Abstract
Severe asthma is characterized by major impairment of quality of life, poor symptom control and frequent exacerbations. Inflammatory, clinical and causative factors identify different phenotypes and endotypes of asthma. In the last few years, new treatment options have allowed for targeted treatments according to the different phenotypes of the disease. To accurately select a specific treatment for each asthmatic variant, the identification of appropriate biomarkers is required. Eosinophilic asthma is a distinct phenotype characterized by thickening of the basement membrane and corticosteroid responsiveness. This review reports the latest evidence on an anti-IL-5 monoclonal antibody, mepolizumab, a new and promising biological agent recently approved by the FDA specifically for the treatment of severe eosinophilic refractory asthma.
Keywords: asthma, costs, effectiveness, IL-5, mepolizumab, phenotype
Introduction
According to recent data, the number of people with asthma around the world has increased from 235 to 334 million between 2011 and 2014, with about 180,000 deaths every year [Global Asthma Network, 2014]. Approximately 20% of these patients are affected by severe persistent asthma, of which one case out of five is inadequately controlled. Patients with uncontrolled severe asthma have a high risk of exacerbations, hospitalization and death [Peters et al. 2006].
Severe asthma is associated with high airway hyperresponsiveness and a variable, usually reversible airflow obstruction; from a clinical point of view, it is characterized by major impairment of quality of life, recurrent symptoms such as breathlessness, chest tightness, wheezing and cough. Airways inflammation is the most important pathophysiological mechanism of asthma and inflammatory, clinical and causative factors characterize different phenotypes and endotypes of asthma [Wenzel, 2012]. Many asthmatic subjects develop irreversible or partially reversible airflow obstruction and experience an accelerated rate of lung function decline, mostly in absence of adequate therapy. In these patients, all the components of the bronchial wall undergo irreversible structural changes defined as ‘airway remodeling’, with an increased thickness compared with normal airways. Remodeling is present in subphenotypes with irreversible airflow obstruction and high airway hyperresponsiveness, which are often associated with increased disease severity [Al-Muhsen et al. 2011; Homer and Elias, 2005]. Recent studies have shown that an extended treatment with omalizumab significantly reduced airway wall and reticular basement membrane thickness, independently of eosinophilic infiltration [Hoshino and Ohtawa, 2012; Riccio et al. 2012].
Studies on large series of patients with well-characterized asthma have demonstrated that clinical and pathological features correlate either with eosinophilic or neutrophilic inflammation [Fahy, 2009]. On the basis of induced sputum, two other inflammatory phenotypes of asthma have been identified, that is, granulocytic and paucigranulocytic, most common in both adults and children with stable asthma [Simpson et al. 2006]. Eosinophilic asthma is a distinct phenotype characterized by thickening of the basement membrane in the airway mucosa and by corticosteroid responsiveness. In contrast, thickening of the basement membrane is not typical of noneosinophilic asthma, a relatively corticosteroid resistant condition frequently associated with severe disease [Pepe et al. 2005]. Some studies report that recurrent asthma exacerbations can predominate in a subgroup of patients with eosinophilic airway inflammation [Haldar et al. 2008]. Eosinophilic infiltration is a key feature of Th2-driven inflammation, and eosinophils can be a useful biomarker in guiding treatment [Holgate, 2008].
In this review we will discuss the results of mepolizumab treatment for severe refractory asthma on the basis of current evidence and the potential indications of this molecule for other eosinophilic-related disorders.
Eosinophilic inflammation, IL-5 and mepolizumab
Eosinophils have a role in acute and subacute processes involved in airway narrowing and in other important events, such as repair and remodeling associated with chronic eosinophilic inflammation. Eosinophil recruitment and survival in the airways and granule maturation are promoted by interleukin (IL)-3, granulocyte macrophage colony-stimulating factor (GM-CSF) and especially IL-5. IL-5 is a 134-amino acid protein that forms a 52-kDa homodimer related to both GM-CSF and IL-3 [Klein Wolterink et al. 2012; McKinnon et al. 1999; Menzies-Gow et al. 2003].
In addition to stimulating final differentiation of activated B cells to antibody-forming cells, IL-5 also enhances proliferation, differentiation and maturation of eosinophil precursors [Kouro and Takatsu, 2009]; in experimental murine models it seems to be involved in airway remodeling [Tanaka et al. 2004].
Mature eosinophils circulate in the blood for 6–10 h [Becchetti et al. 2011; Berek, 2016] to migrate then in connective tissues and end their life cycle after 8–12 days. Eosinophils have IgE receptors and are involved in the activation of allergic reactions and the defense against parasites and worms. They are able to internalize the antigen–antibody complex and release inflammatory mediators (such as major basic protein) killing microbial agents. Their granules contain different mediators of allergic reactions, such as histaminase and arylsulfatase. A second important activity of eosinophils is the secretion of leukotrienes, which are involved in the pathophysiology of asthma by increasing the secretion of mucus and inducing bronchoconstriction [Becchetti et al. 2011; George and Brightling, 2016; Baptista-dos-Reis et al. 2015]. Eosinophilia is associated with a wide variety of conditions over asthma, such as atopic diseases, helminthic infections, hypersensitivity to drugs and cancer [Curtis and Ogbogu, 2015].
IL-5 is determinant in influencing stimulation, proliferation and differentiation of eosinophils, therefore this molecule and its receptor have been evaluated as a definite target in the treatment of eosinophilic disorders. This cytokine is largely produced by Th2 lymphocytes, Tc2 cells (type 2 cytotoxic T cells), eosinophils, mast cells and γδ-T cells [Klein Wolterink et al. 2012]. Type 2 innate lymphoid cells (ILC2s) have been shown to be a further source, while small amounts of IL-5 can be produced by epithelial cells, natural killer (NK) cells and NK T cells [McKinnon et al. 1999].
The type I receptor IL5Rα is expressed in particular by eosinophils. Consequently, these cells respond primarily to IL-5, whose gene is located on chromosome 5, close to the genes encoding IL-3, IL-4 and GM-CSF [Wells et al. 1994; Valent, 1994; Toba et al. 1999]. The IL-5 receptor includes an α and a βc chain; the first one is specific for the IL-5, while the βc subunit is also recognized by IL-3 and GM-CSF [Menzies-Gow et al. 2003; Kouro and Takatsu, 2009]. Antagonizing the IL-5 with specific monoclonal antibodies (mAbs) may have beneficial advantage, given that the eosinophil may also serve a pathogenetic role in the mixed Th2/Th1/Th17 endotype of severe asthma [Caruso et al. 2013a].
The expression of IL-5 is regulated by several transcription factors including GATA3. Produced by TH2 lymphocytes and activated mast cells, IL-5 is an activator of eosinophils and represents a link between the T-cell activation and inflammatory responses mediated by eosinophils. The latter express on their surface specific receptors for the Fc fragment of IgE antibodies and are able to recognize and bind microorganisms (mainly helminths) opsonized by IgE.
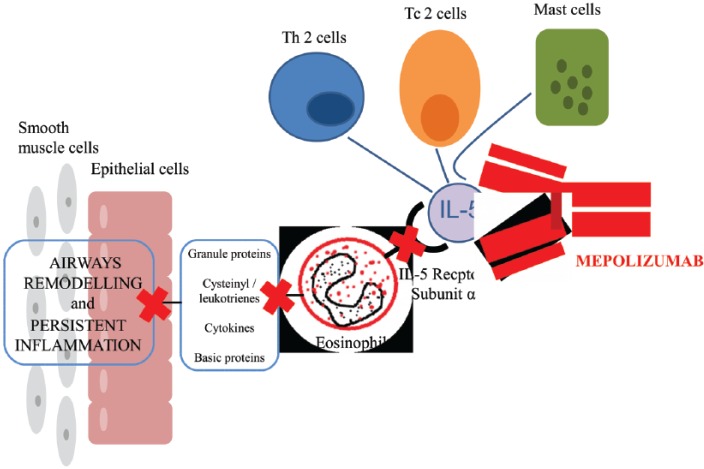
Mepolizumab (SB-240563, GlaxoSmithKline) was the first biological anti IL-5 agent designed and tested in randomized clinical trials (RCTs) on eosinophilic asthma and other eosinophilic diseases. Mepolizumab is a N-glycosylated IgG1 kappa humanized mAb formed by two light chains and two heavy chains bound by a disulphide bridge, with a molecular weight of 149.2 kDa including 3 kDa carbohydrate residues [Zia-Amirhosseini et al. 1999]. This drug binds IL-5 [Zia-Amirhosseini et al. 1999] with both high specificity (IC50 < 1 nM) and affinity (Kd = 4.2 pM), preventing binding to the alpha chain of the IL-5 receptor complex expressed on the eosinophil cell surface [Lopez et al. 1988; Ohnishi et al. 1993; Clutterbuck and Sanderson, 1990; Hart et al. 2001] (Figure 1). In November 2015 the US Food and Drug Administration (FDA) committee approved mepolizumab with the brand name Nucala® at the dose of 100 mg administered subcutaneously once every 4 weeks for the treatment of severe eosinophilic asthma [FDA, 2015].
Figure 1.
Mepolizumab prevents the binding to the alpha chain of the IL receptor on the eosinophil cell surface, with a reduction in inflammatory cascade.
Finally, in December 2015, the European Medicines Agency (EMA) approved a marketing authorization valid throughout the European Union as ‘medicine under additional monitoring’ [EMA, 2009].
Skepticism after the first clinical trials
The initial research studies on mepolizumab produced contradictory results in the clinical setting and several authors questioned the efficacy of this new molecule in asthma treatment [Leckie et al. 2000; Flood-Page et al. 2003]. One of the studies showed nonsignificant data in terms of airway hyperreactivity (AHR), peak expiratory flow (PEF) and forced expiratory volume in 1 s (FEV1) in spite of a decrease in airways and blood eosinophilia [Leckie et al. 2000; Flood-Page et al. 2003]. Another important question was the heterogeneity in the severity and clinical patterns of asthma in several studies, investigating from mild to moderate atopic asthma, persistent asthma and eosinophilic asthma with recurrent exacerbations. These doubts have been considered in a recent Cochrane systematic review [Powell et al. 2015]. A RCT had the aim of investigating the effect of three intravenous infusions of mepolizumab, 250 or 750 mg at monthly intervals, on clinical outcome measures in 362 patients with asthma experiencing persistent symptoms despite inhaled corticosteroid therapy [Flood-Page et al. 2007]. Mepolizumab was associated with a significant reduction in blood and sputum eosinophils in both treatment groups, but an incorrect sample selection (i.e. that did not take into account the level of eosinophilic inflammation in the airways) did not allow the study to reach statistically significant changes in any of the clinical endpoints measured. There was a nonsignificant trend for decrease in exacerbation rates in the mepolizumab 750 mg treatment group, therefore this treatment did not appear to add significant clinical benefit [Flood-Page et al. 2007]. As a matter of fact, early studies did not show a significant improvement in lung function, probably due to lack of efficacy in removing tissue eosinophils and pro-inflammatory cytokines [Powell et al. 2015; Simpson et al. 2006]. Only when an ‘eosinophilic phenotype’ was identified, a subgroup of potential mepolizumab responsive patients could be selected [Ortega et al. 2014a].
Another limitation is the possibility to define reliably the effectiveness and safety of mepolizumab because of a relatively small number of patients and a short duration of studies in literature. In the MENSA trial [Ortega et al. 2014b], 194 participants were treated with mepolizumab 100 mg SC for 32 weeks, while in the SIRIUS trial [Bel et al. 2014] 69 participants were treated with mepolizumab 100 mg SC for 20 weeks.
The majority of trials investigated intravenous mepolizumab, a formulation not licensed at the moment. The evidence for the currently licensed subcutaneous route is limited to two studies evaluating patients with severe eosinophilic asthma. Further research is actually needed on specific biomarkers to identify which subphenotypes of eosinophilic asthma can benefit from mepolizumab.
Another limitation is that reliable studies are lacking on patients of African descent or teenagers and children to obtain meaningful data about the net health benefits of mepolizumab in these subgroups. For this reason, the FDA required two post-marketing studies specifically in children aged 6–11 years. The optimal dosage and duration of treatment are still to be determined [Powell et al. 2015].
Clinical effectiveness data in asthma
Early studies failed their objectives probably because of improper selection of the study population. Leckie and colleagues performed the first randomized, double-blinded, placebo-controlled study on humans. There were nonsignificant improvements in term of AHR, PEF and FEV1 in spite of decreased airways and blood eosinophilia after 4 and 16 weeks [Leckie et al. 2000]. A subsequent RCT in 362 patients with uncontrolled asthma despite inhaled corticosteroid therapy had the aim of evaluating the effect of three intravenous infusions of mepolizumab (250 or 750 mg every month), on clinical outcome measures [Flood-Page et al. 2007]. As already said, the study failed the clinical endpoints despite a significant reduction in blood and sputum eosinophils in both treatment groups [Flood-Page et al. 2007] (Table 1).
Table 1.
Key results of principal anti-interleukin (IL)-5 monoclonal antibodies studies in asthma.
| Study | Population | Study design | Anti-IL-5 dosage | Results |
|---|---|---|---|---|
| Haldar et al. [2009] | Refractory eosinophilic asthma and a history of recurrent severe exacerbations | Randomized, double-blind, placebo-controlled, parallel-group studyn = 61 | 750 mg (mepolizumab) | Reduction in rate of exacerbation, improving in AQLQ score, reduction of blood and sputum eosinophils |
| Nair et al. [2009] | Prednisone-dependent asthma with sputum eosinophilia | Randomized, double-blind, parallel-group trial n = 20 | 750 mg (mepolizumab) | Reduction in the number of blood and sputum eosinophils, prednisone sparing |
| Castro et al. [2011] | Eosinophilic asthma | Randomized, double-blind, placebo-controlled studyn = 106 | 3.0 mg/kg (reslizumab) | Significant reduction in sputum eosinophils, improvements in airway function |
| Pavord et al. [2012] | Severe eosinophilic asthma | Multicenter, double-blind, placebo controlled trial (DREAM) n = 621 | 75 mg, 250 mg, or 750 mg (mepolizumab) | Effectiveness and well tolerated mepolizumab treatment, risk of asthma exacerbations reduction |
| Ortega et al. [2014] ortega [2014b] |
Severe eosinophilic asthma | Randomized, double-blind, double-dummy trial (MENSA) n = 576 | 75 mg, 100 mg (mepolizumab) | Reduction in rate of exacerbations, improving in ACQ-5 and SGRQ-Q score |
| Bel et al. [2014] | Severe eosinophilic asthma | Randomized, double-blind, trial study (SIRIUS) n = 135 | 100 mg (mepolizumab) | Reduction in the glucocorticoid-dose stratum, reduction of the annualized rate of exacerbations |
| Bjermer et al. [2014] | Severe persistent asthma with elevated eosinophils | Multicenter, placebo-controlled, double-blind, 16-week study n = 311 | 0.3 or 3.0 mg/kg (reslizumab) | Significant improvement of overall FEV1 and ACQ score |
| Castro et al. [2014] | Severe eosinophilic asthma | Randomized, controlled, double-blind, dose-ranging phase IIb study n = 324 | 2 mg, 20 mg, or 100 mg (benralizumab) | Reduce asthma exacerbations and blood eosinophils |
| Pham et al. [2016] | Eosinophilic asthma | Two independent study (phase I n = 14 and phase IIa n = 24) randomized, double-blind, placebo-controlled | 100 or 200 mg (benralizumab) | Eosinophil depletion and significant reduction of eosinophil derived neurotoxin, eosinophil cationic protein |
| Gevaert et al. [2011] | Subjects with bilateral nasal polyps | Double-blind, placebo-controlled, randomized, two-center safety and pharmacokinetic study | 3 mg/kg or 1 mg/kg | Reduction in blood eosinophil numbers and concentrations of eosinophil cationic protein and size reduction of nasal polyps |
ACQ, Asthma Control Questionnaire; AQLQ, Asthma Quality of Life Questionnaire; FEV1, forced expiratory volume in 1 s; SGRQ, St. George’s Respiratory Questionnaire.
Two clinical trials demonstrated mepolizumab efficacy. The first was a RCT on 61 subjects who had refractory eosinophilic asthma and a history of recurrent severe exacerbations [Haldar et al. 2009]. Patients received infusions of either mepolizumab or placebo at monthly intervals for 1 year. Mepolizumab was associated with significantly fewer severe exacerbations than placebo, with a significant improvement in the score of the Asthma Quality of Life Questionnaire (AQLQ) and a decrease of eosinophil counts in the blood and sputum. However, there were no significant differences between the two groups with respect to symptoms, post-bronchodilator FEV1 or airway hyperresponsiveness. The second report was another RCT involving patients with persistent sputum eosinophilia and symptoms despite prednisone treatment [Nair et al. 2009]. Nine patients were assigned to receive mepolizumab, administered in five monthly infusions of 750 mg, and 11 patients to placebo. There were 12 asthma exacerbations in 10 patients who received placebo while only one patient who received mepolizumab had an asthma exacerbation. The use of mepolizumab was associated with a significant decrease in the number of sputum and blood eosinophils. Moreover, subjects who received mepolizumab were able to reduce their prednisone dose; improvements in eosinophil numbers, asthma control and FEV1 were maintained for 8 weeks after the last infusion (Table 1).
A subsequent and conclusive study was the Dose Ranging Efficacy and Safety with Mepolizumab (DREAM) trial [Pavord et al. 2012]. It was a large multicenter, double-blind, placebo-controlled trial recruiting 621 subjects with a history of recurrent severe asthma exacerbations, and signs of eosinophilic inflammation. Patients were randomly assigned to receive one of three doses of intravenous mepolizumab (75, 250 or 750 mg) or matched placebo (100 ml 0.9% NaCl). Mepolizumab significantly reduced the number of asthma exacerbations in patients with severe eosinophilic asthma compared with placebo. In addition, treatment lowered blood and sputum eosinophil counts and was well tolerated for 12 months, despite a small effect on FEV1, AQLQ and Asthma Control Questionnaire (ACQ) scores compared with the placebo group. These studies have represented an important advance for the selection and treatment of those patients affected by severe asthma with frequent exacerbations and persistent eosinophilia, accounting for about 40% of severe asthmatics [Rothenberg et al. 2008]. A post hoc analysis of the DREAM had the aim to assess the effect of mepolizumab treatment on reduction of exacerbations in atopic and nonatopic subjects, on seasonal patterns of response by subgroup and the changes in lung function and exhaled nitric oxide fraction (FeNO) according to subgroup [Simpson et al. 2006]. A reduction in exacerbations was observed, irrespective of atopy or IgE levels and more frequent in winter months, however treatment response was unaffected by season or atopy. A subsequent supervised cluster analysis was applied to data of the DREAM study to identify characteristics that maximized the differences among subgroups [Ortega et al. 2014c]. Three main parameters were identified in four primary clusters: blood eosinophils, airway reversibility and body mass index (BMI). The reduction in exacerbations and significant therapeutic benefit were higher in patients with eosinophilic inflammation who received mepolizumab, confirming the eosinophilic phenotype as the one with the greatest response to therapy.
In the MENSA trial 576 patients were selected with recurrent asthma exacerbations and eosinophilic inflammation despite high doses of inhaled glucocorticoids to one of three study groups [Ortega et al. 2014b]. Patients were assigned to receive treatment with mepolizumab, administered as either a 75 mg intravenous dose or a 100 mg subcutaneous dose, or placebo every 4 weeks for 32 weeks. The primary endpoint was the rate of exacerbations, other outcomes were FEV1, scores on the St. George’s Respiratory Questionnaire (SGRQ) and the five-item Asthma Control Questionnaire (ACQ-5). The rate of exacerbations was reduced by 47% among patients receiving intravenous doses and by 53% among those receiving subcutaneous administration. At the end of the study, the mean increase from baseline in FEV1 was 100 ml greater in patients receiving intravenous mepolizumab than in those receiving placebo and 98 ml greater in patients receiving subcutaneous mepolizumab than in the placebo group. There were also significant improvements in the SGRQ and ACQ-5 scores in the intravenous and subcutaneous mepolizumab than in the placebo groups, with a safety profile of mepolizumab comparable with placebo. Also, this study confirmed the efficacy of mepolizumab administered either intravenously or subcutaneously in terms of reduction of asthma exacerbations but, unlike other studies, improvements in markers of asthma control, including FEV1, were demonstrated too.
Bel and colleagues performed a randomized, double-blind trial (SIRIUS study) involving 135 patients with severe eosinophilic asthma, with the aim to compare with placebo the glucocorticoid-sparing effect of mepolizumab 100 mg administered subcutaneously every 4 weeks for a duration of 20 weeks [Bel et al. 2014]. Patients in the mepolizumab group had a reduction in the glucocorticoid dose 2.39 times greater than in the placebo group (95% confidence interval [CI] 1.25–4.56; p = 0.008). Patients had also a relative reduction of 32% in the annualized rate of exacerbations (1.44 versus 2.12, p = 0.04) and a significant reduction of asthma symptoms (p = 0.004) (Table 1).
Another study examined a subpopulation of 188 oral corticosteroid (OCS)-dependent patients enrolled in the DREAM trial who continued to have frequent exacerbations. These subjects had received regular OCS (5–35 mg/day) for ⩾6 months and an additional controller without efficacy. Mepolizumab lowered the peripheral eosinophils and showed a significant glucocorticoid-sparing effect in the non-OCS and OCS groups, reducing exacerbation rate in the 52-week treatment period, particularly in the OCS group [Prazma et al. 2014].
A multicenter, placebo-controlled, double-blind, parallel-group study is currently underway to evaluate the effect of mepolizumab on health-related quality of life and other measures of asthma control, primarily lung function, in patients with severe eosinophilic asthma [ClinicalTrials.gov identifier: NCT02281318].
Finally, but not least, in an interesting study on bronchial remodeling a partial eosinophil reduction was obtained with mepolizumab treatment, associated with significant reductions in tenascin and lumican deposition in the reticular basement membrane [Phipps et al. 2004].
The other side of anti-IL-5 mAbs: benralizumab and reslizumab
In the last 15 years several clinical trials were conducted in asthma with anti-IL-5 mAbs other than mepolizumab, that is, reslizumab and benralizumab.
Reslizumab is a humanized anti-IL-5 monoclonal (IgG4/κ) antibody targeting circulating IL-5 with a high affinity, thus preventing its binding to specific receptor [Kips et al. 2003]. An early study (Sch 55700) showed a long-term effect in reducing both pulmonary eosinophilia and airway hyperresponsiveness in allergic mice, monkeys and rabbits [Egan et al. 1999].
Similarly to mepolizumab, early RCTs on patients with severe uncontrolled asthma despite standard treatment were not encouraging [Kips et al. 2003], because of the lack of improvement in FEV1 or symptoms. However, also in this case a significant reduction in circulating and sputum eosinophils was shown. Once identified the hypereosinophilic asthmatic phenotype (sputum eosinophils >3% or blood eosinophils >400/μl) clinical outcomes have shown an improving trend. In a phase II trial on asthmatic patients with nasal polyposis (NP) a significant amelioration of asthma symptoms was observed (p = 0.012) along with a slight improvement in asthma control measured by ACQ [Gevaert, 2006]. In subsequent phase III RCTs significant improvements in FEV1 and ACQ score were found [Bjermer et al. 2014], particularly in the subgroup with NP [Castro et al. 2011].
Benralizumab (MEDI-563) is an IgG1, afucosylated anti-IL-5Ra mAb that binds to an epitope on IL-5Rα close to the IL-5 binding site. In a study on nonhuman primates, MEDI-563 depleted blood eosinophils and eosinophil precursors in the bone marrow, by induction of antibody-dependent cell-mediated cytotoxicity (ADCC) of eosinophils and basophils [Kolbeck et al. 2010]. In phase IIB RCTs on patients affected by severe hypereosinophilic asthma with a peripheral blood eosinophil counts >300 cells/μl, 20 and 100 mg benralizumab administered subcutaneously showed interesting clinical results, in particular a significant reduction in exacerbations compared with placebo [Ghazi et al. 2012; Castro et al. 2014].
A very recent study had the aim to evaluate the effects of benralizumab on eosinophil counts and activity. Sera were collected from asthma patients enrolled in two clinical phase I and phase IIa studies. A modulation and a significant reduction of blood eosinophils, IL-5, eosinophil-derived neurotoxin (EDN) and eosinophil cationic protein (ECP) were found, confirming a significant anti-inflammatory effect by acting on these mediators [Pham et al. 2016].
The search for predictive biomarkers
In clinical practice asthma management and follow up are often based on symptoms and lung function tests to assess airway obstruction and hyperresponsiveness. Unfortunately, these data do not correlate with the level of airway inflammation. Fiberoptic bronchoscopy with biopsies and bronchoalveolar lavage (BAL) have been considered until now the gold standard to evaluate airway inflammation, but they have the disadvantage of being invasive and not easy to apply in real-life settings [Lommatzsch et al. 2013; Djukanović, 1996].
For these reasons, since the advent of biological therapies with omalizumab, the interest has further increased to identify new biomarkers potentially useful for patient selection and treatment guidance [Caruso et al. 2013b].
At present, serum IgE levels are the only applicable biomarker in clinical practice with good value, in predicting response to omalizumab for severe uncontrolled allergic asthma. Total serum IgE in adult patients with persistent allergic asthma showed almost always levels >150 IU/ml variable over time [Davila et al. 2015]. Some authors reported a significant association between total serum IgE and asthma or airflow limitation severity in a high percentage of patients with IgE >400 IU/ml, confirming the important role of IgE in this subgroup [Davila et al. 2015].
Omalizumab inhibits the binding of IgE to high affinity FcƐRI receptors on mast cell and basophil surface, and reduces the free IgE levels in a short time [Djukanović et al. 2004; Gould and Sutton, 2008]. As a consequence, the expression of FcƐRI is downregulated on inflammatory cells, such as basophils, mast cells and dendritic cells [Prussin and Metcalfe, 2006; MacGlashan et al. 1997; Lin et al. 2004] with subsequent inhibition of inflammatory mediator release. Another effect is the reduction of inflammatory cell recruitment, particularly eosinophils in sputum and tissues [Djukanović et al. 2004] and bronchial remodeling [Humbles et al. 2004].
Baseline IgE is the only parameter with a significant predictive value based on multivariate analysis from the INNOVATE study. A lower baseline IgE was associated with reduced clinical benefits [Bousquet et al. 2007] but analysis of data from patients enrolled in seven clinical trials indicated that omalizumab decreased asthma exacerbation rates across all IgE level quartiles but reached a statistical significance in the three upper IgE quartiles only (p < 0.001) [Bousquet et al. 2007]. In this group, total emergency visit rates declined and, the AQLQ score and the FEV1 improved. In contrast, specific allergen IgE did not show any significant correlation with the clinical response to omalizumab [Wahn et al. 2009].
Unfortunately, to date we have no other reliable biomarkers in clinical practice, with a promising exception represented by periostin [Hanania et al. 2013] for the selection of suitable patients to therapy with omalizumab and lebrikizumab.
The main parameters assessed in human studies including RCTs to identify patients with eosinophilic asthma have been the level of eosinophils in induced sputum and blood, applied as a biomarker for selection and of response to treatment [Pavord et al. 2012; Castro et al. 2011; Wenzel et al. 2013]. Unfortunately, despite being considered a method of choice for establishing eosinophilic lung inflammation, induced sputum is not always practical to obtain and standardize.
In the DREAM study, blood eosinophil counts correlated with the response to mepolizumab, but the same did not happen with sputum eosinophilia [Pavord et al. 2012]. In a post hoc analysis, this important study showed that in patients with frequent exacerbations a single peripheral blood eosinophil count at screening ⩾150 cells/ml was a good predictor of response [Katz et al. 2014]. In contrast, those subjects with baseline eosinophil count <150 cells/ml had a limited reduction of asthma exacerbations. Since blood eosinophil levels can show spontaneous variations over time, a single measurement might not be sufficient to evaluate patients accurately, although this study has shown that a single analysis can be acceptable in RCTs.
In the MENSA trial [Ortega et al. 2014b] the authors enrolled subjects with a peripheral eosinophil count of at least 150 cells/µl at screening or at least 300 cells/µl sometime in the previous year. In this study a subanalysis was conducted on a group of patients with blood eosinophil counts >500 cells/µl, who had a better clinical response to mepolizumab.
Another and easier option is the expired FeNO. Levels of FeNO have a close correlation with eosinophil numbers in the airways as shown in several studies [Jatakanon et al. 1998]. Data and results are anyway controversial, because according to some authors [Schleich et al. 2010] FeNO has a good correlation with sputum eosinophils and can theoretically be used as a less-invasive biomarker supporting the use of IL-5 antagonizing agents [Westerhof et al. 2015]. Unfortunately, in another study FeNO reflected sputum eosinophilia in only 78% of patients [Wagener et al. 2015], increasing uncertainty on the use of this biomarker.
Serum periostin [or osteoblast-specific factor (OSF-2)] is an extracellular matrix protein originally identified in mesenchymal cells as osteoblasts, osteoblast-derived cells and periosteum. Periostin is implicated with many disease states and it is closely related with the biological activity of IL-13 [Izuhara et al. 2016]. So far, it has been studied mainly to predict responses and for the monitoring of treatment to lebrikizumab (an anti-IL-13 mAb) [Izuhara et al. 2016] and to omalizumab [Hanania et al. 2013; Tajiri et al. 2016]. Also in this case, however, the correlation with eosinophilic airway inflammation is not so clear, as well as the ability to accurately distinguish between eosinophilic and noneosinophilic asthma. Available data are contradictory, because in a study serum periostin levels were the best predictor of airway eosinophilia [Jia et al. 2012], while in another study periostin and total IgE were not able to discriminate between eosinophilic and noneosinophilic phenotypes of asthma [Westerhof et al. 2015; Wagener et al. 2015].
Treatment monitoring
The lack of long-term studies on mepolizumab does not allow to accurately define the appropriate timing and method of follow up. To the best of the authors’ knowledge there are only few follow-up studies and none longer than 12 months [Haldar et al. 2014].
In our experience, evaluations useful for an effective and practical monitoring are represented by blood and sputum eosinophil counts, exacerbation rate and score of asthma control questionnaires, such as ACQ or Asthma Control Test (ACT). In clinical studies no significant differences in FEV1 or FeNO have ever been reported. The ideal timing for clinical and laboratory monitoring could be initially after 4 weeks and then every 3 months, based on the pharmacokinetic and pharmacodynamic characteristics [Haldar et al. 2014; Ortega et al. 2014a].
When to stop treatment with mepolizumab or other biological agents is still a matter of debate. Despite a long experience with omalizumab a definite response has not been agreed yet, because the relevant studies conducted so far are few and with small numbers of patients. The available data indicate that withdrawal of omalizumab may cause severe asthma exacerbations in most patients, with return to a pretreatment clinical state [Kupryś-Lipińska and Kuna, 2014]. The long-term efficacy of omalizumab was investigated after a treatment of 6 years in a group of 18 patients with severe IgE-mediated asthma and a withdrawal of 1 year [Nopp et al. 2007] and 3 years [Nopp et al. 2010]. In both cases a stabilization was shown of asthma-related symptoms, similar to that observed during the treatment period with omalizumab, associated with a downregulation of basophil allergen sensitivity. Anyway, until these data will be confirmed on large case series, the general trend is to continue the treatment indefinitely.
To the best of the authors’ knowledge, there is only one study that evaluated the effects of mepolizumab discontinuation [Haldar et al. 2014], that is, an unblinded, prospective, 1-year follow-up analysis in a series of 27 subjects on anti-IL-5 treatment in the previous 12 months. Withdrawal of mepolizumab was associated with a progressive increase of blood eosinophils to baseline counts over 6 months and with a significant rise in frequency of severe exacerbations after 12 months from cessation. A RCT is currently recruiting participants with the primary objective to evaluate whether patients with severe eosinophilic asthma who have received mepolizumab for at least 3 years need to continue this treatment to maintain benefit [ClinicalTrials.gov identifier: NCT02555371]. Secondary outcomes of the study are the ratio to baseline in blood eosinophil count, the time to a decrease in asthma control and, finally, the time to first exacerbation requiring hospitalization or emergency department (ED) visit.
Cost-effectiveness and comparative value
After the introduction of new treatment options for severe asthma such as omalizumab, bronchial thermoplasty and mepolizumab, the analysis of the economic impact needs greater attention because of the significant increase of direct costs. These could be partly offset by reduction in costs incurred by the health service (in particular, for hospitalizations), a reduction of indirect costs and improvement of quality of life as shown in some studies [Menzella et al. 2012, 2014]. Given the very recent introduction of mepolizumab in clinical practice, there are few data about pharmacoeconomic aspects. Analysts and some reports estimate a cost from US$10,000 to US$15,000 per patient per year of treatment [NHS, 2015]. After FDA approval, the real price tag is US$32,500 a year per patient and approximately US$2700 for a single 4-week injection. A recent and very interesting cost-effectiveness analysis (CEA) conducted by the ICER Group was based on a simulation model of asthma outcomes and costs in a representative population of suitable patients to mepolizumab therapy [ICER, 2015]. The incremental cost-effectiveness of mepolizumab was evaluated using drug cost estimates derived from current prices and estimates of reductions in asthma exacerbations and OCS use from relevant clinical trial data. In a scenario analysis the price of mepolizumab was determined that would produce cost-effectiveness results at willingness-to-pay thresholds of US$50,000/quality-adjusted life year (QALY), US$100,000/QALY and US$150,000/QALY, respectively. Mepolizumab should have a value-based cost between US$7800 and US$12,000 a year, while the full list price per patient in the US is US$32,500 a year. Based on current acquisition prices the cost-effectiveness estimates are unfavorable, since they exceed commonly cited thresholds and other doubts arise from the lack of clinical trials evaluating benefits in the long term. To obtain a value correlated to the clinical benefit a discount of two-thirds to three-quarters from the current price list of mepolizumab would be necessary.
Another recent and very interesting study had the purpose to determine the cost-effectiveness of newest medical treatment strategies for severe refractory asthma, such as biological therapies (omalizumab and mepolizumab) and follow-on bronchial thermoplasty [Bogart et al. 2015]. The authors used a model based on US healthcare perspective, a hypothetical cohort of 10,000 adults affected by refractory asthma, an annual cycle and 10-year time horizon. In patient responders to biological treatment, the addition of bronchial thermoplasty was not cost-effective. Mepolizumab without bronchial thermoplasty was the most cost-effective option for biological responders, with a 10-year-per-patient cost of US$116,776 and 5.46 QALYs gained (ICER US$21,388). In the group of nonresponders to biological treatment, bronchial thermoplasty proved to be a cost-effective treatment option (US$33,161/QALY).
Eosinophilic-related diseases other than asthma
Eosinophil-related diseases are very heterogeneous conditions such as hypereosinophilic syndromes (HES), atopic dermatitis (AD), eosinophilic granulomatosis with polyangiitis (EGBPA), eosinophilic esophagitis (EoE), severe NP and bronchial asthma.
Several studies and clinical trials have been performed in the last years, and many others are currently underway on monoclonal anti-IL-5 antibodies (Table 2). Most studies conducted until now have shown in general a reduction of eosinophil levels in the peripheral blood and sputum but clinical results seem often inconsistent [Leckie et al. 2000; Flood-Page et al. 2007; Haldar et al. 2009; Nair et al. 2009; Pavord et al. 2012].
Table 2.
Main fields of application of mepolizumab in eosinophilic-related diseases.
| Study | Disease | Study design | Dosage | Observations/results |
|---|---|---|---|---|
| Rothenberg et al. [2008] | HES | International, randomized, double-blind, placebo-controlled trial | 750 mg i.v | Corticosteroid-sparing for patients negative for FIP1L1-PDGFRA who have the hypereosinophilic syndrome |
| Roufosse et al. [2013] | Open-label extension study | 750 mg i.v | Mepolizumab was well tolerated and effective as a long-term corticosteroid-sparing agent in PDGFRA-negative HES | |
| Oldhoff et al. [2005] | AD | Randomized double-blind, placebo-controlled, parallel group study | 750 mg i.v | Two single doses of 750 mg mepolizumab did not result in clinical success in patients with AD, despite a significant decrease in peripheral blood eosinophils |
| Moosig et al. [2011] | EGBPA | Single-center, phase II, uncontrolled, investigator-initiated trial | 750 mg i.v | Eight of ten patients had a remission and it was possible a reduction of OCS |
| Kim et al. [2010] | Open-label pilot study | 750 mg i.v | Mepolizumab is a safe and well-tolerated therapy in patients with CSS, offering clinical benefit by enabling corticosteroid tapering while maintaining clinical stability |
|
| Kahn et al. [2010] | Case study | 750 mg i.v | Mepolizumab reduced asthma symptoms and both blood and airway eosinophilia | |
| Otani et al. [2013] | EoE | International, randomized, blinded, multicenter pediatric | 750 mg i.v | Pediatric EoE patients had significantly fewer mast cells, IL-9+ cells, and mast cell–eosinophil couplets in the esophageal epithelium following anti-IL-5 therapy. eosinophils were one source of IL-9, they may support esophageal mastocytosis |
| Straumann et al. [2010] | Randomized, placebo-controlled, double-blind trial | 750 mg i.v | Mepolizumab significantly reduced eosinophil numbers in esophageal tissues in adult patients with active EoO, and changes in the expression of molecules associated with esophageal remodeling were reversed. Minimal clinical improvement was achieved in a subgroup of patients with EoO. Mepolizumab had an acceptable safety profile, even at the high 1500 mg dose level | |
| Gevaert et al. [2011] | NP | Randomized, double-blind, placebo-controlled | 750 mg i.v | Mepolizumab produced a significant reduction in total polyp score in 12 of 20 patients, also confirmed by CT scan evaluations |
| ClinicalTrials.gov identifier: NCT02105961 | COPD | Multi-centered, randomized, placebo-controlled, double-blind, parallel group, trial | 100 mg i.v and 300 mg i.v | Ongoing |
| ClinicalTrials.gov identifier: NCT01463644 | Multicenter, randomized, placebo-controlled, double-blind, parallel group, trial | 750 mg i.v | Completed. Results still not available | |
| ClinicalTrials.gov identifier: NCT02105948 | Multicenter, randomized, placebo-controlled, double-blind, parallel group, trial | 100 mg i.v | Ongoing |
AD, atopic dermatitis; CSS, Churg–Strauss syndrome; COPD, chronic obstructive pulmonary disease; EGBPA, eosinophilic granulomatosis with polyangiitis; EoE, eosinophilic esophagitis; EoO, eosinophilic oesophagitis; FIP1L1-PDGFRA, FIP1-like 1-platelet-derived growth factor receptor α; HES, hypereosinophilic syndromes; IL, interleukin; OCS, oral corticosteroids; NP, nasal polyposis.
The potential therapeutic effect of mepolizumab for HES (Table 2) has been investigated by various authors. An interesting study performed by Rothenberg and colleagues [Rothenberg et al. 2008] showed that treatment with mepolizumab had a corticosteroid-sparing effect in patients affected by HES and negative for FIP1-like 1-platelet-derived growth factor receptor α (FIP1L1-PDGFRA) fusion protein gene, confirming the effectiveness of the drug in this disease. An open-label multicenter study had the aim to evaluate long-term safety and efficacy of mepolizumab 750 mg i.v. every 4 weeks for 36 weeks. The anti-IL-5 molecule was safe and effective as a corticosteroid-sparing agent in treating adults with FIP1L1/PDGFRA negative HES [Roufosse et al. 2013].
It is well known that in AD, tissue and blood eosinophils are very activated, with delayed programmed cell death [Leiferman, 2001; Wedi et al. 1997], whereby mepolizumab was considered a potential treatment [Gnanakumaran and Babu, 2003]. A randomized double-blind, placebo-controlled, parallel group study (Table 2) enrolled 18 patients with AD to receive two single doses of 750 mg mepolizumab and 22 on placebo treatment. Peripheral blood eosinophils were significantly reduced in the treatment group compared with placebo but no clinical success was reached by the physician’s global assessment (PGA), objective SCORAD and itch scoring [Oldhoff et al. 2005].
In EGBPA [also known as Churg–Strauss syndrome (CSS)], mepolizumab was used in an open-label study on 10 patients with refractory or relapsing EGBPA: the authors reported a remission in most subjects with a reduction of OCS [Moosig et al. 2011]. A later case-series studied seven patients with EGBPA, treated with only four doses of mepolizumab. A reduction of OCS dose was possible in all patients, associated with a stability of symptoms and an optimal safety profile during the entire course of the study [Kim et al. 2010]. As expected, after cessation of treatment, EGBPA symptoms reappeared with a progressive increase of eosinophils and the need to resume OCS, confirming that it is mandatory to continue mepolizumab for a long time, until new studies will concentrate on the optimal duration of therapy.
Furthermore, a new case study with refractory EGBPA patients showed how monthly doses of mepolizumab (750 mg i.v.) reduced asthma symptoms and eosinophilia in blood and airways [Kahn et al. 2010] (Table 2).
In another fascinating study, authors investigated whether mepolizumab treatment can reduce esophageal mast cell accumulation in 43 pediatric EoE biopsy specimens from a previous anti-IL-5 RCT. Response to anti-IL-5 (defined as <15 eosinophils per high power field following mepolizumab therapy) was found in the 40% of patients and 77% of all subjects had significantly fewer mast cells, IL-9+ cells and mast cell–eosinophil couplets in the esophageal epithelium following anti-IL-5 therapy [Otani et al. 2013]. A clinical correlation was also noted between the reduction of eosinophils and epithelial mast cells and symptoms, with a decrease in stomach and chest/throat pain, along with relief of swallowing discomfort.
In a larger study, 226 children and adolescents with EoE were treated with reslizumab. A median reduction from baseline to the end of therapy was shown in peak esophageal eosinophil counts (59%, 67% and 64% in the 1, 2 and 3 mg/kg reslizumab groups, respectively) versus placebo (24%, p < 0.001 for all comparisons). PGA scores improved in all treatment groups, but without statistically significant differences [Spergel et al. 2012].
In another study on adult patients [Straumann et al. 2010] a mild to moderate improvement was observed in eosinophil counts, but primary endpoints (resolution of eosinophilia and symptoms at comparison between active treatment and placebo) were not met.
Finally, in severe NP, a significant study on 30 patients with 3 or 4 grade disease or recurrence after surgery refractory to corticosteroid therapy showed interesting results [Gevaert et al. 2011]. The subjects were randomized to receive either two single intravenous injections of 750 mg mepolizumab or placebo; the primary outcome was the reduction in NP score at 8 weeks after the first dosing. Secondary outcomes included changes in CT scan scores and functional assessments, such as nasal peak inspiratory flow (nPIF) or symptom score. Mepolizumab produced a significant reduction in total polyp score (TPS) in 12 among 20 patients, as confirmed by CT scan evaluations.
In the studies conducted so far, mepolizumab has been used in a broad range of eosinophilic related disorders, with a good safety profile and with no evidence of specific immune complex formation [Abonia and Putman, 2011].
Future perspectives
New potential areas of interest could extend the indications of anti-IL5 mAbs in the forthcoming years.
Chronic eosinophilic pneumonia (CEP) is an idiopathic condition defined by peripheral eosinophilia, eosinophilic infiltrates in the lung parenchyma occasionally associated with asthma. The pathophysiology of CEP remains unknown, but available data suggest that recruitment of eosinophils into the lung airspaces is a multifactorial process and eosinophil release of IL-5 and cytotoxic granular proteins are involved in the pathogenesis of tissue damage typical of the disorder [Akuthota and Weller, 2012]. On the grounds of this mechanism, an eosinophil-targeted therapy with mepolizumab deserves investigation in CEP patients [Akuthota and Weller, 2012].
In severe chronic obstructive pulmonary disease (COPD) patients, the eosinophil-predominant phenotype is associated with frequent exacerbations despite maximal standard therapy and high doses of OCS. The potential role of mepolizumab in COPD has not been determined yet (Table 1). Three phase III RCTs to evaluate the efficacy and safety of mepolizumab as an add-on treatment in COPD are ongoing and are enrolling patients [ClinicalTrials.gov identifiers: NCT02105961, NCT01463644, NCT02105948]. The primary endpoints of these studies are to evaluate the effect of mepolizumab in decreasing exacerbation rates, lowering sputum eosinophils from baseline and improving lung function, as documented for other eosinophilic-driven airway diseases.
A subgroup of COPD patients as potential candidates for mepolizumab treatment might be those with an eosinophil trait labeled as affected by the asthma–COPD overlap syndrome (ACOS) [Hizawa, 2016].
The possible overlap between omalizumab and mepolizumab
At present, omalizumab is the gold-standard treatment for severe allergic asthma; main clinical outcomes are represented by a reduction of exacerbations, an improvement of quality of life and a limited prescription of systemic corticosteroids [Normansell et al. 2014].
The effectiveness of omalizumab has been recently demonstrated not only in allergic asthmatic patients on long-term treatment but even in intrinsic asthma and chronic spontaneous orticaria-angioedema [Lommatzsch et al. 2014; Zhao et al. 2016]. These data support the hypothesis of a local production of IgE even without systemic sensitization [Forester and Calabria, 2010] and confirm that there is still much to understand about this biologic agent.
It is well known that the proportion of patients with severe asthma and IgE >400 UI/ml is significantly greater than in patients with moderate asthma [Davila et al. 2015]. Baseline IgE was the only predictor of efficacy of omalizumab in the INNOVATE trial and in other six studies on severe atopic asthmatics in which statistical significance was reached in the upper IgE quartile only (p < 0.001) [Humbert et al. 2005; Bousquet et al. 2007]. In the DREAM study, mepolizumab treatment allowed a significant reduction in severe asthma exacerbations irrespective of the baseline IgE levels or atopic status [Pavord et al. 2012]. In light of the partial overlap between the populations eligible for mepolizumab or omalizumab, a multicenter RCT has been planned with the aim of evaluating the effect of mepolizumab in patients with severe eosinophilic asthma not optimally controlled with omalizumab [ClinicalTrials.gov identifier: NCT02654145]. Hopefully, the results will help clinicians to understand in which phenotype of severe allergic asthma anti-IgE or anti-IL-5 mAbs should be the first choice treatment. As reported previously, a recent study demonstrated that the anti-IL-5 agents can be effective even in patients who were nonresponders to omalizumab, with a steroid-sparing effect [Prazma et al. 2014].
In the EXTRA study, carried out on 850 patients, omalizumab was more effective in patients with higher blood eosinophils (>260 cells/μl), high FeNO and periostin levels [Hanania et al. 2013]. In this phenotype of patients anti-IL-5 drugs should be studied as the first choice treatment in nonresponders to anti-IgE molecules.
Conversely, the DREAM study [Pavord et al. 2012] demonstrated that a significant efficacy of mepolizumab was associated with two variables, that is, higher baseline peripheral blood eosinophils and frequent exacerbations. In patients properly selected according to these characteristics, mepolizumab should be considered as the first choice, also considering an important steroid-sparing effect allowing a consistent reduction of related side effects in the medium and long term.
Conclusion
The options provided to date for severe uncontrolled asthma (step 5 treatment of the GINA guidelines) are anti-IgE mAbs and OCS. OCS are associated with several adverse effects, especially in chronic therapy and should be used at the lowest possible dose [GINA, 2015]. A very recent study found that 93% of subjects with severe asthma had one or more pathologic conditions related to systemic corticosteroids, that is, type II diabetes, osteoporosis, dyspeptic disorders, including gastric/duodenal ulceration, cataracts, obesity, hypertension, etc. [Sweeney et al. 2016]. As expected, the relative risk was higher in corticosteroid-dependent asthma (CSD). Therefore, the search for new treatments must take into account as a priority the possibility to reduce the use of systemic steroids.
Advancing knowledge has led to a significant increase of therapeutic options, targeting different phenotypes of the disorder. However, it is not easy to select the right approach to the right patient due to the complexity of asthma pathogenesis and inflammatory pathways.
After the FDA approval of mepolizumab a new option is available for the treatment of severe asthma, after a decade of omalizumab as the only biologic agent. The positive but sometimes conflicting results from anti-IL-5 clinical trials make even more evident the need for careful phenotyping and endotyping of patients.
The potential responders to anti-IL-5 therapy are subjects with eosinophilia (>0.3 × 109/l in blood, >3% in sputum), possibly steroid-responsive, who manifest poor symptom control and frequent exacerbations. Anyway, persistent systemic and airway eosinophilia may not be sufficient to identify the appropriate candidates, particularly in systemic corticosteroid-dependent asthmatic patients, but a multimodal approach is necessary, based also on clinical experience. The development of specific reliable and useful biomarkers beyond eosinophilia is required to more accurately select specific asthma phenotypes and targeted treatments.
The minimum duration of treatment with anti-IL-5 mAbs is still questionable. Even for omalizumab, whose introduction into real-life clinical setting dates back to several years ago, there is no clear response yet. The limited data the minimum duration of treatment with anti-IL-5 mAbs is still questionable. Even for omalizumab, whose introduction into real-life clinical setting dates back to several years ago, there is no clear response yet. The limited data available at present would suggest to continue indefinitely. New studies underway or in the pipeline will probably allow a convincing answer to that topic and other open questions, such as the criteria for the selection of phenotypes with the best chance of response to treatment.
Severe asthma accounts for the largest part of the direct and indirect costs for the disease, mainly due to poor symptom control causing absence from school or work and serious psychological problems such as stress, anxiety and depression impacting heavily on quality of life [Accordini et al. 2013]. Despite an increasing evidence of clinical efficacy, monoclonal anti-IL-5 antibodies however imply a considerable increase of the asthma-related healthcare expenditure. Cost-effectiveness is therefore a fundamental issue of biological therapies to better define real-life utility of these agents and to establish their appropriate position in treatment guidelines.
Pharmacoeconomic studies are quite promising but it will be necessary to reduce the purchase costs to allow equity of access to biological treatments such as mepolizumab in our case.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Francesco Menzella and Luigi Zucchi participated in contracted research and clinical trials for Novartis, Sanofi and GlaxoSmithKline. The other authors report no conflicts of interest in this work.
Contributor Information
Francesco Menzella, Department of Cardio-Thoracic-Vascular and Intensive Care Medicine, Pneumology Unit, IRCCS - Arcispedale Santa Maria Nuova, Viale Risorgimento 56, 42123 Reggio Emilia, Italy.
Mirco Lusuardi, Unit of Respiratory Rehabilitation, AUSL Reggio Emilia, S. Sebastiano Hospital, Correggio, Italy.
Carla Galeone, Department of Cardio-Thoracic-Vascular and Intensive Care Medicine, Pneumology Unit, IRCCS - Arcispedale Santa Maria Nuova, Viale Risorgimento 56, 42123 Reggio Emilia, Italy.
Sofia Taddei, Department of Cardio-Thoracic-Vascular and Intensive Care Medicine, Pneumology Unit, IRCCS - Arcispedale Santa Maria Nuova, Viale Risorgimento 56, 42123 Reggio Emilia, Italy.
Nicola Facciolongo, Department of Cardio-Thoracic-Vascular and Intensive Care Medicine, Pneumology Unit, IRCCS - Arcispedale Santa Maria Nuova, Viale Risorgimento 56, 42123 Reggio Emilia, Italy.
Luigi Zucchi, Department of Cardio-Thoracic-Vascular and Intensive Care Medicine, Pneumology Unit, IRCCS - Arcispedale Santa Maria Nuova, Viale Risorgimento 56, 42123 Reggio Emilia, Italy.
References
- Abonia J., Putnam P. (2011) Mepolizumab in eosinophilic disorders. Expert Rev Clin Immunol 7: 411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accordini S., Corsico A., Braggion M., Gerbase M., Gislason D., Gulsvik A., et al. (2013) The cost of persistent asthma in Europe: an international population-based study in adults. Int Arch Allergy Immunol 160: 93–101. [DOI] [PubMed] [Google Scholar]
- Akuthota P., Weller P. (2012) Eosinophilic pneumonias. Clin Microbiol Rev 25: 649–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Muhsen S., Johnson J., Hamid Q. (2011) Remodeling in asthma. J Allergy Clin Immunol 128: 451–462; quiz 463–464. [DOI] [PubMed] [Google Scholar]
- Baptista-dos-Reis R., Muniz V., Neves J. (2015) Multifaceted roles of cysteinyl leukotrienes in eliciting eosinophil granule protein secretion. Biomed Res Int 2015: 848762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becchetti E., Bani D., Baroni T. (2011) Istologia Umana. Napoli: Idelson-Gnocchi, pp. 393–394. [Google Scholar]
- Bel E., Wenzel S., Thompson P., Prazma C., Keene O.et al. for the SIRIUS Investigators (2014) Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med 25: 1189–1197. [DOI] [PubMed] [Google Scholar]
- Berek C. (2016) Eosinophils: important players in humoral immunity. Clin Exp Immunol 183: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjermer C., Maspero J., Ciesielska M., O’Brien C., Zangrilli J. (2014) A randomized phase 3 study of the efficacy and safety of reslizumab in subjects with asthma with elevated eosinophils. Eur Respir J 44: 299.25082909 [Google Scholar]
- Bogart M., Roberts A., Wheeler S. (2015) Cost-effectiveness of refractory asthma treatment strategies: a decision tree analysis. In: ISPOR 20th Annual International Meeting, Philadelphia, PA, USA. [Google Scholar]
- Bousquet J., Rabe K., Humbert M., Chung K., Berger W., Fox H., et al. (2007) Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respir Med 101: 1483–1492. [DOI] [PubMed] [Google Scholar]
- Caruso M., Crisafulli E., Demma S., Holgate S., Polosa R. (2013a) Disabling inflammatory pathways with biologics and resulting clinical outcomes in severe asthma. Expert Opin Biol Ther 13: 393–402. [DOI] [PubMed] [Google Scholar]
- Caruso M., Crisafulli E., Lizzio R., Polosa R. (2013b) Biologic therapy for atopic asthma and beyond. Curr Opin Allergy Clin Immunol 13: 677–685. [DOI] [PubMed] [Google Scholar]
- Castro M., Mathur S., Hargreave F., Boulet L., Xie F., Young J., et al. (2011) Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med 184: 1125–1132. [DOI] [PubMed] [Google Scholar]
- Castro M., Wenzel S., Bleecker E., Pizzichini E., Kuna P., Busse W., et al. (2014) Benralizumab, an anti-interleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med 2: 879–890. [DOI] [PubMed] [Google Scholar]
- Clutterbuck E., Sanderson C. (1990) Regulation of human eosinophil precursor production by cytokines: a comparison of recombinant human interleukin-1 (rhIL-1), rhIL-3, rhIL-5, rhIL-6, and rh granulocyte-macrophage colony-stimulating factor. Blood 75: 1774–1779. [PubMed] [Google Scholar]
- Curtis C., Ogbogu P. (2015) Evaluation and differential diagnosis of persistent marked Eosinophilia. Immunol Allergy Clin N Am 35: 387–402. [DOI] [PubMed] [Google Scholar]
- Davila I., Valero A., Entrenas L., Valveny N., Herráez L.; SIGE Study Group. (2015) Relationship between serum total IgE and disease severity in patients with allergic asthma in Spain. J Invest Allergol Clin Immunol 25: 120–127. [PubMed] [Google Scholar]
- Djukanović R. (1996) Bronchoscopy as a research tool for the study of asthma pathogenesis and effects of antiasthma drugs. J Allergy Clin Immunol 98(5 Pt 2): S41–S45; discussion S64–S66. [DOI] [PubMed] [Google Scholar]
- Djukanović R., Wilson S., Kraft M., Jarjour N., Steel M., Chung K., et al. (2004) Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med 15: 583–593. [DOI] [PubMed] [Google Scholar]
- Egan R., Athwal D., Bodmer M., Carter J., Chapman R., Chou C., et al. (1999) Effect of Sch 55700, a humanized monoclonal antibody to human interleukin-5, on eosinophilic responses and bronchial hyper reactivity. Arzneimittelforschung 49: 779–790. [DOI] [PubMed] [Google Scholar]
- EMA (2009) Withdrawal assessment report for Bosatria. Document reference EMEA/454803/2009. London: European Medicines Agency; Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003860/human_med (accessed 9 May 2016). [Google Scholar]
- Fahy J. (2009) Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc 1: 256–259. [DOI] [PubMed] [Google Scholar]
- FDA (2015) FDA approves Nucala to treat severe asthma. US Food and Drug Administration (FDA) Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm471031.htm (accessed 10 January 2016). [Google Scholar]
- Flood-Page P., Menzies-Gow A., Kay A., Robinson D. (2003) Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med 15: 199–204. [DOI] [PubMed] [Google Scholar]
- Flood-Page P., Swenson C., Faiferman I., Flood-Page P., Swenson C., Faiferman I., et al. (2007) A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med 176: 1062–1071. [DOI] [PubMed] [Google Scholar]
- Forester J., Calabria C. (2010) Local production of IgE in the respiratory mucosa and the concept of entopy: does allergy exist in nonallergic rhinitis? Ann Allergy Asthma Immunol 105: 249–255. [DOI] [PubMed] [Google Scholar]
- Ghazi A., Trikha A., Calhoun W. (2012) Benralizumab: a humanized mAb to IL-5Ralpha with enhanced antibody-dependent cell-mediated cytotoxicity—a novel approach for the treatment of asthma. Expert Opin Biol Ther 12: 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George L., Brightling C. (2016) Eosinophilic airway inflammation: role in asthma and chronic obstructive pulmonary disease. Ther Adv Chronic Dis 7: 34–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevaert P., Lang-Loidolt D., Lackner A., Stammberger H., Staudinger H., Van Zele T., et al. (2006) Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol 118: 1133–1141. [DOI] [PubMed] [Google Scholar]
- Gevaert P., Van Bruaene N., Cattaert T., Van Steen K., Van Zele T., Acke F., et al. (2011) Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol 128: 989–995. [DOI] [PubMed] [Google Scholar]
- GlaxoSmithKline (2014) Efficacy and safety study of Mepolizumab adjunctive therapy in participants with severe Eosinophilic Asthma on markers of asthma control. ClinicalTrials.gov NCT02281318. [Google Scholar]
- GlaxoSmithKline (2014) Efficacy and safety of Mepolizumab as an add-on treatment in Chronic Obstructive Pulmonary Disease (COPD). Available at: https://clinicaltrials.gov/ct2/show/NCT02105961 (accessed 13 July 2016).
- GlaxoSmithKline (2014) Study to evaluate efficacy and safety of Mepolizumab for frequently exacerbating Chronic Obstructive Pulmonary Disease (COPD) Patients. Available at: https://clinicaltrials.gov/ct2/show/NCT02105948 (accessed 13 July 2016).
- Global Asthma Network (2014) The Global Asthma Report 2014. Available at: http://www.globalasthmareport.org/burden/burden.php (accessed 30 January 2016).
- GINA (2015) Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA) Available at: http://www.ginasthma.org (accessed 5 July 2016). [Google Scholar]
- Gnanakumaran G., Babu K. (2003) Technology evaluation: mepolizumab, GlaxoSmithKline. Curr Opin Mol Ther 5: 321–325. [PubMed] [Google Scholar]
- Gould H., Sutton B. (2008) IgE in allergy and asthma today. Nat Rev Immunol 8: 205–217. [DOI] [PubMed] [Google Scholar]
- Haldar P., Brightling C., Hargadon B., Haldar P., Brightling C., Hargadon B., et al. (2009) Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med 360: 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar P., Brightling C., Singapuri A., Hargadon B., Gupta S., Monteiro W., et al. (2014) Outcomes after cessation of mepolizumab therapy in severe eosinophilic asthma: a 12-month follow-up analysis. J Allergy Clin Immunol 133: 921–923. [DOI] [PubMed] [Google Scholar]
- Haldar P., Pavord I., Shaw D., Berry M., Thomas M., Brightling C., et al. (2008) Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med 1: 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanania N., Wenzel S., Rosén K., Hsieh H., Mosesova S., Choy D., et al. (2013) Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med 187: 804–811. [DOI] [PubMed] [Google Scholar]
- Hart T., Cook R., Zia-Amirhosseini P., Minthorn E., Sellers T., Maleeff B., et al. (2001) Preclinical efficacy and safety of mepolizumab (SB-240563), a humanized monoclonal antibody to IL-5, in cynomolgus monkeys. J Allergy Clin Immunol 108: 250–257. [DOI] [PubMed] [Google Scholar]
- Hizawa N. (2016) Clinical approaches towards asthma and chronic obstructive pulmonary disease based on the heterogeneity of disease pathogenesis. Clin Exp Allergy 46: 678-687. [DOI] [PubMed] [Google Scholar]
- Holgate S. (2008) Pathogenesis of asthma. Clin Exp Allergy 38: 872–897. [DOI] [PubMed] [Google Scholar]
- Homer R., Elias J. (2005) Airway remodeling in asthma: therapeutic implications of mechanisms. Physiology 20: 28–35. [DOI] [PubMed] [Google Scholar]
- Hoshino M., Ohtawa J. (2012) Effects of adding omalizumab, an antiimmunoglobulin E antibody, on airway wall thickening in asthma. Respiration 83: 520–528. [DOI] [PubMed] [Google Scholar]
- Humbert M., Beasley R., Ayres J., Slavin R., Hébert J., Bousquet J., et al. (2005) Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 60: 309–316. [DOI] [PubMed] [Google Scholar]
- Humbles A., Lloyd C., McMillan S., Friend D., Xanthou G., McKenna E., et al. (2004) A critical role for eosinophils in allergic airways remodeling. Science 17: 1776–1779. [DOI] [PubMed] [Google Scholar]
- Institute for Clinical and Economic Review (2015) ICER Draft Reports on Nucala® (Mepolizumab) for Asthma and Tresiba® (Insulin Degludec) for Diabetes Posted for Public Comment Edit. Available at: http://www.icer-review.org/icer-draft-reports-on-nucala-mepolizumab-forasthma-and-tresiba-insulin-degludec-for-diabetes-posted-for-public-comment (accessed 23 January 2016).
- Izuhara K., Conway S., Moore B., Matsumoto H., Holweg C., Matthews J., et al. (2016) Roles of periostin in respiratory disorders. Am J Respir Crit Care Med 193: 949-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatakanon A., Lim S., Kharitonov S., Chung K., Barnes P. (1998) Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax 53: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G., Erickson R., Choy D., Mosesova S., Wu L., Solberg O., et al. (2012) Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol 130: 647–654. e10 10.1016/j.jaci.2012.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Gleich G., Hartley B., Yancey S., Ortega H. (2014) Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac 11: 531–536. [DOI] [PubMed] [Google Scholar]
- Kahn J., Grandpeix-Guyodo C., Marroun I., Catherinot E., Mellot F., Roufosse F., et al. (2010) Sustained response to mepolizumab in refractory Churg–Strauss syndrome. J Allergy Clin Immunol 125: 267–270. [DOI] [PubMed] [Google Scholar]
- Kim S., Marigowda G., Oren E., Israel E., Wechsler M. (2010) Mepolizumab as a steroid-sparing treatment option in patients with Churg–Strauss syndrome. J Allergy Clin Immunol 125: 1336–1343. [DOI] [PubMed] [Google Scholar]
- Kips J., O’Connor B., Langley S., Woodcock A., Kerstjens H., Postma D., et al. (2003) Effect of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: a pilot study. Am J Respir Crit Care Med 167: 1655–1659. [DOI] [PubMed] [Google Scholar]
- Klein Wolterink R., Kleinjan A., van Nimwegen M., Bergen I., Kerstjens M., Levani Y., et al. (2012) Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol 42: 1106–1116. [DOI] [PubMed] [Google Scholar]
- Kolbeck R., Kozhich A., Koike M., Peng L., Andersson C., Damschroder M. (2010) MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol 125: 1344–1353. [DOI] [PubMed] [Google Scholar]
- Kouro T., Takatsu K. (2009) IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol 21: 1303–1309. [DOI] [PubMed] [Google Scholar]
- Kupryś-Lipińska I., Kuna P. (2014) Loss of asthma control after cessation of omalizumab treatment: real life data. Postepy Dermatol Alergol 31: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckie M., ten Brinke A., Khan J., Diamant Z., O’Connor B., Walls C., et al. (2000) Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 356: 2144–2148. [DOI] [PubMed] [Google Scholar]
- Leiferman K. (2001) A role for eosinophils in atopic dermatitis. J Am Acad Dermatol 45: S21–S24. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M., Korn S., Buhl R., Virchow J. (2014). Against all odds: anti-IgE for intrinsic asthma? Thorax 69: 94–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommatzsch S., Martin R., Good J., Jr. (2013) Importance of fiberoptic bronchoscopy in identifying asthma phenotypes to direct personalized therapy. Curr Opin Pulm Med 19: 42–48. [DOI] [PubMed] [Google Scholar]
- Lin H., Boesel K., Griffith D., Prussin C., Foster B., Romero F., et al. (2004) Omalizumab rapidly decreases nasal allergic response and FcepsilonRI on basophils. J Allergy Clin Immunol 113: 297–302. [DOI] [PubMed] [Google Scholar]
- Lopez A., Sanderson C., Gamble J., Campbell H., Young I., Vadas M. (1988) Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med 167: 219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGlashan D., Jr, Bochner B., Adelman D., Jardieu P., Togias A., McKenzie-White J., et al. (1997) Down-regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol 158: 1438–1445. [PubMed] [Google Scholar]
- McKinnon M., Bank M., Solari R., Robinson G. (1999) Interleukin-5 and the interleukin receptor: targets for drug discovery in asthma. In: Sanderson C. (ed.), Interleukin-5: From Molecule to Drug Target for Asthma. New York: Marcel Dekker, Inc., pp. 299–320. [Google Scholar]
- McMaster University (2011) Mepolizumab in Chronic Obstructive Pulmonary Diseases (COPD) With Eosinophilic Bronchitis. Available at: https://clinicaltrials.gov/ct2/show/NCT02281318 (accessed 1 June 2015) and https://clinicaltrials.gov/ct2/show/NCT01463644 (accessed 13 July 2016).
- Menzella F., Facciolongo N., Piro R., Formisano D., Roggeri A., Simonazzi A., et al. (2012) Clinical and pharmacoeconomic aspects of omalizumab: a 4-year follow-up. Ther Adv Respir Dis 6: 87–95. [DOI] [PubMed] [Google Scholar]
- Menzella F., Zucchi L., Piro R., Galeone C., Castagnetti C., Facciolongo N. (2014) A budget impact analysis of bronchial thermoplasty for severe asthma in clinical practice. Adv Ther 31: 751–761. [DOI] [PubMed] [Google Scholar]
- Menzies-Gow A., Flood-Page P., Sehmi R., Burman J., Hamid Q., Robinson D., et al. (2003) Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J Allergy Clin Immunol 111: 714–719. [DOI] [PubMed] [Google Scholar]
- Moosig F., Gross W., Herrmann K., Bremer J., Hellmich B. (2011) Targeting interleukin-5 in refractory and relapsing Churg–Strauss syndrome. Ann Intern Med 155: 341–343. [DOI] [PubMed] [Google Scholar]
- Nair P., Pizzichini M., Kjarsgaard M., Inman M., Efthimiadis A., Pizzichini E., et al. (2009) Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med 360: 985–993. [DOI] [PubMed] [Google Scholar]
- NHS (2015) UK Medicines Information. New Drugs Online Report for Mepolizumab. Available at: http://www.ukmi.nhs.uk/applications/ndo/record_view_open.asp?newDrugID=4675 (accessed 24 January 2016).
- Nopp A., Johansson S., Adédoyin J., Ankerst J., Palmqvist M., Oman H. (2010) After 6 years with Xolair: a 3-year withdrawal follow-up. Allergy 65: 56–60. [DOI] [PubMed] [Google Scholar]
- Nopp A., Johansson S., Ankerst J., Palmqvist M., Oman H. (2007) CD-sens and clinical changes during withdrawal of Xolair after 6 years of treatment. Allergy 62: 1175–1181. [DOI] [PubMed] [Google Scholar]
- Normansell R., Walker S., Milan S., Walters E., Nair P. (2014) Omalizumab for asthma in adults and children. Cochrane Database Syst Rev 1: CD003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T., Sur S., Collins D., Fish J., Gleich G., Peters S. (1993) Eosinophil survival activity identified as interleukin-5 is associated with eosinophil recruitment and degranulation and lung injury twenty-four hours after segmental antigen lung challenge. J Allergy Clin Immunol 92: 607–615. [DOI] [PubMed] [Google Scholar]
- Oldhoff J., Darsow U., Werfel T., Oldhoff J., Darsow U., Werfel T., et al. (2005) Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy 60: 693–696. [DOI] [PubMed] [Google Scholar]
- Ortega H., Chupp G., Bardin P., Bourdin A., Garcia G., Hartley B., et al. (2014a) The role of mepolizumab in atopic and nonatopic severe asthma with persistent eosinophilia. Eur Respir J 44: 239–241. [DOI] [PubMed] [Google Scholar]
- Ortega H., Liu M., Pavord I., Brusselle G., FitzGerald J., Chetta A., et al. (2014b) Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 25: 1198–1207. [DOI] [PubMed] [Google Scholar]
- Ortega H., Li H., Suruki R., Albers F., Gordon D., Yancey S. (2014c) Cluster analysis and characterization of response to mepolizumab: a step closer to personalized medicine for patients with severe asthma. Ann Am Thorac Soc 11: 1011–1017. [DOI] [PubMed] [Google Scholar]
- Otani I., Arjun A., Anilkumar R. (2013) Anti–IL-5 therapy reduces mast cell and IL-9 cell numbers in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol 131: 1576–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani I., Anilkumar A., Newbury R., Bhagat M., Beppu L., Dohil R., et al. (2013) Anti-IL-5 therapy reduces mast cell and IL-9 cell numbers in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol 131: 1576–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavord I., Korn S., Howarth P., Pavord I., Korn S., Howarth P., et al. (2012) Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 380: 651–659. [DOI] [PubMed] [Google Scholar]
- Pepe C., Foley S., Shannon J., Lemiere C., Olivenstein R., Ernst P., et al. (2005) Differences in airway remodeling between subjects with severe and moderate asthma. J Allergy Clin Immunol 116: 544–549. [DOI] [PubMed] [Google Scholar]
- Peters S., Ferguson G., Deniz Y., Reisner C. (2006) Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med 100: 1139–1151. [DOI] [PubMed] [Google Scholar]
- Pham T., Damera G., Newbold P., Ranade K. (2016) Reductions in eosinophil biomarkers by benralizumab in patients with asthma. Respir Med 111: 21–29. [DOI] [PubMed] [Google Scholar]
- Phipps S., Flood-Page P., Menzies-Gow A., Ong Y., Kay A. (2004) Intravenous anti-IL-5 monoclonal antibody reduces eosinophils and tenascin deposition in allergen-challenged human atopic skin. J Invest Dermatol 122: 1406–1412. [DOI] [PubMed] [Google Scholar]
- Powell C., Milan S., Dwan K., Bax L., Walters N. (2015) Mepolizumab versus placebo for asthma. Cochrane Database Syst Rev 7: CD010834. [DOI] [PubMed] [Google Scholar]
- Prazma C., Wenzel S., Barnes N., Douglass J., Hartley B., Ortega H. (2014) Characterisation of an OCS-dependent severe asthma population treated with mepolizumab. Thorax 69: 1141–1142. [DOI] [PubMed] [Google Scholar]
- Prussin C., Metcalfe D. (2006) IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol 117(Suppl. 2): S450–S456. [DOI] [PubMed] [Google Scholar]
- Riccio A., Dal Negro R., Micheletto C., De Ferrari L., Folli C., Chiappori A., et al. (2012) Omalizumab modulates bronchial reticular basement membrane thickness and eosinophil infiltration in severe persistent allergic asthma patients. Int J Immunopathol Pharmacol 25: 475–484. [DOI] [PubMed] [Google Scholar]
- Rothenberg M., Klion A., Roufosse F., Kahn J., Weller P., Simon H., et al. (2008) Mepolizumab HES Study Group: treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med 20: 1215–1228. [DOI] [PubMed] [Google Scholar]
- Roufosse F., Kahn J., Gleich G., Schwartz L., Singh A., Rosenwasser L., et al. (2013) Long-term safety of mepolizumab for the treatment of hypereosinophilic syndromes. J Allergy Clin Immunol 131: 461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleich F., Seidel L., Sele J., Manise M., Quaedvlieg V., Michils A., et al. (2010) Exhaled nitric oxide thresholds associated with a sputum eosinophil count ⩾3% in a cohort of unselected patients with asthma. Thorax 65: 1039–1044. [DOI] [PubMed] [Google Scholar]
- Simpson J., Scott R., Boyle M., Gibson P. (2006) Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology 11: 54–61. [DOI] [PubMed] [Google Scholar]
- Spergel J., Rothenberg M., Collins M., Furuta G., Markowitz J., Fuchs G., III, et al. (2012) Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol 129: 456–463. [DOI] [PubMed] [Google Scholar]
- Straumann A., Conus S., Grzonka P., Kita H., Kephart G., Bussmann C., et al. (2010) Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut 59: 21–30. [DOI] [PubMed] [Google Scholar]
- Sweeney J., Patterson C., Menzies-Gow A., Niven R., Mansur A., Bucknall C., et al. for the British Thoracic Society Difficult Asthma Network (2016) Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax 71: 339–346. [DOI] [PubMed] [Google Scholar]
- Tajiri T., Matsumoto H., Gon Y., Ito R. (2016) Utility of serum periostin and free IgE levels in evaluating responsiveness to omalizumab in patients with severe asthma. Allergy. DOI: 10.1111/all.12922. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Komai M., Nagao K., Ishizaki M., Kajiwara D., Takatsu K., et al. (2004) Role of interleukin-5 and eosinophils in allergen-induced airway remodeling in mice. Am J Respir Cell Mol Biol 31: 62–68. [DOI] [PubMed] [Google Scholar]
- Toba K., Koike T., Shibata A., Hashimoto S., Takahashi M., Masuko M., et al. (1999) Novel technique for the direct flow cytofluorometric analysis of human basophils in unseparated blood and bone marrow, and the characterization of phenotype and peroxidase of human basophils. Cytometry 35: 249–259. [PubMed] [Google Scholar]
- Valent P. (1994) The phenotype of human eosinophils, basophils, and mast cells. J Allergy Clin Immunol 94: 1177–1183. [DOI] [PubMed] [Google Scholar]
- Wagener A., de Nijs S., Lutter R., Sousa A., Weersink E., Bel E., et al. (2015) External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax 70: 115–120. [DOI] [PubMed] [Google Scholar]
- Wahn U., Martin C., Freeman P., Blogg M., Jimenez P. (2009) Relationship between pretreatment specific IgE and the response to omalizumab therapy. Allergy 64: 1780–1787. [DOI] [PubMed] [Google Scholar]
- Wedi B., Raap U., Lewrick H., Kapp A. (1997) Delayed eosinophil programmed cell death in vitro: a common feature of inhalant allergy and extrinsic and intrinsic atopic dermatitis. J Allergy Clin Immunol 100: 536–543. [DOI] [PubMed] [Google Scholar]
- Wells T., Graber P., Proudfoot A., Arod C., Jordan S., Lambert M., et al. (1994) The three-dimensional structure of human interleukin-5 at 2.4-angstroms resolution: implication for the structures of other cytokines. Ann N Y Acad Sci 28: 118–127. [DOI] [PubMed] [Google Scholar]
- Westerhof G., Korevaar D., Amelink M., de Nijs S., de Groot J., Wang J., et al. (2015) Biomarkers to identify sputum eosinophilia in different adult asthma phenotypes. Eur Respir J 46: 688–696. [DOI] [PubMed] [Google Scholar]
- Wenzel S. (2012) Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy 42: 650–658. [DOI] [PubMed] [Google Scholar]
- Wenzel S., Ford L., Pearlman D., Spector S., Sher L., Skobieranda F., et al. (2013) Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med 368: 2455–2466. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Ji C., Yu W., Meng L., Hawro T., Wei J., et al. (2016) Omalizumab for the treatment of chronic spontaneous urticaria: a meta-analysis of randomized clinical trials. J Allergy Clin Immunol 137: 1742–1750. [DOI] [PubMed] [Google Scholar]
- Zia-Amirhosseini P., Minthorn E., Benincosa L., Hart T., Hottenstein C., Tobia L., et al. (1999) Pharmacokinetics and pharmacodynamics of SB-240563, a humanized monoclonal antibody directed to human interleukin-5, in monkeys. J Pharmacol Exp Ther 291: 1060–1067. [PubMed] [Google Scholar]