Abstract
Misregulated transcription factors play prominent roles in human disease, but their dynamic protein-protein interaction network has long made the goal of transcription-targeted therapeutics impractical. Recent advances in technologies for modulating protein interaction networks mean that the end of the quest is in sight.
The existence of endogenous factors that functionally connect DNA-encoded information and protein levels was first postulated by Monod and Jacob in 1961(ref. 1). In the ensuing decades, evidence has mounted that these factors—transcription factors—are also critical drivers of human disease. The overexpression, underexpression and formation of fusion proteins of transcription factors underpin a range of human diseases, and thus these proteins have high intrinsic value as therapeutic targets. A classic example is the transcriptional activator c-Myc, a linchpin in many cellular processes ranging from proliferation to apoptosis. c-Myc expression is dysregulated in many human cancers, and disruption of c-Myc function leads to tumor regression, underscoring its importance as a target for drug discovery2. Other high-profile examples include c-Myb, a transcription factor that is often required for the transformation of myeloid progenitor cells into a leukemic state3; the tumor suppressor p53, which is misregulated in more than half of all human malignancies4; and the Ets family of transcription factors, which affect the status of growth receptors such as EGFR and Her2 and contribute to metastasis5. Given the fundamental importance of these proteins, the logical questions are why there are no drugs that directly target these transcription factors and why there are so few quality probe molecules to further dissect the function of these factors. These questions are particularly urgent given the avalanche of new data regarding transcription factor localization through the transformative technological advances in sequencing and genetic manipulation that have occurred in the past decade.
Despite their potential, transcription factor–targeting molecules have remained elusive; in more conventional terms, transcription factors have largely been classified as undruggable. The origin of this description becomes clear as one examines the possible avenues for altering transcription factor activity. Except for nuclear receptors, transcription factors do not have native small-molecule ligands; thus, the primary options for their alteration involve manipulation of the complex network of protein-protein and protein–nucleic acid interactions by which transcription factors function. The past decade has shown that the ‘undruggable’ characterization is not entirely accurate, both because of conspicuous success in targeting one class of transcription factor protein-protein interactions (PPIs) and because of a growing recognition that the characteristics that render transcription factor complexes challenging also offers powerful opportunities for manipulation5.
An examination of the PPI network of the prototypical transcriptional activator p53 reveals the wide range of affinity and surface area of p53 complexes (Fig. 1a). The complexes formed between p53 and its regulatory partners such as MDM2 are typically high affinity with a relatively small surface area, and their interaction energy largely resides in a small number of residues (Fig. 1a, upper left image). These are structurally well-organized interfaces and are highly amenable to structural characterization. As with many activators, p53 typically functions as a multimer, and the oligomerization interface is another high-affinity, well-organized interface, although it takes place over a considerably larger binding surface (Fig. 1a, upper right image). The final group of PPIs that activators such as p53 utilize are those with coactivator proteins, typically involved as part of the assembly of the transcriptional machinery in the early stages of transcription. These interactions are the least well characterized, likely because the binding partners are conformationally dynamic, inhibiting high-resolution structural studies, and many are of only moderate affinity.
Figure 1.
The chemical space of protein-protein interactions. (a) Protein-protein interactions can be effectively classified by the strength of the complex (y-axis) and the surface area over which the interaction occurs (x-axis). Using p53 as an example, transcriptional activator interaction networks span a broad range of strength and surface area. PBD ID codes for each structure are as follows: p53-Mdm2 repressor, PDB 1YCR; p53 dimer, PDB 1PET; GACKIX domain of CBP with proposed binding residues, PDB 2LQH. (b) Recent advances in protein-protein interaction inhibitor design and screening methodologies have led to the discovery of a number of new small-molecule inhibitors, although success in targeting high-affinity, small-surface-area interactions has far outpaced broader or weaker interactions. SA, surface area. See refs. 7 and 8 for a more detailed discussion.
Building on this example, the interaction between p53 and MDM2 serves as a useful case study for the successful targeting of transcriptionally relevant PPIs. This is a high-affinity complex that masks the p53 activation domain, preventing p53 from functioning as a transcriptional activator and regulating its lifetime through its ubiquitylation state. It is also highly ordered, with a focused interaction surface area of <1,800 A2 that has been amenable to structural characterization and has characteristics similar to those of receptor-ligand interactions. As a result, p53–MDM2 and the closely related p53–MDMx complex have been readily targeted by small molecules with several scaffolds in clinical trials4. Thus, transcription factor PPIs characterized by high affinity and a small interaction surface area are very targetable, largely owing to the advances in PPI inhibitor discovery strategies over the past decade6. This reflects the overall success in targeting high-affinity, small-surface-area PPIs in many functional contexts (Fig. 1b).
Perhaps not surprisingly, PPI complexes that utilize broader interaction surfaces and occur with weaker affinity have been targeted far less successfully7,8. As mentioned above, these broad and weak interactions lead to distinct networks of PPIs being used to assemble the transcriptional machinery. The transient and conformationally dynamic complexes are formed with various coactivators and coactivator complexes. Traditional probe discovery or design methods are ill equipped to target these complexes because one or both binding partners are classified as intrinsically disordered proteins, and the complexes often form transiently in the cell, with affinities one to two orders of magnitude weaker than p53-MDM2–type interactions7,9. Further, the interaction surface is often considerably larger, with interaction energies shared over a greater number of amino acids, defying hotspot analysis.
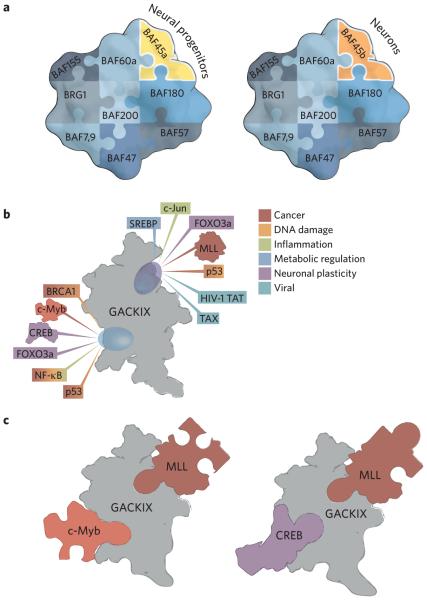
Despite the challenges, the ability to manipulate transcription factor–coactivator complexes offers two exciting possibilities. The first is context specificity. Many of the complexes involved in transcription are dynamic in composition and comprise a core enzymatic function flanked by scaffolding proteins and exchangeable modules. The BAF-type chromatin remodeling complexes that play a key role in transcription initiation are an excellent example. Most share a core enzymatic subunit (the ATPase Brg), but there are exchangeable subunits that vary according to tissue (Fig. 2a)10. In the past ten years, evidence has emerged that transcription factors target both the enzymatic component and exchangeable modules in such complexes as part of the assembly of the transcriptional machinery. In yeast, the application of covalent chemical cross-linking to map contacts with the BAF-type complex Swi–Snf revealed that the transcription factors Gal4 and VP16 each contact the core enzymatic functionality (Snf1) in addition to auxiliary factor Snf5 (ref. 11). Thus, one could imagine blocking a PPI that would affect the localization of a complex at particular gene promoters but would leave the core enzyme unaltered, providing functional capacity in other contexts. A second group of context-dependent transcription factor–coactivator PPIs are those used by viruses to hijack the transcriptional apparatus in infected tissues. Arora and Pan recently demonstrated that an inhibitor of a complex formed between the human papillomavirus transcription factor E6 and the coactivator p300 restores the ability of p53 to function in human papillomavirus–positive head and neck cancers and, in doing so, blocks tumorigenicity12. A challenge for the coming decade is to provide a more comprehensive map of the network of transcription factor PPIs at gene promoters as there remain more questions than answers for most transcription factors. Even in the case of the well-studied transcription factor p53, it is not clear which of its coactivator complexes are the most critical to block, either alone or in combination; considering its interaction with the master coactivator CBP and its close relative p300 alone, p53 binds in vitro with four of the activator interaction motifs within CBP, but the functional relevance of each of those interactions is not yet defined13. This is an area in which chemical biology tools such as covalent chemical capture or high-quality small-molecule probes will be invaluable.
Figure 2.
Transcription complexes are dynamic in composition and conformation. (a) The BAF chromatin remodeling complexes contain the same enzymatic core (BRG1) but have exchangeable subunits such as BAF45a and BAF45b that define tissue specificity. See ref. 10 for a more complete discussion of the composition and function of these complexes. (b) The GACKIX (Gal11, Arc105, CBP/p300, kinase-inducible domain interacting) motif of CBP/p300 uses two binding sites to interact with more than 10 distinct transcriptional activators. (c) Each ternary complex has a signature conformation with the two binding sites in allosteric communication. The structure and function of the GACKIX motif were recently reviewed in ref. 14.
In addition to compositional dynamics within transcription factor complexes, many of the individual subunits exhibit significant conformational plasticity as a means to interact specifically with a variety of transcription factors. The low energy barriers between individual conformations mean that each participant can use the same group of amino acids to recognize a variety of binding partners, with each complex requiring a distinct conformation. As such, synthetic regulation of this conformational plasticity to direct complex assembly represents a second exciting opportunity to specifically target transcription pathways. A foundational example of this phenomenon is the GACKIX (Gal11, Arc105, CBP/p300, kinase-inducible domain interacting) domain of the master coactivators and histone acetyltransferases CBP and p300. GACKIX is highly plastic, and its two transcription factor–binding surfaces can accommodate more than 15 distinct transcription factor binding partners, in the context of binary or ternary complexes, that are involved in a variety of physiological processes and implicated in diseases from cancer to neuropathic pain (Fig. 2b)14. The two binding surfaces are allosterically connected, allowing for cooperative binding of particular transcription factor–GACKIX pairs with a wide range of enhancement (Fig. 2c). Experimental and computational studies indicate that the mechanistic origin of the variable cooperativity is differential stabilization of the ternary complex15–17. Thus by intercepting a particular conformer, a small molecule could have either a positive or a negative influence on the binary and ternary transcription factor–coactivator assembly process. Certainly the field can be guided by the success seen in targeting particular conformational states of kinases or of the protein folding machinery for enhanced selectivity and context-specific effects on downstream processes8,18,19.
Screening techniques that directly address the conformational plasticity of coactivators have been effective for identifying small-molecule modulators that capture distinct conformers. The site-directed fragment screening strategy of tethering first developed at Sunesis is one such strategy. When applied to the conformationally dynamic GACKIX motif, for example, researchers identified chemical cochaperones that stabilize a range of GACKIX conformations and dictate the formation of particular GACKIX–transcription factor assemblies either positively or negatively20. Standard binding screens can also be adapted to discover modulators that capture unique coactivator conformations through the triaging of primary screening hits that mimic native binding partners. Using this process, the natural products sekikaic acid and the related lobaric acid capture a conformation of the GACKIX motif that showed greatly attenuated ternary complex formation21. One advantage from this type of strategy is that small-molecule modulators are likely to exhibit enhanced specificity because they are targeting unique coactivator conformations; this is true of sekikaic acid and lobaric acid. A second advantage is that it presents a generalizable mechanism by which allosteric modulators of highly challenging binding interfaces can be discovered. Again using GACKIX as an example, the binding site used by the oncogene c-Myb is shallow and lacks significant topology. However, the second binding site within GACKIX, which is targeted by sekikaic acid and lobaric acid, is smaller and better defined. By targeting a more druggable binding surface, allosteric networks within the coactivator can be exploited to affect less amenable distal binding sites.
Outlook
In the past decade of chemical biology, there has been enormous progress in targeting protein-protein interactions, and this has fueled successes in developing small-molecule modulators of a key subset of transcriptional activator PPIs. The recognition that the dynamic composition and structure of transcription factor–coactivator complexes offers advantages in terms of specificity and function in recent years now opens the door for similar success in the coming decade. As molecules move toward clinical applications, a question that has yet to be answered is one of potency. At least in vitro, transcription factor–coactivator complexes are an order of magnitude or more weaker than complexes such as p53–Mdm2. Whether it is possible to obtain synthetic modulators that exceed the affinity of the native ligands is still an open question. This is an area in which allosteric modulators may offer significant advantages. NMR screening techniques that rely on conformational dynamics, such as protein-observed fluorine (PrOF) NMR spectroscopy, have proven powerful for focusing on molecules that capture coactivators in a particular conformational space and will be a key discovery tool22. In addition, screening formats such as small-molecule microarrays23 that facilitate the interrogation of transcription factor binding in the presence of endogenous competing factors enable specificity to be assessed much earlier in the discovery process24. Looking toward the future, ion mobility mass spectrometry may be especially effective in a high-throughput capacity as it allows small molecule–induced changes in conformation to be readily detected25. There is still significant untapped potential in the broader network of transcription factor interactions as the field continues to define these critical connections. Approaches such as targeting transcription factor dimerization motifs or preventing promoter localization by blocking DNA binding of transcription factors are additional strategies being explored that may also allow for the successful modulation of transcriptional processes for mechanistic insight or therapeutic gain7. There is thus considerable cause for optimism that the goal of transcription-targeted therapeutic agents is no longer out of reach.
Acknowledgments
The authors thank J. Gestwicki, J. Wells, P. Arora and L. Kiessling for illuminating discussions.
Footnotes
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Jacob F, Monod J. J. Mol. Biol. 1961;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- 2.Lin CY, et al. Cell. 2012;151:56–67. doi: 10.1016/j.cell.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pattabiraman DR, et al. Blood. 2014;123:2682–2690. doi: 10.1182/blood-2012-02-413187. [DOI] [PubMed] [Google Scholar]
- 4.Khoo KH, Verma CS, Lane DP. Nat. Rev. Drug Discov. 2014;13:217–236. doi: 10.1038/nrd4236. [DOI] [PubMed] [Google Scholar]
- 5.Kar A, Gutierrez-Hartmann A. Crit. Rev. Biochem. Mol. Biol. 2013;48:522–543. doi: 10.3109/10409238.2013.838202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arkin MR, Tang Y, Wells JA. Chem. Biol. 2014;21:1102–1114. doi: 10.1016/j.chembiol.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson AD, Dugan A, Gestwicki JE, Mapp AK. ACS Chem. Biol. 2012;7:1311–1320. doi: 10.1021/cb300255p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cesa LC, Mapp AK, Gestwicki JE. Frontiers Bioeng. Biotechnol. 2015;3:119. doi: 10.3389/fbioe.2015.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuxreiter M, et al. Nat. Chem. Biol. 2008;4:728–737. doi: 10.1038/nchembio.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hargreaves DC, Crabtree GR. Cell Res. 2011;21:396–420. doi: 10.1038/cr.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnamurthy M, et al. ACS Chem. Biol. 2011;6:1321–1326. doi: 10.1021/cb200308e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie X, et al. Oncogene. 2014;33:1037–1046. doi: 10.1038/onc.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CW, Ferreon JC, Ferreon AC, Arai M, Wright PE. Proc. Natl. Acad. Sci. USA. 2010;107:19290–19295. doi: 10.1073/pnas.1013078107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakur JK, Yadav A, Yadav G. Nucleic Acids Res. 2014;42:2112–2125. doi: 10.1093/nar/gkt1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shammas SL, Travis AJ, Clarke J. Proc. Natl. Acad. Sci. USA. 2014;111:12055–12060. doi: 10.1073/pnas.1405815111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Law SM, Gagnon JK, Mapp AK, Brooks CL., III Proc. Natl. Acad. Sci. USA. 2014;111:12067–12072. doi: 10.1073/pnas.1405831111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang N, Lodge JM, Fierke CA, Mapp AK. Proc. Natl. Acad. Sci. USA. 2014;111:12061–12066. doi: 10.1073/pnas.1406033111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonard SE, Register AC, Krishnamurty R, Brighty GJ, Maly DJ. ACS Chem. Biol. 2014;9:1894–1905. doi: 10.1021/cb500371g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cesa LC, et al. ACS Chem. Biol. 2013;8:1988–1997. doi: 10.1021/cb400356m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang N, et al. J. Am. Chem. Soc. 2013;135:3363–3366. doi: 10.1021/ja3122334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majmudar CY, et al. Angew. Chem. 2012;51:11258–11262. doi: 10.1002/anie.201206815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gee CT, Koleski EJ, Pomerantz WC. Angew. Chem. 2015;54:3735–3739. doi: 10.1002/anie.201411658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradner JE, McPherson OM, Koehler AN. Nat. Protoc. 2006;1:2344–2352. doi: 10.1038/nprot.2006.282. [DOI] [PubMed] [Google Scholar]
- 24.Pop MS, et al. Mol. Cancer Ther. 2014;13:1492–1502. doi: 10.1158/1535-7163.MCT-13-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niu S, Rabuck JN, Ruotolo BT. Curr. Opin. Chem. Biol. 2013;17:809–817. doi: 10.1016/j.cbpa.2013.06.019. [DOI] [PubMed] [Google Scholar]