Abstract
Background:
Gastric outlet obstruction (GOO) can occur with locally invasive or metastatic cancer involving the upper gastrointestinal tract at the pylorus or the duodenum. Endoscopic management with self-expanding metal stents (SEMSs) is often the preferred palliative approach. Stent occlusion is a common reason for failure and reintervention. We set out to determine whether the location of the malignant obstruction is associated with the angulation of the stent and can predict stent occlusion.
Methods:
We performed a retrospective review of consecutive patients who underwent successful duodenal stenting with SEMS for malignant GOO between 2006 and 2015 at a large advanced endoscopy referral center. We determined the location of obstruction, the stent angle, and the rate of technical and clinical success of stent placement. We then identified cases of subsequent stent occlusion confirmed by endoscopic evaluation.
Results:
A total of 100 consecutive patients were included in the study; 91 of these patients had enough data to evaluate SEMS occlusion. A total of 21 patients (23%) developed stent occlusion with a median time of 39 days. The risk of occlusion sequentially increased as the obstruction occurred more distally from the antrum to the third or fourth portion of the duodenum (p = 0.006). This relationship was maintained after controlling for stent angle (p = 0.05).
Conclusions:
A distal location of malignant GOO was strongly predictive of stent occlusion, independent of stent angle. This may be due to longer and more complex distal obstructions, along with foreshortening of the stent during placement and tumor infiltration. If replicated, these results will have implications for endoscopic practice and future device development.
Keywords: malignant duodenal obstruction, self-expanding metal stent, stent occlusion
Introduction
Gastric outlet obstruction (GOO) can be caused by obstruction at the level of the distal stomach or duodenum, preventing normal gastric emptying. In the setting of malignancy, this complication most frequently occurs in patients with advanced gastric, duodenal, pancreatic, or biliary cancers, and is generally associated with poorer prognosis. Up to 20% of patients with pancreatic cancer will develop GOO [Lopera et al. 2004]. Malignant GOO leads to vomiting, inability to tolerate oral intake, weight loss, malnutrition, and carries a median survival of 3–4 months [Oh et al. 2015]. Traditional treatment for GOO is surgical bypass, however, this can be associated with a high morbidity and long recovery in patients with inherently poor functional status [Jeurnink et al. 2007].
Self-expanding metal stents (SEMSs) were developed to allow endoluminal bypass of a gastrointestinal obstruction, obviating or delaying the need for surgery. SEMSs consist of interwoven metal mesh cylinders that exert a radial, self-expansive force while reaching a maximal, fixed diameter. They are compressed, packaged, and then deployed endoscopically across a stricture under fluoroscopic guidance. SEMSs come in different lengths and diameters and can be used for obstructions in the esophagus, stomach, small bowel, or colon. Certain stents have flared ends to help prevent migration or can be covered with silicone to prevent tumor ingrowth [Park et al. 2015; Varadarajulu et al. 2011].
A comparison of SEMS versus surgery for GOO showed an earlier transition to oral intake and decreased hospital length of stay (LOS) in patients who underwent stent placement. Additionally, SEMS placement is less costly compared with surgery [No et al. 2013; Jeurnink et al. 2010]. A systematic review and meta-analysis of 13 studies with 514 patients, who underwent either SEMS placement or gastrojejunostomy to palliate GOO, demonstrated that endoscopic stenting showed significant improvement in tolerability of oral intake, shorter time to oral intake, shorter LOS, and fewer medical complications compared with surgery [Ly et al. 2010]. While these studies are inherently prone to selection bias, endoluminal stenting is now considered a mainstay of therapy for malignant GOO [Holt et al. 2004].
The technical success of SEMS placement for GOO is exceedingly high. The clinical success of stenting, however, is variable. Most patients can advance their diet quickly after stent placement [Ding et al. 2013]. Stent migration and occlusion, however, occurs in 1.9% and 15% of patients, respectively. In these patients, reintervention is usually feasible and successful in re-establishing lumen patency [Tringali et al. 2014]. Repeat endoscopy, however, is costly and carries its own risks.
Due to the widespread acceptance of SEMS for malignant GOO, understanding the reasons for clinical failure of stent placement should be a priority [Goldberg, 2014]. A previous study showed that severe angulation increased the risk of stent occlusion [Lee et al. 2011]. The angle of a stent placed in the third and fourth portions of the duodenum must conform to the ascension of the duodenum to its tether at the Ligament of Treitz, therefore, stent position in this location may require more stent angulation than in other locations. We set out to determine whether the location of the malignant obstruction and stent angulation were associated with stent occlusion requiring reintervention. We hypothesized that SEMS placement for GOO caused by obstruction in the distal portions of the duodenum would have a higher association with stent occlusion due to the more acute angles noted during stent placement.
Materials and methods
We identified a cohort of patients with malignant GOO who underwent successful endoluminal stenting between 2006 and 2015 in the Center for Advanced Endoscopy at Beth Israel Deaconess Medical Center. All patients had biopsy-proven malignancy, clinical evidence of GOO, and confirmation of GOO on endoscopy. Patients were excluded if they had undergone stenting at another institution and those for whom initial stent placement was not technically feasible. We reviewed and extracted predictor variables and outcomes from the electronic health record and endoscopic report. Information collected included: patient demographics, primary malignancy, site of obstruction, length of stent, pre- and postprocedure oral intake, procedural complications, chemotherapy, need for reintervention due to stent occlusion, LOS, and death. We used a computer software program to digitally measure the final angle of the endoluminal stent (MB Ruler 1.52, Markus Bader, Iffezheim, Germany). The angle was graded numerically: mild >90°, moderate 15–90°, and severe <15°.
Our primary outcome of interest was stent occlusion requiring reintervention. Secondary outcomes were initial clinical success of stent placement, procedural complications of stent placement, hospital LOS, and mortality. Initial clinical success was determined by using a subjective, standardized measure of oral intake, the Gastric Outlet Obstruction Scoring System (GOOSS). This assigns a point for each level of oral intake. Patients who eat nothing, have liquids, soft solids, or low-residue foods are assigned 0, 1, 2, and 3 points, respectively. This scoring system has been used in previous studies of SEMS for malignant GOO [Adler and Baron, 2002].
All patients were sedated using either general anesthesia or monitored anesthesia care (MAC), under the care of an anesthesiologist or a supervised, certified nurse anesthetist. Endoscopy was carried out using a therapeutic gastroscope (GIF-XTQ160, Olympus America, Melville, NY, USA) or therapeutic duodenoscope (TJF180, Olympus America, Melville, NY, USA). The malignant stricture was characterized by a combination of endoscopic and fluoroscopic evaluations. A stricture that was unable to be traversed by the scope could be dilated based on endoscopist preference to help facilitate stent placement or a biliary procedure. In most cases, a hydrophilic guidewire (Jag guidewire, 450 cm length and 0.89 mm diameter, Microvasive, Natick, MA, USA) was placed across the stricture, the bowel was opacified with contrast to confirm wire placement and stricture location, and a stent was advanced into position and deployed over the wire. Stenting was carried out using a WallFlex duodenal stent (Boston Scientific, Natick, MA, USA; 90 and 120 mm lengths; 10F delivery system). The diameter of the stent was 22 mm in all cases (Figure 1). A previous study showed the safety and efficacy of this Wallflex stent [Kanno et al. 2013]. If the patient had concomitant biliary obstruction, a biliary stent was placed during the same procedure if feasible. Otherwise, percutaneous transhepatic biliary drainage (PTBD) was performed by interventional radiology. After the procedure, the patient was placed on a liquid diet, which was then progressed to low-residue foods, as determined by the treating physician.
Figure 1.
(a) Malignant duodenal obstruction, (b) Jag guidewire passing through the obstruction, (c) Wallflex duodenal stent is deployed, and (d) post-stenting X-ray confirms stent placement, here with a severe angle <15°.
Statistical analysis was carried out with SAS™ 9.4 data software (Cary, NC, USA). For binary outcome data, odds ratios (ORs) were determined using Fisher’s exact test. Chi-square test for trend was used to look at ordered, mutually exclusive categories, including obstruction location. Normally distributed, continuous variables were analyzed using Student’s t-test. Skewed data were analyzed using the Wilcoxon rank sum test. Logistic regression was performed on binary variables that had an initial p value < 0.2; variables with more than two outcomes were analyzed with a referent value. Kaplan–Meier curves were used to show the time to reintervention and analyzed by the log-rank test. For statistical tests, a two-tailed p value of ⩽0.05 was considered significant. The study was approved by the Institutional Review Board for Human Subject Research.
Results
We evaluated 100 patients over the study duration that met our inclusion criteria (Table 1). The average age of our study population was 69.7 years and 43% were male. The majority of patients (44%) had GOO from pancreatic cancer. Other sites of malignancy causing obstruction included gastric (15%), duodenal (8%), biliary (14%), and other metastatic sites (19%). The location of GOO included: antrum (12%), duodenal bulb (44%), second portion of duodenum (D2, 34%), and third or fourth portion of the duodenum (D3/D4, 10%). A majority of GOOs (57%) were caused by extraluminal tumor compression of the duodenum. In our study, 11% of patients had dilation immediately prior to stent placement to facilitate biliary stent placement, while 46% had a biliary stent either in situ or concomitantly placed during endoscopy. The number of patients who received chemotherapy after stent placement was 29.6%, however almost all patients had been in a chemotherapy protocol prior to stent placement.
Table 1.
Descriptive statistics and univariate analysis from 100 patients with SEMS placement for malignant gastric outlet obstruction.
| Number (%) | Odds ratio (95% CI) | p value | |
|---|---|---|---|
| Mean age (SD), years | 69.7 (14.6) | – | 0.31 |
| Male | 43 (43.0) | 0.88 (0.69–1.12) | 0.32 |
| Mean BMI (SD), kg/m2 | 27.0 (7.7) | – | 0.63 |
| Primary tumor type | |||
| Gastric cancer | 15 (15.0) | – | 0.50 |
| Duodenal cancer | 8 (8.0) | ||
| Pancreatic cancer | 44 (44.0) | ||
| Biliary cancer | 14 (14.0) | ||
| Other cancer | 19 (19.0) | ||
| Location of obstruction | |||
| Antrum | 12 (12.0) | – | 0.006 |
| Duodenal bulb | 44 (44.0) | ||
| Second part of duodenum | 34 (34.0) | ||
| Third/fourth part of duodenum | 10 (10.0) | ||
| Extraluminal obstruction | 57 (57.0) | 0.96 (0.76–1.22) | 0.80 |
| Chemotherapy | 26 (29.5) | 1.19 (0.87–1.63) | 0.26 |
| Radiation therapy | 18 (19.4) | 0.99 (0.74–1.33) | 1.00 |
| Prestent dilation | 11 (11.0) | 0.98 (0.68–1.42) | 1.00 |
| Biliary stenting | 46 (46.0) | 1.00 (0.80–1.27) | 1.00 |
| Stent angulation | |||
| Mild (>90°) | 13 (15.5) | – | 0.49 |
| Moderate (15–90°) | 32 (38.1) | ||
| Severe (<15°) | 39 (46.4) | ||
| Length of stent | |||
| 22 × 90 mm | 55 (55.0) | 1.10 (0.40–3.00) | 1.00 |
| 22 × 120 mm | 43 (43.0) | ||
Bold values are significant at a two-tailed p value < 0.05.
SD, standard deviation; CI, confidence interval; BMI, body mass index.
Patients who received endoluminal stents were hospitalized, on average, for 5 days. Poststenting GOOSS also increased (1.01–2.28). A total of 76% of patients had died by the time of data extraction in October 2015. The median time from stent placement to death was 53.5 days (Table 2).
Table 2.
Length of hospital stay, gastric outlet obstruction score, death, and stent occlusion after SEMS placement.
| Variable | Result |
|---|---|
| Median hospitalization time (IQR), days | 5 (1.5–11) |
| Mean GOOSS score prior to stent (SD) | 1.01 (0.91) |
| Mean GOOSS score after stent (SD) | 2.28 (0.81) |
| Death | 76 (76%) |
| Median time to death (IQR), days | 53.5 (24–170.5) |
| Stent occlusion | 21 (23%) |
| Median time to occlusion (IQR), days | 39.0 (10–136) |
IQR, interquartile range; SD, standard deviation; GOOSS, gastric outlet obstruction scoring system.
We determined the rate of procedural complications in our cohort. Out of 100 procedures, there were 12 procedure-related complications: gastrointestinal bleeding (4), duodenal perforation (2), aspiration (3), postprocedure pancreatitis (2), and Boorhave’s syndrome (1); in two cases, these complications led to periprocedural death. These complications were directly related to the SEMS placement and not other circumstances like prestent dilation. We analyzed whether one of these adverse events during stent placement was associated with changes in LOS, death, or time from stent placement to death compared with patients who did not suffer a procedural complication. Our analysis showed that an adverse event during stent placement was not associated with a significant increase in hospital LOS or the overall risk of death during the study period. An adverse event, however, did decrease the median time from stent placement to death or censoring (49.5–29.5 days, p = 0.047).
We also determined whether stent placement was successful at relieving GOO. Postprocedural GOOSS was significantly increased from baseline (p ⩽ 0.0001). The mean GOOSS prior to stenting was 1.0, or equivalent to taking liquids only. After stenting, the mean GOOSS was 2.3, equivalent to taking at least soft solids (Table 2).
We then performed an analysis of our primary outcome of stent occlusion. A total of 91% patients had adequate follow up to determine whether they developed stent occlusion requiring reintervention. We obtained angle measurements on 84 of these patients; patients with inadequate poststenting imaging were unable to have an accurate angle measurement. The stent angle was mild (>90°), moderate (15°–90°), and severe (<15°) in 15.5%, 38.1%, and 46.4% of patients, respectively. A total of 21 patients developed stent occlusion; we had angulation data on 17 of these patients. The median time to stent occlusion was 39 days (Table 1). Stent occlusion was not associated with an increase in hospital LOS (p = 0.804).
The location of luminal obstruction was highly associated with stent occlusion (Table 1). In more specific terms, the risk of occlusion increased incrementally when the stent was placed more distally in the gastrointestinal tract from the antrum to duodenal bulb to second and third portions of the duodenum (p = 0.006). We were underpowered to detect a significant difference in the time to stent occlusion among tumor locations (Figure 2, p = 0.11). Stent angulation and other factors, including age, body mass index (BMI), gender, prestent dilation, biliary stenting, primary tumor type, chemotherapy, radiation, and stent length, were not associated with stent occlusion (Table 1).
Figure 2.
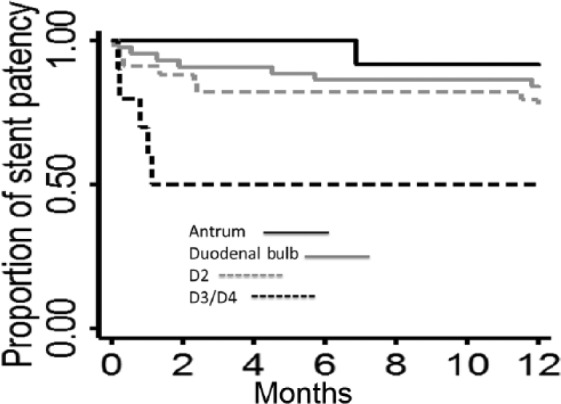
The time to stent occlusion stratified by the location of malignant obstruction did not meet significance (log-rank p = 0.11).
D2, second portion of the duodenum; D3, third portion of the duodenum; D4, fourth portion of the duodenum.
We used logistic regression to determine whether the association between the location of obstruction and stent occlusion was confounded by the stent angle (Table 3). In comparison to a referent value (obstruction in D3/D4), the OR of stent occlusion in antral obstructions was 0.084 when controlling for stent angle (p = 0.05). This trend, although not significant, remained for obstruction in the duodenal bulb and D2, when compared with D3/D4 and controlled for stent angle (p = 0.09, 0.38, respectively). No other variables from our univariate analysis reached the a priori p value of 0.2 to include in the regression model.
Table 3.
Controlling for stent angle, a stent traversing malignant GOO in the antrum had a lower rate of occlusion compared with a stent traversing a distal duodenal malignancy.
| Variable | Odds ratio (95% CI) | p value |
|---|---|---|
| Antrum versus D3/D4 obstruction | 0.084 (0.007–0.99) | 0.05 |
| Duodenal bulb versus D3/D4 obstruction | 0.24 (0.04–1.26) | 0.09 |
| D2 versus D3/D4 obstruction | 0.46 (0.08–2.59) | 0.38 |
| D3/D4 obstruction | Ref | Ref |
| Mild versus severe stent angle | 1.25 (0.23–6.93) | 0.31 |
| Moderate versus severe stent angle | 0.52 (0.15–1.85) | 0.80 |
| Severe stent angle | Ref | Ref |
Bold values are significant at a two-tailed p value <0.05.
D2, second portion of the duodenum; D3, third portion of the duodenum; D4, fourth portion of the duodenum; CI, confidence interval; Ref, reference value.
Discussion
Our results suggest malignant obstruction from more distal duodenal narrowing is associated with an increased risk of SEMS occlusion, requiring reintervention. Although stent angulation has been previously demonstrated as a potential risk factor for occlusion, our results suggest that location of obstruction is more important. We also showed that stent placement has a high rate of clinical success and acceptable procedural risk when compared with the alternative of surgical bypass [Van Halsema et al. 2015].
Our median time to stent occlusion was 39 days, which is a shorter duration of stent patency compared with similar studies. Our patient population was sick and debilitated, as evidenced by the median survival of just over 50 days. These patients had aggressive, diffuse tumors and most had exhausted chemotherapeutic options due to their poor performance status. Because of this, tumor ingrowth seemed to occur quickly and aggressively. Additionally, given the small sample size, the median time to stent occlusion was largely affected by early occlusion. Table 4 gives the time to occlusion and the etiology of the occlusion for each patient. As predicted, most early occlusions were due to technical failures of stent placement (kinking, migration), whereas later obstructions were almost wholly related to tumor ingrowth. If we remove stent occlusion that occurred within 7 days, then the median time of stent patency increases to 70 days, which is similar to other studies.
Table 4.
The time to occlusion and etiology of occlusion for 21 patients undergoing SEMS placement.
| Patient | Location of obstruction | Days to occlusion | Reason for occlusion |
|---|---|---|---|
| 1 | Bulb | 57 | Tumor ingrowth |
| 2 | D3 | 34 | Tumor ingrowth |
| 3 | Bulb | 172 | Tumor ingrowth |
| 4 | D3 | 24 | Foodstuffs |
| 5 | D2 | 346 | Tumor ingrowth |
| 6 | D2 | 41 | Migration |
| 7 | D2 | 7 | Distal stricture |
| 8 | Bulb | 136 | Tumor ingrowth |
| 9 | D3 | 7 | Stent kinking |
| 10 | D2 | 3 | Foodstuffs |
| 11 | D3 | 31 | Tumor ingrowth |
| 12 | D2 | 1316 | Tumor ingrowth |
| 13 | Bulb | 39 | Tumor ingrowth |
| 14 | Bulb | 6 | Migration |
| 15 | D2 | 10 | Nonexpansion |
| 16 | D2 | 72 | Tumor ingrowth |
| 17 | D2 | 70 | Tumor ingrowth |
| 18 | Bulb | 355 | Tumor ingrowth |
| 19 | Bulb | 17 | Stent kinking |
| 20 | D3 | 6 | Stent kinking |
| 21 | Antrum | 207 | Tumor ingrowth |
D2, second portion of duodenum; D3, third portion of duodenum.
We hypothesize that distal obstructions are more likely to be long and complex. Placing SEMS for distal duodenal obstruction is more technically demanding. Additionally, fixed-cell braided stents like the WallFlex have a high axial force and the propensity to straighten after deployment, which can bury the distal flange of the stent into the tumor bed. Therefore, stent occlusion is likely to occur as a result of both patient factors and stent dynamics. It has previously been shown that stent kinks can be prevented by using a minimum SEMS length and ensuring that the stent traverses the full length of the obstruction before deployment [Sasaki et al. 2013]. Additionally, other enteral stent types are commercially available. A study using a flexible, unfixed cell Niti-S enteral stent (TaeWoong Medical, Gyeonggi-do, South Korea) encountered a stent occlusion rate of 2.7%. This stent exerts a high radial force and a lower axial force and, therefore, seems to do a better job maintaining its conformation [Maetani et al. 2007]. Our results may not be applicable to other stent types; however, we still claim that, in general, SEMS placement should be tailored to the location of obstruction.
Limitations of our study include its retrospective design, with a relatively small number of patients. We only deployed one type of through-the-scope stent that has the technical characteristics as listed above. Complete data collection was impossible for some patients who only came to our hospital for a procedural round trip. Also, defining the anatomical location of obstruction, especially in the distal portions of the duodenum, is somewhat arbitrary. However, our findings persisted even when simplifying the anatomic delineation and comparing preduodenal bulb and postduodenal bulb stent occlusion (p = 0.01).
This is one of the few studies looking at the relationship between location of obstruction and stent occlusion. A study by Jung and colleagues looked at clinical success and complications of SEMSs for malignant GOO, based on the type of stent used and the location of the obstruction [Jung et al. 2015]. In their study, 104 patients had obstruction in the gastric antrum, whereas 109 were obstructed in the duodenum. The study found more improvement in oral intake in those with proximal stents versus distal stents. However, unlike our study, this was not related to differences in stent occlusion by location (gastric outlet 25%, duodenum 22.9%; p = 0.724). Additionally, a meta-analysis found that covered SEMSs had a higher rate of migration, especially at the gastric outlet, but favorable rates of occlusion compared with uncovered stents [Pan et al. 2014]. We have shown that stent occlusion may occur more commonly in the distal duodenum. Therefore, our study is complementary to previous studies on SEMSs for GOO and may elucidate an algorithm for future stent placement.
In conclusion, previous analyses have found predictors of stent occlusion to be stent underexpansion during delivery, poor patient performance status, peritoneal carcinomatosis, and ascites [Hori et al. 2015; Sasaki et al. 2012]. We now have shown that a distal location of malignant GOO was strongly predictive of stent occlusion independent of stent angle. This may be due to longer, more complex distal strictures. While more research is needed, endoscopists may be able to reduce the rate of stent occlusion by ensuring that the distal end of the stent, especially in the distal duodenum, completely traverses the tumor bed. If replicated, these results will have implications for endoscopic practice and future device development.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Drs Chuttani, Berzin, and Pleskow have received consulting honoraria for Boston Scientific. No other authors have any relevant disclosures.
Contributor Information
Douglas Grunwald, Division of Gastroenterology, Beth Israel Deaconess Medical Center and Harvard Medical School, 330 Brookline Avenue, Boston, MA 02215, USA.
Jonah Cohen, Center for Advanced Endoscopy, Division of Gastroenterology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA.
Anthony Bartley, Center for Advanced Endoscopy, Division of Gastroenterology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA.
Jennifer Sheridan, Center for Advanced Endoscopy, Division of Gastroenterology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA.
Ram Chuttani, Center for Advanced Endoscopy, Division of Gastroenterology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA.
Mandeep S. Sawhney, Center for Advanced Endoscopy, Division of Gastroenterology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA
Douglas K. Pleskow, Center for Advanced Endoscopy, Division of Gastroenterology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA
Tyler M. Berzin, Center for Advanced Endoscopy, Division of Gastroenterology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA
Meir Mizrahi, Center for Advanced Endoscopy, Division of Gastroenterology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA.
References
- Adler D., Baron T. (2002). Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients. Am J Gastroenterol 97: 72–78. [DOI] [PubMed] [Google Scholar]
- Ding N., Alexander S., Swan M., Hair C., Wilson P., Clarebrough E., et al. (2013). Gastroduodenal outlet obstruction and palliative self-expandable metal stenting: a dual-centre experience. J Oncol 2013: 167851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg E. (2014). Palliative treatment of gastric outlet obstruction in terminal patients: SEMS. Stent every malignant stricture! Gastrointest Endosc 79: 76–78. [DOI] [PubMed] [Google Scholar]
- Holt A., Patel M., Ahmed M. (2004). Palliation of patients with malignant gastroduodenal obstruction with self-expanding metallic stents: the treatment of choice? Gastrointest Endosc 60: 1010–1017. [DOI] [PubMed] [Google Scholar]
- Hori Y., Naitoh I., Ban T., Narita K., Nakazawa T., Hayashi K., et al. (2015). Stent under-expansion on the procedure day, a predictive factor for poor oral intake after metallic stenting for gastric outlet obstruction. J Gastroenterol Hepatol 30: 1246–1251. [DOI] [PubMed] [Google Scholar]
- Jeurnink S., Steyerberg E., Hof G., van Eijck C., Kuipers E., Siersema P. (2007). Gastrojejunostomy versus stent placement in patients with malignant gastric outlet obstruction: a comparison in 95 patients. J Surg Oncol 96: 389–396. [DOI] [PubMed] [Google Scholar]
- Jeurnink S., Steyerberg E., van Hooft J., van Eijck C., Schwartz M., Vleggaar F., et al. (2010). Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc 71: 490–499. [DOI] [PubMed] [Google Scholar]
- Jung K., Ahn J., Jung H., Cho C., Na H., Jung K., et al. (2015). Outcomes of endoscopically inserted self-expandable metal stents in malignancy according to the type of stent and the site of obstruction. Surg Endosc 30: 4001–4010. [DOI] [PubMed] [Google Scholar]
- Kanno Y., Ito K., Fujita N., Noda Y., Kobayashi G., Horaguchi J., et al. (2013). Efficacy and safety of a WallFlex enteral stent for malignant gastric obstruction. Dig Endosc 25: 386–391. [DOI] [PubMed] [Google Scholar]
- Lee E., Bourke M., Williams S., Alrubaie A., Kwan V., Bailey A., et al. (2011). Severity of initial stent angulation predicts reintervention after successful palliative enteral stenting for malignant luminal obstruction. J Gastroenterol Hepatol 26: 484–491. [DOI] [PubMed] [Google Scholar]
- Lopera J., Brazzini A., Gonzales A., Castaneda-Zuniga W. (2004). Gastroduodenal stent placement: current status. Radiographics 24: 1561–1573. [DOI] [PubMed] [Google Scholar]
- Ly J., O’Grady G., Mittal A., Plank L., Windsor J. (2010). A systematic review of methods to palliate malignant gastric outlet obstruction. Surg Endosc 24: 290–297. [DOI] [PubMed] [Google Scholar]
- Maetani I., Isayama H., Mizumoto Y. (2007). Palliation in patients with malignant gastric outlet obstruction with a newly designed enteral stent: a multicenter study. Gastrointest Endosc 66: 355–360. [DOI] [PubMed] [Google Scholar]
- No J., Kim S., Lim C., Kim J., Cho Y., Park J., et al. (2013). Long-term outcome of palliative therapy for gastric outlet obstruction caused by unresectable gastric cancer in patients with good performance status: endoscopic stenting versus surgery. Gastrointest Endosc 78: 55–62. [DOI] [PubMed] [Google Scholar]
- Oh S., Edwards A., Mandelson M., Ross A., Irani S., Larsen M., et al. (2015). Survival and clinical outcome after endoscopic duodenal stent placement for malignant gastric outlet obstruction: comparison of pancreatic cancer and nonpancreatic cancer. Gastrointest Endosc 82: 460–468. e2. [DOI] [PubMed] [Google Scholar]
- Pan Y., Pan J., Guo L., Qiu M., Zhang J. (2014). Covered versus uncovered self-expandable metallic stents for palliation of malignant gastric outlet obstruction: a systematic review and meta-analysis. BMC Gastroenterol 14: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Jeong S., Lee D. (2015). Recent advances in gastrointestinal stent development. Clin Endosc 48: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Isayama H., Maetani I., Nakai Y., Kogure H., Kawakubo K., et al. (2013). Japanese multicenter estimation of WallFlex duodenal stent for unresectable malignant gastric outlet obstruction. Dig Endosc 25: 1–6. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Isayama H., Nakai Y., Togawa O., Kogure H., Kawakubo K., et al. (2012). Predictive factors of solid food intake in patients with malignant gastric outlet obstruction receiving self-expandable metallic stents for palliation. Dig Endosc 24: 226–230. [DOI] [PubMed] [Google Scholar]
- Tringali A., Didden P., Repici A., Spaander M., Bourke M., Williams S., et al. (2014). Endoscopic treatment of malignant gastric and duodenal strictures: a prospective, multicenter study. Gastrointest Endosc 79: 66–75. [DOI] [PubMed] [Google Scholar]
- Van Halsema E., Rauws E., Fockens P., van Hooft J. (2015). Self-expandable metal stents for malignant gastric outlet obstruction: a pooled analysis of prospective literature. World J Gastroenterol 21: 12468–12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadarajulu S., Banerjee S., Barth B., Desilets D., Kaul V., Kethu S., et al. (2011). Enteral stents. Gastrointest Endosc 74: 455–464. [DOI] [PubMed] [Google Scholar]



