Abstract
Stable suppression of gastric acid secretion is a crucial factor in Helicobacter pylori eradication. Vonoprazan is a potassium-competitive acid blocker recently approved for use in Japan. As vonoprazan has a long duration of action and causes rapid and strong inhibition of gastric acid secretion, it has gained clinical attention for treating erosive oesophagitis, peptic ulcers, and H. pylori infection. In this review, we discuss the recent knowledge regarding the safety and efficacy of vonoprazan, focusing on its use in H. pylori eradication. The latest literature and our clinical experience have shown that vonoprazan-based therapies have satisfactory eradication rates. Additionally, vonoprazan-based therapies are associated with similar rates of adverse events as standard triple therapies with conventional proton-pump inhibitors.
Keywords: Vonoprazan, H. pylori, PPI, Takecab, potassium-competitive acid blocker, P-CAB, TAK-438
Helicobacter pylori eradication: current status and problems
Helicobacter pylori infection is the most common infectious disease, affecting half of the world population and causing peptic ulcers. In addition, the World Health Organization classified H. pylori as a group 1 carcinogen [Park et al. 2014]. In 2012, H. pylori infection was estimated as the cause of approximately 774,000 new cases of gastric cancer worldwide [Arikawa et al. 2012; Ferlay et al. 2014; Forman et al. 2014]. Eradication of H. pylori reduced the incidence of gastric cancer by over 30% in China since 2000 [Ma et al. 2012]. In Japan, H. pylori eradication was found to significantly decrease the incidence of secondary cancers after endoscopic resection of early gastric cancer [Fukase et al. 2008]. H. pylori eradication also leads to a favourable long-term outcome of gastric mucosa-associated lymphoid tissue lymphoma [Nakamura et al. 2014] and idiopathic thrombocytopenic purpura [Satake et al. 2007]. Using economic models and based on a threshold of US$50,000 per life year saved, H. pylori screening and treatment strategies were found to be cost effective [Park et al. 2014]. The Japanese government approved H. pylori eradication for the treatment of peptic ulcers and for patients with endoscopically proven gastritis in 2013, a measure expected to save lives and reduce medical costs due to gastric cancers [Asaka, 2014].
For over 20 years, the first-line therapy for H. pylori infection has comprised a combination of a proton-pump inhibitor (PPI) and two antibiotics, usually amoxicillin and clarithromycin. The commonly used PPIs include lansoprazole, omeprazole, rabeprazole, and esomeprazole (S-isomer of omeprazole), which are called ‘conventional PPIs’ in this review [Asaka et al. 2010; Megraud, 2012]. Recently, the eradication rates of H. pylori infection with first-line treatment have fallen below 80% in many countries, reaching less than 70% in some regions [Figura et al. 2016; Shinozaki et al. 2016]. One cause of the eradication failure is the growing resistance of H. pylori to the antibiotics used in the treatment regimens [Broutet et al. 2003; Graham et al. 2010; Megraud, 2012]. A clarithromycin-resistant strain of H. pylori has been reported in Japan and southern Europe, accounting for 30% and 20%, respectively, of the H. pylori eradication failures in those areas [Megraud, 2007]. In some regions, H. pylori strains resistant to other antibiotics have increased significantly. For example, in Singapore, H. pylori resistance to metronidazole has increased from 24.8% to 48.2% in the last 15 years [Ang et al. 2016]. Efforts to overcome H. pylori eradication failure include treatment with various antibiotics. For instance, a 14-day quadruple therapy with bismuth, rabeprazole, minocycline, and amoxicillin as first-line therapy has resulted in an H. pylori eradication rate of over 90% [Song et al. 2016]. In addition, sequential treatment has been proposed, involving a two-step therapy, starting with a PPI and amoxicillin or clarithromycin for 5 days, followed by triple therapy with a PPI, clarithromycin, and a nitroimidazole, for an additional 5 days [Graham et al. 2010].
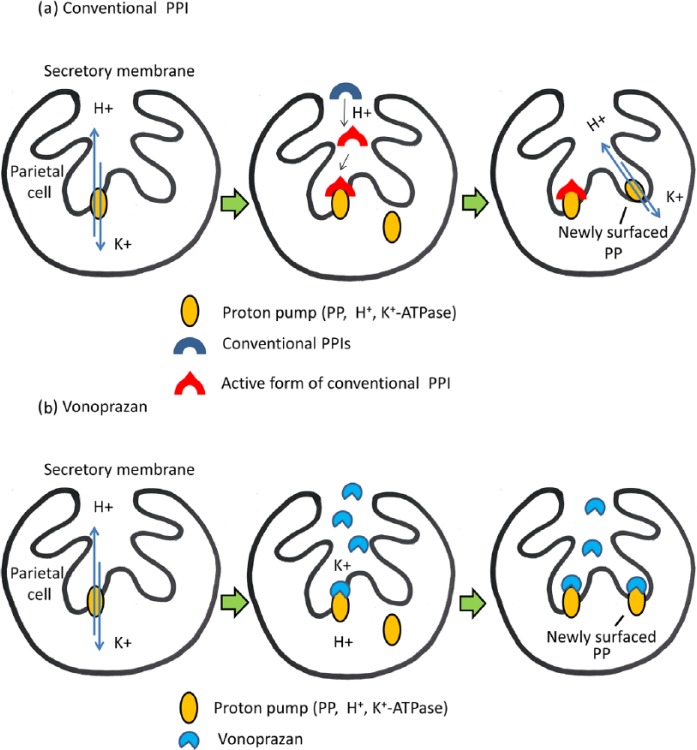
Another critical problem in H. pylori eradication is maintaining the pH of the gastric mucosa to preserve antibiotic function [Sugimoto et al. 2007]. The 24 h gastric pH ranges of 5.0–7.6 and 2.2–6.2 have been reported in patients with successful and failed H. pylori eradication, respectively, indicating that a relatively high gastric pH is optimal for successful H. pylori eradication [Sugimoto et al. 2007]. The membrane-bound H+/K+-ATPase (proton pump) maintains the acidity in the stomach. H+/K+-ATPase belongs to a P-type ATPase family and surfaces from the cytosol to the secretory membrane of the parietal cells when food is present in the stomach, pumping H+ ions out of the cells and into the canaliculi in exchange for K+ ions [Scott et al. 2015] [Figure 1(a)]. Conventional PPIs have been used to suppress intragastric acid secretion during H. pylori eradication for decades. Conventional PPIs are prodrugs, which are activated by acid and covalently bind to the H+/K+-ATPase [Abelo et al. 2000; Hunt et al. 2015] [Figure 1(a)]. Activated PPIs are not stable in acidic condition, whereas H+/K+-ATPase surfacing is stimulated by every food intake. Thus repeated PPI administration for several days is required to attain the maximum effect [Figure 1(a)] [Hunt et al. 2015]. Furthermore, conventional PPIs, especially lansoprazole and omeprazole, are extensively metabolized in the liver by cytochrome P450 2C19 (CYP2C19) genotype [Miki et al. 2003; Furuta et al. 2010]. It has been reported that H. pylori eradication rates of different PPIs vary significantly owing to CYP2C19 polymorphism: the rate of eradication tends to decrease in ‘rapid metabolizers’ because such individuals cannot achieve sufficiently high plasma concentration of PPIs to maintain high gastric pH during the therapy [Sugimoto et al. 2007].
Figure 1.
Simplified schematic description: differences between ‘conventional’ proton-pump inhibitors (PPIs) (a) and vonoprazan (b). (a) The H+/K+-ATPase is located on the secretary membrane of parietal cells and maintains the acidity in the stomach. The enzyme is responsible for pumping H+ ions out of the cells into the canaliculi, in exchange for K+ ions. Conventional PPIs are absorbed in the small intestine and subsequently reach the gastric parietal cells where they are converted to their active forms upon acid exposure, and covalently bind to the H+/K+-ATPase. Since conventional PPIs are unstable in canaliculi and are rapidly degraded, they are not able to inhibit new proton pumps (PPs) that surface after administration of the drug. Thus they require a few days to reach their maximum effect. (b) Vonoprazan, a potassium-competitive acid blocker, does not require acid activation. Vonoprazan is rapidly absorbed in the small intestine and accumulates in the canalicular membranes of parietal cells, binding to H+/K+-ATPase in a K+-competitive manner. Vonoprazan is more stable than conventional PPIs in the canaliculi, allowing fast and stable inhibition of gastric acid secretion.
Vonoprazan, a new H+/K+-ATPase inhibitor
Potassium-competitive acid blockers (PCABs) inhibit acid secretion in gastric parietal cells by competitively inhibiting the binding of potassium ion to H+/K+-ATPase [Parsons et al. 2005]. PCABs, including SCH28080l, have been developed since the 1990s and have been clinically tested [Mendlein et al. 1990; Dent et al. 2008]. However, in clinical trials, PCABs were shown to be hepatotoxic and did not exhibit significant superiority in acid suppression compared with PPIs [Berg et al. 2008; Dent et al. 2008].
Vonoprazan (1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamin monofumarate, initially called TAK-438) is a new PCAB that has been clinically available since 2015 in Japan [Arikawa et al. 2012; Garnock-Jones, 2015; Sakurai et al. 2016a]. Vonoprazan is a basic compound with pKa 9.06–9.3, which is significantly higher than the pKas of conventional PPIs (lansoprazole, pKa 3.8) and previously developed PCABs (SCH28080l, pKa 5.6) [Roche, 2006; Shin et al. 2011; Scott et al. 2015; Takeda Ltd, 2016]. The higher basicity of vonoprazan compared with that of conventional PPIs enables its concentration in low pH-secretory canaliculi. In addition, vonoprazan dissociates slowly from the H+/K+-ATPase [Hori et al. 2010; Scott et al. 2015]. Other advantages of vonoprazan are that it does not require acid activation [Hori et al. 2010], is rapidly absorbed in the intestine, and leads to fast inhibition of acid secretion [Scott et al. 2015] [Figure 1(b)]. In addition, vonoprazan is more stable at neutral pH compared with conventional PPIs: the half maximal inhibitory concentration (IC50) values at pH 7.5 were 66 and 0.028 μM for a conventional PPI and vonoprazan, respectively, in a study employing porcine H+/K+-ATPase [Hori et al. 2010]. Plasma half life of 5.7 and 7 h was reported for vonoprazan (20 mg) after a single dose and on the seventh day of administration in humans, respectively [Jenkins et al. 2015; Takeda Ltd, 2016], longer than the half life of conventional PPIs (<2 h). Importantly, as vonoprazan is mainly metabolized by CYP3A4, its acid inhibitory effect is least influenced by CYP2C19 haplotypes [Sakurai et al. 2015]. These features allow vonoprazan to exert rapid, strong, and stable inhibition of H+/K+-ATPase. Vonoprazan increased intragastric pH to over 4.0 within 4 h after the first administration in humans [Jenkins et al. 2015], creating conditions in which amoxicillin and clarithromycin are stable [Erah et al. 1997]. In addition, amoxicillin and clarithromycin are growth-dependent antibiotics, exerting optimal effects against H. pylori at pH 6–7. However, lower pH values suppresses growth of H. pylori, leading to antibiotic resistance [Hassan et al. 1999; Scott et al. 2015]. A recent study in humans showed that intragastric pH greater than 5 holding time ratio was 99% with vonoprazan at 20 mg twice daily and 84% with esomeprazole at 20 mg twice daily when administered for 7 days [Kagami et al. 2016]. In the same study, the acid inhibitory effect of vonoprazan was superior to that of esomeprazole. As expected, the effect of vonoprazan was not influenced by the CP2C19 genotype. Further, a single administration of vonoprazan raised the gastric pH to over 6 for several hours [Jenkins et al. 2015]. Similar pharmacodynamics of vonoprazan were reported in studies from Japan and the UK [Jenkins et al. 2015]. Taken together, these observations imply the improved potential of vonoprazan for eradicating H. pylori compared with that of conventional PPIs, as discussed in the next section.
Helicobacter pylori treatment with vonoprazan
Published data on the efficacy and safety of vonoprazan in treating H. pylori infection are still limited. An eradication regimen of 20 mg vonoprazan + 750 mg amoxicillin + 200 or 400 mg clarithromycin, twice daily for 7 days, has been approved and has been covered under health insurance for first-line therapy in Japan since 2015. In a phase III clinical study, Murakami and colleagues observed an H. pylori eradication rate of 92.6% with vonoprazan against a 75.9% rate with lansoprazole, as part of first-line therapy [Murakami et al. 2016]. In addition, a retrospective study showed that the eradication rate with vonoprazan-based therapy was significantly higher than the rate obtained with a lansoprazole-based therapy in both an intention-to-treat analysis (89.1% versus 70.9%) and a per protocol analysis (92.1% versus 71.7%) [Suzuki et al. 2016]. Another study that retrospectively reviewed the medical records of 573 patients with triple therapy employing either rabeprazole (10 mg), lansoprazole (30 mg), esomeprazole (20 mg), or vonoprazan (20 mg) showed a superior eradication rate of vonoprazan compared with lansoprazole and rabeprazole (vonoprazan 83%, lansoprazol 66%, and rabeprazole 67%; p < 0.01) [Shinozaki et al. 2016]. However, in this study, the eradication rates using esomeprazole and vonoprazan did not differ significantly. Nevertheless, vonoprazan showed better eradication rates in patients with gastric atrophy, a condition with high risk for gastric cancer. As severe gastric atrophy leads to decreased acid secretion, the stability of vonoprazan at high pH might have contributed to higher eradication rates in these patients. Further clinical studies are required to elucidate this point. In our single-centre study involving 669 patients, H. pylori eradication was achieved in 614 patients (91.4%) [Fukuda et al. 2016] using the vonoprazan-based triple regimen, a result comparable to previously reported rates [Murakami et al. 2016]. Other recent reports from Japan have also indicated H. pylori eradication rates ranging from 88% to 94% with the government approved vonoprazan-based first-line triple therapy [Matsumoto et al. 2016; Murayama et al. 2016; Sue et al. 2016].
The efficacy of vonoprazan after H. pylori eradication failure with first-line treatment is largely unknown. However, in the study by Murakami and colleagues second-line triple therapy with 250 mg metronidazole + 750 mg amoxicillin + 20 mg vonoprazan, twice daily for 7 days (approved and covered under health insurance in Japan as second-line therapy), resulted in an eradication rate of 98% (n = 50). Similarly, in our latest study, a 100% eradication rate was achieved using this second-line therapy (n = 95, 100%) [Akazawa et al. in press]. Inaba and colleagues investigated the effects of a 1-week treatment with amoxicillin, clarithromycin, and vonoprazan, following the failure of a first-line 1-week treatment with amoxicillin, clarithromycin, and rabeprazole. The results of the study showed that, for 70.2% of the cases in which the rabeprazole-based therapy had failed, eradication was achieved with the vonoprazan-based therapy [Inaba et al. 2016]. Interestingly, Murakami and colleagues reported that, in Japanese patients carrying clarithromycin-resistant H. pylori strains, an 82% eradication rate was achieved with first-line treatment using clarithromycin, amoxicillin, and vonoprazan compared with a 42% eradication rate with clarithromycin, amoxicillin, and lansoprazole [Murakami et al. 2016]. Why did the vonoprazan-based treatment show a relatively high eradication rate against clarithromycin-resistant H. pylori? A plausible explanation is that, since vonoprazan, amoxicillin, and clarithromycin are metabolized by CYP3A4, a combined treatment with these three drugs can delay their clearance. In addition, the strong and fast-acting acid inhibitory effect of vonoprazan allowed the antibiotics, especially amoxicillin, to eradicate the H. pylori. These possibilities raise a question: is dual therapy with amoxicillin and vonoprazan sufficient for H. pylori eradication? A very recent 1-week study with a small number of patients implied this possibility. In the study, eradication rates of 95% (n = 19/20) and 90% (n = 18/20) were achieved with the dual therapy as first- and second-line treatment, respectively. The regimen used was 20 mg vonoprazan twice daily + 500 mg amoxicillin three times a day [Furuta et al. 2016]. Although this regimen required a higher dosing frequency for amoxicillin, the three doses per day exerted a sufficient effect. Further, administering the dual treatment for longer periods (10 or 14 days) might result in even better H. pylori eradication outcomes [Graham, 2016]. Low H. pylori resistance to amoxicillin makes the suggested treatments promising. In addition, amoxicillin is available worldwide and has been proven to be relatively safe [Furuta et al. 2010]. Excluding clarithromycin from H. pylori treatment regimens would attenuate newly developing clarithromycin-resistant H. pylori strains. This paradigm should be examined carefully in a larger number of patients and the effects of ethnicity should be investigated.
According to literature and our experience with 1118 patients, adverse effects of vonoprazan include erythema and gastrointestinal symptoms, without any life-threatening events reported so far [Jenkins et al. 2015; Sakurai et al. 2016b; Kamiya et al. 2016]. During a randomized study on erosive oesophagitis by Ashida and colleagues treatment-emergent adverse events (TEAEs) associated with a dose of up to 20 mg vonoprazan which led to discontinuation of the drug were comparable to those associated with 30 mg lansoprazole [Ashida et al. 2016]. In addition, the report by Murakami and colleagues showed a comparable rate of overall TEAEs between lansoprazole and vonoprazan. The most frequent adverse event was diarrhoea [Murakami et al. 2016]. In our studies on H. pylori eradication in the Japanese population using vonoprazan-based therapies, the overall rate of grade 2 adverse events was 2.1%. The side effects included diarrhoea, nausea and vomiting, constipation, abdominal pain, skin rash, and heartburn [Akazawa et al. in press]. One case of erythema multiforme, which required oral steroid treatment, has been reported during first-line treatment with vonoprazan [Kamiya et al. 2016]. Another study reported a slightly higher rate of skin rash in vonoprazan-treated patients compared with patients treated with conventional PPIs [Suzuki et al. 2016]. Nonetheless, caution should be exercised when drugs metabolized by CYP3A4 are coadministered with vonoprazan in patients with liver diseases. Vonoprazan has been reported to induce higher serum gastrin concentrations compared with conventional PPIs, with a stronger feedback mechanism triggered by the hypoacidity it causes reported as a possible cause [Murakami et al. 2016]. This phenomenon may be more concerning in patients with endocrine tumours who require long-term treatment [Jianu et al. 2012]. In fact, serum gastrin levels after 8 weeks of therapy were not statistically different between patients treated with vonoprazan and conventional PPIs [Murayama et al. 2016]. Taken together, the data from our studies and other reports show that the adverse effects of vonoprazan-based therapies are comparable to conventional PPI-based therapies. However, long-term studies on the effects of vonoprazan are required.
Conclusion
Although various strategies for eradicating H. pylori focus on overcoming the increasing antibiotic resistance, studies have indicated that eradication failure can be largely overcome by maintaining high pH levels in the stomach, which can be achieved by the use of an efficient acid blocker such as vonoprazan. Thus far, the data available from various studies have shown vonoprazan-based therapies to be efficient and safe for the treatment of H. pylori infection.
Acknowledgments
We thank Dr Moto Kitayama for helping with the graphic design.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: YA and the Department of Gastroenterology and Hepatology (Nagasaki University Hospital) received research grants and personal fees from the following companies: Eisai Co. Ltd, AstraZeneca, Kyorin Pharmaceutical Co. Ltd, Daiichi Sankyo Co. Ltd, Takeda Pharmaceutical Co. Ltd, Otsuka Pharmaceutical Co. Ltd, Astellas Pharma Inc., Zeria Pharmaceutical Co. Ltd, Abbott Japan Co. Ltd, Mitsubishi Tanabe Pharma, Abbvie, JIMRO Co. Ltd, Kyowa Hakko Kirin Co. Ltd, Asahi Kasei Medical Co. Ltd, Merck Sharp & Dohme and The Japanese Society of Gastroenterology. DF, YF, and the Fukuda Yutaka Surgery clinic received fees from Takeda Pharmaceutical Co. Ltd during the clinical trial of vonoprazan against reflux esophagitis and peptic ulcers.
Contributor Information
Yuko Akazawa, Department of Gastroenterology and Hepatology, Nagasaki University Hospital, 1-7-1 Sakamoto, Nagasaki 852-8501, Japan.
Daisuke Fukuda, Fukuda Yutaka Surgery Clinic, Nagasaki, Japan.
Yutaka Fukuda, Fukuda Yutaka Surgery Clinic, Nagasaki, Japan.
References
- Abelo A., Eriksson U., Karlsson M., Larsson H., Gabrielsson J. (2000) A turnover model of irreversible inhibition of gastric acid secretion by omeprazole in the dog. J Pharmacol Exp Ther 295: 662–669. [PubMed] [Google Scholar]
- Ang T., Fock K., Ang D., Kwek A., Teo E., Dhamodaran S. (2016) The changing profile of Helicobacter pylori antibiotic resistance in Singapore: a 15-year study. Helicobacter 21: 261–265. [DOI] [PubMed] [Google Scholar]
- Arikawa Y., Nishida H., Kurasawa O., Hasuoka A., Hirase K., Inatomi N., et al. (2012) Discovery of a novel pyrrole derivative 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1h-pyrrol-3-Yl]-N-methylmethanamin E fumarate (TAK-438) as a potassium-competitive acid blocker (P-Cab). J Med Chem 55: 4446–4456. [DOI] [PubMed] [Google Scholar]
- Asaka M. (2014) Strategy to eliminate gastric cancer deaths in Japan. International Agency for Research on Cancer (IARC Working Group Reports, No 8: 21–23). Available at http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk28/index.php (accessed 5 May 2016).
- Asaka M., Kato M., Takahashi S., Fukuda Y., Sugiyama T., Ota H., et al. (2010) Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter 15: 1–20. [DOI] [PubMed] [Google Scholar]
- Ashida K., Sakurai Y., Hori T., Kudou K., Nishimura A., Hiramatsu N., et al. (2016) Randomised clinical trial: vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis. Aliment Pharmacol Ther 43: 240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg A., Bottcher G., Andersson K., Carlsson E., Lindstrom A., Huby R., et al. (2008) Early stellate cell activation and veno-occlusive-disease (VOD)-like hepatotoxicity in dogs treated with AR-H047108, an imidazopyridine proton pump inhibitor. Toxicol Pathol 36: 727–737. [DOI] [PubMed] [Google Scholar]
- Broutet N., Tchamgoue S., Pereira E., Lamouliatte H., Salamon R., Megraud F. (2003) Risk factors for failure of Helicobacter pylori therapy – results of an individual data analysis of 2751 patients. Aliment Pharmacol Ther 17: 99–109. [DOI] [PubMed] [Google Scholar]
- Dent J., Kahrilas P., Hatlebakk J., Vakil N., Denison H., Franzen S., et al. (2008) A randomized, comparative trial of a potassium-competitive acid blocker (AZD0865) and esomeprazole for the treatment of patients with nonerosive reflux disease. Am J Gastroenterol 103: 20–26. [DOI] [PubMed] [Google Scholar]
- Erah P., Goddard A., Barrett D., Shaw P., Spiller R. (1997) The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: relevance to the treatment of Helicobacter pylori infection. J Antimicrob Chemother 39: 5–12. [DOI] [PubMed] [Google Scholar]
- Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., et al. (2014) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136: E359–E386. [DOI] [PubMed] [Google Scholar]
- Figura N., Moretti E., Vaglio L., Langone F., Vernillo R., Vindigni C., et al. (2016) Factors modulating the outcome of treatment for the eradication of Helicobacter pylori infection. New Microbiol 35: 335–340. [PubMed] [Google Scholar]
- Forman D., Sierra M. (2014) The current and projected global burden of gastric cancer. International Agency for Research on Cancer (IARC Working Group Reports, No 8): 5–14. Available at http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk18/index.php (accessed 5 May 2016).
- Fukase K., Kato M., Kikuchi S., Inoue K., Uemura N., Okamoto S., et al. (2008) Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 372: 392–397. [DOI] [PubMed] [Google Scholar]
- Fukuda D., Akazawa Y., Fukuda Y. (2016) Safety and efficacy of vonoprazan-based triple therapy against Helicobacter pylori infection: a single-center experience with 1118 patients. Ther Adv Gastroenterol 9: 747–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta T., Graham D. (2010) Pharmacologic aspects of eradication therapy for Helicobacter pylori infection. Gastroenterol Clin N Am 39: 465–480. [DOI] [PubMed] [Google Scholar]
- Furuta T., Sahara S., Ichikawa H., Kagami T., Uotani T., Yamade M., et al. (2016) Dual therapy with vonoprazan and amoxicillin is as effective as standard PPI-based triple therapy with amoxicillin and clarithromycin or metronidazole in Japan. Gastroenterology 150: S877. [Google Scholar]
- Garnock-Jones K. (2015) Vonoprazan: first global approval. Drugs 75: 439–443. [DOI] [PubMed] [Google Scholar]
- Graham D. (2016) Vonoprazan Helicobacter pylori eradication therapy: ethical and interpretation issues. Gut 7 April 2016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D., Fischbach L. (2010) Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 59: 1143–1153. [DOI] [PubMed] [Google Scholar]
- Hassan I., Stark R., Greenman J., Millar M. (1999) Activities of β-lactams and macrolides against Helicobacter pylori. Antimicrob Agents Chemother 43: 1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori Y., Imanishi A., Matsukawa J., Tsukimi Y., Nishida H., Arikawa Y., et al. (2010) 1-[5-(2-Fluorophenyl)-1-(Pyridin-3-ylsulfonyl)-1h-pyrrol-3-Yl]-N-methylmethanamin E monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J Pharmacol Exp Ther 335: 231–238. [DOI] [PubMed] [Google Scholar]
- Hunt R., Scarpignato C. (2015) Potassium-competitive acid blockers (P-CABs): are they finally ready for prime time in acid-related disease? Clin Transl Gastroenterol 6: e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba T., Iwamuro M., Toyokawa T., Okada H. (2016) Letter: promising results of Helicobacter pylori eradication with vonoprazan-based triple therapy after failure of proton pump inhibitor-based triple therapy. Aliment Pharmacol Ther 43: 179–180. [DOI] [PubMed] [Google Scholar]
- Jenkins H., Sakurai Y., Nishimura A., Okamoto H., Hibberd M., Jenkins R., et al. (2015) Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther 41: 636–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jianu C., Fossmark R., Viset T., Qvigstad G., Sordal O., Marvik R., et al. (2012) Gastric carcinoids after long-term use of a proton pump inhibitor. Aliment Pharmacol Ther 36: 644–649. [DOI] [PubMed] [Google Scholar]
- Kagami T., Sahara S., Ichikawa H., Uotani T., Yamade M., Sugimoto M., et al. (2016) Potent acid inhibition by vonoprazan in comparison with esomeprazole with reference to CYP2C19 genotype. Aliment Pharmacol Ther 43: 1048–1059. [DOI] [PubMed] [Google Scholar]
- Kamiya K., Nishio E., Horio A., Tokura Y. (2016) Erythema multiforme caused by triple therapy with amoxicillin, clarithromycin and vonoprazan for Helicobacter pylori. J Dermatol 43: 340–341. [DOI] [PubMed] [Google Scholar]
- Ma J., Zhang L., Brown L., Li J., Shen L., Pan K., et al. (2012) Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst 104: 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H., Kamada T., Oosawa M., Katsumata R., Fujita M. (2016) Potassium-competitive acid blocker antisecretory therapy improves H. pylori eradication. Gastroenterolgy 150: S878. [Google Scholar]
- Megraud F. (2007) Helicobacter pylori and antibiotic resistance. Gut 56: 1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megraud F. (2012) The challenge of Helicobacter pylori resistance to antibiotics: the comeback of bismuth-based quadruple therapy. Ther Adv Gastroenterol 5: 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendlein J., Sachs G. (1990) Interaction of a K+-competitive inhibitor, a substituted imidazo [1,2a] pyridine, with the phospho-and dephosphoenzyme forms of H+, K+-ATPase. J Biol Chem 265: 5030–5036. [PubMed] [Google Scholar]
- Miki I., Aoyama N., Sakai T., Shirasaka D., Wambura C., Maekawa S., et al. (2003) Impact of clarithromycin resistance and CYP2C19 genetic polymorphism on treatment efficacy of Helicobacter pylori infection with lansoprazole-or rabeprazole-based triple therapy in Japan. Eur J Gastroenterol Hepatol 15: 27–33. [DOI] [PubMed] [Google Scholar]
- Murakami K., Sakurai Y., Shiino M., Funao N., Nishimura A., Asaka M. (2016) Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut 65: 1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama M., Kubota D., Miyajima M., Kimura T., Tokutake K., Imai R. (2016) A randomized controlled trial comparing the first-line eradication rateusing vonoprazan or PPI for Helicobacter pylori infectious gastritis. Gastroenterology 150: S1269. [Google Scholar]
- Nakamura S., Sugiyama T., Matsumoto T., Iijima K., Ono S., Tajika M., et al. (2014) Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan. Gut 61: 507–513. [DOI] [PubMed] [Google Scholar]
- Park J., Greenberg E., Parsonnet J., Wild C., Forman D., Herrero R. (2014) Summary of IARC Working Group Meeting on Helicobacter pylori eradication as a strategy for preventing gastric cancer: Lyon, France. International Agency for Research on Cancer (IARC Working Group Reports, No 8): 1–4. Available at http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk8/index.php (accessed 5 May 2016).
- Parsons M., Keeling D. (2005) Novel approaches to the pharmacological blockade of gastric acid secretion. Expert Opin Invest Drugs 14: 411–421. [DOI] [PubMed] [Google Scholar]
- Roche V. (2006) The chemically elegant proton pump inhibitors. Am J Pharm Educ 70: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y., Mori Y., Okamoto H., Nishimura A., Komura E., Araki T., et al. (2016a) Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 Mg in healthy adult male subjects: a randomised open-label cross-over study. Aliment Pharmacol Ther 42: 719–730. [DOI] [PubMed] [Google Scholar]
- Sakurai Y., Nishimura A., Kennedy G., Hibberd M., Jenkins R., Okamoto H., et al. (2015) Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (vonoprazan) doses in healthy male Japanese/non-Japanese subjects. Clin Transl Gastroenterol 6: e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y., Shiino M., Okamoto H., Nishimura A., Nakamura K., Hasegawa S. (2016b) Pharmacokinetics and safety of triple therapy with vonoprazan, amoxicillin, and clarithromycin or metronidazole: a phase 1, open-label, randomized, crossover study. Adv Ther: 1–17. [DOI] [PubMed] [Google Scholar]
- Satake M., Nishikawa J., Fukagawa Y., Akashi K., Okamoto T., Yoshida T., et al. (2007) The long-term efficacy of Helicobacter pylori eradication therapy in patients with idiopathic thrombocytopenic purpura. J Gastroenterol Hepatol 22: 2233–2237. [DOI] [PubMed] [Google Scholar]
- Scott D., Munson K., Marcus E., Lambrecht N., Sachs G. (2015) The binding selectivity of vonoprazan (TAK-438) to the gastric H+, K+-ATPase. Aliment Pharmacol Ther 42: 1315–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Inatomi N., Munson K., Strugatsky D., Tokhtaeva E., Vagin O., et al. (2011) Characterization of a novel potassium-competitive acid blocker of the gastric H,K-ATPase, 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1h-pyrrol-3-Yl]-N-methylmethanamin E monofumarate (TAK-438). J Pharmacol Exp Ther 339: 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki S., Nomoto H., Kondo Y., Sakamoto H., Hayashi Y., Yamamoto H., et al. (2016) Comparison of vonoprazan and proton pump inhibitors for eradication of Helicobacter pylori. Kaohsiung J Med Sci 32: 255–260. [DOI] [PubMed] [Google Scholar]
- Song Z., Suo B., Zhang L., Zhou L. (2016) Rabeprazole, minocycline, amoxicillin, and bismuth as first-line and second-line regimens for Helicobacter pylori eradication. Helicobacter. 6 April 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Sue S., Arima I., Kuwashima I., Sanga K., Oka I. (2016) Efficacy and safety of novel class of acid suppressants: P-Cab-based amoxicillin and clarithromycin 1 week triple therapy as first line eradication of H. pylori in Japan. A multicenter study. Gastroenterology 150: S880. [Google Scholar]
- Sugimoto M., Furuta T., Shirai N., Kodaira C., Nishino M., Ikuma M., et al. (2007) Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter 12: 317–323. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Gotoda T., Kusano C., Iwatsuka K., Moriyama M. (2016) The efficacy and tolerability of a triple therapy containing a potassium-competitive acid blocker compared with a 7-day PPI-based low-dose clarithromycin triple therapy. Am J Gastroenterol 111: 949–956. [DOI] [PubMed] [Google Scholar]
- Takeda Ltd (2016) The Interview Form for Vonoprazan Fumarate (Takecab Tablets 10 mg and 20 mg). Version 7 (in Japanese). Available at: http://www.takeda.co.jp/ (accessed 5 May 2016).