Abstract
Hepatocellular adenoma (HCA) is a rare, benign liver tumor. Discovery of this tumor is usually as an incidental finding, correlated with the use of oral contraceptives, or pregnancy. Treatment options have focused on conservative management for the straightforward, smaller lesions (<5 cm), with resection preferred for larger lesions (>5 cm) that pose a greater risk of hemorrhage or malignant progression. In recent years, a new molecular subclassification of HCA has been proposed, associated with characteristic morphological features and loss or increased expression of immunohistochemical markers. This subclassification could possibly provide considerable benefits in terms of patient stratification, and the selection of treatment options. In this review we discuss the decision-making processes and associated risk analyses that should be made based on lesion size, and subtype. The usefulness of this subclassification system in terms of the procedures instigated as part of the diagnostic work-up of a suspected HCA will be outlined, and suitable treatment schemes proposed.
Keywords: adenoma, immunohistochemistry, liver, MRI
Introduction
Hepatocellular adenomas (HCAs) are an uncommon, solid, benign tumor of the liver, with an estimated incidence of 3–4 per 100,000 women [Bioulac-Sage et al. 2010]; this frequency is based on population research including women using oral contraceptives (OCs) [Baum et al. 1973]. A causal role for hormone activity in HCA growth is suggested by data linking adenoma regression to the cessation of OC use, and growth associated with pregnancy [Cobey and Salem, 2004].
Typically, HCAs are treated conservatively, with patients advised to avoid oral contraception. The risks of growth and rupture of HCAs during pregnancy has to be underlined, especially in larger HCAs. Tumor progression, suggested by internal bleeding and malignant transformation, necessitates a more aggressive therapeutic approach, with lesions larger than 5 cm considered as the primary risk factor [Marrero et al. 2014]. The introduction of a new subclassification system for HCA has been suggested to help clinicians to stratify patients according to imaging criteria, expression of associated immunohistochemical markers or molecular findings. These data may influence the treatment selected [Marrero et al. 2014] since certain subtypes of HCA pose a greater risk of progression to hepatocellular carcinoma (HCC) than others. For example, a subtype of HCA defined by the reduced expression of liver fatty acid-binding protein (LFABP) ordinarily indicates a subtype with a less aggressive course and a tendency towards a benign phenotype.
Based on the recent literature, we describe the impact of this newly instigated HCA subclassification, and discuss whether this knowledge, combined with imaging data, improves our risk analyses for patients with HCA. Furthermore, we outline the different therapeutic options indicated by each HCA subtype.
The Bordeaux classification of HCA
In recent years, four distinct subtypes of HCA have been recognized: inflammatory HCA (40–50%, IHCA), HNF1A-mutated HCA (30–40%, H-HCA), β-catenin activated HCA (10–15% b-HCA), and unclassified HCA (10–25%, UHCA) [Nault et al. 2013]. In these different subtypes, several genetic mutations are identified, causing (benign) proliferation of hepatocytes and in some HCA, malignant transformation [Pilati et al. 2014].
Patients presenting with IHCA demonstrate both serum, and lesional indicators of an active inflammatory response. In these lesions, increased expression is seen of the markers serum amyloid A and C-reactive protein, both classic indicators of the acute phase response [Bioulac-Sage et al. 2007a]. Patients within this HCA category frequently demonstrate increased body weight, and a high alcohol intake [Bioulac-Sage et al. 2007b, 2009; Paradis et al. 2007]. In approximately 10–20% of these lesions, a β-catenin mutation is found [van Aalten et al. 2011b].
A second subtype of HCA, H-HCA, is characterized by a downregulation of the LFABP; this phenotype, which is not apparent in the other HCA subtypes, rarely leads to malignant progression [Zucman-Rossi et al. 2006].
Subtype b-HCA is typified by activating mutations of β-catenin that resist phosphorylation-mediated down-regulation by the GSKB/APC/AXIN complex [Nault et al. 2013]. Particularly the exon 3 mutation of β-catenin plays a significant role in malignant progression in contrast to exon 7/8 mutations [Pilati et al. 2014]. The result is an accumulation of nuclear β-catenin which, combined with deletion of APC, favors progression to HCC [Nault et al. 2013]. The comparatively small number of β-catenin positive nuclei can lead to this phenotype being overlooked in small biopsies [van Aalten et al. 2011b]. The b-HCA subtype also demonstrates an overexpression of glutamate-ammonia ligase (GLUL) encoding glutamine synthase (GS), which can be used as a sensitive diagnostic biomarker for this subtype [van Aalten et al. 2011b].
The final subtype, UHCA, is not yet defined by any specific genetic mutation, but is instead characterized by various histologic criteria that are unusual in the other subtypes; the underlying pathogenesis of this subtype remains unclear [Blanc et al. 2015].
Magnetic resonance imaging of the different subtypes of HCA
The primary differential diagnosis for HCA is focal nodular hyperplasia (FNH). If in doubt, a biopsy should be taken, especially for larger lesions, as the clinical management will differ for either pathology. In most cases these diagnoses can be differentiated according to signal intensity and dynamic vascular patterns after intravenous aspecific gadolinium injection [(conventional magnetic resonance imaging (MRI)] [van Aalten et al. 2011a]. Different patterns can be used for confident diagnosis as proposed by Thomeer and colleagues [Thomeer et al. 2014a].
In more challenging cases, specific hepatobiliary contrast agents can be used. Currently there are two agents available, gadobenate dimeglumine, and gadoxetate disodium [Grazioli et al. 2013; Thomeer et al. 2014a; McInnes et al. 2015]. If the lesion turns hypointense to the surrounding liver in the hepatobiliary phase, FNH can be excluded in most cases. If the lesion becomes iso- to hyperintense the differential diagnosis is FNH or in exceptional cases HCC. However, it should be noted that IHCA can also be isointense in the hepatobiliary phase [Agarwal et al. 2014; Thomeer et al. 2014b]. This might be explained by the presence of internal bile duct proliferation, previously thought to be only visible in FNH. This diagnostic pitfall can be visualized when using gadobenate dimeglumine [Thomeer et al. 2014b], or gadoxetate disodium [Agarwal et al. 2014]. A recent systematic review about the value of gadoxetate disodium has shown that apart from this pitfall, adequate differentiation is possible in most cases [McInnes et al. 2015]. It was reported that the hepatobiliary phase has a sensitivity of 91–100% and a specificity of 87–100% for differentiating HCA from FNH. In the largest study this was only seen in 13% of cases [Bieze et al. 2012].
In conclusion, in the vast majority lesions can easily be differentiated based on a combination of typical findings on conventional MRI and features on hepatobiliary phase MRI.
Some typical MRI features allow us to discriminate different subtypes of HCA (Table 1): IHCA can be hyperintense on T2-weighted images, with persistent enhancement on delayed imaging in the venous phase [Laumonier et al. 2008]. Ronot and colleagues validated this feature as being highly specific for IHCA, with a sensitivity of 82% [28/34, confidence interval (CI) 65–93%], and an optimal specificity of 100% (12/12, CI 75–100%) [Ronot et al. 2011]. Another diagnostic indicator for IHCA is the atoll-sign [van Aalten et al. 2011a], a hyperintense rim on T2-weighted images at the periphery of the lesion (resembling an atoll) that is enhanced late in the venous phase. This feature is present in 27% of IHCAs in this study [van Aalten et al. 2011a].
Table 1.
Typical MRI findings according to subtypes of HCA.
| Subtype | Most typical MRI signs |
|---|---|
| IHCA | Hyperintense on T2-weighted images, with persistent enhancement in the venous phase; atoll sign |
| H-HCA | Diffuse and homogenous fat deposition (Figure 1) |
| b-HCA | (Vaguely demarcated scar) |
| UHCA | No typical sign |
b-HCA, β-catenin activated HCA; HCA, hepatocellular adenoma; H-HCA, HNF1A-mutated HCA; IHCA, inflammatory HCA; MRI, magnetic resonance imaging; UHCA, unclassified HCA.
Whilst H-HCAs are typically characterized by a large amount of aberrant fat which can be readily appreciated by out-of-phase imaging, or on a fat-saturated T1-weighted image [Laumonier et al. 2008; van Aalten et al. 2011a], van Aalten and colleagues failed to detect fat by MRI for as many as 22% of cases (2/9) [van Aalten et al. 2011a]. Ronot and colleagues validated the diagnostic feature of diffuse and homogeneous signal dropout on out-of-phase T1 weighted imaging, with a reported sensitivity of 90% (10/11, CI 58–99), and specificity of 88% (32/36, CI 73–96) [Ronot et al. 2011]. The main drawback of this marker is that diffuse intralesional steatosis may also be present in up to 11% (4/34) of IHCAs [Ronot et al. 2011], although, according to the authors, this does not represent a major pitfall as fat is usually distributed heterogeneously within IHCAs (Figure 1).
Figure 1.
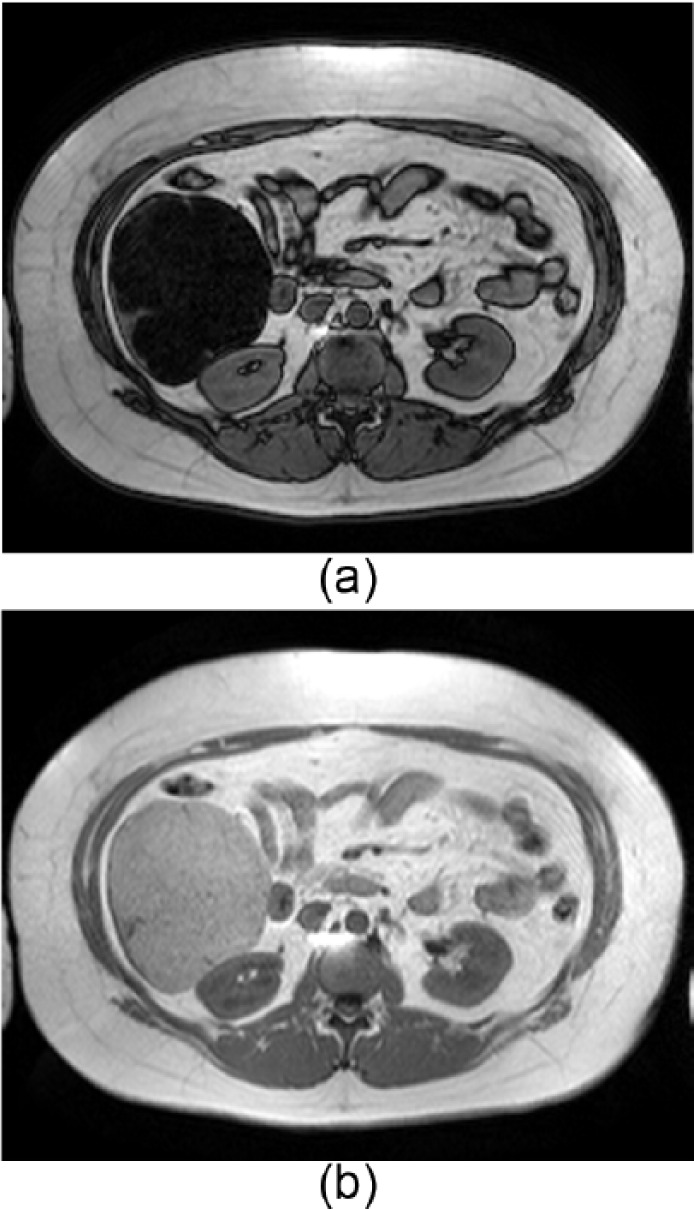
In- and out-of-phase MRI of a typical case of histochemistry proved H-HCA which was resected. Note the diffuse and homogenous signal drop-off on the out-of-phase image (a) versus the in-phase image (b). This correlates with diffuse intralesional fat identified by histology. MRI differentiation between H-HCA and IHCA would not be possible when the signal drop is more heterogeneous.
HCA, hepatocellular adenoma; H-HCA, HNF1A-mutated HCA; IHCA, inflammatory HCA; MRI, magnetic resonance imaging.
The MRI features of b-HCA are not well defined, principally because these lesions are comparatively rare. Van Aalten and colleagues reported poorly delimited, high-signal intensity areas, to be typical of this subtype (5/7, 71%), but additional investigations are warranted [van Aalten et al. 2011a]. Table 2 shows the various MRI features reported in the literature for b-HCA, although, where reported, these features are inconsistent. Despite significant numbers of false negatives, the specificity of these MRI features is very high, leading us to conclude that if any one of these signs are present, a diagnosis of the corresponding MRI subtype can be made with some certainty. Larger datasets will be needed to determine the true value of MRI in HCA imaging for all subtypes; currently, this technique is of most use in evaluating prognosis.
Table 2.
Recently published b-HCAs with their typical characteristics defined by MRI. Note the low prevalence in the literature of MRI data, with inconsistent findings.
| Authors | Year of publication | Number of β-catenin HCA | MRI findings |
|---|---|---|---|
| [Van Aalten et al. 2011a] | 2012 | 7 | Vaguely defined scar on T2W sequences (3 cases) |
| [Laumonier et al. 2008] | 2008 | 5 | Marked hyperintensity on T2W sequences and persistent delayed enhancement (3 cases) Isointensity on T2W sequences, with strong arterial enhancement and delayed washout (2 cases) |
| [Yoneda et al. 2012] | 2012 | 1 | Vaguely defined scar on T2W sequences |
b-HCA, β-catenin positive HCA; HCA, hepatocellular adenoma; MRI, magnetic resonance imaging; T2W, T2-weighted.
Reviewing the known complications
Intralesional bleeding
On reviewing the recent literature, van Aalten and colleagues detected evidence of hemorrhage in 27.2% of all patients (315/1160) with one or more HCAs, giving a 15.8% chance of hemorrhage for every HCA (118/748) [van Aalten et al. 2012]. Acute rupture and intraperitoneal bleeding were reported in 17.5% of patients. A size for the smallest HCAs showing hemorrhage was reported for 13 of the 28 articles reviewed; hemorrhage generally arose in the larger lesions (>5 cm), although smaller lesions could also bleed (Table 3), albeit at much lower rates. These data should be interpreted with caution, as only the resected cases were included. The actual chance of bleeding in the different subtypes is likely to be significantly lower. The risk of bleeding was inconsistent across the subtypes of HCA: IHCA showed a higher propensity for macroscopic hemorrhage (30%), than H-HCA (8%) [Dokmak et al. 2009] which can presumably be attributed to the larger number of venous structures, or telangiectatic changes in this subtype.
Table 3.
Summary of the findings of an earlier review of 12 articles in which the percentage of hemorrhaged HCAs, and minimal lesion sizes, were reported. Hemorrhage occurred mostly in larger HCAs (>5 cm; minimally 42.2%), but smaller lesions also showed some bleeding (range 8.3–11.5%).
| Series | Patients with hemorrhaged HCA | Size of smallest HCA (cm) | Percentage <5 cm of total (%) |
|---|---|---|---|
| [Reddy et al. 2001] | 3 of 25 | 4 | – |
| [Hung et al. 2001] | 4 of 25 | 4.2 | – |
| [Toso et al. 2005] | 10 of 25 | 1.7 | – |
| [Cho et al. 2008] | 12 of 41 | 1 | 8.3 (1/12) |
| [Bioulac-Sage et al. 2009] | 23 of 128 | <5 | – |
| [Edmondson et al. 1976; Dokmak et al. 2009] | 26 of 122 | <5 | 11.5 (3/26) |
| [Edmondson et al. 1976] | 10 of 42 | >5 | 0 |
| [Leese et al. 1988] | 2 of 24 | 5 | 0 |
| [Ault et al. 1996] | 4 of 12 | 6 | 0 |
| [Closset et al. 2000] | 7 of 16 | 7 | 0 |
| [Deneve et al. 2009] | 31 of 124 | >5 | 0 |
| [Chung et al. 1995] | – | 5 | 0 |
HCA, hepatocellular adenoma.
Although there may be a difference in prevalence of internal bleeding, all subtypes bear this intrinsic risk [Laumonier et al. 2008; Dokmak et al. 2009; Ronot et al. 2011; van Aalten et al. 2011b], which diminishes the utility of subtype classification in terms of the clinical management of this risk. Furthermore, more data are needed to prove any correlate between reduced bleeding and the H-HCA subtype.
Bieze and colleagues described a series of 45 patients with 195 lesions. In this cohort, there was a tendency to an enhanced risk of bleeding when the lesion was located in the left lateral liver (11/32 versus 31/163 in other regions), and showed exophytic growth (16/24 versus 9/82) [Bieze et al. 2014] (Figure 1). The latter phenomenon is probably due to the subcapsular location, with no intrinsic capsule, and minimal surrounding liver with which to prevent rupture of the hematoma into the abdominal cavity. However, no other data are available to support this theory, and preventive treatment in these cases does not appear to be warranted.
As for the clinical application of these findings, there is no evidence that supports the use of subtype classification in the stratification and management of individual patients. Moreover, size still remains the most important marker to predict those at risk for larger bleeding in follow up.
Malignant transformation
Malignant transformation of HCA to HCC is rarely reported, but is an accepted risk, particularly when the diameter of the adenoma exceeds 5 cm (Figure 2) [Stoot et al. 2010; Grazioli et al. 2013]. In a systematic review, Stoot and colleagues reported an overall frequency of malignant transformation of 4.2% for HCAs [Stoot et al. 2010] (67 of 1635 HCAs, CI 0–100%). Only three cases showed malignant transformation for tumors <5 cm in diameter, which represented 4.4% of the total number of HCCs arising from HCAs (3 out of 67). As suggested for the internal bleeding data, these reports should be interpreted with caution.
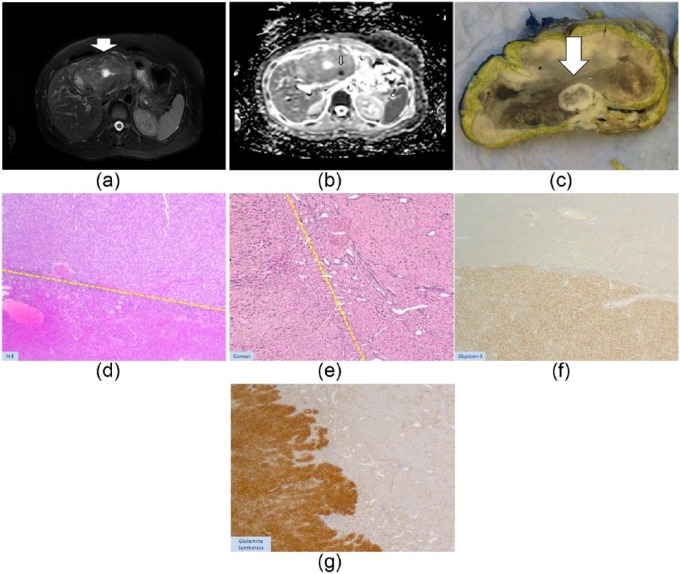
Figure 2.
A 32-year-old female using oral contraceptives with a 11 cm lesion in the liver. Based on MRI this lesion was compatible with a HCA. However, both on T2-weighting (Figure 1(a)) as on the images after contrast injection the lesion appeared heterogeneous with a focus of diffusion restriction (typically a low ADC value, (b)). Diffusion restriction is thought to be a typical sign of malignancy in liver lesions. Based on the findings above and because the lesion was larger than 5 cm, the lesion was resected 3 months later. On gross pathology there was a focal nidus (Figure 1(c), arrow, concordant with the nidus on MRI) which appeared to be an HCC in a HCA (‘nodule-into-nodule’). On histology (H–E × 25, (d)) at the interface HCA/HCC, the upper part of the tumor showed proliferation of hepatocytes without obvious cytological anomalies, intermingled with thin/isolated vessels (down side of dotted line), favoring an HCA. ‘Nodule-into-nodule’ consists of clearer cells with mild atypia (above dotted-line, (d)), disorganized or decreased reticulin fibers (e) and obvious positivity for Glypican-3 (f), favoring an HCC (well differentiated). (g) GS-staining pattern at the periphery of the HCA. Glypican-3, Serum Amyloid A and CRP were negative in the HCA. β-catenin staining showed only membranous expression. Based on the above we interpreted this HCA as a b-HCA.
CRP, C-reactive protein; HCA, hepatocellular adenoma; HCC, hepatocellular carcinoma; GS staining, glutamine synthetase immunostaining indicative of b-HCA; MRI, magnetic resonance imaging.
Of the four HCA subtypes, (exon 3) b-HCAs are known to trigger a potent mitogenic signaling pathway that is prominent in HCC [Zucman-Rossi et al. 2007; Chu and Moon, 2013; Pilati et al. 2014], which suggests a positive correlate between the two. Zucman-Rossi and colleagues reported an incidence of HCCs, or borderline malignant tumors in b-HCAs, of up to 46%; this malignant progression was seldom seen in other subtypes [Zucman-Rossi et al. 2007], and was over-represented for male patents (5 cases, 38%; p = 0.02) [Hussain et al. 2006]. Since the β-catenin pathway can also be activated in IHCA [van Aalten et al. 2011b], both the b-HCA and IHCA subtypes may necessitate more aggressive treatment than either the H-HCA or UHCA, although the clinical relevance of this determination has yet to be broadly accepted. In follow up, malignant progression of HCA to HCC has only rarely been demonstrated, with questionable quality of the imaging data for those rare, reported cases. Therefore it is presently difficult to prove that HCC is a transition from HCA, although the presence of β-catenin has been suggested as a criterion for the selection of HCA, or well-defined HCC, for resection [Bioulac-Sage et al. 2013]. Interestingly, Figure 2 shows a lesion with a typical nodule-in-nodule appearance which suggests a form of transition from HCA to HCC. Another problem is that corroboration of the pathology is seldom available, due to the fact that biopsies of HCA are rarely performed, with diagnoses generally made with MRI [Hussain et al. 2006]. A final diagnosis of b-HCA based solely on MRI findings would be helpful, but the MRI findings published to date for this subtype are sparse (Table 2). Finally, it should be mentioned that HCA shows a higher risk of malignant transformation in men [Farges et al. 2011]. In these cases, the possibility of hepatitis, an underlying glycogen storage disease (Figure 3), or sex steroid hormone abuse, should all be considered as all predispose to HCC [Yoneda et al. 2012]. A more aggressive treatment is advised in such cases, even for lesions <5 cm.
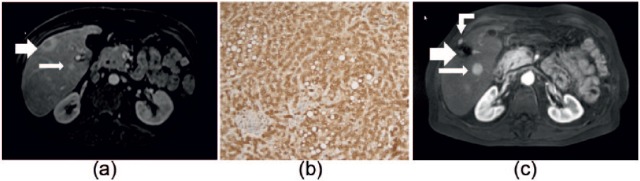
Figure 3.
A 50-year-old male with multiple hypervascular lesions. These lesions were diagnosed as HCA or HCC based on imaging and clinical (glycogen storage disease) findings. (a) An axial MR image, with T1 weighting, after contrast injection in the arterial phase. In segment 5, a small lesion with a cystic central portion (large arrow) was biopsied, and subsequently diagnosed as HCC following positive GS staining with negative β-catenin staining. Posteriorly, a second, smaller lesion was visualized (<1 cm, small arrow). Histologic sample of a lesion with diffuse GS-positivity (b). Axial MR image with T1-weighing after contrast injection in the arterial phase (c). In this image, taken 3 years later, the second lesion has grown (now 3 cm, small arrow). The large arrow shows the resection/ablated part of the liver (from lesion 1). A new hypervascular lesion (curved arrow) was also detected outside the liver, which proved to be a trajectory metastasis. These lesions (large arrow, curved arrow) were successfully ablated. This patient is currently being followed at regular, short intervals, and is on the waiting list for a liver transplantation.
GS staining, glutamine synthetase immunostaining indicative of b-HCA, even with a negative β-catenin staining; HCA, hepatocellular adenoma; HCC, hepatocellular carcinoma; MR, magnetic resonance.
Finally, according to the recent literature, H-HCA almost never degenerate into HCC, although some very rare cases have been reported [Stueck et al. 2015]. The low risk of H-HCA degeneration may help to simplify the management of liver adenomas as will be discussed later.
As for clinical application, mainly b-HCA and IHCA are prone to malignant degeneration, and mostly if >5 cm. In these instances, invasive treatment is recommended.
Pregnancy
Women with HCA who are pregnant, or wish to become pregnant (Figure 4), should be closely monitored for HCA size (with ultrasound or MRI) during their pregnancy, due to the tendency of the lesion to grow, especially during the third trimester when high levels of estrogens are present [Cobey and Salem, 2004]. Hormone-induced growth, and possible rupture, may result in potentially lethal complications for the mother and unborn child. Treatment of HCA during pregnancy may be indicated when the lesion shows signs of growth or bleeding, however specific figures for the risk of HCA complication during pregnancy are not yet available.
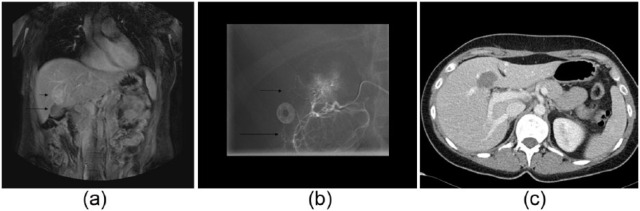
Figure 4.
A 25-year-old female with a MRI diagnosis of single HCA, probably inflammatory subtype. As the patient wished to become pregnant, despite growth of her HCA, a decision to treat with TAE was taken. Coronal MR image with T1-weighting of the upper abdomen (a). A hypervascular HCA is indicated (small arrow), adjacent to the gallbladder (long arrow). Ablation was contraindicated due to the close proximity of the gallbladder. Angiogram (b) before TAE showing an arterial tumor ‘blush’ in the HCA (short arrow), with the gallbladder perfused by the same local hepatic artery division (long arrow). This finding contraindicated TAE due to the possibility of gallbladder necrosis following infarction. Instead, a decision to operate was made, with resection of the gallbladder, and subsequent intraoperative RFA of the HCA. Axial postoperative CT image after contrast injection in the venous phase (c). The gallbladder was resected, in combination with intraoperative RFA (arrow).
CT, computerized tomography; HCA, hepatocellular adenoma; RFA, radiofrequency ablation; TAE, transcatheter arterial embolization.
Whether some subtypes are more prone to complications during pregnancy is not known, mainly because the majority is diagnosed non-invasively.
The choice of follow up, surgery, radiofrequency ablation (RFA) or transcatheter arterial embolization (TAE) for the treatment of HCAs in pregnancy is often a matter of debate. Surgery of lesions located at the periphery of the liver can be performed safely within the first or second trimester, and will usually be indicated by the size of the lesion, and its change in size. Radiation exposure or exposure to iodinated contrast media during RFA or TAE may be contraindicated during the early phase of pregnancy, with the treatment of smaller lesions not being indicated. Given the increased risk of hemorrhage in larger HCAs (>5 cm), or when a previous pregnancy was complicated by either minor or major bleeding, we currently advocate a preemptive treatment strategy before pregnancy, as proposed by Broker and colleagues [Broker et al. 2012]. Whenever a HCA is discovered during pregnancy, the second trimester is the optimal moment for invasive treatment, if indicated, as anesthesia is well tolerated at this stage, and the fetus is not yet so large as to interfere with liver surgery [Parangi et al. 2007].
Liver adenomatosis
Hepatic adenomatosis (HCAs more than 10) is regarded by some authors as a different entity [Barthelmes and Tait, 2005; Frulio et al. 2014]. There seems not to be a strong association with estrogen or anabolic steroid use [Chiche et al. 2000; Grazioli et al. 2000]. However, there is a strong association with glycogen storage disease [Chiche et al. 2000; Frulio et al. 2014]. Mostly, these adenomas are of the H-HCA and IHCA subtypes [Frulio et al. 2014]. The nodules in hepatocellular adenomatosis are often of the same subtypes. Although one might assume that multiple HCAs increase the propensity for lesional bleeding, previous data have shown no significant difference in macroscopic bleeding between single and multiple HCAs (p < 0.155) [Dokmak et al. 2009]. According to literature there seems to be no indication to suggest that the risk of malignant transformation is increased in hepatic adenomatosis compared with solitary HCAs [Barthelmes and Tait, 2005]. However, hepatic adenomatosis are more often found in glycogen storage disease and in man [Chiche et al. 2000], and as such at risk for increased malignant potential. Presently, there is no systematic review available which evaluates the malignant potential of hepatic adenomatosis. As for clinical management, and there are no data suggesting that hepatic adenomatosis should be treated differently from solitary HCAs.
Biopsy in the management of HCA
Since the introduction of the HCA subclassification system, several authors have attempted to further refine the diagnostic work-up using additional techniques, including immunohistochemistry [Zucman-Rossi et al. 2006]. The primary motivation for the introduction of additional biopsies was the prospect of identifying HCAs with greater malignant potential (such as exon-3 β-catenin mutated HCAs). There seem to be no unique MRI features with which to assign a b-HCA subtype risk, which offers one argument for the expansion of the use of diagnostic biopsy in order to arrive at a correct diagnosis.
However, at present there is no consensus regarding the diagnostic work-up of HCA [Nault et al. 2013; Marrero et al. 2014]. Nault and colleagues regard histologic analysis as the backbone of HCA diagnosis, with the detection or exclusion of b-HCA as the main input [Nault et al. 2013]. They argue that biopsy should be offered in all cases of HCA <5 cm with no typical MRI sign of H-HCA. Lesions >5 cm do not require biopsy since they are all preferably resected. In our opinion, and in accordance with recent American College of Gastroenterology guidelines for liver lesions, the diagnostic workup of suspected HCA should be based primarily on MRI findings, with biopsy in cases where the lesion cannot be clearly differentiated from FNH [Marrero et al. 2014]. Other indications for biopsy are an atypical presentation of the HCA on imaging, with the main differential diagnosis being HCC in a noncirrhotic liver [Marrero et al. 2014].
The biopsy of all HCAs (with the exclusion of typical H-HCAs based on MRI) found by imaging would be impractical. Most patients with HCA are young, with minor symptoms on malignant progression; invasive procedures should preferably be avoided. While the risk of bleeding complications is very low when using an 18G core needle biopsy (0.6%) [Haage et al. 1999; Kadri Aribas et al. 2010; Aribas et al., 2012], the risks are not negligible, and deaths due to bleeding complications have been reported [Stattaus et al. 2007]. In our practice a biopsy has not been performed to date, except where the diagnosis of a specific adenoma subtype was expected to alter clinical management.
Unquestionably, a biopsy for further characterization may add important information in well-defined cases. For example, a biopsy with the additional help of immunostaining could facilitate better discrimination between HCA and FNH, as shown in a large retrospective study in France where the investigators compared biopsies against a final diagnosis based on surgical resection [Bioulac-Sage et al. 2012]. A total of 239 needle biopsies were compared with the final diagnosis made on resection. A difference in sensitivity of 74.3% with immunostaining versus 58.6% achieved with routine analyses without GS or other molecular features was reported [Bioulac-Sage et al. 2012]. These data suggest that immunostaining should be made available in centers that routinely treat HCAs.
What is the best approach in cases where differentiation between HCA and HCC is not evident based on MRI? In cases where there is a major suspicion of malignancy (e.g. HCC in noncirrhotic liver) based on a combination of clinical findings, size of the lesion, increased serum α-fetoprotein, and MRI findings (such as heterogeneous presentation with heterogeneous enhancement, washout, and true capsule) (Figure 2), resection without prior biopsy can be recommended. Although biopsy of each suspect lesion would undoubtedly help in detecting HCC, this approach may be impractical due to the significant level of false-negative findings, the chances of seeding (Figure 3), and the enhanced risk of bleeding, which is particularly relevant when multiple biopsies are taken. Furthermore, in cases with a typical presentation, a biopsy will not influence the decision to remove the lesion. Therefore, we suggest to biopsy in selected cases only. Interestingly, the HCC literature documents a similar debate on whether it is acceptable to diagnose HCC in a cirrhotic liver based solely on MRI findings, or whether the use of routine biopsies should be advocated for all suspected lesions in patients with liver cirrhosis [Sherman and Bruix, 2015; Torbenson and Schirmacher, 2015]. Even in high grade dysplastic nodes, follow up by imaging is still preferred above biopsies.
As for daily practice, we recommend biopsy only in very selected cases where HCA cannot be differentiated from FNH with any imaging modality. The clinical repercussion of a wrong diagnosis of either HCA or FNH can have a large influence on a patient’s future in terms of treatment and prognosis. When there are signs of malignancy patients should preferentially be forwarded to an experienced referral center for further evaluation. One should be aware that in some cases MRI or biopsy will be unable to differentiate between HCA and well-differentiated HCA.
Treatment options for HCA
Historically, HCAs were treated with a wait-and-see policy, with surgical intervention preferred for larger (>5 cm) growing tumors. Current management options for HCAs may also include RFA, and TAE, mainly due to the advantages of these minimally-invasive techniques. In the following section we will discuss routine, as well as less commonly used HCA treatment options, with a focus on minimally-invasive, image-guided, treatment options.
Conservative treatment
When HCAs are <5 cm, or regress (to <5 cm) following cessation of OCs, with no further growth detected, a wait-and-see policy is warranted. Although no widely accepted approach has yet been published, we prefer to schedule a patient for follow up, including MRI, or ultrasound in a yearly follow up until menopause.
Surgery
Surgery has long been considered the treatment of choice because complete surgical removal of the lesion can be achieved in a controlled and relatively safe manner. Elective surgical resection is considered for all lesions >5 cm in diameter. With a mortality of 1.1% (review by Lin and colleagues, n = 170), surgery is a relatively safe procedure. In one review, 93% of patients with ruptured, or nonruptured HCAs, were primarily treated with surgery, with complications that included two deaths, one biloma, one bile leakage, one infection, and one case of sepsis [Lin et al. 2011]. In another, single-center retrospective analysis of 41 cases, no perioperative mortality was found, and only minor complications arose. These included pleural effusion requiring drainage (n = 2), pneumonia (n = 1), and wound infection (n = 1) [Cho et al. 2008].
In the latter study, nine cases were operated on laparoscopically, a technique that is increasingly popular, where appropriate. De’Angelis and colleagues described 62 HCA patients who underwent either an open procedure or laparoscopy [De’Angelis et al. 2014]. They found no difference in postoperative morbidity and zero mortality, with no longterm complications or recurrence of HCA. However, patients with smaller lesions were preferentially treated with laparoscopy (68 versus 9). These authors concluded that laparoscopic liver resections may be limited by lesion size and location, and that the technique requires advanced surgical skills. Given the precision required, robotic surgery may prove to be useful in the future, and could reduce complications; we await further evaluation of its efficacy [Jackson et al. 2015].
In rare circumstances, the treatment of HCA may also involve liver transplantation, a procedure described in a case report by Venarecci and colleagues [Vennarecci et al. 2013]. Obesity, steatosis, and diabetes, are frequent co-morbidities in patients with HCAs, particularly the inflammatory subtype. These factors, and especially obesity, make surgery less attractive. For those patients who are poor candidates for surgery (centrally-located lesions, multiple adenomas, or morbid obesity), RFA and TAE may instead be offered.
Radiofrequency ablation
RFA is a minimally invasive technique used in the treatment of HCC, other liver lesions such as colorectal metastases [Solbiati et al. 2001; Cabibbo et al. 2013], and HCAs [van Aalten et al. 2010; van Vledder et al. 2011]. Laparoscopic RFA, or perioperative RFA, may also be considered when the anatomical location [i.e. close proximity to the bowel or gallbladder (Figure 3)] leads to an increased risk using a percutaneous approach. The use of RFA in the treatment of HCAs has only been described in small case series.
Van Vledder and colleagues described one case series including 45 HCAs, in 18 patients, that were ablated in 32 RFA sessions (open, n = 4; percutaneous, n = 28) [Van Vledder et al. 2011]. A total of 26 of 45 HCAs were successfully treated in one RFA session, with no visible residual disease. A further nine HCAs were totally ablated following a second RFA session. There were 3 HCAs that required 3 or more RFA sessions, with all but 7 of the 45 HCAs totally ablated after 3 or more sessions. The treated HCAs had a median size of 3.0 cm (ranging from 0.8 to 7.3 cm). Only minor complications were attributable to the RFA procedure; none of which required additional intervention (class A according to the Society of Interventional Radiology scoring system for complications). A single class D major complication was reported; a cerebrovascular accident during open surgery combined with RFA. Though severe, this complication was linked to anesthesiological and hemodynamic changes during laparotomy, rather than the RFA procedure. In conclusion, RFA can be effectively and safely used in the treatment of HCA, although multiple sessions may be required for larger lesions.
In a review of HCA cases reported between 1998–2008, Lin and colleagues identified 356 HCA patients in reports from China, Europe, North America, and South-East Asia [Lin et al. 2011]. Only 14 (3.9%) of these cases were treated with RFA, and no severe complications were reported. However, no results in terms of efficacy were provided. In 2008, Rhim and colleagues. assessed the therapeutic efficacy and safety of RFA for HCAs [Rhim et al. 2008], and reported their initial experience in 10 patients with 12 HCAs. Tumor sizes ranged from 1.5 to 4.5 cm. As no complications were reported after RFA, and no progression or recurrence was noted, RFA was considered a safe and effective treatment option. A minimal ablative margin of 5 mm is recommended during the radical treatment of lesions when using thermal ablation of HCCs. It is presently unclear if a similar margin should be applied to HCAs, as these lesions are assumed to be benign. No data are yet available regarding the ideal ablative margin during thermal ablation of HCAs. In our opinion, volume reduction is more important than an ablation margin, as the former correlates strongly with a risk of bleeding, and malignant transformation. Follow-up imaging after both RFA, and TAE, is routinely performed in our institution by MRI, 6 months after treatment.
As HCAs requiring treatment are generally large (>5 cm), a promising alternative for RFA may be microwave ablation (MWA). Based on preliminary data, MWA was shown to produce larger ablation zones, in less time, in patients treated for HCC and colorectal metastases. MWA delivers high frequency microwaves (0.9–2.4 GHz) into tumor tissue, which causes fast spinning of molecules and thus destroys tissue. No data are available concerning efficacy and complications after MWA. MWA has specific advantages over RFA, such as larger ablation zones, higher treatment temperatures, and less susceptibility to local cooling by adjacent large blood vessels (heatsink). Although larger zones of ablation can probably be achieved by using single-electrode needles and MWA to treat HCAs, to the best of our knowledge, no data currently exists to substantiate this idea.
Transcatheter arterial embolization
As HCAs are hypervascular arterial lesions, bleeding may be treated by selective transcatheter arterial embolization (TAE) in cases where patients present with hemodynamic instability. Embolization of HCAs is a safe but relatively challenging procedure due to multiple small feeding vessels [van Aalten et al. 2010]. Nonetheless, in cases of spontaneous rupture and bleeding, TAE should be considered as a first line treatment as it is highly successful and minimally invasive in an acute setting. Although high success rates have been described for TAE, there is only a sparse literature comparing TAE with either surgery, or conservative management. In one study Karkar and colleagues described 52 patients with 100 HCAs, of which 37% were treated with TAE [Karkar et al. 2013]. In most of these cases TAE was performed in a (semi) elective setting, with rupture and hemorrhage as indications in 20%, and suspected malignancy in 56%. Success rates of up to 92% were claimed for TAE (32), and of the 37 HCAs embolized, only 3 required secondary interventions (8.1%). All other embolized lesions were treated successfully; some disappeared (5/34), most decreased in size (22/34), or remained stable (7/34). Recurrence rates were also low. It is worth noting that the HCAs embolized were relatively small, with a median diameter of 2.6 cm. However, we feel that resection is indicated if malignancy is suspected and no contraindications for surgery exist. In a report by Erdogan and colleagues, six HCAs were primarily embolized [Erdogan et al. 2007], four because of bleeding, and two electively, 1 year after bleeding. No complications were reported, and all HCAs ceased bleeding. A total of two of the lesions were resected after embolization, two regressed visibly on follow-up imaging, and two HCAs were only seen after resorption of hematoma on follow-up imaging. These last two patients were managed with a wait-and-see policy. In a retrospective study by Dheodar and colleagues, 17 embolizations were successfully performed in eight patients [Deodhar et al. 2011], with five patients undergoing more than one embolization. The mean size of the treated HCAs was 3.6 cm, and regression was noted in all embolized HCAs after embolization. As noted by Karkar and colleagues, TAE may also be used in an elective setting where no acute intervention is needed [Karkar et al. 2013]. This approach is of clinical interest and deserves further consideration.
Proposed management strategy
One of the major discussions on the management strategy of hepatocellular adenomas involves the clinical application of these recent findings in the dynamic field of adenoma subtyping. How should we take into account these new insights into daily practice? In our view, more data are needed to implement this subclassification in the diagnosis and treatment of adenomas, balancing the risk of an invasive liver biopsy with the additional benefits in terms of individualized therapy and prognostic stratification. A major effort should be made by expert centers involved in the diagnosis and treatment of hepatocellular adenomas to work on this collaboratively, preferably in research setting, to gather more data on the potential benefits for an individual patient.
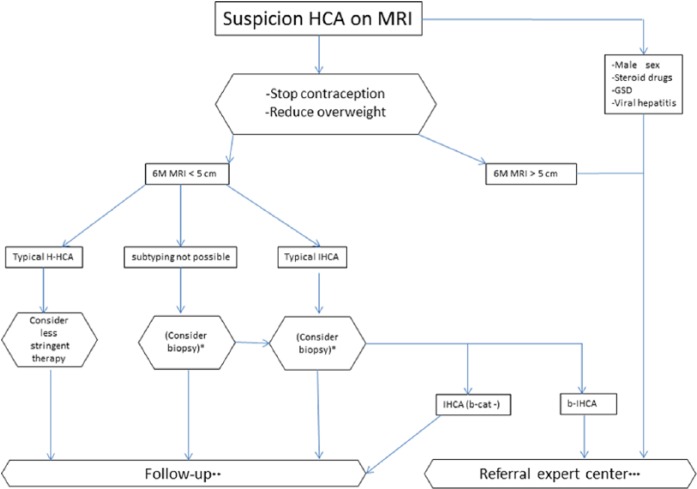
Based on our review of the current literature, we propose a management strategy applicable to most cases in which there is a suspicion of HCA (Figure 5). This decision tree may not be appropriate for all patients; for some, a more customized approach may be required. In standard situations, mainly when a lesion is >5 cm, OCs should be stopped and MRI performed after 6 months. If the lesion has contracted to <5 cm, clear signs of an H-HCA should be ruled in or out (Figure 1). In scenarios where H-HCAs are subsequently identified, therapy can be less aggressive as inherent malignant progression is very low. Follow up is then advised, initially every 6 months, and if the lesion shows no further alteration, follow up can be stopped or simply repeated yearly until menopause [Marrero et al. 2014]. Since typical H-HCAs are easily identified using out-of-phase MRI sequences, intravenous contrast can be obviated at follow up. A second option is to apply sonography during follow up which is cheaper and less inconvenient for patients. For small lesions (<5 cm) that are categorized as IHCA, therapy should ordinarily not be altered (in standard cases). However, some may opt for a biopsy in order to exclude β-catenin mutation. This could also be the case if a subclassification cannot be made with MRI. For larger lesions (>5cm) with a β-catenin mutation or if the patient has an aggravating status such as male sex, steroid use, glycogen storage disease, or underlying viral hepatitis, intervention may be the first alternative. Treatment can be primarily surgical, and, in selected cases, RFA or TAE may be used. Depending on the underlying risks (obesity, diabetes, centrally located tumor), the best option would be to evaluate these patients in an expert referral center.
Figure 5.
The management decision tree used in our tertiary academic medical center. This decision tree may not be appropriate for all patients; for some, a customized approach should be considered.
*One option is to biopsy those lesions where a subtyping diagnosis by imaging is impossible to achieve, or those lesions with typical signs of IHCA. Currently, this option is considered impractical given the large number of biopsies involved.
**Follow up is advised initially, at 6-monthly intervals. Thereafter, if the lesion shows no further alteration, follow up can be stopped, or repeated yearly until menopause. If the lesion is a typical H-HCA, follow up can be less stringent, possibly involving sonography, or MRI without contrast.
***Referral to an expert center is advised for the evaluation of any indication requiring intervention. This decision should be taken with consideration of contraindications (obesity, diabetes, centrally located tumor, ASA classification). Treatment can be primarily surgical, and in selected cases, RFA or local embolization.
b-HCA, β-catenin activated HCA; GSD, glycogen storage disease; HCA, hepatocellular adenoma; H-HCA, HNF1A-mutated HCA; IHCA, inflammatory HCA; M, months; RFA, radiofrequency ablation.
Conclusion
MRI is the preferred tool in the management of HCA, its current principle use being size evaluation (cutoff 5 cm), identification of signs of malignancy, and exclusion of H-HCAs, recognized for their benign course and permitting a conservative approach. Until now, there is no reliable MRI characteristic to diagnose non-invasively b-HCA, being the most important lesion to diagnose as it may have the highest malignant potential.
Conservative management remains the strategy of choice for uncomplicated small HCAs, and surgery may be indicated if imaging shows heterogeneous signal and growing smaller lesions suspected of being highly-differentiated HCC. Further prospective cohort studies are warranted to support the choices made between these treatment strategies and to determine the role of biopsy in the subclassification of HCAs. In cases where a HCA requires treatment, and surgical resection of smaller lesions (<3 cm) carries an unacceptable risk, RFA or TAE may be considered.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Maarten G. Thomeer, Department of Radiology, Erasmus Medical Center Rotterdam, P.O Box 2040, 3015 CE Rotterdam, The Netherlands.
Mirelle Broker, Department of Surgery, Erasmus MC University Medical Center, Rotterdam, The Netherlands.
Joanne Verheij, Department of Pathology, Amsterdam University Medical Center, Amsterdam, The Netherlands.
Michael Doukas, Department of pathology, Erasmus MC University Medical Center, Rotterdam, The Netherlands.
Turkan Terkivatan, Department of Surgery, Erasmus MC University Medical Center, Rotterdam, The Netherlands.
Diederick Bijdevaate, Department of Radiology, Erasmus MC University Medical Center, Rotterdam, The Netherlands.
Robert A. De Man, Department of Gastroenterology and Hepatology, Erasmus MC University Medical Center, Rotterdam, The Netherlands
Adriaan Moelker, Department of Radiology, Erasmus MC University Medical Center, Rotterdam, The Netherlands.
Jan N. IJzermans, Department of Surgery, Erasmus MC University Medical Center, Rotterdam, The Netherlands
References
- Agarwal S., Fuentes-Orrego J., Arnason T., Misdraji J., Jhaveri K., Harisinghani M., et al. (2014) Inflammatory hepatocellular adenomas can mimic focal nodular hyperplasia on gadoxetic acid-enhanced MRI. AJR Am J Roentgenol 203: W408–414. [DOI] [PubMed] [Google Scholar]
- Aribas B., Arda K., Ciledag N., Aktas E., Yakut F., Kavak S., et al. (2012) Accuracy and safety of percutaneous US-guided needle biopsies in specific focal liver lesions: comparison of large and small needles in 1300 patients. Panminerva Med 54: 233–239. [PubMed] [Google Scholar]
- Ault G., Wren S., Ralls P., Reynolds T., Stain S. (1996) Selective management of hepatic adenomas. Am Surg 62: 825–829. [PubMed] [Google Scholar]
- Barthelmes L., Tait I. (2005) Liver cell adenoma and liver cell adenomatosis. HPB (Oxford) 7:186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum J., Bookstein J., Holtz F., Klein E. (1973) Possible Association between benign hepatomas and oral contraceptives. Lancet 2:926–929. [DOI] [PubMed] [Google Scholar]
- Bieze M., Phoa S., Verheij J., van Lienden K., van Gulik T. (2014) Risk factors for bleeding in hepatocellular adenoma. Br J Surg 101: 847–855. [DOI] [PubMed] [Google Scholar]
- Bieze M., van Den Esschert J., Nio C., Verheij J., Reitsma J., Terpstra V., et al. (2012) Diagnostic accuracy of mri in differentiating hepatocellular adenoma from focal nodular hyperplasia: prospective study of the additional value of gadoxetate disodium. AJR Am J Roentgenol 199: 26–34. [DOI] [PubMed] [Google Scholar]
- Bioulac-Sage P., Balabaud C., Bedossa P., Scoazec J., Chiche L., Dhillon A., et al. (2007a) Pathological diagnosis of liver cell adenoma and focal nodular hyperplasia: Bordeaux update. J Hepatol 46: 521–527. [DOI] [PubMed] [Google Scholar]
- Bioulac-Sage P., Balabaud C., Zucman-Rossi J. (2010) Focal nodular hyperplasia, hepatocellular adenomas: past, present, future. Gastroenterol Clin Biol 34: 355–358. [DOI] [PubMed] [Google Scholar]
- Bioulac-Sage P., Cubel G., Taouji S., Scoazec J., Leteurtre E., Paradis V., et al. (2012) Immunohistochemical markers on needle biopsies are helpful for the diagnosis of focal nodular hyperplasia and hepatocellular adenoma subtypes. Am J Surg Pathol 36: 1691–1699. [DOI] [PubMed] [Google Scholar]
- Bioulac-Sage P., Laumonier H., Couchy G., Le Bail B., Sa Cunha A., Rullier A., et al. (2009) Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology 50: 481–489. [DOI] [PubMed] [Google Scholar]
- Bioulac-Sage P., Rebouissou S., Thomas C., Blanc J., Saric J., Sa Cunha A., et al. (2007b) Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology 46: 740–748. [DOI] [PubMed] [Google Scholar]
- Bioulac-Sage P., Sempoux C., Possenti L., Frulio N., Laumonier H., Laurent C., et al. (2013) Pathological diagnosis of hepatocellular cellular adenoma according to the clinical context. Int J Hepatol 2013: 253261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc J., Frulio N., Chiche L., Sempoux C., Annet L., Hubert C., et al. (2015) Hepatocellular adenoma management: call for shared guidelines and multidisciplinary approach. Clin Res Hepatol Gastroenterol 39: 180–187. [DOI] [PubMed] [Google Scholar]
- Broker M., Ijzermans J., van Aalten S., De Man R., Terkivatan T. (2012) The management of pregnancy in women with hepatocellular adenoma: a plea for an individualized approach. Int J Hepatol 2012: 725735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabibbo G., Maida M., Genco C., Alessi N., Peralta M., Butera G., et al. (2013) Survival of patients with hepatocellular carcinoma (HCC) treated by percutaneous radio-frequency ablation (RFA) is affected by complete radiological response. PLoS One 8: e70016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiche L., Dao T., Salame E., Galais M., Bouvard N., Schmutz G., et al. (2000) Liver adenomatosis: reappraisal, diagnosis, and surgical management: eight new cases and review of the literature. Ann Surg 231: 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S., Marsh J., Steel J., Holloway S., Heckman J., Ochoa E., et al. (2008) Surgical management of hepatocellular adenoma: take it or leave it? Ann Surg Oncol 15: 2795–2803. [DOI] [PubMed] [Google Scholar]
- Chu H., Moon W. (2013) Beta-catenin activated hepatocellular adenoma. Clin Mol Hepatol 19: 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K., Mayo-Smith W., Saini S., Rahmouni A., Golli M., Mathieu D. (1995) Hepatocellular adenoma: MR imaging features with pathologic correlation. AJR Am J Roentgenol 165: 303–308. [DOI] [PubMed] [Google Scholar]
- Closset J., Veys I., Peny M., Braude P., van Gansbeke D., Lambilliotte J., et al. (2000) Retrospective analysis of 29 patients surgically treated for hepatocellular adenoma or focal nodular hyperplasia. Hepatogastroenterology 47: 1382–1384. [PubMed] [Google Scholar]
- Cobey F., Salem R. (2004) A review of liver masses in pregnancy and a proposed algorithm for their diagnosis and management. Am J Surg 187: 181–191. [DOI] [PubMed] [Google Scholar]
- De’Angelis N., Memeo R., Calderaro J., Felli E., Salloum C., Compagnon P., et al. (2014) Open and laparoscopic resection of hepatocellular adenoma: trends over 23 years at a specialist hepatobiliary unit. HPB (Oxford) 16: 783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneve J., Pawlik T., Cunningham S., Clary B., Reddy S., Scoggins C., et al. (2009) Liver cell adenoma: a multicenter analysis of risk factors for rupture and malignancy. Ann Surg Oncol 16: 640–648. [DOI] [PubMed] [Google Scholar]
- Deodhar A., Brody L., Covey A., Brown K., Getrajdman G. (2011) Bland embolization in the treatment of hepatic adenomas: preliminary experience. J Vasc Interv Radiol 22: 795–799, quiz 800. [DOI] [PubMed] [Google Scholar]
- Dokmak S., Paradis V., Vilgrain V., Sauvanet A., Farges O., Valla D., et al. (2009) A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology 137: 1698–1705. [DOI] [PubMed] [Google Scholar]
- Edmondson H., Henderson B., Benton B. (1976) Liver-cell adenomas associated with use of oral contraceptives. N Engl J Med 294: 470–472. [DOI] [PubMed] [Google Scholar]
- Erdogan D., van Delden O., Busch O., Gouma D., van Gulik T. (2007) Selective transcatheter arterial embolization for treatment of bleeding complications or reduction of tumor mass of hepatocellular adenomas. Cardiovasc Intervent Radiol 30: 1252–1258. [DOI] [PubMed] [Google Scholar]
- Farges O., Ferreira N., Dokmak S., Belghiti J., Bedossa P., Paradis V. (2011) Changing trends in malignant transformation of hepatocellular adenoma. Gut 60: 85–89. [DOI] [PubMed] [Google Scholar]
- Frulio N., Chiche L., Bioulac-Sage P., Balabaud C. (2014) Hepatocellular adenomatosis: what should the term stand for? Clin Res Hepatol Gastroenterol 38: 132–136. [DOI] [PubMed] [Google Scholar]
- Grazioli L., Federle M., Ichikawa T., Balzano E., Nalesnik M., Madariaga J. (2000) Liver adenomatosis: clinical, histopathologic, and imaging findings in 15 patients. Radiology 216: 395–402. [DOI] [PubMed] [Google Scholar]
- Grazioli L., Olivetti L., Mazza G., Bondioni M. (2013) MR imaging of hepatocellular adenomas and differential diagnosis dilemma. Int J Hepatol 2013: 374170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haage P., Piroth W., Staatz G., Adam G., Gunther R. (1999) [CT-guided percutaneous biopsies for the classification of focal liver lesions: a comparison between 14 g and 18 g puncture biopsy needles]. CT-Gesteuerte Perkutane Biopsien zur Klassifizierung von Fokalen Leberlasionen: Vergleich Zwischen 14 G- und 18 G-Stanzbiopsienadeln. Rofo 171: 44–48. [DOI] [PubMed] [Google Scholar]
- Hung C., Changchien C., Lu S., Eng H., Wang J., Lee C., et al. (2001) Sonographic features of hepatic adenomas with pathologic correlation. Abdom Imaging 26: 500–506. [DOI] [PubMed] [Google Scholar]
- Hussain S., van Den Bos I., Dwarkasing R., Kuiper J., den Hollander J. (2006) Hepatocellular adenoma: findings at state-of-the-art magnetic resonance imaging, ultrasound, computed tomography and pathologic analysis. Eur Radiol 16: 1873–1886. [DOI] [PubMed] [Google Scholar]
- Jackson N., Hauch A., Hu T., Buell J., Slakey D., Kandil E. (2015) The safety and efficacy of approaches to liver resection: a meta-analysis. JSLS 19: e2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadri Aribas B., Dingil G., Sahin G., Nil Unlu D., Dogan K., et al. (2010) Accuracy and safety of percutaneous us-guided needle biopsies in liver metastasis and hemangiomas. Minerva Gastroenterol Dietol 56: 377–382. [PubMed] [Google Scholar]
- Karkar A., Tang L., Kashikar N., Gonen M., Solomon S., Dematteo R., et al. (2013) Management of Hepatocellular adenoma: comparison of resection, embolization and observation. HPB (Oxford) 15: 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumonier H., Bioulac-Sage P., Laurent C., Zucman-Rossi J., Balabaud C., Trillaud H. (2008) Hepatocellular adenomas: magnetic resonance imaging features as a function of molecular pathological classification. Hepatology 48: 808–818. [DOI] [PubMed] [Google Scholar]
- Leese T., Farges O., Bismuth H. (1988) Liver cell adenomas. a 12-year surgical experience from a specialist hepato-biliary unit. Ann Surg 208: 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., van Den Esschert J., Liu C., van Gulik T. (2011) Systematic review of hepatocellular adenoma in china and other regions. J Gastroenterol Hepatol 26: 28–35. [DOI] [PubMed] [Google Scholar]
- Marrero J., Ahn J., Rajender Reddy K. American College of Gastroenterology (2014) ACG clinical guideline: the diagnosis and management of focal liver lesions. Am J Gastroenterol 109: 1328–1347; quiz 1348. [DOI] [PubMed] [Google Scholar]
- McInnes M., Hibbert R., Inacio J., Schieda N. (2015) Focal nodular hyperplasia and hepatocellular adenoma: accuracy of gadoxetic acid-enhanced MR imaging: a systematic review. Radiology 277: 413–423. [DOI] [PubMed] [Google Scholar]
- Nault J., Bioulac-Sage P., Zucman-Rossi J. (2013) Hepatocellular benign tumors-from molecular classification to personalized clinical care. Gastroenterology 144: 888–902. [DOI] [PubMed] [Google Scholar]
- Paradis V., Champault A., Ronot M., Deschamps L., Valla D., Vidaud D., et al. (2007) Telangiectatic adenoma: an entity associated with increased body mass index and inflammation. Hepatology 46: 140–146. [DOI] [PubMed] [Google Scholar]
- Parangi S., Levine D., Henry A., Isakovich N., Pories S. (2007) Surgical Gastrointestinal Disorders During Pregnancy. Am J Surg 193: 223–232. [DOI] [PubMed] [Google Scholar]
- Pilati C., Letouze E., Nault J., Imbeaud S., Boulai A., Calderaro J., et al. (2014) Genomic profiling of hepatocellular adenomas reveals recurrent FRK-activating mutations and the mechanisms of malignant transformation. Cancer Cell 25: 428–441. [DOI] [PubMed] [Google Scholar]
- Reddy K., Kligerman S., Levi J., Livingstone A., Molina E., Franceschi D., et al. (2001) Benign and solid tumors of the liver: relationship to sex, age, size of tumors, and outcome. Am Surg 67: 173–178. [PubMed] [Google Scholar]
- Rhim H., Lim H., Kim Y., Choi D. (2008) Percutaneous radiofrequency ablation of hepatocellular adenoma: initial experience in 10 patients. J Gastroenterol Hepatol 23: e422–e427. [DOI] [PubMed] [Google Scholar]
- Ronot M., Bahrami S., Calderaro J., Valla D., Bedossa P., Belghiti J., et al. (2011) Hepatocellular adenomas: accuracy of magnetic resonance imaging and liver biopsy in subtype classification. Hepatology 53: 1182–1191. [DOI] [PubMed] [Google Scholar]
- Sherman M., Bruix J. (2015) Biopsy for liver cancer: how to balance research needs with evidence-based clinical practice. Hepatology 61: 433–436. [DOI] [PubMed] [Google Scholar]
- Solbiati L., Livraghi T., Goldberg S., Ierace T., Meloni F., Dellanoce M., et al. (2001) Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology 221: 159–166. [DOI] [PubMed] [Google Scholar]
- Stattaus J., Kuhl H., Hauth E., Kalkmann J., Baba H., Forsting M. (2007) [Liver biopsy under guidance of multi slice computed tomography: comparison of 16g and 18g biopsy needles]. Leberbiopsie Mit Hilfe der Mehrschichtcomputertomographie: Vergleich Zwischen 16-G- und 18-G-Biopsienadeln. Radiologe 47: 430–438. [DOI] [PubMed] [Google Scholar]
- Stoot J., Coelen R., de Jong M., Dejong C. (2010) Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford) 12: 509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stueck A., Qu Z., Huang M., Campreciós G., Ferrell L., Thung S. (2015) Hepatocellular carcinoma arising in an HNF-1α-mutated adenoma in a 23-year-old woman with maturity-onset diabetes of the young: a case report. Semin Liver Dis 35: 444–449. [DOI] [PubMed] [Google Scholar]
- Thomeer M., Me E., de Lussanet Q., Biermann K., Dwarkasing R., de Man R., et al. (2014a) Genotype-phenotype correlations in hepatocellular adenoma: an update of MRI findings. Diagn Interv Radiol 20: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomeer M., Willemssen F., Biermann K., El Addouli H., de Man R., Ijzermans J., et al. (2014b) MRI features of inflammatory hepatocellular adenomas on hepatocyte phase imaging with liver-specific contrast agents. J Magn Reson Imaging 39: 1259–1264. [DOI] [PubMed] [Google Scholar]
- Torbenson M., Schirmacher P. (2015) Liver cancer biopsy: back to the future? Hepatology 61: 431–433. [DOI] [PubMed] [Google Scholar]
- Toso C., Majno P., Andres A., Rubbia-Brandt L., Berney T., Buhler L., et al. (2005) Management of hepatocellular adenoma: solitary-uncomplicated, multiple and ruptured tumors. World J Gastroenterol 11: 5691–5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aalten S., de Man R., IJzermans J., Terkivatan T. (2012) Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br J Surg 99: 911–916. [DOI] [PubMed] [Google Scholar]
- Van Aalten S., Terkivatan T., de Man R., van Der Windt D., Kok N., Dwarkasing R., et al. (2010) Diagnosis and treatment of hepatocellular adenoma in the netherlands: similarities and differences. Dig Surg 27: 61–67. [DOI] [PubMed] [Google Scholar]
- Van Aalten S., Thomeer M., Terkivatan T., Dwarkasing R., Verheij J., de Man R., et al. (2011a) Hepatocellular adenomas: correlation of mr imaging findings with pathologic subtype classification. Radiology 261: 172–181. [DOI] [PubMed] [Google Scholar]
- Van Aalten S., Verheij J., Terkivatan T., Dwarkasing R., de Man R., Ijzermans J. (2011b) Validation of a liver adenoma classification system in a tertiary referral centre: implications for clinical practice. J Hepatol 55: 120–125. [DOI] [PubMed] [Google Scholar]
- Van Vledder M., van Aalten S., Terkivatan T., de Man R., Leertouwer T., Ijzermans J. (2011) Safety and efficacy of radiofrequency ablation for hepatocellular adenoma. J Vasc Interv Radiol 22: 787–793. [DOI] [PubMed] [Google Scholar]
- Vennarecci G., Santoro R., Antonini M., Ceribelli C., Laurenzi A., Moroni E., et al. (2013) Liver transplantation for recurrent hepatic adenoma. World J Hepatol 5: 145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda N., Matsui O., Kitao A., Kozaka K., Gabata T., Sasaki M., et al. (2012) Beta-catenin-activated hepatocellular adenoma showing hyperintensity on hepatobiliary-phase gadoxetic-enhanced magnetic resonance imaging and overexpression of OATP8. Jpn J Radiol 30: 777–782. [DOI] [PubMed] [Google Scholar]
- Zucman-Rossi J., Benhamouche S., Godard C., Boyault S., Grimber G., Balabaud C., et al. (2007) Differential Effects of Inactivated Axin1 and Activated Beta-Catenin Mutations in Human Hepatocellular Carcinomas. Oncogene 26: 774–780. [DOI] [PubMed] [Google Scholar]
- Zucman-Rossi J., Jeannot E., Nhieu J., Scoazec J., Guettier C., Rebouissou S., et al. (2006) Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology 43: 515–524. [DOI] [PubMed] [Google Scholar]