Abstract
This study applied a case-control approach to investigate the association between low-grade inflammation, defined by high values within the normal range of C-reactive protein (CRP) and interleukin-6 (IL-6), and urinary markers of nucleic acid oxidation. No differences in excretion of urinary markers of nucleic acid oxidation between cases and controls were found and multivariable linear regression analysis showed no association between urinary markers of nucleic acid oxidation and inflammatory markers. Post-hoc multivariable linear regression analysis showed significant associations between nucleic acid oxidation and various iron status markers and especially a close relationship between nucleic acid oxidation and ferritin. This study shows no association between low-grade inflammation and urinary markers of nucleic acid oxidation in a population of elderly Italian people. The results suggest that low-grade inflammation only has a negligible impact on whole body nucleic acid oxidation, whereas iron status seems to be of great importance.
Keywords: DNA oxidation; inflammation; iron; 8-hydroxy-2´-deoxyguanosine; 8-oxo-7,8-dihydro-2 ’-deoxyguanosine; 8-oxodG; 8-oxo-7,8-dihydroguanosine; 8-oxoGuo
Introduction
Previous studies have demonstrated a strong association between chronic inflammation and increased risk of diseases such as cancer and cardiovascular disease [1–5]. One suggested link between inflammation and human disease is the increased production of free radicals at the site of inflammation and the resulting molecular changes, including DNA oxidation.
Several lines of evidence suggest an association between inflammation and DNA oxidation [6–8] and some studies have found positive associations between markers of DNA oxidation and inflammatory markers in peripheral blood [9–12]. One of the most thoroughly studied markers of DNA oxidation is the guanine modification 8-oxo-7,8-dihydro- 2’-deoxyguanosine (8-oxodG). 8-oxodG and the RNA ribonucleoside counterpart 8-oxo-7,8-dihydroguanosine (8-oxoGuo) are excreted in urine and represent rate estimates of the DNA and RNA oxidation in an intact organism [13].
The relationship between low-grade inflammation and nucleic acid oxidation has not been adequately investigated. In a cohort consisting of elderly people we applied a case-control approach to investigate the association between excretion of markers of nucleic acid oxidation (8-oxodG and 8-oxoGuo) in urine and low-grade inflammation, reflected in high levels within the normal range of serum C-reactive protein (CRP) as well as interleukin-6 (IL-6) in peripheral blood.
Materials and methods
Study population
InCHIANTI (Invecchiare in Chianti, ageing in the Chianti area) is an epidemiological study of risk factors contributing to the declining ability to walk in late life. The study was performed in two small towns located in Tuscany (Italy): Greve in Chianti and Bagno a Ripoli, between September 1998 and April 2000. The design and data collection methods of InCHIANTI are described in detail elsewhere [14]. One thousand two hundred and seventy persons 65 years or older were randomly selected from the population. Of these, 220 subjects were included in the present study. One hundred and ten cases were selected from the top tertile of both serum CRP and IL-6 and were matched by age and sex with 110 controls found in the lowest tertile of serum IL-6 and CRP. For four cases and five controls the remaining urine volume was insufficient and these nine subjects were not included in the analyses.
Participants received an extensive description of the study and participated after providing written informed consent. The Italian National Institute of Research and Care on Ageing Ethical Committee approved the study protocol, which complied with the principles stated in the Declaration of Helsinki.
Measures
Fasting blood samples were drawn using standardized conditions. Samples were stored at −80°C. High-sensitivity C-reactive protein was measured in duplicate using an enzyme-linked immunosorbent assay (ELISA) and colourimetric competitive immunoassay that uses purified protein and polyclonal anti-C-reactive protein antibodies. Levels of IL-6 were assessed using ELISA with ultrasensitive commercial kits (Human Ultrasensitive, BIOSOURCE International Inc, Camarillo, CA). Besides CRP and IL-6, the following inflammatory markers in peripheral blood were analysed: Fibrinogen, interleukin-1β (IL-1β), interleukin-1 receptor antagonist (IL-1RA), interleukin-6 receptor (IL-6R), interleukin-18 (IL-18) and tumour necrosis factor-alpha (TNFα). To account for the possible confounding effect of iron status, markers of iron status in serum (iron, haematocrit, haemoglobin, ferritin and soluble transferrin receptor (sTfR)) were analysed as well. Measurement of the supplementary inflammatory markers and markers of iron status are described in detail elsewhere [15,16].
Twenty-four-hour urine samples were assayed for the oxidatively modified nucleosides 8-oxodG and 8-oxoGuo using high-performance liquid chromatography-tandem mass spectrometry (HPLC MS/MS). Chromatographic separation was performed on a Perkin Elmer Series 200 HPLC equipped with two pumps, autosampler, solvent cabinet and vacuum degasser (Perkin Elmer, Norwalk, CT). The column used was a Phenomenex Prodigy ODS HPLC column (100 × 2 mm, 3 µ) protected with a C18 (ODS) guard column (4 × 2 mm), both obtained from Phenomenex (Torrance, CA). The mass spectrometric detection was performed on an API 3000 triple quadrupole mass spectrometer (Sciex, Toronto, Canada) equipped with a turboionspray source (Turbospray). Details of the analysis are described elsewhere [17].
Completeness of urine collection was evaluated using 24-h urinary creatinine excretion. Subjects with urinary creatinine level < 6 mmol/day plus total urine volume < 1000 mL/day or with a urinary creatinine level < 5 mmol/day were identified as having incomplete urine [18].
Statistical analysis
The distribution of basic characteristics, inflammation markers and markers of iron status and nucleic acid oxidation was evaluated for the cases and controls by median and inter-quartile range for continuous variables and by frequency and within-group percentage for categorical variables. Statistical significance of the differences between cases and controls was assessed by the p-value of a Mann-Whitney test for continuous variables and of a chi-squared test for categorical variables.
The association between nucleic acid oxidation and inflammation status was assessed in a conditional logistic regression model to account for the matching on sex and age and effect estimates were furthermore adjusted for body weight and height, iron concentration and smoking status. Additionally, for each of the matching variables, the covariates interaction with nucleic acid oxidation was investigated in the conditional logistic regression model.
The association between the numerical values of the nucleic acid oxidation and the various inflammation markers and iron status markers was assessed by the regression coefficient of a multivariate regression analysis. The Spearman rank correlation coefficient was calculated to assess the association between ferritin and urinary 8-oxodG and 8-oxoGuo concentrations and the association between urinary 8-oxodG and 8-oxoGuo. To adjust for multiple testing we controlled for the false discovery rate at 5% [19]. All statistical analyses were performed using the SAS software version 9.1 (SAS Institute Inc. Cary, NC). Statistical significance was defined as p < 0.05.
Results
The basic characteristics of the study population are shown in Table I. Smoking status and body weight differed significantly between cases and controls. Control subjects had higher body weight and a higher proportion of cases were smokers. Cases had significantly lower levels of iron and significantly higher levels of soluble transferrin receptor than controls.
Table I.
Characteristics of the InChianti case-control study population (n = 211).
| Variable | Cases (n = 106) | Controls (n = 105) | pa |
|---|---|---|---|
| Basic characteristics | |||
| n (male/female) | 106 (54/52) | 105 (54/51) | 0.9438 |
| Age (years) | 73 (69–76) | 72 (68–75) | 0.4939 |
| Smoking habits, n (%) | 0.0046 | ||
| Never smoked | 43 (42.2) | 63 (60.0) | |
| Ex-smoker | 30 (29.4) | 30 (28.6) | |
| Current smoker | 29 (28.4) | 12 (11.4) | |
| Weight (kg) | 69.0 (60.5–76.5) | 72.6 (65.5–81.0) | 0.0306 |
| Height (cm) | 159 (154–167) | 160 (153–168) | 0.8182 |
| BMI (kg/m2) | 26.8 (24.2–28.8) | 27.5 (25.3–30.1) | 0.0609 |
| Inflammatory markers | |||
| CRP (µg/ml) | 8.62 (5.68–14.10) | 0.97 (0.62–1.27) | < 0.0001 |
| IL-6 (pg/ml) | 3.22 (2.31–4.88) | 0.63 (0.46–0.82) | < 0.0001 |
| Fibrinogen (mg/dl) | 407 (354–464) | 315 (284–343) | < 0.0001 |
| IL-1β (pg/ml) | 0.12 (0.08–0.21) | 0.11 (0.08–0.16) | 0.0945 |
| IL-1RA (pg/ml) | 174.56 (118.77–262.99) | 103.57 (80.73–144.82) | < 0.0001 |
| IL-6R (ng/ml) | 108.94 (77.0–137.54) | 84.57 (60.08–109.79) | 0.0009 |
| IL-18 (pg/ml) | 408.71 (326.76–478.73) | 344.34 (253.15–454.72) | 0.0035 |
| TNF-α (pg/ml) | 4.97 (3.63–6.96) | 3.89 (2.97–5.25) | < 0.0001 |
| Iron status | |||
| Iron (µg/dl) | 71 (58–92) | 91 (78–104) | < 0.0001 |
| Haematocrit (%) | 41.5 (38.7–43.9) | 41.3 (38.9–43.4) | 0.4985 |
| Haemoglobin (g/dl) | 14.0 (13.0–14.9) | 13.95 (13.00–15.10) | 0.9737 |
| Ferritin (ng/ml) | 117.5 (55.0–195.0) | 116 (60–221) | 0.5691 |
| sTfR (nmol/l) | 16.8 (13.6–20.0) | 15.2 (12.7–17.6) | 0.0082 |
| Nucleic acid oxidation markers | |||
| 8-oxodG (nmol/24 h) | 14.28 (10.10–19.03) | 15.64 (11.07–20.53) | 0.0939 |
| 8-oxoGuo (nmol/24 h) | 24.36 (19.69–32.76) | 26.93 (20.12–35.11) | 0.2694 |
| 8-oxodG (nmol/kg/24 h) | 0.20 (0.15–0.28) | 0.22 (0.16–0.29) | 0.3687 |
| 8-oxoGuo (nmol/kg/24 h) | 0.39 (0.29–0.47) | 0.37 (0.27–0.49) | 0.9952 |
Data are medians (interquartile range) unless otherwise stated.
Significance (p-value) of the difference between cases and controls was assessed with a Mann-Whitney test for continuous variables and with a chi-squared test for categorical variables.
BMI, Body Mass Index; CRP, C-reactive protein; IL-6, Interleukin-6; IL-1β, Interleukin-1β; IL-1RA, Interleukin-1 receptor antagonist; IL-6R, Interleukin-6 receptor; IL-18, Interleukin-18; TNFα, Tumour necrosis factor-alpha; sTfR, Soluble Transferrin Receptor; 8-oxodG, 8-oxo-7,8-dihydro-2’-deoxyguanosine; 8-oxoGuo, 8-oxo-7,8-dihydroguanosine.
Using urinary creatinine to identify incomplete 24-h urine collection, no difference in completeness of 24-h urine collection was found between cases and controls (10% vs 14% incomplete, respectively, p = 0.30).
We found no statistical significant difference in the excretion of 8-oxodG and 8-oxoGuo between cases and controls, regardless of adjustment for weight Table I), although analysis of unadjusted data showed a tendency towards higher excretion of 8-oxodG p = 0.09) in controls.
Table II shows the results of the logistic regression analysis of the case-control data, where the excretion of nucleic acid oxidation markers were adjusted for gender, age, body weight, height, iron concentration and smoking status. The adjusted analysis confirms our results of the unadjusted analysis, revealing no difference in the excretion of 8-oxodG and 8-oxoGuo between cases and controls. None of the variables sex, age, smoking status, iron status, body weight and height showed significant interaction with nucleic acid oxidation (data not shown).
Table II.
Conditional logistic regression analysis of the case-control data 1:1 matched for sex and age.
| Tertiles of nucleic acid oxidation | ||||
|---|---|---|---|---|
| Nucleic acid oxidation | Low OR (95%CI) | Middle ORa (95%CI) | High ORa (95%CI) | pb |
| 8-oxodG (nmol/24 h) | 1.000 | 0.880 (0.387;2.001) | 0.566 (0.230;1.392) | 0.4430 |
| 8-oxoGuo (nmol/24 h) | 1.000 | 1.370 (0.565;3.318) | 0.757 (0.295;1.945) | 0.4214 |
| 8-oxodG (nmol/kg/24 h) | 1.000 | 0.875 (0.379;2.022) | 0.498 (0.219;1.137) | 0.2162 |
| 8-oxoGuo (nmol/kg/24 h) | 1.000 | 1.446 (0.603;3.465) | 0.604 (0.244;1.498) | 0.1664 |
The association of each of four nucleic acid oxidation evaluations (8-oxodG and 8-oxoGuo both unadjusted and per kg of body weight, each represented as categorical variables with its tertiles as categories) with high inflammation status (case). An odds ratio greater than 1 indicates greater probability of being case.
The analysis is matched on sex and age and furthermore adjusted for body weight and height and iron concentration (all dichotomised at the median) and smoking status.
Wald Chi-squared test for the overall significance of the nucleic acid oxidation variables.
8-oxodG, 8-oxo-7,8-dihydro-2’-deoxyguanosine; 8-oxoGuo, 8-oxo-7,8-dihydroguanosine.
Multivariable linear regression analysis of the nucleic acid oxidation variables on various inflammation markers (Table III) showed significant association between 8-oxoGuo (nmol/24 h) and TNF-α(p = 0.02), but when expressed per body weight and/or corrected for multiple testing this was not significant. No significant association was found between nucleic acid oxidation and any of the other inflammatory markers.
Table III.
Multivariable linear regression analysis of the nucleic acid oxidation variables on various inflammation markers.
| Nucleic acid oxidation | Regression coefficient a of the inflammation marker (95%CI) | pb |
|---|---|---|
| CRP (µg/ml) | ||
| 8-oxodG (nmol/24 h) | −0.0539 (−0.1526;0.0448) | 0.2828 |
| 8-oxoGuo (nmol/24 h) | 0.1994 (−0.0432;0.4421) | 0.1066 |
| 8-oxodG (nmol/kg/24 h) | −0.0006 (−0.0020;0.0007) | 0.3577 |
| 8-oxoGuo (nmol/kg/24 h) | 0.0030 (−0.0007;0.0066) | 0.1120 |
| IL-6 (pg/ml) | ||
| 8-oxodG (nmol/24 h) | −0.2880 (−0.7651;0.1891) | 0.2352 |
| 8-oxoGuo (nmol/24 h) | −0.1605 (−0.9778;0.6568) | 0.6987 |
| 8-oxodG (nmol/kg/24 h) | −0.0036 (−0.0101;0.0030) | 0.2852 |
| 8-oxoGuo (nmol/kg/24 h) | −0.0033 (−0.0156;0.0090) | 0.5870 |
| Fibrinogen (mg/dl) | ||
| 8-oxodG (nmol/24 h) | −0.0030 (−0.0173;0.0113) | 0.6804 |
| 8-oxoGuo (nmol/24 h) | 0.0091 (−0.0162;0.0345) | 0.6987 |
| 8-oxodG (nmol/kg/24 h) | −0.0000 (−0.0002;0.0002) | 0.6905 |
| 8-oxoGuo (nmol/kg/24 h) | 0.0001 (−0.0003;0.0005) | 0.5809 |
| IL-1β (pg/ml) | ||
| 8-oxodG (nmol/24 h) | −0.1917 (−1.1975;0.8141) | 0.7073 |
| 8-oxoGuo (nmol/24 h) | −0.5753 (−2.1573;1.0067) | 0.4738 |
| 8-oxodG (nmol/kg/24 h) | −0.0008 (−0.0146;0.0130) | 0.9094 |
| 8-oxoGuo (nmol/kg/24 h) | −0.0059 (−0.0296;0.0179) | 0.6273 |
| IL-1RA (pg/ml) | ||
| 8-oxodG (nmol/24 h) | 0.0025 (−0.0039;0.0088) | 0.4460 |
| 8-oxoGuo (nmol/24 h) | 0.0026 (−0.0079;0.0132) | 0.6203 |
| 8-oxodG (nmol/kg/24 h) | 0.0000 (−0.0000;0.0001) | 0.3924 |
| 8-oxoGuo (nmol/kg/24 h) | 0.0000 (−0.0001;0.0002) | 0.5598 |
| IL-6R (ng/ml) | ||
| 8-oxodG (nmol/24 h) | −0.0182 (−0.0414;0.0050) | 0.1235 |
| 8-oxoGuo (nmol/24 h) | −0.0199 (−0.0596;0.0198) | 0.3242 |
| 8-oxodG (nmol/kg/24 h) | −0.0002 (−0.0006;0.0001) | 0.1446 |
| 8-oxoGuo (nmol/kg/24 h) | −0.0002 (−0.0008;0.0003) | 0.4144 |
| IL-18 (pg/ml) | ||
| 8-oxodG (nmol/24 h) | 0.0018 (−0.0065;0.0100) | 0.6715 |
| 8-oxoGuo (nmol/24 h) | 0.0009 (−0.0124;0.0143) | 0.8892 |
| 8-oxodG (nmol/kg/24 h) | 0.0000 (−0.0001;0.0001) | 0.9319 |
| 8-oxoGuo (nmol/kg/24 h) | −0.0000 (−0.0002;0.0002) | 0.9397 |
| TNF-α (pg/ml) | ||
| 8-oxodG (nmol/24 h) | 0.0435 (−0.0333;0.1204) | 0.2648 |
| 8-oxoGuo (nmol/24 h) | 0.1380 (−0.0196;0.2565) | 0.0227 |
| 8-oxodG (nmol/kg/24 h) | 0.0005 (−0.0006;0.0015) | 0.3845 |
| 8-oxoGuo (nmol/kg/24 h) | 0.0016 (−0.0002;0.0034) | 0.0754 |
The analysis is adjusted for age, body weight and height and iron concentration (all continuously valued), sex and smoking status.
T-test for the significance of the regression coefficient. To control for the false discovery rate at 5%, p-values lower than 0.0016 are considered significant.
CRP, C-reactive protein; IL-6, Interleukin-6; IL-1β, Interleukin-1; IL-1RA, Interleukin-1 receptor antagonist; IL-6R, Interleukin-6 receptor; IL-18, Interleukin-18; TNFα, Tumour necrosis factor-alpha; 8-oxodG, 8-oxo-7,8-dihydro-2’-deoxyguanosine; 8-oxoGuo, 8-oxo-7, 8-dihydroguanosine.
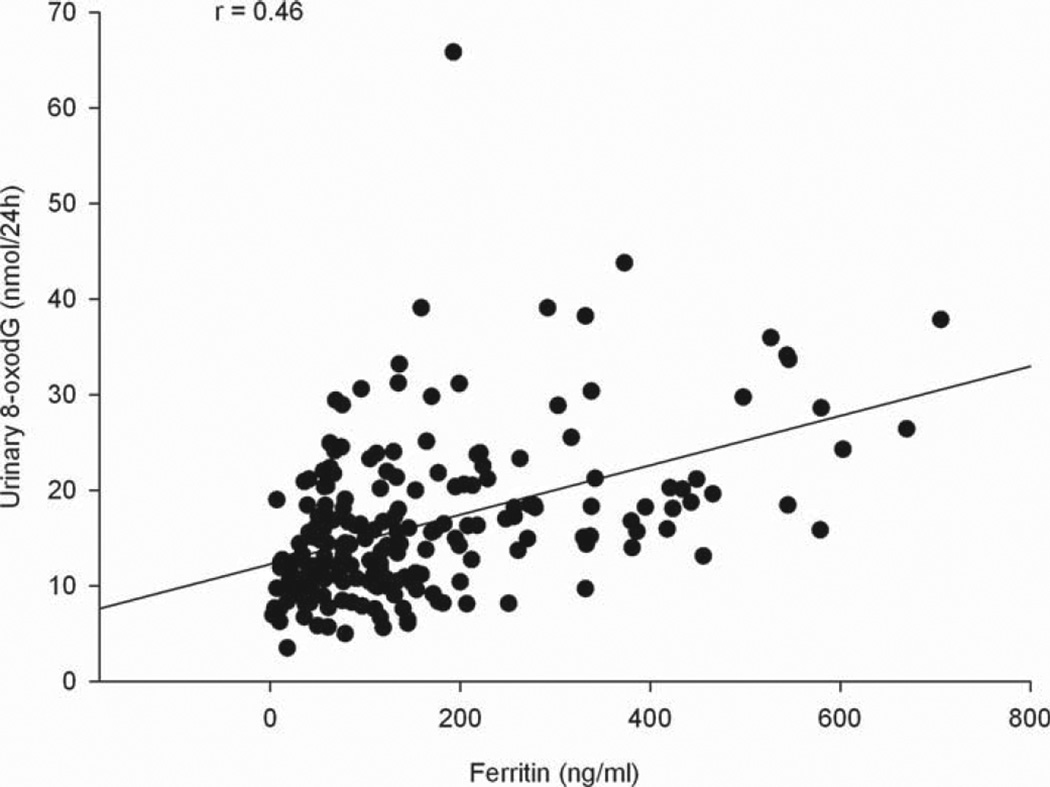
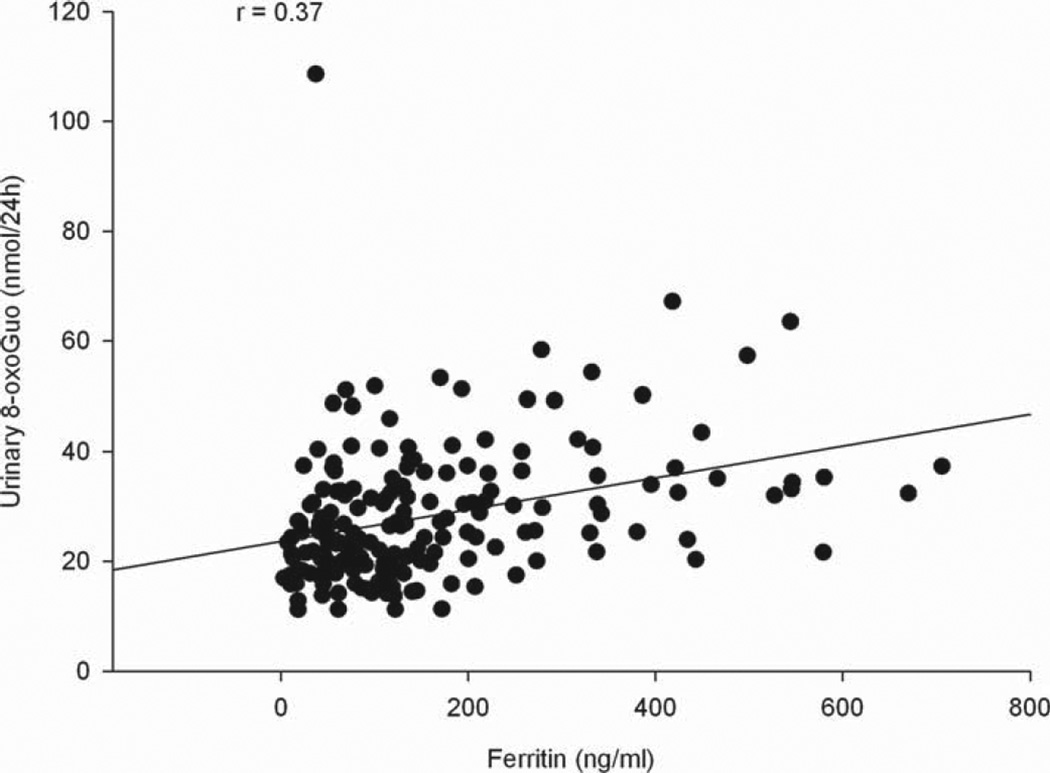
Multivariable linear regression analysis of the nucleic acid oxidation variables on iron status markers (Table IV) revealed significant association between nucleic acid oxidation and iron, ferritin and soluble transferrin receptor concentration. A highly significant positive association between ferritin and nucleic acid oxidation markers was shown (all nucleic acid oxidation variables, p < 0.0001), with the Spearman rank correlation coefficient being 0.46 and 0.37 for 8-oxodG (nmol/24 h) and 8-oxoGuo (nmol/24 h), respectively (Figures 1 and 2). 8-oxodG was significantly associated with iron (8-oxodG (nmol/24 h), p = 0.0003 and 8-oxodG (nmol/kg/24 h), P= 0.0006), and a significant association between iron and 8-oxoGuo (nmol/24 h) was shown (p = 0.01). An inverse association between the RNA oxidation marker 8-oxoGuo and soluble TfR was observed (8-oxoGuo (nmol/24 h), p = 0.01 and 8-oxoGuo (nmol/kg/24 h), p = 0.02). In addition, we found significant associations between 8-oxoGuo (nmol/kg/24 h) and iron, 8-oxodG (nmol/24 h and nmol/kg/24 h) and haemoglobin and a significant inverse association between 8-oxodG (nmol/24 h) and sTfR, but when corrected for multiple testing these associations were no longer significant.
Table IV.
Multivariable linear regression analysis of the nucleic acid oxidation variables on various markers of iron status.
| Nucleic acid oxidation | Regression coefficienta of the marker of iron status (95%CI) | pb |
|---|---|---|
| Iron (µg/dl) | ||
| 8-oxodG (nmol/24 h) | 0.0853 (0.0399;0.1307) | 0.0003 |
| 8-oxoGuo (nmol/24 h) | 0.0958 (0.0221;0.1694) | 0.0111 |
| 8-oxodG (nmol/kg/24 h) | 0.0011 (0.0005;0.0017) | 0.0006 |
| 8-oxoGuo (nmol/kg/24 h) | 0.0012 (0.0001;0.0023) | 0.0366 |
| Haematocrit (%) | ||
| 8-oxodG (nmol/24 h) | 0.3399 (−0.0801;0.7598) | 0.1121 |
| 8-oxoGuo (nmol/24 h) | 0.4453 (−0.2344;1.1250) | 0.1977 |
| 8-oxodG (nmol/kg/24 h) | 0.0044 (−0.0014;0.0101) | 0.1333 |
| 8-oxoGuo (nmol/kg/24 h) | 0.0062 (−0.0040;0.0163) | 0.2311 |
| Haemoglobin (g/dl) | ||
| 8-oxodG (nmol/24 h) | 1.2161 (0.0847;2.3474) | 0.0353 |
| 8-oxoGuo (nmol/24 h) | 1.6297 (−0.2059;3.4652) | 0.0815 |
| 8-oxodG (nmol/kg/24 h) | 0.0156 (0.0001;0.0311) | 0.0484 |
| 8-oxoGuo (nmol/kg/24 h) | 0.0223 (−0.0051;0.0497) | 0.1105 |
| Ferritin (ng/ml) | ||
| 8-oxodG (nmol/24 h) | 0.0230 (0.0157;0.0302) | < 0.0001 |
| 8-oxoGuo (nmol/24 h) | 0.0268 (0.0143;0.0392) | < 0.0001 |
| 8-oxodG (nmol/kg/24 h) | 0.0003 (0.0002;0.0004) | < 0.0001 |
| 8-oxoGuo (nmol/kg/24 h) | 0.0004 (0.0002;0.0006) | < 0.0001 |
| sTfR (nmol/l) | ||
| 8-oxodG (nmol/24 h) | −0.1913 (−0.3782;−0.0044) | 0.0449 |
| 8-oxoGuo (nmol/24 h) | −0.3795 (−0.6730;−0.0859) | 0.0116 |
| 8-oxodG (nmol/kg/24 h) | −0.0025 (−0.0051;0.0001) | 0.0551 |
| 8-oxoGuo (nmol/kg/24 h) | −0.0053 (−0.0097;−0.0009) | 0.0180 |
The analysis is adjusted for age, body weight and height (all continuously valued), sex and smoking status.
T-test for the significance of the regression coefficient. To control for the false discovery rate at 5%, p-values lower than 0.0225 are considered significant.
sTfR, Soluble Transferrin Receptor; 8-oxodG, 8-oxo-7,8-dihydro-2’-deoxyguanosine; 8-oxoGuo, 8-oxo-7,8-dihydroguanosine.
Figure 1.
Relationship between ferritin concentration and urinary 8-oxodG excretion.
Figure 2.
Relationship between ferritin concentration and urinary 8-oxoGuo excretion.
The two nucleic acid oxidation markers 8-oxodG and 8-oxoGuo were shown to be closely correlated (p <0.0001), with the Spearman rank correlation coefficient being 0.68.
Discussion
No association between inflammation and urinary markers of nucleic acid oxidation was shown in this study on normal elderly people, with inflammatory markers within the normal range. This is not in accordance with the general perception that inflammation and oxidative stress are inseparable phenomena [20–24] and indicates that a threshold of inflammation must be reached before an association between inflammation and urinary markers of nucleic acid oxidation is seen.
Studies of the association between urinary 8-oxodG and inflammation are inconclusive [10,11,25,26].
Studies using different methodological approaches have shown results that are in agreement with ours. Two studies of the relationship between Helicobacter pylori infection and urinary 8-oxodG did not show association between inflammation and 8-oxodG. In a study of children aged 5–18 years, Siomek et al. [25] did not find a difference in urinary 8-oxodG (HPLC/GC/MS) between the tree study groups: Control group (n = 20), gastritis with H. pylori infection (n = 23) and gastritis without H. pylori infection (n = 20). Shimizu et al. [26] could not find an association between the degree of inflammatory cell infiltration and the level of urinary 8-oxodG (ELISA) in 15 children and 13 parents suffering from H. pylori infection.
Other studies have shown results that are more in accordance with the generally accepted understanding of the relationship between oxidative stress and inflammation.
Sakano et al. [11] found a significant positive association between urinary 8-oxodG (ELISA) and CRP in females (p = 0.006), but not in males (p = 0.595) or in the whole study group (p = 0.067) in 677 healthy Japanese people aged 20–67 years. Crohns et al. [10] reported significant positive associations between urinary 8-oxodG (HPLC/GC/MS) and CRP in a study group consisting of 36 non-cancer controls (aged 18–75 years) and 65 lung cancer patients (aged 48–83 years). The correlation between urinary 8-oxodG and CRP was significant in the lung cancer group (p = 0.016) but not in the whole study group (p = 0.06) and the correlation in the control group was not mentioned in the work by Crohns et al.
The discordance between these findings of positive associations between urinary 8-oxodG and inflammation and our results can be explained in several ways: The positive association between urinary 8-oxodG and CRP was only found in sub-groups. When looking at the association in the whole study group, there was actually no discordance between our study and the studies by Sakano et al. [11] and Crohns et al. [10], since no significant associations were found here.
Different methodological approaches could account for some inconsistency. Sakano et al. [11] used ELISA for the determination of 8-oxodG. ELISA is less specific than HPLC and the ELISA kit may be cross-reactive towards other compounds. The collection of urine was different in the studies. In our study we used 24-h collection, while Crohns et al. [10] and Sakano et al. [11] used collection for shorter periods normalized to creatinine concentration.
The population in the study by Crohns et al. [10] consisted of lung cancer patients. Controls were patients referred for bronchoscopy due to prolonged cough, who had several pathological conditions including acute and chronic inflammation, whereas our study population were primarily healthy subjects. A comparison between patients (with or without cancer) and primarily healthy subjects is problematic, as different levels of inflammation and nucleic acid oxidation and repair are expected. In this case, possible explanations for the difference in results could be that an association may only hold for ‘ill’ patients (or ‘ill’ vs ‘healthy’) or that the range of nucleic acid oxidation and inflammatory markers in our study of healthy subjects is too small and any association is masked by natural variation.
Further studies are needed to examine the impact of higher levels of inflammation on urinary markers of nucleic acid oxidation.
Some limitations of our study should be noted. First, the size of our study population is limited to 220 persons, which could result in too little statistical power to detect small differences. Our study had 80% power to detect an effect size of 0.35, reflecting a small-to-medium difference. Second, only elderly people were included in our study population. This makes it difficult to extrapolate our results to the population in general. Differences in morbidity, hormonal status, etc., between young and older people could have an impact on the relationship between oxidative stress and inflammation.
One interpretation of our results is that urinary 8-oxodG does not reflect inflammation at all, but, considering the substantial evidence of an association between oxidative stress and inflammation, we suggest another explanation. As urinary 8-oxodG reflects whole body DNA oxidation, it is possible that the small variability at low-grade inflammation leads to a lack of association. The substantial inter-individual variability in the urinary excretion of 8-oxodG [27] makes it likely that a small contribution of nucleic acids oxidation resulting from low-grade inflammation is masked by the overall variation and that a significant association is seen only at a greater inflammatory burden, resulting in higher levels of inflammatory markers in blood.
The relationship between chronic inflammation and iron status is well described [28] and studies indicate that body iron storage is an important determinant of oxidative damage to DNA [29]. To control our analysis for the possible confounding effect of iron status on nucleic acid oxidation, our analyses were adjusted for iron concentration and, to further elucidate the relationship between nucleic acid oxidation and iron status, we performed a post-hoc multivariable linear regression analysis of the nucleic acid oxidation variables on iron status markers. In our study the previously shown close positive association between ferritin and 8-oxodG/8-oxoGuo [29–32] was confirmed. In addition, a positive association between nucleic acid oxidation and iron was demonstrated and the expected inverse association between nucleic acid oxidation and soluble transferring receptor was shown, although this was only significant for RNA-oxidation. These results indicate that nucleic acid oxidation is tightly linked to iron status, where storage iron, as reflected by ferritin levels, seems to be of greatest importance. This also suggests that the possible confounding effect of iron status should be taken into account whenever possible, when conducting clinical studies on nucleic acid oxidation.
In conclusion, this study shows no association between low-grade inflammation and urinary markers of nucleic acid oxidation in a population of elderly Italian people. The present findings suggest that low-grade inflammation only has a negligible impact on whole body nucleic acid oxidation and that at low-grade inflammation other factors are more significant determinants of nucleic acid oxidation, where iron status seems to be of great importance.
Acknowledgments
Chemist Peter R Hillestrøm and lab technicians Mads Sabroe and Bjørg Lindblom performed the LC-MS analysis.
This study was supported by research funding from the Research Committee at Copenhagen University Hospital–Rigshospitalet. The authors alone are responsible for the content and writing of the paper.
Footnotes
Declaration of interest
The authors report no conflicts of interest.
References
- 1.Kaptoge S, Di AE, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB. Inflammatory markers and the risk of coronary heart disease in men and women. N Eng J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 3.Boekholdt SM, Hack CE, Sandhu MS, Luben R, Bingham SA, Wareham NJ, Peters RJG, Jukema JW, Day NE, Kastelein JJP, Khaw KT. C-reactive protein levels and coronary artery disease incidence and mortality in apparently healthy men and women: the EPIC-Norfolk prospective population study 1993–2003. Atherosclerosis. 2006;187:415–422. doi: 10.1016/j.atherosclerosis.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 5.Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol. 2009;27:2217–2224. doi: 10.1200/JCO.2008.19.8440. [DOI] [PubMed] [Google Scholar]
- 6.Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol Chem. 2006;387:365–372. doi: 10.1515/BC.2006.049. [DOI] [PubMed] [Google Scholar]
- 7.Altindag O, Karakoc M, Kocyigit A, Celik H, Soran N. Increased DNA damage and oxidative stress in patients with rheumatoid arthritis. Clin Biochem. 2007;40:167–171. doi: 10.1016/j.clinbiochem.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Bolukbas C, Bolukbas FF, Kocyigit A, Aslan M, Selek S, Bitiren M, Ulukanligil M. Relationship between levels of DNA damage in lymphocytes and histopathological severity of chronic hepatitis C and various clinical forms of hepatitis B. J Gastroenterol Hepatol. 2006;21:610–616. doi: 10.1111/j.1440-1746.2005.04069.x. [DOI] [PubMed] [Google Scholar]
- 9.Haghdoost S, Maruyama Y, Pecoits-Filho R, Heimburger O, Seeberger A, Anderstam B, Suliman ME, Czene S, Lindholm B, Stenvinkel P, Harms-Ringdahl M. Elevated serum 8-oxo-dG in hemodialysis patients: a marker of systemic inflammation? Antiox Redox Signal. 2006;8:2169–2173. doi: 10.1089/ars.2006.8.2169. [DOI] [PubMed] [Google Scholar]
- 10.Crohns M, Saarelainen S, Erhola M, Alho H, Kellokumpu-Lehtinen P. Impact of radiotherapy and chemotherapy on biomarkers of oxidative DNA damage in lung cancer patients. Clin Biochem. 2009;42:1082–1090. doi: 10.1016/j.clinbiochem.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Sakano N, Wang DH, Takahashi N, Wang BL, Sauriasari R, Kanbara S, Sato Y, Takigawa T, Takaki J, Ogino K. Oxidative stress biomarkers and lifestyles in Japanese healthy people. J Clin Biochem Nutr. 2009;44:185–195. doi: 10.3164/jcbn.08-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demirbag R, Yilmaz R, Gur M, Kocyigit A, Celik H, Guzel S, Selek S. Lymphocyte DNA damage in patients with acute coronary syndrome and its relationship with severity of acute coronary syndrome. Mutat Res Fund Mol Mech Mutagenesis. 2005;578:298–307. doi: 10.1016/j.mrfmmm.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Poulsen HE. Oxidative DNA modifi cations. Exp Toxicol Pathol. 2005;57(Suppl 1):161–169. doi: 10.1016/j.etp.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Ferrucci L, Bandinelli S, Benvenuti E, Di IA, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 15.McDermott MM, Guralnik JM, Corsi A, Albay M, Macchi C, Bandinelli S, Ferrucci L. Patterns of inflammation associated with peripheral arterial disease: the InCHIANTI study. Am Heart J. 2005;150:276–281. doi: 10.1016/j.ahj.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 16.Ferrucci L, Guralnik JM, Bandinelli S, Semba RD, Lauretani F, Corsi A, Ruggiero C, Ershler WB, Longo DL. Unexplained anaemia in older persons is characterised by low erythropoietin and low levels of pro-inflammatory markers. Br J Haematol. 2007;136:849–855. doi: 10.1111/j.1365-2141.2007.06502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weimann A, Belling D, Poulsen HE. Quantification of 8-oxo-guanine and guanine as the nucleobase, nucleoside and deoxynucleoside forms in human urine by high-performance liquid chromatography-electrospray tandem mass spectrometry. Nucl Acids Res. 2002;30:E7. doi: 10.1093/nar/30.2.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murakami K, Sasaki S, Takahashi Y, Uenishi K, Watanabe T, Kohri T, Yamasaki M, Watanabe R, Baba K, Shibata K, Takahashi T, Hayabuchi H, Ohki K, Suzuki J. Sensitivity and specificity of published strategies using urinary creatinine to identify incomplete 24-h urine collection. Nutrition. 2008;24:16–22. doi: 10.1016/j.nut.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Benjamini Y, Hochberg Y. Controlling the false discovery rate - A practical and powerful approach to multiple testing. J Roy Stat Soc Ser B Methodol. 1995;57:289–300. [Google Scholar]
- 20.Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 21.Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456–1462. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 22.Vaziri ND, Rodriguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006;2:582–593. doi: 10.1038/ncpneph0283. [DOI] [PubMed] [Google Scholar]
- 23.Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121:2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 24.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 25.Siomek A, Rytarowska A, Szaflarska-Poplawska A, Gackowski D, Rozalski R, Dziaman T, Czerwionka-Szaflarska M, Olinski R. Helicobacter pylori infection is associated with oxidatively damaged DNA in human leukocytes and decreased level of urinary 8-oxo-7,8-dihydroguanine. Carcinogenesis. 2006;27:405–408. doi: 10.1093/carcin/bgi238. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu T, Lee T, Shoji H, Kudo T, Satoh Y, Yamashiro Y. Urinary 8-hydroxydeoxyguanosine excretion in children before and after therapy for eradication of Helicobacter pylori infection. Acta Paediatr. 2003;92:1026–1028. doi: 10.1080/08035250310004766. [DOI] [PubMed] [Google Scholar]
- 27.Kasai H, Iwamoto-Tanaka N, Miyamoto T, Kawanami K, Kawanami S, Kido R, Ikeda M. Life style and urinary 8-hydroxydeoxyguanosine, a marker of oxidative dna damage: effects of exercise, working conditions, meat intake, body mass index, and smoking. Jpn J Cancer Res. 2001;92:9–15. doi: 10.1111/j.1349-7006.2001.tb01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganz T, Nemeth E. Iron sequestration and anemia of inflammation. Semin Hematol. 2009;46:387–393. doi: 10.1053/j.seminhematol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hori A, Mizoue T, Kasai H, Kawai K, Matsushita Y, Nanri A, Sato M, Ohta M. Body iron store as a predictor of oxidative DNA damage in healthy men and women. Cancer Sci. 2010;101:517–522. doi: 10.1111/j.1349-7006.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakano M, Kawanishi Y, Kamohara S, Uchida Y, Shiota M, Inatomi Y, Komori T, Miyazawa K, Gondo K, Yamasawa I. Oxidative DNA damage (8-hydroxydeoxyguanosine) and body iron status: a study on 2507 healthy people. Free Radic Biol Med. 2003;35:826–832. doi: 10.1016/s0891-5849(03)00432-5. [DOI] [PubMed] [Google Scholar]
- 31.Broedbaek K, Poulsen HE, Weimann A, Kom GD, Schwedhelm E, Nielsen P, Boger RH. Urinary excretion of biomarkers of oxidatively damaged DNA and RNA in hereditary hemochromatosis. Free Radic Biol Med. 2009;47:1230–1233. doi: 10.1016/j.freeradbiomed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Tuomainen TP, Loft S, Nyyssonen K, Punnonen K, Salonen JT, Poulsen HE. Body iron is a contributor to oxidative damage of DNA. Free Radic Res. 2007;41:324–328. doi: 10.1080/10715760601091642. [DOI] [PubMed] [Google Scholar]