Abstract
Fimbriae are protein-based filamentous appendages that protrude from the bacterial cell surface and facilitate host adhesion. Two types of fimbriae, FimA and Mfa1, of the periodontal pathogen Porphyromonas gingivalis are responsible for adherence to other bacteria and to host cells in the oral cavity. Both fimbrial forms are composed of 5 proteins, but there is limited information about their polymerization mechanisms. Here, the authors evaluated the function of Mfa5, one of the Mfa1 fimbrial accessory proteins. Using mfa5 gene disruption and complementation studies, the authors revealed that Mfa5 affects the incorporation of other accessory proteins, Mfa3 and Mfa4, into fibers and the expression of fimbriae on the cell surface. Mfa5 is predicted to have a C-terminal domain (CTD) that uses the type IX secretion system (T9SS), which is limited to this organism and related Bacteroidetes species, for translocation across the outer membrane. To determine the relationship between the putative Mfa5 CTD and the T9SS, mutants were constructed with in-frame deletion of the CTD and deletion of porU, a C-terminal signal peptidase linked to T9SS-mediated secretion. The ∆CTD-expressing strain presented a similar phenotype to the mfa5 disruption mutant with reduced expression of fimbriae lacking all accessory proteins. The ∆porU mutants and the ∆CTD-expressing strain showed intracellular accumulation of Mfa5. These results indicate that Mfa5 function requires T9SS-mediated translocation across the outer membrane, which is dependent on the CTD, and subsequent incorporation into fibers. These findings suggest the presence of a novel polymerization mechanism of the P. gingivalis fimbriae.
Keywords: periodontal disease, microbiology, bacterial virulence, bacteria, biofilm, molecular biology
Introduction
Periodontal disease is a chronic gingival inflammatory disease caused by a multispecies biofilm in the gingival crevice (Socransky and Haffajee 2002). Porphyromonas gingivalis, a gram-negative anaerobic bacterium, is a component of dental plaque biofilms in humans and plays a key role in the initiation and progression of chronic periodontitis (Hajishengallis and Lamont 2012; Hajishengallis 2015). P. gingivalis has many virulence factors, including fimbriae, arginine- (Rgp) and lysine-gingipains (Kgp) (cysteine proteases), and lipopolysaccharides (Lamont and Jenkinson 1998). Fimbriae are protein-based filamentous appendages that protrude from the cell surface and facilitate adhesion to host cells or tissues and to other bacteria. P. gingivalis expresses 2 forms of fimbriae, FimA and Mfa1 (Yoshimura et al. 2009), which regulate attachment to oral commensal bacteria, biofilm formation, and interaction with host cells (Amano 2003).
The 2 fimbrial types are genetically distinct and expressed from separate gene clusters (Yoshimura et al. 2009). Despite very low sequence similarity, they have similar architecture and each comprise 5 proteins: proteins FimA–E for FimA and proteins Mfa1–5 for Mfa1 (Fig. 1A). Mfa1, the first protein encoded by the mfa gene cluster, is the major subunit and polymerizes into the fibrillar shaft. We reported that Mfa2, the second protein, is an outer membrane protein that regulates fimbrial length and identified Mfa3–5 as accessory proteins associated with Mfa1 fimbriae (Hasegawa et al. 2009). Mfa3 was reported to be located at the fimbrial tip with the Mfa3–5 complex functioning as an adhesin (Hasegawa et al. 2013). Direct mutagenesis of the mfa3 and mfa4 genes individually led to the absence of all accessory proteins in fimbriae, suggesting that Mfa3 and Mfa4 are required to form the accessory protein complex on the Mfa1 fimbrial fiber (Hasegawa et al. 2013; Ikai et al. 2015). Because mfa4 deficiency caused leakage of Mfa5 into the culture supernatant without incorporation into fibers, Mfa4 was suggested to interact with Mfa5 during their integration (Ikai et al. 2015). However, little was known about the assembly mechanism or how these fimbrial proteins are translocated to the outer membrane and polymerized in Mfa1 fimbriae. Understanding of fimbrial polymerization in general is based on the Escherichia coli type 1 fimbriae, which polymerize via a chaperone-usher mechanism (Thanassi et al. 1998; Gerlach and Hensel 2007). Upon polymerization, which is assisted by the membrane-bound usher, the chaperone β-strand is displaced by a donor strand from the next fimbrial subunit. In P. gingivalis, several of the fimbrial proteins are processed in 2 steps: first by signal peptidase II, which removes the signal peptide, and second by Rgp, which cleaves the proteins to yield the mature forms (Shoji et al. 2004). A recent study revealed the crystal structure of the precursor form of Mfa4 and proposed that the N-terminus of the mature protein functions as a donor strand in the polymerization of P. gingivalis fimbriae (Kloppsteck et al. 2016).
Figure 1.
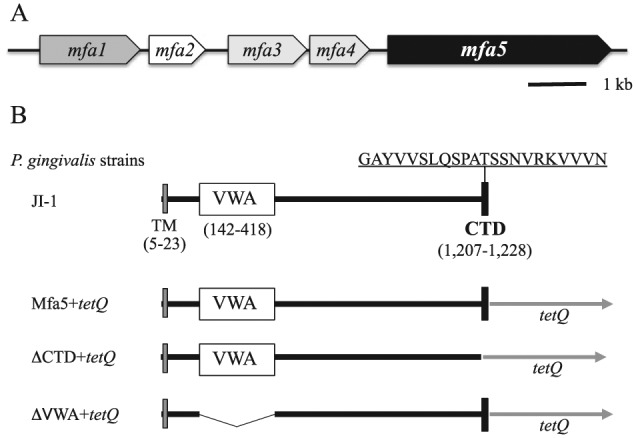
Schematic diagram of the mfa gene cluster (A) and the N-terminus (amino acids 1 to 1,228) of Mfa5 and in-frame deletions constructed in the present study (B). CTD, C-terminal domain; tetQ, tetracycline resistance gene; TM, transmembrane; VWA, von Willebrand factor A domain. The consensus CTD sequence (residues 1,207 to 1,228) is shown. Porphyromonas gingivalis Mfa5+tetQ was constructed as a control strain with tetQ inserted immediately downstream of the intact mfa5.
The type IX secretion system (T9SS) is distributed among some members of the Bacteroidetes phylum, including oral periodontal pathogens such as P. gingivalis and Tannerella forsythia (Nakayama 2015). The P. gingivalis T9SS comprises more than 10 proteins, including PorK, PorL, PorM, PorN, PorP, PorQ, PorT, PorU, PorV, PorW, and Sov (Nakayama 2015). The proteins secreted by the T9SS all have conserved C-terminal domains (CTDs) that are removed by PorU, a C-terminal signal peptidase (Glew et al. 2012) when these proteins are exported and attached to the cell surface. RgpA, RgpB, and Kgp are examples of CTD-containing virulence factors that are translocated across the outer membrane by the T9SS (Sato et al. 2010; Shoji et al. 2011; Nakayama 2015). In T. forsythia, mutants deficient in genes orthologous to the T9SS-encoding genes porK, porT, and sov lacked the surface layer (S-layer) and expressed less-glycosylated versions of the S-layer glycoproteins TfsA and TfsB (Narita et al. 2014). However, a link between the T9SS and fimbrial proteins has not been reported to our knowledge.
Based on amino acid sequence analysis, Mfa5 (corresponding to PGN_0291), the last gene of the mfa cluster, is predicted to have the only CTD in the mfa gene cluster (Hasegawa and Murakami 2014) and a von Willebrand factor type A (VWA) domain, which is widely distributed among archaea, bacteria, and eukaryotes. Well-studied examples of VWA domains occur in some integrins, extracellular matrix proteins, and magnesium chelatases and perform diverse functions, usually for protein-protein interaction or cell adhesion (Whittaker and Hynes 2002). In bacteria, VWA-containing proteins at the tips of fimbrial fibers in a few gram-positive pathogenic bacteria have been reported to play important roles in host-cell adhesion (Konto-Ghiorghi et al. 2009; Nielsen et al. 2012).
In this study, we characterized Mfa5 and its role in assembly of Mfa1 fimbriae. In addition, we analyzed the putative CTD and VWA domains in Mfa5 for their roles in Mfa5 function by in-frame mutations. We also investigated the role of T9SS in the translocation of Mfa5 by direct gene mutagenesis targeting porU and propose a novel polymerization mechanism for Mfa1 fimbriae.
Materials and Methods
Bacterial Strains and Growth Conditions
The bacterial strains used in this study are shown in Appendix Table 1. All P. gingivalis strains were cultivated on Brucella HK agar (Kyokuto Pharmaceutical Industrial) supplemented with 5% (v/v) laked rabbit blood, 2.5 µg/mL hemin, 5 µg/mL menadione, and 1% (w/v) dithiothreitol (DTT) at 37°C under anaerobic conditions. Liquid cultures of P. gingivalis were in trypticase soy broth supplemented with 0.25% (w/v) yeast extract, 2.5 µg/mL hemin, 5 µg/mL menadione, and 1% DTT (sTSB). When necessary, 5 µg/mL chloramphenicol, 20 µg/mL erythromycin, or 1 µg/mL tetracycline was added to the medium. E. coli were grown in Luria-Bertani medium supplemented, when necessary, with 50 µg/mL kanamycin or 50 µg/mL ampicillin.
Construction of In-Frame Deletion Mutants of mfa5 and porU Deletion Mutants
In-frame deletion mutants of mfa5 and porU deletion mutants were generated using a polmerase chain reaction (PCR)–based overlap extension method by replacement with tetQ as previously described (Hasegawa et al. 2013). The primers and their annealing sites are shown in Appendix Table 2 and Appendix Figures 1–4, respectively. Details are described in the Appendix.
Construction of Complemented Strain of FMFA5C
Genetic complementation strain FMFA5C was constructed using the expression vector pTCBex2 (Nagano et al. 2007). In brief, the mfa5 region in ATCC 33277 was amplified by PCR using cMfa5FX and cMfa5RN with XbaI and NotI restriction sites, respectively (Appendix Table 2). The PCR product was then cloned downstream of the ragA promoter region in pTCBex2 after the vector was digested with XbaI and NotI restriction enzymes. The resulting vector was introduced into FMFA5 (Ikai et al. 2015) via conjugation from E. coli S17-1, and P. gingivalis FMFA5C was isolated.
Preparation of Protein Samples
According to Murakami’s protocol (Murakami et al. 2002), whole-cell lysate was separated into soluble (periplasm/cytoplasm) and insoluble (membrane) fractions. The culture supernatant was concentrated by ammonium sulfate precipitation (70% saturation) as described previously (Hasegawa et al. 2003). Details are provided in the Appendix.
Purification of Mfa1 Fimbriae
Mfa1 fimbriae were purified as described previously (Hasegawa et al. 2013). The degree of purity and identity of Mfa1 fimbriae were verified by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and mass spectrometry as described previously (Kishi et al. 2012). Details are described in the Appendix.
SDS-PAGE and Western Blotting
SDS-PAGE and Western blotting were performed as previously described (Ikai et al. 2015). Details are described in the Appendix.
Filtration Enzyme-Linked Immunosorbent Assay
Filtration enzyme-linked immunosorbent assay (ELISA) was performed as previously described (Ikai et al. 2015). Details are described in the Appendix.
Data are reported as mean ± standard deviation (SD). One-way analysis of variance and Dunnett’s multiple-comparison test were used to evaluate differences between groups. Significance was defined as P < 0.01.
Results
Localization of Mfa5 Protein of P. gingivalis
We analyzed the deduced amino acid sequence of Mfa5 using PSORT (http://psort.hgc.jp/form.html). The result showed that Mfa5 contained an N-terminal signal peptide region predictive of an outer membrane or periplasmic protein. Mfa5 was previously predicted to have a CTD (Hasegawa and Murakami 2014) that used the T9SS for translocation across the outer membrane (Nakayama 2015). A C-terminal amino acid alignment constructed using Mfa5 and other CTD proteins (Appendix Fig. 5) showed that the consensus CTD sequence (Sato et al. 2013) was conserved in Mfa5 residues 1,207 to 1,228 (Fig. 1B). The Mfa5 protein was also annotated with a VWA domain in the P. gingivalis ATCC 33277 database (Naito et al. 2008). The putative VWA domain comprises N-terminal residues 142 to 418 (Fig. 1B).
To determine the localization of Mfa5 protein, whole-cell lysate from P. gingivalis JI-1 (∆fimA) was prepared, and various cell fractions were subjected to SDS-PAGE and immunoblotting with anti-Mfa5 antiserum (Fig. 2). The calculated molecular mass of the Mfa5 protein (1,228 amino acids) is 134 kDa, which agrees closely with the observed mass in the whole-cell lysate (130 kDa). A 130-kDa immunoreactive protein was detected mainly in the periplasm/cytoplasm fraction and not in the culture supernatant or membrane fraction. Mfa5 was originally identified as the accessory protein of Mfa1 fimbriae purified from the soluble (periplasm/cytoplasm) fraction (Hasegawa et al. 2009). Because CTD-containing proteins are translocated across the outer membrane by the T9SS (Nakayama 2015), we hypothesized that Mfa5 is translocated from the periplasm across the outer membrane by T9SS, mediated by the CTD, and subsequently integrated into Mfa1 fibers.
Figure 2.
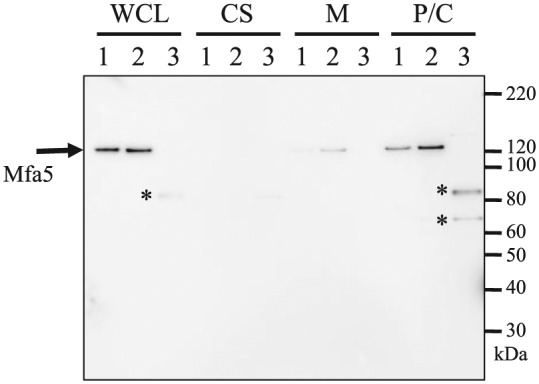
Immunoblot analysis with an antiserum against Mfa5 protein. Proteins in the culture supernatant (CS) were concentrated by ammonium sulfate precipitation, while whole-cell lysates (WCLs) were fractionated into periplasm/cytoplasm (P/C) and membrane (M) fractions. Lanes: 1, JI-1; 2, Mfa5+tetQ; 3, ∆CTD+tetQ. Possible degradation products are indicated with asterisks.
Functions of Mfa5 in Expression of Mfa1 Fimbriae
To investigate the function of Mfa5 in expression of Mfa1 fimbriae, we constructed an Mfa5 mutant (FMFA5) in which mfa5 was disrupted by ermF-B (Ikai et al. 2015). After confirming expression of the major subunit of Mfa1 in the whole-cell lysates of JI-1 and FMFA5 (Appendix Fig. 6), fiber expression on the cell surface was examined by filtration ELISA (Fig. 3A). FMFA5 showed significantly reduced fiber expression compared with JI-1. Mfa1 fimbriae were purified from FMFA5 and JI-1 to examine the effect of mfa5 deficiency on composition and assembly. JI-1 fimbriae exhibited several protein bands corresponding to Mfa1 and Mfa3–5 (Fig. 3B) (Ikai et al. 2015), whereas FMFA5 fimbriae exhibited the ladder of Mfa1 bands between 50 and 75 kDa observed in JI-1 but did not contain the Mfa3–5 accessory components. Schemata of the possible fimbrial structures are shown in Figure 3C. To confirm the relationship between mfa5 and cell-surface expression of fimbriae and incorporation of accessory proteins into fibers, we constructed a complemented strain FMFA5C that expresses wild-type mfa5 in trans. The resulting FMFA5C strain exhibited expression of intact fimbriae similar to the parent strain (Fig. 3). Absence or overexpression of Mfa5 protein in FMFA5 or FMFA5C, respectively, was confirmed by immunoblot analysis (Appendix Fig. 7).
Figure 3.
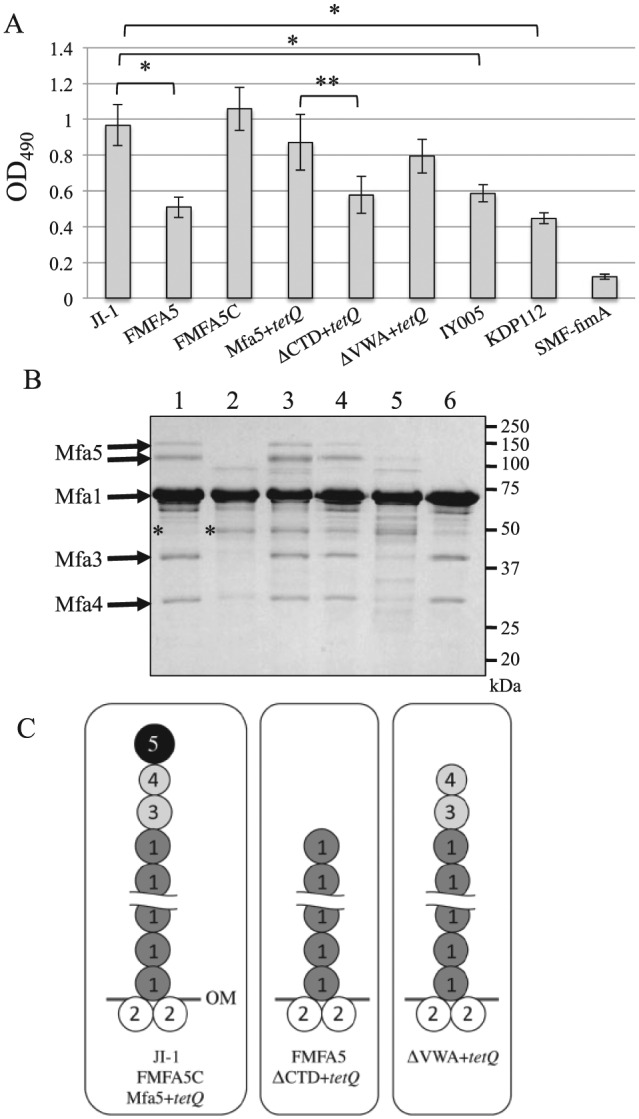
Mfa1 fimbriae of Porphyromonas gingivalis strains. (A) Analysis of cell surface expression of Mfa1 fimbriae by filtration enzyme-linked immunosorbent assay of intact cells. Various P. gingivalis cells were applied over filters in a filtration plate at 1 × 107 cells/well. Bacterial cells were reacted with antibodies against Mfa1 fimbriae and then with peroxidase-conjugated goat anti-rabbit IgG. Data are mean ± SD from triplicates. *P < 0.01 vs. JI-1 or **P < 0.01 vs. Mfa5+tetQ based on Dunnett’s test. (B) Analysis of the components of Mfa1 fimbriae. Mfa1 fimbriae purified from P. gingivalis strains derived from JI-1 (∆fimA) to avoid contamination of FimA fimbriae were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and stained with Coomassie brilliant blue R-250. Lanes: 1, JI-1; 2, FMFA5; 3, FMFA5C; 4, Mfa5+tetQ; 5, ∆CTD+tetQ; 6, ∆VWA+tetQ. Protein bands identified as PGN_1808 by mass spectrometry are indicated with asterisks. (C) Scheme of a possible structure model of Mfa1 fimbria. Mfa1 fimbriae from JI-1, FMFA5C, and Mfa5+tetQ are composed of a central Mfa1 polymer associated with the accessory proteins Mfa3, Mfa4, and Mfa5 at the tip (left). Mfa2 also associates with the Mfa1 filament base in the outer membrane (OM). No accessory proteins were present in FMFA5 and ∆CTD+tetQ fimbriae (center). In contrast, only Mfa5 was not incorporated in ∆VWA+tetQ (right).
The 50-kDa bands in the SDS-PAGE gels for purified fimbriae from JI-1 and FMFA5 (Fig. 3B) were identified by mass spectrometry. Both 50-kDa proteins were identified as gene products of PGN_1808 that are annotated as conserved hypothetical proteins in the 33277 database. BLASTP analysis showed that this protein was highly conserved among many Porphyromonas and Bacteroides species. The C-terminal region of the protein is homologous to corresponding regions of type IV fimbriae in Bacillus species, suggesting that it is somewhat related to fimbriae.
Functional Characterization by In-Frame Deletion Mutations
To examine the contribution of Mfa5 domains to protein function, we constructed 2 in-frame deletion mutants by deleting the CTD and VWA domains: ∆CTD+tetQ (deletion of residues 1,207 to 1,228) and ∆VWA+tetQ (deletion of residues 142 to 418) (Fig. 1B). After confirming Mfa1 protein expression in the whole-cell lysates of ∆VWA+tetQ, ∆CTD+tetQ, and Mfa5+tetQ, a control strain, by immunoblot analysis (Appendix Fig. 6), filtration ELISA was performed (Fig. 3A). ∆VWA-expressing cells produced the same amount of fimbriae as Mfa5+tetQ, whereas ∆CTD-expressing cells produced significantly fewer (i.e., almost half). We next analyzed the fimbrial components of the purified fimbriae from these mutants by SDS-PAGE and confirmed that tetQ insertion immediately downstream of intact mfa5 had no effect (Fig. 3B, C). However, the purified Mfa1 fimbriae from ∆CTD-expressing cells contained no accessory proteins (Fig. 3B, C). Importantly, the phenotype of the fimbrial structure of ∆CTD-expressing cells was similar to that of FMFA5. In contrast, ∆VWA-expressing cells showed incorporation of Mfa3 and Mfa4 into the fiber but no Mfa5 (Fig. 3B, C).
Expression and localization of Mfa5 in the in-frame deletion mutants were examined by immunoblot analysis (Fig. 2). In ∆CTD-expressing cells, immunoreactive bands with molecular weights 81 and 65 kDa were detected in whole-cell and periplasm/cytoplasm fractions, suggesting that Mfa5 in the ∆CTD-expressing cells accumulated intracellularly, without incorporation in fibers, and was degraded.
Effect of porU Mutation on Mfa5 Localization
The results described above suggested that the CTD of Mfa5 was necessary for T9SS-mediated translocation across the outer membrane. To examine this possibility, porU deletion mutants were constructed from 33277 (IY002) and JI-1 (IY005) to abolish the T9SS. The phenotypic change of the porU deletion mutants was observed through colony color because the T9SS is strongly associated with the colonial black pigmentation of P. gingivalis (Appendix Fig. 8) (Nakayama 2015). By filtration ELISA, IY005 showed significantly reduced fimbriae compared with JI-1 (Fig. 3A). In immunoblot analysis using anti-Mfa5 antiserum, Mfa5 was detected in IY002 and IY005 as 3 bands with molecular weights of 170, 79, and 63 kDa mainly in the whole-cell lysate and periplasm/cytoplasm fractions but not in the culture supernatant (Fig. 4). The 130-kDa Mfa5 was not detected in any fraction. We attempted to purify fimbriae from IY005 to determine porU function in translocation/integration of Mfa5 into fimbriae by analyzing fimbrial components; however, fimbriae could not be purified. To solve this problem, we made an mfa4/porU double mutant (IY011) because deficiency of mfa4 caused leakage of Mfa5 into the culture supernatant without incorporation into fibers (Ikai et al. 2015). If porU were involved in Mfa5 translocation, inactivation of porU would result in accumulation of Mfa5 in the cell. In FMFA4, Mfa5 was detected with molecular weights of 80 to 130 kDa in the culture supernatant but not the whole-cell lysate, membrane, or periplasm/cytoplasm fractions (Fig. 4). Analysis of IY011 showed 170-, 79-, and 63-kDa bands mainly in the whole-cell lysate and the periplasm/cytoplasm fraction but not in the culture supernatant, as observed for IY002 and IY005. These data suggested that PorU functions in Mfa5 translocation across the outer membrane. Importantly, 2 low-molecular-weight bands of Mfa5 (~79 and 63 kDa) were detected in ∆CTD-expressing cells in all of the porU mutants (Fig. 2), suggesting degradation of Mfa5 caused by intracellular Mfa5 accumulation.
Figure 4.
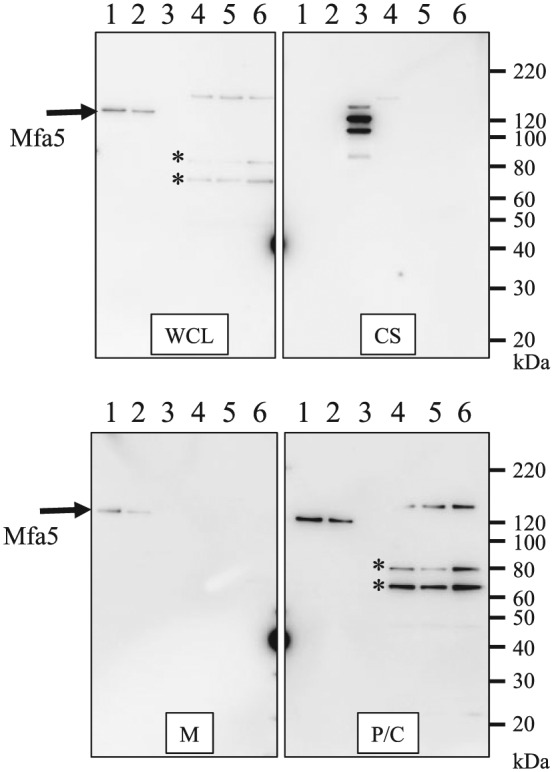
Effect of porU mutation on expression of Mfa5. Proteins in the culture supernatant (CS) were concentrated by ammonium sulfate precipitation, while whole-cell lysates (WCL) were fractionated into periplasm/cytoplasm (P/C) and membrane (M) fractions. Samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and reacted with Mfa5 antibodies by immunoblotting. Possible degradation products are indicated with asterisks. Lanes: 1, 33277; 2, JI-1; 3, FMFA4; 4, IY002; 5, IY005; 6, IY011.
Involvement of Rgps in Mfa5 Maturation
Rgps are involved in the maturation of fimbrial proteins such as Mfa1, Mfa3, and Mfa4 (Shoji et al. 2004; Hasegawa and Murakami 2014). Since T9SS is necessary for the secretion and activity of gingipains (Sato et al. 2010), we considered the possibility that gingipains contribute to Mfa5 processing. To test this notion, we analyzed Mfa5 expression in KDP112, an rgpA/B double-deficient mutant (Nakayama et al. 1995), by immunoblotting (Appendix Fig. 9). The 130-kDa Mfa5 band was detected in the whole-cell lysate of KDP112 and in JI-1. Since KDP112 could produce a mature Mfa5 protein, it is likely that Rgps are not essential for Mfa5 maturation. However, the 170-kDa Mfa5 band detected in all porU mutants was also detected in KDP112 (Appendix Fig. 9); thus, Rgps might partially affect Mfa5 maturation.
Discussion
In this study, we demonstrated that Mfa5 is necessary for expression of intact Mfa1 fimbriae and for integration of Mfa3 and Mfa4 into fimbriae. The ∆porU mutants and ∆CTD-expressing strain showed intracellular accumulation of Mfa5. In addition, phenotypic similarities between FMFA5 and ∆CTD-expressing cells indicate that translocation of Mfa5 through the outer membrane is important for Mfa5 functions. To our knowledge, this is the first report to link T9SS and fimbriae expression.
Recently, Kloppsteck et al. (2016) solved the crystal structure of the precursor form of Mfa4 and proposed that the N-terminus of the mature protein functions as a donor strand in the polymerization of P. gingivalis Mfa1 fimbriae (Fig. 5)—that is, Mfa1, Mfa3, and Mfa4 are transported to the periplasmic space via the Sec system in the inner membrane and thereafter to the outer membrane through an unknown system. The N-termini of Mfa1, Mfa3, and Mfa4 are cleaved by gingipains to yield the mature forms, and the resulting Nmature strands are inserted into neighboring Mfa1, Mfa3, and Mfa4 based on a donor-strand complementation mechanism. However, experimental evidence demonstrating the involvement of T9SS and Mfa5 was not reported. In this study, we clearly showed that Mfa5 is transported across the outer membrane by T9SS and integrated into fimbriae. Since Rgps are not essential for Mfa5 maturation (Appendix Fig. 9), incorporation of Mfa5 may occur through an independent polymerization pathway as for Mfa1, Mfa3, and Mfa4. In addition, because all accessory proteins except Mfa5 were integrated into Mfa1 fibers in ΔVWA-expressing cells (Fig. 3), Mfa5 likely associates with fimbrial proteins via the VWA domain. Mfa5 was released in the culture supernatant without integration into fimbriae in FMFA4 (Fig. 4), suggesting that the VWA domain is the functional region for Mfa4 interaction in the integration of Mfa5 into fimbriae. However, to our knowledge, there is no particular evidence to show direct physical interactions between Mfa5 and accessory proteins.
Figure 5.
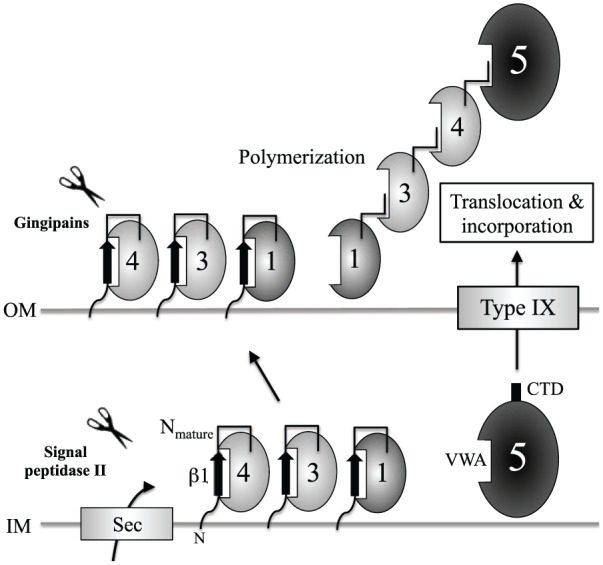
Polymerization model for Mfa1 fimbriae. Mfa1, Mfa3, and Mfa4 are transported to the periplasmic space via the Sec system in the inner membrane (Hasegawa and Murakami 2014) and thereafter across the outer membrane. β1-strands at the N-termini of Mfa1, Mfa3, and Mfa4 are processed by gingipains, and the resulting Nmature strands are inserted into neighboring Mfa1, Mfa3, and Mfa4 based on a donor-strand complementation mechanism (Kloppsteck et al. 2016). In this report, we demonstrated that Mfa5 is transported through the outer membrane via the type IX secretion system via C-terminal domain (CTD) recognition, the CTD is removed by PorU, and the mature protein may be incorporated into Mfa1 fimbriae through von Willebrand factor type A (VWA)–mediated interactions. Modified from (Kloppsteck et al. 2016).
Mfa5 incorporated into Mfa1 fimbriae was dissociated and migrated as 2 bands with molecular weights of 130 and 150 kDa (Fig. 3B). As the first corresponds to the calculated molecular mass of 134 kDa, the second appears to be partially modified after morphogenesis. Notably, CTD proteins are extensively modified with anionic lipopolysaccharide (A-LPS) after T9SS-dependent translocation and therefore migrate as diffuse bands in SDS-PAGE with molecular masses generally 20 kDa higher than expected from the sequence (Glew et al. 2012). A-LPS modification of CTD proteins has suggested a role for CTDs in export, at the modification site after their release, and cell surface attachment (Seers et al. 2006; Shoji et al. 2011). In this study, a possible precursor form of Mfa5 was also detected as 170-kDa bands in both the porU mutant and KDP112 (Fig. 4; Appendix Fig. 9). Further work is needed to determine the relationship between posttranslational modification and integration of Mfa5 into fimbriae.
Incorporation of accessory components into mature fimbriae is common among gram-negative pathogenic bacteria such as uropathogenic E. coli, Neisseria meningitidis, and Pseudomonas aeruginosa (Carbonnelle et al. 2006; Giltner et al. 2010; Heiniger et al. 2010). The roles of some of these components are known. For example, accessory components that cap fimbrial tips facilitate adhesion, as in type 1 fimbriae and P pili in E. coli (Kuehn et al. 1992; Jones et al. 1995). In P. gingivalis, it has been demonstrated that the accessory proteins FimC–E are essential for maintaining the structure and adhesive properties of FimA fimbriae (Nishiyama et al. 2007). Binding of the C-type lectin DC-SIGN to Mfa1 fimbriae allows P. gingivalis to survive within dendritic cells (El-Awady et al. 2015); the Mfa3–5 fimbrial tip complex is thought to be involved in this interaction. Therefore, our findings have implications for potential antibacterial therapeutic intervention for periodontitis.
Author Contributions
Y. Hasegawa, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; Y. Iijima, contributed to conception and data analysis, drafted and critically revised the manuscript; K. Persson, K. Nagano, Y. Yoshida, R.J. Lamont, T. Kikuchi, A. Mitani, and F. Yoshimura, contributed to design and data interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Supplementary Material
Acknowledgments
The authors thank Mikie Sato for analyzing mass spectrometry data.
Footnotes
This work was supported by JSPS KAKENHI grant 25861 752 to Y.H., and DE012505 from the National Institutes of Health to R.J.L.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Amano A. 2003. Molecular interaction of Porphyromonas gingivalis with host cells: implication for the microbial pathogenesis of periodontal disease. J Periodontol. 74(1):90–96. [DOI] [PubMed] [Google Scholar]
- Carbonnelle E, Helaine S, Nassif X, Pelicic V. 2006. A systematic genetic analysis in Neisseria meningitidis defines the Pil proteins required for assembly, functionality, stabilization and export of type IV pili. Mol Microbiol. 61(6):1510–1522. [DOI] [PubMed] [Google Scholar]
- El-Awady AR, Miles B, Scisci E, Kurago ZB, Palani CD, Arce RM, Waller JL, Genco CA, Slocum C, Manning M, et al. 2015. Porphyromonas gingivalis evasion of autophagy and intracellular killing by human myeloid dendritic cells involves DC-SIGN-TLR2 crosstalk. PLoS Pathog. 10(2):e1004647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach RG, Hensel M. 2007. Protein secretion systems and adhesins: the molecular armory of Gram-negative pathogens. Int J Med Microbiol. 297(6):401–415. [DOI] [PubMed] [Google Scholar]
- Giltner CL, Habash M, Burrows LL. 2010. Pseudomonas aeruginosa minor pilins are incorporated into type IV pili. J Mol Biol. 398(3):444–461. [DOI] [PubMed] [Google Scholar]
- Glew MD, Veith PD, Peng B, Chen YY, Gorasia DG, Yang Q, Slakeski N, Chen D, Moore C, Crawford S, et al. 2012. PG0026 is the C-terminal signal peptidase of a novel secretion system of Porphyromonas gingivalis. J Biol Chem. 287(29):24605–24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. 2015. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 15(1):30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Lamont RJ. 2012. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 27(6):409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Iwami J, Sato K, Park Y, Nishikawa K, Atsumi T, Moriguchi K, Murakami Y, Lamont RJ, Nakamura H, et al. 2009. Anchoring and length regulation of Porphyromonas gingivalis Mfa1 fimbriae by the downstream gene product Mfa2. Microbiology. 155(Pt 10):3333–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Murakami Y. 2014. Porphyromonas gingivalis fimbriae: recent developments describing the function and localization of mfa1 gene cluster. J Oral Biosci. 56(3):86–90. [Google Scholar]
- Hasegawa Y, Nagano K, Ikai R, Izumigawa M, Yoshida Y, Kitai N, Lamont RJ, Murakami Y, Yoshimura F. 2013. Localization and function of the accessory protein Mfa3 in Porphyromonas gingivalis Mfa1 fimbriae. Mol Oral Microbiol. 28(6):467–480. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Nishiyama S, Nishikawa K, Kadowaki T, Yamamoto K, Noguchi T, Yoshimura F. 2003. A novel type of two-component regulatory system affecting gingipains in Porphyromonas gingivalis. Microbiol Immunol. 47(11):849–858. [DOI] [PubMed] [Google Scholar]
- Heiniger RW, Winther-Larsen HC, Pickles RJ, Koomey M, Wolfgang MC. 2010. Infection of human mucosal tissue by Pseudomonas aeruginosa requires sequential and mutually dependent virulence factors and a novel pilus-associated adhesin. Cell Microbiol. 12(8):1158–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikai R, Hasegawa Y, Izumigawa M, Nagano K, Yoshida Y, Kitai N, Lamont RJ, Yoshimura F, Murakami Y. 2015. Mfa4, an accessory protein of Mfa1 fimbriae, modulates fimbrial biogenesis, cell auto-aggregation, and biofilm formation in Porphyromonas gingivalis. PLoS One. 10(10):e0139454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CH, Pinkner JS, Roth R, Heuser J, Nicholes AV, Abraham SN, Hultgren SJ. 1995. FimH adhesin of type 1 pili is assembled into a fibrillar tip structure in the Enterobacteriaceae. Proc Natl Acad Sci U S A. 92(6):2081–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi M, Hasegawa Y, Nagano K, Nakamura H, Murakami Y, Yoshimura F. 2012. Identification and characterization of novel glycoproteins involved in growth and biofilm formation by Porphyromonas gingivalis. Mol Oral Microbiol. 27(6):458–470. [DOI] [PubMed] [Google Scholar]
- Kloppsteck P, Hall M, Hasegawa Y, Persson K. 2016. Structure of the fimbrial protein Mfa4 from Porphyromonas gingivalis in its precursor form: implications for a donor-strand complementation mechanism. Sci Rep. 6:22945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konto-Ghiorghi Y, Mairey E, Mallet A, Duménil G, Caliot E, Trieu-Cuot P, Dramsi S. 2009. Dual role for pilus in adherence to epithelial cells and biofilm formation in Streptococcus agalactiae. PLoS Pathog. 5(5):e1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn MJ, Heuser J, Normark S, Hultgren SJ. 1992. P pili in uropathogenic E. coli are composite fibres with distinct fibrillar adhesive tips. Nature. 356(6366):252–255. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Jenkinson HF. 1998. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 62(4):1244–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Imai M, Nakamura H, Yoshimura F. 2002. Separation of the outer membrane and identification of major outer membrane proteins from Porphyromonas gingivalis. Eur J Oral Sci. 110(2):157–162. [DOI] [PubMed] [Google Scholar]
- Nagano K, Murakami Y, Nishikawa K, Sakakibara J, Shimozato K, Yoshimura F. 2007. Characterization of RagA and RagB in Porphyromonas gingivalis: study using gene-deletion mutants. J Med Microbiol. 56(Pt 11):1536–1548. [DOI] [PubMed] [Google Scholar]
- Naito M, Hirakawa H, Yamashita A, Ohara N, Shoji M, Yukitake H, Nakayama K, Toh H, Yoshimura F, Kuhara S, et al. 2008. Determination of the genome sequence of Porphyromonas gingivalis strain ATCC 33277 and genomic comparison with strain W83 revealed extensive genome rearrangements in P. gingivalis. DNA Res. 15(4):215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K. 2015. Porphyromonas gingivalis and related bacteria: from colonial pigmentation to the type IX secretion system and gliding motility. J Periodontal Res. 50(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Kadowaki T, Okamoto K, Yamamoto K. 1995. Construction and characterization of arginine-specific cysteine proteinase (Arg-gingipain)–deficient mutants of Porphyromonas gingivalis. Evidence for significant contribution of Arg-gingipain to virulence. J Biol Chem. 270(40):23619–23626. [DOI] [PubMed] [Google Scholar]
- Narita Y, Sato K, Yukitake H, Shoji M, Nakane D, Nagano K, Yoshimura F, Naito M, Nakayama K. 2014. Lack of a surface layer in Tannerella forsythia mutants deficient in the type IX secretion system. Microbiology. 160(Pt 10):2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen HV, Guiton PS, Kline KA, Port GC, Pinkner JS, Neiers F, Normark S, Henriques-Normark B, Caparon MG, Hultgren SJ. 2012. The metal ion-dependent adhesion site motif of the Enterococcus faecalis EbpA pilin mediates pilus function in catheter-associated urinary tract infection. MBio. 3(4):e00177-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama S, Murakami Y, Nagata H, Shizukuishi S, Kawagishi I, Yoshimura F. 2007. Involvement of minor components associated with the FimA fimbriae of Porphyromonas gingivalis in adhesive functions. Microbiology. 153(Pt 6):1916–1925. [DOI] [PubMed] [Google Scholar]
- Sato K, Naito M, Yukitake H, Hirakawa H, Shoji M, McBride MJ, Rhodes RG, Nakayama K. 2010. A protein secretion system linked to bacteroidete gliding motility and pathogenesis. Proc Natl Acad Sci U S A. 107(1):276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Yukitake H, Narita Y, Shoji M, Naito M, Nakayama K. 2013. Identification of Porphyromonas gingivalis proteins secreted by the Por secretion system. FEMS Microbiol Lett. 338(1):68–76. [DOI] [PubMed] [Google Scholar]
- Seers CA, Slakeski N, Veith PD, Nikolof T, Chen YY, Dashper SG, Reynolds EC. 2006. The RgpB C-terminal domain has a role in attachment of RgpB to the outer membrane and belongs to a novel C-terminal-domain family found in Porphyromonas gingivalis. J Bacteriol. 188(17):6376–6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M, Naito M, Yukitake H, Sato K, Sakai E, Ohara N, Nakayama K. 2004. The major structural components of two cell surface filaments of Porphyromonas gingivalis are matured through lipoprotein precursors. Mol Microbiol. 52(5):1513–1525. [DOI] [PubMed] [Google Scholar]
- Shoji M, Sato K, Yukitake H, Kondo Y, Narita Y, Kadowaki T, Naito M, Nakayama K. 2011. Por secretion system-dependent secretion and glycosylation of Porphyromonas gingivalis hemin-binding protein 35. PLoS One. 6(6):e21372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD. 2002. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 28:12–55. [DOI] [PubMed] [Google Scholar]
- Thanassi DG, Saulino ET, Hultgren SJ. 1998. The chaperone/usher pathway: a major terminal branch of the general secretory pathway. Curr Opin Microbiol. 1(2):223–231. [DOI] [PubMed] [Google Scholar]
- Whittaker CA, Hynes RO. 2002. Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol Biol Cell. 13(10):3369–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F, Murakami Y, Nishikawa K, Hasegawa Y, Kawaminami S. 2009. Surface components of Porphyromonas gingivalis. J Periodontal Res. 44(1):1–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


