Abstract
Myeloproliferative neoplasms (MPNs) are clonal disorders of hematopoiesis characterized by a high frequency of genetic alterations and include chronic myeloid leukemia (CML) and the BCR-ABL1-negative MPNs. Herein we summarize recent advances and controversies in our understanding of the biology and therapy of these disorders, as discussed at the 8th post-American Society of Hematology CML-MPN workshop. The principal areas addressed include the breakthrough discovery of CALR mutations in patients with JAK2/MPL wild type MPN, candidate therapies based on novel genetic findings in leukemic transformation and new therapeutic targets in MPNs, and an appraisal of bone marrow histopathology in MPNs with a focus on the potential new clinical entity of “ masked ” polycythemia vera. An update on clinical trials of Janus kinase (JAK) inhibitors is presented as well as current understanding regarding the definitions and mechanisms of resistance to JAK inhibitors, and updated information on the safety and efficacy of discontinuation of tyrosine kinase inhibitors in patients with CML.
Introduction
The chronic myeloproliferative neoplasms (MPNs) include chronic myeloid leukemia (CML), primary myelofibrosis (PMF), essential thrombocythemia (ET) and polycythemia vera (PV). As knowledge on these disorders continues to evolve, new preclinical and clinical challenges have been identified [1]. Following the unraveling of the molecular pathogenesis of CML in the 1980s, tyrosine kinase inhibitors (TKIs) entered the clinic for patients with chronic myeloid leukemia (CML) [2–4]. While the results have proven very impressive, it is easy to forget the considerable initial skepticism about the possible clinical value of TKIs in the early 1990s. Today the estimated long-term survival for CML patients receiving TKIs approaches ~85%. With this backdrop, when the genomic landscape in the BCR-ABL1-negative MPNs began to unfold in 2005, there was much enthusiasm surrounding the entry of the JAK inhibitors into the clinics for patients with myelofibrosis (MF) [5,6]. So far these compounds have met a qualified success [7,8]. Here, we briefly summarize recent advances in knowledge related to MPN biology and indicate where they may have important therapeutic implications as discussed at the 8th post-American Society of Hematology CML-MPN workshop, which took place in New Orleans, Louisiana, USA on December 10–11, 2013 and updated at the 16th John Goldman European School of Hematology CML Conference, Philadelphia, PA on 5 – 7 September 2014.
Evolving insights into the BCR-ABL1-negative myeloproliferative neoplasm genomic landscape
Although the precise details of the initiating events in BCR – ABL1-negative MPNs remain elusive, mutations in JAK2 and MPL have been associated with this and the pivotal importance of the JAK – signal transducer and activator of transcription (STAT) pathway is now recognized [9]. A considerable proportion of cases with PMF and ET diagnosis, however, do not contain JAK2V617F, JAK2 exon 12 or MPL mutations. These patients with JAK2 and MPL wild-type were the focus of two independent whole-exome sequencing studies that identified mutations in the CALR gene encoding calreticulin (Figure 1) [10,11]. CALR mutations are present in ~ 35% of cases of PMF and 25% of ET, all of which are wild-type for JAK2 and MPL. Thus, JAK2, CALR and MPL mutations are present in _ 90% of all cases of MPN. With the exception of a few patients with refractory anemia with ringed sideroblasts associated with marked thrombocytosis (RARS-T), CALR mutations have rarely been reported in patients with PV and other leukemias or solid tumors. CALR mutations cluster in exon 9 and consist of deletions and insertions of variable size. Two CALR mutations (del52 and ins5, also known as type 1 and type 2 mutations) are present in 85% of all CALR mutant cases, but many other lower frequency insertion/deletions have been identified [12]. These mutations are typically present at ~ 50% allele burden, although type 2 mutations can be rendered homozygous by mitotic recombination. Clonal analysis of individual hematopoietic colonies from patients with MPN suggests that CALR mutations tend to occur early during disease evolution.
Figure 1.
CALR gene. (A) Calreticulin (CALR) domain structure. CALR protein has three distinctive domains (N, P, C). The P-domain is involved in the chaperone function. The C-domain is rich in acidic amino acids and contains the high capacity, low affinity calcium binding site. The last four C-terminal amino acids (KDEL) are the endoplasmic reticulum retention signal. (B) Variability of CALR exon 9 insertion/deletion mutations found in MPN. Type 1 and type 2 mutations are most frequent and together they are present in over 85% of CALR mutated cases. (C) Impact of MPN associated mutations on CALR protein structure. Despite the diversity of mutations found in the CALR gene exon 9, the impact on the protein structure is very similar. The two most common CALR insertion/deletion mutations found in MPN (type 1 and type 2) are shown, which frameshift to the same alternative reading frame. This results in a novel C-terminus of the mutant CALR that turns into a positively charged peptide lacking the calcium binding and KDEL regions.
Despite the mutational variability, the impact on CALR protein sequence is relatively uniform, as the CALR insertions and deletions result in a frameshift to a specific alternative reading frame of exon 9. This frameshift generates a novel C-terminus of the mutant CALR protein rich in arginine and methionine that coverts the net charge from negative to positive. In addition, the mutant CALR lacks the last four C-terminal amino acids’ (KDEL) endoplasmatic reticulum (ER) retention signal, which results in dislocation of at least a proportion of the mutant CALR from the ER. Mutations in CALR activate JAK – STAT signaling, although the underlying mechanism remains unclear. Consistent with JAK2 activation, a JAK2 inhibitor impaired the proliferation of Ba/F3 cells expressing mutant CALR. Thus, the hallmark of all three major MPN-associated mutations is activation of the JAK – STAT signaling pathway. In both PMF and ET, some patients with CALR mutation tend to be younger at diagnosis, have less severe cytopenias and appear to have a better prognosis.
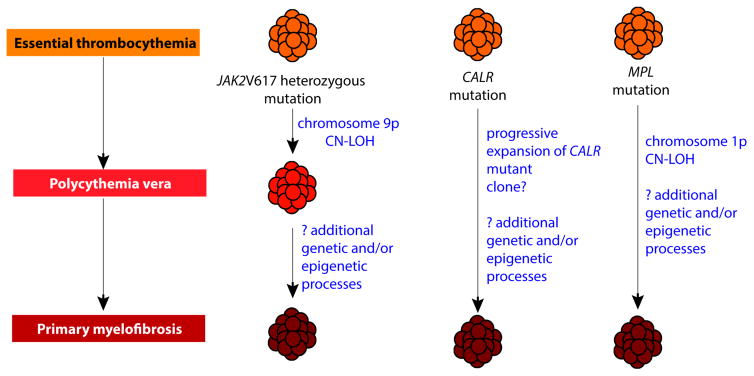
Cazzola and Kralovics recently hypothesized that each MPN genotypic entity arises from an initial ET phenotype [12]. In this model, clonal evolution of JAK2V617F –mutant MPNs may involve a phenotypic switch from ET to PV, and later PV to MF. Moreover, MPL- and CALR-mutant MPNs may be responsible, possibly with some other cooperating mutations, for a phenotypic switch from ET to MF (Figure 2). This model, based on the role of megakaryocytes in the pathophysiology of MPNs, would support the notion of an initial ET phenotype, which later may transit from one subtype to another, with MF being the result of late stage evolution. Skoda and colleagues very recently demonstrated, in a murine model, how the deletion of STAT1 in the presence of JAK2 V617F alters the phenotypic manifestations by reducing megakaryopoiesis and promoting erythropoiesis, most likely via the interferon gamma/STAT1 pathway [13]. Clearly much additional work is required to validate (or refute) the various hypotheses.
Figure 2.
Proposed pathophysiology of the BCR – ABL1 negative classic myeloproliferative neoplasms (MPNs) based on new genetic insights. In this model, proposed by Mario Cazzola and Robert Kralovics, all MPNs originate in a state most consistent with essential thrombocythemia (ET). This occurs in patients with heterozygous JAK2 V617F mutations, CALR mutations or MPL mutations. Patients with heterozygous JAK2 V617F mutations that undergo copy neutral loss-of-heterozygosity (CN-LOH) of the locus of the JAK2 V617F mutation (on chromosome 9p) undergo a phenotypic switch from an ET phenotype to a phenotype most consistent with polycythemia vera (PV). Further genetic and/or epigenetic alterations may then result in further evolution from PV to myelofibrosis (MF). In contrast, patients with a MPL or CALR mutation either remain with a phenotype consistent with ET or undergo evolution directly to MF. Genetic alterations known to be associated with transition from ET to MF include CN-LOH of the locus of MPL mutation (on chromosome 1p). Additionally, genetic data suggest that progressive expansion of CALR mutant clones is associated with transformation from ET to MF.
New insights into mechanisms of leukemic transformation in myeloproliferative neoplasms
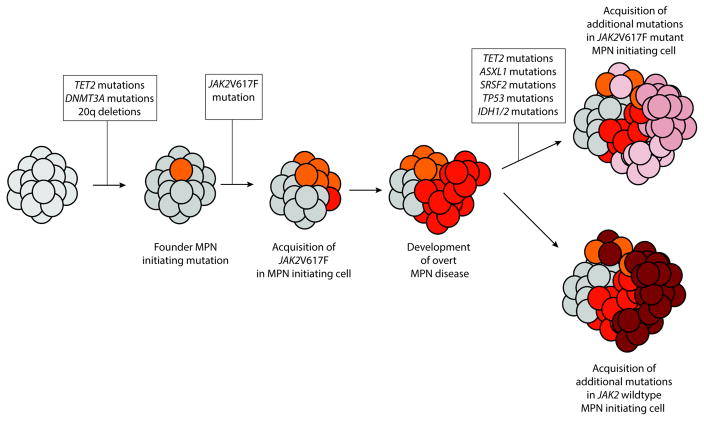
Transformation to acute leukemia may occur at a late stage in the evolution of all MPNs. In CML, the risk of transformation has been reduced to be 5% or less, with the introduction of TKIs [14]. In contrast, the current risk of transformation in BCR-ABL1-negative MPNs remains unchanged, at around 4–8% for PV and ET and 23% for MF [15,16]. Leukemic transformation is associated with adverse clinical outcome, characterized by a poor response and early resistance to conventional therapies. Recent research efforts have addressed the role of mutations frequently identified in leukemic transformation of MPNs (Figure 3) and the candidacy of adenosine deaminase acting on RNA (ADAR) in promoting resistance and relapse in blast crisis CML [17,18]. To study this further, Rampal and colleagues developed a murine model in which JAK2V617F is combined with Tp53 loss in vivo [19]. Retroviral transduction of JAK2V617F cDNA in mouse bone marrow (BM) cells deficient for Tp53 resulted in an acute leukemia in recipient mice resembling an erythroleukemia. Notably, disease from JAK2V617F/Tp53−/− cells, but not JAK2V617F/Tp53+/+ cells, was transplantable into secondary recipients consistent with increased self-renewal in vivo. This model represents the first model of leukemic transformation of JAK2V617F mutant MPNs for preclinical use. To this end, the authors tested the utility of JAK inhibitors alone and in combination with other drugs in cells from these mice in vitro and in vivo. Notably, the combination of INCB18424 and decitabine was associated with synergistic inhibitory effects in vitro. Moreover, in vivo testing of INCB18424 and the HSP90 inhibitor PUH-71 was carried out in secondary recipients. Treatment with either PUH-71 or INCB18424 resulted in significant prolongation of survival and reduction of organomegaly compared with control mice. In addition, treatment with PUH-71 extended survival significantly compared with INCB18424. These observations validated the murine model and further studies are ongoing.
Figure 3.
Model of current understanding of genetic events responsible for leukemic transformation of chronic BCR – ABL1 negative myeloproliferative neoplasms (MPNs). Although JAK2 V617F mutations are sufficient for development of MPN phenotype, a large amount of evidence suggests that earlier genetic events predate development of the JAK2 V617F mutations to establish a “pre-leukemic” MPN initiating cell. Mutations in TET2 as well as DNMT3A have been most frequently described as predating JAK2V617F mutations in patients with MPN. Acquisition of the JAK2 V617F mutation then results in overt MPN clinical disease. Later, acquisition of further mutations, either in a cell bearing the JAK2 mutation or a JAK2 wild type cell results in transformation to acute leukemia. Currently, few studies regarding leukemic transformation of CALR-mutant chronic MPN patients have been described.
Research conducted by Jamieson and colleagues identified that RNA editing by the adenosine deaminase acting on RNA (ADAR) as an important driver of resistance and relapse in blast crisis CML [18]. Through whole transcriptome sequencing of normal, chronic phase, and serially transplantable blast crisis CML progenitor samples, the authors identified increased IFN-γ pathway gene expression in concert with BCR-ABL amplification, enhanced expression of the IFN-responsive ADAR1 p150 isoform, and a propensity for increased adenosine-to-inosine RNA editing during CML progression, particularly in the context of primate specific Alu sequences. Serial transplant and lentiviral shRNA studies demonstrated that ADAR1 knockdown impaired in vivo self-renewal capacity of blast crisis CML progenitors. Together these data provide a compelling rationale for developing ADAR1-based therapeutic strategies for CML. To this end, more recently Jamieson and colleagues studied a humanized RAG2−/−γc−/− mouse model of blast crisis CML. In this model, a potent BCR-ABL1 inhibitor, dasatinib, combined with the selective JAK2 inhibitor, fedratinib (SAR302503), reduced phospho-JAK2 mediated ADAR1 expression and activity as well as serial transplantation potential. While ADAR1 activation promotes proliferation of normal human hematopoietic stem cells in a tightly regulated manner, increased inflammatory stimuli emanating from the malignant microenvironment combined with heightened BCR-ABL1 oncogene-mediated sensitivity to TNF, IFN and JAK2 signaling promotes RNA editing of self-renewal regulatory transcripts and altered expression of microRNAs involved in reprogramming. Notably, aberrant RNA recoding was effectively reduced in CML progenitors with a combination of BCR-ABL1 and JAK2 inhibition that expunges malignant self-renewal capacity in vivo. Targeted reversal of RNA recoding and malignant reprogramming in inflammatory microenvironments that promote progenitor senescence may enhance cancer stem cell (CSC) eradication in a broad array of human malignancies and provides a strong rationale for reducing both extrinsic and intrinsic JAK2 signaling as a vital component of CSC targeted clinical trials.
Does the order of mutations or the mutations’ burden in MPNs matter?
There has been considerable debate as to the determinants of the MPN phenotype. Prchal and colleagues presented whole-exome sequencing and DNA copy-number analysis of 31 JAK2 V617F-positive patients and further investigated the evolution of somatic mutations using longitudinal samples. Five different patterns of 9paUPD (acquired uniparental disomy) were observed [20]. Almost one-half of the patients were heterozygous for JAK2 V617F without 9paUPD (subgroup I); the remaining patients had a duplicate JAK2 V617F allele via mitotic recombination to produce 9paUPD (subgroup II). Ten percent of patients acquired 9paUPD first, followed by JAK2 V617F mutation, yielding patients in subgroup III. In a single female patient, they observed almost complete 9paUPD with a low JAK2 V617F allelic burden (0.24), indicating that the majority of the PV clone was composed of 9paUPD (subgroup IV; this patient was probably in a transient state from 9paUPD with wild-type JAK2 to subgroup III). About 3% of patients with PV exhibited trisomy of 9p, generating two copies of the JAK2 V617F allele (subgroup IV). The genes with recurrent loss of wild-type germline alleles within the aUPD regions could be under selection for the PV phenotype. Forty-eight genes lost their wild-type alleles in at least three patients. Among them, nine genes are related to cell division, seven to transcriptional regulation, four are involved in epigenetic regulation and three are potential tumor suppressors. KDM4C and SMARCA2, which are involved in histone modification and chromatin remodeling, are among them.
In addition to JAK2 V617F and 9pUPD, they identified frequent recurrent somatic mutations in ASXL1, TET2, DNMT3A, SF3B1 and NF1. Forty-two percent of patients had a somatic mutation in at least one epigenetic modifier gene. In four of 31 patients, variant allele abundance suggested that mutation of JAK2 V617F was preceded by other somatic mutations, including ASXL1, DNMT3A and SF3B1. Strikingly, in four patients pre-JAK2 variants were detected at COSMIC codons in one or more PV-related genes, in which somatic mutations across the cohort were discovered in T-cells. To determine whether these COSMIC mutations are truly germline or are acquired and constitute the pre-JAK2 PV clone, but are not sufficient for PV phenotype, these investigators analyzed non-hematopoietic tissues, such as skin or archival tumor biopsies of non-hematological tumors, and observed that in some patients these mutations were indeed germline, i.e. present in non-hematological tissues. This suggests that some pre-JAK2 V617F rare COSMIC mutations are truly germline, and candidates for PV predisposition observed in some families with multiple affected members [21]. It is also of interest that the order of mutation acquisition has been shown to dictate the phenotype of MPN [22]. These observations collectively highlight some of the complexity of PV pathogenesis and support the notion of additional work to clarify matters further.
Is bone marrow histology really important in MPNs?
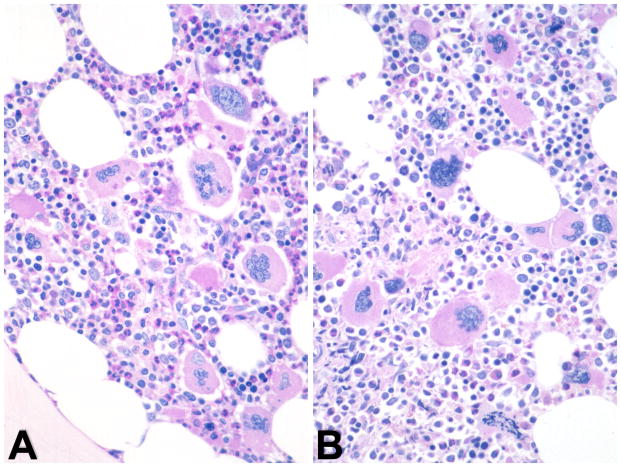
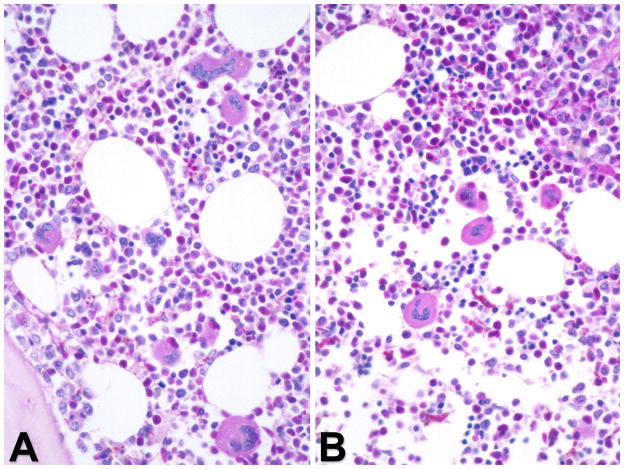
The current World Health Organization (WHO) MPN diagnostic criteria includes BM morphology as a major criterion in ET and PMF and a minor criterion in PV. The WHO MPN subcommittee defines characteristic BM patterns, often constellations of diverse parameters and not single histological features [23]. This can pose challenges in early MPN stages that may present with thrombocytosis and clinically mimic ET [24]. Prefibrotic/early PMF is another example of a diagnostic challenge but recent studies underlined the stepwise evolution of disease pathogenesis in PMF with significant differences in survival, progression, and complications in comparison to ET [24,25]. Although evolution to overt MF may occur in ET, the incidence is much lower than in prefibrotic PMF. Histopathology of BM biopsy samples in prefibrotic/early PMF reveals hypercellularity with prominent granulocytic and megakaryocytic proliferation in the absence of fibrosis. Therefore, the demonstration of reticulin fibrosis, although characteristic, is not a required criterion for diagnosis of PMF [26]. The megakaryocytes in PMF are characterized by a more pronounced degree of cytological atypia, compared to those in ET or PV (Figure 4). Conversely, BM histopathology in initial ET is usually consistent with an age-matched cellularity and a predominant megakaryopoiesis without a significant erythroid or neutrophilic myeloproliferation (Figure 5) [24,27]. Gross disturbances of megakaryocyte morphology such as extensive dense clustering or prominent lining of bony trabeculae are normally not detectable [28]. Another area of diagnostic challenge is ‘masked’ PV (discussed below) [29]. Collectively, there appears to be persuasive evidence that the current WHO BM criteria are reproducible and clinically useful [30,31].
Figure 4.
Bone marrow morphology in early/prefibrotic PMF is characterized by hypercellular BM displaying a prominent megakaryocytic and granulocytic myeloproliferation. (A) Megakaryocytes are frequently endosteal-paratrabecularly dislocated and show small to large dense clusters. There is a high variability in size ranging from small to giant forms. Other prominent features are signify cant aberrations of nuclear organization such as marked hypolobulation, irregular foldings and condensed chromatin pattern thus creating bulbous or so-called cloud-like/balloon-shaped nuclei with increased nuclear-cytoplasmic ratio. (B) Hematoxylin and eosin staining.
Figure 5.
Bone marrow morphology in ET showing an increase in number of megakaryocytes with a random distribution throughout the marrow space or loose clustering of large to giant cells. (A) Megakaryocytes reveal deeply folded nuclei surrounded by a corresponding area of mature cytoplasm, i.e. no evidence of nuclear-cytoplasmic abnormalities. (B) Periodic acid – Schiff staining.
The introduction of JAK inhibition in MPN has led to considerable interest in MPN BM histology. A recent international European LeukemiaNet (ELN) project has proposed a set of BM features that characterize therapy response by BM morphology. In analogy to the WHO grading concept for fibrosis [23], this proposal defines reproducible scoring systems for the grading of collagen deposition and osteosclerosis [32]. The proposed criteria include response categories that suggest disease modification, as well as those that provide objective quantification of drug activity in improving major MPN-associated BM features. Efforts are also assessing the potential clinical impact of histological responses.
What is ‘masked’ polycythemia vera?
There has been an ongoing debate amongst experts in PV regarding the possible existence of a smoldering or ‘masked’ phase of the disease and the contribution of BM morphology in this diagnosis [33]. Barbui and colleagues examined the BM features and clinical correlates of 140 patients with JAK2V617F mutant PV, who met all WHO diagnostic criteria, with the sole exception of the hemoglobin (Hb) levels [29]. In contrast to the WHO Hb levels, stipulated as >18.5 g/dl for men and >16.5 g/dl for women, these investigators’ study cohort, operationally referred to as ‘masked’ PV, had Hb value of <18.5 g/dl for men and <16.5 g/dl for women. They compared the clinical and hematological features of masked PV patients to those of 257 JAK2V617F-positive PV patients, who satisfied the full WHO diagnostic criteria. The masked PV patients were found to be predominantly men, had platelet counts >450 × 109/L, had experienced more arterial thrombosis, demonstrated significantly higher risk of leukemic transformation and had an inferior survival, compared with the ‘control’ group. The BM trephine morphology of the masked PV patients demonstrated trilineage hypercellularity (pan-myelosis). These observations suggest that masked PV represents a heterogenous hematological disorder, with some patients sharing features of ET while others resembling overt PV with no patient fulfilling current WHO diagnostic criteria [34]. Thus, masked PV is proposed to be a variant of PV despite lower Hb levels at diagnosis, and has been suggested to be included in the new WHO classification.
Resistance to JAK inhibitors in MF - Myths and Facts
Although various definitions for response and resistance to ruxolitinib have been proposed in the setting of clinical trials in patients with MF, none have been validated in clinical practice. It is assumed that primary resistance entails the absence or minor reduction in spleen size and constitutional symptoms, while spleen regrowth and recurrence of symptoms after a period with good response establish secondary resistance. Several biological mechanisms of resistance have been described. In particular, acquisition of new mutations in the predicted ruxolitinib-binding region was previously shown to confer resistance to JAK inhibitors in vitro [35–37]. Kiladjian and colleagues studied 41 consecutive MF patients treated with ruxolitinib in a single centre, and aimed to characterize criteria for resistance as well as a molecular signature of resistance [38]. The JAK2 mutation status was determined in all patients with MF. Overall, 16/39 (41%) of patients were considered ruxolitinib-resistant, with only 4/16 exhibiting primary resistance (<10% reduction in spleen size). Median spleen size reduction was 60% in the whole cohort, 50% in patients who developed secondary resistance to ruxolitinib, and 80% in non-resistant patients. Secondary resistance was defined as regrowth of spleen either alone, or associated with recurrence of symptoms or with marked leukocytosis. Median ruxolitinib exposure was longer in ruxolitinib-resistant patients compared to non-resistant patients (median of 383 vs. 292 days). Median starting dose was similar in both groups (15 mg BID), but a higher proportion of patients in the ruxolitinib-resistant group had to reduce the dose to <10 mg BID during follow-up. Among ruxolitinib-resistant patients, there was a higher proportion of patients with high IPSS (56% vs. 39% in non-resistant; p=0.06), and of post-ET MF (38% vs. 26%; p=0.08). Molecular profiling of patients developing ruxolitinib-resistance showed that 31% of them had no mutation detectable at diagnosis in JAK2, MPL, TET2 and SRSF2, compared to 9% of patients in the non ruxolitinib-R group (p=0.003). Sequencing of the JAK2 kinase domain in samples taken at the time of resistance in 14/16 ruxolitinib resistant patients did not detect any new mutations, suggesting that such a mechanism is rarely involved in clinical resistance to ruxolitinib. An interesting possibility, which may merit further investigation, is reactivation of the JAK – STAT signaling following long-term exposure to ruxolitinib [39,40].
What can JAK inhibition offer patients with myelofibrosis in 2014?
Ruxolitinib, a JAK1/2 inhibitor, is the only JAK inhibitor licensed for the treatment of MF. The drug accords a substantial and durable improvement in constitutional symptoms, reduction of splenomegaly and prolongs survival, but does not appear to eliminate the disease [7,8,41]. Mature outcome data confirm the durability of reduction in splenomegaly and improvements in constitutional symptoms [42]. There are early intriguing data on the drug’s effects on the BM fibrosis in some patients [43]. The adverse events attributable to ruxolitinib appear to be relatively mild and generally manageable. Anemia is the most common side effect, sometimes with thrombocytopenia, and may require dose adjustments.
Fedratintib (previously known as SAR302503), a selective JAK2 inhibitor, has been tested in MF patients and the final results of a randomized placebo-control phase-III trial (JAKARTA) are available [44,45]. The results show a significant improvement in splenomegaly and constitutional symptoms, in addition to some molecular responses and improvements in bone marrow fibrosis. Of interest, results of JAKARTA-2, a phase II study assessing the efficacy and safety of fedratinib in MF patients resistant/refractory to ruxolitib, also demonstrate the drug’s efficacy in according improvements in splenomegaly and symptoms [46]. However, despite these positive results, fedratinib was found to be associated with development of Wernicke’s encephalopathy in a small proportion of patients, leading to a cessation of further clinical development of the drug in MF until firm causal mechanism can be ascertained. It is of some interest that although the blood thiamine levels of affected patients have not been reported so far, in vitro studies suggest that fedratinib, but not ruxolitinib, inhibits the carrier-mediated uptake and transcellular flux of thiamine in Caco-2 cells [47]. It remains to be seen whether this unique inhibition of thiamine affects other candidate JAK inhibitors.
Preliminary results from several other JAK inhibitors in MF studies, in particular pacritinib (SB1518), momelotinib (CYT387), LY2784544, and BMS911543, also demonstrate benefits, and appear to be relatively safe [48–51]. Pacritinib, a JAK2/FLT3 inhibitor, is currently being tested in two phase III clinical trials (PERSIST-1 and PERSIST-2), both of which compare pacritinib with best available therapy. Momelotinib is being compared to ruxolitinib, with the hopes of identifying an improvement in momelotinib with regards to anemia. The interim results from a phase II study of a selective JAK1 inhibitor, INCB039110, are less encouraging [52]. The rationale for this trial was to try to distinguish the impact of JAK1 inhibition alone versus combined JAK1/JAK2 inhibition. Major findings are little myelosuppression and considerable improvements in constitutional symptoms, but also only modest reduction of splenomegaly.
There is also interest in a novel telomerase inhibitor, imetelstat, in patients with advanced MF [53]. Early results show improvements in splenomegaly and constitutional symptoms, in addition to improvements in bone marrow fibrosis. Unfortunately, this was associated with a high incidence of severe myelosuppression and hepatic insufficiency, leading to a partial cessation of current clinical trials, which were reopened in June 2014 [54].
Discontinuation of TKI treatment in CML patients and deep molecular responses
The notion of being able to discontinue TKI therapy safely and effectively is a critical landmark in clinical studies of CML patients today. This is of substantial interest due to impact on quality of life issues, but also related to economic costs of the drug [55,56]. Currently, most specialists advocate discontinuation only within the framework of a clinical trial, with close molecular monitoring. A principal goal therefore is to define robust molecular criteria, such as a major molecular response (MMR), defined as a 3-log reduction of BCR-ABL1 transcripts compared to baseline, and a complete molecular response (MR), defined rather diversely as a 4- (MR4), 4.5- (MR4.5), or 5- (MR5) log reduction of BCR-ABL1 transcripts. Current treatment guidelines propose the use of MMR after 3 months of TKI therapy as an indicator of an optimal response and a predictor for MR, which is now a candidate marker for long-term success and discontinuation of therapy (50). There are several ongoing efforts to improve these milestones further by assessing the dynamics of the EMR [58].
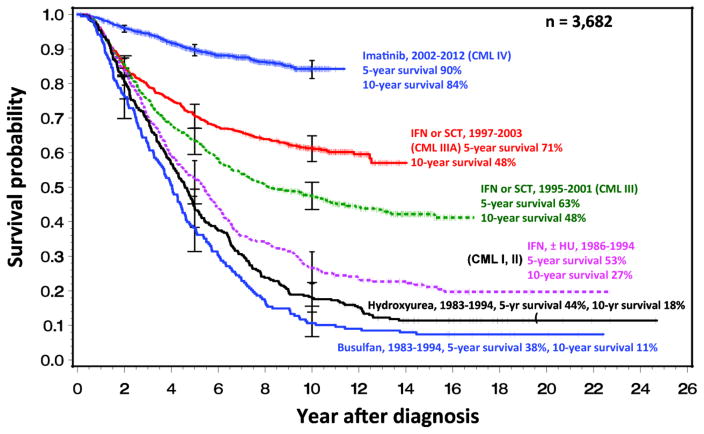
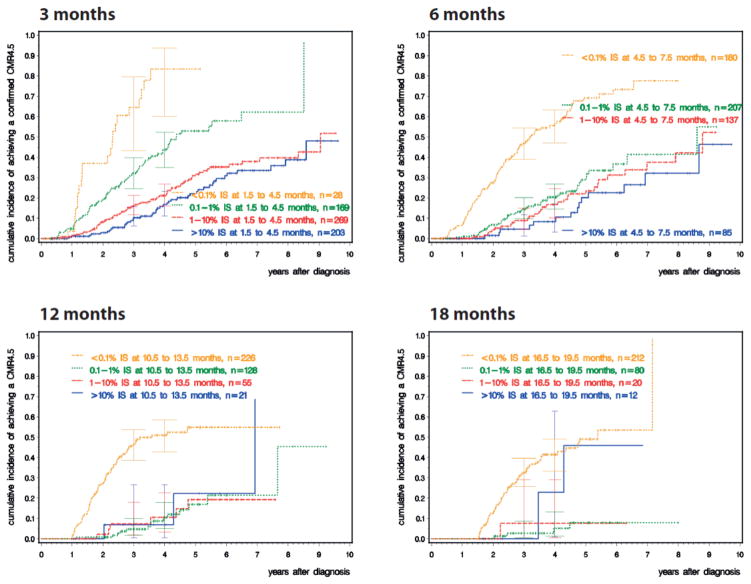
In order to assess the impact of MR on survival, following an optimized dose of imatinib, the German CML study group conducted a five-arm randomized study (Study IV) comparing imatinib 400 mg vs. imatinib plus cytarabine vs. imatinib plus interferon alfa (IFN) vs. imatinib after IFN failure vs. imatinib 800 mg [59]. There is additional interest in such an approach in view of the widely anticipated global increase in the use of generic imatinib in early 2015, and its associated positive pharmacoeconomic impact. After a median observation time of 67.5 months, the estimated 10-year survival was 83% (Figure 6), and the cumulative incidences of MR4.5 were significantly higher in the optimized imatinib cohort; the cumulative response rates of the study cohort, comparing MR4.5 with CCyR, MMR and MR4 are depicted in Figure 7. The CCyR and MR4.5 rates were ~80% and ~70%, respectively, after 9 years. A landmark analysis at 4 years shows that confirmed MR4.5 is a significantly better predictor of survival than a CCyR, with no progression to the advanced phase and an estimated 5-year overall survival of 97%. This study also confirmed the notion of MMR by 3 months affording the highest probability of a subsequent MR4.5, and potential to discontinue therapy fastest. The study, therefore, supports the notion of using MR4.5 as a new molecular predictor of long-term outcome in CML patients on TKIs, though overall survival of patients achieving MR4.5 is not significantly different from those achieving CCyR. Parenthetically, though MR4.5 was reached by the significant majority of imatinib-treated patients, this landmark was achieved faster with the optimized imatinib 800 mg dosing schedule, and it should be of interest to compare this with the second generation TKIs.
Figure 6.
Survival with CML over time: the German CML-study group experience. The data comprise 3682 patients from five randomized trials over the period 1983 – 2013.
Figure 7.
Cumulative incidence of deep molecular response (MR 4.5) dependent on BCR – ABL1 transcript levels at 3, 6, 12 and 18 months. The fastest and highest level of MR 4.5 is achieved in patients who have achieved a major molecular response (MMR, <0.1% IS) at the respective time points.
Conclusion
It is remarkable to witness how basic and preclinical studies continue to define MPNs, and how many of the lessons learned from CML are now being applied to improve our knowledge of BCR-ABL1-negative MPNs. For patients with CML, the introduction of TKIs resulted in multiple achievements. First, it established that the BCR-ABL1 translocation is of principal pathogenic importance in the disease. Second, it established the usefulness of TKIs to accord a survival benefit to the majority of patients with CML in chronic phase. Indeed, the natural history of all BCR-ABL1+ leukemias has been improved by the introduction of TKI therapy. Dasatinib and nilotinib produce CCyR and MMR at higher rates and at a much faster pace than imatinib and current results suggest superior rates of freedom from progression. Furthermore most, though not all, side effects are easily manageable [60]. Drug resistance, despite BCR – ABL1 inhibition, however, remains a problem. Recent efforts to better understand underlying molecular mechanisms of TKI resistance suggest a role of cytoplasmic mislocalization of p27, a nuclear cyclin dependent kinase (Cdk), and further work is ongoing [61,62]. For patients with BCR-ABL1-negative MPNs, the introduction of JAK inhibitors have not resulted in such impressive results, rather a qualified progress has been made. In patients with MF, JAK inhibition provides substantial symptomatic benefits, reduction in splenomegaly and improved survival, but no significant impact on the malignant clone. Thus, efforts to improve the natural history of the disease through targeting of JAK kinases and other targets continue [63].
Acknowledgments
The members of the 8th Post-ASH CML-MPN workshop comprised of: Chairs: J. Goldman*, T. Mughal, A. Tefferi; Faculty: O. Abdel-Wahab, R. Arlinghaus, T. Barbui, G. Barosi, R. Bhatia, J. Crispino, M. Deininger, C. Gambacort-Passerini, A. Green, O. Hantschel, R. Hehlmann, T. Holyoake, C. Jamieson, JJ Kiladjian, R. Kralovics, H.M. Kvasnicka, F. Mahon, G. Martinelli, R. Mesa, A. Mullaly, D. Perrotti, J. Prchal, F. Rasool, K. Bhalla, T. Odenike, H. Pahl, H. Gisslinger, M. Mauro, R. Rampal, G. Saglio, T. Shimoda, R. Silver, R. Skoda, T. Skorski, E. Solary, S. Soverini, J. Thiele, R. Tibes, R.A. Van Etten. The members wish to thank Alpine Oncology Foundation, in particular Dr. Alpa Parmar, who helped organize the workshop and Incyte Corporation (USA), Bristol-Myers Squibb Oncology and Gilead Corporation for their unrestricted educational support. *Dr. J. Goldman, who had been a co-chair with Dr. T. Mughal, since the inaugural workshop in 2006, died on 24th December 2013.
Footnotes
Dedicated to John M. Goldman
References
- 1.Mughal TI, Vannucchi AM, Soverini S, et al. Current pre-clinical and clinical advances in the BCR-ABL1-positive and -negative chronic myeloproliferative neoplasms. Haematologica. 2014;99:797–803. doi: 10.3324/haematol.2013.097832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Druker B, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1042. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 3.Cortes J, Hochhaus A, Kim DW, et al. Four-year (yr) follow-up of patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) receiving dasatinib or imatinib: efficacy based on early response. Blood. 2013;122(Suppl 1):Abstract 65. [Google Scholar]
- 4.Saglio G, Hochhaus A, Hughes T, et al. ENESTnd update: nilotinib (NIL) vs imatinib (IM) in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) and the impact of early molecular response (EMR) and Sokal risk at diagnosis on long-term outcomes. Blood. 2013;122(Suppl 1):Abstract 92. [Google Scholar]
- 5.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 6.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebocontrolled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 9.Rampal R, Al-Shahrour F, Abdel-Wahab O, et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood. 2014;123:e123–e133. doi: 10.1182/blood-2014-02-554634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klampf T, Gisslinger H, Hanutyunyan A, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379–2390. doi: 10.1056/NEJMoa1311347. [DOI] [PubMed] [Google Scholar]
- 11.Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutationsin myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369:2391–2405. doi: 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cazzola M, Kralovics R. From Janus kinase 2 to calreticulin: the clinically relevant genomic landscape of myeloproliferative neoplasms. Blood. 2014;123:3714–3719. doi: 10.1182/blood-2014-03-530865. [DOI] [PubMed] [Google Scholar]
- 13.Duek A, Lundberg P, Shimizu T, et al. Loss of Stat1 decreases megakaryopoiesis and favors erythropoiesis in a JAK2-V617F driven mouse model of myeloproliferative neoplasms. Blood. 2014;123:3943–3950. doi: 10.1182/blood-2013-07-514208. [DOI] [PubMed] [Google Scholar]
- 14.Mughal TI, Goldman JM. Why does CML evolve from a chronic phase to blast phase? Front Biosci. 2006;1:198–208. doi: 10.2741/1791. [DOI] [PubMed] [Google Scholar]
- 15.Tam CS, Nussenzveig RM, Popat U, et al. The natural history and treatment outcome of blast phase BCR-ABL-myeloproliferative neoplasms. Blood. 2008;112:1628–1637. doi: 10.1182/blood-2008-02-138230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennedy JA, Atenafu EG, Messner HA, et al. Treatment outcomes following leukemic transformation in Ph-negative myeloproliferative neoplasms. Blood. 2013;121:2725–2733. doi: 10.1182/blood-2012-10-464248. [DOI] [PubMed] [Google Scholar]
- 17.Zhang SJ, Rampal R, Manshouri T, et al. Genetic analysis of patients with leukemic transformation of myeloproliferative neoplasms shows recurrent SRSF2 mutations that are associated with adverse outcome. Blood. 2012;119:4480–4485. doi: 10.1182/blood-2011-11-390252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Q, Crews LA, Barrett CL, et al. ADAR-1 promotes malignant progenitor programming in chronic myeloid leukemia. Proc Natl Acad Sci USA. 2013;110:1041–1046. doi: 10.1073/pnas.1213021110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rampal R, Pandey S, Abdel-Wahab O, et al. Development of a murine model for leukemic transformation of myeloproliferative neoplasms for preclinical therapeutic studies. Blood. 2012;120(Suppl 1):Abstract 808. [Google Scholar]
- 20.Wang L, Swierczek SI, Lanikova L, et al. The relationship of JAK2V617F and acquired UPD at chromosome 9p in polycythemia vera. Leukemia. 2014;28:938–941. doi: 10.1038/leu.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Swierczek SI, Drummond J, et al. Whole-exome sequencing of polycythemia vera revealed driver genes and somatic mutation shared by T cells and granulocytes. Leukemia. 2014;28:935–938. doi: 10.1038/leu.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kent D, Green AR. Order matters: mutation order influences tumour evolution and stem cell potency by altering transcriptional consequences of the second mutation. Haematologica. 2014 EHA Abstract LBA2434. [Google Scholar]
- 23.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 24.Kvasnicka HM, Thiele J. Prodromal myeloproliferative neoplasms: the 2008 WHO classification. Am J Hematol. 2008;85:62–69. doi: 10.1002/ajh.21543. [DOI] [PubMed] [Google Scholar]
- 25.Barosi G, Poletto V, Massa M, et al. JAK2 V617F genotype is a strong determinant of blast transformation in primary myelofibrosis. PLoS One. 2013;8:e59791. doi: 10.1371/journal.pone.0059791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thiele J, Kvasnicka HM, Vardiman J. Bone marrow histopathology in the diagnosis of chronic myeloproliferative disorders: a forgotten pearl. Best Pract Res Clin Haematol. 2006;19:413–437. doi: 10.1016/j.beha.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Thiele J, Kvasnicka HM, Diehl V. Standardization of bone marrow features - does it work in hematopathology for histological discrimination of different disease patterns? Histol Histopathol. 2005;20:633–644. doi: 10.14670/HH-20.633. [DOI] [PubMed] [Google Scholar]
- 28.Thiele J, Kvasnicka HM, Mullauer L, et al. Essential thrombocythemia versus early primary myelofibrosis: a multicenter study to validate the WHO classification. Blood. 2011;117:5710–5718. doi: 10.1182/blood-2010-07-293761. [DOI] [PubMed] [Google Scholar]
- 29.Barbui T, Thiele J, Gisslinger H, et al. Masked polycythemia vera (mPV): results of an international study. Am J Hematol. 2014;89:52–54. doi: 10.1002/ajh.23585. [DOI] [PubMed] [Google Scholar]
- 30.Ejerblad E, Kvasnicka HM, Thiele J, et al. Diagnosis according to World Health Organization determines the long-term prognosis in patients with myeloproliferative neoplasms treated with anagrelide: results of a prospective long-term follow-up. Hematology. 2013;18:8–13. doi: 10.1179/1607845412Y.0000000023. [DOI] [PubMed] [Google Scholar]
- 31.Gisslinger H, Gotic M, Holowiecki J, et al. Anagrelide compared with hydroxyurea in WHO-classified essential thrombocythemia: the ANAHYDRET study, a randomized controlled trial. Blood. 2013;121:1720–1728. doi: 10.1182/blood-2012-07-443770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thiele J, Orazi A, et al. European LeukemiaNet (ELN) consensus criteria for therapy response based on bone marrow features in patients with myelofibrosis. J Clin Oncol to Leukemia. in press. [Google Scholar]
- 33.Spivak JL. Polycythemia vera: myths, mechanisms, and management. Blood. 2002;100:4272–4290. doi: 10.1182/blood-2001-12-0349. [DOI] [PubMed] [Google Scholar]
- 34.Barbui T, Thiele J, Carobbio A, et al. Masked polycythemia vera diagnosed according to WHO and BCSH classification. Am J Hematol. 2014;89:199–202. doi: 10.1002/ajh.23617. [DOI] [PubMed] [Google Scholar]
- 35.Deshpande A, Reddy MM, Schade GOM, et al. Kinase domain mutations confer resistance to novel inhibitors targeting JAK2V617F in myeloproliferative neoplasms. Leukemia. 2012;26:708–715. doi: 10.1038/leu.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marty C, Saint-Martin C, Pecquet C, et al. Germ-line JAK2 mutations in the kinase domain are responsible for hereditary thrombocytosis and are resistant to JAK2 and HSP90 inhibitors. Blood. 2014;123:1372–1383. doi: 10.1182/blood-2013-05-504555. [DOI] [PubMed] [Google Scholar]
- 37.Meyer S, Levine R. Molecular pathways: molecular basis for sensitivity and resistance to JAK kinase inhibitors. Clin Cancer Res. 2014;20:2051–2059. doi: 10.1158/1078-0432.CCR-13-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andreoli A, Verger E, Robin M, et al. Clinical resistance to ruxolitinib is more frequent in patients without MPN-associated mutations and is rarely due to mutations in the JAK2 kinase drug binding domain. Blood. 2013;122(Supp 1):Abstract 1591. [Google Scholar]
- 39.Weigert O, Lane A, Bird J, et al. Genetic resistance to JAK2 enzymatic inhibitors is overcome by HSP90 inhibition. J Exp Med. 2012;209:259–273. doi: 10.1084/jem.20111694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koppikar P, Bhagwat N, Kilpivaara O, et al. Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature. 2012;489:155–159. doi: 10.1038/nature11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cervantes F, Vannucchi AM, Kiladjian J-J, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122:4047–4053. doi: 10.1182/blood-2013-02-485888. [DOI] [PubMed] [Google Scholar]
- 42.Verstovsek S, Mesa R, Gotlib J, et al. Long-term outcomes of ruxolitinib therapy in patients with myelofibrosis: 3-year update from COMFORT-I. Blood. 2013;122(Suppl 1):Abstract 396. [Google Scholar]
- 43.Kvasnicka HM, Thiele J, Bueso-Ramos CE, et al. Effects of five-years of ruxolitinib therapy on bone marrow morphology in patients with myelofibrosis and comparison with best available therapy. Blood. 2013;122(Suppl 1) Abstract 4055. [Google Scholar]
- 44.Harrison CN, Cortes JE, Cervantes F, Mesa, et al. Results of a randomized, double-blind, placebo-controlled phase III study (JAKARTA) of the JAK2-selective inhibitor fedratinib (SAR302503) in patients with myelofibrosis (MF) Blood. 2013;122(Suppl 1) Abstract 393. [Google Scholar]
- 45.Mesa R, Cortes JE, Cervantes F, et al. Symptom burden and health-related quality of life (HRQoL) in patients with myelofibrosis (MF) treated with fedratinib (SAR302503) in a phase III study (JAKARTA) Blood. 2013;122(Suppl 1) Abstract 4061. [Google Scholar]
- 46.Harrison C, Schaap NP, Zweegman S, et al. Efficacy and safety of fedratinib (SAR302503/TG101348) in patients with intermediate or high-risk myelofibrosis (MF), post polycythemia vera (PV) MF, or post-essential thrombocythemia (ET) MF previously treated with ruxolitinib: interim results from a phase II study (JAKARTA-2) Blood. 2013;122(Suppl 1) Abstract 661. [Google Scholar]
- 47.Zhang Q, Zhang Y, Diamond S, et al. The Janus kinase 2 inhibitor fedratinib inhibits thiamine uptake: a putative mechanism for the onset of Wernicke’ s encephalopathy. Drug Metab Dispos. 2014;42:1656–1662. doi: 10.1124/dmd.114.058883. [DOI] [PubMed] [Google Scholar]
- 48.Dean JP, Cernohous P, Komrokji RS, et al. Pacritinib, a dual JAK2/FLT3 inhibitor: an integrated efficacy and safety analysis of phase II trial data in patients with primary and secondary myelofibrosis (MF) and platelet counts <100,000/μl. Blood. 2013;122(Suppl 1) Abstract 395. [Google Scholar]
- 49.Pardanani A, Laborde RR, Lasho TL, et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia. 2013;27:1322–1327. doi: 10.1038/leu.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verstovsek S, Mesa RA, Salama ME, et al. Phase I study of LY2784544, a JAK2 selective inhibitor, in patients with myelofibrosis (MF), polycythemia vera (PV), and essential thrombocythemia (ET) Blood. 2013;122(Suppl 1) Abstract 665. [Google Scholar]
- 51.Pardanani A, Roberts AW, Seymour JF, et al. BMS-911543, a selective JAK2 inhibitor: a multicenter phase 1/2a study in myelofibrosis. Blood. 2013;122(Suppl 1) Abstract 664. [Google Scholar]
- 52.Mascarenhas J, Talpaz M, Gupta V, et al. An open-label, phase II study of the JAK1 inhibitor INCB039110 in patients with myelofibrosis. Blood. 2013;122(Suppl 1) Abstract 663. [Google Scholar]
- 53.Tefferi A, Begna K, Laborde RR, et al. Imetelstat, a telomerase inhibitor, induces morphologic and molecular remissions in myelofibrosis and reversal of bone marrow fibrosis. Blood. 2013;122(Suppl 1) Abstract 662. [Google Scholar]
- 54.Birgegard G. Does anything work for anemia in myelofibrosis? Best Pract Res Clin Haematol. 2014;27:175–185. doi: 10.1016/j.beha.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 55.Mahon FX, Etienne G. Deep molecular response in chronic myeloid leukemia: the new goal therapy? Clin Cancer Res. 2014;20:310–322. doi: 10.1158/1078-0432.CCR-13-1988. [DOI] [PubMed] [Google Scholar]
- 56.Experts in Chronic Myeloid Leukemia. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood. 2013;121:4439–4442. doi: 10.1182/blood-2013-03-490003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baccarani M, Soverini S. Molecular response in CML: where is the bar? Blood. 2014;124:469–471. doi: 10.1182/blood-2014-06-578617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hehlmann R, Muller MC, Lauseker M, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high dose imatinib: results from the randomized CML-Study IV. J Clin Oncol. 2014;32:415–423. doi: 10.1200/JCO.2013.49.9020. [DOI] [PubMed] [Google Scholar]
- 60.Kantarjian HM, Cortes JE, Kim DW, et al. Bosutinib safety and management of toxicity in leukemia patients with resistance or intolerance to imatinib and other tyrosine kinase inhibitors. Blood. 2014;123:1309–1318. doi: 10.1182/blood-2013-07-513937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agarwal A, Bumm TG, Corbin AS, et al. Absence of SKP2 expression attenuates BCR-ABL-induced myeloproliferative disease. Blood. 2008;112:1960–1970. doi: 10.1182/blood-2007-09-113860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roy A, Banerjee S. p 27 and leukemia: cell cycle and beyond. J Cell Physiol. 2014 Sep 10; doi: 10.1002/jcp.24819. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 63.Stein BL, Swords R, Hochhaus A, et al. Novel myelofibrosis treatment strategies: potential partners for combination therapies. Leukemia. 2014 Jun 3; doi: 10.1038/leu.2014.176. Epub ahead of print. [DOI] [PubMed] [Google Scholar]