Abstract
Primary cilia, present on most mammalian cells, function as a sensor to sense the environment change and transduce signaling. Loss of primary cilia causes a group of human pleiotropic syndromes called Ciliopathies. Some of the ciliopathies display skeletal dysplasias, implying the important role of primary cilia in skeletal development and homeostasis. Emerging evidence has shown that loss or malfunction of primary cilia or ciliary proteins in bone and cartilage is associated with developmental and function defects. Intraflagellar transport (IFT) proteins are essential for cilia formation and/or function. In this review, we discuss the role of primary cilia and IFT proteins in the development of bone and cartilage, as well as the differentiation and mechanotransduction of mesenchymal stem cells, osteoblasts, osteocytes, and chondrocytes. We also include the role of primary cilia in tooth development and highlight the current advance of primary cilia and IFT proteins in the pathogenesis of cartilage diseases, including osteoarthritis, osteosarcoma, and chondrosarcoma.
Keywords: ciliary proteins, cellular mechanotransduction, osteoarthritis, osteosarcoma, chondrosarcoma, skeletal development
Introduction
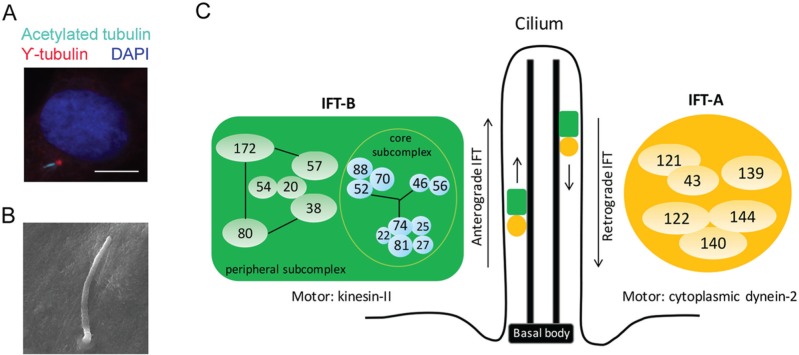
Primary cilia are microtubule-based organelles that present on the surface of almost all mammalian cells (Fig. 1A, B). Centriole, which becomes the basal body in cilium, provides the template to build the axoneme, the microtubular backbone of cilium. Hundreds of proteins reside in the cilium; however, cilium itself does not synthesize any proteins. All ciliary proteins are synthesized in the cytosol and transported to cilium by intraflagellar transport (IFT), the bidirectional transport system operated by IFT proteins and motors. IFT proteins function as adaptors to assemble cargo proteins and IFT motors together (Ishikawa and Marshall 2011; Taschner et al. 2012). IFT proteins form 2 complexes named complex A (IFT-A) and complex B (IFT-B). IFT-A has 6 proteins (IFT43, IFT121, IFT122, IFT139, IFT140, and IFT144) and is associated with retrograde transport, which binds with dynein-2 motor to move proteins from cilium tip to cell body. IFT-B contains 16 IFT proteins and is involved in anterograde transport, which binds with kinesin-II motor to transport proteins from basal body to cilium tip. IFT proteins in IFT-B complex form 2 subcomplexes named core subcomplex and peripheral subcomplex. Core subcomplex is formed by IFT22, IFT25, IFT27, IFT46, IFT52, IFT56, IFT70, IFT74, IFT81, and IFT88. Peripheral subcomplex is formed by IFT20, IFT38, IFT54, IFT57, IFT80, and IFT172. Two subcomplexes connect through the interaction of IFT38, IFT52, IFT57, and IFT88 (Katoh et al. 2016; Taschner et al. 2016; Fig. 1C).
Figure 1.
Primary cilium structure and intraflagellar transport proteins. (A) Immunofluorescence micrograph of primary cilium located on primary osteoblasts derived from mouse calvarial bone. Primary cilium was stained with γ-tubulin (basal body; red) and acetylated tubulin (axoneme; cyan) antibody. Nuclear was stained with DAPI (blue). Scale bars represent 10 μm. (B) Scanning electron microscopic image of primary cilium present on mouse mesenchymal stem cell. Scale bars represent 2 μm. (C) Schema of primary cilium structure and intraflagellar transport (IFT) complexes. Adapted from Katoh et al. (2016). This figure is available in color online at http://jdr.sagepub.com.
Primary cilia were observed in chondrocytes about 40 y ago and later found in mesenchymal stem cells, osteoblasts, and osteocytes (Yuan et al. 2015). Mutations of IFT proteins and cilia-related proteins cause cilium loss and malfunction, leading to a group of disorders called Ciliopathies—some of which display skeletal dysplasias, such as Jeunes syndrome, short rib–polydactyly, and Ellis–van Creveld syndrome (reviewed in Yuan and Yang 2015a). Therefore, interest has been growing in discovering the role of primary cilia and IFT proteins in skeletal development and homeostasis. Indeed, primary cilia are essential for hedgehog (Hh) signaling transduction (Huangfu et al. 2003), and Hh signaling is required for bone development. Further studies suggested that primary cilia and IFT proteins regulate platelet-derived growth factor and Wnt signaling, highlighting the role of primary cilia and IFT proteins in development. In addition to the chemical-sensing role, the mechanosensory role of primary cilia has been extensively studied (reviewed in Ruhlen and Marberry 2014). Bone is a highly dynamic organ, and how bone cells sense and respond to the mechanical signaling affects bone formation and homeostasis. Primary cilia project from the cell surface to the extracellular space, making them suitable to sense and transduce mechanical stimuli in bone, such as interstitial fluid flow. Studies began to uncover the responsible channels and proteins in primary cilia that mediate mechanotransduction (Kwon et al. 2010; He et al. 2015; Lee et al. 2015).
In this review, we summarize the recent studies of primary cilia and IFT proteins in bone and cartilage development and maintenance. We also discuss the current advance of primary cilia and IFT proteins in tooth development and the pathogenesis of osteoarthritis, osteosarcoma, and chondrosarcoma.
Role of Primary Cilia/IFT in Bone
Osteoblasts, the main bone-forming cells, are derived from mesenchymal progenitors and characterized by producing alkaline phosphatase, osteocalcin, and type I collagen. Runx2 and osterix are essential for osteoblast differentiation and maturation. Primary cilia have been shown to regulate osteogenic differentiation of bone marrow–derived mesenchymal stem cells (Tummala et al. 2010) and adipose-derived stem cells (Bodle et al. 2013). Primary cilia in adipose-derived stem cells elongate in response to osteogenic induction with osteogenic media, but the mechanism and the consequence are largely unknown (Bodle et al. 2013).
A small subset of osteoblasts is trapped by the bone matrix and becomes osteocytes. Osteocytes account for the majority of bone cells in the mature bone tissue (Dallas et al. 2013). Osteocytes are multifunctional cells that serve as the sensor of mechanical loading, the regulator of bone formation and resorption, and the reservoir of calcium in bone (Dallas et al. 2013).
Several recent studies, which we summarize below, suggested the essential role of primary cilia and IFT proteins in osteoblast differentiation, bone development, and osteocyte function.
Primary Cilia/IFT in Osteogenesis and Bone Formation
Studies in our laboratory and others have shown that several IFT proteins (IFT80 and IFT88), IFT motor protein (KIF3A), and other ciliary-related proteins (Evc and polycystin) regulate osteogenesis (Table; reviewed in Yuan et al. 2015). Mouse models with Kif3a (Qiu, Xiao, et al. 2012), Evc2 (Pacheco et al. 2012), or pkd1 (encoding polycystin-1; Qiu et al. 2010) mutations display impaired osteogenesis and bone development. Mutation of Kif3a causes cilium loss in osteoblasts with impaired Hh signaling and Wnt signaling. Silence of Evc2 does not affect cilium formation but inhibits Hh signaling (Pacheco et al. 2012). Those data suggest that ciliary protein regulate Hh signaling transduction in both cilia-dependent and cilia-independent manners. Moreover, loss of pkd1 (Qiu et al. 2010) or Kif3a (Qiu, Xiao, et al. 2012) enhances adipogenesis, indicating that ciliary proteins balance osteogenesis and adipogenesis.
Table.
Primary Cilia and Intraflagellar Transport in Bone and Cartilage Formation.
| Name |
Study |
Reference |
|
|---|---|---|---|
| Bone development | |||
| IFT80 | Majority of IFT80gt/gt(gene-trap line) mice died during embryonic stage, while only 2% of the mice could survive to postnatal stages with multiple bone defects and Hh signaling defect | Rix et al. 2011 | |
| Silencing IFT80 in C3H10T1/2 and bone marrow–derived stromal cells blocks cilia formation and osteoblast differentiation | Yang and Wang 2012 | ||
| OSX-Cre;IFT80f/f mice show decreased bone mass with impaired osteoblast differentiation; loss of IFT80 blocks canonical Hh-Gli signaling transduction while elevating noncanonical Hh-Gαi-RhoA signaling | Yuan et al. 2016 | ||
| IFT88 | Oc-Cre;Ift88 mice show decreased osteoblast number and bone density at 9 mo old on a mixed genetic background, however, on an inbred C57BL/6J genetic background, deletion of Ift88 leads to an increased bone volume/total volume ratio and trabecular thickness at 1 mo old. | Session S. 2010a | |
| Kif3a | Kif3aOc-cKOmice display osteopenia phenotype with impaired osteoblast function; deletion of Kif3a in osteoblasts inhibits osteogenesis but promotes proliferation and adipogenesis; the intracellular calcium response in response to fluid flow shear stress is blocked as well as the Wnt and Hh signaling in Kif3a-deficiency osteoblasts | Qiu, Xiao, et al. 2012 | |
| Kif3aflox/nullmice show normal osteogenesis but impaired adipogenesis with decreased peroxisome proliferator-activated receptor γ expression and increased Gli2 expression | Qiu et al. 2010 | ||
| Colα1(I) 2.3–Cre;Kif3afl/flmice have normal bone development but reduced bone formation in response to a cyclic ulnar loading | Temiyasathit et al. 2012 | ||
| Evc | Evc-/- mice display short limbs and ribs with defective bone collar development, which is associated with impaired osteoblast differentiation and Hh signaling | Pacheco et al. 2012 | |
| Reduced expression of Evc2 in C3H10T1/2 cells inhibits Sonic hedgehog–induced Gli expression and osteogenic differentiation | Dorn et al. 2012 | ||
| PKD | Pkd1null mice display abnormal skeletal phenotypes, including spina bifida occulta and osteochondrodysplasia | Lu et al. 2001 | |
|
Pkd1m1Bei/m1Bei mice are embryonically lethal with a mineralized defect in calvaria and long bones; Pkd1m1Bei/+ mice can survive but have defects in both osteoblast and osteoclast differentiation |
Xiao et al. 2006 | ||
| Oc-Cre;Pkd1flox/m1Bei mice show reduced bone mineral density and impaired osteoblast differentiation with reduced phosphatidylinositol 3-kinase–AKT–GSK3β–β-catenin signaling pathways | Xiao et al. 2010 | ||
| Stable knockdown of PKD1 in MG-63 cells increases cell proliferation and adipogenesis but impairs osteogenesis | Qiu, Zhou,et al. 2012 | ||
| Cartilage development | |||
| IFT80 | Silencing IFT80 in bone marrow–derived stromal cells disrupts chondrogenic differentiation with impaired Hh signaling and enhanced Wnt signaling | Wang et al. 2013 | |
| Deletion of IFT80 in embryonic stage in Col2α1;IFT80f/f model shows shortened cartilage and limbs at birth; deletion of IFT80 in postnatal stage causes reduced growth plate length but increased articular cartilage thickness | Yuan and Yang 2015b | ||
| IFT88 | Tg737orpk mice have defects in appositional and endochondral growth with smaller growth plates and disorganized articular cartilage | McGlashan et al. 2007 | |
| Col2αCre;Ift88fl/flmice display thicker articular cartilage with reduced apoptosis in chondrocytes | Chang and Serra 2013 | ||
| BBS proteins | Bbs mutant mice (Bbs1, Bbs2, or Bbs6) display early signs of osteoarthritis with significantly reduced articular joint thickness and proteoglycan content saturation | Kaushik et al. 2009 | |
| Evc | Evc1-/- mice show advanced chondrocyte maturation in the growth plate and delayed bone collar formation; in the growth plate of Evc1-/-, Ihh expression is normal, but Ihh transduction is blocked | Ruiz-Perez et al. 2007 | |
| Evc1-/- mice show cranial base defects with impaired chondrocyte proliferation and hypertrophy | Pacheco et al. 2012 | ||
| Kif3a | Kif3a;Col2α1-Cre mice show postnatal dwarfism with disorganized growth plate and altered chondrocyte orientation; deletion of Kif3a inhibits cell proliferation but accelerates hypertrophic differentiation, leading to the premature close of growth plate | Song et al. 2007 | |
Hh, hedgehog; IFT, intraflagellar transport; Ihh, Indian Hh.
Presented at American Society for Bone and Mineral Research 2010 Annual Meeting, Toronto, Canada.
Our laboratory uncovered that silence of IFT80 in osteoblast precursor cells disrupts primary cilia and blocks osteoblast differentiation (Yang and Wang 2012). Most recently, we found the novel role of IFT80 in skeletal development using an IFT80 conditional knockout mouse model (Yuan et al. 2016). Osx-Cre-mediated IFT80 deletion in osteoblast precursor cells leads to markedly decreased bone mass with impaired osteoblast differentiation. Interestingly, loss of IFT80 not only blocks canonical Hh-Gli signaling transduction but also elevates cilia-independent noncanonical Hh-Gαi-RhoA signaling by increasing Smo and Gαi binding, resulting in excess stress fiber that further blocks osteoblast differentiation. In addition, inhibiting noncanonic Hh signaling disrupts actin stress fiber and enhances cilium formation in IFT80-deficient osteoblast precursor cells, therefore partially rescuing osteoblast differentiation. These findings suggest that IFT80 regulates osteoblast differentiation by balancing canonical Hh-Gli and noncanonical Hh-Gαi-RhoA pathways (Yuan et al. 2016).
Primary Cilia/IFT in Bone Mechanosensing
Bone adaptively remodels according to the mechanical stimulation, maintaining bone homeostasis (Xiao and Quarles 2015). Recently, Chen et al. (2015) transplanted bone marrow cells from GFP-expressing mice into normal mice that were subjected to mechanical loading. By tracking GFP-positive cells, they demonstrated that biophysical stimulus promotes the homing and attachment of bone marrow cells to bone surface and enhances osteogenic differentiation. Disruption of cilium formation by deleting Kif3a decreases the bone formation upon mechanical stimulation, confirming that primary cilium is a key player in sensing and transducing mechanical signals.
Oscillatory fluid flow (OFF) promotes cyclooxygenase 2 (COX-2) and osteopontin expression and increases prostaglandin E2 (PGE2) secretion in osteoblasts. Disruption of cilia in murine osteoblast MLO-A5 by chloral hydrate inhibits OFF-induced PGE2 release and calcium matrix deposition (Delaine-Smith et al. 2014). Application of OFF to MLO-A5 for 5 consecutive days reduces the cilium length as well as cilium incidence (Delaine-Smith et al. 2014). Cilium disassembly in response to loading has also been reported in chondrocytes, and this mechanism was proposed to avoid overloading (McGlashan et al. 2010). Whether all the bone cells reduce cilium incidence and length in response to loading and how the cilium disassembly is regulated during loading are still unclear.
Osteocytes are thought to be a major player in bone mechanotransduction, as they have greater sensitivity to mechanical stress than osteoblasts. Osteocytes are embedded in the bone matrix and form an interconnected network via gap junction–coupled cell processes passing through canaliculi to sense environment change and transduce signaling (Xiao and Quarles 2015; Fig. 2). Although the precise molecular mechanisms regarding the osteocytes sense that the mechanical stimuli have not yet been fully uncovered, it is believed that mechanical stimulation increases intracellular Ca2+ and that Ca2+ mobilization is required for the release of nitric oxide and prostaglandins (Dallas et al. 2013). Primary cilia have been shown to mediate mechanosensing in osteocyte-like cells. In response to dynamic flow, intracellular levels of cyclic adenosine monophosphate (cAMP) decrease rapidly in MLO-Y4 cells (Malone et al. 2007; Kwon et al. 2010; Fig. 2). Silence of IFT88 blocks cilium formation in osteocytes and inhibits flow-induced cAMP decrease. Further studies highlight the role of adenylyl cyclase 6 (AC6), a membrane-bound enzyme converting adenosine triphosphate (ATP) to cAMP, in mechanotransduction. AC6 is preferentially localized in the primary cilia of MLO-Y4 cells. In response to flow, increased Ca2+ blocks the function of AC6 and decreases the level of cAMP, which promotes the expression of COX-2 (Kwon et al. 2010; Fig. 2).
Figure 2.
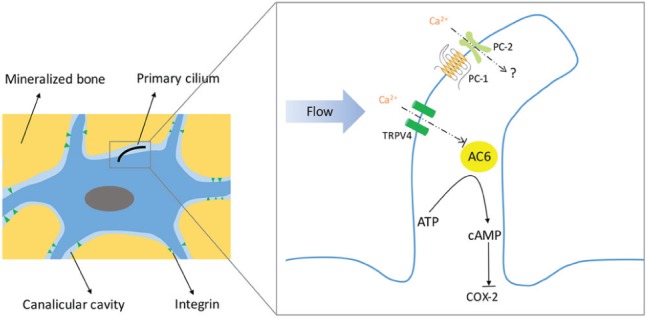
Illustration of the cilium-mediated mechanosensing in osteocytes. Osteocytes reside within the mineralized bone matrix. Primary cilia are projected from osteocytes into lacunar cavity to sense the flow of interstitial fluid generated by mechanical stimuli applied to bone. In response to dynamic flow, cilia deflect, and Ca2+ increases in cilia through transient receptor potential vanilloid 4 (TRPV4). Adenylyl cyclase 6 (AC6), an enzyme converting adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP), is preferentially localized in the primary cilia of osteocytes. Increased Ca2+ level by flow inhibits AC6 function and therefore decreases cAMP, which subsequently increases cyclooxygenase 2 (COX-2) expression and activates osteogenesis. Integrins are transmembrane proteins that form attachments between the osteocyte cell process and the canalicular wall. Integrins connect the cytoskeleton to the extracellular matrix and function as mechanosensors (Kwon et al. 2010; Nguyen and Jacobs 2013; Vaughan et al. 2015).
Most recently, Lee et al. (2015) used a fluorescence resonance energy transfer–based Ca2+ biosensor to monitor Ca2+ in cilia by fusing biosensor to ciliary protein Arl13b. The Ca2+ biosensor has a calmodulin (CaM) region with 4 Ca2+-binding domains. Binding of Ca2+ caused conformational change and increased fluorescence resonance energy transfer signaling. Using this approach, they found that in MLO-Y4 cells, fluid flow increases both ciliary and cytosolic Ca2+ signaling. Primary cilium forms a Ca2+ microdomain that is distinct from the cytosol, and this ciliary Ca2+ microdomain depends on Ca2+ entry through transient receptor potential vanilloid 4 (TRPV4; Fig. 2). TRPV4 also facilitates COX-2 expression in response to flow stimulation. Silence of pkd2 (encoding polycystin-2 [PC-2]) could not block the increase of ciliary Ca2+ and COX-2 expression with flow stimulation (Lee et al. 2015). Their findings suggested that flow-induced ciliary Ca2+ mobilization and mechanotrasduction are dependent on TRPV4, not PC-2, in osteocytes. However, in primary cilia, PC-2 (a Ca2+-permeable channel) colocalizes with polycystin-1 (PC-1, a G protein–coupled receptor) to form a mechanosensory complex, which senses physical change and regulates bone mass in osteoblasts (Xiao and Quarles 2015; Fig. 2). Thus, primary cilia–mediated mechanotransductional mechanism may differ among various cells.
Recently, Vaughan et al. (2015) developed a fluid structure model to predict how integrin and primary cilia interact with the surrounding fluid flow stimulus and transduce mechanical signaling (Fig. 2). Under in vitro fluid flow stimulation, primary cilium deflects and arises large membrane strains at the ciliary base. Longer cilium shows greater increase in cell membrane strain and therefore has more potential to mediate mechanotransduction. The mechanosensor role of primary cilium in vivo depends on its configuration. A short free-standing primary cilium does not serve as a sensor. Only the primary cilium that discretely attaches the lacunar wall could be highly stimulated by flow in vivo. Additionally, integrin attachments can be highly stimulated with flow both in vitro and in vivo. Whether the integrin and primary cilium interact in vivo and whether primary cilium forms an attachment to the extracellular matrix within the lacunar cavity need to be further studied.
Role of Primary Cilia/IFT in Cartilage
Chondrocyte is a unique cell type that produces and maintains the extracellular matrix of cartilage. Condensation of mesenchymal cells leads chondrogenesis, which includes proliferation, maturation, hypertrophy, terminal differentiation, and apoptosis (Kronenberg 2003; Zuscik et al. 2008). The role of primary cilia in chondrocytes—including orientation, extracellular matrix secretion, endocytosis, osmotic response, and apoptosis—has been extensively studied (reviewed in Ruhlen and Marberry 2014 and Yuan et al. 2015).
Primary Cilia/IFT in Cartilage Development
IFT proteins (IFT80 and IFT88), IFT motor proteins (KIF3A), and ciliary proteins (Evc and BBS proteins) have been found to regulate chondrocyte differentiation and cartilage development (Table; reviewed in Yuan et al. 2015). Deletion of IFT88 in chondrocyte lineage (Col2αCre;ift88fl/fl mouse model) increases thickness of articular cartilage with reduced apoptosis in chondrocytes (Chang et al. 2012). The mechanical properties of cartilage, particularly in the deeper zones, is significantly reduced (Tummala et al. 2010). Moreover, IFT88 regulates cytoskeleton in the articular cartilage, and IFT88orpk mice (hypomorphic mutation of IFT88) display increased F-actin staining in the proliferative zone of the growth plate (McGlashan et al. 2007). Chondrocytes from IFT88orpk mice consistently display increased acto-myosin stress fiber organization when compared with that in normal chondrocytes. Micropipette aspiration in conjunction with the standard linear solid model shows reduced membrane bleb formation in IFT88orpk chondrocytes, followed by reduced reformation rate of the actin cortex, demonstrating that IFT88 regulates actin organization and the stiffness of the actin cortex (Wang et al. 2016). However, IFT88 deficiency causes cilium loss, which might also contribute to the altered cytoskeletal character in IFT88orpk chondrocytes.
Besides IFT88, our laboratory studied the role of IFT80 in cartilage development (Yuan and Yang 2015b). Mutation of IFT80 was found in Jeune asphyxiating thoracic dystrophy or short rib–polydactyly syndrome type III with narrow thoracic cavity and cartilage defects. We used a Col2α1-CreER;IFT80f/f mouse model to study the role of IFT80 in cartilage development in embryonic and postnatal stages by injection of tamoxifen at different time points. Deletion of IFT80 disrupts cilium formation in cartilage, alters Hh and Wnt signaling, and eventually blocks chondrocyte differentiation. IFT80-deficient mice show shortened cartilage and, therefore, short limbs at birth. Deletion of IFT80 in the postnatal stage causes reduced growth plate length but increased articular cartilage thickness (Yuan and Yang 2015b). The aberrant Hh and Wnt signaling transduction was also found in the growth plate of Col2αCre;Ift88fl/fl mice (Chang and Serra 2013). Secreted frizzled-related protein 5 (Sfrp5, an extracellular antagonist of Wnt signaling pathway) was suggested to bridge the Hh and Wnt signaling in chondrocytes, as disruption of Hh signaling reduces the expression of Sfrp5, which subsequently upregulates Wnt signaling (Chang and Serra 2013). Both IFT88orpk mice (McGlashan et al. 2007) and Col2αCre;Ift88fl/fl mice (Song et al. 2007) display cilium loss and smaller growth plates, similar to Col2α1-CreER;IFT80f/f mouse. Therefore, it is possible that these cartilage abnormalities are due to cilium loss. However, it is worth noting that IFT80 gene trap mice with lower levels of IFT80 expression and normal cilium formation also display defects in growth plate and long bone formation (Rix et al. 2011), indicating that IFT80 has its unique function independent of the cilia.
Primary Cilia/IFT in Chondrocyte Mechanosensing
Primary cilia play an important role in mechanotransduction in chondrocytes. Most recently, He et al. (2015) found that moderate mechanical loading inhibits matrix metalloproteinase 1 (MMP-1) and MMP-13 expression by activating Cbp/p300 interacting transactivator with ED-rich tail 2 (CITED2; He et al. 2015). Strain activates CITED2 in a primary cilia–dependent way in human chondrocytes. Knockdown of IFT88 in articular chondrocytes by intra-articular injection of IFT88 siRNA diminishes treadmill-induced CITED2 expression. Further study showed that extracellular ATP, P2 purinergic receptor, Ca2+ signaling, ERK1/2 phosphorylation, and transcription factors hypoxia-inducible factor 1α (HIF1α) and specificity protein 1 are also involved in strain-induced CITED2 activation. A putative shear stress response element (SSRE) was identified in the CITED2 promoter region and proved to participate in strain-induced CITED2 transactivation. The proposed CITED2 activation in chondrocytes is summarized in Figure 3.
Figure 3.
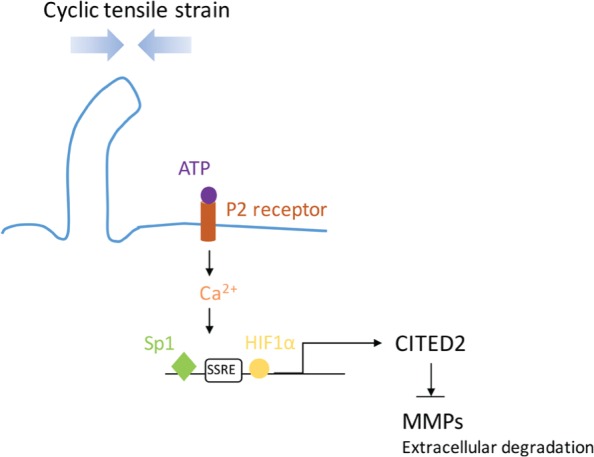
Proposed CITED2 (Cbp/p300 interacting transactivator with ED-rich tail 2)–mediated matrix metalloproteinase (MMP) regulation during moderate mechanical loading. Cyclic tensile strain is sensed by primary cilia and transduced by extracellular ATP-induced P2 purinergic receptors and calcium signaling, which, together with hypoxia-inducible factor 1α (HIF1α) and specificity protein 1 (Sp1), transactivate CITED2. CITED2 activation inhibits MMP expression to protect extracellular matrix degradation. Adapted from He et al. (2015).
In adult bovine articular chondrocytes, cyclic tensile strain (10% strain) activates Hh signaling and promotes the expression of A disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS-5; a catabolic enzyme that causes the degeneration of cartilage) in primary cilia–dependent manner. High strain significantly induces histone deacetylase 6 (a tubulin deacetylase)–mediated cilium disassembly and abolishes loading-induced Hh activation and ADAMTS-5 expression (Thompson et al. 2014). A conflict result was reported recently by Rais et al. (2015). They found that the ciliogenesis is significantly increased in the growth plate of young chickens harnessed with small bags weighing 10% of their body weight for 4 d, suggesting that cilium formation is enhanced in response to loading. Whether the contrary observation is caused by different loading systems and/or strain needs to be further studied.
Role of Primary Cilia/IFT in Bone Diseases
Primary Cilia/IFT in Bone Tumors
Bone tumors range from benign tumor, such as osteochondroma, to malignant chondrosarcoma (Bovee et al. 2010). Osteochondroma is the most common benign bone tumor. It forms along the growth plate in bone and cartilage and has similar histologic features in growth plate (Benoist-Lasselin et al. 2006; de Andrea et al. 2010). The development of osteochondroma is a process of misorientation (de Andrea et al. 2015), and primary cilium has been considered as a regulator of chondrocyte orientation (Yuan et al. 2015). Therefore, the relationship between cilia and osteochondroma has been studied. Cilium formation is normal within osteochondroma, but the cilium orientation largely alters as compared with normal growth plate, in which polarized chondrocytes in proliferating and hypertrophic zones orient the cilia parallel to the growth axis (Fig. 4). This unique orientation of cilia in chondrocytes is disrupted in osteochondromas, as primary cilia randomly locate on the cell surface in most chondrocytes in osteochondromas, which might contribute to the loss of chondrocyte rotation and stacked columns (Fig. 4; de Andrea et al. 2010).
Figure 4.
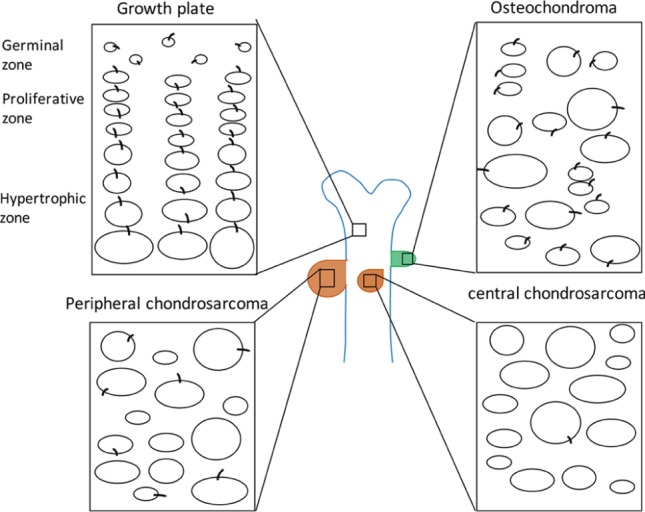
Schematic representation shows the cilium orientation in the growth plate and bone tumors. The growth plate is highly organized and divided into 3 distinct regions. In the germinal zone (or resting zone), there are nonpolarized committed stem cells with irregularly arranged cilia. In the proliferative zone, chondrocytes undergo rapid proliferation but develop into orderly columns. In the hypertrophic zone, chondrocytes stop proliferation, increase in size, and prepare for mineralization of the surrounding matrix. In both the proliferative and hypertrophic zones, the cilium orientation is parallel to the longitudinal axis of the bone, which represents the axis of chondrocyte polarity inside the growth plate. However, in osteochondroma, chondrocytes are irregularly arranged without acquired columnar organization. Hypertrophic-like chondrocytes are found within the resting and proliferative zones. Cilia are randomly located on the cell surface (de Andrea et al. 2010; de Andrea and Hogendoorn 2012). In human malignant chondrosarcomas, cilium incidence in neoplastic chondrocytes is only 12.4% (Ho et al. 2013). In the mouse peripheral chondrosarcoma, primary cilia are absent in the majority of chondrocytes (de Andrea et al. 2015).
Chondrosarcoma is a malignant tumor and exhibits lobular and invasive growth patterns. In human chondrosarcomas, the percentage of ciliated neoplastic chondrocytes is only 12.4%, which is a very low frequency as compared with 67.7% in normal articular cartilage (Ho et al. 2013). In the mouse peripheral chondrosarcoma, primary cilia are absent on the majority of chondrocytes, which might contribute to the loss of polarity of chondrocytes in peripheral chondrosarcoma (de Andrea et al. 2015). Compared with chondrosarcoma, osteochondroma does not show invasive growth patterns. The growth of osteochondromas stops when the growth plate closes (Bovee et al. 2010). Primary cilia are present on the osteochondroma chondrocytes but just lose their orientation. However, in chondrosarcoma, decreased cilium incidence might further contribute to the invasive tumor growth (Fig. 4). These observations suggested that primary cilia might serve as a biomarker to distinguish the low- and high-grade bone tumors.
Hh gradient is important for chondrocyte proliferation and differentiation. In osteochondroma, Hh gradient is disrupted, showing homogeneous pattern (Benoist-Lasselin et al. 2006). Chondrocyte-specific overexpression of Gli2 (Col2α1-Gli2, activated Hh signaling in growth plate) leads to cartilage tumors in mice (Ho et al. 2013). Similar to Col2α1-Gli2 mice, IFT88+/- mice (partial loss IFT88 leads a partial loss of ciliogenesis) also develop cartilage tumors. More evidence demonstrates that the disruption of cilia (by decreasing IFT88 expression or treating with choral hydrate) in Gli2-overexpressed chondrosarcomas results in further activation of the Hh signaling pathway and promotes tumor growth, suggesting that cilia act as an inhibitor of Hh signaling in neoplastic chondrocytes (Ho et al. 2013). A recent study suggested that loss of primary cilia in chondrocytes creates a proliferative block and might help to select those cells that have lost cell-cycle regulatory genes, which promotes tumor growth (de Andrea et al. 2015). These results are controversial with the previously suggested inhibitory role of cilia in malignant tumors. How cilia are involved in bone tumor progress needs to be carefully investigated in the future.
Primary Cilia/IFT in Osteoarthritis
Osteoarthritis (OA) is a chronic joint disease characterized by articular cartilage degeneration (McGlashan et al. 2008). Primary cilia are present on mild and severe OA tissue. However, the incidence and length of the cilia increase in the eroding articulating surface (McGlashan et al. 2008), which is associated with increased Hh signaling (Lin et al. 2009). In addition, the orientation of cilia is changed in the surface of articular cartilage in OA. Instead of clearly being oriented away from the articulating surface, osteoarthritic cells have their primary cilia oriented toward the center of abnormal cell clusters (Fig. 5; McGlashan et al. 2008). The relationship between cilium misorientation and cartilage degradation is still unknown.
Figure 5.
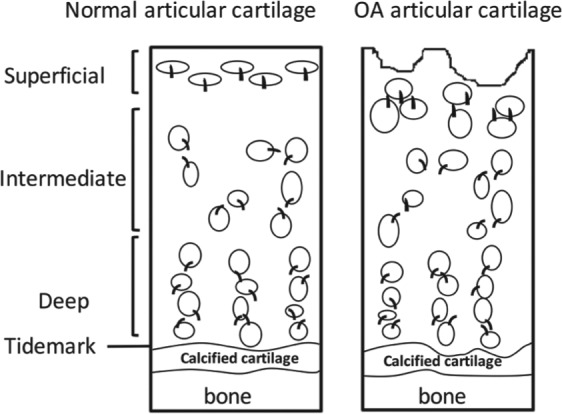
Cilium orientation of normal and osteoarthritis (OA) articular cartilage chondrocytes. The articular cartilage is divided into superficial, intermediate, and deep zones, with the tidemark and calcified cartilage, which is connected to the bone. In the superficial zone of normal articular cartilage, the chondrocytes are ellipsoid and parallel to the articular surface. The cilia are consistently oriented away from the articular surface. Chondrocytes often form a pair of chondrons in the intermediate zone, and chondrocytes are usually perpendicular to the articular surface in the deep zone. The cilium orientation in the intermediate and deep zone is either between 2 cells within the chondron or on the medial or lateral cell membranes along the longitudinal axis parallel to the long bone. However, in the OA articular cartilage, the smooth surface becomes pitted and frayed. Chondrocytes were present in clusters along the eroding surface. Cilia are oriented toward the center of the cluster at the eroding surface. The cilia orientation in the deep zone of OA cartilage is similar to that in normal cartilage (McGlashan et al. 2008).
Interestingly, deletion of IFT88 in cartilage (IFT88;Col2α-Cre mouse model) causes OA phenotype with reduced stiffness and upregulated expression levels of OA markers, including MMP-13, Adamts5, ColX, and Runx2 (Chang et al. 2012). Further study showed that primary cilia are required for processing full-length Gli3 to its repressor form. Loss of Gli3 repressor leads to upregulation of Hh signaling, thus causing the OA in IFT88;Col2α-Cre mice (Chang et al. 2012). Modulation of Hh signaling by pharmacologic or genetic inhibition of Hh signaling reduces the severity of OA (Lin et al. 2009). However, a recent study suggested that Indian Hh (Ihh) does not cause extracellular matrix degradation in healthy cartilage even with the inflammatory cytokine interleukin-1β (IL-1β; Thompson et al. 2015). Therefore, the role of Hh in OA pathogenesis needs to be further clarified. Although it is well known that primary cilia are essential for transducing Hh signaling, how primary cilia are involved in Hh signaling transduction in the OA is largely unknown.
IL-1, highly expressed in OA, induces the elongation of the cilia via a PKA-dependent mechanism in chondrocytes (Wann and Knight 2012). In addition, IL-1 stimulates the secretion of nitric oxide, PGE2, and inflammatory chemokines. However, inhibition of IL-1-induced cilium elongation by PKA inhibitor or cilium disruption (deleting IFT88) significantly attenuates IL-1-induced inflammatory response in chondrocytes (Wann and Knight 2012). Interestingly, cilium elongation in response to IL-1 is companied with a transient HIF-2α accumulation in cilia (Wann et al. 2013). The ciliary sequestration of HIF-2α provides a negative regulation of the HIF signaling pathway during inflammation (Wann et al. 2013). These results provide the evidence that primary cilium takes part in an inflammatory response and could be a novel therapeutic target for inflammatory disease such as OA.
Primary Cilia/IFT in Tooth Development
In addition to bone and cartilage, primary cilia have been found on periodontal ligament stem cells, dental pulp stem cells, ameloblasts, and odontoblasts (Haycraft and Serra 2008; Thivichon-Prince et al. 2009; Martinez et al. 2011), and growing studies have begun to uncover the role of primary cilia in tooth development.
Very interestingly, IFT88orpk mice develop an ectopic molar mesial to the first molar that correlates with increased Shh signaling in the diastema mesenchyme, suggesting the role of primary cilia in tooth patterning (Zhang et al. 2003; Ohazama et al. 2009). Dental anomalies have consistently been reported in several ciliopathies, such as Ellis–van Creveld syndrome (Haycraft and Serra 2008; Nakatomi et al. 2013). Evc-/- mice develop smaller first molars (Ruiz-Perez et al. 2007), resulting from the progressive loss of Shh signaling that causes delayed differentiation (Nakatomi et al. 2013). Deletion of Kif3a in dental mesenchyme (Wnt1-Cre;Kif3afl/fl) totally disrupts incisor development but enlarges molars with reduced Hh signaling and increased Wnt signaling, implying that primary cilia balance the Hh and Wnt signaling between dental epithelia and mesenchyme for tooth development (Kolpakova-Hart et al. 2007; Liu et al. 2014). Our laboratory found that IFT80 regulates odontoblast proliferation in molar root development. Deletion of IFT80 in dental pulp stem cells disrupts osteogenic differentiation as well as fibroblast growth factor–induced proliferation (unpublished data). Future study should address how primary cilia are involved in the differentiation of ameloblasts and odontoblasts.
Primary cilia were found to align parallel to the dentin walls, with the cilium tip pointed toward the dental pulp (Magloire et al. 2004; Thivichon-Prince et al. 2009). Moreover, dentinal fluid flow keeps dentin healthy. However, it is not clear how this cilium orientation is formed and maintained and whether primary cilia sense dentinal fluid and transduce the calcium signaling. Although primary cilia were proposed to be involved in tooth pain transmission because they are close to nerve fibers (Magloire et al. 2004), currently there is no direct evidence showing the interaction between primary cilia and nerve fibers.
Concluding Remarks
The past decade has witnessed remarkable progress in uncovering the role of primary cilia in bone and cartilage development and functionality. Despite substantial progress in discovering the essential functions of primary cilia and IFT proteins in bone cells, little is known about the involved signaling pathways. Most of the current studies are focused on the Hh and Wnt signaling. How cilia regulate other pathways, such as fibroblast growth factor, platelet-derived growth factor, TGF-β, and Notch signaling in bone and cartilage, is largely unknown. Another challenging issue is detecting primary cilia in bone cells within mineralized bone tissue. A recent immunohistochemistry study showed that primary cilia are present in approximately 4% of osteocytes, 4.6% of bone-lining cells, and 1% bone marrow cells of ovine cervical vertebrae (Coughlin et al. 2015). However, an early study suggested that primary cilia are present on 94% of the osteocytes of young rats (Uzbekov et al. 2012). Whether the conflict results are caused by different species or different technical approaches needs to be clarified in the future.
In conclusion, this review reconfirms the essential role of primary cilia in skeleton development and maintenance; however, future studies are still needed to uncover the molecular mechanisms that eventually lead to the finding of therapeutic targets for bone and other diseases.
Author Contributions
X. Yuan, S. Yang, contributed to conception, design, and data acquisition, drafted and critically revised the manuscript. Both authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
The authors acknowledge the researchers whose work has had a direct association with this review but could not be cited due to limitations imposed by journal guidelines.
Footnotes
Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Institute of Aging, part of the National Institutes of Health (awards DE023105, AR066101, and AG048388 to S.Y.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Benoist-Lasselin C, de Margerie E, Gibbs L, Cormier S, Silve C, Nicolas G, LeMerrer M, Mallet JF, Munnich A, Bonaventure J, et al. 2006. Defective chondrocyte proliferation and differentiation in osteochondromas of mhe patients. Bone. 39(1):17–26. [DOI] [PubMed] [Google Scholar]
- Bodle JC, Rubenstein CD, Phillips ME, Bernacki SH, Qi J, Banes AJ, Loboa EG. 2013. Primary cilia: the chemical antenna regulating human adipose-derived stem cell osteogenesis. PloS One. 8(5):e62554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovee JV, Hogendoorn PC, Wunder JS, Alman BA. 2010. Cartilage tumours and bone development: molecular pathology and possible therapeutic targets. Nat Rev Cancer. 10(7):481–488. [DOI] [PubMed] [Google Scholar]
- Chang CF, Ramaswamy G, Serra R. 2012. Depletion of primary cilia in articular chondrocytes results in reduced Gli3 repressor to activator ratio, increased hedgehog signaling, and symptoms of early osteoarthritis. Osteoarthritis Cartilage. 20(2):152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CF, Serra R. 2013. Ift88 regulates hedgehog signaling, Sfrp5 expression, and beta-catenin activity in post-natal growth plate. J Orthop Res. 31(3):350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Hoey DA, Chua M, Bellon R, Jacobs CR. 2015. Mechanical signals promote osteogenic fate through a primary cilia-mediated mechanism. FASEB J. 30(4):1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin TR, Voisin M, Schaffler MB, Niebur GL, McNamara LM. 2015. Primary cilia exist in a small fraction of cells in trabecular bone and marrow. Calcif Tissue Int. 96(1):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas SL, Prideaux M, Bonewald LF. 2013. The osteocyte: an endocrine cell . . . and more. Endocr Rev. 34(5):658–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andrea CE, Hogendoorn PC. 2012. Epiphyseal growth plate and secondary peripheral chondrosarcoma: the neighbours matter. J Pathol. 226(2):219–228. [DOI] [PubMed] [Google Scholar]
- de Andrea CE, Wiweger M, Prins F, Bovee JV, Romeo S, Hogendoorn PC. 2010. Primary cilia organization reflects polarity in the growth plate and implies loss of polarity and mosaicism in osteochondroma. Lab Invest. 90(7):1091–1101. [DOI] [PubMed] [Google Scholar]
- de Andrea CE, Zhu JF, Jin H, Bovee JV, Jones KB. 2015. Cell cycle deregulation and mosaic loss of ext1 drive peripheral chondrosarcomagenesis in the mouse and reveal an intrinsic cilia deficiency. J Pathol. 236(2):210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaine-Smith RM, Sittichokechaiwut A, Reilly GC. 2014. Primary cilia respond to fluid shear stress and mediate flow-induced calcium deposition in osteoblasts. FASEB J. 28(1):430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn KV, Hughes CE, Rohatgi R. 2012. A smoothened-Evc2 complex transduces the hedgehog signal at primary cilia dev. Cell. 23:823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, Serra R. 2008. Cilia involvement in patterning and maintenance of the skeleton. Curr Top Dev Biol. 85:303–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Leong D, Zhuo Z, Majeska R, Cardoso L, Spray D, Goldring M, Cobelli N, Sun H. 2015. Strain-induced mechanotransduction through primary cilia, extracellular ATP, purinergic calcium signaling, and ERK1/2 transactivates CITED2 and downregulates MMP-1 and MMP-13 gene expression in chondrocytes. Osteoarthritis Cartilage. 24(5):892–901. [DOI] [PubMed] [Google Scholar]
- Ho L, Ali SA, Al-Jazrawe M, Kandel R, Wunder JS, Alman BA. 2013. Primary cilia attenuate hedgehog signalling in neoplastic chondrocytes. Oncogene. 32(47):5388–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. 2003. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 426(6962):83–87. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Marshall WF. 2011. Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol. 12(4):222–234. [DOI] [PubMed] [Google Scholar]
- Katoh Y, Terada M, Nishijima Y, Takei R, Nozaki S, Hamada H, Nakayama K. 2016. Overall architecture of the intraflagellar transport (IFT)-B complex containing Cluap1/IFT38 as an essential component of the IFT-B peripheral subcomplex. J Biol Chem. 291:10962–10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik AP, Martin JA, Zhang Q, Sheffield VC, Morcuende JA. 2009. Cartilage abnormalities associated with defects of chondrocytic primary cilia in Bardet-Biedl syndrome mutant mice. J Orthop Res. 2009;27(8):1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpakova-Hart E, Jinnin M, Hou B, Fukai N, Olsen BR. 2007. Kinesin-2 controls development and patterning of the vertebrate skeleton by Hedgehog- and Gli3-dependent mechanisms. Dev Biol. 309(2):273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg HM. 2003. Developmental regulation of the growth plate. Nature. 423(6937):332–336. [DOI] [PubMed] [Google Scholar]
- Kwon RY, Temiyasathit S, Tummala P, Quah CC, Jacobs CR. 2010. Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic amp in bone cells. FASEB J. 24(8):2859–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KL, Guevarra MD, Nguyen AM, Chua MC, Wang Y, Jacobs CR. 2015. The primary cilium functions as a mechanical and calcium signaling nexus. Cilia. 4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AC, Seeto BL, Bartoszko JM, Khoury MA, Whetstone H, Ho L, Hsu C, Ali SA, Alman BA. 2009. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat Med. 15(12):1421–1425. [DOI] [PubMed] [Google Scholar]
- Liu B, Chen S, Cheng D, Jing W, Helms JA. 2014. Primary cilia integrate hedgehog and wnt signaling during tooth development. J Dent Res. 93(5):475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Shen X, Pavlova A, Lakkis M, Ward CJ, Pritchard L, Harris PC, Genest DR, Perez-Atayde AR, Zhou J. 2001. Comparison of Pkd1-targeted mutants reveals that loss of polycystin-1 causes cystogenesis and bone defects. Hum Mol Genet. 10:2385–2396. [DOI] [PubMed] [Google Scholar]
- Magloire H, Couble M-L, Romeas A, Bleicher F. 2004. Odontoblast primary cilia: facts and hypotheses. Cell Biol Int. 28(2):93–99. [DOI] [PubMed] [Google Scholar]
- Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, Jacobs CR. 2007. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 104(33):13325–13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez C, Smith PC, Rodriguez JP, Palma V. 2011. Sonic hedgehog stimulates proliferation of human periodontal ligament stem cells. J Dent Res. 90(4):483–488. [DOI] [PubMed] [Google Scholar]
- McGlashan SR, Cluett EC, Jensen CG, Poole CA. 2008. Primary cilia in osteoarthritic chondrocytes: from chondrons to clusters. Dev Dyn. 237(8):2013–2020. [DOI] [PubMed] [Google Scholar]
- McGlashan SR, Haycraft CJ, Jensen CG, Yoder BK, Poole CA. 2007. Articular cartilage and growth plate defects are associated with chondrocyte cytoskeletal abnormalities in Tg737orpk mice lacking the primary cilia protein polaris. Matrix Biol. 26(4):234–246. [DOI] [PubMed] [Google Scholar]
- McGlashan SR, Knight MM, Chowdhury TT, Joshi P, Jensen CG, Kennedy S, Poole CA. 2010. Mechanical loading modulates chondrocyte primary cilia incidence and length. Cell Biol Int. 34(5):441–446. [DOI] [PubMed] [Google Scholar]
- Nakatomi M, Hovorakova M, Gritli-Linde A, Blair HJ, MacArthur K, Peterka M, Lesot H, Peterkova R, Ruiz-Perez VL, Goodship JA, et al. 2013. Evc regulates a symmetrical response to Shh signaling in molar development. J Dent Res. 92(3):222–228. [DOI] [PubMed] [Google Scholar]
- Nguyen AM, Jacobs CR. 2013. Emerging role of primary cilia as mechanosensors in osteocytes. Bone. 54(2):196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohazama A, Haycraft CJ, Seppala M, Blackburn J, Ghafoor S, Cobourne M, Martinelli DC, Fan CM, Peterkova R, Lesot H, et al. 2009. Primary cilia regulate Shh activity in the control of molar tooth number. Development. 136(6):897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco M, Valencia M, Caparros-Martin JA, Mulero F, Goodship JA, Ruiz-Perez VL. 2012. Evc works in chondrocytes and osteoblasts to regulate multiple aspects of growth plate development in the appendicular skeleton and cranial base. Bone. 50(1):28–41. [DOI] [PubMed] [Google Scholar]
- Qiu N, Cao L, David V, Quarles LD, Xiao Z. 2010. Kif3a deficiency reverses the skeletal abnormalities in Pkd1 deficient mice by restoring the balance between osteogenesis and adipogenesis. PloS One. 5(12):e15240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu N, Xiao Z, Cao L, Buechel MM, David V, Roan E, Quarles LD. 2012. Disruption of Kif3a in osteoblasts results in defective bone formation and osteopenia. J Cell Sci. 125(Pt 8): 1945–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu N, Zhou H, Xiao Z. 2012. Downregulation of PKD1 by shRNA results in defective osteogenic differentiation via cAMP/PKA pathway in human MG-63 cells. J Cell Biochem. 2012;113(3):967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rais Y, Reich A, Simsa-Maziel S, Moshe M, Idelevich A, Kfir T, Miosge N, Monsonego-Ornan E. 2015. The growth plate’s response to load is partially mediated by mechano-sensing via the chondrocytic primary cilium. Cell Mol Life Sci. 72(3):597–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rix S, Calmont A, Scambler PJ, Beales PL. 2011. An Ift80 mouse model of short rib polydactyly syndromes shows defects in hedgehog signalling without loss or malformation of cilia. Hum Mol Genet. 20(7):1306–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhlen R, Marberry K. 2014. The chondrocyte primary cilium. Osteoarthritis Cartilage. 22(8):1071–1076. [DOI] [PubMed] [Google Scholar]
- Ruiz-Perez VL, Blair HJ, Rodriguez-Andres ME, Blanco MJ, Wilson A, Liu YN, Miles C, Peters H, Goodship JA. 2007. Evc is a positive mediator of ihh-regulated bone growth that localises at the base of chondrocyte cilia. Development. 134(16):2903–2912. [DOI] [PubMed] [Google Scholar]
- Song B, Haycraft CJ, Seo HS, Yoder BK, Serra R. 2007. Development of the post-natal growth plate requires intraflagellar transport proteins. Dev Biol. 305(1):202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschner M, Bhogaraju S, Lorentzen E. 2012. Architecture and function of IFT complex proteins in ciliogenesis. Differentiation. 83(2):S12–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschner M, Weber K, Mourao A, Vetter M, Awasthi M, Stiegler M, Bhogaraju S, Lorentzen E. 2016. Intraflagellar transport proteins 172, 80, 57, 54, 38, and 20 form a stable tubulin-binding IFT-B2 complex. EMBO J. 35(7):773–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temiyasathit S, Tang WJ, Leucht P, Anderson CT, Monica SD, Castillo AB, Helms JA, Stearns T, Jacobs CR. 2012. Mechanosensing by the primary cilium: deletion of Kif3A reduces bone formation due to loading. PLoS One. 7(3):e33368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thivichon-Prince B, Couble ML, Giamarchi A, Delmas P, Franco B, Romio L, Struys T, Lambrichts I, Ressnikoff D, Magloire H, et al. 2009. Primary cilia of odontoblasts: possible role in molar morphogenesis. J Dent Res. 88(10):910–915. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Chapple JP, Knight MM. 2014. Primary cilia disassembly down-regulates mechanosensitive hedgehog signalling: a feedback mechanism controlling ADAMTS-5 expression in chondrocytes. Osteoarthritis Cartilage. 22(3):490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CL, Patel R, Kelly T-AN, Wann AK, Hung CT, Chapple JP, Knight MM. 2015. Hedgehog signalling does not stimulate cartilage catabolism and is inhibited by interleukin-1β. Arthritis Res Ther. 17(1):373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummala P, Arnsdorf EJ, Jacobs CR. 2010. The role of primary cilia in mesenchymal stem cell differentiation: a pivotal switch in guiding lineage commitment. Cell Mol Bioeng. 3(3):207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzbekov RE, Maurel DB, Aveline PC, Pallu S, Benhamou CL, Rochefort GY. 2012. Centrosome fine ultrastructure of the osteocyte mechanosensitive primary cilium. Microsc Microanal. 18(6):1430–1441. [DOI] [PubMed] [Google Scholar]
- Vaughan TJ, Mullen CA, Verbruggen SW, McNamara LM. 2015. Bone cell mechanosensation of fluid flow stimulation: a fluid-structure interaction model characterising the role integrin attachments and primary cilia. Biomech Model Mechanobiol. 14(4):703–718. [DOI] [PubMed] [Google Scholar]
- Wang C, Yuan X, Yang S. 2013. IFT80 is essential for chondrocyte differentiation by regulating hedgehog and Wnt signaling pathways. Exp Cell Res. 319(5):623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wann AK, Thompson CL, Hassen A, Wang W, Knight MM. 2016. IFT88 influences chondrocyte actin organization and biomechanics. Osteoarthritis Cartilage. 24(3):544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wann AK, Knight MM. 2012. Primary cilia elongation in response to interleukin-1 mediates the inflammatory response. Cell Mol Life Sci. 69(17):2967–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wann AK, Thompson CL, Chapple JP, Knight MM. 2013. Interleukin-1beta sequesters hypoxia inducible factor 2alpha to the primary cilium. Cilia. 2(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Quarles LD. 2015. Physiological mechanisms and therapeutic potential of bone mechanosensing. Rev Endocr Metab Disord. 16(2):115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Zhang S, Cao L, Qiu N, David V, Quarles LD. 2010. Conditional disruption of Pkd1 in osteoblasts results in osteopenia due to direct impairment of bone formation. J Biol Chem. 285:1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Zhang S, Mahlios J, Zhou G, Magenheimer BS, Guo D, Dallas SL, Maser R, Calvet JP, Bonewald L, et al. 2006. Cilia-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression. J Biol Chem. 281:30884–30895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Wang C. 2012. The intraflagellar transport protein ift80 is required for cilia formation and osteogenesis. Bone. 51(3):407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Cao J, He X, Serra R, Qu J, Cao X, Yang S. 2016. Ciliary ift80 balances canonical versus non-canonical hedgehog signalling for osteoblast differentiation. Nature Commun. 7:11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Serra RA, Yang S. 2015. Function and regulation of primary cilia and intraflagellar transport proteins in the skeleton. Ann N Y Acad Sci. 1335:78–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Yang S. 2015a. Cilia/IFT protein and motor-related bone diseases and mouse models. Front Biosci. 20:515–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Yang S. 2015b. Deletion of IFT80 impairs epiphyseal and articular cartilage formation due to disruption of chondrocyte differentiation. PloS One. 10(6):e0130618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Murcia NS, Chittenden LR, Richards WG, Michaud EJ, Woychik RP, Yoder BK. 2003. Loss of the Tg737 protein results in skeletal patterning defects. Dev Dyn. 227(1):78–90. [DOI] [PubMed] [Google Scholar]
- Zuscik MJ, Hilton MJ, Zhang X, Chen D, O’Keefe RJ. 2008. Regulation of chondrogenesis and chondrocyte differentiation by stress. J Clin Invest. 118(2):429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]