Abstract
Purpose
To identify explanatory factors of fear of recurrence (FOR) in breast cancer survivors of different sexual orientations and their caregivers, and to assess the directionality in the survivor and caregiver dyads’ FOR.
Methods
We recruited survivors of non-metastatic breast cancer of different sexual orientations and invited their caregivers into this study. Using a telephone survey, we collected data from 167 survivor and caregiver dyads. Using simultaneous equation models and a stepwise selection process, we identified the significant determinants of survivors and caregivers’ FOR and determined the directionality of survivors and caregivers’ FOR. Weighting the model by the inverse propensity score ensured that differences by sexual orientation in age and proportion of life in the caregiver-survivor relationship were accounted for.
Results
Caregivers’ FOR predicted survivors’ FOR, and sexual orientation had a significant effect on survivors’ FOR, in that sexual minority women reported less FOR than heterosexual women. Other determinants of survivors’ FOR included their medical characteristics, co-residence with caregivers, and caregivers’ social support and use of counseling. Caregivers’ FOR was related to their social support and survivors’ medical characteristics.
Conclusions
This study suggests a need for caregiver interventions. Because survivors’ FOR is affected by caregivers’ FOR caregiver interventions will likely benefit survivors’ FOR.
Implications for Cancer Survivors
Both sexual minority and heterosexual breast cancer survivors’ FOR are affected by their caregivers’ FOR, which suggests the caregivers of breast cancer survivors are central for the survivors’ well-being and shall therefore be integrated into the care process.
Keywords: Breast cancer, caregiving, dyads, fear of recurrence, psychological needs
Introduction
In the United States, approximately 14.5 million cancer survivors were alive in 2014 [1]. Due to advances in cancer screening and cancer treatments, it is expected that this number will increase to 19 million cancer survivors by 2024 [1]. Forty-one percent of female cancer survivors report a history of breast cancer [1]. With increased survival rates, the field of cancer survivorship aims to understand the concerns and needs of cancer survivors over the long term. One of the psychosocial problems most commonly reported by breast cancer survivors is fear of recurrence (FOR) [2, 3], which is defined as worry and concern about recurrence of cancer. Prevalence estimates of breast cancer survivors’ FOR vary from 25% to 97% [3, 4], due to the different measures of FOR used in studies. Despite the absence of a defined clinically relevant level of FOR, consensus exists that FOR is the most common unmet need for breast cancer survivors in part due to consistent evidence linking FOR to anxiety, depression, and decreased quality of life [3] and follow-up care [5].
Based on several review studies summarizing decades of research on FOR [3, 6, 7], demographic and cancer characteristics as well as other medical factors have been identified as determinants of FOR [3, 7]. Among the demographic factors, younger age has consistently been associated with greater FOR [3, 7], while the evidence for other demographic factors has been more mixed. Some, yet not all, studies have found lower education to be associated with greater FOR [3, 7]. Furthermore, the association between race or ethnicity, marital status, income or employment and FOR was inconsistent [3, 7]. In these reviews, no mention was made of sexual orientation and its relationship to FOR. With respect to the cancer-related variables, time since diagnosis was generally unrelated to FOR, and the evidence for a relationship between cancer stage and cancer treatments was mostly inconsistent, with some evidence pointing to a relationship between comorbid disease and FOR [3, 7].
Studies also indicate that FOR is not limited to survivors, leading to the evaluation of FOR among survivors’ partners, spouses, and family members [3, 8]. In one review, five of nine studies indicated that caregivers’ FOR was greater than that of survivors [3]. Generally there was consensus that survivors and their partner or family member were interdependent in terms of FOR, in that FOR experienced by one influenced the FOR by the other [9]. However, studies to date were limited in several ways. First, heterosexuality was assumed in that studies focused on breast cancer survivors and their husbands and family members. Yet breast cancer also occurs in the underserved population of women who report being lesbian, bisexual, or have a preference for a female partner, which we refer to as sexual minority women (SMW) [10]. Omitting SMW from this body of research is a significant oversight due the growing body of evidence of SMW’s greater risk factors for breast cancer [11–25] and some evidence suggesting SMW have higher incidence [26], and greater breast cancer mortality [27] compared to heterosexual women (HSW). To date, a number of studies examining SMW breast cancer survivors have concluded that quality of life and levels of anxiety, depression, and mood disturbance are similar to those among HSW with breast cancer [28].
With the exception of one prior study, studies of the caregivers to SMW breast cancer survivors have been lacking [29]. The only caregiver study indicated that caregivers reported significantly less support compared to the SMW breast cancer survivors, while SMW survivors and caregivers’ distress was similar [29]. FOR was not considered in this study and no comparison sample of heterosexual women (HSW) and their caregivers was available. To address this gap in research on FOR among SMW and their caregivers, we conducted a study that recruited SMW and HSW with breast cancer. Each participating woman with breast cancer was asked to put us in contact with her partner or spouse if partnered, while survivors without a partner were asked to identify the most important support person to them, as we explained in more detail elsewhere [30]. Having survey data from survivors of different sexual orientations and their participating caregivers allows for a detailed examination of the explanatory factors of survivors and their caregivers’ FOR. Moreover, the current study acknowledges the interdependence between survivors (actors) and their caregivers’ (partners) FOR. Using an analytic approach common in econometrics [31], yet entirely novel in this context, this study aims to identify directionality of the interdependence, or whether the actor-partner’s FOR is mutual, and whether the actor’s FOR impacts the partner’s FOR or vice versa.
Methods
All aspects of this study were approved by the Institutional Review Board of Boston University.
Recruitment
Because this study sought to learn about heterosexual women (HSW) and sexual minority women (SMW) with breast cancer and their caregivers, the recruitment focused on SMW and HSW with breast cancer first. To obtain a sample of SMW and HSW with breast cancer, we initially re-contacted participants from an earlier comparative study of 257 HSW and 181 SMW, who were recruited from a cancer registry [32]. Additionally, we used community-based recruitment, by inviting SMW and HSW who were ineligible for a study of advanced breast cancer for which we recruited concurrently. For this non-probability recruitment, we used the Love/AVON Army of Women (AOW) – an online recruitment resource designed to partner a diverse group of women with the research community in an effort to accelerate breast cancer research. Eligibility criteria for women with breast cancer were a diagnosis of non-metastatic (stage in situ to III) and non-recurrent breast cancer after age 21. Eligible SMW and HSW were invited to participate in a 45-minute telephone survey. At the end of the survey, each participant was given the opportunity to invite her partner, if partnered, or her primary support person, if unpartnered, to participate in a similarly structured phone survey. Any individual who was identified as the partner or the primary support for a woman with breast cancer was invited to participate in a telephone survey of approximately the same length and asked the same or similar questions as we asked the breast cancer survivors.
From May to July 2012, we recruited 297 breast cancer survivors of whom 203 (68.4%) provided contact information for their caregiver, while 82 (27.6%) refused to provide contact information, and 12 (4.0%) indicated they did not have a caregiver. When we contacted the 203 caregivers for whom we had contact information, 167 (82.3%) participated in the survey, 25 (12.3%) exceeded the number of contact attempts to complete the survey, 7 (3.4%) responded, yet were unable to participate before the end of the study period, and 4 (2.0%) refused participation. For this study, we relied on the 167 breast cancer survivors and their caregivers for whom we had complete data.
Measures
The outcome, FOR, was measured by the self-report measure developed by Northouse in 1981 [6]. This 22-item instrument is available for survivors and family caregivers [33]. This scale uses negatively and positively worded items to determine the amount of worry and concern survivors and their caregivers have about the cancer recurring. Higher scores indicate greater FOR. The Cronbach alpha for the FOR scale in our sample of survivors and caregivers was 0.94 and 0.93, respectively.
The main independent measure, sexual orientation, distinguished heterosexual survivors and caregivers from sexual minority survivors and caregivers. Sexual minorities were comprised of lesbian, gay, bisexual individuals, and individuals reporting a preference for same-sex partners. Demographic, cancer-related, and medical factors were considered as independent variables, consistent with the literature on fear of recurrence [3, 7]. Because of our focus on sexual orientation, we also considered discrimination experiences and the social support resources of survivors and caregivers, which were relevant factors explaining the experiences of sexual minority survivors in prior studies [29, 32, 34].
Demographic data consisted of age, race, education, employment, and income for all participants. For survivors, we assessed marital status, having a partner, living alone, and health insurance. From survivors’ addresses we derived two measures of neighborhood socioeconomic status. Using Census 2000 data, we obtained: (1) Poverty level, the percentage of the population in a census block living under the Federal poverty level and (2) the census block’s median household income. In the context of the demographic data, we asked survivors and caregivers if they ever felt discriminated against because of their age, race/ethnicity, gender, sexual orientation, appearance, income level, or having had cancer, allowing for yes or no answers. Each affirmative discrimination experience was then counted [35, 36].
Medical information included survivors’ cancer-related experiences such as time since diagnosis (calculated from the diagnosis to the survey date), stage, and cancer treatments, from which we derived measures of surgery, radiation, chemotherapy, and receipt of anti-estrogen (tamoxifen or aromatase inhibitor) therapy. For both survivor and caregiver participants, we determined comorbidities using a measure developed by Ganz [37].
We measured multiple aspects of social support. We used a six-item short form of the Interpersonal Support Evaluation List (ISEL) (Cronbach alpha=0.72) [38–40]. To determine in more detail the sources of social support, we also used a 12-item social support instrument (MPSS) (Cronbach alpha=0.90), which assesses support from three sources, family, friends, and a significant other [41]. Using single items, we inquired about survivors’ and caregivers’ use of cancer support groups and assessed mental health counseling prior to and after the breast cancer diagnosis. As dyadic characteristics, we assessed survivor and caregiver’s co-residence, the type of survivor-caregiver relationship, and the duration of the relationship from which we determined the proportion of life they were in this relationship. Another dyadic characteristic was a five item scale that measures dyadic cohesion (Cronbach alpha=0.76), which was derived from the dyadic assessment scale [42]. Finally, from a single item adapted from a study on couples [43], we determined breast cancer’ effect on closeness of the relationship.
Statistical Analysis
Survivors and caregivers’ demographic, clinical, and dyadic characteristics were compared, using t-tests and chi-square tests, to examine differences by survivors’ sexual orientation. To account for potential selection biases and confounding that may influence the outcomes, survivors’ and their caregivers’ FOR, we used propensity scores. Of the significant factors related to sexual orientation in our sample, we used caregiver age and proportion of life in relationship with the survivor in the propensity score model, since both are unmodifiable risk factors and highly unbalanced in our sample. Propensity scores weighted all estimators by their inverse-probability and were generated using a multivariable logistic regression model for the dichotomous outcome (SMW vs HSW). To identify independent factors associated with each actor’s FOR, we used a stepwise selection process in regressions, always including survivors’ sexual orientation. P-values less than 0.1 were considered significant enough to be included in the simultaneous equation model. For this step, all variables in Table 1 were considered, with the exception of caregiver gender and sexual orientation because there was complete overlap between these variables and the main independent variable of interest, survivor sexual orientation. Since each actor’s FOR were both outcomes and predictors for the other actor’s FOR, we had “endogeneity” [44] causing the following two problems: 1. a linear regression model that had FOR of the other actor as a determinant would give invalid inference due to the significant correlation between the determinant and the error term, and 2. their coefficients would be biased. To address the inference and bias problems caused by endogeneity, we used simultaneous equation models (SIM), which are widely used in econometrics, stem from economic theory and have a causal interpretation. All analyses were performed in SAS 9.4
Table 1.
Characteristics of the Sample
| Characteristic | Heterosexual Survivor N=43 |
Sexual Minority Survivor N=124 |
p-value | Caregiver to Heterosexual Survivor N=43 |
Caregiver to Sexual Minority Survivor N=124 |
p-value |
|---|---|---|---|---|---|---|
|
| ||||||
| Demographics | ||||||
|
| ||||||
| Gender: Female | 43 | 124 | 7 (16.3) | 116 (93.5) | <.0001 | |
| vs. Male | 0 | 0 | 36 (83.7) | 8 (6.5) | ||
|
| ||||||
| Sexual Orientation | <.0001 | |||||
| Heterosexual | 43 | 0 | 43 ( 100) | 18 (14.5) | ||
| Sexual Minority | 0 | 124 | 0 | 106 (85.5) | ||
|
| ||||||
| Age at survey | 60.8 (8.4) | 56.3 (8.6) | 0.0037 | 62.4 (8.0) | 55.8 (9.3) | <.0001 |
| Mean (SD) | ||||||
|
| ||||||
| Race: White | 38 (88.4) | 114 (91.9) | 0.5379 | 42 (97.7) | 111 (89.5) | 0.0962 |
| vs. Other | 5 (11.6) | 10 ( 8.1) | 1 ( 2.3) | 13 (10.5) | ||
|
| ||||||
| Marital Status | <.0001 | |||||
| Never Married | 3 ( 7.0) | 50 (40.7) | ||||
| Married | 35 (81.4) | 55 (44.7) | ||||
| Sep/Div/Widowed | 5 (11.6) | 18 (14.6) | ||||
| Missing | 0 | 1 | ||||
|
| ||||||
| Having a partner/spouse | 0.7801 | |||||
| No | 7 (16.3) | 18 (14.5) | ||||
| Yes | 36 (83.7) | 106 (85.5) | ||||
|
| ||||||
| Lives alone | 0.4266 | |||||
| No | 37 (86.0) | 100 (80.6) | ||||
| Yes | 6 (14.0) | 24 (19.4) | ||||
|
| ||||||
| Education | 0.5187 | 0.1506 | ||||
| High School or less/Techn Training | 3 ( 7.0) | 3 ( 2.4) | 3 ( 7.0) | 3 ( 2.4) | ||
| Some College | 4 ( 9.3) | 9 ( 7.3) | 6 (14.0) | 14 (11.3) | ||
| Graduated College | 16 (37.2) | 49 (39.5) | 15 (34.9) | 31 (25.0) | ||
| Completed Grad School | 20 (46.5) | 63 (50.8) | 19 (44.2) | 76 (61.3) | ||
|
| ||||||
| Currently employed | 0.1307 | 0.0024 | ||||
| Yes | 24 (55.8) | 85 (68.5) | 20 (47.6) | 90 (73.2) | ||
| No | 19 (44.2) | 39 (31.5) | 22 (52.4) | 33 (26.8) | ||
| Missing | 1 | 1 | ||||
|
| ||||||
| Health insurance | 1 | |||||
| Yes | 43 ( 100) | 122 (98.4) | ||||
| No | 0 | 2 ( 1.6) | ||||
|
| ||||||
| Individual income | 0.8781 | 0.4057 | ||||
| Less than $30K | 8 (20.0) | 27 (22.3) | 5 (12.5) | 27 (22.1) | ||
| $30K – <$70K | 14 (35.0) | 45 (37.2) | 18 (45.0) | 47 (38.5) | ||
| $70K or more | 18 (45.0) | 49 (40.5) | 17 (42.5) | 48 (39.3) | ||
| Missing | 3 | 3 | 3 | 2 | ||
|
| ||||||
| Census poverty level | 0.7826 | -- | -- | |||
| <5% | 19 (44.2) | 58 (46.8) | ||||
| 5% – <10% | 12 (27.9) | 36 (29.0) | ||||
| 10% – <20% | 10 (23.3) | 21 (16.9) | ||||
| 20%+ | 2 ( 4.7) | 9 ( 7.3) | ||||
|
| ||||||
| Census median household income | 0.2278 | -- | -- | |||
| Low $43,846 or less | 12 (27.9) | 31 (25.0) | ||||
| Mid $43,847 – $74,313 | 20 (46.5) | 74 (59.7) | ||||
| High > $74,313 | 11 (25.6) | 19 (15.3) | ||||
|
| ||||||
| Discrimination | 0.8 (1.1) | 1.5 (1.6) | 0.0023 | 0.5 (0.8) | 1.5 (1.5) | <.0001 |
| Mean (SD) | ||||||
|
| ||||||
| Clinical | ||||||
|
| ||||||
| Years since diagnosis | 5.8 (3.9) | 7.3 (3.6) | 0.0314 | NA | NA | |
| Mean (SD) | ||||||
|
| ||||||
| Stage of cancer at time of diagnosis | 0.9269 | NA | NA | |||
| In situ (ductal carcinoma) | 8 (19.0) | 26 (21.0) | ||||
| Stage I | 18 (42.9) | 46 (37.1) | ||||
| Stage II | 12 (28.6) | 40 (32.3) | ||||
| Stage III | 4 ( 9.5) | 12 ( 9.7) | ||||
| Missing | 1 | 0 | ||||
|
| ||||||
| Surgical Treatment | 0.1404 | NA | NA | |||
| Lumpectomy | 41 (95.3) | 105 (84.7) | ||||
| Mastectomy Only | 0 ( 0.0) | 10 ( 8.1) | ||||
| Mastectomy and Reconstruction | 2 ( 4.7) | 9 ( 7.3) | ||||
|
| ||||||
| Radiation | 0.2338 | NA | NA | |||
| No | 11 (25.6) | 44 (35.5) | ||||
| Yes | 32 (74.4) | 80 (64.5) | ||||
|
| ||||||
| Anti-estrogen Treatment | 0.8883 | NA | NA | |||
| No | 12 (27.9) | 36 (29.0) | ||||
| Yes | 31 (72.1) | 88 (71.0) | ||||
|
| ||||||
| Comorbidities | 0.9519 | 0.2659 | ||||
| Mean (SD) | 2.5 (1.6) | 2.5 (1.7) | 2.5 (1.7) | 2.2 (1.5) | ||
|
| ||||||
| Number of Comorbidities | 0.3199 | 0.3433 | ||||
| 0 | 3 ( 7.1) | 13 (10.7) | 5 (11.6) | 15 (12.4) | ||
| 1 | 11 (26.2) | 18 (14.8) | 8 (18.6) | 31 (25.6) | ||
| 2 | 8 (19.0) | 33 (27.0) | 7 (16.3) | 29 (24.0) | ||
| 3+ | 20 (47.6) | 58 (47.5) | 23 (53.5) | 46 (38.0) | ||
|
| ||||||
| Social support | ||||||
|
| ||||||
| ISEL-SF Mean (SD) | 22.3 (2.0) | 22.5 (2.3) | 0.7815 | 21.7 (2.6) | 22.5 (1.9) | 0.0743 |
|
| ||||||
| Multidimensional Perceived Social Support Scale: Total Score | 6.0 (0.5) | 6.0 (0.8) | 0.5568 | 5.8 (0.8) | 6.0 (0.6) | 0.2529 |
|
| ||||||
| Multidimensional Perceived Social Support Scale: Significant Other Subscale | 6.2 (0.5) | 6.3 (0.9) | 0.0924 | 6.0 (0.9) | 6.3 (0.6) | 0.0256 |
|
| ||||||
| Multidimensional Perceived Social Support Scale: Family Subscale | 6.0 (5.5, 6.0) | 6.0 (5.0, 6.8) | 0.103 | 5.8 (0.9) | 5.6 (1.2) | 0.1389 |
|
| ||||||
| Multidimensional Perceived Social Support Scale: Friends Subscale | 6.1 (0.5) | 6.3 (0.8) | 0.0256 | 5.9 (0.9) | 6.2 (0.6) | 0.0132 |
|
| ||||||
| Ever cancer support group? | 0.0154 | 0.2715 | ||||
| No | 30 (69.8) | 60 (48.4) | 39 (90.7) | 104 (83.9) | ||
| Yes | 13 (30.2) | 64 (51.6) | 4 ( 9.3) | 20 (16.1) | ||
|
| ||||||
| Counseling to deal with breast cancer? | 0.069 | 0.0533 | ||||
| No | 32 (74.4) | 73 (58.9) | 41 (95.3) | 103 (83.7) | ||
| Yes | 11 (25.6) | 51 (41.1) | 2 ( 4.7) | 20 (16.3) | ||
|
| ||||||
| Counseling before breast cancer? | 0.0008 | 0.0002 | ||||
| No | 24 (55.8) | 34 (27.4) | 30 (69.8) | 45 (36.6) | ||
| Yes | 19 (44.2) | 90 (72.6) | 13 (30.2) | 78 (63.4) | ||
|
| ||||||
| Dyadic | ||||||
|
| ||||||
| Relationship to Survivor | 0.0233 | |||||
| Spouse/Partner | 36 (83.7) | 106 (85.5) | ||||
| Child | 3 (7.0) | 0 | ||||
| Sibling | 2 (4.7) | 3 (2.4) | ||||
| Parent | 1 (2.3) | 3 (2.4) | ||||
| Friend | 1 (2.3) | 12 (9.7) | ||||
|
| ||||||
| Spouse /partner | 36 (83.7) | 106 (85.5) | 0.7801 | |||
| vs. other Caregiver | 7 | 18 | ||||
|
| ||||||
| Survivor –Caregiver Co residence | 36 (83.7) | 97 (78.2) | 0.4406 | |||
|
| ||||||
| Years of Rel with Survivor | <0.0001 | |||||
| Mean (SD) | 31.6 (13.1) | 18.6 (12.0) | ||||
|
| ||||||
| Proportion of life been in relationship with Survivor Mean (SD) | <0.0001 | |||||
| 0.5 (0.2) | 0.3 (0.2) | |||||
|
| ||||||
| Dyadic cohesion Mean (SD) | 17.5 (2.6) | 18.1 (3.0) | 0.284 | 17.5 (2.9) | 18.2 (2.5) | 0.1036 |
|
| ||||||
| Breast cancer | 0.3812 | 0.7525 | ||||
| Brought you closer, | 29 (69.0) | 88 (72.7) | 33 (76.7) | 94 (77.7) | ||
| Distanced you from her, | 1 (2.4) | 8 (6.6) | 2 (4.7) | 3 (2.5) | ||
| Had no effect on your relationship | 12 (28.6) | 25 (20.7) | 8 (18.6) | 24 (19.8) | ||
|
| ||||||
| Outcome variable | ||||||
|
| ||||||
| Fear of Recurrence Scale | 0.2316 | |||||
| Mean (SD) | 73.9 (15.4) | 70.9 (16.4) | 0.2955 | 75.2 (13.6) | 71.8 (16.5) | |
Results
Table 1 presents survivors’ characteristics by sexual orientation and the caregivers’ characteristics by the sexual orientation of the survivor. We first tested for differences between HSW and SMW. SMW were on average younger, 56 years, compared to HSW who were on average 61 years old. There were no differences with respect to race, in that about 90% of all survivors reported white race. Survivors’ marital status differed, with 41% of SMW reporting never having been married compared to 7% of HSW. About 85% of SMW and HSW had a partner and less than 20% of survivors reported living alone. There were no differences in socioeconomic status, in that survivors were highly educated, about half reported having completed graduate school, more than 40% had an income of $70,000 or more, more than 40% resided in low poverty-level neighborhoods, and about 25% lived in neighborhoods with a low median household income ($43,846 or lower). Compared to HSW, SMW reported twice the amount of discrimination experiences. Survivors were similar with respect to their clinical characteristics, with about 60% reporting in situ or stage I breast cancer. More than 80% were treated with breast conserving surgery, more than 60% received radiation therapy, and about 70% received anti-estrogen therapy. The average years since diagnosis was seven for SMW compared to six for HSW. Both survivor groups shared similar levels of comorbidities, 2.5 on average, with almost half of survivors reporting three or more comorbidities. Survivors’ overall social support scores were similar but SMW reported receiving significantly more support from friends than reported by HSW. Compared to HSW, SMW reported significantly greater use of cancer support groups and greater use of counseling prior to their breast cancer, yet survivors’ use of counseling after the breast cancer diagnosis was similar.
The caregivers significantly differed by gender and sexual orientation, in that 84% of HSW caregivers were male and all self-reported as heterosexual, while 94% of caregivers to SMW were female and 86% self-reported as sexual minority. Caregivers to SMW were significantly younger, on average 56 years old, compared to HSW’ caregivers, who were on average 62 years old. Both groups of caregivers reported mostly white race, similar income levels, and high educational attainment (more than 75% reported a college or graduate degree). Caregivers to SMW were significantly more likely employed (73% vs. 48%) and reported three times the discrimination reported by HSW’ caregivers. Both groups of caregivers reported less than three medical comorbidities on average. Caregivers’ social support was similar overall, yet SMW’ caregivers reported significantly more support from significant others and from friends than HSW caregivers. Caregivers to SMW were significantly more likely to report having used counseling prior to the survivors’ cancer, while the use of support groups and counseling to deal with the survivors’ cancer was similar by caregiver group.
When comparing survivor-caregiver’s dyadic characteristics, both survivor groups’ were 85% spouse or partner caregivers, yet caregivers to HSW were significantly more likely to be family members while caregivers to SMW were more likely to be friends. The duration of the survivor-caregiver relationship was significantly longer for HSW than for SMW (31.6 vs. 18.6 years). Dyads of SMW and their caregivers were similar to HSW caregiver dyads in cohesion and closeness. Finally, when assessing the outcomes, survivors’ FOR did not differ by sexual orientation, and both caregiver groups reported similar FOR. As expected, there was a significant correlation between caregiver FOR and survivor FOR (rho=0.29, p-value<.0001).
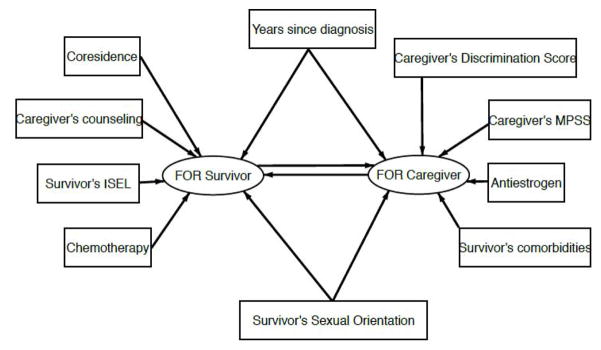
Figure 1 provides a path diagram of the determinants we included in the SIM. Rectangles denote independent variables, ellipses denote dependent variables. Determinants associated with the survivors’ FOR (p<.10) consisted of years since diagnosis, co-residence with the caregiver, caregivers’ counseling, survivors’ social support (ISEL), chemotherapy treatment, and survivors’ sexual orientation. In addition, we considered caregivers’ FOR as a determinant of survivors’ FOR. The determinants associated with the caregivers’ FOR (p<.10) were years since diagnosis, caregivers’ discrimination, caregivers’ social support (MPSS), and survivors’ anti-estrogen therapy, comorbidities, and sexual orientation. As before, we added the survivors’ FOR as a determinant of the caregivers’ FOR.
Figure 1.
Path diagram of SIM model.
Table 2 presents the results of the two-staged SIM analyses, showing the regression coefficients and their t-statistics, all adjusted using propensity scores to account for the differences in age and proportion of life in the relationship with the survivor. Columns 3 and 4 of Table 2 show the direct effects for each outcome. The direct effects for each outcome account for all independent factors found significant in the model selection step along with the effect of the other actor’s FOR. These results indicate that actor-partner FOR is unidirectional, in that caregivers’ FOR significantly impacts survivors’ FOR but not the other way around. Covariates that were significant and included in the SIM for caregiver FOR were anti-estrogen therapy, years since diagnosis, caregiver discrimination score, survivors’ comorbidities, caregivers’ total social support scores (MPSS). Covariates that were significant and included in the SIM for survivors’ FOR were years since diagnosis, chemotherapy, whether caregivers sought mental health counseling to deal with diagnosis, co-residence, and survivors’ ISEL.
Table 2.
Explanatory factors of FOR in survivor and caregiver dyads of different sexual orientations
| Direct Effects | Total Effects | ||||
|---|---|---|---|---|---|
| Survivor FOR | Caregiver FOR | Survivor FOR | Caregiver FOR | ||
| Sexual Orientation (SM vs HS) | Estimate | −0.13 | −0.15 | −0.21 | −0.16 |
| Std Err | 0.08 | 0.09 | 0.07 | 0.08 | |
| t-value | −1.76 | −1.68 | −2.95 | −1.96 | |
| p-value | 0.0779 | 0.0924 | 0.0032 | 0.0503 | |
| Years Since Diagnosis | Estimate | −0.17 | −0.24 | −0.30 | −0.25 |
| Std Err | 0.08 | 0.09 | 0.07 | 0.08 | |
| t-value | −2.08 | −2.76 | −4.27 | −3.32 | |
| p-value | 0.0374 | 0.0058 | <.0001 | 0.0009 | |
| Anti-estrogen therapy (Yes vs No) | Estimate | 0.22 | 0.11 | 0.22 | |
| Std Err | 0.07 | 0.04 | 0.07 | ||
| t-value | 3.10 | 2.52 | 3.30 | ||
| p-value | 0.0019 | 0.0117 | 0.0010 | ||
| Survivor Comorbidities | Estimate | 0.24 | 0.12 | 0.25 | |
| Std Err | 0.07 | 0.05 | 0.07 | ||
| t-value | 3.21 | 2.60 | 3.48 | ||
| p-value | 0.0013 | 0.0092 | 0.0005 | ||
| Chemotherapy (Yes vs No) | Estimate | 0.16 | 0.16 | 0.01 | |
| Std Err | 0.06 | 0.07 | 0.03 | ||
| t-value | 2.46 | 2.50 | 0.37 | ||
| p-value | 0.0138 | 0.0123 | 0.7117 | ||
| Caregiver Sought Mental Health counseling to deal with diagnosis (Yes vs No) | Estimate | 0.31 | 0.32 | 0.02 | |
| Std Err | 0.07 | 0.06 | 0.05 | ||
| t-value | 4.76 | 4.98 | 0.37 | ||
| p-value | <.0001 | <.0001 | 0.7102 | ||
| CORESIDENCE (Yes vs No) | Estimate | 0.22 | 0.23 | 0.01 | |
| Std Err | 0.07 | 0.07 | 0.04 | ||
| t-value | 3.35 | 3.44 | 0.37 | ||
| p-value | 0.0008 | 0.0006 | 0.7108 | ||
| Caregiver Discrimination Score | Estimate | 0.14 | 0.07 | 0.15 | |
| Std Err | 0.07 | 0.04 | 0.08 | ||
| t-value | 1.90 | 1.74 | 1.93 | ||
| p-value | 0.0575 | 0.0827 | 0.0541 | ||
| Survivor ISEL | Estimate | −0.19 | −0.19 | −0.01 | |
| Std Err | 0.06 | 0.07 | 0.03 | ||
| t-value | −2.90 | −2.94 | −0.37 | ||
| p-value | 0.0038 | 0.0032 | 0.7110 | ||
| Caregiver MPSS TOTAL | Estimate | −0.23 | −0.12 | −0.24 | |
| Std Err | 0.07 | 0.05 | 0.07 | ||
| t-value | −3.34 | −2.57 | −3.41 | ||
| p-value | 0.0008 | 0.0102 | 0.0006 | ||
| Caregiver FOR | Estimate | 0.49 | 0.51 | 0.03 | |
| Std Err | 0.15 | 0.16 | 0.08 | ||
| t-value | 3.37 | 3.15 | 0.36 | ||
| p-value | 0.0008 | 0.0017 | 0.7180 | ||
| Survivor FOR | Estimate | 0.06 | 0.03 | 0.06 | |
| Std Err | 0.15 | 0.08 | 0.16 | ||
| t-value | 0.38 | 0.36 | 0.36 | ||
| p-value | 0.7074 | 0.7180 | 0.7154 | ||
The total effects of each predictor on the outcome of interest, FOR, while accounting for age and proportion of life in relationships with survivors, are shown in columns 5 and 6 of Table 2. This total effects model accounts for both direct effects on FOR as well as indirect effects. Indirect effects result from the causality loop, in that beyond the direct effects of caregiver FOR on survivor FOR, all determinants for caregiver FOR affect survivor FOR. Sexual orientation had a significant effect on survivor FOR, with SMW having a small but statistically significantly lower FOR than HSW. There was a similar effect of sexual orientation on caregiver FOR, but it was not statistically significant. Caregiver FOR also had a positive relationship with survivor FOR, with higher caregiver FOR resulting in higher survivor FOR. The opposite relationship between actors (survivors and caregivers) was also positive but not statistically significant. Significant factors that increased survivors’ FOR included receipt of anti-estrogen therapy, caregivers who sought mental health counseling to deal with diagnosis, co-residence, survivors’ comorbidities, and receipt of chemotherapy. Significant factors that were negatively correlated with survivor FOR included years since diagnosis, survivors’ social support (ISEL), and caregivers’ social support (MPSS). Significant factors that increased caregiver FOR included receipt of anti-estrogen therapy and survivors’ comorbidities. Significant factors that were negatively correlated with caregivers’ FOR included years since diagnosis and caregivers’ social support (MPSS).
Discussion
In many ways this study is consistent with existing knowledge about FOR, indicating that even five or more years after diagnosis, FOR is a persistent concern [45]. As indicated by previous studies, social support, medical comorbidities, time since diagnosis and cancer treatments related to FOR [3, 7, 45]. While prior studies linked demographic factors to FOR [3, 7], the contribution of this study are the links between the survivors’ sexual orientation and the survivors’ and caregivers’ FOR. Moreover, the significant correlation between survivors’ and caregivers’ FOR that we identified confirms survivor-caregiver interdependence [3, 8, 9]. While there is consensus that FOR experienced by one dyad member influences the FOR of the other [9], this study’s novelty and unique contribution is to reveal greater details about the dynamics within caregiver-survivor dyads that have implications for interventions for survivors and caregivers related to FOR.
This study illuminates a more complex picture, mostly suggesting paths by which caregivers increase survivors’ FOR. First, this study determined that survivor-caregiver co-residence increased survivors’ FOR but was unrelated to caregivers’ FOR. We suggest that living together may allow survivors to closely observe their caregivers’ reactions, coping, and mood, which may then increase survivors’ FOR. Second, caregivers who sought counseling to deal with the cancer diagnosis increased survivors’, but was unrelated to caregivers’ FOR. On the other hand, caregivers with more social support decreased survivors’ FOR. These findings may suggest that survivors perceive their caregivers’ help-seeking behavior as a weakness or as an inability to cope, whereas caregivers who have social support, possibly through sources other than a therapist decreased survivors’ FOR. These dynamics suggest needs for further studies into survivor-caregiver communication and possible later opportunities for communication and cognitive behavioral stress management interventions for these dyads [46–49]. For example, our findings of survivors being reactive to caregivers may make it necessary to develop cognitive behavioral interventions focused on reassuring survivors about their caregivers’ ability to cope or changing survivors’ cognitions to perceive their caregivers’ help seeking behaviors as strengths and appropriate self-care.
Most importantly, these findings go beyond interdependence of survivor-caregiver FOR to demonstrate how the dynamics were unidirectional, in that caregivers’ FOR directly affected survivors’ FOR, while survivors’ FOR did not affect caregivers’ FOR. Our interpretation of these findings is that they reveal a common feature of survivors being highly reactive to their caregivers. The unidirectional dynamic of caregivers’ FOR directly affecting survivors’ FOR and caregivers’ social support reducing survivors’ and caregivers’ FOR provide opportunities for caregiver interventions that may improve both dyad members’ FOR. While specific medical and cancer characteristics affected both dyad members, they are not modifiable and therefore unsuitable for interventions. In contrast, social support and discrimination experiences are mutable factors suitable for interventions to reduce FOR. The importance of social support as a mechanism suitable for interventions to reduce caregivers and survivors’ FOR has already been suggested by others [50].
With respect to sexual orientation and FOR, we found that SMW and HSW had similar FOR as did the caregivers of SMW and HSW. However, in the final fully adjusted models, we found that SMW who had similar characteristics as HSW had slightly less FOR compared to HSW. Additionally, caregivers of SMW, who had similar characteristics as the caregivers of HSW, had somewhat lower FOR compared to caregivers of HSW. These differences in FOR by sexual orientation are an indication that our study did not fully account for all differences between dyads of HSW and their caregivers and the dyads of SMW and their caregivers. Future studies are needed to identify and explain these differences in FOR by sexual orientation. We suggest unmeasured factors which might account for SMW somewhat lower FOR are SMW’ protective factors such as being connected to the LGBT community [51] and having developed resilience through other life circumstances such as experiences with discrimination [52]. We found that compared to HSW survivors, SMW survivors reported more discrimination, consistent with our prior studies [32, 53]. While we linked caregivers’ discrimination experiences to caregivers and survivors’ FOR initially, in our final models the effect of discrimination experiences diminished when other factors were accounted for. However, we used only a cursory assessment of discrimination experiences. Future studies will need to assess in greater detail the specifics of SMW survivors and their caregivers’ experiences of discrimination, including whether any of these experiences are linked to their treating providers, nurses, or administrators. Given our approach of restricting the caregiver choice for partnered survivors to the partner or spouse, caregivers to HSW were more likely men compared to the mostly female caregivers to SMW. Also, this study focused on the dyad, therefore an inclusion criterion of this study was to have a participating caregiver, implying that we only included partnered survivors who had a participating spouse or partner. As we explained elsewhere [30], this criterion was easier met by partnered SMW survivors than by partnered HSW survivors, in that fewer male partners agreed to participate. SMW’ caregivers, the majority of whom self-reported a sexual minority identity themselves, were more likely employed, and shared with the SMW survivor greater experiences of discrimination and use of counseling before the cancer diagnosis compared HSW and their caregivers. Similar to survivors, we found that caregivers to SMW were more likely to report discrimination experiences compared to caregivers of HSW. This confirms a recent review of sexual minority patients’ caregivers, which pointed to discrimination within health and social services systems [54].
This study has limitations worth noting. For example, caregiver gender and sexual orientation could not be considered in this study. If future studies allow survivors to identify any person as their caregiver, irrespective of the relationship to the caregiver, it will be possible to better understand gender effects, assuming some HSW survivors will identify female caregivers rather than a male partner or spouse. This may provide additional insights with respect to the effects of caregiver gender and caregiver role (partner versus other). Our sample lacked diversity in terms of race and ethnicity preventing us from examining the intersectionality of race and sexual orientation.
Despite these limitations, this study’s ability to reveal dynamics in caregiver and survivor dyads is a major strength and innovation. Other strengths include consideration of the sexual orientation of breast cancer survivors, involving caregivers who were and were not partners, and our novel analytic approach that allowed us to identify a causal relationship in that caregivers’ FOR affected survivors’ FOR, while survivors’ FOR did not affect caregivers’ FOR. Therefore, findings of this study point to the importance of caregiver interventions to reduce FOR in survivors and their caregivers and thereby enhance their well-being.
Acknowledgments
Funding: This study was funded by the American Cancer Society (RSGT-06-135-01-CPPB).
Support for this research was provided by the American Cancer Society, Grant No. RSGT-06-135-01-CPPB PI: U. Boehmer. Additional supplemental funding was made available by a Boston University School of Public Health pilot grant and by funding from NCI 3R01CA181392-02S1. The authors are grateful to the participants who took the time to respond to our questions and complete the survey.
Footnotes
Compliance with Ethical Standards:
Conflict of Interest: All authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.American Cancer Society. Cancer Treatment and Survivorship Facts & Figures 2014–2015. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 2.Hewitt M, Herdman R, Holland J, editors. Institute of Medicine. Meeting Psychosocial Needs of Women with Breast Cancer. National Cancer Policy Board (NCPB), Institute of Medicine (IOM); Washington, D.C: The National Academies Press; 2004. [PubMed] [Google Scholar]
- 3.Simard S, Thewes B, Humphris G, Dixon M, Hayden C, Mireskandari S, et al. Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. J Cancer Surviv. 2013;7:300–22. doi: 10.1007/s11764-013-0272-z. [DOI] [PubMed] [Google Scholar]
- 4.Koch L, Bertram H, Eberle A, Holleczek B, Schmid-Hopfner S, Waldmann A, et al. Fear of recurrence in long-term breast cancer survivors-still an issue. Results on prevalence, determinants, and the association with quality of life and depression from the cancer survivorship--a multi-regional population-based study. Psychooncology. 2014;23:547–54. doi: 10.1002/pon.3452. [DOI] [PubMed] [Google Scholar]
- 5.Kenyon M, Mayer DK, Owens AK. Late and long-term effects of breast cancer treatment and surveillance management for the general practitioner. J Obstet Gynecol Neonatal Nurs. 2014;43:382–98. doi: 10.1111/1552-6909.12300. [DOI] [PubMed] [Google Scholar]
- 6.Thewes B, Butow P, Zachariae R, Christensen S, Simard S, Gotay C. Fear of cancer recurrence: a systematic literature review of self-report measures. Psychooncology. 2012;21:571–87. doi: 10.1002/pon.2070. [DOI] [PubMed] [Google Scholar]
- 7.Crist JV, Grunfeld EA. Factors reported to influence fear of recurrence in cancer patients: a systematic review. Psychooncology. 2013;22:978–86. doi: 10.1002/pon.3114. [DOI] [PubMed] [Google Scholar]
- 8.Schmid-Buchi S, Halfens RJ, Dassen T, van den Borne B. A review of psychosocial needs of breast-cancer patients and their relatives. J Clin Nurs. 2008;17:2895–909. doi: 10.1111/j.1365-2702.2008.02490.x. [DOI] [PubMed] [Google Scholar]
- 9.Mellon S, Kershaw TS, Northouse LL, Freeman-Gibb L. A family-based model to predict fear of recurrence for cancer survivors and their caregivers. Psychooncology. 2007;16:214–23. doi: 10.1002/pon.1074. [DOI] [PubMed] [Google Scholar]
- 10.Institute of Medicine. The Health of Lesbian, Gay, Bisexual, and Transgender People: Building a Foundation for Better Understanding. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 11.Brown JP, Tracy JK. Lesbians and cancer: an overlooked health disparity. Cancer Causes Control. 2008;19:1009–20. doi: 10.1007/s10552-008-9176-z. [DOI] [PubMed] [Google Scholar]
- 12.Case P, Bryn Austin S, Hunter DJ, Manson JE, Malspeis S, Willett WC, et al. Sexual Orientation, Health Risk Factors, and Physical Functioning in the Nurses’ Health Study II. J Womens Health. 2004;13:1033–47. doi: 10.1089/jwh.2004.13.1033. [DOI] [PubMed] [Google Scholar]
- 13.Aaron D, Markovic N, Danielson M, Honnold J, Janosky J, Schmidt N. Behavioral risk factors for disease and preventive health practices among lesbians. Am J Public Health. 2001;91:972–5. doi: 10.2105/ajph.91.6.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dibble SL, Roberts SA, Robertson PA, Paul SM. Risk factors for ovarian cancer: lesbian and heterosexual women. Oncol Nurs Forum. 2002;29:E1–7. doi: 10.1188/02.ONF.E1-E7. [DOI] [PubMed] [Google Scholar]
- 15.Valanis BG, Bowen DJ, Bassford T, Whitlock E, Charney P, Carter RA. Sexual orientation and health: Comparisons in the women’s health initiative sample. Arch Fam Med. 2000;9:843–53. doi: 10.1001/archfami.9.9.843. [DOI] [PubMed] [Google Scholar]
- 16.Boehmer U, Bowen DJ, Bauer GR. Overweight and obesity in sexual minority women: evidence from population-based data. Am J Public Health. 2007;97:1134–40. doi: 10.2105/AJPH.2006.088419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boehmer U, Bowen DJ. Examining factors linked to overweight and obesity in women of different sexual orientations. Prev Med. 2009;48:357–61. doi: 10.1016/j.ypmed.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Burgard SA, Cochran SD, Mays VM. Alcohol and tobacco use patterns among heterosexually and homosexually experienced California women. Drug Alcohol Depend. 2005;77:61–70. doi: 10.1016/j.drugalcdep.2004.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cochran SD, Keenan C, Schober C, Mays VM. Estimates of alcohol use and clinical treatment needs among homosexually active men and women in the U.S. population. J Consult Clin Psychol. 2000;68:1062–71. doi: 10.1037//0022-006x.68.6.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drabble L, Midanik LT, Trocki K. Reports of alcohol consumption and alcohol-related problems among homosexual, bisexual and heterosexual respondents: results from the 2000 National Alcohol Survey. J Stud Alcohol. 2005;66:111–20. doi: 10.15288/jsa.2005.66.111. [DOI] [PubMed] [Google Scholar]
- 21.Gilman SE, Cochran SD, Mays VM, Hughes M, Ostrow D, Kessler RC. Risk of psychiatric disorders among individuals reporting same-sex sexual partners in the National Comorbidity Survey. Am J Public Health. 2001;91:933–9. doi: 10.2105/ajph.91.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cochran SD, Mays VM, Bowen D, Gage S, Bybee D, Roberts SJ, et al. Cancer-related risk indicators and preventive screening behaviors among lesbians and bisexual women. Am J Public Health. 2001;91:591–7. doi: 10.2105/ajph.91.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rankow EJ, Tessaro I. Mammography and Risk Factors for Breast Cancer in Lesbian and Bisexual Women. Am J Health Behav. 1998;22:403–10. [Google Scholar]
- 24.Kavanaugh-Lynch MHE, White E, Daling JR, Bowen DJ. Correlates of lesbian sexual orientation and the risk of breast cancer. J Gay Lesbian Med Assoc. 2002;6:91–5. [Google Scholar]
- 25.Dibble SL, Roberts SA, Nussey B. Comparing breast cancer risk between lesbians and their heterosexual sisters. Womens Health Issues. 2004;14:60–8. doi: 10.1016/j.whi.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Boehmer U, Ozonoff A, Timm A. County-level association of sexual minority density with breast cancer incidence: results from an ecological study. Sex Res Social Policy. 2011;8:139–45. [Google Scholar]
- 27.Cochran SD, Mays VM. Risk of Breast Cancer Mortality Among Women Cohabiting with Same Sex Partners: Findings from the National Health Interview Survey, 1997–2003. J Womens Health (Larchmt) 2012;21:528–33. doi: 10.1089/jwh.2011.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boehmer U. Breast cancer in lesbian and bisexual women. In: Boehmer U, Elk R, editors. Cancer and the LGBT Community: Unique Perspectives from Risk to Survivorship. Cham: Springer; 2015. pp. 141–57. [Google Scholar]
- 29.Boehmer U, Freund KM, Linde R. Support providers of sexual minority women with breast cancer: who they are and how they impact the breast cancer experience. J Psychosom Res. 2005;59:307–14. doi: 10.1016/j.jpsychores.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 30.Bazzi AR, Clark MA, Winter M, Tripodis Y, Boehmer U. Recruitment of breast cancer survivors and their caregivers: implications for dyad studies. doi: 10.1007/s13142-016-0400-1. forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hausman JA. Specification and Estimation of Simultaneous Equation Models, Handbook of Econometrics. Amsterdam: Elsevier Science; 1983. [Google Scholar]
- 32.Boehmer U, Glickman M, Milton J, Winter M. Health-related quality of life in breast cancer survivors of different sexual orientations. Qual Life Res. 2012;21:225–36. doi: 10.1007/s11136-011-9947-y. [DOI] [PubMed] [Google Scholar]
- 33.Northouse LL. Mastectomy patients and the fear of cancer recurrence. Cancer Nurs. 1981;4:213–20. [PubMed] [Google Scholar]
- 34.Boehmer U, Glickman M, Winter M, Clark MA. Breast cancer survivors of different sexual orientations: Which factors explain survivors’ quality of life and adjustment? Ann Oncol. 2013;24:1622–30. doi: 10.1093/annonc/mdt035. [DOI] [PubMed] [Google Scholar]
- 35.Krieger N. Racial and gender discrimination: risk factors for high blood pressure? Soc Sci Med. 1990;30:1273–81. doi: 10.1016/0277-9536(90)90307-e. [DOI] [PubMed] [Google Scholar]
- 36.Stuber J, Galea S, Ahern J, Blaney S, Fuller C. The Association between Multiple Domains of Discrimination and Self-assessed Health: A Multilevel Analysis of Latinos and Blacks in Four Low-Income New York City Neighborhoods. Health Serv Res. 2003;38:1735–60. doi: 10.1111/j.1475-6773.2003.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. J Natl Cancer Inst. 2002;94:39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 38.Cohen S, Hoberman H. Positive events and social supports as buffers of life change stress. J Appl Soc Psychol. 1983;13:99–125. [Google Scholar]
- 39.McGregor BA, Carver CS, Antoni MH, Weiss S, Yount SE, Ironson G. Distress and internalized homophobia among lesbian women treated for early stage breast cancer. Psychol Women Q. 2001;25:1–9. [Google Scholar]
- 40.Arena PL, Carver CS, Antoni MH, Weiss S, Ironson G, Durán RE. Psychosocial responses to treatment for breast cancer among lesbian and heterosexual women. Women Health. 2006;44:81– 102. doi: 10.1300/j013v44n02_05. [DOI] [PubMed] [Google Scholar]
- 41.Zimet GD, Dahlem NW, Zimet SG, Farley GK. Multidimensional Scale of Perceived Social Support (MSPSS) In: Corcoran K, Fischer J, editors. Measures for Clinical Practice. New York: The Free Press; 2000. pp. 502–3. [Google Scholar]
- 42.Spanier GB. Measuring dyadic adjustment: New scales for assessing the quality of marriage or similar dyads. J Marriage Fam. 1976;38:15–28. [Google Scholar]
- 43.Dorval M, Guay S, Mondor M, Masse B, Falardeau M, Robidoux A, et al. Couples who get closer after breast cancer: frequency and predictors in a prospective investigation. J Clin Oncol. 2005;23:3588–96. doi: 10.1200/JCO.2005.01.628. [DOI] [PubMed] [Google Scholar]
- 44.Duncan GJ, Magnuson KA, Ludwig J. The endogeneity problem in developmental studies. Res Hum Dev. 2004;1:59–80. [Google Scholar]
- 45.Koch L, Jansen L, Brenner H, Arndt V. Fear of recurrence and disease progression in long-term (>/= 5 years) cancer survivors--a systematic review of quantitative studies. Psychooncology. 2013;22:1–11. doi: 10.1002/pon.3022. [DOI] [PubMed] [Google Scholar]
- 46.Manne SL, Taylor KL, Dougherty J, Kemeny N. Supportive and negative responses in the partner relationship: their association with psychological adjustment among individuals with cancer. J Behav Med. 1997;20:101–25. doi: 10.1023/a:1025574626454. [DOI] [PubMed] [Google Scholar]
- 47.Manne SL, Ostroff JS, Norton TR, Fox K, Goldstein L, Grana G. Cancer-related relationship communication in couples coping with early stage breast cancer. Psychooncology. 2006;15:234–47. doi: 10.1002/pon.941. [DOI] [PubMed] [Google Scholar]
- 48.Manne S, Badr H. Intimacy and relationship processes in couples’ psychosocial adaptation to cancer. Cancer. 2008;112:2541–55. doi: 10.1002/cncr.23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Q, Loke AY. A literature review on the mutual impact of the spousal caregiver-cancer patients dyads: ‘communication’, ‘reciprocal influence’, and ‘caregiver-patient congruence’. Eur J Oncol Nurs. 2014;18:58–65. doi: 10.1016/j.ejon.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Badr H, Carmack CL, Diefenbach MA. Psychosocial interventions for patients and caregivers in the age of new communication technologies: opportunities and challenges in cancer care. J Health Commun. 2015;20:328–42. doi: 10.1080/10810730.2014.965369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frost DM, Meyer IH. Measuring community connectedness among diverse sexual minority populations. J Sex Res. 2012;49:36–49. doi: 10.1080/00224499.2011.565427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyer IH. Couns Psychol. 2010. Identity, Stress, and Resilience in Lesbians, Gay Men, and Bisexuals of Color; p. 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boehmer U, Clark MA, Timm A, Glickman M, Sullivan M. Comparing sexual minority cancer survivors recruited through a cancer registry to convenience methods of recruitment. Womens Health Issues. 2011;21:345–52. doi: 10.1016/j.whi.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 54.Washington KT, McElroy J, Albright D, Oliver DP, Lewis A, Meadows S, et al. Experiences of Sexual and Gender Minorities Caring for Adults with Non-AIDS-Related Chronic Illnesses. Soc Work Res. 2015;39:71–81. [Google Scholar]