Abstract
Background
The accuracy of physical activity (PA) monitors to discriminate between PA, sedentary behavior, and non-wear in extremely obese (EO) adolescents is unknown.
Methods
Twenty-five subjects (9 male/16 female; age=16.5±2.0 y; BMI=51±8 kg/m2) wore three activity monitors (StepWatch [SAM], Actical [AC], Actiheart [AH]) during a 400 meter walk test (400MWT), two standardized PA bouts of varying duration, and one sedentary bout.
Results
For the 400MWT, percent error between observed and monitor recorded steps was 5.5±7.1% and 82.1±38.6% for the SAM and AC steps, respectively (observed vs. SAM steps: −17.2±22.2 steps; observed vs. AC steps: −264.5±124.8 steps). All activity monitors were able to differentiate between PA and sedentary bouts but only SAM steps and AH heart rate were significantly different between sedentary behavior and non-wear (p<0.001). For all monitors, sedentary behavior was characterized by bouts of zero steps/counts punctuated by intermittent activity steps/counts; non-wear was represented almost exclusively by zero steps/counts.
Conclusion
Of all monitors tested, the SAM was most accurate in terms of counting steps and differentiating levels of PA, and thus, most appropriate for EO adolescents. The ability to accurately characterize PA intensity in EO adolescents critically depends on activity monitor selection.
Keywords: validation, obesity, sedentary
Introduction
National survey data indicate that approximately 12.5 million U.S. children and adolescents (age 2–19 y) are obese, 1.8 million of whom are considered severely obese (body mass index [BMI] > 120% of the 95th percentile or ≥35 kg/m2).1, 2 Obesity established by adolescence strongly predicts obesity for the remainder of adult life,3 and the consequences of pediatric obesity are potentially medically and economically devastating. Obese children potentially face a lifelong burden of co-morbid medical and psychosocial conditions.
Few successful treatments are available for moderate to severe obesity in children and adolescents, and both behavioral and non-surgical medical treatment options have had limited success.4 Though still relatively rare, adolescent bariatric surgery is gaining acceptance as an appropriate treatment course for extremely obese adolescents (EO, BMI ≥ 99th percentile for age and gender) who have failed traditional intervention options. In general, surgery-induced weight loss is far superior to other treatment options for extreme obesity. However, there is great variability in terms of initial weight loss and weight loss maintenance.5–7 Given the important role that physical activity (PA) plays in weight management, and in particular, in long-term weight maintenance following non-surgical weight loss,8 it is hypothesized that long-term successful weight loss following bariatric surgery is highly dependent upon physical activity behavior. Thus, the ability to objectively and accurately monitor PA is critically important in determining factors that influence the long-term effectiveness of bariatric surgery, as well as other treatments for EO adolescents.
Due to weight-related gait abnormalities and slower walking speed associated with severe obesity,9–11 there is reason to question the ability of most activity monitors to accurately measure PA behavior in this unique population.12–14 Given the highly sedentary nature of the EO population,15 of particular concern, is the ability of activity monitors to accurately assess low-intensity PA, which has health benefits when it replaces sedentary behaviors.16 Thus, a critical analytical issue associated with interpretation of PA monitor data is differentiating between low-intensity PA, and sedentary behaviors, as well as monitor non-wear and/or monitor error.17–20
To date, there are no published data comparing the ability of objective activity monitoring to measure moderate-intensity PA or differentiate between low-intensity PA, sedentary behavior, and non-wear time in EO adolescents. This study sought to address these limitations in the literature by 1)evaluating the ability of activity monitors to accurately characterize total steps during a well-defined 400 meter walk test (400MWT) protocol, and 2) determining whether periods of monitor non-wear, sedentary behaviors, low-intensity PA, and moderate-intensity PA could be accurately identified from the activity monitor outputs of EO adolescents. Three activity monitors were evaluated. The StepWatch was selected because it has been shown to be accurate at slow walking speeds, in obese adults, and adults with usual gaits.21 The Actical monitor was selected because it has been previously used in the extremely obese adolescent population.22 The Actiheart monitor was selected because of its ability to assess heart rate, which is a good indicator of monitor non-wear. This study provides a basis for scientifically valid interpretation of monitor data collected during interventions for weight loss in EO populations.
Methods
The Teen Longitudinal Assessment of Bariatric Surgery (Teen-LABS) is a multi-center observational study examining the outcomes of EO adolescents undergoing weight loss surgery.23 The focus of the study includes safety and efficacy, with an emphasis on both metabolic and psychosocial outcomes. Subjects were identified from a cohort of EO adolescents seeking weight loss surgery at the Surgical Weight Loss Program for Teens (SWLPT) program at Cincinnati Children’s Hospital Medical Center (CCHMC). Sequential subjects were recruited during their pre-operative treatment phase, and the study visit was scheduled to coincide with a pre-operative clinical visit during a 3–6 month period of preparation prior to bariatric surgery. Based on the anticipated association between recorded and manually counted steps, 22 subjects were estimated to be required to detect a correlation coefficient of 0.6 between these two measures with 80% power, assuming an alpha of 0.017. Although 22 subjects were estimated to be required, a total of 25 subjects were included in the study to offset any potentially incomplete data (i.e., device malfunction); this sample size is sufficient to detect a correlation as low as 0.57 with 80% power. Inclusion criteria were age 13–19 years and established clinical criteria (e.g., presence of EO with obesity related co-morbid conditions) for acceptance into the surgical program.24 Subjects were excluded if they experienced any of the following: 1) inability to attempt the walk tests due to use of a wheelchair, walker, or cane; 2) elevated blood pressure (systolic blood pressure >180 mmHg or diastolic blood pressure >100 mmHg); 3) resting heart rate >130 or <40 beats per minute; 4) significant electrocardiogram (ECG) findings in the last 12 months; or 5) other contraindication for exertion. The CCHMC Institutional Review Board approved this study, and informed consent/assent was obtained from each subject and at least one of his/her parents or legal guardians.
Subjects were asked to wear three activity monitors, the StepWatch (SAM, Orthocare Innovations, Oklahoma City, OK), the Actical (AC, Philips Resprionics, Murrysville, PA), and the Actiheart (AH, CamNtech Inc. Boerne,TX). A standardized protocol was employed that included varying levels of PA intensity utilizing available clinic space to mimic real life movements. The SAM is a dual axial accelerometer (75×50×20 mm) and is worn at the ankle; its measurements are recorded in steps. The AC tri-axial accelerometer is a small (approximately 30 mm2) device that is worn on the hip and measures changes in the wearer’s acceleration and converts these measures to both steps (meant to represent actual steps taken) and activity counts (i.e. summed measures of movement velocity in multiple planes). The AH activity monitor is strapped to the chest and measures activity counts and heart rate. Both steps and activity counts are used in the literature to characterize movement in free-living populations. Heart rate, measured in beats per minute (bpm), can be used to describe PA intensity when evaluated as a percentage of maximum heart rate, which we estimated as 200-(age*0.5).25
To assess the performance of the electronic monitors under conditions that simulate real-life scenarios, data were captured during the following five defined conditions : 1) a 400 meter walk test (400MWT); 2) a short bout of PA represented by a “lifestyle walk” to the blood pressure assessment station, simulating movement from one room to another in a home (approximately 15 meters, completion time approximately 1 minute); 3) a longer bout of PA with stairs represented by a walk down and up one flight of stairs to the hospital pharmacy, simulating walking between classes at school (approximately 250 meters, completion time approximately 5 minutes); 4) an observed period of sedentary behavior in which subjects remained seated in the clinic examination room for at least 15 minutes while talking to a member of the clinical team; and 5) non-wear, with the fully operational monitors placed on a counter-top in the clinic examination room for at least 15 minutes simulating time during which subjects using the monitors in their home might remove the monitor for bathing. Monitors were programmed with subject data (clinical height and weight) the morning of the study visit and synchronized for time using monitor software and a laptop personal computer using standard settings. All monitor data were reduced to 1-minute epochs prior to analysis.
The 400MWT is a valid measure of cardio-respiratory fitness and mobility deficits used extensively in geriatric populations;26 its use is gaining acceptance for the pediatric population27 and was selected in the present study as an alternative to treadmill testing due to the subjects’ generally limited ability to maintain pace or balance. During the 400MWT, subjects were instructed to walk, at their usual pace, a total of ten, consecutive 40 m laps. Heart rate was recorded at baseline (prior to the test) and immediately following completion of the walk. Time to test completion and number of times needed to rest (if any) during the test were also recorded. A research staff member walked slightly behind the subject counting total steps during the 400MWT with a metal mechanical counter (Staples, Boston MA).
Data were analyzed using Stata Statistical Analysis software version 11 (StataCorp, College Station, TX). Basic descriptive statistics were calculated for all demographic information (sex, race, age, weight, height and calculated BMI). Repeated measures analysis of variance (ANOVA) was used to examine the association between observed steps and activity monitor steps during the 400MWT. Percent error was calculated as ([monitor steps − observed steps]/observed steps) × 100%. Oneway ANOVA was used to examine percent error by race (African American vs. not African-Amercican) gender, and severity of obesity (extreme obesity defined as BMI ≥50 kg/m2 vs. BMI below 50 kg/m2). Bland-Altman analysis was used to examine level of agreement and potential bias between observed and recorded steps. Repeated measures ANOVA was used to examine the ability of the monitors to differentiate between various levels of PA and non-wear in terms of steps, activity counts, and heart rate. Statistical significance was defined as p<0.05.
Results
Basic descriptive statistics for all subjects are provided in Table 1. The mean BMI of these subjects was 50.7±7.8 kg/m2 (40% (n=10) of subjects had a BMI ≥ 50 kg/m2). A total of 25 subjects completed the study, with 24 having complete SAM data (one monitor failure), 19 having complete AC data (monitor shipping delay at start of study), and 25 having complete AH data.
Table 1.
Descriptive Statistics for Subjects by Gender (mean ± SD unless otherwise noted)
| Males (n=9) | Females (n=16) | Total (N=25) | p (for gender) | |
|---|---|---|---|---|
| Age, yr | 16.8 ± 2.3 | 16.3 ± 1.9 | 16.5 ±2.1 | 0.59 |
| Weight, kg | 158.3 ± 28.0 | 143.1 ± 22.4 | 148.6 ± 25.1 | 0.15 |
| Height, cm | 176.8 ± 6.5 | 167.9 ± 9.1 | 171.1 ± 9.2 | 0.01 |
| BMI, kg/m2 | 50.7 ± 9.2 | 50.7 ± 7.2 | 50.7 ± 7.8 | 0.98 |
|
Race, n (%) African American |
2 (22.2) | 4 (25.0) | 6 (24.0) | 0.88 |
SD=Standard Deviation, BMI=body mass index, HR=heart rate, bpm=beats per minute
Validation against observed steps
During the 400MWT, the average number of steps was 327.9 ± 35.2 steps, 343.75 ± 35.7 steps, and 587.05 ± 133.2 steps for observed steps, SAM steps, and AC steps, respectively. Total SAM steps, but not AC steps, were not significantly different from observed steps (SAM: F1,22=40.5, p<0.001; AC: F1,17=2.92, p=0.10) for the 400MWT. However, mean differences between observed steps and the two monitors differed greatly (observed vs. SAM steps: −17.2 ± 22.2 steps; observed vs. AC steps: −264.5 ± 124.8 steps). Percent error between observed and monitor recorded steps was 5.5 ± 7.1 % and 82.1 ± 38.6 % for the SAM and AC steps, respectively. Percent errors did not significantly differ by gender, race, or extreme obesity status (p>0.05). Bland-Altman analysis indicated that there was no bias (F1,22=0.01, p=0.91) between observed and SAM steps (Figure 1). There was, however, significant bias (F1,17=90.1, p<0.001) in AC steps versus observed steps (Figure 2), with increasing differences between the two measures as the number of steps increased.
Figure 1.
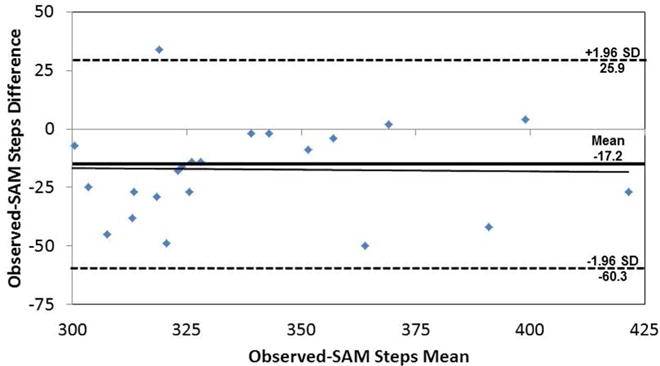
Bland-Altman analysis for observed versus SAM steps for 400MWT. The regression line is represented as a solid black line, and the mean difference is represented by a solid, bold line with ± 1.96 standard deviations represented by the dashed lines.
Figure 2.
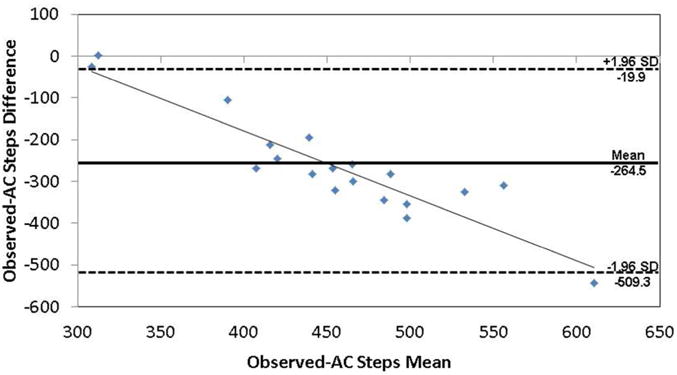
Bland-Altman analysis for observed versus AC steps for 400MWT. The regression line is represented as a solid black line, and the mean difference is represented by a solid, bold line with ± 1.96 standard deviations represented by the dashed lines.
Using heart rate to differentiate activity levels and non-wear
Mean heart rate recorded by the AH monitor is shown in Figure 3. Mean heart rate and activity intensity, as defined by percentage of age-predicted maximum heart rate, were not significantly different (p>0.05) between the short (106.2 ± 20.9 bpm, 53.4 ± 10.5%) and longer duration activity with stairs (111.2 ± 34.0 bpm, 58.1 ± 13.1%) and the 400MWT (104.9 ± 37.4 bpm, 54.9 ± 15.5%), all of which would be considered moderate-intensity PA based on the recorded heart rates. Heart rate for sedentary behavior (83.0 ± 21.0 bpm, 41.7 ± 10.6%) in this adolescent EO population was significantly lower than all three active conditions (p<0.01). As expected, average heart rate output during non-wear was effectively non-detectable (2.5 ± 5.0 bpm).
Figure 3.
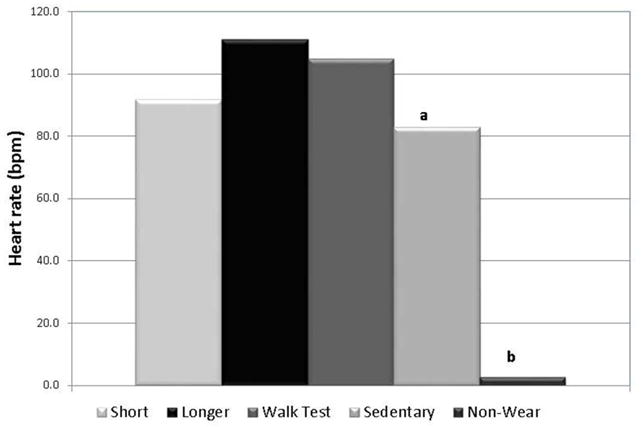
Heart rate recorded by the AH monitor across activity bouts. a=significantly different from longer duration activity (p<0.01); b=significantly different from all other activity levels.
Ability to differentiate between activity levels
Figure 4, panels A–D, show differences in average recorded steps and counts across the various levels of activity and non-wear for each of the monitors examined. Mean steps or activity counts per minute were significantly higher for the longer activity bout with stairs compared to the short activity, sedentary and non-wear bouts (p<0.01). Mean steps/activity counts per minute, as determined by the SAM and AC, were significantly higher for the short activity bout compared to the sedentary and non-wear bouts (p<0.01). Only the AH activity counts demonstrated no significant difference between the short activity and sedentary bouts, primarily due to the large variability in AH counts for the short activity bout.
Figure 4.
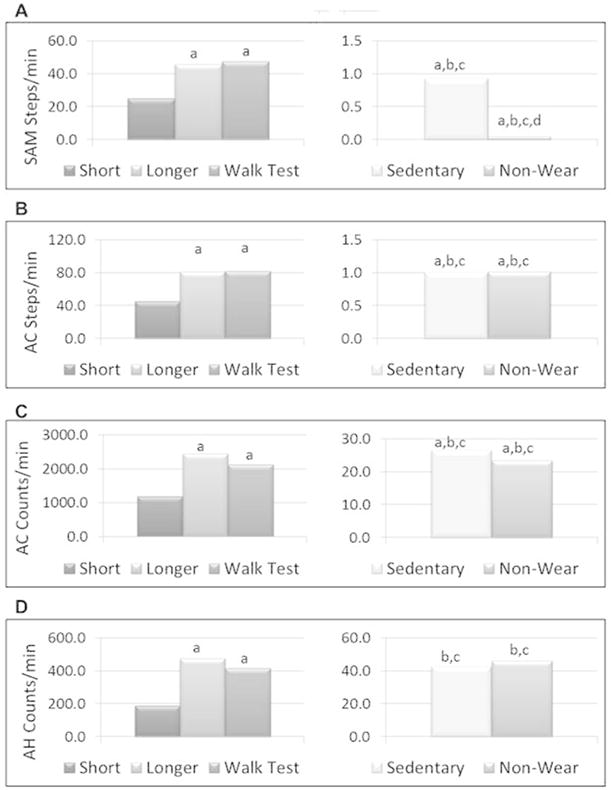
Ability of step- and count-based monitors to differentiate across activity bouts: (A) SAM steps; (B) AC steps; (C) AC activity counts; (D) AH activity counts. a=significantly different from low activity (p<0.01); b=significantly different from longer duration activity (p<0.01); c=significantly different from walk test (p<0.01); d=significantly different from sedentary behavior (p<0.01).
Differentiating sedentary behavior from non-wear
Mean steps per minute, as determined by the SAM, were significantly higher for the sedentary bout compared to the non-wear bout (Figure 4A, p<0.001). Surprisingly, steps and/or activity counts were not significantly different between sedentary and non-wear bouts for any other monitor (Figure 4B–D, p>.05). Nevertheless, the percent of total time in which the monitors read zero steps or counts during the sedentary compared to the non-wear bout was significantly different (p<0.001) for all monitors (Table 2).
Table 2.
Percentage of Monitor Time with Zero Steps or Counts (mean ± SD)
| Sedentary Behavior | Non-wear | |
|---|---|---|
| SAM Steps | 77.4% ± 14.5% | 99.3% ± 3.1%* |
| AC Steps | 94.0% ± 3.4% | 96.3% ± 5.7%* |
| AC Activity Counts | 72.8% ± 16.1% | 94.0% ± 7.7%* |
| AH Activity Counts | 31.6% ± 21.0% | 86.5% ± 20.0%* |
p≤0.001 compared to sedentary behavior; SD = Standard Deviation, SAM = Stepwatch Activity Monitor, AC = Actical, AH = Actiheart
Discussion
The present study provides an objective assessment of three activity monitors in a controlled setting in which periods of sedentary behavior, short and longer bursts of activity, and non-wear were carefully defined and correlated to monitor output. In this study, a substantial difference in the agreement of the SAM and AC monitors with observed steps was observed, with significant error bias and overestimation noted for AC steps but not for SAM steps. All monitors were able to differentiate between bouts of sedentary behavior and moderate-intensity PA in terms of steps, activity counts and/or heart rate beats per minute. Nevertheless, only SAM steps per minute and AH heart rate beats per minute were significantly different between bouts of sedentary behavior and non-wear. For all three monitors the percentage of time with zero counts/steps differed between behaviors, with a significantly lower percent time when the monitors were registering zero counts for sedentary behavior compared to non-wear. Thus, defining non-wear may be improved by taking into account the number of steps/counts in an individual minute, as well as examining the pattern of steps/counts over time.
The treatment of extreme obesity in adolescents is immensely challenging, and this population is expanding at an alarming rate. Presently, it is not known to what extent the basic components of energy balance (i.e., physical activity and nutrition) influence the development of extreme, early-onset obesity and/or response to obesity interventions in EO adolescents. Recent systematic reviews provided evidence that weight loss following bariatric surgery was positively associated with PA level and that PA level tended to increase following weight loss surgery.28, 29 However, all of the reviewed studies were observational and many were cross-sectional. Only one of the reviewed studies that examined the relationship between PA and weight loss utilized objective monitoring (pedometers); thus, the studies provided primarily qualitative self-assessments of PA and pointed to the need for better, more unbiased tools for assessing total activity as well as activity patterns.30
Like their adult counterparts,31 EO adolescents are hypothesized to experience long periods of sedentary behaviors associated with the physical limitations related to massive weight, making characterization of their activity patterns before and after obesity treatment particularly problematic. Accurately assessing the movement patterns of individuals with very low-intensity or infrequent PA is highly dependent upon monitor accuracy and sensitivity, as well as the processing algorithms used to interpret the monitor data. Potential sources of activity monitor error include sampling time frame, data reduction strategies, monitor function/placement, and the characteristics of the individuals being measured (e.g., large waist circumference, abnormal gait in EO individuals).
In terms of sampling time frame, the present study utilized a relatively short period of activity assessment to examine the ability of the monitors to differentiate between activity levels in a controlled environment. Within this time frame, all monitors were able to differentiate short and longer durations of moderate intensity from sedentary behavior. However, there is evidence that for free-living activity assessment, longer time frames may be needed to more accurately characterize activity patterns, in particular sedentary behavior and non-wear. For example, when investigating differences in accelerometer wear and non-wear patterns in 49 adults and 76 youth who spent 24h in a whole-room calorimeter, Choi et al.32 found that investigating the non-zero counts in the 30 minutes before or after a specified number of zero counts improved the estimation of time spent in sedentary behaviors.32 Our results also suggest that activity count/step patterns (number of zeros combined with punctuated activity) are important to consider, as steps/counts per minute between sedentary and non-wear bouts were not significantly different for some monitors, while percentage of time with no steps/counts was.
Data reduction algorithms are another possible source of error and have a tremendous potential to bias the ability to differentiate when a subject is sedentary for a long period of time from when they actually remove the monitor. In the adult bariatric surgery literature, the cut points of uninterrupted zero steps or counts for the characterization of non-wear range from 30 minutes15 to 2 hours33, possibly reflecting the use of different monitors. Using the SAM (which measures steps per minute), King et al.17 examined the impact of applying various minimum durations of monitor inactivity (i.e., 60, 90, 120, 150 minutes) to estimate non-wear on physical activity parameters. The investigators demonstrated that applying a ≥ 60 minute duration of inactivity to define non-wear produced unlikely non-wear patterns, while a two hour duration produced more reasonable wear and non-wear estimates. Importantly, there was a significant difference between physical activity parameters (e.g. minutes active/day, percentage time active/day) between data processed with the various non-wear rules.17 In contrast, Miller et al.34 concluded the opposite; they reported only minimal differences in activity patterns when different algorithms were utilized to identify non-wear in a cohort of overweight and obese individuals with type 2 diabetes.
Presently, the majority of data reduction algorithms are population-specific, and there is a need for standardized methods to analyze/reduce and interpret the data that are obtained from activity monitors.35–39 Data reduction algorithms typically require 3–5 days of monitor data with a minimum of 8–10 hours of wear time to characterize PA patterns.19 Tudor-Lock et al. has shown that some reduction techniques in adults19 and in children40 create a bias towards under-representing less active individuals. Unfortunately, analyses from uncensored data may also produce unrealistic results, suggesting, on average, adult Americans achieve 10,000 steps per day.19 Determining appropriate data processing algorithms is important to unbiased interpretation of the data collected.
Activity monitors have been demonstrated to vary widely in their accuracy in assessing physical movement. Issues related to the type and intensity of activity, as well as the placement and angle of the monitors, may be important in accurately assessing movement, particularly for obese individuals. Tyo et al.41 compared two pedometers (New Lifestyles NL-2000 and the DigiWalker SW-200) to the SAM in 74 normal weight, overweight weight and obese adults and reported that the NL-2000 and DigiWalker pedometers both underestimated the number of steps compared to the SAM (in contrast to our study in which the AC produced more steps than both observed or SAM steps). Significantly greater error was observed for the obese group (BMI 33.6 ± 6.6 kgm2), compared to normal and overweight individuals,41 suggesting waist-worn monitors might be more affected by weight status than ankle-mounted monitors. In another study, the ActiGraph GT1M, NL-2000, AC, and SAM were examined for accuracy in assessing treadmill walking across a range of BMI and walking speeds.42 Walking speed but not BMI was found to influence the accuracy of the ActiGraph GT1M, NL-2000, and AC, such that monitors underestimated actual steps at slower walking speeds. The SAM most accurately assessed steps across a wide range of walking speeds, and was the only monitor to not differ significantly from observed steps at the slowest walking speed.42 Kinnunen et al. measured activity levels of overweight and obese pregnant women and reported significant correlations between pedometers and accelerometers; however, the direction of the relationship and amount correlation was highly dependent on intensity of activity, and the agreement between the two measures varied widely making it difficult to characterize the women as active or inactive.43
In terms of monitor placement, Feito et al. reported that an absolute tilt angle <10 degrees for the AC monitor resulted in significantly higher activity counts in obese compared to the normal weight individuals (P=0.01).44 In a study of normal weight, overweight, and obese children (N=77, 10–12 y), varying the physical placement of pedometers produced significant differences in monitor accuracy, with anterior thigh and right side of the waist providing the most accurate assessments of steps compared to navel, back or posterior thigh.45 The studies above illustrate the challenges in objectively assessing PA in EO adolescents, which are compounded by the extremely sedentary nature this population.
Consistent with the studies described above, the SAM was most accurate in assessing actual steps and in differentiating various levels of activity and non-wear in our EO population. The SAM may be a better device in obese adolescents compared to other types of monitors due to its placement at the ankle, particularly for assessing walking behavior. For other types of behavior, the use of multiple monitors that assess different types of parameters (eg., movement and heart rate) may provide more accurate characterization of differences in PA.
Only the SAM steps per minute and the AH heart rate beats per minute were able to differentiate between non-wear and observed sedentary behavior. More research is therefore needed to understand why and how monitors may continue to accumulate steps and/or counts during periods of non-wear. In our study, activity counts registered while the monitors were recording but were not worn may represent the monitors’ sensitivity to vibration or other movement not associated with PA. This has implications for study design as some studies include transfer of monitors to and from participants via shipping services.
Limitations and Strengths
This study used existing clinic time, activities and space to assess PA. While the design minimized subject burden, it allowed for variation in the distances traveled within a specified condition, (e.g. distances from one exam room to the blood pressure monitoring station were not uniform). In addition, as determined by heart rate assessment, the design did not include a low-intensity condition (i.e. <50% of maximum heart rate), although the short duration condition was walked, on average, at a cadence of 25 steps/min according to the SAM. In addition, this study used “standard” sensitivity settings with the SAM instead of setting the monitor’s sensitivity settings per the individuals walking style, which may have reduced its accuracy in counting steps. Strengths of the study include an objective assessment of three different types of activity monitors in a controlled setting in which “real life” activity behaviors were modeled and correlated to monitor output in an understudied population. Although the sample size was small and not all participants wore all monitors, this study provides substantial insight in the ability of objective monitoring to assess sedentary behavior in EO adolescents.
Conclusions
Given the role of PA in optimizing weight loss and preventing weight regain, assessment tools are needed to accurately characterize both PA and sedentary behavior, particularly in obese individuals. All activity monitors evaluated in this study could differentiate between sedentary and moderate-intensity activity. Step-based activity monitors, with and without heart rate monitoring, varied in their accuracy for assessing sedentary behavior. In EO adolescents, sedentary behavior was represented by a substantial percentage of zero steps/activity counts punctuated by periods of counts that were distinct from non-wear. These results suggest that the low activity counts observed in the EO participants may represent true sedentary behavior. Future advances in monitor technology may minimize wear/non-wear issues, such as removal for bathing or water activities. For example, the Sensewear Armband™ includes sensors that detect skin temperature and sweat rate, in addition to movement, potentially providing more accurate information about non-wear versus sedentary behavior. Unfortunately, until the point where technology can completely overcome all human influences, appropriate interpretation of activity monitor data is crucial for understanding human activity patterns. Especially important is differentiating low levels of activity versus sedentary activities. This study has shown that concerns about differentiating sedentary behavior and non-wear can be mitigated through monitor choice (e.g. SAM) or through the use of a combination of activity and heart rate monitors.
Acknowledgments
This study was conducted as a cooperative agreement and funded by the National Institute of Diabetes and Digestive and Kidney Diseases with a grant to Cincinnati Children’s Hospital Medical Center (Dr. Thomas Inge, PI; U01 DK072493) and Supplement (American Recovery and Reinvestment Act of 2009 (“Recovery Act” or “ARRA” and Data Coordinating Center U01 DK095710). We gratefully acknowledge the significant contributions made by the Teen-LABS Consortium, as well as our parent study LABS Consortium (U01 DK066557).
Funding Source
This study was conducted as a cooperative agreement and funded by the National Institute of Diabetes and Digestive and Kidney Diseases with a grant to Cincinnati Children’s Hospital Medical Center (Dr. Thomas Inge, PI; U01 DK072493 and DCC grant) and Supplement (American Recovery and Reinvestment Act of 2009 (“Recovery Act” or “ARRA”).
Contributor Information
Renee M. Jeffries, Email: rmjeffreys@gmail.com, Cincinnati Children’s Hospital Medical Center.
Thomas H. Inge, Email: Thomas.Inge@cchmc.org, Cincinnati Children’s Hospital Medical Center.
Todd M Jenkins, Email: todd.jenkins@cchmc.org, Cincinnati Children’s Hospital Medical Center.
Wendy King, Email: KingW@edc.pitt.edu, University of Pittsburgh.
Vedran Oruc, Email: orucved@uab.edu, University of Alabama at Birmingham Medical School.
Andrew D. Douglas, Email: A346andy@uab.edu, University of Alabama at Birmingham School of Public Health.
Molly Bray, Email: mbray@uab.edu, Professor, Dept of Epidemiology, Director, Heflin Center for Genomic Sciences Genomics Core Lab, University of Alabama at Birmingham, Physical address: 720 20th Street S, Kaul 420A, Mailing address: 1720 2nd Avenue S, RPHB 230H, Birmingham, AL 35294, Office: (205) 975-7651, Fax: (205) 934-8665.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. Jama. 2006 Apr 5;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Wang YC, Gortmaker SL, Taveras EM. Trends and racial/ethnic disparities in severe obesity among US children and adolescents, 1976–2006. Int J Pediatr Obes. 2010 Mar 17; doi: 10.3109/17477161003587774. [DOI] [PubMed] [Google Scholar]
- 3.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007 Jan;150(1):12–17 e12. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 4.Inge TH, Krebs NF, Garcia VF, et al. Bariatric surgery for severely overweight adolescents: concerns and recommendations. Pediatrics. 2004 Jul;114(1):217–223. doi: 10.1542/peds.114.1.217. [DOI] [PubMed] [Google Scholar]
- 5.Christou NV, Look D, Maclean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006 Nov;244(5):734–740. doi: 10.1097/01.sla.0000217592.04061.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005 Apr 5;142(7):547–559. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 7.Still CD, Wood GC, Chu X, et al. Clinical factors associated with weight loss outcomes after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring) 2013 Jun 26; doi: 10.1002/oby.20529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saris WH, Blair SN, van Baak MA, et al. How much physical activity is enough to prevent unhealthy weight gain? Outcome of the IASO 1st Stock Conference and consensus statement. Obes Rev. 2003 May;4(2):101–114. doi: 10.1046/j.1467-789x.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 9.de Souza SA, Faintuch J, Valezi AC, et al. Gait cinematic analysis in morbidly obese patients. Obes Surg. 2005 Oct;15(9):1238–1242. doi: 10.1381/096089205774512627. [DOI] [PubMed] [Google Scholar]
- 10.Hills AP, Byrne NM, Wearing S, Armstrong T. Validation of the intensity of walking for pleasure in obese adults. Prev Med. 2006 Jan;42(1):47–50. doi: 10.1016/j.ypmed.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Mattsson E, Larsson UE, Rossner S. Is walking for exercise too exhausting for obese women? Int J Obes Relat Metab Disord. 1997 May;21(5):380–386. doi: 10.1038/sj.ijo.0800417. [DOI] [PubMed] [Google Scholar]
- 12.Cyarto EV, Myers AM, Tudor-Locke C. Pedometer accuracy in nursing home and community-dwelling older adults. Med Sci Sports Exerc. 2004 Feb;36(2):205–209. doi: 10.1249/01.MSS.0000113476.62469.98. [DOI] [PubMed] [Google Scholar]
- 13.Foster RC, Lanningham-Foster LM, Manohar C, et al. Precision and accuracy of an ankle-worn accelerometer-based pedometer in step counting and energy expenditure. Prev Med. 2005 Sep-Oct;41(3–4):778–783. doi: 10.1016/j.ypmed.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Melanson EL, Knoll JR, Bell ML, et al. Commercially available pedometers: considerations for accurate step counting. Prev Med. 2004 Aug;39(2):361–368. doi: 10.1016/j.ypmed.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 15.Bond DS, Jakicic JM, Vithiananthan S, et al. Objective quantification of physical activity in bariatric surgery candidates and normal-weight controls. Surg Obes Relat Dis. 2009 Sep 10; doi: 10.1016/j.soard.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raynor HA, Bond DS, Freedson PS, Sisson SB. Sedentary behaviors, weight, and health and disease risks. Journal of obesity. 2012;2012:852743. doi: 10.1155/2012/852743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King WC, Li J, Leishear K, Mitchell JE, Belle SH. Determining activity monitor wear time: an influential decision rule. J Phys Act Health. 2011 May;8(4):566–580. doi: 10.1123/jpah.8.4.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller GD, Jakicic JM, Rejeski WJ, et al. Effect of Varying Accelerometry Criteria on Physical Activity: The Look AHEAD Study. Obesity (Silver Spring) 2012 May 4; doi: 10.1038/oby.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tudor-Locke C, Johnson WD, Katzmarzyk PT. Accelerometer-determined steps per day in US adults. Med Sci Sports Exerc. 2009 Jul;41(7):1384–1391. doi: 10.1249/MSS.0b013e318199885c. [DOI] [PubMed] [Google Scholar]
- 20.Tudor-Locke CE, Myers AM. Challenges and opportunities for measuring physical activity in sedentary adults. Sports Med. 2001 Feb;31(2):91–100. doi: 10.2165/00007256-200131020-00002. [DOI] [PubMed] [Google Scholar]
- 21.Boone DA, Coleman J., Jr Use of a step activity monitor in determining outcomes. J Prosthet Ortho. 2006;18:86–92. [Google Scholar]
- 22.Hay J, Maximova K, Durksen A, et al. Physical activity intensity and cardiometabolic risk in youth. Arch Pediatr Adolesc Med. 2012 Nov;166(11):1022–1029. doi: 10.1001/archpediatrics.2012.1028. [DOI] [PubMed] [Google Scholar]
- 23.Inge TH, Zeller M, Harmon C, et al. Teen-Longitudinal Assessment of Bariatric Surgery: methodological features of the first prospective multicenter study of adolescent bariatric surgery. J Pediatr Surg. 2007 Nov;42(11):1969–1971. doi: 10.1016/j.jpedsurg.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inge TH, Garcia V, Daniels S, et al. A multidisciplinary approach to the adolescent bariatric surgical patient. J Pediatr Surg. 2004 Mar;39(3):442–447. doi: 10.1016/j.jpedsurg.2003.11.025. discussion 446–447. [DOI] [PubMed] [Google Scholar]
- 25.Miller WC, Wallace JP, Eggert KE. Predicting max HR and the HR-VO2 relationship for exercise prescription in obesity. Med Sci Sports Exerc. 1993 Sep;25(9):1077–1081. [PubMed] [Google Scholar]
- 26.Dimonsick E, Fan E, Fleg J. Estimating Cardiorespiratory Fitness in Well-Functioning Older Adults: Treadmill Validation of the Long Disance Corridor Walk. Journal of the American Geriatrics Society. 2006;54:127–132. doi: 10.1111/j.1532-5415.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- 27.Kist K, Jeffreys, Claytor . Amercian College of Sports Medicine. Baltimore, MD: 2010. 400MWT for Obese Children and Adolescents Participating in a Clinical Weight Management Program. [Google Scholar]
- 28.Jacobi D, Cianguira C, Couet C, Oppert JM. Physical activity and weight loss following bariatric surgery. Obesity Reviews. 2010;12:366–377. doi: 10.1111/j.1467-789X.2010.00731.x. [DOI] [PubMed] [Google Scholar]
- 29.Livhits M, Mercado C, Yermilov I, et al. Exercise following bariatric surgery: systematic review. Obes Surg. 2010 May;20(5):657–665. doi: 10.1007/s11695-010-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zunker C, King W. Psychosocial Assessment and Treatment of Bariatric Surgery Patients. In: Mitchell J, de Zwaan M, editors. Physical Activity Pre- and Post Bariatric Surgery. London: Psychology Press and Routledge, part of the Taylor and Francis Group; 2011. pp. 131–158. [Google Scholar]
- 31.Bond DS, Unick JL, Jakicic JM, et al. Objective assessment of time spent being sedentary in bariatric surgery candidates. Obes Surg. 2011 Jun;21(6):811–814. doi: 10.1007/s11695-010-0151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011 Feb;43(2):357–364. doi: 10.1249/MSS.0b013e3181ed61a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King WC, Belle SH, Eid GM, Dakin GF, Inabet WB, Mitchell JE, Patterson EJ, Courcoulas AP, Flum DR, Chapman WH, Wolfe BM. Physical Activity Levels of Patients Undergoing Bariatric Surgery in the Longitdinal Assessment of Bariatric Surgery (LABS) Study. Surgery for Obesity and Related Diseases. 2008;4(6):721–728. doi: 10.1016/j.soard.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller GD, Jakicic JM, Rejeski WJ, et al. Effect of varying accelerometry criteria on physical activity: the look ahead study. Obesity (Silver Spring) 2013 Jan;21(1):32–44. doi: 10.1038/oby.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Vries SI, Bakker I, Hopman-Rock M, Hirasing RA, van Mechelen W. Clinimetric review of motion sensors in children and adolescents. J Clin Epidemiol. 2006 Jul;59(7):670–680. doi: 10.1016/j.jclinepi.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Masse LC, Fuemmeler BF, Anderson CB, et al. Accelerometer data reduction: a comparison of four reduction algorithms on select outcome variables. Med Sci Sports Exerc. 2005 Nov;37(11 Suppl):S544–554. doi: 10.1249/01.mss.0000185674.09066.8a. [DOI] [PubMed] [Google Scholar]
- 37.Reilly JJ, Penpraze V, Hislop J, Davies G, Grant S, Paton JY. Objective measurement of physical activity and sedentary behaviour: review with new data. Arch Dis Child. 2008 Jul;93(7):614–619. doi: 10.1136/adc.2007.133272. [DOI] [PubMed] [Google Scholar]
- 38.Trost SG, PhD. Measurment of Physical Activity in Children and Adolescents. American Journal of Lifestyle Medicine. 2007;1(4):299–314. [Google Scholar]
- 39.Ward DS, Evenson KR, Vaughn A, Rodgers AB, Troiano RP. Accelerometer use in physical activity: best practices and research recommendations. Med Sci Sports Exerc. 2005 Nov;37(11 Suppl):S582–588. doi: 10.1249/01.mss.0000185292.71933.91. [DOI] [PubMed] [Google Scholar]
- 40.Tudor-Locke C, Johnson WD, Katzmarzyk PT. Accelerometer-determined steps per day in US children and youth. Med Sci Sports Exerc. 2010 Dec;42(12):2244–2250. doi: 10.1249/MSS.0b013e3181e32d7f. [DOI] [PubMed] [Google Scholar]
- 41.Tyo BM, Bassett DR, Jr, Coe DP, Feito Y, Thompson DL. Effect of BMI on pedometers in early adolescents under free-living conditions. Med Sci Sports Exerc. 2013 Mar;45(3):569–573. doi: 10.1249/MSS.0b013e3182746aa5. [DOI] [PubMed] [Google Scholar]
- 42.Feito Y, Bassett DR, Thompson DL, Tyo BM. Effects of body mass index on step count accuracy of physical activity monitors. J Phys Act Health. 2012 May;9(4):594–600. doi: 10.1123/jpah.9.4.594. [DOI] [PubMed] [Google Scholar]
- 43.Kinnunen TI, Tennant PW, McParlin C, Poston L, Robson SC, Bell R. Agreement between pedometer and accelerometer in measuring physical activity in overweight and obese pregnant women. BMC public health. 2011;11:501. doi: 10.1186/1471-2458-11-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feito Y, Bassett DR, Tyo B, Thompson DL. Effects of body mass index and tilt angle on output of two wearable activity monitors. Med Sci Sports Exerc. 2011 May;43(5):861–866. doi: 10.1249/MSS.0b013e3181fefd40. [DOI] [PubMed] [Google Scholar]
- 45.Graser SV, Pangrazi RP, Vincent WJ. Effects of placement, attachment, and weight classification on pedometer accuracy. J Phys Act Health. 2007 Oct;4(4):359–369. doi: 10.1123/jpah.4.4.359. [DOI] [PubMed] [Google Scholar]


