Abstract
The role of inflammation in neurological disorders is increasingly recognised. Inflammatory processes are associated with the aetiology and clinical progression of migraine, psychiatric conditions, epilepsy, cerebrovascular diseases, dementia and neurodegeneration, such as seen in Alzheimer’s or Parkinson’s disease. Both central and systemic inflammatory actions have been linked with the development of brain diseases, suggesting that complex neuro-immune interactions could contribute to pathological changes in the brain across multiple temporal and spatial scales. However, the mechanisms through which inflammation impacts on neurological disease are improperly defined. To develop effective therapeutic approaches, it is imperative to understand how detrimental inflammatory processes could be blocked selectively, or controlled for prolonged periods, without compromising essential immune defence mechanisms. Increasing evidence indicates that common risk factors for brain disorders, such as atherosclerosis, diabetes, hypertension, obesity or infection involve the activation of NLRP3, NLRP1, NLRC4 or AIM2 inflammasomes, which are also associated with various neurological diseases. This review focuses on the mechanisms whereby inflammasomes, which integrate diverse inflammatory signals in response to pathogen-driven stimuli, tissue injury or metabolic alterations in multiple cell types and different organs of the body, could functionally link vascular- and neurological diseases and hence represent a promising therapeutic target.
Keywords: Inflammation, brain disease, inflammasome, systemic, neurodegeneration
Introduction
The role of innate immune and vascular inflammatory mechanisms in common neurological disorders
Induction of inflammation in any organ or tissue includes rapid vascular changes in parallel with the activation of innate immune cells that sense pathogens or tissue injury via pattern recognition receptors (PRRs). Recognition of pathogen-derived molecules, and those released by the compromised cells and tissues of the host (which induce sterile inflammation), triggers an inflammatory response that initially includes the activation of largely overlapping immune cell populations in different organs, such as monocytes, macrophages, dendritic cells or granulocytes. These cells express multiple PRRs, and some PRRs recognise both host- and pathogen-derived molecules. As an example, Toll-like receptor 4 (TLR4) is activated by both bacterial lipopolysaccharide (LPS) and high mobility group box protein 1 (HMGB1), which is released from injured cells and acts as an endogenous danger molecule.1,2 In this way, monocytes, neutrophils or tissue macrophages can react rapidly to harmful stimuli of different origin and mount an inflammatory response that has several common features, which are not stimulus-specific. Injured tissues contribute to sterile inflammation via releasing danger signals and alarmins such as ATP, interleukin-α (IL-1α), HMGB1 and many other cytoplasmic proteins as well as nuclear or mitochondrial DNA. Following the development of the appropriate immune response, and when the harmful stimulus is no longer present, resolution of inflammation takes place and tissue regeneration is initiated.3,4 The normal inflammatory response to injury or infection commonly involves systemic changes including increases in circulating inflammatory cytokine and acute phase protein levels, activation of the HPA axis and the autonomic nervous system, which resolve soon after tissue regeneration or clearance of infection. In contrast, non-resolving infection, impaired wound healing or lasting metabolic alterations such as high blood glucose, uric acid or triglyceride levels are associated with chronic inflammation. Excessive or uncontrolled inflammation can result in tissue injury and is often associated with systemic inflammatory changes. These include prolonged upregulation of circulating cytokines, acute phase proteins and inflammation in different vascular beds of the body. Systemic inflammation is also associated with vascular changes and activation of glial cells in the nervous system and perhaps not surprisingly, diabetes, hyperlipidaemia, atherosclerosis, hypertension, obesity and infection are risk factors for cerebrovascular and neurodegenerative diseases. Preceding systemic inflammation also leads to worse clinical outcome in acute cerebrovascular diseases.5–9 Evidence indicates that systemic inflammation is associated with inflammatory changes in the brain before neurological symptoms develop. For example, patients with multiple risk factors for stroke and chronically elevated C-reactive protein (CRP), but without history of a previous cerebrovascular event and in the absence of any obvious brain pathology as assessed by neuroradiologists on MR scans, show microglial activation in the brain. This also occurs in mice or rats with chronic vascular disease.10 Vascular and microglial activation and lipid deposition in the brain are also seen in mice fed an atherogenic diet.10,11 Acute or chronic inflammation in the CNS could also be induced by local signals. ATP, IL-1α, HMGB1 and DNA are released from injured neurons or glia, whereas amyloid-beta (Aβ) oligomers, tau and aggregated α-synuclein are potent inducers of glial activation and inflammatory cytokine production.12–17 Emerging data indicate that blockade of inflammation is protective in common neurological diseases. Interestingly, non-steroidal anti-inflammatory drugs (NSAIDs) and statins, which have diverse anti-inflammatory actions, appear to be protective in Alzheimer’s (AD) and Parkinson’s disease (PD).18–25
IL-1 and IL-18: Mediators of inflammation, vascular disease and brain injury
One of the main inflammatory mediators that contributes to a wide range of vascular, metabolic and neurological diseases is IL-1. IL-1 family cytokines are key mediators of inflammation, and the proinflammatory forms IL-1α and IL-1β are involved in both central and systemic inflammatory mechanisms.3,8,9,16,17,21,22,26 Blockade of IL-1 actions is markedly protective against brain injury in animal models.27,28 IL-18 is another inflammatory cytokine whose involvement in acute and chronic inflammatory conditions and common brain diseases has been documented.29 In particular, IL-18 is elevated in the brain and/or the circulation of patients with AD, schizophrenia, multiple sclerosis (MS), depression or cerebral ischaemia.30,31 Both IL-1β and IL-18 production are regulated by caspase-1 activation. Patients with mutations in the NLRP3 gene, which controls the activity of caspase-1, have been found to secrete more IL-1β and IL-18 and suffer from systemic inflammatory disease. This is associated with high circulating concentrations of IL-6, serum amyloid A and CRP.32 Recent research has revealed that the molecular machinery regulating IL-1β and IL-18 production in response to signalling via multiple PRRs includes the formation of inflammasomes.
Structure and activation of inflammasomes
Inflammasomes are large multi-molecular protein complexes that form in response to inflammatory stimuli and that are responsible for the processing of the inactive precursors of IL-1β and IL-18.33 Inflammasomes are composed of a sensor molecule, adaptor proteins and pro-inflammatory caspases. The sensor molecule is a PRR that senses pathogen (PAMPs), or damage associated molecular patterns (DAMPs), which are markers of infection or cell stress/injury, respectively. Inflammasomes are defined by the PRR, and although several have been described,33 those that have been associated with brain injury are NLRP1,34–36 NLRP3,37–39 NLRC440 and AIM2.40,41 The endogenous ligand for NLRP1 (NLR family pyrin domain containing 1) is unknown. NLRP3 (NLR family pyrin domain containing 3) senses a diverse array of both PAMP and DAMP stimuli.33 NLRC4 (NLR family CARD domain containing 4) senses bacterial ligands through co-receptors called NAIP proteins (NLR family apoptosis inhibitory protein),42,43 and AIM2 (absent in melanoma 2) directly binds to double stranded DNA.44,45 Upon PAMP or DAMP sensing NLRP3 nucleates the ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) adaptor molecule to oligomerise and form a large inflammasome speck within the cell based on a prion like aggregation.46,47 Pro-caspase-1 is also recruited to this complex where it becomes activated. Active caspase-1 then processes pro-IL-1β and pro-IL-18 to mature forms that are rapidly secreted from the cell.48 Once formed these inflammasome specks can also become extracellular and propagate further inflammatory responses.49,50 The mechanism of IL-1β release after inflammasome activation has been poorly understood but is now known to require cleavage of the cytoplasmic protein gasdermin D (Gsdmd).51,52 Gsdmd is cleaved by caspase-1 and results in a destabilisation of the plasma membrane facilitating release of IL-1β.51–53 Similar to the effects of knocking out Gsdmd, we have recently reported that membrane stabilising agents such as the complex polyphenol punicalagin are able to inhibit the release of IL-1β specifically without affecting inflammasome activation.54 Interestingly, punicalagin has also been shown to be neuroprotective in rodent models of stroke.55,56
Emerging role for inflammasomes in vascular disease, neuroinflammation and brain injury
Recent studies suggest that inflammasomes are involved in a wide range of pathophysiological processes in the brain and in chronic diseases that are risk factors for neurodegenerative or cerebrovascular disease. IL-1β and/or NLRP3 polymorphisms and changes in gene expression are associated with depression, migraine, MS, AD, PD and ischaemic stroke in patients.28,57–63 Similarly, NLRP3 polymorphisms are associated with risk of hypertension and age-related increase of blood pressure, type 2 diabetes, coronary heart disease and acute vascular events.64–67 Increased caspase-1 activation is seen in the brain of AD patients and the NLRP3 inflammasome was found to contribute to disease progression and changes in cognitive function in rodents.21,68 The NLRP3 inflammasome is also involved in the pathogenesis of CNS demyelination and prion disease in animal models69–72 and contributes to amyotrophic lateral sclerosis (ALS).73 Acute brain injury following cerebral ischaemia and traumatic brain injury (TBI) as well as spinal cord injury involve ASC-dependent mechanisms mediated by the NLRP1, AIM2 or NLRC4 inflammasomes.34–36,40 Since IL-1- and inflammasome-mediated actions contribute to inflammation and injury in both the periphery and the brain, it is important to understand the cellular mechanisms involved in order to develop effective therapeutic strategies for CNS disease.
Cell type-specific activation of inflammasomes links systemic inflammation and brain disease
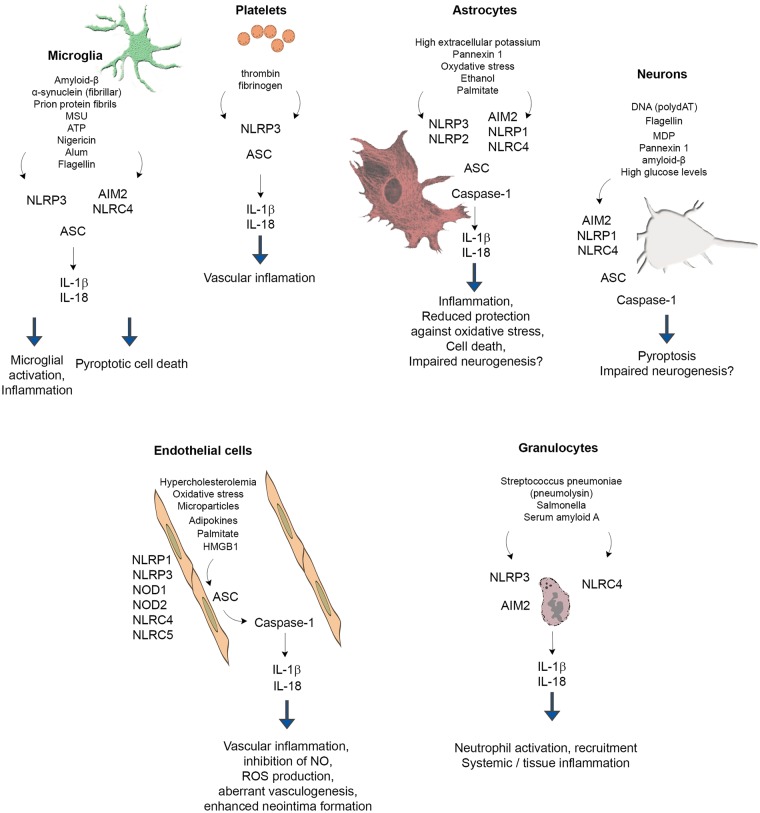
Inflammasome research has largely focused on monocytes and macrophages, since these cells not only respond rapidly to a diverse array of DAMPs and PAMPs, but are also considered to be key inducers of inflammation via IL-1 and IL-18 in different tissues. However, recent research has revealed that endothelial cells, granulocytes, neurons and astrocytes (and other cells) also express functional inflammasomes that contribute to both systemic inflammation and pathophysiological processes in the CNS (Figure 1). Because of the extensive literature available, this section focuses on inflammasome-mediated actions in different cell types in the context of neuroinflammation and risk factors for brain disease. Inflammasome signalling in monocytes and macrophages are not discussed in detail here but are reviewed extensively elsewhere.3,33,74,75
Figure 1.
Inflammasome signalling in different cell types involved in brain inflammation and injury. Because of the extensive literature data available, inflammasome signalling in monocytes and macrophages is not discussed in detail only pathways confirmed in the case of microglia. See detailed explanation in the text. Please note that triggers, pathways and downstream events of inflammasome activation were depicted in the figure only if experimental evidence is available for the given cell type.
Microglia
Similarly to other organs, injury in the CNS causes the release of DAMPs leading to microglial activation and IL-1 production. Although a comprehensive comparison of microglial inflammasome signalling with that of monocytes and macrophages has not been performed, the available evidence indicates that common PAMPs and DAMPs activate microglial inflammasomes. For example, LPS, HMGB1 and the acute phase protein serum amyloid A act as a priming stimulus for microglia, whereas classical DAMPs such ATP and monosodium ureate (MSU) crystals induce the activation of the NLRP3 inflammasome, leading to the release of mature IL-1β.76–79
Aggregated amyloid-β can activate the NLRP3 inflammasome in microglia, which release IL-1β upon stimulation following LPS priming. This is associated with lysosomal damage and release of cathepsin B.78,80 In turn, caspase-1 expression is observed in the human brain in patients with mild cognitive impairment and AD, whereas NLRP3- or caspase-1-deficient mice carrying mutations associated with familial AD are largely protected from loss of spatial memory.68 Deposition of α-synuclein is one of the main pathological changes seen in the brain in PD. Both monomeric and fibrillar forms of α-synuclein were found to induce the synthesis of IL-1β through TLR2, whereas fibrillar α-synuclein induced IL-1β secretion via NLRP3 in microglia.12 In another study, stimulation of microglia with α-synuclein was sufficient to induce a modest priming effect, but did not result in the release of active IL-1β.78 α-synuclein added to THP1 cells (a human monocyte cell line) induces activation of TLR2 and the NLRP3 inflammasome leading to IL-1β secretion.81,82 Prion protein fibrils induce the activation of NLRP3 inflammasome and IL-1β production from microglia via lysosome destabilisation, ultimately leading to the release neurotoxic mediators and neuronal death.83 In brain tissues from patients with Rasmussen’s encephalitis (an inflammatory encephalopathy of unknown cause defined by seizures with progressive neurological disabilities), increased expression inflammasome-associated genes (IL-1β, IL-18, NLRP1, NLRP3 and CASP1) was found. IL-1β and caspase-1 expression was associated with white matter myeloid cells.84
Neurons and astrocytes
Neurons express inflammasome components both in vivo and in vitro and neuronal inflammasome activation occurs in response to acute injury, brain trauma, stress and in animal models of neuroinflammation and neurodegeneration. For example, NLRP1, NLRC4, AIM2, caspase-1 and ASC mRNAs are present in cultured neurons, and treatment with the NLRP1 agonist muramyl dipeptide (MDP), the AIM2 agonist poly-(dA:dT) or the NLRC4 agonist recombinant flagellin, induces activation of caspase-1 and caspase-6.85 NLRP1 immunopositive neurons are increased 25- to 30-fold in AD brains compared with non-AD brains.85 Neuronal NLRP1 levels are also upregulated in APPswe/PS1dE9 transgenic mice, and NLRP1-mediated pyroptosis (a caspase-1 dependent, inflammatory form of cell death86) is induced in cultured cortical neurons in response to amyloid-β.87 The NLRP1 inflammasome is also activated in patients with medial temporal lobe epilepsy, and NLRP1 inflammasome activation contributes to neuronal pyroptosis in the amygdala kindling-induced rat model.88
Traumatic brain injury induces expression of caspase-1, caspase-11, ASC and NLRP1 in cortical neurons, which is associated with production of mature IL-1β. Activation of the AIM2 inflammasome is also seen in the human brain after traumatic brain injury and neurons stimulated with synthetic double-stranded DNA (poly-(dA:dT)) undergo AIM2 inflammasome-dependent pyroptosis, which is prevented by inhibition of pannexin-1.41 Systemic metabolic and/or inflammatory changes could also exert direct impact on neuronal inflammasomes. For example, high glucose levels induce the release of mature IL-1β and IL-18 from cultured neurons, whereas increased expression of NLRP1, ASC and caspase-1 is found in neurons both in vitro and in vivo in the cerebral cortex of streptozocin (STZ)-induced diabetic rats.89
Astrocytes are capable of producing IL-1β both in vitro and in vivo and express NLRP3, AIM2, NLRP1 and NLRC4 inflammasomes.90–92 Expression of the NLRP2 inflammasome by astrocytes has also been suggested.93 A growing body of literature suggests that inflammasome activation in astrocytes could contribute to brain inflammation after acute injury and in neurodegenerative diseases. High extracellular potassium levels open pannexin 1 channels leading to caspase-1 activation and IL-1β and IL-18 release from primary neurons and astrocytes.94 Astrocytes play an important role in protection against oxidative stress in the brain and this is linked with inflammasome activation. Lack of uncoupling protein 2 (UCP2), which regulates ROS production, leads to the aggravation of inflammation via activating the NLRP3 inflammasome and at the same time increased endoplasmic reticulum stress in astrocytes. UCP2 knockout mice show exacerbated dopaminergic neuron loss in the 1,2,3,6-methyl-phenyl-tetrahydropyridine (MPTP) murine model of PD, which is accompanied by increased astrocyte activation.95 Ethanol also induces NLRP3 activation and cell death in cultured astrocytes, which is blocked by a mitochondrial ROS scavenger.96 Increased expression of both NLRP1 and NLRP3 is seen in hippocampal neurons and astrocytes in post mortem alcoholic human brain, and inhibition of neurogenesis by ethanol is reversed by a neutralising antibody to IL-1β, or blockade of the IL-1β receptor with IL-1R antagonist in organotypic slice cultures.97 Expression levels of NLRC4 and ASC are significantly elevated in a subgroup of sporadic AD patients, and palmitate (saturated fatty acid, a major component of high fat diet) induces the activation of NLRC4 and production of mature IL-1β in cultured astrocytes.98 In post mortem tissues from patients with amyotrophic lateral sclerosis, increased NLRP3, ASC, caspase-1 and IL-18 levels are found compared with control tissues, and NLRP3 is expressed in spinal cord astrocytes of male SOD1(G93A) mice carrying a mutant human superoxide dismutase 1 (SOD1) variant. In these mice, NLRP3 expression and production of IL-1β were already detectable at a pre-symptomatic stage of the disease.90 Interestingly, another study failed to find substantial IL-1β or IL-18 release from astrocytes after inflammasome activation.78 Thus, it is important to note that some of the neuroprotective effects of inflammasome inhibition could be due to direct actions on neurons or astrocytes and not necessarily to blockade of microglia- or macrophage-dependent mechanisms in different models of brain injury.
Endothelial cells
Human cerebral endothelial cells express various NOD-like receptors (e.g. NOD1, NOD2, NLRC4, NLRC5, NLRP1 and NLRP3) and release IL-1β in a caspase-1-dependent manner upon LPS priming and stimulation with muramyl dipeptide.99 Importantly, inflammasomes in endothelial cells contribute to chronic inflammation and altered vascular responses in different vascular beds. Hypercholesterolemia and oxidative stress result in NLRP3 inflammasome activation in endothelial cells, leading to inhibition of endothelial nitric oxide synthase (eNOS) and coronary endothelial dysfunction.100,101 Adipokines such as visfatin activate the NLRP3 inflammasome in endothelial cells, leading to enhanced neointima formation.102 Similarly, uraemic sera from patients with chronic kidney disease induces NLRP3 activation, caspase-1 activation and ROS production in cultured endothelial cells.103 Palmitate in vitro and high-fat diet in vivo result in NLRP3 inflammasome activation in endothelial cells via thioredoxin-interacting protein (TXNIP)-mediated actions.104 It has also been postulated that the NLRP1 inflammasome is involved endothelial activation as human aortic endothelial cells exposed to sera from patients with peripheral arterial disease show significantly higher expression of NLRP1 than those exposed to sera from healthy individuals.105 Inflammasome activation may lead to aberrant vasculogenesis. In autoimmune diseases like systemic lupus erythematosus (SLE), IL-18 inhibits the differentiation of endothelial progenitor cells and circulating angiogenic cells into mature endothelial cells, which is reversed by antibody neutralisation of IL-18.106 Hemorrhagic shock leads to the activation of the NLRP3 inflammasome in lung endothelial cells in response to HMGB1 stimulation of TLR4, leading to secretion of IL-1β.107 Although the contribution of endothelial inflammasomes to neuroinflammation is presently unclear, circulating DAMPs and PAMPs are known to induce inflammatory changes in multiple organs, including the brain, via direct endothelial signalling mediated by TLRs.108–111
Granulocytes
Granulocytes are key drivers of inflammation and tissue injury in both the periphery and the injured brain. IL-1 is a potent inducer of granulocyte migration to inflamed tissues and granulocytes undergo profound functional changes after transendothelial migration. In the brain, transmigrated neutrophils release proteases and decondensed DNA contributing to neurotoxicity, which can be transferred by neutrophil-conditioned medium in vitro.20,112,113 Interestingly, IL-1 is a key mediator driving the recruitment of neutrophils in the mouse brain but is dispensable in extracerebral tissues including the lung and peritoneum.114 Human and mouse neutrophils also express key inflammasome components and release IL-1β and IL-18. Purified human neutrophils were found to have functional NLRP3 and AIM2 inflammasomes.115 However, unlike macrophages, neutrophils appear to be resistant to pyroptotosis and sustained IL-1β production is not associated with reduced neutrophil viability.116 Pneumolysin (a pore-forming toxin of Streptococcus pneumoniae) induces IL-1β processing in neutrophils through NLRP3 and ASC, a process that takes place independently of pyroptotic cell death or lysosomal destabilisation.117 Similarly, Salmonella infection triggers the activation of the neutrophil NLRC4 inflammasome, which selectively promotes IL-1β maturation without pyroptosis.116 Inflammasomes in neutrophils are also involved in the pathophysiology of non-infectious diseases, such as asthma.118 Interestingly, acute phase proteins can directly induce inflammasome activation in neutrophils. In neutrophils isolated from healthy donors, the acute phase protein serum amyloid A (which also induces microglial priming, see above) promotes the expression of pro-IL-1β, followed by the activation of the NLRP3 inflammasome and caspase-1, leading to the secretion of mature IL-1β.119 Recruitment of granulocytes into the cerebral ventricles as well as microglial and vascular activation in response to diet induced atherosclerosis in mice is also dependent on IL-1β.11 Thus, inflammasome activation in endothelial cells, microglia and granulocytes could link systemic inflammatory actions with diverse neuropathological processes in the brain.
Platelets
Platelets are a major source for IL-1α, IL-1β and IL-18 and contribute to circulating levels of these cytokines. The role of platelet-derived IL-1 in systemic and cerebrovascular inflammation has also been demonstrated.120–123 Blockade of platelet-mediated actions and IL-1 signalling has been found to protect against brain injury after cerebral ischaemia in both naive mice and under systemic inflammatory conditions, when stroke is preceded by Streptococcus pneumoniae infection.124,125 Platelets constitutively express the inflammasome components NLRP3 and ASC and have functional inflammasomes, enabling caspase-1 activation and IL-1β processing.126 Similarly to nucleated cells, platelets can be primed by TLR2- and TLR4-mediated signals, but caspase-1-dependent IL-1β processing does not require a second stimulus. Platelet IL-1β mRNA is induced by thrombin or fibrinogen and also released through platelet microparticles.127 Although the functional role of platelet inflammasomes in disease remains to be investigated in detail, interactions between platelets, circulating immune cells and the vasculature in response to various inflammasome activating stimuli is expected to occur in a wide range of diseases.
Inflammasomes in comorbidities and risk factors for brain disease
Diabetes
High glucose levels are associated with the activation of the NLRP3 inflammasome (Table 1), and type 2 diabetes is accompanied by elevated circulating IL-1β.128,129 Increases in NLRP3, ASC and IL-1β mRNA and protein levels have also been shown ex vivo in monocyte-derived macrophage (MDMs) cultures from newly diagnosed drug-naive type 2 diabetes patients. Exposure of MDMs to ATP, HMGB1, FFAs (free fatty acids), IAPP (islet amyloid polypeptide) or MSU crystals increased production of IL-1β and IL-18.128 The gain-of-function single nucleotide polymorphism (SNP) rs35829419 in the NLRP3 gene (p.Gln705Lys) is associated with increased production of IL-1β and increased risk for macrovascular complications (mainly myocardial infarction) in type 2 diabetes patients.65 In turn, Nlrp3−/− mice show improved glucose tolerance and insulin sensitivity.141
Table 1.
Inflammasomes in vascular diseases and risk factors for brain disease.
| Comorbidity | Inflammasome activated | Trigger |
|---|---|---|
| Type 2 diabetes | NLRP3128,129 | Hyperglyceamia,130 ATP, HMGB1, FFAs, IAPP, MSU,128 SFAs131 |
| Atherosclerosis | NLRP3132,133 | Cholesterol crystals133 |
| Hyperlipidaemia and obesity | NLRP3 | Cholesterol crystals101 |
| NLRP1134a | Ceramide135 | |
| Hypertension | NLRP3 | Hypoxia, high salt levels136–138 |
| Gout and arthritis | NLRP3, NLRC4139 | MSU, CPPD crystals140 |
Inflammasomes involved in different conditions and triggers identified were listed. See explanation in the text.
Inflammasome activation is associated with a protective phenotype.
Atherosclerosis
Atherosclerosis is a progressive artery disease which involves inflammation of the vessel walls and the deposition of lipid-rich plaques. One major component of atherosclerotic lesions is cholesterol. Cholesterol crystals can induce inflammation via activating the NLRP3 inflammasome in vitro and in vivo, a process associated with phagolysosomal damage. It has been demonstrated that NLRP3 is overexpressed in the aorta of patients with atherosclerosis or hypertension.132 LDLR−/− mice fed a high cholesterol diet show markedly decreased early atherosclerosis in parallel with reduction in IL-1β and IL-18 levels after transplantation with NLRP3−/−, ASC−/− or IL-1α/β−/− deficient bone-marrow, whereas mice lacking NLRP3 or IL-1 are resistant to diet-induced atherosclerosis.133 It has been shown that cholesterol crystals could function as both a priming stimulus, and a stimulus to induce IL-1β release. Cholesterol crystals also trigger formation of neutrophil extracellular traps (NETs), which prime macrophages to release cytokines, further amplifying the immune cell recruitment in atherosclerotic lesions.142 Selective blockade of the NLRP3 inflammasome in ApoE2.Ki mice results in the reduction of IL-1β production in peritoneal macrophages and reduced serum lipid (total cholesterol and triglyceride) levels are observed with a shift of macrophage polarisation from M1 to M2 state, which is associated with fewer atherosclerotic lesions.143 The purinergic receptor, P2X7 is coexpressed with NLRP3 in atherosclerotic plaques of ApoE−/− mice and plays a significant role in atherosclerosis by activating the NLRP3 inflammasome via facilitating the phosphorylation of protein kinase R (PKR).144
Hyperlipidaemia and obesity
The NLRP3 inflammasome contributes to obesity-induced inflammation and insulin resistance.135 NLRP3 activation is seen in the subcutaneous adipose tissue of dyslipidaemic, diabetic and obese patients and is associated with the severity of coronary atherosclerosis.145–147 Blockade of the NLRP3 inflammasome resulted in the amelioration of serum markers of diet-induced obesity and hyperlipidaemia and enhanced insulin signalling in parallel with a reduction of inflammation in the adipose tissue and the liver.135,148 Acute hypercholesterolemia increased the expression of caspase-1, NLRP3 and HMGB1 in the coronary arteries and decreased endothelium-dependent vasodilatation was observed in NLRP3+/+ mice compared with NLRP3−/− mice. Treatment with either a caspase-1 or a HMGB1 inhibitor restored the vasodilation response in NLRP3+/+ mice.101 Obesity is associated with vascular dysfunction (reduced aortic relaxation, macrophage accumulation and intimal thickening) in rats via activation of the NLRP3 inflammasome and mitochondrial dysfunction.149 Saturated fatty acids (SFAs), like palmitic acid, are known priming signals of NLRP3 inflammasome activation and IL-1β production via TLR4, leading to impaired insulin signalling, in contrast to unsaturated fatty acids (UFAs), which exert anti-inflammatory actions through inhibition of IL-1β processing.131,150,151 NLRP1 is also implicated in obesity, and similar to IL-18 deficient mice, NLRP1−/− mice are resistant to diet-induced metabolic dysfunctions and have reduced adiposity.134,152
Hypertension
There is an association between the NLRP3 SNP rs7512998 and blood pressure in patients,64 and circulating IL-1β levels are strongly linked with the development of hypertension.153 Expression of NLRP3, caspase-1 and production of IL-1β and IL-18 were confirmed in pulmonary hypertension in rodents.136,154 Mice lacking ASC show substantially reduced pulmonary hypertension and right ventricular remodelling,155 whereas reduced renal inflammation and better outcome are observed in a salt-induced hypertension model.137 High-salt-induced hypertension is associated with increases in NLRP3, caspase-1 and IL-1β levels in the paraventricular nucleus of the hypothalamus, which could be attenuated by inhibition of IL-1β or NF-κB activation suggesting the possible involvement of central inflammasome signalling in hypertension.138,156
Gout and arthritis
Rheumatic disease is associated with higher risk of cerebrovascular disease. For example, according to recent meta-analyses, the risk of stroke is higher among rheaumatoid arthritis patients under 50 years. Moreover, inflammatory arthropathies involve higher risk than non-inflammatory diseases and affect long-term disability after stroke.157,158 Gout also represents a risk factor for both ischaemic and haemorrhagic stroke in younger and older age as well.159,160 Interestingly, stroke itself can also increase the susceptibility for gouty arthritis in patients.161 Hyperuricemia contributes to chronic inflammation in gout. Monosodium urate (MSU) and calcium pyrophosphate dihydrate (CPPD) crystals activate the NLRP3 inflammasome, and macrophages derived from caspase-1, ASC or NLRP3 deficient mice have defective IL-1β production in response to MSU.140 Treatment either with IL-1R antagonist or IL-1 trap is an effective gout therapy.162,163 NLRP3-inflammasome activation has been demonstrated in rheaumatoid arthritis and NLRP3 polymorphisms are linked with the susceptibility to arthritis and the response to anti-TNF treatment.164 ASC has also been implicated to rheaumatoid arthritis. ASC−/− mice showed decreased infiltration of inflammatory cells and cartilage/bone destruction in a collagen-induced arthritis model.165 In an Ag-induced arthritis (AIA) model, ASC−/− mice have decreased synovial IL-1β and serum amyloid A levels. In contrast, NLRP3−/−, NLRC4−/− or caspase-1−/− mice do not show any alteration of joint inflammation compared with controls indicating that the effect of ASC is independent of NLRP3 or NLRC4 inflammasomes.139
Infection
Infection is an established risk factor for CNS disease, for which there is a wealth of literature.7,9 Infectious burden significantly correlates with the risk of ischaemic stroke in patients and there is a strong positive association between bacterial infection and AD.166,167 The role of inflammasomes in infectious diseases that manifest in the CNS is also well-established. For example, meningitis caused by Streptococcus pneumoniae is associated with the activation of NLRP3 via the pore-forming complex pneumolysin.168 NLRP3−/− and ASC−/− mice with pneumococcal meningitis show decreased inflammatory response, which is more pronounced in ASC−/− mice.169 Pertussis toxin induces the formation of a pyrin-dependent inflammasome that cleaves pro-IL-1β into its active form, promoting IL-6 production that facilitates neutrophil intravascular crawling in cerebral capillaries and promotes experimental autoimmune encephalomyelitis (EAE).170 Since recognition of diverse bacterial, fungal or viral PAMPs by different PRRs induces inflammasome activation, infections that manifest in either the periphery or in the brain could potentially contribute to neuroinflammation and brain injury via inflammasome activation in brain cells, circulating leukocytes or different vascular beds in the body. Blockade of inflammasome-mediated actions could have therapeutic benefit in brain diseases. Supporting this, studies from mouse models suggest that inflammasomes are in general dispensable for infectious disease, their absence merely delaying the induction of an adaptive immune response.171
Inflammasome activation is linked with diverse brain diseases in humans and experimental animals
Cerebral aneurysms and intracerebral/subarachnoid haemorrhage
NLRP3, ASC and caspase-1 expression are increased in ruptured aneurysms of patients (Table 2) compared with unruptured ones.172 A significant correlation has also been observed between the NLRP3 SNP rs35829419 and plasma IL-1β levels among patients with abdominal aortic anurysm.173 In animal models, NLRP3 silencing in microglia significantly improved neurological outcome, reduced brain oedema in vivo, and attenuated inflammation both in vivo and in vitro.187 NLRP3 is activated in a collagenase-induced rat model of intracerebral haemorrhage. Silencing the P2X7 receptor with siRNA or selectively inhibiting it with a non-competitive antagonist, Brilliant blue G, reduced expression of NLRP3, IL-1β and IL-18, which resulted in reduced brain oedema, neutrophil infiltration and better neurological outcome. Levels of Nox2 (gp91phox), iNOS and their cytotoxic product, peroxinitrite (ONOO−) were also decreased.174 Mitochondrial dysfunction and ROS production are suggested to activate the NLRP3 inflammasome after subarachnoid haemorrhage in rats.174,188 The inhibition of ROS production either with a mitochondrial permeability transition pore (mPTP) inhibitor (TRO-19622), or a mitochondrial ROS scavenger (Mito-TEMPO), significantly decreases expression of NLRP3, IL-1β and the activation of caspase-1, which is accompanied by reduced neutrophil recruitment after intracerebral haemorrhage in mice.175
Table 2.
Inflammasomes in neurological diseases.
| CNS disorder | Inflammasome activated | Trigger |
|---|---|---|
| Cerebral aneurysms and intracerebral/ subarachnoid haemorrhage | NLRP3172,173 | ROS174,175 |
| Ischemic brain injury | NLRP338,39 | Drop in extracellular pH176 |
| NLRP137,94 | Decrease in cytosolic ATP177 | |
| NLRC440 | Intracellular K+ efflux178,179 | |
| AIM240 | Pannexin 194 | |
| Traumatic brain injury | NLRP134,180,181, | |
| NLRP3182 | ||
| Infection (CNS) | NLRP3169 | Flagellin184 |
| NLRC4 | ||
| AIM2183 | ||
| Alzheimer’s and Parkinson’s disease | NLRP368,80,87,185 | |
| NLRP1185,199 | ||
| NLRC498 |
Inflammasomes involved in different conditions and triggers identified were listed. See details regarding the mechanisms in the text.
Ischaemic brain injury
A number of intracellular changes that occur after ischaemia have been proposed to induce inflammasome activation. These include mitochondrial damage, production of ROS, lysosomal destabilisation, pore formation in the cell membrane, changes in cell volume and increases in intracellular Ca2+ concentration, which eventually activates the NLRP3 inflammasome.178,189,190 In macrophages, ATP-induced release of mitochondrial DNA (particularly its oxidised form) into the cytosol contributes to activation of caspase-1 and IL-18/IL-1β secretion via NLRP3.191,192 DNA also activates the AIM2 inflammasome.45 Acidic extracellular pH, which is typical at sites of ischaemia and inflammation activates the NLRP3 inflammasome, while alkaline pH exerts an inhibitory effect on inflammasome activation.176 HMGB1, an endogenous DAMP released by cellular stress and pathogenic insults, could also potentiate the neuroinflammatory response via NLRP3 activation in microglia in a redox state-dependent manner.193 Ischaemia also causes anoxic depolarisation in neurons, which results in excessive glutamate and ATP release leading to excitotoxicity. The ionotropic P2X7 receptor and the pannexin 1 (Panx1) channel are overactivated by high extracellular ATP and NMDA receptor activation, and blockade of these channels is protective in cerebral ischaemia.189,194,195 ROS are thought to be key mediators of NLRP3 activation, as blocking NOX-dependent ROS production, or scavenging by N-acetylcysteine (NAC), in macrophages inhibits NLRP3 inflammasome activation by interfering the priming step required for the induction of NLRP3 expression.196 It has also been shown that NLRP3 activating DAMPs stimulate an inflammatory response in glia that contributes to brain inflammation after ischaemia.77 High extracellular K+ and reduced intracellular ATP, or increased AMP levels could trigger the activation of NLRP1 during hypoxia or after stroke.177,189
Although many of the processes described above occur after cerebral ischaemia, it is still unclear to what extent ischaemic brain injury is influenced by different inflammasomes in vivo. NLRP3 has been identified as a contributor to brain injury after focal cerebral ischaemia using NLRP3−/− mice.39 Bruton’s tyrosine kinase has also been suggested to physically interact with ASC and NLRP3, to play a role in NLRP3 inflammasome activation and to contribute to ischaemic brain injury.38 However, other studies found that NLRP3 is dispensable for neuronal injury in neonatal197 or adult40 mice after ischaemia. Antibody neutralisation of NLRP1 eliminated the assembly of the NLRP1 inflammasome in neurons and astrocytes and protected against brain injury.37,94 Neutralising antibodies against NLRP1 also reduces the inflammatory response after thromboembolic stroke in mice.35 Our recent studies identified the NLRC4 and AIM2 inflammasomes as major contributors to ischaemic brain injury via ASC-dependent mechanisms. Because of the diversity of the signals involved in inflammasome activation after cerebral ischaemia, it is likely that blockade of the ASC adaptor protein, common for several inflammasomes, could have great therapeutic potential. It is also essential to assess to what extent systemic inflammatory actions contribute to brain injury in certain models of cerebral ischaemia, which could in part explain the differences observed regarding the role of given inflammasomes in stroke outcome. As detailed above, the NLRP3 inflammasome plays a role in diverse systemic inflammatory actions; therefore, its role in experimental models of stroke combined with comorbidities requires further investigation.
Traumatic brain injury
NLRP1 is expressed in cortical neurons and CSF levels of ASC, caspase-1 and NLRP1 are increased in patients after traumatic brain injury.34,180 NLRP3 expression has also been found in the cerebral cortex after TBI in rats.182 Neutralisation of ASC or NLRP1 reduces inflammation and improves outcome in rats after traumatic brain injury.34 Recent data show that NLRP1 inflammasome components are associated with exosomes, which were isolated from the CSF of TBI or spinal cord injured patients.181
AD and PD
IL-1β and IL-18 are increased in the brains of patients with AD and PD and microglia are recruited to senile plaques where they produce IL-1β.31,198 This process includes the phagocytosis of Aβ and subsequent lysosomal damage, NLRP3 inflammasome activation and release of cathepsin B.80
APP/PS1 transgenic mice lacking either the NLRP3 or caspase-1 have decreased plaque deposition, increased Aβ clearance, reduced levels of IL-1β and better memory.68 The NLRP1 inflammasome is also implicated in AD.185 Nonsynonymous polymorphisms in the NLRP1 gene are associated with the susceptibility of AD in humans.186 In APP/PS1 transgenic mice, NLRP1 levels are upregulated in cortical neurons, and cultured neurons undergo caspase-1-dependent pyroptosis in response to Aβ, which is reduced by silencing either the NLRP1 or the caspase-1 gene with si-RNA.87 NLRC4 and ASC are upregulated in post-mortem brain tissues in a subgroup of sporadic AD patients. Addition of conditioned medium derived from NLRC4-silenced astrocytes to primary neurons, significantly reduces the production of IL-1β and generation of Aβ1-42 by neurons.98 It is currently unclear how inflammasome-mediated signals and cellular stress interact to cause neuronal injury. For example, chronic overexpression of IL-1β in itself promotes brain inflammation without overt neurodegeneration199 and could decrease the amount of amyloid plaques while increasing tau hyperphosphorylation in 3xTgAD/IL-1βXAT mice.200
The fibrillar form of α-synuclein is recognised by TLR2 and also activates the NLRP3 inflammasome leading to the release of IL-1β from human monocytes,12 as opposed to the monomer form which does not.82 The activation of NLRP3 is triggered by lysosomal rupture induced by the phagocytosis of α-synuclein fibrils both in isolated monocytes and in monocytic and neuronal cell lines.12,81 The activation of NLRP3 in dopaminergic neurons in the substantia nigra of MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-induced or transgenic mouse models of PD depends largely on Cdk5, as inhibition or deletion of Cdk5 efficiently blocks NLRP3 and neuroinflammation in these models.201 Thus, inflammasome-mediated actions are linked with several major pathological changes in neurodegenerative diseases and hence represent a promising therapeutic target.
Local and systemic inflammatory signals interact in brain disease
Complex interactions between peripheral and central inflammasome-mediated pathways, involving diverse stimuli such as the microbiota, tissue injury and metabolic alterations, could contribute to neuroinflammation and neurodegenerative diseases. Understanding these mechanisms will be instrumental to support both diagnosis and treatment. For example, type 2 diabetes is identified as a risk factor for AD and for cerebrovascular disease in general.5,202,203 Amyloid deposits (misfolded proteins rich in cross-β-sheet structure, which accumulate in various tissues in the form of plaques) are a hallmark of AD, but they are also typical in type 2 diabetes as islet amyloid polypeptide (IAPP) in the pancreas. Bacteria also produce amyloids as a component of their extracellular matrix during biofilm formation. Curli fibres produced by Salmonella enterica serovar Typhimurium and Escherichia coli activate the NLRP3 inflammasome, leading to the production of IL-1β via caspase-1 activation.204 As discussed above, amyloid β activates the NLRP3 inflammasome in microglia, as does IAPP in the pancreas, leading to IL-1β production.205 Furthermore, amyloid β, serum amyloid A (SAA) and IAPP are all recognised by TLR2 leading to IL-1β secretion in response to NLRP3 activation.77,204,206
Microparticles are released from virtually all cell types and their role in inflammatory processes is widely recognised. It is likely that circulating, neuronal- or glial-derived microparticles could induce activation of inflammatory cells in the periphery, whereas peripheral-derived microparticles could stimulate the brain vasculature. It has been shown that microparticles derived from monocytes activate endothelial cells in an IL-1-dependent manner, which leads to increased expression of cell adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and E-selectin. This effect is reduced by knockdown of NLRP3 in monocytes.207
The gut microbiota are involved in brain injury that occurs after stroke208 and acute brain injury leads to changes in the gut microbiota in part via altered autonomic activity.209 Bacterial translocation from the gut to the circulation has been documented after acute brain injury,210 which could induce inflammasome activation via a wide array of PAMPs in different tissues. In fact, we have shown that NLRC4-deficient mice are protected against brain injury, which could either be due to inflammasome activation induced by flagellin of bacteria, or a currently unrecognised endogenous ligand of NLRC4.40 As discussed above, changes in intracellular K+ concentrations after ischaemia trigger NLRP3 activation. Bacterial toxins can also cause K+ efflux, leading to the activation of the NLRP3 inflammasome.178 It needs to be investigated in detail to what extent neuronal and glial inflammasomes may be primed or activated by circulating (or local) bacterial, fungal and viral PAMPs or acute phase proteins in different experimental models. Examples also exist of altered peripheral inflammasome signalling in risk factors for brain disease in humans. For example, upregulation of inflammasome activity and increased gut permeability are associated with obesity in children and adolescents.146 Recent data show that genetic deficiency of caspase-1 decreased depressive- and anxiety-like behaviours and increased locomotor activity in mice, which paralleled changes in the faecal microbiome.211
Aging is an another important factor to consider in the context of systemic inflammation and brain disease. Interestingly, hippocampal lysates from aged rats showed significantly higher levels of NLRP1, ASC, caspase-1, caspase-11, the purinergic receptor P2X7, pannexin-1 and X-linked inhibitor of apoptosis (XIAP) than lysates from younger animals, which was associated with cognitive decline.212 It has been postulated that NLRP3 inflammasome activation links systemic low-grade inflammation to functional decline in aging as it promotes age-related degenerative changes, whereas deficient NLRP3 inflammasome-mediated caspase-1 activity improved glyceamic control and attenuated bone loss and thymic demise.213 It is likely that results from rodent models underestimate the impact of aging and comorbidities on pathological changes in the brain compared with humans, where a high proportion of aged individuals present with multiple chronic diseases and are exposed to an array of medications for several years or decades.
Therapeutic strategies to inhibit inflammasome activation
Drugs targeting the IL-1 pathway downstream of the inflammasome pathway do exist. Biologicals that target IL-1, like IL-1Ra (anakinra) and specific monoclonal antibodies such as canakinumab, are used for the clinical management of several auto-inflammatory disorders and are in trials for others. For example, anakinra has shown efficacy in preclinical models of acute brain disease and is undergoing clinical evaluation.214 However, biologicals such as anakinra may not easily access the brain during brain disease where there is limited disruption of the blood brain barrier. Given their role in vascular disease and associated co-morbidities, and their seemingly dispensible role in infection,171 inflammasomes are an attractive therapeutic target. In fact, in vivo data suggest that knocking out inflammasomes merely delays the resolution of an infection. The exact reason for this is unclear, but it has been suggested that animals maintained inflammasomes over evolution not to defend against vertebrate-adapted pathogens but instead to counteract infection by a plethora of undiscovered opportunistic pathogens residing in the environment.171 Supporting this, increased risk of infection was not observed after experimental stroke in response to inflammasome inhibition or treatment with IL-1Ra34,40,215 and IL-1Ra treatment was not associated with more infections or other major adverse events in patients with myocardial infarction,216 traumatic brain injury217 or stroke.9,218 Furthermore, strategies that specifically target NLRP3 will not compromise the role of pathogen sensing inflammasomes such as AIM2 or NLRC4. Thus, the available data suggest that targeting inflammasomes will be safe. The best characterised inflammasome is NLRP3 and a number of small molecules are being developed and investigated as potential NLRP3 inhibitors (reviewed extensively in Baldwin et al.219). To-date, the most advanced NLRP3 inhibitor is MCC950, which is protective in the rodent EAE model.220 Small molecule inhibitors of the NLRP1 inflammasome are also being developed.219 In addition to the development of novel inflammasome inhibitors, efforts are underway to repurpose existing drugs. For example, nucleoside reverse transcriptase inhibitors effective as an anti-HIV therapy also inhibit the NLRP3 inflammasome independently of reverse transcriptase inhibition.221 Repurposing drugs like this may accelerate the translation of inflammasome targeting strategies clinically. Thus, the development of small molecule inflammasome inhibitors may offer an alternative or an adjunct therapy to existing IL-1 blocking strategies.
Conclusions
In conclusion, inflammasome-mediated actions link systemic and central inflammatory mechanisms and could contribute to major human disorders, including common brain diseases. The association of diverse neurological diseases with inflammasome polymorphisms, the activation of inflammasomes in post mortem brain tissues of patients with common brain diseases and the pivotal role of inflammasomes in major risk factors for brain diseases suggest that inflammasomes could be a promising therapeutic target. This is strengthened by comprehensive preclinical studies suggesting that blockade of inflammasome signalling is effective in a number of conditions and appears to have minor side effects with respect to changing susceptibility to infection or infectious burden. Understanding the molecular mechanisms that contribute to brain diseases and the development of novel inflammasome inhibitors could have a profound impact on the clinical management of common human diseases.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: OTKA K109743 (AD), the Hungarian Brain Research Program KTIA_13_NAP-A-I/2 (AD), and the János Bolyai Research Scholarship and the ‘Lendulet’ program of the Hungarian Academy of Sciences (AD).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Yu M, Wang H, Ding A, et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 2006; 26: 174–179. [DOI] [PubMed] [Google Scholar]
- 2.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine 2008; 42: 145–151. [DOI] [PubMed] [Google Scholar]
- 3.Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol 2012; 4(3): pii: a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol 2005; 6: 1191–1197. [DOI] [PubMed] [Google Scholar]
- 5.Umegaki H. Neurodegeneration in diabetes mellitus. Adv Exp Med Biol 2012; 724: 258–265. [DOI] [PubMed] [Google Scholar]
- 6.Cai H, Cong WN, Ji S, et al. Metabolic dysfunction in Alzheimer's disease and related neurodegenerative disorders. Curr Alzheimer Res 2012; 9: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol 2007; 7: 161–167. [DOI] [PubMed] [Google Scholar]
- 8.Murray KN, Buggey HF, Denes A, et al. Systemic immune activation shapes stroke outcome. Mol Cell Neurosci 2013; 53: 14–25. [DOI] [PubMed] [Google Scholar]
- 9.Smith CJ, Lawrence CB, Rodriguez-Grande B, et al. The immune system in stroke: clinical challenges and their translation to experimental research. J Neuroimmune Pharmacol 2013; 8: 867–887. [DOI] [PubMed] [Google Scholar]
- 10.Drake C, Boutin H, Jones MS, et al. Brain inflammation is induced by co-morbidities and risk factors for stroke. Brain Behav Immun 2011; 25: 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denes A, Drake C, Stordy J, et al. Interleukin-1 mediates neuroinflammatory changes associated with diet-induced atherosclerosis. J Am Heart Assoc 2012; 1: e002006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Codolo G, Plotegher N, Pozzobon T, et al. Triggering of inflammasome by aggregated alpha-synuclein, an inflammatory response in synucleinopathies. PLoS One 2013; 8: e55375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salminen A, Ojala J, Kauppinen A, et al. Inflammation in Alzheimer's disease: amyloid-beta oligomers trigger innate immunity defence via pattern recognition receptors. Prog Neurobiol 2009; 87: 181–194. [DOI] [PubMed] [Google Scholar]
- 14.Wes PD, Easton A, Corradi J, et al. Tau overexpression impacts a neuroinflammation gene expression network perturbed in Alzheimer's disease. PLoS One 2014; 9: e106050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues RJ, Tome AR, Cunha RA. ATP as a multi-target danger signal in the brain. Front Neurosci 2015; 9: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol 2008; 3: 99–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brough D, Denes A. Interleukin-1alpha and brain inflammation. IUBMB Life 2015; 67: 323–330. [DOI] [PubMed] [Google Scholar]
- 18.Lleo A, Galea E, Sastre M. Molecular targets of non-steroidal anti-inflammatory drugs in neurodegenerative diseases. Cell Mol Life Sci 2007; 64: 1403–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minghetti L. Role of inflammation in neurodegenerative diseases. Curr Opin Neurol 2005; 18: 315–321. [DOI] [PubMed] [Google Scholar]
- 20.Denes A, Thornton P, Rothwell NJ, et al. Inflammation and brain injury: acute cerebral ischaemia, peripheral and central inflammation. Brain Behav Immun 2010; 24: 708–723. [DOI] [PubMed] [Google Scholar]
- 21.Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol 2015; 14: 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Najjar S, Pearlman DM, Alper K, et al. Neuroinflammation and psychiatric illness. J Neuroinflammation 2013; 10: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vezzani A, French J, Bartfai T, et al. The role of inflammation in epilepsy. Nat Rev Neurol 2011; 7: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waeber C, Moskowitz MA. Migraine as an inflammatory disorder. Neurology 2005; 64: S9–S15. [DOI] [PubMed] [Google Scholar]
- 25.Schiess M. Nonsteroidal anti-inflammatory drugs protect against Parkinson neurodegeneration: can an NSAID a day keep Parkinson disease away? Arch Neurol 2003; 60: 1043–1044. [DOI] [PubMed] [Google Scholar]
- 26.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011; 117: 3720–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray KN, Parry-Jones AR, Allan SM. Interleukin-1 and acute brain injury. Front Cell Neurosci 2015; 9: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denes A, Pinteaux E, Rothwell NJ, et al. Interleukin-1 and stroke: biomarker, harbinger of damage, and therapeutic target. Cerebrovasc Dis 2011; 32: 517–527. [DOI] [PubMed] [Google Scholar]
- 29.Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol 2003; 73: 213–224. [DOI] [PubMed] [Google Scholar]
- 30.Alboni S, Cervia D, Sugama S, et al. Interleukin 18 in the CNS. J Neuroinflammation 2010; 7: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ojala J, Alafuzoff I, Herukka SK, et al. Expression of interleukin-18 is increased in the brains of Alzheimer's disease patients. Neurobiol Aging 2009; 30: 198–209. [DOI] [PubMed] [Google Scholar]
- 32.Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr 2006; 83: 447S–455S. [DOI] [PubMed] [Google Scholar]
- 33.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 2015; 21: 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Rivero Vaccari JP, Lotocki G, Alonso OF, et al. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab 2009; 29: 1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abulafia DP, de Rivero Vaccari JP, Lozano JD, et al. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J Cereb Blood Flow Metab 2009; 29: 534–544. [DOI] [PubMed] [Google Scholar]
- 36.de Rivero Vaccari JP, Lotocki G, Marcillo AE, et al. A molecular platform in neurons regulates inflammation after spinal cord injury. J Neurosci 2008; 28: 3404–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fann DY, Lee SY, Manzanero S, et al. Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis 2013; 4: e790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ito M, Shichita T, Okada M, et al. Bruton's tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat Commun 2015; 6: 7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang F, Wang Z, Wei X, et al. NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J Cereb Blood Flow Metab 2014; 34: 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Denes A, Coutts G, Lenart N, et al. AIM2 and NLRC4 inflammasomes contribute with ASC to acute brain injury independently of NLRP3. Proc Natl Acad Sci USA 2015; 112: 4050–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adamczak SE, de Rivero Vaccari JP, Dale G, et al. Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J Cereb Blood Flow Metab 2014; 34: 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 2011; 477: 592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen H, Miao EA, Ting JP. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity 2013; 39: 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernandes-Alnemri T, Yu JW, Datta P, et al. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 2009; 458: 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hornung V, Ablasser A, Charrel-Dennis M, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 2009; 458: 514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai X, Chen J, Xu H, et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell 2014; 156: 1207–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu A, Magupalli VG, Ruan J, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 2014; 156: 1193–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1beta secretion. Cytokine Growth Factor Rev 2011; 22: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baroja-Mazo A, Martin-Sanchez F, Gomez AI, et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol 2014; 15: 738–748. [DOI] [PubMed] [Google Scholar]
- 50.Franklin BS, Bossaller L, De Nardo D, et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol 2014; 15: 727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kayagaki N, Stowe IB, Lee BL, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015; 526: 666–671. [DOI] [PubMed] [Google Scholar]
- 52.Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015; 526: 660–665. [DOI] [PubMed] [Google Scholar]
- 53.He WT, Wan H, Hu L, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res 2015; 25: 1285–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin-Sanchez F, Diamond C, Zeitler M, et al. Inflammasome-dependent IL-1beta release depends upon membrane permeabilisation. Cell Death Differ 2016; 23: 1219–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yaidikar L, Byna B, Thakur SR. Neuroprotective effect of punicalagin against cerebral ischemia reperfusion-induced oxidative brain injury in rats. J Stroke Cerebrovasc Dis 2014; 23: 2869–2878. [DOI] [PubMed] [Google Scholar]
- 56.Yaidikar L, Thakur S. Punicalagin attenuated cerebral ischemia-reperfusion insult via inhibition of proinflammatory cytokines, up-regulation of Bcl-2, down-regulation of Bax, and caspase-3. Mol Cell Biochem 2015; 402: 141–148. [DOI] [PubMed] [Google Scholar]
- 57.Forlenza OV, Diniz BS, Talib LL, et al. Increased serum IL-1beta level in Alzheimer's disease and mild cognitive impairment. Dement Geriatr Cogn Disord 2009; 28: 507–512. [DOI] [PubMed] [Google Scholar]
- 58.Ravaglia G, Paola F, Maioli F, et al. Interleukin-1beta and interleukin-6 gene polymorphisms as risk factors for AD: a prospective study. Exp Gerontol 2006; 41: 85–92. [DOI] [PubMed] [Google Scholar]
- 59.Zhu Z, Yan J, Geng C, et al. A polymorphism within the 3'UTR of NLRP3 is associated with susceptibility for ischemic stroke in Chinese population. Cell Mol Neurobiol 2015; 36: 981–988. [DOI] [PubMed] [Google Scholar]
- 60.Arman A, Isik N, Coker A, et al. Association between sporadic Parkinson disease and interleukin-1 beta -511 gene polymorphisms in the Turkish population. Eur Cytokine Netw 2010; 21: 116–121. [DOI] [PubMed] [Google Scholar]
- 61.Schuh E, Lohse P, Ertl-Wagner B, et al. Expanding spectrum of neurologic manifestations in patients with NLRP3 low-penetrance mutations. Neurol Neuroimmunol Neuroinflamm 2015; 2: e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alcocer-Gomez E, Cordero MD. NLRP3 inflammasome: a new target in major depressive disorder. CNS Neurosci Ther 2014; 20: 294–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McGeer PL, Yasojima K, McGeer EG. Association of interleukin-1 beta polymorphisms with idiopathic Parkinson's disease. Neurosci Lett 2002; 326: 67–69. [DOI] [PubMed] [Google Scholar]
- 64.Kunnas T, Maatta K, Nikkari ST. NLR family pyrin domain containing 3 (NLRP3) inflammasome gene polymorphism rs7512998 (C>T) predicts aging-related increase of blood pressure, the TAMRISK study. Immun Ageing 2015; 12: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klen J, Goricar K, Janez A, et al. NLRP3 inflammasome polymorphism and macrovascular complications in type 2 diabetes patients. J Diabetes Res 2015; 2015: 616747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Omi T, Kumada M, Kamesaki T, et al. An intronic variable number of tandem repeat polymorphisms of the cold-induced autoinflammatory syndrome 1 (CIAS1) gene modifies gene expression and is associated with essential hypertension. Eur J Hum Genet 2006; 14: 1295–1305. [DOI] [PubMed] [Google Scholar]
- 67.Varghese GP, Uporova L, Halfvarson J, et al. Polymorphism in the NLRP3 inflammasome-associated EIF2AK2 gene and inflammatory bowel disease. Mol Med Rep 2015; 11: 4579–4584. [DOI] [PubMed] [Google Scholar]
- 68.Heneka MT, Kummer MP, Stutz A, et al. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature 2013; 493: 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gris D, Ye Z, Iocca HA, et al. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J Immunol 2010; 185: 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Inoue M, Williams KL, Gunn MD, et al. NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 2012; 109: 10480–10485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jha S, Srivastava SY, Brickey WJ, et al. The inflammasome sensor, NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. J Neurosci 2010; 30: 15811–15820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi F, Yang L, Kouadir M, et al. The NALP3 inflammasome is involved in neurotoxic prion peptide-induced microglial activation. J Neuroinflammation 2012; 9: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meissner F, Molawi K, Zychlinsky A. Mutant superoxide dismutase 1-induced IL-1beta accelerates ALS pathogenesis. Proc Natl Acad Sci USA 2010; 107: 13046–13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol 2012; 28: 137–161. [DOI] [PubMed] [Google Scholar]
- 75.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell 2014; 157: 1013–1022. [DOI] [PubMed] [Google Scholar]
- 76.Weber MD, Frank MG, Tracey KJ, et al. Stress induces the danger-associated molecular pattern HMGB-1 in the hippocampus of male Sprague Dawley rats: a priming stimulus of microglia and the NLRP3 inflammasome. J Neurosci 2015; 35: 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Savage CD, Lopez-Castejon G, Denes A, et al. NLRP3-inflammasome activating DAMPs stimulate an inflammatory response in glia in the absence of priming which contributes to brain inflammation after injury. Front Immunol 2012; 3: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gustin A, Kirchmeyer M, Koncina E, et al. NLRP3 inflammasome is expressed and functional in mouse brain microglia but not in astrocytes. PLoS One 2015; 10: e0130624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burm SM, Zuiderwijk-Sick EA, t Jong AE, et al. Inflammasome-induced IL-1beta secretion in microglia is characterized by delayed kinetics and is only partially dependent on inflammatory caspases. J Neurosci 2015; 35: 678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Halle A, Hornung V, Petzold GC, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 2008; 9: 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Freeman D, Cedillos R, Choyke S, et al. Alpha-synuclein induces lysosomal rupture and cathepsin dependent reactive oxygen species following endocytosis. PLoS One 2013; 8: e62143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gustot A, Gallea JI, Sarroukh R, et al. Amyloid fibrils are the molecular trigger of inflammation in Parkinson's disease. Biochem J 2015; 471: 323–333. [DOI] [PubMed] [Google Scholar]
- 83.Hafner-Bratkovic I, Bencina M, Fitzgerald KA, et al. NLRP3 inflammasome activation in macrophage cell lines by prion protein fibrils as the source of IL-1beta and neuronal toxicity. Cell Mol Life Sci 2012; 69: 4215–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramaswamy V, Walsh JG, Sinclair DB, et al. Inflammasome induction in Rasmussen's encephalitis: cortical and associated white matter pathogenesis. J Neuroinflammation 2013; 10: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaushal V, Dye R, Pakavathkumar P, et al. Neuronal NLRP1 inflammasome activation of Caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated Caspase-6 activation. Cell Death Differ 2015; 22: 1676–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 2009; 7: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tan MS, Tan L, Jiang T, et al. Amyloid-beta induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer's disease. Cell Death Dis 2014; 5: e1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tan CC, Zhang JG, Tan MS, et al. NLRP1 inflammasome is activated in patients with medial temporal lobe epilepsy and contributes to neuronal pyroptosis in amygdala kindling-induced rat model. J Neuroinflammation 2015; 12: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meng XF, Wang XL, Tian XJ, et al. Nod-like receptor protein 1 inflammasome mediates neuron injury under high glucose. Mol Neurobiol 2014; 49: 673–684. [DOI] [PubMed] [Google Scholar]
- 90.Johann S, Heitzer M, Kanagaratnam M, et al. NLRP3 inflammasome is expressed by astrocytes in the SOD1 mouse model of ALS and in human sporadic ALS patients. Glia 2015; 63: 2260–2273. [DOI] [PubMed] [Google Scholar]
- 91.Lau LT, Yu AC. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J Neurotrauma 2001; 18: 351–359. [DOI] [PubMed] [Google Scholar]
- 92.Zeis T, Allaman I, Gentner M, et al. Metabolic gene expression changes in astrocytes in Multiple Sclerosis cerebral cortex are indicative of immune-mediated signaling. Brain Behav Immun 2015; 48: 313–325. [DOI] [PubMed] [Google Scholar]
- 93.Minkiewicz J, de Rivero Vaccari JP, Keane RW. Human astrocytes express a novel NLRP2 inflammasome. Glia 2013; 61: 1113–1121. [DOI] [PubMed] [Google Scholar]
- 94.Silverman WR, de Rivero Vaccari JP, Locovei S, et al. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem 2009; 284: 18143–18151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu M, Sun XL, Qiao C, et al. Uncoupling protein 2 deficiency aggravates astrocytic endoplasmic reticulum stress and nod-like receptor protein 3 inflammasome activation. Neurobiol Aging 2014; 35: 421–430. [DOI] [PubMed] [Google Scholar]
- 96.Alfonso-Loeches S, Urena-Peralta JR, Morillo-Bargues MJ, et al. Role of mitochondria ROS generation in ethanol-induced NLRP3 inflammasome activation and cell death in astroglial cells. Front Cell Neurosci 2014; 8: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zou J, Crews FT. Inflammasome-IL-1beta signaling mediates ethanol inhibition of hippocampal neurogenesis. Front Neurosci 2012; 6: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu L, Chan C. IPAF inflammasome is involved in interleukin-1beta production from astrocytes, induced by palmitate; implications for Alzheimer's Disease. Neurobiol Aging 2014; 35: 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nagyoszi P, Nyul-Toth A, Fazakas C, et al. Regulation of NOD-like receptors and inflammasome activation in cerebral endothelial cells. J Neurochem 2015; 135: 551–564. [DOI] [PubMed] [Google Scholar]
- 100.Chen Z, Wen L, Martin M, et al. Oxidative stress activates endothelial innate immunity via sterol regulatory element binding protein 2 (SREBP2) transactivation of microRNA-92a. Circulation 2015; 131: 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Y, Li X, Pitzer AL, et al. Coronary endothelial dysfunction induced by nucleotide oligomerization domain-like receptor protein with pyrin domain containing 3 inflammasome activation during hypercholesterolemia: beyond inflammation. Antioxid Redox Signal 2015; 22: 1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xia M, Boini KM, Abais JM, et al. Endothelial NLRP3 inflammasome activation and enhanced neointima formation in mice by adipokine visfatin. Am J Pathol 2014; 184: 1617–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martin-Rodriguez S, Caballo C, Gutierrez G, et al. TLR4 and NALP3 inflammasome in the development of endothelial dysfunction in uraemia. Eur J Clin Invest 2015; 45: 160–169. [DOI] [PubMed] [Google Scholar]
- 104.Mohamed IN, Hafez SS, Fairaq A, et al. Thioredoxin-interacting protein is required for endothelial NLRP3 inflammasome activation and cell death in a rat model of high-fat diet. Diabetologia 2014; 57: 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bleda S, de Haro J, Varela C, et al. NLRP1 inflammasome, and not NLRP3, is the key in the shift to proinflammatory state on endothelial cells in peripheral arterial disease. Int J Cardiol 2014; 172: e282–e284. [DOI] [PubMed] [Google Scholar]
- 106.Kahlenberg JM, Thacker SG, Berthier CC, et al. Inflammasome activation of IL-18 results in endothelial progenitor cell dysfunction in systemic lupus erythematosus. J Immunol 2011; 187: 6143–6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xiang M, Shi X, Li Y, et al. Hemorrhagic shock activation of NLRP3 inflammasome in lung endothelial cells. J Immunol 2011; 187: 4809–4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Khakpour S, Wilhelmsen K, Hellman J. Vascular endothelial cell Toll-like receptor pathways in sepsis. Innate Immun 2015; 21: 827–846. [DOI] [PubMed] [Google Scholar]
- 109.Curtiss LK, Tobias PS. Emerging role of Toll-like receptors in atherosclerosis. J Lipid Res 2009; 50 Suppl: S340–S345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pandey S, Agrawal DK. Immunobiology of Toll-like receptors: emerging trends. Immunol Cell Biol 2006; 84: 333–341. [DOI] [PubMed] [Google Scholar]
- 111.Miranda-Hernandez S, Baxter AG. Role of toll-like receptors in multiple sclerosis. Am J Clin Exp Immunol 2013; 2: 75–93. [PMC free article] [PubMed] [Google Scholar]
- 112.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013; 13: 159–175. [DOI] [PubMed] [Google Scholar]
- 113.Allen C, Thornton P, Denes A, et al. Neutrophil cerebrovascular transmigration triggers rapid neurotoxicity through release of proteases associated with decondensed DNA. J Immunol 2012; 189: 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Giles JA, Greenhalgh AD, Davies CL, et al. Requirement for interleukin-1 to drive brain inflammation reveals tissue-specific mechanisms of innate immunity. Eur J Immunol 2015; 45: 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bakele M, Joos M, Burdi S, et al. Localization and functionality of the inflammasome in neutrophils. J Biol Chem 2014; 289: 5320–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen KW, Gross CJ, Sotomayor FV, et al. The neutrophil NLRC4 inflammasome selectively promotes IL-1beta maturation without pyroptosis during acute Salmonella challenge. Cell Rep 2014; 8: 570–582. [DOI] [PubMed] [Google Scholar]
- 117.Karmakar M, Katsnelson M, Malak HA, et al. Neutrophil IL-1beta processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux. J Immunol 2015; 194: 1763–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Simpson JL, Phipps S, Baines KJ, et al. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur Respir J 2014; 43: 1067–1076. [DOI] [PubMed] [Google Scholar]
- 119.Migita K, Izumi Y, Jiuchi Y, et al. Serum amyloid A induces NLRP-3-mediated IL-1beta secretion in neutrophils. PLoS One 2014; 9: e96703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lindemann S, Tolley ND, Dixon DA, et al. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol 2001; 154: 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ahmad R, Iannello A, Samarani S, et al. Contribution of platelet activation to plasma IL-18 concentrations in HIV-infected AIDS patients. AIDS 2006; 20: 1907–1909. [DOI] [PubMed] [Google Scholar]
- 122.Thornton P, McColl BW, Greenhalgh A, et al. Platelet interleukin-1alpha drives cerebrovascular inflammation. Blood 2010; 115: 3632–3639. [DOI] [PubMed] [Google Scholar]
- 123.Morrell CN, Aggrey AA, Chapman LM, et al. Emerging roles for platelets as immune and inflammatory cells. Blood 2014; 123: 2759–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kleinschnitz C, Pozgajova M, Pham M, et al. Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation 2007; 115: 2323–2330. [DOI] [PubMed] [Google Scholar]
- 125.Denes A, Pradillo JM, Drake C, et al. Streptococcus pneumoniae worsens cerebral ischemia via interleukin 1 and platelet glycoprotein Ibalpha. Ann Neurol 2014; 75: 670–683. [DOI] [PubMed] [Google Scholar]
- 126.Hottz ED, Lopes JF, Freitas C, et al. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood 2013; 122: 3405–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hottz ED, Monteiro AP, Bozza FA, et al. Inflammasome in platelets: allying coagulation and inflammation in infectious and sterile diseases? Mediators Inflamm 2015; 2015: 435783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee HM, Kim JJ, Kim HJ, et al. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes 2013; 62: 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dixit VD. Nlrp3 inflammasome activation in type 2 diabetes: is it clinically relevant? Diabetes 2013; 62: 22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ruscitti P, Cipriani P, Di Benedetto P, et al. Monocytes from patients with rheumatoid arthritis and type 2 diabetes mellitus display an increased production of interleukin (IL)-1beta via the nucleotide-binding domain and leucine-rich repeat containing family pyrin 3(NLRP3)-inflammasome activation: a possible implication for therapeutic decision in these patients. Clin Exp Immunol 2015; 182: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.L'Homme L, Esser N, Riva L, et al. Unsaturated fatty acids prevent activation of NLRP3 inflammasome in human monocytes/macrophages. J Lipid Res 2013; 54: 2998–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zheng F, Xing S, Gong Z, et al. NLRP3 inflammasomes show high expression in aorta of patients with atherosclerosis. Heart Lung Circ 2013; 22: 746–750. [DOI] [PubMed] [Google Scholar]
- 133.Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010; 464: 1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Murphy AJ, Kraakman MJ, Kammoun HL, et al. IL-18 Production from the NLRP1 inflammasome prevents obesity and metabolic syndrome. Cell Metab 2016; 23: 155–164. [DOI] [PubMed] [Google Scholar]
- 135.Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 2011; 17: 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Villegas LR, Kluck D, Field C, et al. Superoxide dismutase mimetic, MnTE-2-PyP, attenuates chronic hypoxia-induced pulmonary hypertension, pulmonary vascular remodeling, and activation of the NALP3 inflammasome. Antioxid Redox Signal 2013; 18: 1753–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Krishnan SM, Dowling JK, Ling YH, et al. Inflammasome activity is essential for one kidney/deoxycorticosterone acetate/salt-induced hypertension in mice. Br J Pharmacol 2016; 173: 752–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Qi J, Yu XJ, Shi XL, et al. NF-kappaB blockade in hypothalamic paraventricular nucleus inhibits high-salt-induced hypertension through NLRP3 and caspase-1. Cardiovasc Toxicol Epub ahead of print 5 October 2015. DOI: 10.1007/s12012-015-9344-9. [DOI] [PubMed] [Google Scholar]
- 139.Kolly L, Karababa M, Joosten LA, et al. Inflammatory role of ASC in antigen-induced arthritis is independent of caspase-1, NALP-3, and IPAF. J Immunol 2009; 183: 4003–4012. [DOI] [PubMed] [Google Scholar]
- 140.Martinon F, Petrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006; 440: 237–241. [DOI] [PubMed] [Google Scholar]
- 141.Zhou R, Tardivel A, Thorens B, et al. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 2010; 11: 136–140. [DOI] [PubMed] [Google Scholar]
- 142.Warnatsch A, Ioannou M, Wang Q, et al. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015; 349: 316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Abderrazak A, Couchie D, Mahmood DF, et al. Anti-inflammatory and antiatherogenic effects of the NLRP3 inflammasome inhibitor arglabin in ApoE2.Ki mice fed a high-fat diet. Circulation 2015; 131: 1061–1070. [DOI] [PubMed] [Google Scholar]
- 144.Peng K, Liu L, Wei D, et al. P2X7R is involved in the progression of atherosclerosis by promoting NLRP3 inflammasome activation. Int J Mol Med 2015; 35: 1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yin Z, Deng T, Peterson LE, et al. Transcriptome analysis of human adipocytes implicates the NOD-like receptor pathway in obesity-induced adipose inflammation. Mol Cell Endocrinol 2014; 394: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rainone V, Schneider L, Saulle I, et al. Upregulation of inflammasome activity and increased gut permeability are associated with obesity in children and adolescents. Int J Obes (Lond) 2016. [DOI] [PubMed] [Google Scholar]
- 147.Bando S, Fukuda D, Soeki T, et al. Expression of NLRP3 in subcutaneous adipose tissue is associated with coronary atherosclerosis. Atherosclerosis 2015; 242: 407–414. [DOI] [PubMed] [Google Scholar]
- 148.Honda H, Nagai Y, Matsunaga T, et al. Isoliquiritigenin is a potent inhibitor of NLRP3 inflammasome activation and diet-induced adipose tissue inflammation. J Leukoc Biol 2014; 96: 1087–1100. [DOI] [PubMed] [Google Scholar]
- 149.Liu P, Xie Q, Wei T, et al. Activation of the NLRP3 inflammasome induces vascular dysfunction in obese OLETF rats. Biochem Biophys Res Commun 2015; 468: 319–325. [DOI] [PubMed] [Google Scholar]
- 150.Yan Y, Jiang W, Spinetti T, et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013; 38: 1154–1163. [DOI] [PubMed] [Google Scholar]
- 151.Wen H, Gris D, Lei Y, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 2011; 12: 408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Netea MG, Joosten LA. The NLRP1-IL18 connection: A stab in the back of obesity-induced inflammation. Cell Metab 2016; 23: 6–7. [DOI] [PubMed] [Google Scholar]
- 153.Dalekos GN, Elisaf M, Bairaktari E, et al. Increased serum levels of interleukin-1beta in the systemic circulation of patients with essential hypertension: additional risk factor for atherogenesis in hypertensive patients? J Lab Clin Med 1997; 129: 300–308. [DOI] [PubMed] [Google Scholar]
- 154.Tang B, Chen GX, Liang MY, et al. Ellagic acid prevents monocrotaline-induced pulmonary artery hypertension via inhibiting NLRP3 inflammasome activation in rats. Int J Cardiol 2015; 180: 134–141. [DOI] [PubMed] [Google Scholar]
- 155.Cero FT, Hillestad V, Sjaastad I, et al. Absence of the inflammasome adaptor ASC reduces hypoxia-induced pulmonary hypertension in mice. Am J Physiol Lung Cell Mol Physiol 2015; 309: L378–L387. [DOI] [PubMed] [Google Scholar]
- 156.Qi J, Zhao XF, Yu XJ, et al. Targeting Interleukin-1 beta to suppress sympathoexcitation in hypothalamic paraventricular nucleus in dahl salt-sensitive hypertensive rats. Cardiovasc Toxicol 2015; 16: 298–306. [DOI] [PubMed] [Google Scholar]
- 157.Wiseman SJ, Ralston SH, Wardlaw JM. Cerebrovascular disease in rheumatic diseases: A systematic review and meta-analysis. Stroke 2016; 47: 943–950. [DOI] [PubMed] [Google Scholar]
- 158.Nguyen-Oghalai TU, Wu H, McNearney TA, et al. Functional outcome after stroke in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum 2008; 59: 984–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Clarson LE, Hider SL, Belcher J, et al. Increased risk of vascular disease associated with gout: a retrospective, matched cohort study in the UK clinical practice research datalink. Ann Rheum Dis 2015; 74: 642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Seminog OO, Goldacre MJ. Gout as a risk factor for myocardial infarction and stroke in England: evidence from record linkage studies. Rheumatology (Oxford) 2013; 52: 2251–2259. [DOI] [PubMed] [Google Scholar]
- 161.Lin YH, Hsu HL, Huang YC, et al. Gouty arthritis in acute cerebrovascular disease. Cerebrovasc Dis 2009; 28: 391–396. [DOI] [PubMed] [Google Scholar]
- 162.So A, De Smedt T, Revaz S, et al. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther 2007; 9: R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Terkeltaub R, Sundy JS, Schumacher HR, et al. The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study. Ann Rheum Dis 2009; 68: 1613–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mathews RJ, Robinson JI, Battellino M, et al. Evidence of NLRP3-inflammasome activation in rheumatoid arthritis (RA); genetic variants within the NLRP3-inflammasome complex in relation to susceptibility to RA and response to anti-TNF treatment. Ann Rheum Dis 2014; 73: 1202–1210. [DOI] [PubMed] [Google Scholar]
- 165.Yamazaki H, Takeoka M, Kitazawa M, et al. ASC plays a role in the priming phase of the immune response to type II collagen in collagen-induced arthritis. Rheumatol Int 2012; 32: 1625–1632. [DOI] [PubMed] [Google Scholar]
- 166.Maheshwari P, Eslick GD. Bacterial infection and Alzheimer's disease: a meta-analysis. J Alzheimers Dis 2015; 43: 957–966. [DOI] [PubMed] [Google Scholar]
- 167.Miller EC, Elkind MS. Infection and stroke: an update on recent progress. Curr Neurol Neurosci Rep 2016; 16: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hoegen T, Tremel N, Klein M, et al. The NLRP3 inflammasome contributes to brain injury in pneumococcal meningitis and is activated through ATP-dependent lysosomal cathepsin B release. J Immunol 2011; 187: 5440–5451. [DOI] [PubMed] [Google Scholar]
- 169.Geldhoff M, Mook-Kanamori BB, Brouwer MC, et al. Inflammasome activation mediates inflammation and outcome in humans and mice with pneumococcal meningitis. BMC Infect Dis 2013; 13: 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dumas A, Amiable N, de Rivero Vaccari JP, et al. The inflammasome pyrin contributes to pertussis toxin-induced IL-1beta synthesis, neutrophil intravascular crawling and autoimmune encephalomyelitis. PLoS Pathog 2014; 10: e1004150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Maltez VI, Miao EA. Reassessing the evolutionary importance of inflammasomes. J Immunol 2016; 196: 956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang D, Yan H, Hu Y, et al. Increased expression of NLRP3 inflammasome in wall of ruptured and unruptured human cerebral aneurysms: Preliminary results. J Stroke Cerebrovasc Dis 2015; 24: 972–979. [DOI] [PubMed] [Google Scholar]
- 173.Roberts RL, Van Rij AM, Phillips LV, et al. Interaction of the inflammasome genes CARD8 and NLRP3 in abdominal aortic aneurysms. Atherosclerosis 2011; 218: 123–126. [DOI] [PubMed] [Google Scholar]
- 174.Feng L, Chen Y, Ding R, et al. P2X7R blockade prevents NLRP3 inflammasome activation and brain injury in a rat model of intracerebral hemorrhage: involvement of peroxynitrite. J Neuroinflammation 2015; 12: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ma Q, Chen S, Hu Q, et al. NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann Neurol 2014; 75: 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rajamaki K, Nordstrom T, Nurmi K, et al. Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. J Biol Chem 2013; 288: 13410–13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liao KC, Mogridge J. Activation of the Nlrp1b inflammasome by reduction of cytosolic ATP. Infect Immun 2013; 81: 570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Munoz-Planillo R, Kuffa P, Martinez-Colon G, et al. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 2013; 38: 1142–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Petrilli V, Papin S, Dostert C, et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ 2007; 14: 1583–1589. [DOI] [PubMed] [Google Scholar]
- 180.Adamczak S, Dale G, de Rivero Vaccari JP, et al. Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: clinical article. J Neurosurg 2012; 117: 1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.de Rivero Vaccari JP, Brand F, III, Adamczak S, et al. Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochem 2016; 136 Suppl 1: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu HD, Li W, Chen ZR, et al. Expression of the NLRP3 inflammasome in cerebral cortex after traumatic brain injury in a rat model. Neurochem Res 2013; 38: 2072–2083. [DOI] [PubMed] [Google Scholar]
- 183.Hanamsagar R, Aldrich A, Kielian T. Critical role for the AIM2 inflammasome during acute CNS bacterial infection. J Neurochem 2014; 129: 704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jamilloux Y, Pierini R, Querenet M, et al. Inflammasome activation restricts Legionella pneumophila replication in primary microglial cells through flagellin detection. Glia 2013; 61: 539–549. [DOI] [PubMed] [Google Scholar]
- 185.Saresella M, La Rosa F, Piancone F, et al. The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer's disease. Mol Neurodegener 2016; 11: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pontillo A, Catamo E, Arosio B, et al. NALP1/NLRP1 genetic variants are associated with Alzheimer disease. Alzheimer Dis Assoc Disord 2012; 26: 277–281. [DOI] [PubMed] [Google Scholar]
- 187.Yuan B, Shen H, Lin L, et al. Recombinant adenovirus encoding NLRP3 RNAi attenuate inflammation and brain injury after intracerebral hemorrhage. J Neuroimmunol 2015; 287: 71–75. [DOI] [PubMed] [Google Scholar]
- 188.Li J, Chen J, Mo H, et al. Minocycline protects against NLRP3 inflammasome-induced inflammation and P53-associated apoptosis in early brain injury after subarachnoid hemorrhage. Mol Neurobiol 2015; 53: 2668–2678. [DOI] [PubMed] [Google Scholar]
- 189.Bonora M, Wieckowsk MR, Chinopoulos C, et al. Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition. Oncogene 2015; 34: 1608. [DOI] [PubMed] [Google Scholar]
- 190.Horng T. Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends Immunol 2014; 35: 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shimada K, Crother TR, Karlin J, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012; 36: 401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nakahira K, Haspel JA, Rathinam VA, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 2011; 12: 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Frank MG, Weber MD, Fonken LK, et al. The redox state of the alarmin HMGB1 is a pivotal factor in neuroinflammatory and microglial priming: A role for the NLRP3 inflammasome. Brain Behav Immun 2015; 55: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bargiotas P, Krenz A, Hormuzdi SG, et al. Pannexins in ischemia-induced neurodegeneration. Proc Natl Acad Sci USA 2011; 108: 20772–20777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cisneros-Mejorado A, Gottlieb M, Cavaliere F, et al. Blockade of P2X7 receptors or pannexin-1 channels similarly attenuates postischemic damage. J Cereb Blood Flow Metab 2015; 35: 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bauernfeind F, Bartok E, Rieger A, et al. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol 2011; 187: 613–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ystgaard MB, Sejersted Y, Loberg EM, et al. Early upregulation of NLRP3 in the brain of neonatal mice exposed to hypoxia-ischemia: No early neuroprotective effects of NLRP3 deficiency. Neonatology 2015; 108: 211–219. [DOI] [PubMed] [Google Scholar]
- 198.Blum-Degen D, Muller T, Kuhn W, et al. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer's and de novo Parkinson's disease patients. Neurosci Lett 1995; 202: 17–20. [DOI] [PubMed] [Google Scholar]
- 199.Shaftel SS, Carlson TJ, Olschowka JA, et al. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J Neurosci 2007; 27: 9301–9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ghosh S, Wu MD, Shaftel SS, et al. Sustained interleukin-1beta overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer's mouse model. J Neurosci 2013; 33: 5053–5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang P, Shao XY, Qi GJ, et al. Cdk5-dependent activation of neuronal inflammasomes in Parkinson's disease. Mov Disord 2016; 31: 366–376. [DOI] [PubMed] [Google Scholar]
- 202.Holscher C. Diabetes as a risk factor for Alzheimer's disease: insulin signalling impairment in the brain as an alternative model of Alzheimer's disease. Biochem Soc Trans 2011; 39: 891–897. [DOI] [PubMed] [Google Scholar]
- 203.Vagelatos NT, Eslick GD. Type 2 diabetes as a risk factor for Alzheimer's disease: the confounders, interactions, and neuropathology associated with this relationship. Epidemiol Rev 2013; 35: 152–160. [DOI] [PubMed] [Google Scholar]
- 204.Rapsinski GJ, Wynosky-Dolfi MA, Oppong GO, et al. Toll-like receptor 2 and NLRP3 cooperate to recognize a functional bacterial amyloid, curli. Infect Immun 2015; 83: 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Masters SL, Dunne A, Subramanian SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol 2010; 11: 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Niemi K, Teirila L, Lappalainen J, et al. Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J Immunol 2011; 186: 6119–6128. [DOI] [PubMed] [Google Scholar]
- 207.Wang JG, Williams JC, Davis BK, et al. Monocytic microparticles activate endothelial cells in an IL-1beta-dependent manner. Blood 2011; 118: 2366–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Benakis C, Brea D, Caballero S, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med 2016; 22: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Houlden A, Goldrick M, Brough D, et al. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun Epub ahead of print 6 April 2016. DOI: 10.1016/j.bbi.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Caso JR, Hurtado O, Pereira MP, et al. Colonic bacterial translocation as a possible factor in stress-worsening experimental stroke outcome. Am J Physiol Regul Integr Comp Physiol 2009; 296: R979–R985. [DOI] [PubMed] [Google Scholar]
- 211.Wong ML, Inserra A, Lewis MD, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry 2016; 21: 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mawhinney LJ, de Rivero Vaccari JP, Dale GA, et al. Heightened inflammasome activation is linked to age-related cognitive impairment in Fischer 344 rats. BMC Neurosci 2011; 12: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Youm YH, Grant RW, McCabe LR, et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab 2013; 18: 519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Brough D, Rothwell NJ, Allan SM. Interleukin-1 as a pharmacological target in acute brain injury. Exp Physiol 2015; 100: 1488–1494. [DOI] [PubMed] [Google Scholar]
- 215.Maysami S, Wong R, Pradillo JM, et al. A cross-laboratory preclinical study on the effectiveness of interleukin-1 receptor antagonist in stroke. J Cereb Blood Flow Metab 2016; 36: 596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Abbate A, Kontos MC, Abouzaki NA, et al. Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies). Am J Cardiol 2015; 115: 288–292. [DOI] [PubMed] [Google Scholar]
- 217.Helmy A, Guilfoyle MR, Carpenter KL, et al. Recombinant human interleukin-1 receptor antagonist in severe traumatic brain injury: a phase II randomized control trial. J Cereb Blood Flow Metab 2014; 34: 845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Emsley HC, Smith CJ, Georgiou RF, et al. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry 2005; 76: 1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Baldwin AG, Brough D, Freeman S. Inhibiting the inflammasome: A chemical perspective. J Med Chem 2016; 59: 1691–1710. [DOI] [PubMed] [Google Scholar]
- 220.Coll RC, Robertson AA, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 2015; 21: 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fowler BJ, Gelfand BD, Kim Y, et al. Nucleoside reverse transcriptase inhibitors possess intrinsic anti-inflammatory activity. Science 2014; 346: 1000–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]