Abstract
Background
Compatible solutes are natural substances that are known to stabilize cellular functions. Preliminary ex vivo and in vivo studies demonstrated that the compatible solute ectoine restores natural apoptosis rates of lung neutrophils and contributes to the resolution of lung inflammation. Due to the low toxicity and known compatibility of the substance, an inhalative application as an intervention strategy for humans suffering from diseases caused by neutrophilic inflammation, like COPD, had been suggested. As a first approach to test the feasibility and efficacy of such a treatment, we performed a population-based randomized trial.
Objective
The objective of the study was to test whether the daily inhalation of the registered ectoine-containing medical device (Ectoin® inhalation solution) leads to a reduction of neutrophilic cells and interleukin-8 (IL-8) levels in the sputum of persons with mild symptoms of airway disease due to lifelong exposure to environmental air pollution.
Methods
A double-blinded placebo-controlled trial was performed to study the efficacy and safety of an ectoine-containing therapeutic. Prior to and after both inhalation periods, lung function, inflammatory parameters in sputum, serum markers, and quality-of-life parameters were determined.
Results
While the other outcomes revealed no significant effects, sputum parameters were changed by the intervention. Nitrogen oxides (nitrate and nitrite) were significantly reduced after ectoine inhalation with a mean quotient of 0.65 (95% confidence interval 0.45–0.93). Extended analyses considering period effects revealed that the percentage of neutrophils in sputum was significantly lower after ectoine inhalation than in the placebo group (P=0.035) even after the washout phase.
Conclusion
The current study is the first human trial in which the effects of inhaled ectoine on neutrophilic lung inflammation were investigated. Besides demonstrating beneficial effects on inflammatory sputum parameters, the study proves the feasibility of the therapeutic approach in an aged study group.
Keywords: osmolytes, extremolytes, molecular prevention, neutrophil apoptosis, SALIA cohort, air pollution
Introduction
Neutrophilic lung inflammation induced by environmental air pollution is a major cause of chronic diseases of the lung. The release of inflammatory mediators, reactive oxygen species, and matrix-destructing enzymes is responsible for tissue degradation and subsequent induction of end points like emphysema and COPD.1–3 Increased inflammatory markers in these diseases correlate well with the decrease in lung function. Chronic lung inflammation may contribute to the development of degenerative end points like emphysema by accelerating the aging of the lung tissue.4,5 Neutrophils, rather than other inflammatory cells, are considered to be the most prominent and determining cell type in these events.6 Therefore, the modulation of recruitment, perpetuation, and clearance in the lungs of patients are potential targets for therapy of inflammatory obstruction and for prevention of premature lung aging.7,8
Unfortunately, glucocorticoids, which are the most effective therapeutics for other inflammatory diseases, are of minor importance for the therapy of chronic neutrophilic inflammation and COPD.9,10 Neutrophilic granulocytes are less sensitive to glucocorticoids than other immune cells.9,10 Moreover, an acquired resistance in neutrophils against glucocorticoids is frequently observed in COPD patients.11 Recently, several therapeutic approaches intended to resolve neutrophilic inflammation by modulating the life span of neutrophils are considered and tested.12,13 In earlier ex vivo studies, we were able to demonstrate that neutrophils in the presence of ectoine are protected against antiapoptotic stimuli coming not only from environmental pollution but also from proinflammatory mediators like GM-CSF and LTB4.14,15 This strategy prevented the expansion of the life span of neutrophils which occurs in the environment of an ongoing inflammation. The underlying molecular events of this effect were investigated by a couple of in vivo and in vitro studies which demonstrated that the mechanism of ectoine action is based on the stabilization of membrane-coupled signaling platforms.16 Stress-dependent activation of membrane receptors was specifically prevented by ectoine. Subsequently, the activation of signaling pathways for specific pathogenic outcomes including inflammation was reduced.17,18 In animal studies in which neutrophilic lung inflammation was induced by single or repetitive application of environmental model particles, a mild but significant reduction of the inflammatory response was observed in the presence of ectoine, which could be attributed to the accelerated decline of the inflammation due to the reduce neutrophilic life span.15,17,19,20
Compatible solutes (also known as osmolytes or extremolytes) are a group of naturally occurring, mostly zwitterionic substances. They are produced as stabilizers of cellular function by the microorganisms which live under extreme conditions.21 Due to their specific properties in aqueous solutions, compatible solutes foster the stability and functionality of macromolecules by influencing their hydration layer.22 The promising data obtained so far, which demonstrate the prevention of cell stress reactions in the airways by ectoine and that it is well tolerated by cells even at high concentrations, suggest that this compound can be used for the treatment of humans who suffer from airway diseases associated with chronic neutrophilic lung inflammation.
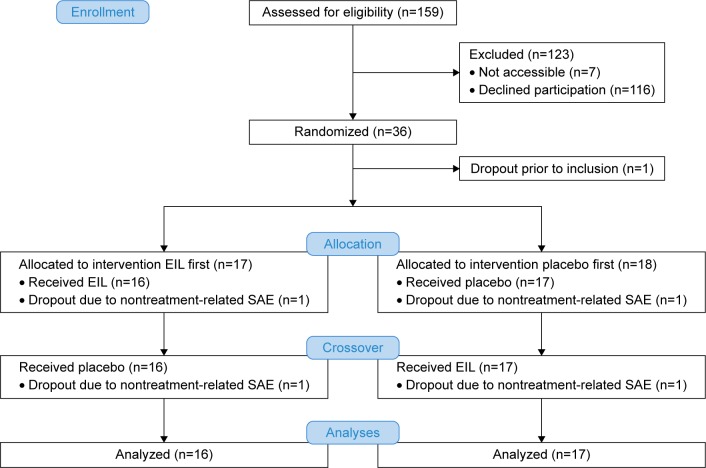
As a first approach to test the feasibility and efficacy of such a treatment, we performed a population-based randomized trial. Persons with mild lung function impairment and accelerated inflammatory parameters due to lifelong exposure to environmental air pollution, which were identified during earlier studies, were selected for a randomized intervention trial (Figure 1).23 The recent study was entitled “An efficacy and feasibility study to investigate the effect of Ectoin® inhalation solution (EIL) in subjects with inflammation and airway obstruction” (EFECT). The objective of the study was to test whether the application of ectoine is able to reduce markers of chronic lung inflammation. Specifically, the hypothesis that a daily inhalation of the registered ectoine-containing medical device (EIL) for 28 days, in comparison to physiological saline as a placebo, leads to a reduction of neutrophilic cells and interleukin-8 (IL-8) levels in the sputum was tested. Additional inflammatory markers were analyzed in sputum as secondary parameters. In addition, treatment with EIL was expected to have no adverse effects.
Figure 1.
CONSORT flow scheme of the EFECT study.
Abbreviations: EFECT, An efficacy and feasibility study to investigate the effect of EIL in subjects with inflammation and airway obstruction; SAE, serious adverse events; EIL, ectoine inhalation solution.
Methods
Design
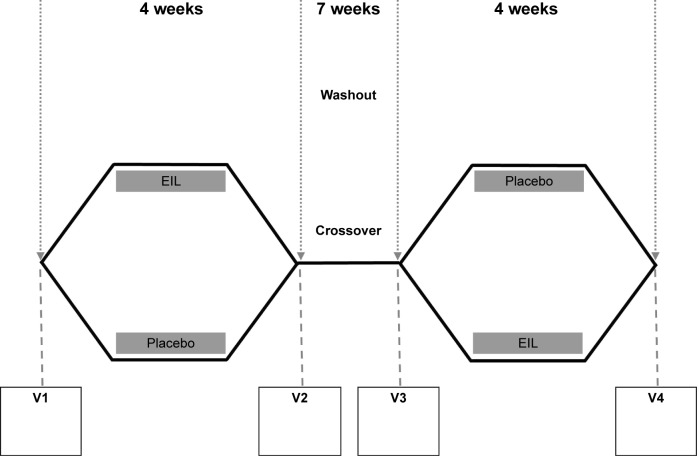
In order to estimate safety and feasibility, a double-blinded, placebo-controlled crossover study was performed in compliance with the ethical principles of the Declaration of Helsinki and amendments and Good Clinical Practice guidelines, and in accordance with EN ISO 14155-1 and EN ISO 14155-2 (approved by the local ethical committee of the Heinrich-Heine-University, Duesseldorf, study number MPG-MO-2). It is internationally registered at ClinicalTrials.gov with the study number NCT01225965. EIL or placebo was inhaled once daily during two treatment phases of 28 days which were separated by a 7-week washout phase during which verum (EIL) and placebo groups were exchanged (Figure 2). No changes to the study design and methods have been made after the commencement of the trial.
Figure 2.
Study design of the double-blinded placebo-controlled crossover study.
Abbreviations: V1–V4, visits at the study center; EIL, ectoine inhalation solution.
Study settings
The study was performed at the IUF Leibniz-Research Institute for Environmental Medicine. During the visits, a physician performed general examinations and took blood samples. The study participants were trained for using the inhalation device to accomplish daily inhalation at home. Questionnaires were filled by trained interviewers. Sputum sampling and lung function measurements were performed by trained nurses. During four visits (visit 1 to visit 4 [V1–V4]), prior to and after the treatment phases, volunteers were examined and sputum as well as blood samples were taken at the study center.
Interventions
Samples of EIL (1.3% ectoine in saline) or physiological saline (placebo), each 2.5 mL, were administered by inhalation using the eFlow® rapid nebuliser system (PARI GmbH, Stranberg, Germany). Compliance was monitored by online recall of inhalation times and durations from personal digital assistants. An inhalation was defined as valid when the inhalation duration was longer than 150 seconds.
Eligibility criteria for participants
The study subjects were recruited from the SALIA (Study on the Influence of Air pollution on Lung function, Inflammation, and Aging) study group. The study cohort was initiated in the early 1980s to investigate the effects of air pollution exposure on women living in two rural and an industrial area in West Germany.2 Until 2008, several consecutive cross-sectional studies were performed in which, among other end points, lung function and inflammatory parameters in sputum were correlated to air quality parameters at residence. Subjects exhibiting signs of mild COPD, defined as a Tiffeneau index (forced expiratory volume in 1 second/forced vital capacity [FEV1/FVC]) below 0.79 and sputum parameters (tumor necrosis factor-alpha [TNF-α] and number of neutrophils) above the median during the last examination of SALIA, were considered for inclusion (Figure 1). From this group, 33 individuals characterized as postmenopausal nonsmoking women were recruited. Consent forms together with additional information were supplied to the volunteers prior to the commencement of the study. After obtaining their written consent, these individuals were included in the study. The main exclusion criteria were additional severe concomitant disease, myocardial infarction or apoplexy during the last year, uncontrolled arterial hypertension (systolic blood pressure >200 mmHg, diastolic blood pressure >120 mmHg), diagnosed aortic aneurysm, current respiratory tract disorder other than COPD, and live attenuated vaccination within 14 days prior to inclusion.
Randomization
Randomization was performed as blocked randomization using a computerized random number generator by the sponsor of the study (bitop AG, Witten, Germany). Samples of the two groups (A: EIL first, B: placebo first) were packed and labeled with a similar appearance. Blinding was guaranteed as personal parameters of the individuals were not available for the sponsor, and the results of randomization were not known by the volunteers and the study personnel until unblinding.
Assessment of respiratory health by pulmonary function
Spirometry was performed using a MasterScope® spirometer (VIASYS Healthcare, Hoechberg, Germany) before and after application of the bronchodilator salbutamol (0.2 mg). Acceptable spirograms were obtained from a minimum of three forced expirations. American Thoracic Society/European Respiratory Society quality criteria were applied.24 All staff were specifically trained, and the same measuring device was used throughout the study.
Sputum samples
Sputum samples were collected after inhalation of 2.5 mL of saline. Samples were liquefied by dithiotreitol.25 Cells were collected by centrifugation (15 minutes, 200× g). Supernatants were stored as aliquots at −80°C. Differential cell counting from at least 200 cells (up to 800 cells) was performed by a trained person. Random samples were checked by a second person to determine the percentages of cell types as a quality control. In addition to the percentage of neutrophilic cells, the ratio of neutrophilic cells to mononuclear cells (monocytes and macrophages) was calculated.
Inflammatory parameters
All the inflammatory parameters from sputum (IL-8, TNF-α) and serum (C-reactive protein [CRP]) were measured in triplicate using commercially available enzyme-linked immunosorbent assays (BD, Franklin Lakes, NJ, USA) according to the manufacturer’s instructions. Nitrogen oxide (nitrite and nitrate) levels in sputum were detected by measuring nitrate and nitrite content using the Griess assay (Cayman Chemical, Ann Arbor, MI, USA) as recommended by the manufacturer.
Quality of life
Respiratory health was also assessed by a standardized self-administered quality-of-life questionnaire which included the following questions: Do you usually cough in the morning, when you get up or during the day? If yes: Do you produce phlegm when you have this cough?
Statistical methods
Two end points were chosen as primary outcomes: IL-8 level and percentage of neutrophils in sputum. For these end points, a confirmatory data analysis was planned. To ensure an overall type I error rate (α) of 5%, a fixed sequence testing method was adopted.26
End points were analyzed within the per-protocol population (N=33). Demographical, baseline, and safety data were analyzed in the intention-to-treat population. In general, missing data were not substituted. The only exception was, if one concentration value of the four possible values of inflammatory biomarker was missing, the missing value was replaced by the another value from the same period. A sensitivity analysis without substitutions was performed. Values below the detection limit were replaced by values two-third of the detection limit. Inflammatory markers were determined in triplicate. Single determinations lying outside the range of ±3 standard deviation (SD) were removed and the mean value without that outlier was used. All concentration values, cell counts and cell percentages, FEV1, and FVC were approximately normally distributed after logarithmic transformation. Therefore, these variables were analyzed after logarithmic transformation. The effect measure was the ratio of after treatment to baseline of the study (V1). FEV1/FVC ratio was normally distributed. This variable as well as the variables characterizing the quality of life was analyzed linearly. The effect measure was the difference between after treatment and baseline of the study (V1). The main question of the analysis was whether this effect measure was statistically more pronounced in the group treated with EIL compared to that treated with placebo. In case of log-normally distributed variables, we tested whether the ratio of the effect measure after EIL to the effect measure after placebo was different from one (repeated measures Student’s t-test). In case of normally distributed variables, we tested whether the difference between the effect measure after EIL and the effect measure after placebo was different from zero (repeated measures Student’s t-test). Time period effects, considered as hints for carryover effects, were also estimated. All statistical analyses were performed using the SAS software package (version 9.2; SAS Institute Inc., Cary, NC, USA).
Determination of sample size
Based on the scheduled statistical analyses, sample size was calculated to provide adequate power to test the hypothesis that EIL is superior to placebo. We considered a 30% lower concentration of inflammatory markers as relevant after using EIL compared to placebo. Such a difference would be significant (α=5% and power 80%), given a log SD of 0.215 as found in the SALIA study,2,27 when investigating 30 women in the first phase of the study.
Ancillary statistical analyses
In the case of indicated period effects, the analysis was repeated but restricted to the first inhalation period. In this case, additionally, the first period was extended to include the washout period. In these analyses, the baseline measure of the second period (before the specific inhalation) was compared to that of the first period. Differences between EIL and placebo in this analysis demonstrated a carryover effect. The chosen crossover design adequately takes main effects of possible confounding variables into account. We did not consider interactions as relevant in this fairly homogeneous group of study participants.
Results
Study population
Inclusion criteria were fulfilled by 159 persons (Figure 1). Of these, 36 persons agreed to participate and were randomly assigned to the study groups. After one dropout prior to treatment, 17 volunteers in the “EIL first” group and 18 volunteers in the “placebo first” group remained. Two additional dropouts due to nontreatment-related serious adverse events (SAEs, one in each group) led to a final sample size of 33 individuals who were treated per protocol. The study was performed in 2010 and 2011 (first inhalation phase: October 25–December 6, 2010 and second inhalation phase: January 10–February 21, 2011). The volunteer characteristics were analyzed after unblinding (baseline data are summarized in Table 1). No significant differences with respect to the selected criteria were observed between the two groups. Baseline inflammatory levels at the first visit (V1) did not differ significantly between the two groups (Table 2). Consistent with the findings of other studies, these values were in the range that is usually observed in healthy volunteers.28 During both inhalation periods, high numbers of valid inhalations (mean: 27.5 and 27.3 for EIL, 26.9 and 27.9 for placebo; maximum reachable was 28) were achieved. A priori compliance has been defined as more than 20 valid inhalations per period. All individuals reached that value; we therefore achieved a compliance rate of 100%.
Table 1.
Volunteer characteristics (PP population)a
| Placebo first | EIL first | Total | |
|---|---|---|---|
| Subjects (n) | 17 | 16 | 33 |
| Age (years) | 76.61 (73.83–80.88) | 76.08 (71.53–80.26) | 76.35 (71.53–80.88) |
| Females (%) | 100 | 100 | 100 |
| Height (cm) | 158.24 (149.00–170.00) | 157.69 (149.00–165.00) | 157.97 (149.00–170.00) |
| Weight (kg) | 72.80 (49.70–99.60) | 70.33 (60.30–92.00) | 71.60 (49.70–99.60) |
| Never smoker (n) | 16 | 15 | 31 |
| Exsmoker (n) | 1 | 1 | 2 |
| FEV1 (l) | 1.97 (1.14–2.99) | 2.03 (1.31–2.68) | 2.00 (1.14–2.99) |
| FVC (l) | 2.70 (1.93–3.86) | 2.75 (1.81–3.54) | 2.72 (1.81–3.86) |
| FEV1/FVC (Tiffeneau) | 0.73 (0.59–0.81) | 0.74 (0.68–0.82) | 0.73 (0.59–0.82) |
Note:
Arithmetic mean (minimum – maximum).
Abbreviations: EIL, ectoine inhalation solution; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PP, per-protocol.
Table 2.
Descriptive statistics given as geometric means (SD) (PP population)
| EIL first
|
Placebo first
|
|||||||
|---|---|---|---|---|---|---|---|---|
| EIL
|
Placebo
|
Placebo
|
EIL
|
|||||
| V1 | V2 | V3 | V4 | V1 | V2 | V3 | V4 | |
| Subjects (n) | 16 | 16 | 16 | 16 | 17 | 17 | 17 | 17 |
| IL-8 (pg/mL) in sputum | 711.1 (2.8) | 452.1 (4.2) | 382.4 (6.1) | 491.2 (5.6) | 903.3 (3.4) | 819.6 (2.8) | 840.6 (5.9) | 1,206.6 (5.3) |
| Neutrophils (%) | 17.6 (4.0) | 8.9 (4.5) | 5.2 (4.8) | 6.6 (3.9) | 21.5 (2.5) | 19.4 (4.0) | 18.7 (4.9) | 21.6 (4.1) |
| Neutrophils/macrophagesc | 3.0 (2.5) | 2.1 (2.8) | 3.7 (3.7) | 4.6 (2.0) | 2.1 (2.8) | 2.8 (2.5) | 4.7 (2.8) | 5.5 (3.1) |
| TNF-α (pg/mL) in sputum | 19.5 (1.9) | 33.7 (2.3) | 20.8 (1.5) | 23.5 (2.3) | 20.5 (2.7) | 28.8 (2.4) | 28.0 (2.5) | 27.8 (3.2) |
| Nitrogen oxides (μM) in sputum (nitrate + nitrite) | 45.3 (1.8) | 32.4 (2.1) | 42.8 (1.8) | 43.6 (2.6) | 34.0 (1.9) | 36.8 (2.1) | 54.7 (1.9) | 35.3 (1.9) |
| CRP (μg/mL) in serum | 1.0 (2.9) | 1.1 (2.7) | 1.1 (3.0) | 1.1 (2.8) | 0.9 (3.6) | 0.7 (3.0) | 0.9 (3.1) | 0.9 (3.1) |
| FEV1 (l)a | 2.0 (1.2) | 2.0 (1.2) | 2.0 (1.2) | 1.9 (1.3) | 1.9 (1.3) | 1.9 (1.3) | 1.9 (1.2) | 1.8 (1.3) |
| FVC (l)a | 2.7 (1.2) | 2.7 (1.2) | 2.6 (1.2) | 2.6 (1.2) | 2.7 (1.2) | 2.7 (1.2) | 2.6 (1.2) | 2.6 (1.2) |
| Tiffeneaua,b | 0.74 (0.04) | 0.74 (0.05) | 0.74 (0.05) | 0.73 (0.04) | 0.73 (0.05) | 0.72 (0.06) | 0.73 (0.06) | 0.71 (0.06) |
| QoL coughb | 1.4 (1.3) | 1.6 (1.2) | 1.8 (1.4) | 1.2 (1.3) | 2.7 (1.8) | 1.9 (1.6) | 2.1 (1.0) | 1.8 (1.4) |
| QoL phlegmb | 0.7 (1.1) | 1.3 (1.1) | 1.3 (1.6) | 0.9 (1.0) | 2.2 (1.7) | 1.8 (1.3) | 2.0 (1.1) | 1.5 (1.3) |
| QoL tightness in chestb | 0.6 (0.9) | 0.2 (0.5) | 0.5 (0.9) | 0.3 (0.6) | 1.3 (1.8) | 0.7 (1.2) | 0.9 (1.3) | 1.1 (1.3) |
| QoL breathlessnessb | 1.4 (1.6) | 1.6 (1.5) | 1.6 (1.5) | 1.5 (1.5) | 2.8 (1.6) | 2.7 (1.5) | 2.7 (1.5) | 2.5 (1.3) |
Notes:
All values are given as measures before salbutamol, no significant changes were observed after salbutamol inhalation;
arithmetic means (SD), as these values exhibited normal distributions;
monocytes and macrophages.
Abbreviations: CRP, C-reactive protein; EIL, ectoine inhalation solution; PP, per-protocol; QoL, quality of life; SD, standard deviation; TNF-α, tumor necrosis factor-alpha; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; IL, interleukin; V1–V4, visits at the study center.
Outcomes and estimations
After the first treatment period (V1–V2) with EIL, the sputum levels of IL-8, nitrogen oxides (nitrate and nitrite), and neutrophils were markedly reduced (Table 2). After the second treatment period (V3–V4), however, such an effect was only observed for the nitrogen oxide levels. Therefore, the statistical analysis of this crossover study, combining the effects from the first and second inhalation period, showed a significant effect of EIL on the nitrogen oxide levels in sputum (Table 3, P=0.02).
Table 3.
Changes after application of EIL inhalation solution
| End point | Mean quotienta/differencec | 95% CI (LL) | 95% CI (UL) | P-value |
|---|---|---|---|---|
| IL-8 (pg/mL) in sputuma | 0.88 | 0.48 | 1.63 | 0.68 |
| % neutrophilsa | 0.71 | 0.37 | 1.39 | 0.31 |
| Neutrophils/macrophagesa,d | 0.69 | 0.41 | 1.16 | 0.15 |
| TNF-α (pg/mL) in sputuma | 1.04 | 0.58 | 1.84 | 0.9 |
| Nitrogen oxides (μM) in sputum (nitrate + nitrite)a | 0.65 | 0.45 | 0.93 | 0.02 |
| CRP (μg/mL) in seruma | 1.17 | 0.80 | 1.71 | 0.40 |
| FEV1 (l)a,b | 0.98 | 0.95 | 1.02 | 0.32 |
| FVC (l)a,b | 0.98 | 0.96 | 1.00 | 0.10 |
| Tiffeneaua,b | 0.003 | −0.017 | 0.023 | 0.757 |
| QoL coughc | 0.69 | −0.09 | 1.37 | 0.08 |
| QoL phlegmc | 0.44 | −0.14 | 1.02 | 0.13 |
| QoL tightness in chestc | 0.35 | −0.15 | 0.84 | 0.16 |
| QoL breathlessnessc | 0.16 | −0.33 | 0.64 | 0.51 |
Notes:
A quotient <1 indicates a reduction by EIL treatment compared to placebo;
before salbutamol;
mean differences <1 would indicate an improvement by EIL treatment compared to placebo;
monocytes and macrophages.
Abbreviations: EIL, ectoine inhalation solution; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; TNF-α, tumor necrosis factor-alpha; CI, confidence interval; IL, interleukin; CRP, C-reactive protein; QoL, quality of life; UL, upper limit; LL, lower limit.
The primary end points, IL-8 level and percentage of neutrophils, showed no combined effect. For these variables, however, period effects (IL-8, P=0.06; percentage of neutrophils, P=0.08) were indicated. Serum levels of CRP as well as lung function remained unchanged during the whole study. Interestingly, inhalation of the placebo (physiological saline) appeared to reduce cough and phlegm in both treatment periods. Although this effect was only marginally significant (P=0.08), it might be specific. EIL did not influence the other quality-of-life questionnaire variables.
Ancillary statistical analyses
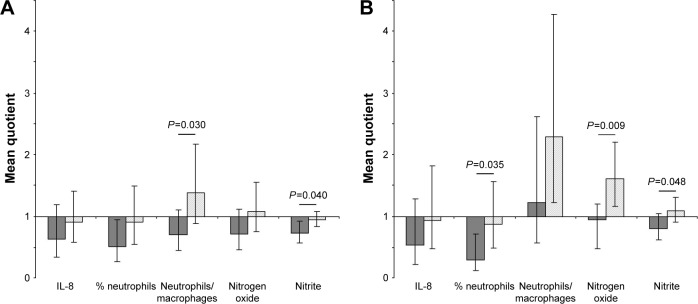
Since period effects were indicated, we investigated whether a carryover effect might be relevant, although such an effect was not anticipated. In the first period, a reduction of neutrophils and total nitrite levels after EIL treatment was significantly greater than after placebo treatment (Figure 3A), and this effect remained stable during the washout period (Figure 3B). This might be an indication for a long-lasting ectoine effect.
Figure 3.
Effect of EIL in comparison with the effect of placebo inhalation on inflammatory markers. (A) Effects after the end of the first inhalation period (V1/V2). (B) Effects after the end of the washout phase before starting the second period (V1/V3). Dark gray bars, EIL; light gray bars, placebo. Geometric mean values of individual quotients with 95% confidence intervals are presented. P-values of Student’s t-tests for the EIL–placebo comparison are given if P<0.05.
Abbreviations: EIL, ectoine inhalation solution; IL, interleukin; V1–V3, visits at the study center.
Adverse events
During the study, 52 adverse events (AEs) were reported. Two events were assessed as SAEs which, after the final diagnosis, were not found to be related to the treatment and led to dropouts, one in each group. A list of all AEs is given in Table S1. Approximately, 23 events occurred in 14 subjects under treatment with placebo and 29 events occurred in 16 subjects under treatment with EIL. Two AEs (both cough and irritation, both of mild intensity) were assessed as “probably or possibly related” to EIL treatment compared to no similar AEs under placebo treatment. Under placebo treatment, three AEs (cold and cough, cough, and unclear diagnoses of slight irritation, all of mild intensity) were considered as “not assessable”. All other events observed were assessed as “not related” to study treatment, and the most common event was cold. A comparison of the occurrence of AEs in both the groups revealed no significant association with the kind of intervention (EIL or placebo).
Discussion
The current randomized double-blinded crossover trial demonstrated that daily inhalation of ectoine had beneficial effects on inflammatory sputum parameters in elderly volunteers. A significant reduction of nitrogen oxides (nitrate and nitrite) in sputum by the inhalation intervention was observed. Lung function and quality of life were not influenced. Ancillary analyses of carryover effects indicated a long-lasting reduction of neutrophilic inflammation by ectoine, which even lasted into the second inhalation period.
Efficacy
Owing to these carryover effects, which were not anticipated, the effect estimates for our primary end points (IL-8 level and percentage of neutrophils in sputum) were not significant, when combining the results from both periods, which was the a priori test strategy. However, the significant effects in markers which were not considered as primary efficacy variables give clear indications for beneficial effects of the inhalation of EIL. This effect is particularly evident by the robust reduction of nitrogen oxides in sputum after the treatment (Table 3). In earlier investigations of the SALIA cohort, these NO derivatives correlated well with the exposure to environmental air pollution.27,29 Nitrogen oxides (nitrate and nitrite) in sputum have earlier been described to be relevant inflammatory parameters in COPD patients, which correlate with disease markers including tissue-destructing metalloproteinases.30,31 It is assumed that due to the oxidative stress, nitrogen monoxide is converted into nitrogen metabolites. Thus, nitrogen oxides in sputum are considered as markers of oxidative and nitrosative stress.32 This is also reflected by the fact that the levels of nitrosothiols, reaction products of peroxynitrite, are elevated in patients with stable COPD.33 A significant reduction in the total nitrogen oxide levels in the sputum samples of both groups who inhaled ectoine can therefore be considered as a beneficial effect on nitrosative and oxidative stress resulting from chronic neutrophilic lung inflammation.
In our ancillary analyses, a significant reduction of neutrophils in the sputum after EIL inhalation was visible (Figure 3). This can be due to a reduction of the chronic neutrophilic inflammation in the volunteers. Moreover, these results corroborate our earlier mechanistic studies in which we demonstrated that the compatible solute ectoine reduces lung inflammation at the level of lung neutrophils by preventing proinflammatory cell reactions triggered by different stimuli.14,15
Safety and feasibility
The data presented here demonstrate that a daily inhalation of 2.5 mL of EIL for 28 days has no severe adverse effects on a female study population with a mean age of 76.53 (±71.53–80.88) years. The authors consider the extraordinarily high compliance in an elderly population as an indicator for the feasibility of this kind of intervention strategy.
Limitations of the study
The EFECT study is the first in which a compatible solute was applied to the respiratory tract of humans. Therefore, our estimates of the effective doses as well as the length of the washout phase were based on toxicological studies, kinetics, and mechanistic experiments performed in animal systems. The observed period effects indicate that the inhalation of EIL has an enduring effect on the respiratory tract, which leads to a reduction of inflammatory markers associated with neutrophils which were still significantly reduced after the washout phase. This fact might be considered as a limitation of the current protocol which should be avoided in further studies. However, this finding could also be an indication that the ectoine treatment led to significant interference with the vicious circle of inflammation and destruction and provided a sustainable beneficial effect in the individuals.
Conclusion
The beneficial effect of ectoine on neutrophilic lung inflammation had so far only been demonstrated in several human ex vivo and animal in vivo studies. Using a population-based design with volunteers who exhibited mild impairments of airway function, we were able to observe a significant reduction of nitrogen oxides (nitrate and nitrite) in sputum by the inhalative intervention. Lung function and quality of life were not influenced in this short-term study. Ancillary analyses of carryover effects indicated a long-lasting reduction of neutrophilic inflammation by ectoine, which even lasted into the second inhalation period. Considering these specific effects of ectoine inhalation, it should be possible to treat patients suffering from severe forms of COPD dominated by neutrophilic lung inflammation. The feasibility, documented by high compliance, and safety of the treatment, with almost no treatment-related adverse effects, in a probably sensitive elderly study group indicate that this strategy can be tested in clinical studies. Moreover, the results may be considered as relevant for environmentally induced lung aging processes. Based on clinical observations, COPD can be considered as a phenotype of accelerated lung aging.4,34,35 Using carbon particles as a model substance for combustion-derived environmental pollution, we recently described that this kind of exposure is in fact able to trigger cellular senescence.36 Intervention studies with ectoine may aid in investigating the role of environmental air pollution in extrinsic lung aging.
Supplementary material
Table S1.
List of adverse events reported during V1–V4 periods of the EFECT study
| Treatment | Reported adverse event; ICD-10 code; verbatim term | Severitya | Relation to treatmentb |
|---|---|---|---|
| EIL | Diseases of the eye and adnexa; H57.9; inflammation of the eye | 1 | 4 |
| EIL | Diseases of the genitourinary system; N30.9; cystitis | 1 | 4 |
| EIL | Diseases of the genitourinary system; N30.9; cystitis | 2 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold, cough | 1 | 4 |
| EIL | Symptoms, signs, and abnormal clinical and laboratory findings, not elsewhere classified; R05; cough and irritation | 1 | 3 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the circulatory system; I10; arterial hypertension (>200) and cardiac arrhythmia | 2 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Pressure and pain in chest area. According to the subject known for a long time and no therapy possible | 3 | 4 |
| EIL | Symptoms, signs, and abnormal clinical and laboratory findings, not elsewhere classified; R05; cough and irritation during inhalation of EIL or placebo, whichever applies | 1 | 2 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J30.1; hay fever | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Symptoms, signs, and abnormal clinical and laboratory findings, not elsewhere classified; R05; cough | 1 | 6 |
| PLAC | Diseases of the musculoskeletal system and connective tissue; M19.94; arthrosis in right hand | 2 | 4 |
| PLAC | Diseases of the digestive system; K52.9; diarrhea | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Unclear. Volunteer reports on mild irritation. A fast recovery was achieved by sage tea | 1 | 6 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold and cough | 1 | 6 |
| PLAC | Diseases of the respiratory system; J06.9; cough and cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the musculoskeletal system and connective tissue; M54.99; back pain | 1 | 4 |
| PLAC | Diseases of the musculoskeletal system and connective tissue; M25.59; pain in joint | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold, sniffles, flu | 2 | 4 |
| EIL | Diseases of the digestive system; K31.9; gastric disorders Neoplasm; C16.0 | 4 | 4 |
| PLAC | Diseases of the circulatory system; I48; cardiac arrhythmia and atrial fibrillation | 4 | 4 |
Notes: AEs in the washout phase are assigned to period 1 and AEs on the last study day (V4) are assigned to period 2.
Severity: 1= mild, 2= moderate, 3= severe, 4= life-threatening, 5= lethal;
Relation to study treatment: 1= definitely, 2= probably, 3= possibly, 4= not related, 5= not assessed, 6= not assessable;
Outcome: 4= in-patient hospitalization or prolongation of existing hospitalization.
Abbreviations: AE, adverse event; EIL, ectoin inhalation solution; EFECT, an efficacy and feasibility study to investigate the effect of EIL in subjects with inflammation and airway obstruction; PLAC, placebo inhalation solution; V1–V4, visits at the study center; ICD-10, International Classification of Diseases-10th Revision.
Acknowledgments
The authors wish to acknowledge the excellent technical assistance provided by Gabriele Seitner-Sorge, Ragnhild Wirth, and Winfried Brock. The human study was sponsored by bitop AG (Witten, Germany). EIL solution was produced and provided by bitop AG. The publication of this article was funded by the Open Access fund of the Leibniz Association.
Footnotes
Author contributions
All authors contributed substantially to study design, data acquisition, data analysis, and data interpretation. They critically revised the manuscript and agree to be accountable for all aspects of this publication.
Disclosure
AB is an employee of bitop AG, the sponsor of the study. JK acts as a consultant for bitop AG. The groups of KU and UK have received research grants from bitop AG. The other authors report no conflicts of interest in this work.
References
- 1.Karrasch S, Holz O, Jorres RA. Aging and induced senescence as factors in the pathogenesis of lung emphysema. Respir Med. 2008;102(9):1215–1230. doi: 10.1016/j.rmed.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Schikowski T, Sugiri D, Ranft U, et al. Long-term air pollution exposure and living close to busy roads are associated with COPD in women. Respir Res. 2005;6:152. doi: 10.1186/1465-9921-6-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macnee W. Pathogenesis of chronic obstructive pulmonary disease. Clin Chest Med. 2007;28(3):479–513. v. doi: 10.1016/j.ccm.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 4.MacNee W. Accelerated lung aging: a novel pathogenic mechanism of chronic obstructive pulmonary disease (COPD) Biochem Soc Trans. 2009;37(Pt 4):819–823. doi: 10.1042/BST0370819. [DOI] [PubMed] [Google Scholar]
- 5.MacNee W. Aging, inflammation, and emphysema. Am J Respir Crit Care Med. 2011;184(12):1327–1329. doi: 10.1164/rccm.201110-1764ED. [DOI] [PubMed] [Google Scholar]
- 6.O’Donnell R, Breen D, Wilson S, Djukanovic R. Inflammatory cells in the airways in COPD. Thorax. 2006;61(5):448–454. doi: 10.1136/thx.2004.024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louis R, Djukanovic R. Is the neutrophil a worthy target in severe asthma and chronic obstructive pulmonary disease? Clin Exp Allergy. 2006;36(5):563–567. doi: 10.1111/j.1365-2222.2006.02493.x. [DOI] [PubMed] [Google Scholar]
- 8.Quint JK, Wedzicha JA. The neutrophil in chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2007;119(5):1065–1071. doi: 10.1016/j.jaci.2006.12.640. [DOI] [PubMed] [Google Scholar]
- 9.Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2013;131(3):636–645. doi: 10.1016/j.jaci.2012.12.1564. [DOI] [PubMed] [Google Scholar]
- 10.Belvisi MG. Regulation of inflammatory cell function by corticosteroids. Proc Am Thorac Soc. 2004;1(3):207–214. doi: 10.1513/pats.200402-002MS. [DOI] [PubMed] [Google Scholar]
- 11.Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373(9678):1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- 12.Hallett JM, Leitch AE, Riley NA, Duffin R, Haslett C, Rossi AG. Novel pharmacological strategies for driving inflammatory cell apoptosis and enhancing the resolution of inflammation. Trends Pharmacol Sci. 2008;29(5):250–257. doi: 10.1016/j.tips.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Birrell MA, Wong S, McCluskie K, et al. Second-generation inhibitors demonstrate the involvement of p38 mitogen-activated protein kinase in post-transcriptional modulation of inflammatory mediator production in human and rodent airways. J Pharmacol Exp Ther. 2006;316(3):1318–1327. doi: 10.1124/jpet.105.093310. [DOI] [PubMed] [Google Scholar]
- 14.Autengruber A, Sydlik U, Kroker M, et al. Signalling-dependent adverse health effects of carbon nanoparticles are prevented by the compatible solute mannosylglycerate (firoin) in vitro and in vivo. PLoS One. 2014;9(11):e111485. doi: 10.1371/journal.pone.0111485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sydlik U, Peuschel H, Paunel-Gorgulu A, et al. Recovery of neutrophil apoptosis by ectoine: a new strategy against lung inflammation. Eur Respir J. 2013;41(2):433–442. doi: 10.1183/09031936.00132211. [DOI] [PubMed] [Google Scholar]
- 16.Peuschel H, Sydlik U, Grether-Beck S, et al. Carbon nanoparticles induce ceramide and lipid raft-dependent signalling in lung epithelial cells: a target for a preventive strategy against environmentally-induced lung inflammation. Part Fibre Toxicol. 2012;9:48. doi: 10.1186/1743-8977-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sydlik U, Gallitz I, Albrecht C, Abel J, Krutmann J, Unfried K. The compatible solute ectoine protects against nanoparticle-induced neutrophilic lung inflammation. Am J Respir Crit Care Med. 2009;180(1):29–35. doi: 10.1164/rccm.200812-1911OC. [DOI] [PubMed] [Google Scholar]
- 18.Peuschel H, Sydlik U, Haendeler J, et al. c-Src-mediated activation of Erk1/2 is a reaction of epithelial cells to carbon nanoparticle treatment and may be a target for a molecular preventive strategy. Biol Chem. 2010;391(11):1327–1332. doi: 10.1515/BC.2010.131. [DOI] [PubMed] [Google Scholar]
- 19.Kroker M, Sydlik U, Autengruber A, et al. Preventing carbon nanoparticle-induced lung inflammation reduces antigen-specific sensitization and subsequent allergic reactions in a mouse model. Part Fibre Toxicol. 2015;12:20. doi: 10.1186/s12989-015-0093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unfried K, Kroker M, Autengruber A, Gotic M, Sydlik U. The compatible solute ectoine reduces the exacerbating effect of environmental model particles on the immune response of the airways. J Allergy (Cairo) 2014;2014:708458. doi: 10.1155/2014/708458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yancey PH. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol. 2005;208(Pt 15):2819–2830. doi: 10.1242/jeb.01730. [DOI] [PubMed] [Google Scholar]
- 22.Street TO, Bolen DW, Rose GD. A molecular mechanism for osmolyte-induced protein stability. Proc Natl Acad Sci U S A. 2006;103(38):13997–14002. doi: 10.1073/pnas.0606236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schikowski T, Ranft U, Sugiri D, et al. Decline in air pollution and change in prevalence in respiratory symptoms and chronic obstructive pulmonary disease in elderly women. Respir Res. 2010;11:113. doi: 10.1186/1465-9921-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pellegrino R, Viegi V, Brusasco RO, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 25.Woolhouse IS, Bayley DL, Stockley RA. Effect of sputum processing with dithiothreitol on the detection of inflammatory mediators in chronic bronchitis and bronchiectasis. Thorax. 2002;57(8):667–671. doi: 10.1136/thorax.57.8.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huque MF, Alosh M. A flexible fixed-sequence testing method for hierarchically ordered correlated multiple endpoints in clinical trials. J Stat Plan Inference. 2008;138(2):321–335. [Google Scholar]
- 27.Vossoughi M, Schikowski T, Vierkotter A, et al. Air pollution and subclinical airway inflammation in the SALIA cohort study. Immun Ageing. 2014;11(1):5. doi: 10.1186/1742-4933-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balbi B, Pignatti P, Corradi M, et al. Bronchoalveolar lavage, sputum and exhaled clinically relevant inflammatory markers: values in healthy adults. Eur Respir J. 2007;30(4):769–781. doi: 10.1183/09031936.00112306. [DOI] [PubMed] [Google Scholar]
- 29.Teichert T, Vossoughi M, Vierkotter A, et al. Investigating the spillover hypothesis: analysis of the association between local inflammatory markers in sputum and systemic inflammatory mediators in plasma. Environ Res. 2014;134:24–32. doi: 10.1016/j.envres.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 30.Beeh KM, Beier J, Koppenhoefer N, Buhl R. Increased glutathione disulfide and nitrosothiols in sputum supernatant of patients with stable COPD. Chest. 2004;126(4):1116–1122. doi: 10.1378/chest.126.4.1116. [DOI] [PubMed] [Google Scholar]
- 31.Ziora D, Dworniczak S, Kozielski J. Induced sputum metalloproteinases and their inhibitors in relation to exhaled nitrogen oxide and sputum nitric oxides and other inflammatory cytokines in patients with chronic obstructive pulmonary disease. J Physiol Pharmacol. 2008;59(Suppl 6):809–817. [PubMed] [Google Scholar]
- 32.Ziora D, Dworniczak S, Kaczmarczyk G, Jastrzebski D, Krzywiecki A, Kozielski J. Correlation of exhaled nitric oxide with nitrogen oxides and selected cytokines in induced sputum of chronic obstructive pulmonary disease patients. J Physiol Pharmacol. 2007;58(Suppl 5, Pt 2):791–799. [PubMed] [Google Scholar]
- 33.Kanazawa H, Shiraishi S, Hirata K, Yoshikawa J. Imbalance between levels of nitrogen oxides and peroxynitrite inhibitory activity in chronic obstructive pulmonary disease. Thorax. 2003;58(2):106–109. doi: 10.1136/thorax.58.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence exacerbates pulmonary inflammation in patients with chronic obstructive pulmonary disease. Respiration. 2010;80(1):59–70. doi: 10.1159/000268287. [DOI] [PubMed] [Google Scholar]
- 35.Faner R, Rojas M, Macnee W, Agusti A. Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186(4):306–313. doi: 10.1164/rccm.201202-0282PP. [DOI] [PubMed] [Google Scholar]
- 36.Buchner N, Ale-Agha N, Jakob S, et al. Unhealthy diet and ultrafine carbon black particles induce senescence and disease associated phenotypic changes. Exp Gerontol. 2013;48(1):8–16. doi: 10.1016/j.exger.2012.03.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
List of adverse events reported during V1–V4 periods of the EFECT study
| Treatment | Reported adverse event; ICD-10 code; verbatim term | Severitya | Relation to treatmentb |
|---|---|---|---|
| EIL | Diseases of the eye and adnexa; H57.9; inflammation of the eye | 1 | 4 |
| EIL | Diseases of the genitourinary system; N30.9; cystitis | 1 | 4 |
| EIL | Diseases of the genitourinary system; N30.9; cystitis | 2 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold, cough | 1 | 4 |
| EIL | Symptoms, signs, and abnormal clinical and laboratory findings, not elsewhere classified; R05; cough and irritation | 1 | 3 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the circulatory system; I10; arterial hypertension (>200) and cardiac arrhythmia | 2 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Pressure and pain in chest area. According to the subject known for a long time and no therapy possible | 3 | 4 |
| EIL | Symptoms, signs, and abnormal clinical and laboratory findings, not elsewhere classified; R05; cough and irritation during inhalation of EIL or placebo, whichever applies | 1 | 2 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J30.1; hay fever | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| EIL | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Symptoms, signs, and abnormal clinical and laboratory findings, not elsewhere classified; R05; cough | 1 | 6 |
| PLAC | Diseases of the musculoskeletal system and connective tissue; M19.94; arthrosis in right hand | 2 | 4 |
| PLAC | Diseases of the digestive system; K52.9; diarrhea | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Unclear. Volunteer reports on mild irritation. A fast recovery was achieved by sage tea | 1 | 6 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold and cough | 1 | 6 |
| PLAC | Diseases of the respiratory system; J06.9; cough and cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the musculoskeletal system and connective tissue; M54.99; back pain | 1 | 4 |
| PLAC | Diseases of the musculoskeletal system and connective tissue; M25.59; pain in joint | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold | 1 | 4 |
| PLAC | Diseases of the respiratory system; J06.9; cold, sniffles, flu | 2 | 4 |
| EIL | Diseases of the digestive system; K31.9; gastric disorders Neoplasm; C16.0 | 4 | 4 |
| PLAC | Diseases of the circulatory system; I48; cardiac arrhythmia and atrial fibrillation | 4 | 4 |
Notes: AEs in the washout phase are assigned to period 1 and AEs on the last study day (V4) are assigned to period 2.
Severity: 1= mild, 2= moderate, 3= severe, 4= life-threatening, 5= lethal;
Relation to study treatment: 1= definitely, 2= probably, 3= possibly, 4= not related, 5= not assessed, 6= not assessable;
Outcome: 4= in-patient hospitalization or prolongation of existing hospitalization.
Abbreviations: AE, adverse event; EIL, ectoin inhalation solution; EFECT, an efficacy and feasibility study to investigate the effect of EIL in subjects with inflammation and airway obstruction; PLAC, placebo inhalation solution; V1–V4, visits at the study center; ICD-10, International Classification of Diseases-10th Revision.