Summary
Data from the National LymphoCare Study (a prospective, multicentre registry that enrolled follicular lymphoma (FL) patients from 2004–2007) were used to determine disease characteristics, treatment patterns, outcomes and prognosis for elderly FL (eFL) patients. Of 2650 FL patients, 209 (8%) were aged >80 years; these eFL patients more commonly had grade 3 disease, less frequently received chemoimmunotherapy and anthracyclines, and had lower response rates when compared to younger patients. With a median follow-up of 6.9 years, 5-year overall survival (OS) for eFL patients was 59%; 38% of deaths were lymphoma-related. No treatment produced superior OS among eFL patients. In multivariate Cox models, anaemia, B-symptoms and male sex predicted worse OS (P < 0·01); a prognostic index of these factors (0, 1 or ≥2 present) predicted OS (hazard ratio [95% confidence interval]: ≥2 vs. 0, 4·72 [2·38–9·33], ; 1 vs. 0, 2·63 [1·39–4·98]), with a higher concordance index (0·63) versus the Follicular Lymphoma International Prognostic Index (0·55). The index was validated in an independent cohort. In the largest prospective US-based eFL cohort, no optimal therapy was identified and nearly 40% of deaths were lymphoma-related, representing baseline outcomes in the modern era.
Keywords: follicular lymphoma, elderly patients, elderly lymphoma, rituximab, chemotherapy
Introduction
Follicular lymphoma (FL) is the most common low-grade non-Hodgkin lymphoma in the USA with more than 14000 cases diagnosed annually (Siegel et al, 2012). Treatment choices often depend on stage, age, performance status (PS), comorbidities and therapeutic goals. Options include watchful waiting, involved field radiotherapy, single-agent chemotherapy, monoclonal antibodies and chemoimmunotherapy (Gribben, 2007; Witzig et al, 2011). Prognostic models predicting survival in FL have been developed, with the most prominent model being the Follicular Lymphoma International Prognostic Index (FLIPI) (Federico et al, 2009; Solal-Celigny et al, 2004). While age is a critical prognostic component in FLIPI, little is known about very elderly FL patients as few studies, if any, have addressed this true elderly population. Although significant improvement in the overall survival (OS) of FL patients has been witnessed in recent years, (Fisher et al, 2005; Swenson et al, 2005) pivotal studies that have shaped our management paradigm and have led to current standards have typically enrolled patients with a median age of ≤60 years (Buske et al, 2006; Marcus et al, 2008; Salles et al, 2011). Given that FL is a disease of the elderly, understanding how modern therapies have been applied to older patient populations is critical. Whether elderly patients are under-treated or are treated less effectively, and whether their disease-related outcomes mimic those of younger patients remains unknown. Furthermore, whether present prognostic models apply to older patients is uncertain. In the absence of prospective studies specifically designed for elderly FL patients, uniform guidelines for treating these patients and assessing their prognosis are lacking.
To better understand patterns of care, prognosis and outcomes in older FL patients in the USA, and to investigate the impact of age on treatment selection and outcomes, we analysed data from the National LymphoCare Study (NLCS), a prospective, multicentre, observational registry that enrolled 2650 newly diagnosed FL patients between 2004 and 2007. To our knowledge, this represents the largest comprehensive prospective data analysis of very elderly FL patients (eFL) reported to date.
Patients and methods
The NLCS registry is a prospective cohort database study of patients with FL in the USA that was developed by Genentech, Inc. (South San Francisco, CA) and Biogen Idec (Cambridge, MA). The NLCS has guidelines that have been previously described (Friedberg et al, 2009). Patients were recruited from academic and community practices between 2004 and 2007 and final selection of participating sites and data collection occurred as previously described (Friedberg et al, 2009). All patients signed an institutional review board-approved written informed consent. All patients who were within 6 months from their initial FL diagnosis and who had no prior history of lymphoma were eligible. Patients were evaluated and treated according to each physician’s standard discretion without study-specific treatments. The treating physician documented each treatment programme, including observation and treatment responses. As this was strictly an observational study, there were no standardized assessments to determine responses and progression and cause of death was by report of the treating physician. No data on comorbidities was collected. Follow-up data on relapses, new treatments and vital status (including cause of death) were prospectively collected every 3 months from the treating physician.
Statistical methods
To investigate the impact of age on treatment selection and outcomes, we divided patients into three age groups (≤60 years, 61–80 years and >80 years). Patients >80 years were considered eFL. The younger patients (<80 years) were separated into two groups because >60 years is a well-known risk factor for poor OS in FL patients and is one of the FLIPI components. Demographics, baseline disease characteristics and initial treatment strategy for eFL in the NLCS were summarized using descriptive statistics (medians and ranges for continuous variables; frequencies for categorical variables). The associations of demographics, baseline disease characteristics and initial treatment strategy with age groups were evaluated using the Pearson chi-square test. Baseline demographics, disease characteristics and response rates to initial therapy were compared between groups of first-line treatment within each age group. The difference was tested using the Pearson chi-square test.
Median progression-free survival (PFS) and OS by treatment group were estimated using Kaplan-Meier methods within each age group. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using Cox regression. For the OS endpoint, two models were fitted; one comparing watchful waiting, rituximab monotherapy and rituximab plus chemotherapy; the other comparing anthracycline- vs. non-anthracycline-based chemotherapies. The models included age at diagnosis, treatment and treatment by age interaction to investigate the difference in treatment effect between age groups. In addition, the Cox models included important demographic and disease characteristics to account for the imbalance between treatment-age groups. Specifically, the following baseline characteristics were adjusted: sex, race/ethnicity, grade, nodal sites, lactate dehydrogenase (LDH), haemoglobin, stage, PS, bone marrow involvement and treatment centre type (community vs. academic). In addition, the use of rituximab maintenance was also included in the Cox models as a time-varying covariate so the impact of rituximab maintenance was accounted for after the maintenance treatment started.
For patients >80 years, a Cox regression model was used to determine the factors that were significantly associated with OS based on a p-value level of ≤ 0·05. The selection of the resulting significant factors was then confirmed via a backward selection Cox regression model. The baseline characteristics included in the model for consideration were sex, histology grade, stage, LDH, haemoglobin, nodal sites, extra-nodal sites, Eastern Cooperative Oncology Group (ECOG) performance score, B symptoms and bone marrow involvement. Stage, LDH, haemoglobin, and nodal sites were dichotomized based on the established FLIPI risk factors. For ECOG performance score, categories 1 and ≥ 2 were grouped together because of the small sample size for the ≥ 2 category. First line treatment and time-varying rituximab-maintenance were also included. From the selected risk factors, the eFL index was created. The prognostic properties of the eFL index were compared to FLIPI risk by HRs and CIs from unadjusted cox regression models for PFS, OS and lymphoma-related mortality (LRM). The model performance was assessed by the concordance index (c-index) (Harrell et al, 1996). The comparison was performed on the same set of patients who had complete FLIPI and no missing values for the selected risk factors.
Validation cohort
To evaluate our proposed prognostic index in a separate eFL population, we utilized a cohort of FL patients who were >80 years from the Surveillance, Epidemiology and End Results (SEER) registry linked with the Medicare database (2001–2009) (http://healthcaredelivery.cancer.gov/seermedicare/). In this dataset, we ascertained anaemia prior to a diagnosis of FL using the International Classification of Diseases (ICD)-9 codes (280·x, 281·x, 283·xx, 284·8, 284·9, 285·2x, 285·9). A patient’s diagnosis of anaemia had to appear on at least two different Medicare inpatient, outpatient or physician claims that were more than 30 days apart in the 12 months before FL diagnosis through the month of diagnosis. This was done to account for the possibility that physicians may have recorded a diagnosis as being present, when the correct coding should have been to “rule out” the condition. To evaluate the performance of the eFL prognostic index in this population, we performed multivariate Cox regression models, using the prognostic index of male sex, B symptoms and anaemia (0 vs. 1 vs. ≥2 present) in eFL patients controlling for FL grade, stage, year of diagnosis, and treatment.
Results
Patient characteristics (Table I)
Table I.
Patient and disease characteristics at enrolment.
| Characteristic, n (%) | ≤60 years (n = 1255, 47%*) | 61–80 years (n = 1186, 45%*) | >80 years (n = 209, 8%*) | P value (Pearson χ2) |
|---|---|---|---|---|
| Female sex | 636 (50·7) | 616 (51·9) | 122 (58·4) | 0·119 |
|
| ||||
| White race | 1107 (88·2) | 1089 (91·8) | 197 (94·3) | 0·010 |
|
| ||||
| Grade 3 disease† | 210 (18·0) | 227 (21·9) | 51 (27·1) | 0·004 |
| Number of missing data | 87 | 151 | 21 | |
|
| ||||
| FLIPI risk category†‡ | < 0·001 | |||
| Good (0–1) | 518 (51·1) | 206 (21·8) | 25 (15·4) | |
| Intermediate (2) | 342 (33·8) | 240 (25·4) | 61 (37·7) | |
| Poor (3–5) | 153 (15·1) | 498 (52·8) | 76 (46·9) | |
| Number of missing data | 242 | 242 | 47 | |
|
| ||||
| Number of FLIPI risk factors excluding age†‡ | 0·030 | |||
| 0–1 | 518 (46·6) | 518 (51·0) | 95 (55·6) | |
| 2–4 | 593 (53·4) | 498 (49·0) | 76 (44·4) | |
| Number of missing data | 144 | 170 | 38 | |
|
| ||||
| Stage III to IV† | 885 (71.1) | 745 (63.7) | 132 (63.2) | <0·001 |
| Number of missing data | 11 | 16 | ||
|
| ||||
| LDH >ULN† | 188 (19·3) | 210 (23·6) | 28 (18·2) | 0·049 |
| Number of missing data | 279 | 295 | 55 | |
|
| ||||
| Haemoglobin <120 g/l† | 182 (15·6) | 266 (23·9) | 72 (37·7) | < 0·001 |
| Number of missing data | 86 | 72 | 18 | |
|
| ||||
| Nodal sites ≥5† | 476 (39·3) | 353 (31·2) | 35 (17·8) | < 0·001 |
| Number of missing data | 43 | 53 | 12 | |
|
| ||||
| ECOG PS† | < 0·001 | |||
| 0 | 672 (75·6) | 504 (62·5) | 59 (45·0) | |
| 1 | 192 (21·6) | 259 (32·1) | 54 (41·2) | |
| ≥2 | 25 (2·8) | 43 (5·3) | 18 (13·7) | |
| Number of missing data | 366 | 380 | 78 | |
|
| ||||
| B-symptoms | 333 (26·5) | 291 (24·5) | 45 (21·5) | 0·229 |
|
| ||||
| Bone marrow involvement† | 419 (41·4) | 304 (34·9) | 36 (32·7) | 0·008 |
| Number of missing data | 243 | 316 | 99 | |
|
| ||||
| Geographic region† | 0·291 | |||
| Midwest | 416 (33·2) | 355 (29·9) | 65 (31·1) | |
| Northeast | 228 (18·2) | 197 (16·6) | 34 (16·3) | |
| Southeast | 351 (28·0) | 384 (32·4) | 65 (31·1) | |
| Southwest | 90 (7·2) | 96 (8·1) | 12 (5·7) | |
| West | 169 (13·5) | 154 (13) | 33 (15.8) | |
| Number of missing data | 1 | |||
|
| ||||
| Center type† | < 0·001 | |||
| Academic | 286 (22·8) | 198 (16·7) | 32 (15·3) | |
| Community | 968 (77·2) | 988 (83·3) | 177 (84·7) | |
| Number of missing data | 1 | |||
ECOG PS, Eastern Cooperative Oncology Group performance status; FLIPI, Follicular Lymphoma International Prognostic Index; LDH, lactate dehydrogenase; ULN, upper limit of normal.
Percentage of the overall sample.
Missing data exist. Missing data are excluded when calculating the percentages.
Modified FLIPI excluding age has fewer missing data than FLIPI because more patients can be classified into the new categories even with missing data in some factors.
A total of 2650 patients were evaluable of whom 209 (8%) were >80 years. Median age at baseline for all patients was 61 years (range 22–97). Although not statistically significant, 58% of patients >80 years were female compared with 51% and 52% in the younger age groups. As shown in Table I, eFL patients more commonly had grade 3 disease (27% vs. 22% in patients 61–80 years and 18% in patients ≤60 years, P = 0·004) and had higher FLIPI score (47% with poor risk vs. 15% in patients ≤60 years, P < 0·0001), mainly driven by age and lower haemoglobin factors. It is worth noting that all patients >80 years had at least one FLIPI risk factor (age >60 years). Therefore, excluding age, in eFL patients, the low FLIPI risk corresponds to a presence of zero risk factor, the intermediate FLIPI risk corresponds to 1 risk factor, and the high risk corresponds to 2–4 risk factors. Comparison of FLIPI excluding age actually shows that older patients are less likely to have poor risk status than younger patients.
Initial treatment regimens by age groups
Treatments varied significantly by age (P < 0·0001). While patients on observation were notably similar between the age groups, when treated, those >80 years were more likely to receive rituximab monotherapy than patients 61–80 years (29% vs. 15%) or ≤60 years (10%). R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) was the most commonly used chemoimmunotherapy regimen in patients ≤60 years (57%) while R-CVP (rituximab, cyclophosphamide, vincristine and prednisone) was the most commonly used chemoimmunotherapy regimen in patients >80 years (53%). Patients >80 years less commonly received chemoimmunotherapy as an initial strategy compared to others (32% vs. 48% [61–80 years], 52% [≤60 years]) (Table II). Among those receiving chemotherapy (with or without rituximab), patients aged >80 years were less likely to receive anthracyclines (28%) than patients aged 61–80 years (58%) or ≤60 years (68%) (P < 0·0001). Only grade 3 histology significantly predicted anthracycline use within all age groups, including eFL. Of note, the use of maintenance rituximab amongst rituximab-treated patients did not vary by age but eFL patients induced with rituximab monotherapy were less likely to receive maintenance rituximab compared with their younger counterparts (Table II).
Table II.
Front-line treatments by age group.
| Characteristic, n (%) | ≤60 years (n = 1255) | 61–80 years (n = 1186) | >80 years (n = 209) | P value (Pearson χ2) |
|---|---|---|---|---|
| Watchful waiting | 241 (19·2) | 265 (22·3) | 51 (24·4) | < 0·001 |
|
| ||||
| R-mono | 121 (9·6) | 174 (14·7) | 60 (28·7) | |
|
| ||||
| R-chemo | 651 (51·9) | 566 (47·7) | 67 (32·1) | |
|
| ||||
| Other treatments | 242 (19·3) | 181 (15·3) | 31 (14·8) | |
| Chemo* | 31 (12·8) | 31 (17·1) | 9 (29·0) | |
| Radiotherapy* | 54 (22·3) | 61 (33·7) | 10 (32·3) | |
| Combined modality: radiotherapy* | 41 (16·9) | 31 (17·1) | 6 (19·4) | |
| Investigational therapy* | 104 (43·0) | 49 (27·1) | 5 (16·1) | |
| Other* | 12 (5·0) | 9 (5·0) | 1 (3·2) | |
|
| ||||
| Anthracycline use among Chemo/R-chemo therapy† | 456 (68·3) | 332 (58·1) | 21 (28·4) | < 0·001 |
|
| ||||
| R-maintenance use‡ | ||||
| After R-mono/R-chemo induction | 274 (46·3) | 230 (44·3) | 37 (48·7) | 0·688 |
| After R-mono induction | 51 (60·7) | 58 (48·3) | 14 (37·8) | 0·048 |
| After R-chemo induction | 223 (43·9) | 172 (43·1) | 23 (59·0) | 0·160 |
Chemo, chemotherapy; R-chemo, rituximab plus chemotherapy; R-maintenance, rituximab maintenance; R-mono, rituximab monotherapy.
Percentages are calculated among patients receiving Other Treatments.
Percentages are calculated among patients receiving front-line Chemo/R-chemo therapy.
Patients who have completed the induction treatment, with a complete response, partial response, or stable disease who have not progressed or started second-line treatment 7 months after the end of induction are included in this analysis.
Overall response rates
Within each treatment category, response rates were similar across age groups (P > 0·05) except for a low response rate in eFL among patients receiving anthracyclines (P = 0·03). In all age categories, treatment with chemoimmunotherapy produced the highest overall response rate (ORR) (92% in ≤60 years, 91% in 61–80 and 84% in >80), which was statistically significant compared with rituximab-monotherapy in patients ≤60 years (P = 0·0002) and 61–80 years (P < 0·0001), but was not statistically significant in eFL patients (P = 0·618). Similar responses were observed amongst eFL patients regardless of anthracycline use while patients <80 years demonstrated statistically significantly higher ORR when receiving anthracyclines (Table III).
Table III.
Overall response rates and adjusted* hazard ratio for PFS and OS by age group based on the treatment programme.
| First-line treatment | ≤60 years | 61–80 years | >80 years |
|---|---|---|---|
| ORR by treatment regimen, % of patients | |||
| All patients | 76·0 | 72·8 | 66·5 |
| R-chemo | 92·3 | 90·9 | 83·9 |
| R-mono | 81·3 | 78·8 | 80·4 |
| P values† (treatment difference) | 0·0002 | < 0·0001 | 0·618 |
| ORR by anthracycline-containing chemotherapy, % of patients | |||
| Anthracycline based | 93·5 | 93·7 | 77·8 |
| Non-anthracycline based | 86·2 | 83·3 | 77·6 |
| P values‡ (treatment difference) | 0·0024 | 0·0001 | 0·98 |
| Adjusted* hazard ratio (95% CI) for PFS | |||
| WW vs. R-mono | 1·30 (0·95–1·79) | 1·33 (1·02–1·74) | 1·00 (0·62–1·64) |
| R-Chemo vs. R-mono | 0·47 (0·35–0·63) | 0·73 (0·58–0·93) | 0·84 (0·54–1·30) |
| Anthracycline-based vs. non-anthracycline-based (regimens containing chemotherapy) | 0·80 (0·61–1·05) | 0·66 (0·51–0·85) | 1·28 (0·67–2·45) |
| Adjusted* hazard ratio (95% CI) for OS | |||
| WW vs. R-mono | 0·72 (0·34–1.52) | 1·05 (0·71–1·55) | 0·94 (0·53–1·66) |
| R-Chemo vs. R-mono | 0·75 (0·39–1·43) | 1.06 (0·76–1·49) | 1·34 (0·83–2·16) |
| Anthracycline- vs. non-anthracycline (regimens containing chemotherapy) | 0·60 (0·36–0·98) | 0·57 (0·42–0·78) | 0·99 (0·49–1·98) |
CI, confidence interval; NR, not reached; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; R-chemo, rituximab plus chemotherapy; R-mono, rituximab monotherapy; WW, watchful waiting.
Cox proportional hazards model includes sex, race/ethnicity, histology grade, nodal sites, LDH, haemoglobin, stage, Eastern Cooperative Oncology Group performance score, bone marrow involvement, centre type, and use of R-maintenance therapy as a time dependent covariate and treatment by age group interaction.
Pearson chi-square test comparing response rate between R-chemo and R-mono within each age groups.
Pearson chi-square test comparing response rate between anthracycline- and non-anthracycline-based regimens within each age group.
PFS and OS (Table III, Fig 1)
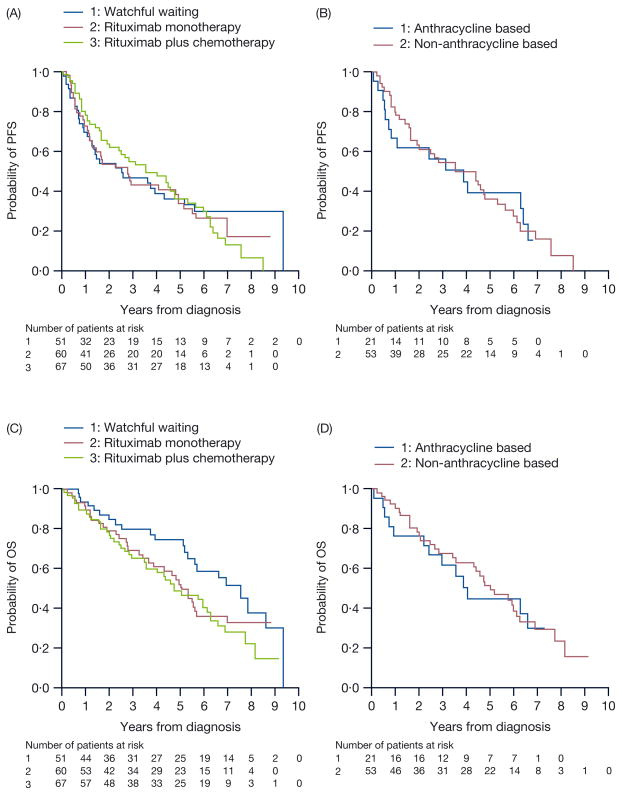
Fig 1.
(A) PFS for FL patients aged >80 years in the NLCS based on their initial treatment strategy. No specific regimen amongst these studied provided these elderly patients with a superior PFS.
(B) PFS for FL patients aged >80 years in the NLCS based on anthracycline use. Anthracyclines did not improve PFS in this elderly cohort.
(C) OS for patients aged >80 years based on initial treatment selection. No particular regimen improved OS in this elderly cohort.
(D) OS for patients aged >80 years based on anthracycline use. Anthracyclines did not improve OS in this elderly cohort.
FL, follicular lymphoma; NLCS, National LymphoCare Study; OS, overall survival; PFS, progression-free survival
With a median follow-up of 6·9 years, median OS was 5·7 years (95% CI; 5·0–6·6) for eFL patients, and was not reached for patients ≤60 and 61–80 years. Median PFS was 7·7 years (95% CI; 6·8–not estimable) in patients ≤60 years, 5·2 years (95% CI; 4·6–6·1) for patients 61–80 years and 3·0 years (95% CI; 2·3–4·4) for patients >80 years. From Cox proportional hazards model, PFS significantly varied by age groups and treatments (P = 0·002 for the age-by-treatment interaction). Among patients >80 years, there was no difference in PFS for patients on watchful waiting (HR; 1·00 [0·62–1·64]) or treated with chemoimmunotherapy (HR; 0.84 [0·54–1·30]) compared with initial treatment with rituximab monotherapy after adjusting for baseline characteristics and rituximab maintenance treatment. In contrast, chemoimmunotherapy appeared to improve PFS in patients ≤60 years (HR; 0·47 [0·35–0·63] compared to rituximab monotherapy, and 0·36 [0·29–0·45] compared to observation) and 61–80 years (HR; 0·73 [0·58–0·93], 0·55 [0·44–0·69], respectively). Use of regimens containing an anthracycline, compared to no anthracycline use, was associated with superior PFS and OS in patients ≤60 years (PFS: HR; 0·80 [0·61–1·05]; OS: HR; 0·60 [0·36–0·98]) and 61–80 years (PFS: HR; 0·66 [0·51–0·85]; OS: HR; 0·57 [0·42–0·78]). However, similar observations were not witnessed in patients >80 years (PFS: HR; 1·28 [0·67–2·45]; OS: HR; 0·99 [0·49–1·98]). While no specific treatment appeared to improve PFS or OS for eFL patients when compared with observation, it is critical to note that the small number of eFL patients receiving rituximab plus chemotherapy might have decreased the ability to detect these differences.
Cause of death
At 5 years, 92.2% (95% CI; 90·5–93·6) of all patients ≤60 years were alive while 58·5% (95% CI; 51·0–65·3) of all patients >80 years remained alive. Interestingly, this significant difference was observed as early as 2 years when 97·2% (95% CI; 96·1– 98·0) of patients ≤60 years were alive but only 79·5% (95% CI; 73·2–84·4) of patients >80 years remained living, implying that some eFL experience early death. Among patients receiving chemoimmunotherapy specifically, 48·9% of patients >80 years were alive at 5 years (95% CI; 35·6–60·9) while 91·2% (95% CI; 88·6–93·2) of patients ≤60 years remained living at the same time point. Of the 112 deaths recorded among patients >80 years, 38·4% were attributed to FL and none were recorded as related to lymphoma treatment toxicity. These numbers were only somewhat lower than patients ≤60 years, of whom 47·5% died due to lymphoma and an additional 5·9% related to lymphoma treatment-related toxicity.
Prognostic index
Among eFL patients, we evaluated factors associated with OS using a Cox regression model. This analysis (Table IV) showed that anaemia (haemoglobin <120 g/l) was the only FLIPI factor that was significantly associated with OS in eFL patients (HR; 2·15 [1·34–3·45]). In addition to anaemia, B-symptoms predicted inferior OS (2·17 [1·29– 3·64]), while female sex was associated with improved OS (HR; 0·55 [0·36–0·84]). From these three risk factors, we proposed a new index for eFL patients with the corresponding risk categories: 0 (Good), 1 (Intermediate) and 2–3 (Poor). The categories of 2 and 3 were collapsed into one due to small sample size.
Table IV.
Prognostic factors OS in patients >80 years (n=197).
| Hazard ratio (95% CI) | P value | P value (Group) | |
|---|---|---|---|
| Sex | |||
| Male | 0·0058 | ||
| Female | 0·55 (0·36–0·84) | 0·0058 | |
|
| |||
| Follicular histology grade | |||
| 1 or 2 | 0·3124 | ||
| 3 | 1·33 (0·84–2·13) | 0·2275 | |
| Missing | 0·77 (0·36–1·63) | 0·4984 | |
|
| |||
| Stage category | |||
| I or II | 0·4398 | ||
| III or IV | 1·21 (0·74–1·98) | 0·4398 | |
|
| |||
| LDH | |||
| Normal | 0·8163 | ||
| > ULN | 1·08 (0·57–2·03) | 0·8229 | |
| Unknown | 1·18 (0·71–1·96) | 0·5273 | |
|
| |||
| Hb | |||
| ≥ 120 g/l | 0.0062 | ||
| < 120 g/l | 2·15 (1·34–3·45) | 0·0014 | |
| Unknown | 1·33 (0·64–2·80) | 0·4466 | |
|
| |||
| Nodal sites | |||
| < 5 | 0·4101 | ||
| ≥ 5 | 0·79 (0·45–1·38) | 0·4101 | |
|
| |||
| Extranodal sites | |||
| None | 0·5799 | ||
| 1 | 1·38 (0·83– 2·31) | 0·2163 | |
| ≥ 2 | 1·23 (0·60–2·53) | 0·5761 | |
| Missing | 0·70 (0·16–3·06) | 0·6314 | |
|
| |||
| ECOG performance score | |||
| 0 | 0·1730 | ||
| ≥ 1 | 0·91 (0·50–1·65) | 0·7626 | |
| Unknown | 1·42 (0·85–2·39) | 0·1810 | |
|
| |||
| B-symptoms | |||
| No | 0·0034 | ||
| Yes | 2·17 (1·29–3·64) | 0·0034 | |
|
| |||
| Bone marrow involvement | |||
| No | 0·4085 | ||
| Yes | 1·32 (0·64–2·.73) | 0·4574 | |
| Missing | 1·40 (0·85–2·32) | 0·1838 | |
|
| |||
| First-line treatment | |||
| R-Mono | 0·8512 | ||
| Watchful waiting | 0·75 (0·39–1·44) | 0·3818 | |
| R-Chemo | 0·95 (0·54–1·65) | 0·8427 | |
| Other | 0·84 (0·39–1·80) | 0·6484 | |
The Cox model was also adjusted for follow-on use of R-maintenance as a time-varying covariate. Group p-values test for overall group differences (not trend).
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; Hb, haemoglobin; LDH, lactate dehydrogenase; R-Chemo, rituximab plus chemotherapy; R-mono, rituximab monotherapy; ULN, upper limit of normal
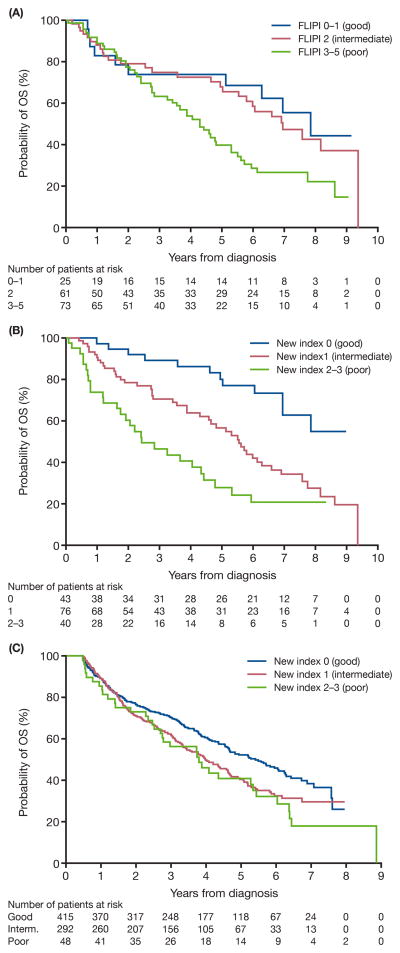
We applied this new index in the eFL patients. As shown in Fig 2, the new index separates eFL patients very well, while the FLIPI risk group does not separate patients with low and intermediate risk. Compared to patients with good risk, those who had intermediate risk or poor risk had inferior OS (HR; 2·63 [95% CI; 1·39–4·98] and 4·72 [2·38–9·33], respectively). In contrast, no differences were observed in OS between FLIPI intermediate risk and good risk groups. Poor risk FLIPI was a significant predictor of inferior OS (HR; 2·09 [1·05–4·15]) compared to good risk, but the magnitude of the HR was much smaller than seen in the new index. The c-index for discrimination of the model (1·0 is perfect and 0·5 indicates no predictive discrimination) was improved, from 0·55 for FLIPI risk group to 0·63 for the new index. The new prognostic index high risk also predicted worsened LRM (HR; 4·03 [1·39–11·69]) and inferior PFS (HR; 2·32 [1·29–4·14]) comparing to low risk (Table V).
Fig 2.
(A) OS for patients aged >80 years based on FLIPI risk group. Log-rank test for group difference, P = 0·0148.
(B) OS for patients aged >80 years based on the new prognostic index of B-symptoms, gender (male) and haemoglobin <120 g/l. Log-rank test for group difference, P < 0·0001.
(C) OS for patients aged >80 years in the validation cohort using the SEER-Medicare database. Log-rank test for group difference, P = 0·0072.
FLIPI, Follicular Lymphoma International Prognostic Index; OS, overall survival; SEER, Surveillance, Epidemiology and End Results
Table V.
Patient and disease characteristics, treatment in the validation cohort using the SEER-Medicare database (n = 1333).
| Patient characteristics | n (%) |
|---|---|
| Female sex | 862 (64·7) |
|
| |
| White race | 1269 (95·2) |
|
| |
| Grade 3 disease | 200 (22·6) |
| Number of missing data | 448 |
|
| |
| Stage III/IV | 478 (39·4) |
| Number of missing data | 120 |
|
| |
| Anaemia | 56 (4·2) |
|
| |
| B-symptoms | 61 (8·2) |
| Number of missing data | 586 |
|
| |
| New prognostic index based on male, anaemia, and B-symptoms | |
| Good | 415 (55·0) |
| Intermediate | 292 (38·7) |
| Poor | 48 (6·4) |
| Number of missing data | 578 |
|
| |
| Geographic region | |
| Midwest | 210 (15·8) |
| Northeast | 326 (24·5) |
| South | 300 (22·5) |
| West | 497 (37·3) |
|
| |
| First-line treatment | |
| Watchful waiting | 690 (51·8) |
| R-mono | 250 (18·8) |
| R-chemo | 172 (12·9) |
| Other | 221 (16·6) |
| Chemo | 67 (30·3) |
| Radiotherapy | 154 (69·7) |
|
| |
| Year of diagnosis | |
| 1999–2001 | 265 (19·9) |
| 2002–2004 | 386 (29·0) |
| 2005–2007 | 446 (33·5) |
| 2008–2009 | 236 (17·7) |
Chemo, chemotherapy; R-chemo, rituximab plus chemotherapy; R-mono, rituximab monotherapy; SEER, Surveillance, Epidemiology and End Results
Validation
We examined this new prognostic index in a SEER-Medicare cohort of 1333 FL patients >80 years that had been analysed previously (Nastoupil et al, 2013). Table V shows patient characteristics of this cohort. Comparing to NLCS eFL cohort, the SEER-Medicare cohort had similar percentages of male gender, white race and grade 3 disease, but lower percentages of stage III/IV, anaemia and B-symptoms. The presence of 0, 1 or 2–3 factors of the new index segregated patients into groups with significantly different outcomes (unadjusted HRs for OS, intermediate 1·32 [1·08–1·62] poor 1·56 [1·09–2·24]) (Table VI, Fig 2c). Even after controlling for FL grade, stage, year of diagnosis, and treatment, the new index predicted for worse OS (HR, intermediate 1·33 [1·08–1·64], poor 1·50 [1·03–2·19]) in this cohort. Since SEER-Medicare does not collect all components of FLIPI, it is not possible to assess/compare the predictive ability of the new index to FLIPI in the validation cohort.
Table VI.
Comparison of PFS, OS, and LRM for patients aged >80 years by FLIPI index and the new prognostic index (based on haemoglobin <120 g/l, presence of B-symptoms and male sex).
| Hazard ratio (95% CI) | ||||
|---|---|---|---|---|
|
| ||||
| Cohort | Outcome | Risk group | New index | FLIPI |
| NLCS | OS | Low | 1 (Reference) | 1 (Reference) |
| Intermediate | 2·63 (1·39–4·98) | 1·16 (0·56–2·41) | ||
| High | 4·72 (2·38–9·33) | 2·09 (1·05–4·15) | ||
|
| ||||
| NLCS | PFS | Low | 1 (Reference) | 1 (Reference) |
| Intermediate | 1·66 (0·98–2·81) | 0·86 (0·46–1·59) | ||
| High | 2·32 (1·29–4·14) | 1·48 (0·83–2·63) | ||
|
| ||||
| NLCS | LRM | Low | 1 (Reference) | 1 (Reference) |
| Intermediate | 2·30 (0·85–6·26) | 1·93 (0·42–8·95) | ||
| High | 4·03 (1·39–11·69) | 4·49 (1·05–19·19) | ||
|
| ||||
| SEER | OS | Low | 1 (Reference) | 1 (Reference) |
| Intermediate | 1·32 (1·08–1·62) | N/A | ||
| High | 1.56 (1·09–2·24) | N/A | ||
CI, confidence interval; FLIPI, Follicular Lymphoma International Prognostic Index; LRM, lymphoma-related mortality; NLCS, National LymphoCare Study; OS, overall survival, PFS, progression-free survival, SEER, Surveillance, Epidemiology and End Results; N/A, not available
Discussion
In the largest prospective study of eFL patients published to date, we show that compared to younger patients, patients with FL who are >80 years less commonly received chemoimmunotherapy or anthracyclines, and more frequently received rituximab monotherapy or were observed. While the ORR was generally lower in eFL patients, responses to specific regimens did not vary by age except for anthracyclines. Furthermore, these variations in treatment selection and response did not impact survival in eFL patients. Another key finding of this study is that 38% of deaths in eFL patients were attributed to disease with a median OS of 5·7 years. In addition, we developed and validated a simple and practical prognostic index composed of anaemia, B-symptoms and male sex in eFL patients, which predicts inferior OS, PFS and LRM. With the caveats of observational study designs, our findings suggest that current approaches to the management of FL in elderly patients are associated with higher than expected LRM and no particular standard chemoimmunotherapy regimen among those evaluated was associated with superior OS compared with observation, although there was limited power to detect an OS difference between regimens. While this suggests that observation remains a viable strategy for elderly patients with FL, it also suggests that novel and more effective therapies are needed for the patients who still frequently die due to FL. Clinical trials that are specifically designed for these patients are therefore warranted.
Understanding disease characteristics, treatment patterns and outcomes in older patients is critical as the US population is aging. The number of persons >80 years has increased by more than 250% between 1960 and 2000. (Jemal et al, 2009) By 2015, this age group is expected to increase by another 50% (Mora & Zucca, 2007). Importantly, the SEER database suggests that approximately 10% of FL patients diagnosed are >80 years (Nabhan et al, 2014) and little is known about these patients and how best to treat their disease. While prognostic models have included age as an adverse factor in lymphomas, (Solal-Celigny et al, 2004; The International Non-Hodgkin’s Lymphoma Prognostic Factors Project, 1993) studies that have led to this conclusion included few patients >80 years and were conducted before the modern chemoimmunotherapy era. Whether old age affects outcome due to different disease biology in the elderly or due to older patients being treated less aggressively remains unknown (Hurria et al, 2009). Another factor that could affect outcome in the elderly patient population is dose density and intensity. Martin et al (2013) demonstrated that FL patients >70 years were less likely to receive 6 cycles of treatment, which could potentially contribute to lower responses and inferior outcomes.
Inferior outcomes in elderly patients may also be partially explained by concomitant morbidities (Extermann & Hurria, 2007). Our study indicates that eFL patients more commonly presented with grade 3 FL and is corroborated by studies in diffuse large B-cell lymphoma, which indicate that older patients may more commonly present with the more aggressive activated B-cell subtype (Armitage et al, 2007; Pfreundschuh, 2010). However, we were unable to assess comorbidity in our cohort.
More patients >80 years were observed compared with patients ≤60 years and when treated, rituximab monotherapy was commonly used. This is aligned with a large retrospective study of 303 lymphoma patients >80 years where 38% of patients with indolent B-cell lymphomas were observed, 21% received rituximab alone and 15% received chemoimmunotherapy (Nabhan et al, 2012). While we demonstrated that treatment-related toxicity did not contribute to increased mortality in eFL patients, early discontinuation might have accounted for inferior PFS in this cohort. Martin et al (2013) evaluated 1165 FL patients who received rituximab plus chemotherapy and showed that age ≥75 years was associated with early discontinuation of therapy. Whether the lack of treatment-related mortality in our eFL cohort as compared with their younger counterparts reflects the fact that they received less aggressive therapies with milder toxicities, remains to be elucidated.
Identifying predictors of inferior outcomes is critical in this era of competing therapies as tailoring treatments to specific individual patients’ characteristics could lead to improved outcomes. While some retrospective studies demonstrated that failure to achieve complete response and loss of activity of daily livings (ADLs) predicted inferior PFS and OS in indolent lymphoma patients >80 years, (Nabhan et al, 2012) our data lacked prospective geriatric assessments to allow better evaluation of the impact of ADLs, geriatric syndromes and organ dysfunction on outcomes. We showed that male sex predicted worse OS. Whether this reflects different toxicities, variable responses to specific regimens, or the fact that females have better OS than males in the general USA population remains unanswered. It is noteworthy to mention that several studies have shown differences in rituximab serum concentrations between men and women in a variety of lymphoma histologies, which intuitively could affect responses and outcomes (Muller et al, 2012; Jager et al, 2012). We subsequently constructed a new prognostic index utilizing lower haemoglobin, B-symptoms and gender, as these were the three critical factors predicting inferior OS. This new simple prognostic index was validated in the SEER-Medicare patient population and predicted inferior OS in this cohort as well (Fig 2c). Arguably, FLIPI is a prognostic index composed of readily available clinical information; however, our prospective data shows that some FL patients do not undergo staging bone marrow biopsies and their LDH values are missing, confounding the true prognostic score for these patients. As this proposed validated prognostic index is specifically designed for FL patients >80 years and as it utilizes anaemia, gender and B-symptoms, all of which are easily available to the treating physician, we believe that it can be used in the design of prospective studies enrolling eFL patients.
In summary, this report represents the largest prospective analysis of FL patients >80 years to date. This report supports the notion that, compared to younger FL patients, those >80 years have inferior outcomes when treated using our most modern approaches and a substantial number of these patients die from their disease. Together, these findings represent a baseline of outcomes in the modern therapeutic era, and support designing prospective trials for this patient population incorporating our prognostic score.
Acknowledgments
Grant Support: P30 008748
This study was funded by Genentech (South San Francisco, CA, USA)/F. Hoffmann-La Roche (Basel, Switzerland). Support for editorial assistance was funded by Genentech.
Footnotes
Author contribution
C.N. designed the concept and research question. CN contributed to the data assembly, analysis and interpretation, writing the manuscript and final approval.
M.B contributed to the data analysis and interpretation, manuscript writing and final approval of manuscript.
J.R.C contributed to the conception and design, data analysis and interpretation, manuscript writing and final approval of the manuscript.
K.D contributed to the data analysis and interpretation, manuscript writing and final approval of manuscript.
C.R.F contributed to the conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing and final approval of the manuscript.
J.W.F contributed to the collection and assembly of data, data analysis and interpretation, manuscript writing and final approval of the manuscript.
B.K.L contributed to the data analysis, manuscript writing and final approval of manuscript.
M.J.M contributed to the data analysis and interpretation, manuscript writing and final approval of manuscript.
A.R contributed to the data analysis and interpretation, manuscript writing and final approval of manuscript.
A.D.Z contributed to the conception and design, data analysis, manuscript writing and final approval of the manuscript.
X.Z contributed to the data analysis and interpretation, manuscript writing and final approval of the manuscript.
Presented in part as a poster at the 54th Annual Meeting of the American Society of Hematology, Atlanta, GA, December 2012, as an oral presentation at the 13th International Congress on Malignant Lymphoma Meeting, Lugano, Switzerland, June, 2013, and as a poster at the 55th Annual Meeting of the American Society of Hematology, New Orleans, LA, December 2013
Conflict-of-interest disclosures:
C.N discloses consulting/advisory board roles for Genentech, Janssen, Celgene, Astellas, and Gilead.
M.B discloses employment and stock options with Genentech.
J.R.C discloses consulting/advisory roles with Genentech.
K.D discloses employment and stock options with Genentech.
C.R.F discloses consulting/advisory roles with Algeta, OptumRx, Biogen Idec, Genentech BioOncology, Roche, Celgene and research funding from Abbott, Celgene, Millennium/Takeda, Spectrum, Gilead, and Janssen/Pharmacyclics.
J.W.F has no relevant conflict of interests to disclose.
B.K.L discloses honoraria from Genentech, consulting and advisory roles with Genentech, AbbVie, Gilead, and research funding from Genentech, Millennium, Pharmacyclics, and Janssen.
M.J.M has no relevant conflict of interests to disclose.
A.R has no relevant conflict of interests to disclose.
A.D.Z discloses consulting /advisory roles with Genentech, Celegene, Gilead, Amgen, Hospira, Reddy Laboratories and research funding from Genentech/Roche, Gilead, and BMS.
X.Z is employed by RTI-HS, which has a research contract with Genentech.
References
- Armitage JO. How I treat patients with diffuse large B-cell lymphoma. Blood. 2007;110:29–36. doi: 10.1182/blood-2007-01-041871. [DOI] [PubMed] [Google Scholar]
- Buske C, Hoster E, Dreyling M, Hasford J, Unterhalt M, Hiddemann W. The Follicular Lymphoma International Prognostic Index (FLIPI) separates high-risk from intermediate- or low-risk patients with advanced-stage follicular lymphoma treated front-line with rituximab and the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) with respect to treatment outcome. Blood. 2006;108:1504–1508. doi: 10.1182/blood-2006-01-013367. [DOI] [PubMed] [Google Scholar]
- Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. Journal of Clinical Oncology. 2007;25:1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- Federico M, Bellei M, Marcheselli L, Luminari S, Lopez-Guillermo A, Vitolo U, Pro B, Pileri S, Pulsoni A, Soubeyran P, Cortelazzo S, Martinelli G, Martelli M, Rigacci L, Arcaini L, Di Raimondo F, Merli F, Sabattini E, McLaughlin P, Solal-Celigny P. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. Journal of Clinical Oncology. 2009;27:4555–4562. doi: 10.1200/JCO.2008.21.3991. [DOI] [PubMed] [Google Scholar]
- Fisher RI, LeBlanc M, Press OW, Maloney DG, Unger JM, Miller TP. New treatment options have changed the survival of patients with follicular lymphoma. Journal of Clinical Oncology. 2005;23:8447–8452. doi: 10.1200/JCO.2005.03.1674. [DOI] [PubMed] [Google Scholar]
- Friedberg JW, Taylor MD, Cerhan JR, Flowers CR, Dillon H, Farber CM, Rogers ES, Hainsworth JD, Wong EK, Vose JM, Zelenetz AD, Link BK. Follicular lymphoma in the United States: first report of the national LymphoCare study. Journal of Clinical Oncology. 2009;27:1202–1208. doi: 10.1200/JCO.2008.18.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribben JG. How I treat indolent lymphoma. Blood. 2007;109:4617–4626. doi: 10.1182/blood-2006-10-041863. [DOI] [PubMed] [Google Scholar]
- Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in Medicine. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hurria A, Wong FL, Pal S, Chung CT, Bhatia S, Mortimer J, Somlo G, Hurvitz S, Villaluna D, Naeim A. Perspectives and attitudes on the use of adjuvant chemotherapy and trastuzumab in older adults with HER-2+ breast cancer: a survey of oncologists. Oncologist. 2009;14:883–890. doi: 10.1634/theoncologist.2009-0056. [DOI] [PubMed] [Google Scholar]
- Jager U, Fridrik M, Zeitlinger M, Heintel D, Hopfinger G, Burgstaller S, Mannhalter C, Oberaigner W, Porpaczy E, Skrabs C, Einberger C, Drach J, Raderer M, Gaiger A, Putman M, Greil R. Rituximab serum concentrations during immuno-chemotherapy of follicular lymphoma correlate with patient gender, bone marrow infiltration and clinical response. Haematologica. 2012;97:1431–1438. doi: 10.3324/haematol.2011.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Center MM, Ward E, Thun MJ. Cancer occurrence. Methods in Molecula Biology. 2009;471:3–29. doi: 10.1007/978-1-59745-416-2_1. [DOI] [PubMed] [Google Scholar]
- Marcus R, Imrie K, Solal-Celigny P, Catalano JV, Dmoszynska A, Raposo JC, Offner FC, Gomez-Codina J, Belch A, Cunningham D, Wassner-Fritsch E, Stein G. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. Journal of Clinical Oncology. 2008;26:4579–4586. doi: 10.1200/JCO.2007.13.5376. [DOI] [PubMed] [Google Scholar]
- Martin P, Byrtek M, Dawson K, Ziemiecki R, Friedberg JW, Cerhan JR, Flowers CR, Link BK. Patterns of delivery of chemoimmunotherapy to patients with follicular lymphoma in the United States: results of the National LymphoCare Study. Cancer. 2013;119:4129–4136. doi: 10.1002/cncr.28350. [DOI] [PubMed] [Google Scholar]
- Mora O, Zucca E. Management of elderly patients with hematological neoplasms. Annals of Oncology. 2007;18:i49–i53. doi: 10.1093/annonc/mdl451. [DOI] [PubMed] [Google Scholar]
- Muller C, Murawski N, Wiesen MH, Held G, Poeschel V, Zeynalova S, Wenger M, Nickenig C, Peter N, Lengfelder E, Metzner B, Rixecker T, Zwick C, Pfreundschuh M, Reiser M. The role of sex and weight on rituximab clearance and serum elimination half-life in elderly patients with DLBCL. Blood. 2012;119:3276–3284. doi: 10.1182/blood-2011-09-380949. [DOI] [PubMed] [Google Scholar]
- Nabhan C, Smith SM, Helenowski I, Ramsdale E, Parsons B, Karmali R, Feliciano J, Hanson B, Smith S, McKoy J, Larsen A, Hantel A, Gregory S, Evens AM. Analysis of very elderly (>/=80 years) non-hodgkin lymphoma: impact of functional status and co-morbidities on outcome. Britsh Journal of Haematology. 2012;156:196–204. doi: 10.1111/j.1365-2141.2011.08934.x. [DOI] [PubMed] [Google Scholar]
- Nabhan C, Aschebrook-Kilfoy B, Chiu BC, Kruczek K, Smith SM, Evens AM. The impact of race, age, and sex in Follicular Lymphoma: A comprehensive SEER analysis across consecutive treatment eras. American Journal of Hematology. 2014;8:633–638. doi: 10.1002/ajh.23708. [DOI] [PubMed] [Google Scholar]
- Nastoupil L, Rai A, Lipscomb J, Nabhan C, Williams JN, Ward K, Howard DH, Flowers CR. Disease Characteristics, Patterns Of Care, and Outcomes Of Follicular Lymphoma (FL) In The Oldest Old. Blood. 2013;122 Abstract 1817. [Google Scholar]
- Pfreundschuh M. How I treat elderly patients with diffuse large B-cell lymphoma. Blood. 2010;116:5103–5110. doi: 10.1182/blood-2010-07-259333. [DOI] [PubMed] [Google Scholar]
- The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. New England Journal of Medicine. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- Salles G, Seymour JF, Offner F, López-Guillermo A, Belada D, Xerri L, Feugier P, Bouabdallah R, Catalano JV, Brice P, Caballero D, Haioun C, Pedersen LM, Delmer A, Simpson D, Leppa S, Soubeyran P, Hagenbeek A, Casasnovas O, Intragumtornchai T, Fermé C, da Silva MG, Sebban C, Lister A, Estell JA, Milone G, Sonet A, Mendila M, Coiffier B, Tilly H. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377:42–51. doi: 10.1016/S0140-6736(10)62175-7. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2012. CA: A Cancer Journal for Clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Solal-Celigny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, Au WY, Bellei M, Brice P, Caballero D, Coiffier B, Conde-Garcia E, Doyen C, Federico M, Fisher RI, Garcia-Conde JF, Guglielmi C, Hagenbeek A, Haïoun C, LeBlanc M, Lister AT, Lopez-Guillermo A, McLaughlin P, Milpied N, Morel P, Mounier N, Proctor SJ, Rohatiner A, Smith P, Soubeyran P, Tilly H, Vitolo U, Zinzani PL, Zucca E, Montserrat E. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- Swenson WT, Wooldridge JE, Lynch CF, Forman-Hoffman VL, Chrischilles E, Link BK. Improved survival of follicular lymphoma patients in the United States. Journal of Clinical Oncology. 2005;23:5019–5026. doi: 10.1200/JCO.2005.04.503. [DOI] [PubMed] [Google Scholar]
- Witzig TE, Fishkin P, Gordon L, Gregory SA, Jacobs S, Macklis R, McLaughlin P, Press O, Zelenetz AD. Treatment recommendations for radioimmunotherapy in follicular lymphoma: a consensus conference report. Leukemia & Lymphoma. 2011;52:1188–1199. doi: 10.3109/10428194.2011.570396. [DOI] [PubMed] [Google Scholar]