T1 up to values over 8 Log CFU/g. The microbial dynamics and subsequently the pH evolution, particularly in the first part of fermentation, may be due to the different ripening temperatures adopted. The effect of proteolysis could therefore be responsible for the pH evolution from T1 to T4. The use of the selected starter culture did not seem to alter pH evolution even if proteolysis was higher in starter-added samples than in control ones. Although the artisanal production could be responsible for differences in microbial population, the evolution of the microbial growth between the two groups was similar: as the LAB increased the rest of bacterial population, including Staphylococcus spp., decreased.
Despite a higher degree of proteolysis observed, the use of selected starter culture had no effect on BA content of the products. The production of BAs in dry sausages is often related to the proteolytic activity during fermentation and ripening (Suzzi and Gardini, 2003) but no direct correlation has been found between proteolytic activity of Staphylococcus xylosus and BA production (Bover-Cid et al., 1999).
In the present study, the most evident increase of total BA concentration was observed between T0 and T1 and may be related, beside the normal catabolism of nitrogenous compounds carried out by the microflora during ripening, to the possible protective activity of BAs against the increase of micro-environmental acidity that has been hypothesized by several authors about BA producing bacteria (Silla Santos, 1996; Suzzi and Gardini, 2003; Wunderlichova et al., 2014). As already reported, such an evident increase in both groups of samples between T0 and T1 was mainly due to TYR and to PUT and CAD to a lesser extent. HIS was observed at higher concentrations in control samples than in starter-added ones where the microorganisms are selected. However, these differences were never significant, with the exception of values at T4. The progressive slight increase of HIS has been consistently observed in fermented sausages by several authors (Rea et al., 2005b) and also the presence of this BA in the latest stages of ripening has been consistently detected in the same and other kinds of Italian salami (Rea et al., 2005a).
Generally, TYR, PUT, CAD and HIS are the most important amines detected in both artisanal and industrial fermented sausages (Latorre-Moratalla et al., 2010). In the present study, these BAs were abundant in both groups of samples at each sampling time, with the exception of SPM whose presence is considered physiological and often normal. Indeed, polyamines SPD and SPM are almost always present in raw meat and the presence in raw fermented sausages is mostly related to the drying process involved in the manufacture of the product (Martuscelli et al., 2000). This reason may explain the irregular presence and/or trend of these two compounds during the ripening of products as already observed in dry fermented sausages (Cecchini et al., 2004; Rea et al., 2005a, 2005b, 2008). The results obtained in the present study showed that the concentration of SPM apparently increased as for all the other BAs but if the values referred to total matter were reported to dry matter, the trend was almost the same for all BAs (obviously at higher values) with the exception of SPM. SPM showed a gradual and constant decrease during the ripening process both in control and in starter-added samples: from 78.51 to 58.42 mg/kg and from 68.54 to 50.60 mg/kg, respectively (data not shown).
Moreover, sometimes B-PHE is related to high concentrations of TYR due to non-specificity of tyrosine decarboxylase even though in the present study B-PHE was lower or around 30 ppm, which is generally considered the limit of acceptance for this BA (Shalaby, 1996; Rea et al., 2005a, 2005b). PUT and CAD showed quite high levels but noticeably lower than those observed by Rea et al. (2005a) in the same products. TYR was always the most represented amine as observed by different authors (Rea et al., 2005b) and at levels similar to those detected by Rea et al. (2005a) in the same type of salami at 0, 25 and 60 days of ripening. However, TYR was never found by those authors at levels close to the range 100-800 ppm considered compatible with the usual good manufacturing practices (Shalaby, 1996; Rea et al., 2005a, 2008). As for the limits of toxicity for PUT, CAD and TYR, higher limits than those cited have been reported by Naila et al. (2010) and the values detected in the present study for these BAs were much lower than such limits: 2000 mg/kg as oral toxicity level for PUT; more than 2000 mg/kg as acute toxicity level for TYR and CAD; 2000 mg/kg as no observed adverse effect level (NOAEL) for TYR, PUT and CAD.
The BAs in food are mainly produced by microbial activity especially in fermented raw products which have undergone lactic acid fermentation (Suzzi and Gardini, 2003; Rea et al., 2005a, 2005b). Indeed, amino acid decarboxylases are enzymes present in many genera of microorganisms of food concern (Bacillus, Pseudomonas, Photobacterium, Enterobacteriaceae, Staphylococcus, Micrococcus), including many LAB (Lactobacillus, Enterococcus, Carnobacterium, Pediococcus, Lactococcus, Leuconostoc), some of which are used as starter cultures (Leuscher and Hammes, 1998; Suzzi and Gardini, 2003; Rea et al., 2005a). Many fermented products are obtained from raw material containing high amounts of proteins that provide the substrate for decarboxylase activity of both starter culture and contaminating microflora during ripening (Shalaby, 1996; Silla Santos, 1996; Avellini et al., 1999; Mascaro et al., 2010), especially meat products like dry fermented sausages (Suzzi and Gardini, 2003). For this reason the selection of starter cultures is particularly important in fermented food, where the use of short fermentations with carefully selected starter cultures may prevent uncontrolled fermentation and the formation of BAs (Silla Santos, 1996). However, variable amounts of BAs have been reported for processed meat, particularly for fermented dry and semi-dry sausages even using BA-negative starter cultures that may limit the growth of natural microflora. This variability can be due to several factors such as ripening process time, residue decarboxylase activity of the natural microflora and/or starter culture, manufacturing process, type and quality of raw meat and other ingredients used, and general compliance with hygiene principles (Maijala et al., 1995; Shalaby, 1996; Martuscelli et al., 2000).
Many BAs are physiologically degraded mainly through oxidative deamination catalyzed by amino oxydases DAO and MAO which are very important for BA metabolism, ubiquitous in plants and also found in animal and human cells – particularly DAO at intestinal, hepatic and renal level (Zaman et al., 2010) as well as MAO in mitochondria of brain cells (Wunderlichova et al., 2014). These enzymes were also observed in several strains of bacteria such as Enterobacteriaceae (Klebsiella, Enterobacter, Escherichia, Salmonella, Serratia, Proteus), Micrococcus, Lactobacillus (L. plantarum, L. sakei, L. pentosus), Staphylococcus xylosus, Bacillus (Leuschner et al., 1998). In particular L. plantarum amino oxidase positive for HIS (but also for TIR, PUT and CAD) was observed in sauerkraut, Staph. xylosus amino oxidase positive for HIS and TYR in salted fermented anchovies (Zaman et al., 2010) and Italian artisanal fermented sausages (Martuscelli et al., 2000), and Bacillus positive for HIS degradation (Zaman et al., 2010) was found in salted fermented anchovies. These enzymes catalyze the oxidative deamination of amines with production of aldehydes, hydrogen peroxide and ammonia thus allowing many microorganisms to use amines as nutrient sources (Leuschner et al., 1998; Martuscelli et al., 2000; Wunderlichova et al., 2014). In fact it was observed for B-FEN in E. coli K12 (Parrott et al., 1987) and for TYR in Kocuria (once Micrococcus) varians (Leuscher and Hammes, 1998; Leuschner et al., 1998) again at the conditions present during sausage fermentation but with a retarded decrease rate in the center of products compared to the peripheral parts (Leuscher and Hammes, 1998). Moreover, a low potential for BA degradation was observed among lactobacilli by Leuschner et al. (1998). Another pathway of degradation peculiar to HIS, which represents the primary of the two pathways according to some authors (Stratton et al., 1991), is the methylation process catalyzed by histamine N-methyltranferase (HNMT) present in endotelial, hepatic and gastric cells of animals to form N-methylhistamine which is then oxidized by MAO to form methyl imidazoleacetic acid. Many bacteria capable of degrading BAs belong to halotolerant or halophilic groups but the studies on BA degradation are often performed in buffer systems and probably their activity may be not the same as when transferred to complex systems which are found in salted and fermented foods (Zaman et al., 2010). Moreover, sometimes the reduction in BA concentration by bacterial strains capable of reducing the production of BAs is not the same when raw material of a different hygienic quality is used for sausage manufacturing (Latorre-Moratalla et al., 2010). Actually, it is not only the inoculation of starters and/or the modification of formulation but also the optimal hygienic conditions of raw materials and processing procedures that contribute to the enhancement of the starter performance and to the reduction of the aminogenesis during traditional fermented sausage manufacture (Latorre-Moratalla et al., 2010). Despite different authors referring to the effects of Staphylococcus xylosus and Lactobacillus plantarum on the BA production or degradation (Martuscelli et al., 2000; Gardini et al., 2002; Ammor and Mayo, 2007), no differences were noticed in this study. The results obtained confirm that these commercial starter cultures cannot prevent BA production absolutely. Indeed, products made using commercial starters sometimes show similar or even higher levels of these compounds than traditional fermented meat products manufactured without starter cultures (Latorre-Moratalla et al., 2010) and also reported by Parente et al. (2001) in different types of Italian dry sausages.
Conclusions
The use of selected starter cultures in PGI Ciauscolo salami has limited effects on the chemical and physical characteristics of the products as well as on the quantity of BAs. Therefore, the use of these starters could be avoided especially if products are consumed after 20 days of ripening. Further studies on the possible effects of different starter culture addition on the sensory properties of the products could be of utmost interest for the producers to define a possible intervention strategy alternative to the strictly artisanal production. From the opposite point of view, the correct compliance with good manufacturing practices and the use of high quality raw materials and ingredients may guarantee high standards of production even in artisanal manufacturing of dry fermented sausages without using standardised starter cultures that sometimes may reduce or hide the peculiarities of such valuable products.
References
- Ammor MS, Mayo B. 2007. Selection criteria for lactic acid bacteria to be used as functional starter cultures in dry sausage production: an update. Meat Sci 76:138-46. [DOI] [PubMed] [Google Scholar]
- AOAC, 1990. Official methods of analysis. 15th ed. Association of Official Analytical Chemists ed., Washington, DC, USA. [Google Scholar]
- Aquilanti L, Santarelli S, Silvestri G, Osimani A, Petruzzelli A, Clementi F, 2007. The microbial ecology of a typical Italian salami during its natural fermentation. Int J Food Microbiol 120:136-45. [DOI] [PubMed] [Google Scholar]
- Avellini P, Clementi F, Trabalza Marinucci M, Cenci Goga BT, Rea S, Branciari R, Cavallucci C, Reali C, Di Antonio E, 1999. Pit cheese: compositional microbiological and sensory characteristics. Ital J Food Sci 11:317-33. [Google Scholar]
- Bendall JR, 1977. Cold-contracture and atpturnover in the red and white musculature of the pig, post mortem. J Sci Food Agric 26:55-71. [DOI] [PubMed] [Google Scholar]
- Bover-Cid S, Izquierdo-Pulido M, Vidal-Carou MC, 1999. Effect of proteolytic starter cultures of Staphylococcus spp. on biogenic amine formation during the ripening of dry fermented sausages. Int J Food Microbiol 46:95-104. [DOI] [PubMed] [Google Scholar]
- Cantoni C, 1995. Ammine biogene di prodotti carnei nazionali. Ind Aliment 34:9-12. [Google Scholar]
- Cecchini S, Rea S, Ricciutelli M, Stocchi R, Loschi AR, 2004. Indagine preliminare sul contenuto di amine biogene in ciauscoli artigianali. Proceedings of the LVIII Congress of the Italian Society of Veterinary Sciences, 23-25 September, pp 197-8. [Google Scholar]
- European Commission, 2009. Commission Regulation of 10 August. 2009, entering a name in the register of protected designations of origin and protected geographical indications (Ciauscolo (PGI), 729/2009/EC. In: Official Journal, L 207, 11 August 2009. [Google Scholar]
- Federici S, Ciarrocchi F, Campana R, Ciandrini E, Blasi G, Baffone W, 2014. Identification and functional traits of lactic acid bacteria isolated from Ciauscolo salami produced in Central Italy. Meat Sci 98:575-84. [DOI] [PubMed] [Google Scholar]
- Gardini F, Martuscelli M, Crudele MA, Paparella A, Suzzi G, 2002. Use of Staphylococcus xylosus as a starter culture in dried sausages: effect on the biogenic amine content. Meat Sci 61:275-85. [DOI] [PubMed] [Google Scholar]
- Hernandez-Jover T, Izquierdo-Pulido M, Veciana-Nogues MT, Vidal-Carou MC, 1996. Biogenic amine sources in cooked cured shoulder pork. J Agric Food Chem 44:3097-101. [Google Scholar]
- Igarashi K, Kashiwagi K. 2010. Modulation of cellular function by polyamines. Int J Biochem Cell Biol 42:39-51. [DOI] [PubMed] [Google Scholar]
- ISO, 2003. Qualità dell’acqua - Ricerca ed enumerazione degli enterococchi intestinali. Parte 2. Metodo di filtrazione su membrana. ISO Norm 7899-2:2003. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- ISO, 2004. Microbiology of food and animal feeding stuffs. Horizontal methods for the detection and enumeration of Enterobacteriaceae check spelling? Part 2: colony-count method. ISO Norm 21528-2:2004. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- Latorre-Moratalla ML, Bover-Cid S, Talon R, Garriga M, Zanardi E, Ianieri A, Fraqueza MJ, Elias M, Drosinos EH, Vidal-Carou MC. 2010. Strategies to reduce biogenic amine accumulation in traditional sausage. Food Sci Technol 43:20-5. [Google Scholar]
- Leroy F, Verluyten J, De Vuist L. 2006. Functional meat starter cultures for improved sausage fermentation. Int J Food Microbiol 106:270-85. [DOI] [PubMed] [Google Scholar]
- Leuschner RG, Heidel M, Hammes WP. 1998. Histamine and tyramine degradation by food fermenting microorganisms. Int J Food Microbiol 39:1-10. [DOI] [PubMed] [Google Scholar]
- Leuschner RGK, Hammes WP, 1998. Tyramine degradation by micrococci during ripening of fermented sausage. Meat Sci 49:289-96. [DOI] [PubMed] [Google Scholar]
- Maijala R, Eerola S, Lievonen S, Hill P, Hirvi T, 1995. Formation of biogenic amines during ripening of dry sausages as affected by starter culture and thawing time of raw materials. J Food Sci 60:1187-90. [Google Scholar]
- Martinez S, Hand M, Da Pra M, Pollack S, Ralston K, Smith T, Vogel S, Clark S, Lohr L, Low S, Newman C, 2010. Local food systems concepts, impacts, and issues. US Department of Agriculture, Economic Research Service, Washington, DC, USA. [Google Scholar]
- Martuscelli M, Crudele MA, Gardini F, Suzzi G, 2000. Biogenic amine formation and oxidation by Staphylococcus xylosus strains from artisanal fermented sausages. Lett Appl Microbiol 31:228-32. [DOI] [PubMed] [Google Scholar]
- Mascaro N, Stocchi R, Ricciutelli M, Cammertoni N, Renzi F, Cecchini S, Loschi AR, Rea S, 2010. Contenuto di amine biogene e caratteristiche chimico-fisiche del Formaggio di Fossa. Rivista dell’Associazione Italiana Veterinari Igienisti 8:49-53. [Google Scholar]
- Muscarella M, Iammarino M, Centonze D, Palermo C, 2005. Measurement of histamine in seafood by HPLC, CE, and ELISA: comparison of three techniques. Vet Res Commun 29(Suppl.2):343-6. [DOI] [PubMed] [Google Scholar]
- Naila A, Flint S, Fletcher G, Bremer P, Meerdink G, 2010. Control of biogenic amines in food. Existing and emerging approaches. J Food Sci 75:139-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson D, 1991. The chemical analysis of food. University of Reading, Reading, UK. [Google Scholar]
- Parente E, Martuscelli M, Gardini F, Greco S, Crudele MA, Suzzi G, 2001. Evolution of microbial population and biogenic amine production in dry sausages produced in Southern Italy. J Appl Microbiol 90:882-91. [DOI] [PubMed] [Google Scholar]
- Parrott S, Jones S, Cooper RA, 1987. 2-Phenylethylamine catabolism by Escherichia coli K12. J Gen Microbiol 133:347-51. [DOI] [PubMed] [Google Scholar]
- Ranucci D, Branciari R, Acuti G, Della Casa G, Trabalza Marinucci M, Miraglia D, 2013. Quality traits of Ciauscolo salami from meat of pigs fed rosemary extract enriched diet. Ital J Food Safety 2:e16. [Google Scholar]
- Rea S, Cecchini S, Stocchi R, Loschi AR, Ricciutelli M, 2005a. Caratteristiche fisico-chimiche e contenuto di amine biogene nel salame Ciauscolo. Ind Aliment 44:38-45. [Google Scholar]
- Rea S, Mascaro N, Cecchini S, Stocchi R, Loschi AR, Ricciutelli M, 2008. Indagine sulla presenza di amine biogene in differenti prodotti alimentari di origine animale. Ind Aliment 47:983-90. [Google Scholar]
- Rea S, Pacifici L, Stocchi R, Loschi AR, Ceccarelli M, 2003. Storia, caratteristiche e tecnologia di produzione del Ciauscolo. Ind Aliment 42:371-7. [Google Scholar]
- Rea S, Ricciutelli M, Cecchini S, Pacifici L, Stocchi R, Loschi AR, 2005b. Biogenic amine concentration in “Lardellato” salami, a traditional product of central Italy, during ripening. Ital J Food Sci 17:211-9. [Google Scholar]
- Shalaby AR, 1996. Significance of biogenic amines to food safety and human health. Food Res Int 29:675-90. [Google Scholar]
- Silla Santos MH, 1996. Biogenic amines: their importance in foods. Int J Food Microbiol 29:213-31. [DOI] [PubMed] [Google Scholar]
- Stratton JE, Hutkins RW, Taylor SL, 1991. Biogenic amines in cheese and other fermented foods: a review. J Food Protect 54:460-70. [DOI] [PubMed] [Google Scholar]
- Suzzi G, Gardini F, 2003. Biogenic amines in dry fermented sausages: a review. Int J Food Microbiol 88:41-54. [DOI] [PubMed] [Google Scholar]
- Trani A, Gambacorta G, Loizzo P, Alviti G, Schena A, Faccia M, Aquilanti L, Santarelli S, Di Luccia A, 2011. Biochemical traits of Ciauscolo, a spreadable typical Italian dry cured sausage. J Food Sci 75:514-24. [DOI] [PubMed] [Google Scholar]
- Veciana-Nogues MT, Mariné-Font A, Vidal-Carou MC, 1997. Biogenic amine as hygienic quality indicators of tuna. Relationships with microbial counts, ATP-related compounds, volatile amines, and organoleptic changes. J Agr Food Chem 45:2036-41. [Google Scholar]
- Visciano P, Schirone M, Tofalo R, Suzzi G, 2012. Biogenic amines on raw and processed seafood. Front Microbiol 3:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlichova L, Bunkova L, Koutny M, Jancova P, Bunka F, 2014. Formation, degradation, and detoxification of putrescine by foodborne bacteria: a review. Comp Rev Food Sci Food Safety 13:1012-30. [Google Scholar]
- Yamamoto S, Itano H, Kataoka H, Makita M, 1982. Gas-liquid chromatographic method for analysis of di- and polyamines in foods. J Agr Food Chem 30:435-9. [DOI] [PubMed] [Google Scholar]
- Zaman MZ, Bakar FA, Selamat J, Bakar J, 2010. Occurrence of biogenic amines and amines degrading bacteria in fish sauce. Czech J Food Sci 28:440-9. [Google Scholar]


