Abstract
This review summarizes articles that have been reported in literature on liposome-based strategies for effective drug delivery across the blood–brain barrier. Due to their unique physicochemical characteristics, liposomes have been widely investigated for their application in drug delivery and in vivo bioimaging for the treatment and/or diagnosis of neurological diseases, such as Alzheimer’s, Parkinson’s, stroke, and glioma. Several strategies have been used to deliver drug and/or imaging agents to the brain. Covalent ligation of such macromolecules as peptides, antibodies, and RNA aptamers is an effective method for receptor-targeting liposomes, which allows their blood–brain barrier penetration and/or the delivery of their therapeutic molecule specifically to the disease site. Additionally, methods have been employed for the development of liposomes that can respond to external stimuli. It can be concluded that the development of liposomes for brain delivery is still in its infancy, although these systems have the potential to revolutionize the ways in which medicine is administered.
Keywords: Alzheimer, Parkinson, stroke, cerebral ischemia, glioma, liposomes, blood–brain barrier
Introduction
In the 1880s, Paul Ehrlich intravenously injected dyes (eg, trypan) into animals, and observed that the dyes were able to stain all organs except for the brain. He concluded that the brain had a lower affinity to the dye when compared to other organs.1 In 1913, Edwin Goldmann, a student of Ehrlich, did the opposite, and injected the very same dyes directly to the cerebrospinal fluid of animal brains. He found that in this case, the dyes readily stained the brain and not the other organs.2 These experiments clearly demonstrated the existence of a separation between the blood and the brain. However, in 1898, Max Lewandowsky was the first to postulate the existence of a specialized barrier at the level of cerebral vessels: the blood–brain barrier (BBB), after he and his colleagues had carried out some experiments to demonstrate that some drugs were neurotoxic when injected directly into the brain and not into the vascular system.3 It was just in the late 1960s that Reese and Karnovsky visualized the fact that the barrier was localized to the endothelium by electron-microscopy studies.4
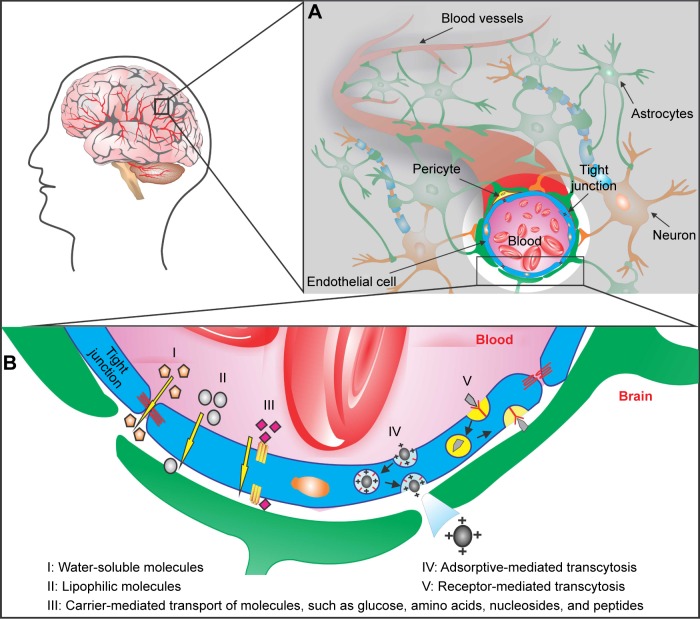
The BBB is composed of polarized endothelial cells connected by tight junctions of the cerebral capillary endothelium and a variety of transporters (Figure 1), which are responsible for its extremely low permeability, limiting the delivery of drugs to the central nervous system (CNS).5,6 BBB functionality is dynamically regulated by an ensemble of different cell types, such as astrocytes, pericytes, and neurons (Figure 1A).7–9 Endothelial cells are surrounded by a basal lamina, which is generally rich in laminin, fibronectin, type IV collagen, and heparin sulfate,5,7–9 which may represent an interesting targeting for drug transport and provides a negatively charged interface.10,11
Figure 1.
Pathways for crossing the blood–brain barrier (BBB).
Notes: The BBB is located at the walls of the blood vessels that supply the central nervous system, including the brain. (A) Cross-section of a cerebral capillary, showing the structure of the BBB. The barrier is composed of a network of astrocytes, pericytes, neurons, and endothelial cells that form the tight junctions. (B) Different mechanisms for drug delivery across the BBB: water-soluble molecules penetrate the BBB through the tight junctions (I); lipid-soluble molecules are able to diffuse across the endothelial cells passively (II); carrier-mediated transport machineries are responsible for transporting peptides and small molecules (III); cationic drug increases its uptake by adsorptive-mediated transcytosis or endocytosis (IV); larger molecules are transported through receptor-mediated transcytosis (V).
Aimed at the development of more efficient therapies for neurological disorders, extensive research is being done into the molecular and cell biology of many of these disorders. To date, human genetic mutations and defective cell-signaling pathways linked to a disease have been identified, and may contribute to the development of mechanism-based therapies and biomarkers for affected patients at early stages in the disease.12,13 Moreover, pharmaceutical companies have spent billions of dollars in the hope that their scientists could develop drugs to defeat the brain disorders, eg, a drug that helps brain-cell growth, repairs damage, or slows down tumor progress, something that is not available now. However, obstacles to effective therapy delivery remain, and one of the most notable obstacles for drugs to penetrate the brain effectively is the BBB.14,15
How to circumvent the blood–brain barrier?
Based on better knowledge of BBB biology, several different strategies for delivering molecules across the barrier have been developed for treating CNS diseases, and can be broadly classified as invasive, pharmacological, and physiological approaches.15–19 The invasive method is based on direct delivery of drugs into the brain tissue through varying techniques, such as the use of polymers or microchip systems, stereotactically guided drug insertion through a catheter, and transient disruption of the BBB. However, these approaches are invasive, leading to risks of infection, damage to brain tissue, and toxicity. Furthermore, invasive approaches are costly and require hospitalization.20–22
The pharmacological method for crossing the BBB is based on modifying, through medicinal chemistry, a drug molecule to enable BBB permeability and making it insusceptible to drug-efflux pumps, such as P-glycoprotein (PgP).17 One early strategy was based on the development of highly lipophilic and small drugs, allowing them to diffuse successfully through the brain’s endothelial cells (Figure 1B). Unfortunately, synthesizing drugs that fulfill this condition eliminate a vast number of potentially useful polar molecules that could be used to treat CNS disorders. A second possibility is to use small water-soluble drugs to facilitate traversal of the BBB by the paracellular hydrophilic diffusion pathway (Figure 1B), though the majority of these molecules are just able to penetrate the interendothelial space of the cerebral vasculature up to the tight junctions, and not beyond. Moreover, modifications to drug structure often result in loss of the drug’s biological activity.23
Among all the approaches employed in drug delivery to the brain tissues, the physiological method is the most advantageous, as it takes advantage of the transcytosis capacity of specific transporting receptors expressed at the BBB surface in order to penetrate the barrier (Figure 1B). For example, the occurrence of low-density lipoprotein receptor-related protein on the BBB is of critical importance for therapeutic proteins or peptides to glial cells or neurons across the whole CNS.24–26 Another method consists in the use of receptor-mediated endocytosis by conjugation of drug molecules to ligands, such as antibodies and peptides, against receptors that are expressed on the surface of endothelial cells of the barrier,6 allowing the drug to be transported into the brain (Figure 1B). In addition, cationic compounds are able to bind to the negatively charged plasma membrane of the endothelial cells by electrostatic interactions.10,11 Therefore, the cationic substance crosses the BBB by adsorption-mediated transcytosis or endocytosis (Figure 1B). However, a low rate of drug dissociation from the ligands, nonspecific drug–receptor interactions, and the limited concentration of cationic substances in the brain are disadvantages for this kind of approach.
Undoubtedly, all three of these approaches have strong disadvantages that limit the successful treatment of neurological diseases. In response to this insufficiency in methods to transport therapeutic drugs across the BBB, aggressive research efforts into the use of nanotechnology to deliver drugs effectively across the BBB without altering their effect is being done. For this purpose, a broad range of nanoparticles with different sizes, architectures, and surface properties have been engineered for brain drug delivery.27,28 These include liposomes,29,30 polymeric nanoparticles,31,32 carbon nanotubes,33,34 nanofibers,35,36 dendrimers,37,38 micelles,39 inorganic nanoparticles made of iron oxide,40 and gold nanoparticles.41 Unfortunately, it is beyond the scope of this article to review potential advantages – or disadvantages – of each of these nanocarriers in the imaging and/or therapy of the brain. For a more detailed overview of nanotechnology-based systems on drug delivery to the CNS, we refer the reader to Vlieghe and Khrestchatisky.27 Here we focus on the one of most promising approaches aimed at improving brain drug targeting and delivery: liposomes and molecules that can selectively target brain tissues. In fact, liposomes are at present the nanoparticle type with the most studies that have been published for delivery to the brain, representing in this way the most advanced material and thus with the highest potential for clinical applications.
Why use liposomes for treating neurological disorders?
Common diseases of the CNS, such as neurodegeneration, multiple sclerosis, stroke, and brain tumors, represent a huge medical need. According to a World Health Organization report, about 1.5 billion people globally are suffering from neurological diseases.42 The prevalence of neurological disorders is expected to have a significant increase in the next decade, as the aging population is highly increasing and living longer. Drug therapies to the brain have been particularly inefficient, especially due to the BBB, as discussed earlier. It would be thus be desirable to gain a better understanding of the molecular mechanism of the disease and the development of improved diagnostic devices and treatments. In this way, liposomes have emerged as promising carriers for CNS delivery.
Liposomes are roughly nano- or microsize vesicles consisting of one or more lipid bilayers surrounding an aqueous compartment. The potential use of these vesicles as a carrier system for therapeutically active compounds was recognized soon after its discovery in the early 1960s. In recent years, liposomes have been explored as carriers of therapeutic drugs, imaging agents, and genes, in particular for treatment and/or diagnosis of neurological diseases.29,43–45 Due to their unique physicochemical characteristics, liposomes are able to incorporate hydrophilic, lipophilic, and hydrophobic therapeutic agents. Hydrophilic compounds may either be entrapped into the aqueous core of the liposomes or be located at the interface between the lipid bilayer and the external water phase. Lipophilic or hydrophobic drugs are generally entrapped almost completely in the hydrophobic core of the lipid bilayers of the liposomes. In addition, the use of cationic lipids allows the adsorption of polyanions, such as DNA and RNA (Figure 2). They also have the advantage of presenting good biocompatibility and biodegradability, low toxicity, drug-targeted delivery, and controlled drug release.46,47 In order to improve blood circulation and brain-specific delivery, the liposome surface can be further modified by the inclusion of macromolecules, such as polymers, polysaccharides, peptides, antibodies, or aptamers (Figure 2). Unfortunately, efficient brain-specific drug delivery by liposomes is not in clinical practice. However, several liposomal drugs are either approved for clinical use or in clinical trial studies (Table 1).48–59
Figure 2.
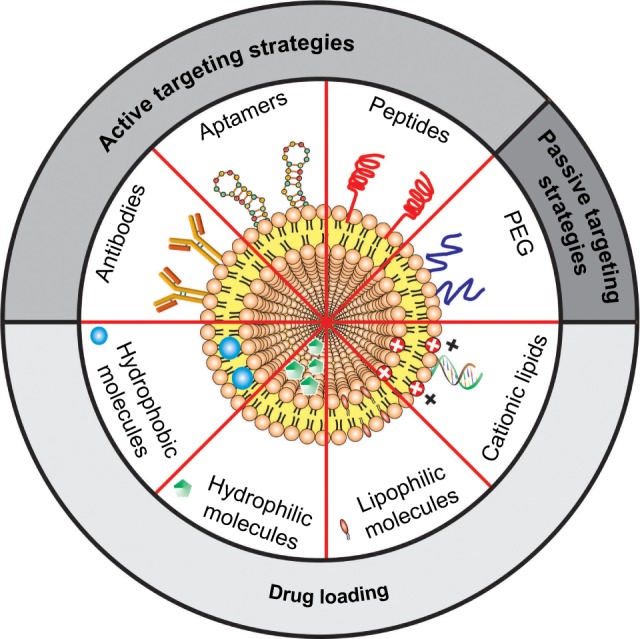
Schematic representation of the main liposomal drugs and targeting agents that improve liposome affinity and selectivity for brain delivery.
Abbreviation: PEG, polyethylene glycol.
Table 1.
Liposome-based drugs on market or in clinical trials for brain-targeted drug delivery
| Commercial name | Compound | Lipid composition | Indications | Trial phase | References |
|---|---|---|---|---|---|
| AmBisome | Amphotericin B | HSPC, DSPG, and cholesterol | Cryptococcal meningitis | NA | 48, 49 |
| Abelcet® | Amphotericin B | DMPC and DMPG | Cryptococcal meningitis | NA | 48, 49 |
| DaunoXome® | Daunorubicin | DSPC and cholesterol | Pediatric brain tumors | I | 50 |
| Depocyt® | Cytarabine | Cholesterol, triolein, DOPC, and DPPG | Lymphomatous meningitis | NA | 51 |
| Doxil®/Caelyx®a | Doxorubicin | HSPC, cholesterol, and DSPE-PEG2,000 | Glioblastoma multiforme | II | 52–55 |
| Pediatric brain tumors | II | 56, 57 | |||
| Myocet® | Doxorubicin | EPC and cholesterol | Glioblastoma multiforme | II | 58 |
Note:
PEGylated liposomal doxorubicin is known as Doxil® in the US and Caelyx® in Europe.
Abbreviations: DMPC, dimyristoylphosphatidylcholine; DMPG, dimyristoylphosphatidylglycerol; DOPC, dioleoylphosphatidylcholine; DPPG, dipalmitoylphosphatidylglycerol; DSPC, distearoylphosphatidylcholine; DSPE, distearoylphosphatidylethanolamine; DSPG, distearoylphosphatidylglycerol; EPC, egg phosphatidylcholine; HSPC, hydrogenated soy phosphatidylcholine; NA, not applicable; PEG, polyethylene glycol.
Optimizing the ideal liposome for crossing the BBB has important implications for the treatment of neurological diseases. Different liposomal formulations and strategies have been developed for enhancing drug delivery across the BBB. The following examples illustrate current strategies using liposomes as brain vectors (Table 2).60–69 Cationic liposomes are successfully used as carriers for the delivery of therapeutic drugs and genes.70–72 Several studies have shown that these cationic nanocarriers are more efficient vehicles for drug delivery to the brain than conventional, neutral, or anionic liposomes, possibly due to the electrostatic interactions between the cationic liposomes and the negatively charged cell membranes, enhancing nanoparticle uptake by adsorptive-mediated endocytosis.60–62 But there is a major drawback to the use of cationic nanocarriers for brain delivery: due to nonspecific uptake by peripheral tissues and their binding to serum proteins that attenuates their surface charge, large amounts of these nanocarriers will be required to reach therapeutic efficacy, and those carriers are potentially cytotoxic. Therefore, there is a need for the development of liposomes that efficiently target diseased areas in the brain.
Table 2.
Means by which liposomes can penetrate the BBB
| Strategies to permeate the BBB | Short description | References |
|---|---|---|
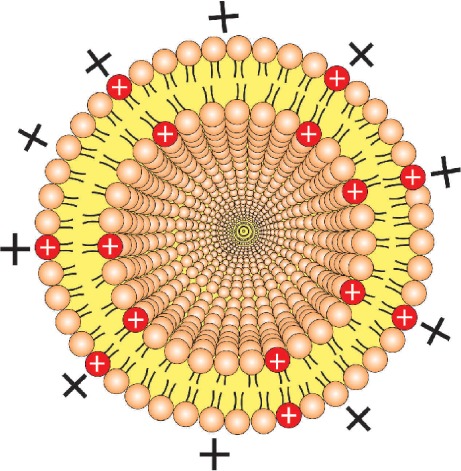
| Cationization of the vector | ||
|
The use of cationic liposomes is an interesting strategy, due to electrostatic interaction between their positive charges and the polyanions present at the BBB, resulting in adsorptive-mediated endocytosis. | 60–62 |
|
| ||
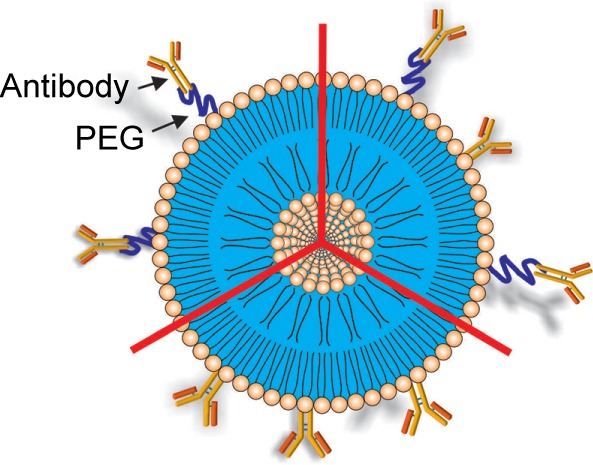
| Targeting ligand | ||
|
To increase liposomal drug accumulation into the brain, the use of ligand-targeted liposomes toward the receptors expressed on brain endothelial cells has been suggested, resulting in receptor-mediated transcytosis. One or more targeting ligands, such as antibodies and aptamers, can be covalently bound over the liposome surface or to the ends of the PEG chains. | 44, 63–65 |
|
| ||
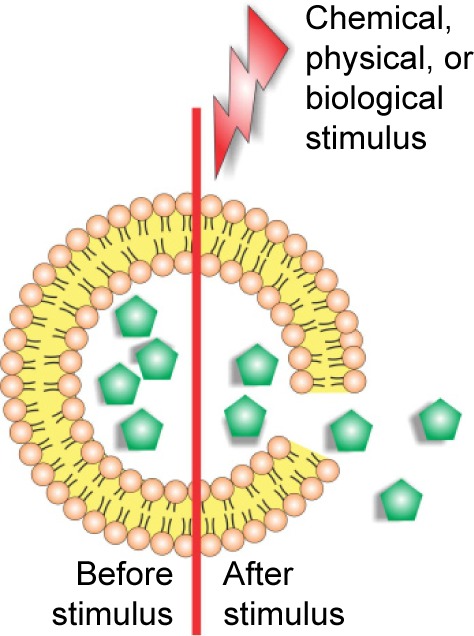
| Triggered drug release | ||
|
Strategies developed for trigged drug release of liposome contents in response to specific external stimuli, such as variations in magnetic field, temperature, ultrasound intensity, light or electric pulses, and others. | 66–68 |
|
| ||
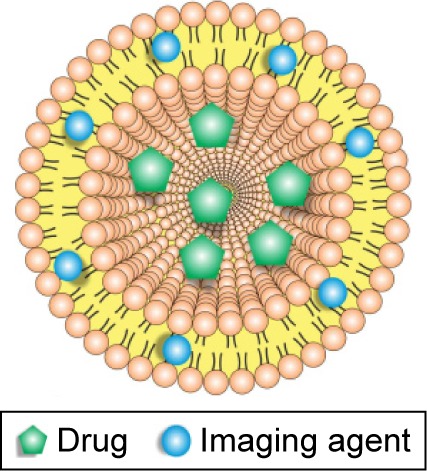
| Theranostic | ||
|
Liposomes are a very well-known carrier for drugs, but they can also incorporate a noninvasive contrast agent. This multifunctional theranostic liposomal drug-delivery system has advantages in diagnosis, real-time monitoring of disease treatment, and pharmacokinetics of liposomes. | 30, 68, 69 |
Abbreviations: BBB, blood–brain barrier; PEG, polyethylene glycol.
Surface-functionalization methodologies improve, at least in part, the pharmacokinetics and biodistribution of liposomes into the brain. For example, the addition of polyethylene glycol (PEG) or polysaccharides forms a protective layer over the surface of liposomes and protects the vehicle from the binding of plasma proteins, preventing the opsonization process and subsequent clearance of liposomes. Even though the PEGylation of liposomes prolongs their circulation time in the body, it does allow liposomes to cross the BBB. Therefore, their functionalization with biologically active ligands, such as peptides, antibodies, aptamers, and others, which specifically bind to receptors that are expressed on the surface of the brain endothelial cells, facilitates their binding and transport across the BBB.73,74
Although actively targeted brain drug delivery has improved the crossing of nanoparticles into the brain, additional properties can be included in liposomal systems to enhance the delivery of drugs at the targeted site in response to specific stimuli, such as variations in temperature, magnetic field, ultrasound intensity, or changes in pH. For example, recent reports introduced the concept of magnetic liposomes as a targeting moiety for delivering of therapeutic molecules across the BBB. In one example, one or more drug molecules could be reversibly bound to the surface of iron oxide nanoparticles, and when encapsulated within the core of liposomes, bypassed an established in vitro model of the BBB by action of an external magnetic field.67 Furthermore, it has been shown that magnetic liposomes can also be taken up into human monocytes, followed by the entry of nonmagnetic monocytes into the brain.67 Although this approach has not been largely explored for brain delivery, this may become a good strategy for effective drug delivery by stimuli-responsive liposomes.
Furthermore, multifunctional liposomes can be engineered into a single structure, providing a powerful approach to improve disease-specific detection, treatment, and follow-up monitoring.30 The term “theranostic” is used for nanoparticles that incorporate both therapeutic and diagnostic agents onto the same system.75 One example of theranostic agent for brain delivery was described by Wen et al,76 using quantum dots and apomorphine liposomally encapsulated for both brain therapy and imaging. The results showed that theranostic liposomes were transported across the BBB, providing a new and exciting strategy for brain-cancer imaging and therapy.76
It is worth mentioning that various routes of administration have been tested to access the brain for therapeutic purposes. For the delivery of liposomes to the CNS, intravenous injection seems to be the preferred route. The possibility of choosing between alternative routes of administration (oral, ocular, or mucosal) has been largely explored for bypassing the BBB, but it is beyond the scope of this article. For example, intranasal administration provides a practical and noninvasive approach to deliver drugs to the brain, allowing in this way an increase in the amount of drugs delivered across the barrier.77–79 It was shown that a liposomal formulation of rivastigmine was able to prevent degradation of the drug in the nasal cavity and to carry it through the mucosal barriers.80 Furthermore, the ability of cationic liposomes to delivering proteins to the brain via the intranasal route has also been demonstrated.81
In this review, a search of the literature was undertaken to investigate whether the use of liposomes offered any additional benefit than the therapeutic drug alone to treat most significant neurological diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease, stroke, and brain cancer, and discuss its advantages and limitations. As a vast majority of CNS drugs have limited brain uptake, they may benefit from the use of liposomes as a drug-delivery vehicle into the brain. Moreover, liposomes have been widely explored as drug-delivery carriers to increase uptake of such drugs into the CNS. Therefore, there appears to be an obvious need for establishing CNS-penetrant and specific therapeutics to overcome the BBB and to do this in a controlled manner.
Materials and methods
Search strategy
A PubMed and Web of Science search was conducted to identify all known published articles on liposomes in drug development focused on the treatment of neurological disorders up to May 2016.
Study selection
Initially, articles were identified using a combination of the following keywords: 1) “liposomes” and “Alzheimer”; 2) “liposomes” and “Parkinson”; 3) “liposomes” and “Huntington”; 4) “liposomes” and “stroke” or “cerebral ischemia”; and 5) “liposomes” and “glioma”. Reviews, patents, editorial materials, book chapters, conference publications, and articles not published in English were excluded from the literature search. Based on titles/abstracts, only studies that described in vivo experiments were selected for review. The final decision to include/exclude studies was based on full copies of articles.
Data extraction
In vivo studies with liposomes have been performed in most species, including mice, rats, dogs, monkeys, and humans. As in vivo study interpretation of results deserves attention, especially because of the biological differences between species, this was the parameter used to group the studies. Also, the following parameters of the liposome formulation were compared: 1) route of administration, 2) time points, 3) liposome composition, 4) ligands, 5) drug or imaging agent, and 6) particle size. Lately, biological outcome into the CNS has also been reported.
Results and discussion
Neurodegenerative disorders
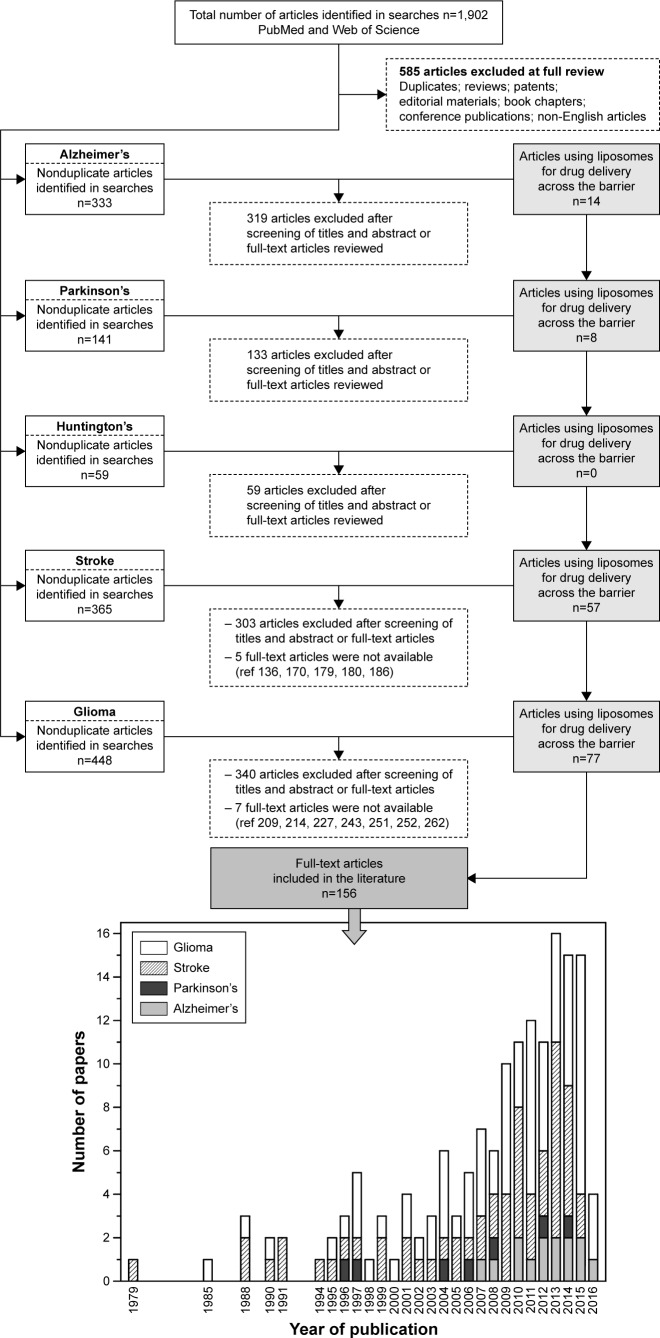
AD, PD, and Huntington’s disease were grouped together in this topic, because a growing number of studies indicates that these disorders share in common some features, such as the accumulation of intracellular or extracellular protein aggregates, selective degeneration of neurons, inclusion-body formation, and inflammation in particular brain regions.82 However, the search for reports on the use of liposomes for delivery of active or imaging compounds against neurological diseases was done individually. A flowchart of the literature search is shown in Figure 3. An initial search yielded a total of 319 articles for AD, 141 articles for PD, and 59 articles for Huntington’s, after excluding duplicate articles found in the PubMed and Web of Science databases. For AD, 26 full-text article reviews were performed, and 12 studies were included for their fulfillment of inclusion criteria (Figure 3, Table 3).80,83–95 For PD, 19 full-text articles were reviewed, and eight articles had all the requisites to be considered here (Figure 3, Table 3).96–103 Unfortunately, for Huntington’s disease, just one full article was analyzed and this article did not show any outcome of interest for this disease, and for this reason was not included here (Figure 3).
Figure 3.
Flow diagram of studies that were identified based on the search terms described in the body of this article.
Abbreviation: ref, reference.
Table 3.
Studies on liposome application in Alzheimer’s and Parkinson’s diseases
| Species | Administration | Time points | Liposome composition | Ligands | Drug/imaging agent | Particle size (nm) | CNS action | References |
|---|---|---|---|---|---|---|---|---|
| Alzheimer’s disease (AD) | ||||||||
| Mice | Intravenous injection | 5–1,440 minutes | DMPC, cholesterol, and DSPE-PEG2,000 EYPC, cholesterol, and DSPE-PEG2,000 |
Glutathione | 111In-VHH-pa2H antibody | 110 | Both ligand-targeted liposomes showed ability for specific brain delivery of single-domain antibody fragments beyond the BBB. | 83 |
| Mice | Intraperitoneal injection | 3 weeks | SM and cholesterol | mApoE and phosphatidic acid | NI | 121 | Bifunctionalized liposomes decreased brain Aβ-plaque deposits, improving mouse impaired memory. | 84 |
| Mice | Intravenous injection | 1, 4, or 24 hours | SM and cholesterol | mApoE and phosphatidic acid | 14C and 3H | 123 | Bifunctionalized liposomes were also able to affect brain Aβ-oligomer aggregation/disaggregation in vivo. | 85 |
| Mice | Intraperitoneal injection or oral administration | 6, 8, and 24 hours | DPPC and cholesterol | NI | RIVA | 3,400 | Acetylcholinesterase inhibition was higher when RIVA liposomes were intraperitoneally injected in mice. | 86 |
| Mice | Intravenous injection | 72 hours | DPPC, DSPE-PEG2,000 and cholesterol | Methoxy-XO4 | Methoxy-XO4 | 150 | Liposomes might cross the BBB, since the nanostructures selectively bind to Aβ-plaque deposits to both parenchymal plates and cerebral amyloid angiopathy. | 87 |
| Rats | Intravenous injection | 0.5, 2, 4, 8, 18, and 24 hours | DSPE-PEG2,000 | Tf | α-Mangostin | 196 | Targeted liposomes crossed the BBB and delivered α-mangostin into rat brain. | 88 |
| Rats | Intranasal administration | 7 days | EPC, DSPE-PEG2,000 and cholesterol | NI | H102 | 112 | Liposomes might have great potential for AD, since they ameliorate spatial memory impairment in rats. | 112 |
| Rats | Intranasal administration | 15–240 minutes | EPC and cholesterol EPC, DSPE-PEG2,000 and cholesterol | CPP | RIVA | 166 179 |
The drug was efficiently delivered to the brain, especially by targeting liposomes. | 90 |
| Rats | Subcutaneous injection | 3 months | PC, DC-Chol. and cholesterol | NI | RIVA | 67 529 |
Despite liposome sizes, their therapeutic effect was evidenced by nearly preventing amyloid-plaque formation. | 91 |
| Rats | Intranasal administration | 10 hours | PG | NI | GH | NI | Liposomes readily transported GH into brain tissues, suggesting some promise for this approach in brain drug targeting for AD treatment. | 92 |
| Rats | Intranasal administration | 3 weeks | PC, EPC, and cholesterol | NI | QC | NI | The use of liposomes improved learning and memory deficits, possibly by reducing the levels of oxidative stress and acetylcholinesterase activity. | 93 |
| Rats | Intranasal administration | 3 weeks | PC, EPC, and cholesterol | NI | QC | NI | Liposomes containing QC attenuated the death of neurons and cholinergic neuron cells in the hippocampus. | 94 |
| Rats | Intranasal administration | 15–720 minutes | Soy lecithin and cholesterol | NI | RIVA | 10,000 | Liposome-encapsulated RIVA effectively delivered the drug into the brain. | 80 |
| Rats | Intranasal administration | 4 weeks | PC, EPC, and cholesterol | NI | QC | NI | Liposomes increased anxiolytic activity and cognitive enhancement in the animals. | 95 |
| Parkinson’s disease (PD) | ||||||||
| Mice | Intraperitoneal injection | 5 days | HSPC, DSPE-PEG2,000, and cholesterol | Chlorotoxin | Levodopa | 107 | Ligand-targeted liposomes increased the distribution of the drug in the brain, significantly attenuating serious behavioral disorders. | 96 |
| Mice | Intraperitoneal injection | 7 days | EPC and cholesterol | NI | Levodopa | 60 | Liposome-encapsulated levodopa inhibited akinesia more effectively and increased muscle rigidity when compared to the free drug. | 97 |
| Mice | Intraperitoneal injection | 14 days | EPC and cholesterol | NI | Levodopa | 60 | Treatment with levodopa incorporated into liposomes increased the quantity of dopamine in the mouse striatum. | 98 |
| Mice | Intraperitoneal injection | NI | EPC and cholesterol | NI | Levodopa | 60 | An extremely low dose of levodopa containing liposomes increased the rate of dopamine metabolism and altered the metabolism of signal phospholipids in the striatum. | 99 |
| Rats | Intranasal administration | 3–4 weeks | DOPC, cholesterol, and stearylamine | NI | GDNF | 149 | Treatments with liposomes induced a neurotropic effect in the rat brain. | 100 |
| Rats | Intraperitoneal injection | 8 weeks | DPPC, DODAB, and DSPE-PEG2,000 | OX26 | GDNF plasmid | 117 | Sustained therapeutic effects are achieved in experimental PD with the formulation described here. | 101 |
| Rats | Intraperitoneal injection | 430 minutes | DMPC and cholesterol | NI | Levodopa prodrugs | NI | Liposome formulations were demonstrated to be a good delivery and release system for the brain striatum of anti-PD agents. | 102, 103 |
Abbreviations: BBB, blood–brain barrier; CNS, central nervous system; CPP, cell-penetrating peptide; DC-Chol, 3β-(N-[N′,N′-dimethylaminoethane]-carbamoyl)-cholesterol hydrochloride; DCP, dihexadecyl phosphate; DMPC, dimyristoylphosphatidylcholine; DODAB, dioctadecyldimethylammonium bromide; DOPC, dioleoylphosphatidylcholine; DPPC, dipalmitoylphosphatidylcholine; DSPE, distearoylphosphatidylethanolamine; EPC, egg phosphatidylcholine; EYPC, egg-yolk phosphatidylcholine; GH, galantamine hydrobromide; HSPC, hydrogenated soy phosphatidylcholine; methoxy-XO4, 4,4′-[(2-methoxy-1,4-phenylene)di-(1E)-2,1-ethenediyl]bisphenol; mApoE, apolipoprotein E-derived peptide; NI, not informed; OX26, anti-transferrin receptor antibody; PC, phosphatidylcholine; PEG, polyethylene glycol; PG, phosphatidylglycerol; QC, quercetin; RIVA, rivastigmine; SM, N-palmitoyl-d-erythro-sphingosylphosphorylcholine; Tf, transferrin.
Liposomes in the treatment of Alzheimer’s disease
AD is a progressive and irreversible disease of the brain, affecting mainly people aged over 65 years. The neuropathogenesis of AD is a critical unsolved question. Progressive production and accumulation of insoluble protein aggregates, such as neurofibrillary tangles of hyperphosphorylated tau and amyloid-β (Aβ) plaques are thought to underlie the neuropathology of AD, leading to brain atrophy and neurodegeneration.104 In addition, some studies have also suggested that deficits in cholinergic neurotransmitter systems and increased levels of free radicals or proinflammatory cytokines might be involved in AD neuropathogenesis.105–108 More recently, a new potential cause for AD has been found in the behavior of certain immune cells that normally protect the brain instead beginning to consume a vital nutrient: the amino acid arginine.109 This new discovery has implications not only in a new potential cause of the disease but also as a new strategy for targeting disease.
To date, the US Food and Drug Administration (FDA) has approved three acetylcholinesterase inhibitors – rivastigmine, galantamine, and donepezil – for the treatment of AD, which lead to an increase in central cholinergic action in the brain areas affected by the disease.110 However, the administration of these inhibitors is associated with some severe side effects. It would thus be desirable to develop new formulations to avoid these side effects, and all studies proved that the use of liposomes was a good strategy in the treatment of AD.80,86,90–92,111 Intranasal delivery of rivastigmine or galantamine liposomes has been shown to be a viable and effective route to improve drug bioavailability for brain drug targeting.80,90,92 Intranasal delivery was also used as a successful approach for delivery of liposomes containing quercetin, which has antioxidant properties. As oxidative stress plays a very important role in the neuropathogenesis of AD, the use of quercetin liposomes has been shown to decrease neuronal oxidative stress.93–95
Moreover, there have been several studies exploring different strategies to block the effects of Aβ and tau proteins that constitute major hallmarks of AD.84–87,91 Once encapsulated into liposomes, the H102 peptide, a β-sheet breaker, was able to block the early steps of aggregation and misfolding of the soluble Aβ, improving the spatial memory impairment of AD in rats.112 α-Mangostin is a polyphenolic xanthone that exhibits pharmacological effects, such as anti-inflammation, antioxidant, and antitumor effects. When administered intravenously, α-mangostin liposomes have been shown to protect and improve the neurons against Aβ-oligomer toxicity in rats.88
Methoxy-XO4, a highly specific Aβ plaque ligand with the dual role of targeting moiety and fluorescent marker, has been conjugated to liposomes. When administered intravenously, these liposomes were able to cross the BBB in vivo and specifically bind to Aβ-plaque deposits, labeling vascular and parenchymal amyloid deposits in brain tissue.87 For example, glutathione PEGylated liposomes demonstrated efficient encapsulation of an antiamyloid single-domain antibody fragment (VHH-pa2H), increasing its transport from blood into the brain.83 It has also been demonstrated that bifunctionalized liposomes decorated with phosphatidic acid and a modified ApoE-derived peptide are able to cross the BBB in vivo and destabilize Aβ aggregates, suggesting that this approach is a good option for AD treatment.84,85
Although the scope of this review is on liposome-strategies with the aim of facilitating BBB crossing, it is important to mention that other strategies have been developed for the use of liposomes for AD treatment.89,113–116 Curcumin is a natural compound extract from the plant Curcuma longa, and has been reported to be a fluorescent molecule with high affinity for the Aβ peptide and able to reduce Aβ aggregation. In this way, intracranial injection of liposomes encapsulating curcumin efficiently labeled Aβ deposits in both human and mice tissues, proving to be an effective formulation for diagnosis and treatment of AD.113 Also, intraperitoneal injection of liposomes containing phosphatidic acid or cardiolipin was able to reduce Aβ peptides in the plasma and shifted the equilibrium that exists between brain and blood Aβ peptides, slightly affecting the plaques in the brain.89 Lastly, different liposome-based vaccines were developed and directed toward Aβ plaques115,116 and tau.114
Liposomes in the treatment of Parkinson’s disease
PD affects 4 million people worldwide.117 The neuropathogenesis of PD is characterized by motor symptoms, such as tremor, rigidity, slowness of movement, difficulty with walking, and problems with gait. These motor symptoms result primarily from the death of dopamine-generating neurons in an area of the brain called the substantia nigra, leading to the decreasing of dopamine levels.118 Also, misfolding and intracellular aggregation of α-synuclein fibrils, also known as Lewy bodies, are pivotal to PD neuropathogenesis.117,118 Mitochondrial dysfunction and oxidative stress may also be implicated in PD neurodegeneration.118 However, the mechanisms underlying PD pathogenesis have not been fully elucidated.
Currently, available therapies for PD are essentially symptom-directed, having no effect on the disease progression. To date, the natural precursor of dopamine, levodopa or L-dopa, has been used in the clinic for several years.119 However, levodopa cannot be administered alone, since it is converted to dopamine via peripheral dopamine-decarboxylase enzyme and causes such side effects as sleepiness, nausea, and dyskinesia.120 A recently reported study overcame this problem, developing liposomes for site-specific delivery of levodopa into the CNS.96 Chlorotoxin-modified stealth liposomes encapsulating levodopa proved to be an efficient nanocarrier, increasing levodopa concentration into the substantia nigra and striatum.96 In the same way, it was also observed that intraperitoneal injection of liposome formulations encapsulating anti-PD drugs could improve the release of dopamine in the striatum region.96–99,102,103
Also, there are ongoing studies showing that GDNF is able to promote growth, regeneration, and survival of substantia nigra dopamine neurons, preventing the progression of PD if administered in the early stages of the disease.121–124 Recently, a very promising study showed neurotrophic and neuroprotective effects of GDNF protein into the rat brain.100 Although the liposomal preparation of GDNF offered no significant advantage of GDNF alone after intranasal injection, the liposomal formulation might have a protective effect on the protein, preventing it from degradation.100 Another example that demonstrated the improvement of the treatment of the disease with GDNF is reported in Xia et al.101 In this study, intravenous administration of OX26-targeted PEGylated liposomes was used as a nonviral gene-delivery system to deliver GDNF plasmid into the CNS. The expression of GDNF genes, under the influence of a rat tyrosine hydroxylase promoter, was observed in organs where the TH gene is highly expressed, including the substantia nigra, adrenal gland, and liver. Sustained therapeutic delivery was achieved at the neurons of the nigrostriatal tract in experimental PD.101 Lastly, novel liposomal formulations have been characterized and efficacy in PD rats reported after intracerebral injection.125–130 As the injection was at the local site of the disease and did not show any evidence of transposing the BBB, they were not considered in this review article.
Stroke or cerebral ischemia
Unlike the other neurological disorders described so far, stroke has high incidence, disability, and mortality rates in a modern society.131 An ischemic stroke is characterized by the sudden reduction of brain blood flow due to obstruction of cerebral vasculature, damaging the neural tissue (ischemic penumbra zone).132 Unfortunately, the treatment for stroke has its limitations, due to the poor ability to deliver therapeutic agents across the BBB. Therefore, efforts have been made to identify and develop drug-delivery systems to the brain. Liposomes are described as a possible valuable system to achieve better therapeutic effects in the treatment of stroke. The search for reports on the use of liposomes as drug-delivery nanocarriers for the treatment and/or diagnosis of stroke is shown in Figure 3. An initial search yielded a total of 365 articles after excluding duplicate articles found in the PubMed and Web of Science databases. In total, 62 articles were eligible.133–194 Although all articles described new nanocarriers for the delivery of therapeutic molecules into the brain, only 57 studies are included in Table 4, because the full text of five articles139,173,182,183,189 was not available to access.
Table 4.
Studies on liposome application in stroke or cerebral ischemia
| Species | Administration | Time points | Liposome composition | Ligands | Drug/imaging agent | Particle size (nm) | CNS action | References |
|---|---|---|---|---|---|---|---|---|
| Mice | Intraperitoneal injection | 24 hours | Lecithin and cholesterol | NI | Chr | NI | Chr liposomes offered protection against cerebral ischemia-reperfusion injury by reducing either oxidative stress species or apoptosis. | 133 |
| Mice | Intravenous injection | 6 hours and 1, 3, and 7 days | DSPC, DSPE-PEG2,000, DSPE-PEG2,000-Mal, and cholesterol | Anti-ICAM-1 antibody | Gd and Rhb | 210 | Direct in vivo MRI-based detection after stroke was achieved only with ICAM-I-targeted MPIO. | 134 |
| Mice | Via gavage | 7 days | DOPE, CHEMS, and DSPE-PEG2,000 | NI | ATP, PBT, and suramin | 150 | Treatment with the liposomal formulation increased the number of surviving hippocampal CAI neurons, possibly due to the increased antioxidant capacity of the mouse brain. | 135 |
| Mice | Intracarotid injection | 24 hours | PC, DSPE-PEG2,000, DSPE-PEG2,000-Mal, and cholesterol | Anti-NRI-receptor antibody | SOD enzyme | 160 | Ligand-targeted liposomes offered protection against ischemia-reperfusion injury, reduced inflammatory markers, and improved behavior in vivo. | 136 |
| Mice | Intramuscular injection | 1 or 7 days | PS and PC | NI | ATP | 100 | ATP liposomes offered protection for the retina against ischemia-reperfusion injury. | 137 |
| Mice | Intraperitoneal injection | 24 hours | PS and PC | NI | ATP | 100 | The liposomal formulation reduced CNS damaged due to the increased survival of retinal neurons after ischemic injury. | 138 |
| Rats | Intra-arterial injection | 7 days | PC, PE, and cholesterol | NI | Angiogenic peptides and 99mTc | 128 | Liposomes as imaging agents to the delivery of angiogenic peptides hold promise as an indicator of the effectiveness of angiogenic therapy in cerebral ischemia. | 140 |
| Rats | Intravenous injection | 24 hours | Soy lecithin, DSPE-PEG2,000, and cholesterol | TF peptide | ZL006 | 74 | Ligand-targeted liposomes for the delivery of the ZL006 significantly ameliorated neurological deficit and reduced infarct volume induced by ischemia reperfusion. | 141 |
| Rats | Intravenous injection | 7 days | DSPC, DSPE-PEG2,000, and cholesterol | Anti-HSP72 antibody | RhB, Gd, and citicoline | 100 | Treatment with liposomes containing citicoline reduced lesion volumes. | 142 |
| Rats | Intravenous injection | 2 hours | DSPC, DSPE-PEG2,000, and cholesterol | NI | 18F | 100 | Accumulation of PEG liposomes in and around the ischemic region was observed. | 143 |
| Rats | Intravenous injection | 24 hours | DSPC, DSPE-PEG2,000, and cholesterol | NI | Hb | 230 | The delivery of Hb by liposomes reduced cerebral infarct volume. | 144 |
| Rats | Intravenous injection | 22 days | DSPC, DSPE-PEG2,000, and cholesterol | NI | Hb | 250 | Liposome-encapsulated hemoglobin was protective in amygdala SAT in transient whole-brain ischemia. | 145 |
| Rats | Intravenous injection | 24 hours | EPC, DOPC, and cholesterol | NI | NO | 800 | Ultrasound-controlled delivery of NO had potential for improving stroke treatment. | 146 |
| Rats | Intra-arterial infusion | 24 hours | DSPC, DSPE-PEG2,000, and cholesterol | NI | Hb | 200–250 | Liposomal treatment reduced injury by decreasing the effect of MMP9, due to higher production of neutrophils. | 147 |
| Rats | Intravenous injection | 1 hour | DSPC and cholesterol | NI | Dil | 114.5 | Liposomal drug delivery to an ischemic zone was observed, even when cerebral blood circulation was reduced. | 148 |
| Rats | Intravenous injection | 3 or 24 hours | DPPC and DSPE-PEG2,000 | Tacrolimus | Dil | 109 | Reduction of cerebral cell death and improvement in motor function was observed after liposome administration. | 149 |
| Rats | NI | 2, 3, or 5 hours | PC, DSPE-PEG2,000, DPPC, and cholesterol | NI | Xe | NI | For maximal neuroprotection, administration dose of liposome-encapsulated Xe must be 7–14 mg/kg. | 150 |
| Rats | Intravenous injection | 8 hours | DSPC and cholesterol | NI | ISA | 200 | ISA liposomes increased distribution of the drug into the brain and consequently enhanced therapeutic efficacy. | 151 |
| Rats | Intravenous injection | 0, 1, 3, 6, or 24 hours | DSPC and DSPE-PEG2,000 | AEPO | Dil or 125I | NI | This liposomal formulation was able to accumulate in the brain ischemic zone and be retained there for at least 24 hours after injection. Also, liposome treatment significantly reduced cerebral injury and ameliorated motor functions. | 152 |
| Rats | Intravenous injection | 1, 2, 3, 5, and 7 days | DSPC and DSPE-PEG2,000 | AEPO | NI | NI | Liposomes significantly improved the neurological deficit. This might have been due to their neuroprotective properties. | 153 |
| Rats | Intravenous injection | 2 hours and 7 days | DPPC, DSPE-PEG2,000, and cholesterol | NI | DXP | 100 | Treatment with this liposomal formulation significantly improved behavioral outcome in animals after stroke. | 154 |
| Rats | Intravenous or intraperitoneal injection | 1, 3, and 7 days | DSPC, DSPE-PEG2,000, and cholesterol | NI | Citicoline | NI | Intravenously injected liposome-encapsulated citicoline caused a reduction in infarct sizes. | 155 |
| Rats | Intraperitoneal injection | 13 days | Lecithin and cholesterol | NI | Vitamin E and luteolin | 150 | Liposome-encapsulated luteolin reduced brain injuries after postischemic treatment. | 156 |
| Rats | Intravenous injection | 2 and 21 days | POPC, DDAB, and DSPE-PEG2,000 | Tf | VEGF | 100 | Tf-VEGF liposomes demonstrated neuroprotective properties and consequently vascular regeneration in the chronic stage of cerebral infarction. | 157 |
| Rats | Intracarotid injection | 1 and 3 days | DPPC and DOPC | NI | Xe | NI | Xe liposomes promoted improved performance in all behavioral tests of animals. | 158 |
| Rats | Intravenous injection | 30 minutes | PE, cholesterol, and dicetylphosphate | p-Aminophenyl-α-D-mannoside | CDP choline | 60–90 | CDP liposomes exhibited neuroprotection in both young and aged rats by inhibition of mitochondrial injury in moderate cerebral ischemia reperfusion. | 159 |
| Rats | Intravenous injection | 7 days | NI | NI | Hb | 230 | Liposome-encapsulated Hb demonstrated neuroprotective effects against transient cerebral ischemia. | 160 |
| Rats | Intravenous injection | 4 days | PC, cholesterol, and stearic acid | NI | Hb | NI | The treatment with Hb-liposomes suggested that depending on the cellular type of Hb, it is possible to alleviate ischemia in rats. | 161 |
| Rats | Intravenous injection | 24 hours | DSPC, DSPE-PEG2,000, and cholesterol | NI | Hb | 230 | Liposome-encapsulated Hb promoted reduction in size of cerebral infarction in rats. | 162 |
| Rats | Intravenous injection | 1 day | DSPC, DSPE-PEG2,000, and cholesterol | NI | Hb | NI | Liposome-encapsulated Hb treatment significantly reduced edema formation into a large area of the brain. | 163 |
| Rats | Intravenous injection | 60 minutes | DSPC, DSPE-PEG2,000, and cholesterol | NI | Hb and 18F | 211 | For liposome-encapsulated Hb, was shown that the delivery oxygen happens even into the ischemic brain from the periphery toward the core of ischemia. | 164 |
| Rats | Intraventricular injection | 3 hours | PS, PC, and cholesterol | NI | Antisense ODNs | NI | Successful application of liposome-mediated antisense ODNs delivery in vivo demonstrated knockdown of synaptotagmin I, attenuating ischemic brain damage in neonatal rats. | 165 |
| Rats | Intraperitoneal injection | 24 hours | Lecithin | NI | QC | NI | QC-liposome treatment demonstrated neuroprotective effects after ischemia. Also, this study suggested that endogenous brain glutathione is critical in defense mechanisms against stroke. | 166 |
| Rats | Intravenous injection | 24 hours | DSPC, DSPE-PEG2,000, and cholesterol | NI | Hb | 230 | Liposome-encapsulated Hb treatment provided a reduction in the area of infarction in the cortex, but not in the basal ganglia after ischemia. | 167 |
| Rats | Intravenous injection | 30 minutes | PE, dicetylphosphate, and cholesterol | NI | QC | 234 | Liposome-encapsulated QC demonstrated in neuronal cells of young and old rats showed preservation of antioxidant enzymes and inhibition of cellular edema formation. | 168 |
| Rats | Intravenous injection | 24 hours | DPPC, DPPS, GM1 ganglioside, and cholesterol | NI | CDP choline | 50 | CDP choline-liposome treatment reduced cerebral infarction in rats after ischemia. | 169 |
| Rats | Intraventricular injection | DOPC and cholesterol | NI | NGF | NI | NGF-liposome treatment significantly reduced infarct volume after ischemia. | 170 | |
| Rats | Transfusion | 45 minutes | NI | NI | Hb | 220 | Hemodilution with Hb liposomes did not attenuate ischemia in rats. | 171 |
| Rats | Intracarotid injection | 24 hours | EYPC and cholesterol | Antiactin antibody | tPA | 200-250 | tPA-liposome therapy reduced vascular membrane damage and hemorrhagic transformation after ischemia. | 172 |
| Rats | Intrathecal injection | 48 hours | DOTAP | NI | Plasmid | NI | Transfections in vivo resulted in reduction in number of apoptotic cells in the infarct and penumbra area. | 174 |
| Rats | Intraperitoneal injection | 20 minutes | EYPC and cholesterol | NI | L-Ascorbic acid or α-tocopherol | NI | L-Ascorbic acid- or α-tocopherol-containing liposome treatment prevented the production of diene in excess of 2 hours prior to cerebral ischemic insult. | 175 |
| Rats | Intrathecal injection | 24 and 72 hours | HSPC and cholesterol | NI | Fasudil | 110 | Liposome-encapsulated fasudil treatment presented an improvement on neurological outcomes after 24 hours in vivo, due to a reduction in infarct area. | 176 |
| Rats | Intravenous injection | 1 week | DPPC, DPPS, and cholesterol | NI | CDP choline | 49 | Liposome-encapsulated CDP-choline treatment provided a rapid recovery of the damaged membranes of neuronal cells, allowing an enhancement of brain functionality. | 177 |
| Rats | Intravenous injection | 4 hours and 7 days | PC and cholesterol | NI | ALLNaI | NI | Liposome-encapsulated ALLNaI was able to inhibit calpain on neurotoxic damage, which offers an optional treatment for transient forebrain cerebral ischemia. | 178 |
| Rats | Intravenous injection | 8 days | DPPC, DPPS, GM1 ganglioside, and cholesterol | NI | CDP choline | 50 | CDP-choline liposomes were active against ischemic injury, improving survival rates of rats. | 179 |
| Rats | Intravenous injection | 11 days | DPPC, DPPS, GM1 ganglioside, and cholesterol | NI | CDP choline | 50 | CDP-choline liposomes improved the survival rate of rats subjected to ischemia and reperfusion. | 180 |
| Rats | Intravenous injection | 6 days | DPPC, DPPS, GM1 ganglioside, and cholesterol | NI | CDP choline | 50 | CDP-choline liposomes were able to protect the brain against damage induced by ischemia. | 181 |
| Rats | Intracarotid injection | 60 minutes | PC and cholesterol | NI | ATP | NI | The opening of the BBB under certain hypoxic conditions allowed the liposomes to reach the cerebral parenchyma. | 184 |
| Rats | Intravenous injection | 30 minutes | DPPC, cholesterol, and stearylamine | NI | SOD enzyme | NI | Liposomes were able to deliver a higher amount of SOD into the brain. | 185 |
| Rats | Intravenous injection | 1, 2, 8, and 24 hours | PC, stearylamine, and cholesterol | NI | CuZn-SOD enzyme | NI | Liposome-encapsulated SOD treatment reduced infarct sizes for the anterior artery area, middle artery area, and posterior artery area. | 186 |
| Rats | Intracarotid injection | NI | PC and cholesterol | NI | ATP | NI | Liposome-encapsulated ATP increased the number of ischemic episodes tolerated by rats. | 187, 188 |
| Rats | Intravenous injection | 2 hours | DSPC, DSPE-PEG2,000, and cholesterol | NI | 18F | 99 | PEG liposomes radiolabeled with 18F accumulated in and around the ischemic zone into the brain. | 190 |
| Rats | Intracarotid injection | 24 hours | DPPC, DSPE-PEG2,000, DHSG, and cholesterol | NI | Hb | 262–269 | Treatment with Hb liposomes significantly decreased the cerebral infarct volume of the cortex, improving motor-dysfunction score. | 191 |
| Gerbil | Intraperitoneal injection | 2 hours | NI | NI | CuZn SOD | NI | Liposome-entrapped SOD increased endogenous SOD activity in normal brain tissue, and when given at the end of ischemia counteracted both the ischemic reduction in endogenous SOD and the increased peroxidation of mitochondrial membranes. | 192 |
| Monkey | Intravenous injection | 8 days | DSPC, DSPE-PEG2,000, and cholesterol | NI | Hb | 230 | Liposome-encapsulated Hb treatment persistently reduced damage beyond the acute phase of ischemia. | 193, 194 |
Abbreviations: 18F, fluorine-18; 99mTc, technetium-99m; 125I, 125 iodine; AEPO, asialo-erythropoietin; ALLNaI, N-acetyl-leucyl-leucyl-norleucine amide; BBB, blood–brain barrier; CDP, cytidine diphosphate; CHEMS, cholesteryl hemisuccinate; Chr, chrysophanol; CNS, central nervous system; CuZn, copper-zinc couple; DDAB, dimethyldioctadecylammonium bromide; DHSG, 1,5-O-dihexadecyl-N-succinyl-l-glutamate; DiI, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; DOPC, dioleoylphosphatidylcholine; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; DPPC, dipalmitoylphosphatidylcholine; DPPS, 1,2-dipalmitoyl-sn-glycero-3-phosphoserine; DSPC, distearoylphosphatidylcholine; DSPE, distearoylphosphatidylethanolamine; DXP, dexamethasone phosphate; EPC, egg phosphatidylcholine; EYPC, egg-yolk phosphatidylcholine; Gd, gadolinium; Hb, hemoglobin; HSPC, hydrogenated soy phosphatidylcholine; ICAM-1, intercellular adhesion molecule 1; ISA, isopropylidene–shikimic acid; Mal, maleimide; MMP-9, matrix metalloproteinase-9; MPIO, micron-sized iron oxide particles; MRI, magnetic resonance imaging; NGF, nerve growth factor; NI, not informed; NO, nitric oxide; ODNs, oligodeoxynucleotides; PBT, pentobarbital; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PEG, polyethylene glycol; POPC, 1-palmitoyl-2-oleoylphosphatidylcholine; PS, phosphatidylserine; QC, quercetin; RhB, rhodamine B; SOD, superoxide dismutase enzyme; Tf, transferin; tPA, tissue plasminogen activator; VEGF, vascular endothelial growth factor; Xe, xenon; ZL006, 5-(3, 5-dichloro-2-hydroxybenzylamino)-2-hydroxybenzoic acid.
The initial treatment for acute ischemic stroke consists in the administration of the FDA-approved tissue plasminogen activator (tPA), which is effective within the first 3 hours after the event occurs. This drug works on quickly dissolving the blood clot to restore brain perfusion.195 However, its use is limited, due to elevated risk of cerebral hemorrhage, most probably due to the generation of free radicals posttreatment.196 Because oxidative damage is an important aspect of the pathology of stroke and involved in vascular cell-membrane damage, researchers considered the possibility of developing a novel system to deliver tPA efficiently to the ischemic penumbra area in the brain. Actin is already known to be able to bind to antigens present at the surface of cells with damaged membranes. Therefore, actin-targeted liposomes for tPA delivery were developed, and this new drug-delivery system was in fact very efficient in delivering tPA within the brain, reducing hemorrhagic transformation in rats after focal embolic stroke.173 Furthermore, the enzyme SOD was demonstrated to be an excellent biological natural free radical scavenger, and its ability as a neuroprotectant agent was tested. As free enzymes possess no BBB-penetration capacity and degrade rapidly in the serum, SOD encapsulation in liposomes was needed. In vivo experiments demonstrated the efficacy of SOD-loading liposomes to get into the brain, providing significant protection against free radicals.136,139,185,186,189,192
Moreover, a wide debate is ongoing in the literature about new strategies to treat this disease. Neuroprotective and neuroreparative drugs (for example, citicoline) are under development.197 Citicoline, an exogenous form of cytidine-5′-diphosphocholine, is a key intermediate in the biosynthesis of phosphatidylcholine, the primary neuronal membrane phospholipid that is degraded during brain ischemia to free radicals and fatty acids. In addition, citicoline restored Na+/K+ ATPase, inhibited activation of phospholipase A2, and accelerated cerebral edema reabsorption.198 Therefore, citicoline was considered a good candidate for molecular therapy for stroke, since its acts at several points on the ischemic pathway. Unfortunately, due to the drug’s polar nature, crossing the BBB was far lower than desired. It has been observed that liposome-encapsulated citicoline increases its bioavailability within the brain parenchyma and improves its therapeutic efficacy for the treatment of stroke in animals.142,155,159,169,177,179–182
Besides damaged blood vessels in cerebral ischemia, another important process that occurs in stroke is neovascularization or angiogenesis. This is the physiological process of forming new blood vessels from the existing vasculature in healthy brain tissue into areas of ischemic penumbra.199 The outermost cells in the zone of ischemic penumbra slightly restore their metabolism activities, since they have more blood supply when compared to cells more centrally located in the ischemic area. At this site, where blood supply is limited, there is rapid consumption of ATP, due to low levels of oxygen. Therefore, the delivery of exogenous ATP by liposomes could restore the metabolism of ischemic cells and reduce the area of injury.135,137,138,183,184,187,188
As mentioned earlier, cells within the infarcted area of ischemic tissue do not receive enough oxygen or nutrients to generate ATP. For this purpose, liposome-encapsulated hemoglobin (Hb) was engineered as a pharmacological agent able to deliver oxygen for the treatment of ischemic diseases. Several studies reported in the literature suggest the efficacy of Hb liposomes in the treatment of stroke by enhancing the biodistribution of Hb liposomes within the ischemic area in the brain.144,145,147,160–164,167,171,191,193,194 In the same way, liposomes for the delivery of angiogenic peptides140 and VEGF157 to promote angiogenesis in ischemic tissue were developed, and both formulations effectively promoted vascular regeneration.140,157
Over the years, many liposomal formulations have been developed for the treatment of stroke. Moreover, when liposomes were associated with contrast agents, researchers observed that they quickly accumulated in the ischemic zone.134,143,148,152,190 Some formulations have demonstrated their ability to improve in vivo activity of drugs, such as chrysophanol,133 dexamethasone phosphate,154 nerve growth factor,170 Xe,150,158 FK506,149 isopropylidene–shikimic acid,151 asialo-erythropoietin,153 antisense oligonucleotides,165 plasmids,174 quercetin,166,168 fasudil,176 nitric oxide,146 N-acetyl-leucyl-leucyl-norleucine amide,178 and a combination of synergistic drugs.156,175 Very recently, a promising uncoupling new drug – ZL006 (5-(3, 5-dichloro-2-hydroxybenzylamino)-2-hydroxybenzoic acid) – was developed for stroke treatment. Its mechanism of action is based on the selective blocking of the coupling of nitric oxide synthase, and it was also recognized as a neuroprotective drug. As with many other drugs, ZL006 possesses low to BBB-permeability capacity. However, its encapsulation in immunoliposomes targeted the BBB and significantly enhanced the delivery of ZL006 within the brain. A remarkable neuroprotective effect was also observed.141
Brain cancer – glioma
There are more than 100 different types of brain and CNS tumors. In this article, we focused our search on the term “glioma”, which encompasses all tumors that arise from glial cells, including astrocytomas, oligodendrogliomas, ependymomas, and glioblastomas multiforme.200 Glioblastoma multiforme is by far the most common and aggressive cancer form of the glial tumors. The current standard of care for this type of cancer includes surgery, followed by treatment with radiation and/or chemotherapeutic drugs. The current median overall survival of patients with glioblastoma multiforme is less than 15 months after surgery, followed by synergistic combination of radiotherapy and chemotherapy with the anticancer drug temozolomide.201 Treatment for this type of cancer has its limitations, due to the poor ability to deliver therapeutic agents across the two unique barriers present in the brain: the BBB and the blood–brain tumor barrier (BBTB). Moreover, the low accumulation of nanoparticles into brain tumors by the enhanced permeability and retention (EPR) effect should be also taken into account.202 Therefore, efforts have been made to identify and develop drug-delivery systems for the brain. Liposomes are described as a possible valuable system to achieve better therapeutic effects in the treatment of gliomas, since several targeting strategies have been reported showing ability to reach the brain and to target the tumor. The search for reports on the use of liposomes as drug-delivery nanocarriers for the treatment and/or diagnosis of gliomas is shown in Figure 3. An initial search yielded a total of 448 articles after exclusion of duplicate articles found in the PubMed and Web of Science databases. In total, 80 articles were eligible.60,64,65,203–283 Although all described new nanocarriers for the delivery of therapeutic molecules into the brain, only 77 studies are included in Table 5, because the full text of seven articles212,217,230,246,254,255,265 was not available to access.
Table 5.
Studies on liposome application in glioma
| Species | Administration | Time points | Liposome composition | Ligands | Drug/imaging agent | Particle size (nm) | CNS action | References |
|---|---|---|---|---|---|---|---|---|
| Mice | Intravenous injection | >50 days | PC, DSPE-PEG2,000, and cholesterol | PTD HIV1 peptide | EPI plus celecoxib | 108 | Targeted liposomes were able to cross the BBB and accumulate in the tumor, leading to the destruction of glioma vasculogenic mimicry channels. | 203 |
| Mice | Intravenous injection | >50 days | SPC, DSPE-PEG2,000, and cholesterol | Cell-penetrating peptides | PTX | 100–120 | Dual-targeted liposomes exhibited selective targeting and anticancer therapeutic effects. | 204 |
| Mice | Intravenous injection | >60 days | SPC, DSPE-PEG2,000, and cholesterol | TR peptide | PTX | 130 | Ligand-targeted liposomes for the delivery of PTX showed an effective targeting ability to cancer stem cells, destroying the vasculogenic mimicry channels. | 205 |
| Mice | Intravenous injection | 8 hours | DODAP, DSPC, C16 Cer-PEG2,000, and cholesterol | CTX | miR-21 | 190 | Ligand-targeted liposomes were able to efficiently deliver anti-miRNA oligonucleotides into the brain. | 206 |
| Mice | Intravenous injection | 52 days | SPC, DSPE-PEG2,000, and cholesterol | RGD peptide | PTX | 107 | Ligand-targeted liposomes for the delivery of PTX showed an effective targeting ability for brain-cancer stem cells. | 207 |
| Mice | Intravenous injection | 60 minutes | DOPC, DSPE-PEG2,000, and cholesterol | NI | Gd-DTPA | 100 | Liposome-encapsulated Gd-DTPA was able to release the contrast agent in response to a chance in the pH environment. | 208 |
| Mice | Intravenous injection | >77 days | NI | RGD peptide | DOX and iron oxide nanoparticles | 90 | Ligand-targeted liposomes were combined with iron oxide to produce a magnetic field-responsive liposome hybrid. These nanosystems presented enhanced targeting ability and facilitated site-specific drug delivery into the brain. | 209 |
| Mice | Intravenous injection | 50 days | PC, DSPE-PEG2,000, and cholesterol | WGA and TAM | DNR and quinacrine | 104 | Ligand-targeted liposomes for the delivery of DNR plus quinacrine exhibited evident capabilities in crossing the BBB, in killing glioma and glioblastoma stem cells, and in diminishing brain gliomas in mice. | 210 |
| Mice | Intravenous injection | 7 days | SPC, DSPE-PEG2,000, and cholesterol | RGD peptide and Tf | PTX | 128 | Ligand-targeted liposomes for the delivery of PTX were developed and presented high brain distribution. | 211 |
| Mice | Intravenous injection | 4 hours | PC, DSPE-PEG2,000 DSPE-PEG1,000, and cholesterol | TAT-peptide and Tf | DOX | 114 | Ligand-targeted liposomes for the delivery of DOX possessed strong capability of synergistic targeted delivery of the chemotherapeutic drug for brain tumors. | 213 |
| Mice | Vascular and intratumoral injection by CED | >70 days | DSPC, DSPE-PEG2,000, and cholesterol | NI | Irinotecan | NI | Liposome-encapsulated irinotecan treatment demonstrated good antitumor activity on glioblastomas and a higher median survival time of tumor-bearing mice. | 214 |
| Mice | Intravenous injection | 10, 14, and 20 days | DODAP, DSPC, C16 Cer-PEG2,000, and cholesterol | CTX | asOs siRNA | 178 144 | Ligand-targeted liposomes for nucleic acids delivery demonstrated that CTX enhanced liposome internalization into glioma cells. | 215 |
| Mice | Intravenous injection | 55 days | HSPC, DSPE-PEG2,000, and cholesterol | RGERPPR peptide | DOX | 90 | Ligand-targeted liposomes for delivering of DOX showed an enhanced targeted therapeutic effect on glioblastomas. | 216 |
| Mice | Intravenous injection | 30 minutes | DPPC, MSPC, and DSPE-PEG2,000 EPC, DSPE-PEG2,000, and cholesterol |
NI | DOX | 121 123 |
Thermosensitive liposome-encapsulated DOX delivered DOX across the BBB and also improved median survival time of tumor-bearing mice. | 218 |
| Mice | Intravenous injection | >50 days | DPPC and DSPE-PEG2,000 | Angiopep-2 peptide | DOX | 100 | Ligand-targeted liposomes for the delivery of DOX demonstrated antitumor activity and prolonged median survival time of tumor-bearing mice. | 219 |
| Mice | Intravenous injection | 70 days | SPC and DSPE-PEG2,000 | NI | Topotecan | 100 | Liposome-encapsulated topotecan delayed tumor growth and prolonged median survival time of tumor-bearing mice. | 220 |
| Mice | Intravenous injection | 10 days | HSPC, DSPE-PEG2,000, and cholesterol | CTX | DOX | 100 | Ligand-targeted liposomes demonstrated higher accumulation into tumors and antitumor activity. | 221 |
| Mice | Intravenous injection | 4, 12, 24, and 48 hours | DOPC, DOPG, DOGS-NTA-Ni, DSPE-PEG2,000, and cholesterol | Anti-EGFR antibody | BSH | 100 | Ligand-targeted liposomes were an effective tool for delivery of BSH to glioma cells into the brain. | 222 |
| Mice | Intraperitoneal injection | 200 days | DPPC, DSPE-PEG2,000, and cholesterol | IL-13 protein | DOX | 100 | Ligand-targeted liposomes for the delivery of DOX promoted a reduction in tumor size and prolonged median survival time on tumor-bearing mice. | 223 |
| Mice | Intravenous injection | 6, 24, 48 and 72 hours | DPPC, DSPE-PEG2,000, and cholesterol | Tf | 10B | 100 | Ligand-targeted liposomes delivered in a specific way a high concentration of BSH into the tumor tissue. | 224 |
| Mice | Intravenous injection | >60 days | NI | NI | U1snRNA/ribozymes | NI | Delivery of U1snRNA/ribozymes by liposomes inhibited tumor growth and angiogenesis. | 225 |
| Rats | Intravenous injection + FUS | >80 days | HSPC, DOPE, CTAB, didodecyldimethylammonium bromide, and cholesterol | NI | DOX or QD | 187 175 |
Combining the reversible opening of the by FUS and the ability of cationic liposomes to bind to glioma cells, it was possible to improve median survival of tumor-bearing rats. | 226 |
| Rats | Intravenous injection | 9, 14, and 17 days | Liposomal doxorubicin | NI | Ultrasonic MB for delivery of lipo-DOX | 100 | FUS increased tumor drug delivery over time in glioma-tumor model. | 228 |
| Rats | Intravenous injection | 24 days | DSPC, DSPE-PEG2,000, and cholesterol | NI | 188Re | 80 | Liposome-encapsulated 188Re prolonged median survival of tumor-bearing rats. | 229 |
| Rats | Intracarotid injection | 2–5 minutes | DMPC, DOTAP, and cholesterol DMPC and cholesterol DMPG and cholesterol |
NI NI NI |
DiD DiD DiD |
NI NI NI |
The concentration of cationic liposomes was greater into the brain parenchyma compared to anionic and neutral liposomes. Cationic liposomes were also readily observed within glioma tissue after intra-arterial injection. |
60 |
| Rats | Intravenous injection | 48 hours | DSPC, mPEG-DSPE, DSPE-PEG2,000, and cholesterol | Anti-SAv and antiendoglin antibodies | Gd-DTPA | 267 | The two-step endoglin-targeted imaging using biotin–streptavidin interaction was demonstrated to induce intense enhancement of the tumor periphery, which implies that this advanced MR molecular contrast agent may be suitable for accurately delineating glioma tumor margins. Ligand-targeted imaging liposomes demonstrated enhancement of the tumor periphery, demarcating glioma tumor margins accurately. | 231 |
| Rats | Intravenous injection | 4 weeks | DPPC, DSPE-PEG2,000, and cholesterol | NI | Evans blue | 173 | Evans blue liposomes visually delineated invasive glioma margins. | 232 |
| Rats | Intravenous injection | 48 hours | PC, DSPE-PEG2,000, DDAB, and cholesterol | Anti-VEGF antibody | DiI | 163 | Ligand-targeted liposomes demonstrated specific accumulation of liposomes in glioma tumors. | 233 |
| Rats/mice | Intravenous injection | 46 days | PC, DSPE-PEG2,000 | MAN-TPGS1,000, and DQA-PEG2,000-DSPE | PTX and artemether | 90 | Ligand-targeted liposomes for the codelivery of PTX and artemether were able to deliver drugs across the BBB. | 234 |
| Rats | Intravenous injection | >100 days | DSPC, DSPE-PEG2,000, and cholesterol | NI | Irinotecan | 102–113 | Liposome-encapsulated irinotecan prolonged median survival time in tumor-bearing mice. | 235 |
| Rats | Intravenous injection | 18 days | PC, DSPE-PEG, Mal-PEG2,000,-DSPE, DC-Chol | Tf and CTX peptide | plasmid | 120 | Ligand-targeted liposomes for gene delivery were able to increase the transport of plasmid DNA and specifically delivery the gene for glioma cells into the brain. | 236 |
| Rats | Intracarotid injection | 50 days | Lipoplatin or Lipoxal™ | NI | Cis or oxaliplatin | NI | Tumor uptake was higher for Lipoxal than for the free drug oxaliplatin. Oppositely, lipoplatin led to lower tumor uptake compared with free cisplatin. | 237 |
| Rats | Intravenous injection | 30 days | DSPC, DSPE-PEG2,000, and cholesterol | Tf and folate | DOX | 180 | Ligand-targeted liposomes for the delivery of DOX were able to deliver the drug across the BBB and were distributed mainly in brain gliomas. | 238 |
| Rats | Intravenous injection | 24 hours | Lecithin, DSPE-PEG2,000, and cholesterol | Anti-GFAP and anti-E2 extracellular loop of Cx43 antibodies | Dil or Gd- DTPA | 140 | Ligand-targeted liposomes demonstrated suitability as diagnostic agents to the peritumoral invasion zone of glioma. | 239 |
| Rats | CED administration | 59 days | PC, CHEMS, and DSPE- PEG2,000 | NI | Cis | 55 | The formulation was highly neurotoxic after CED administration, and resulted in the death of animals after 24 hours. | 240 |
| Rats | Intravenous injection | >200 days | DSPC, DSPE-PEG2,000, and cholesterol | NI | IB | 160 | Liposome-encapsulated IB treatment followed by liposomal DOX prolonged median survival of tumor-bearing rats. | 241 |
| Rats | CED administration | 120 | DSPC and cholesterol | NI | 186Re | NI | Prolonged survival of tumor-bearing rats was observed after brachytherapy with liposomally encapsulated 186Re. | 242 |
| Rats | Intravenous injection | 1, 2, and 3 weeks | Doxil® | NI | Ultrasonic MB for delivery of lipo-DOX | 100 | Posttreatment MRI revealed that the combination of FUS with liposomal DOX reduced tumor-growth rate. | 243 |
| Rats | Intravenous injection | >100 days | NI | Lactoferrin | DOX | 208 | Ligand-targeted liposomes for the delivery of DOX significantly prolonged median survival time of tumor-bearing rats. | 244 |
| Rats | Intravenous injection | 72 hours | DSPC, DSPE-PEG2,000, and cholesterol | NI | 188Re | 80 | This study showed that 188Re-liposome was a good candidate for further development of a theranostic agent for treating glioma. | 245 |
| Rats | Intravenous injection | 1 hour | DSPC, DSPE-PEG2,000, and cholesterol | Ala-Pro-Arg-Pro- Gly peptide | 18F and Dil | 113 | The smallest tumor among those tested, having a diameter of 1 mm, was successfully imaged by the liposomal 18F. | 247 |
| Rats | Intravenous injection | >129 days | SPC and cholesterol | TAT peptide | DOX | 105 | Ligand-targeted liposomes for the delivery of DOX prolonged median survival of glioma-targeted rats. | 248 |
| Rats | Intravenous injection | 16 days | EPC, DSPE-PEG2,000, and cholesterol | Tf and TAM | EPI | 110 | Evident effect of targeting brain-tumor cells in vitro and extended median survival time in brain glioma-bearing rats. | 249 |
| Rats | Intravenous injection | 14 days | EPC, DSPE-PEG2,000, and cholesterol | MAN and Tf | DNR | 122.8 | Ligand-targeted liposomes for the delivery of DNR improved therapeutic efficacy for gliomas. | 250 |
| Rats | Intravenous injection | 31 days | PC, DSPE-PEG2,000 and cholesterol | TAM and WGA | Topetecan | 100–110 | Ligand-targeted liposomes for the delivery of topotecan prolonged median survival time of brain tumor-bearing rats. | 251 |
| Rats | CED administration | 48 days | DSPC, DSPG, and cholesterol | NI | Topetecan and Gd | 75–120 | Liposomes for codelivery of topotecan and Gd were able to prolong median survival time of glioma-bearing rats. | 252 |
| Rats | Intravenous injection | 30 hours | DSPE-S-S-PEG5,000, DPPC, and cholesterol | Folate | NI | 100 | Ligand-targeted liposomes indicated that masking targeting ligands with cleavable phospholipid-PEG proved to be a good strategy for controlled exposure of targeting ligands to ensure that circulation times remained uncompromised. | 65 |
| Rats | Intravenous injection | 5, 30, 60, and 120 minutes | EYPC, mPEG-DSPE, PDP-PEG-HEPE, and cholesterol | anti-CD 105 antibody | Gd-DTPA | 165.3 | Ligand-targeted liposomes for tumor imaging detected early tumor angiogenesis on MR images. | 253 |
| Rats | CED administration | 6 hours or 2 weeks | DSPC, DSPE-PEG2,000, and cholesterol | NI | DiD | NI | Development of retroconvection-enhanced delivery method increased blood-brain transfer of macromolecules. | 256 |
| Rats | CED administration | >70 days | DSPC, DSPE-PEG2,000, and cholesterol Doxil | NI | Irinotecan and DOX | 95-110 | Convection-enhanced delivery proved a good technique for delivery of liposomes to intracranial tumor-bearing rats. | 257 |
| Rats | Intravenous injection | 42 days | DSPC, DSPE-PEG2,000, and cholesterol | Folate | DOX | 110-115 | Ligand-targeted liposomes for the delivery of DOX prolonged the median survival time of tumor-bearing rats. | 64 |
| Rats | CED administration | >90 days | DSPC and cholesterol Doxil | NI | Topotecan and DOX | 100-120 | Convection-enhanced delivery seemed to be a good technique for delivery of liposomes to intracranial tumor-bearing rats. | 258 |
| Rats | CED administration | > 100 days | DSPC, DSPE-PEG2,000, and cholesterol | NI | Irinotecan | 96-101 | Convection-enhanced delivery was a good technique for the delivery of liposomes to intracranial tumor-bearing rats. | 259 |
| Rats | CED administration | NI | DSPC and cholesterol | NI | Dil and topotecan | 112 | Convection-enhanced delivery of liposomes enabled effective and continuous delivery of the chemotherapeutic drug in tumor-bearing rats. | 260 |
| Rats | CED administration | 24 hours | DOPC and DSPE-PEG2,000 | NI | Gadodiamide and Dil | NI | Convection-enhanced delivery of liposomes allowed them to be spread into the tumor tissue. Greater penetration was observed for smaller liposomes and their coinfusion with mannitol. | 261 |
| Rats | CED administration | 2 hours | DOPC and cholesterol | NI | DOX, Gd, and Dil | 77 | Theranostic liposomes allowed in vivo monitoring of therapeutic distribution of liposomes into brain tumor-bearing rats. | 262 |
| Rats | Intravenous injection | 14 days | NI | Tf | siRNA | 85 | Cationic liposomes for siRNA transfection inhibited luciferase gene expression in brain gliomas up to 5 days after a single intravenous injection of nanoparticles. | 263 |
| Rats | Intravenous injection | 24 hours | DPPC | NI | Photofrin | NI | Liposome-encapsulated Photofrin enhanced the PDT treatment of rat brain tumors. | 264 |
| Rats | Intraperitoneal injection | 24 hours | DPPC | NI | Photofrin | NI | Liposome-encapsulated Photofrin enhanced the PDT treatment of rat brain tumors. | 266 |
| Rats | Intravenous injection | 36 days | DSPC, DSPE-PEG2,000, and cholesterol | NI | DOX | NI | Liposome-encapsulated DOX enhanced the therapeutic effect of the drug. | 267 |
| Rats | Intravenous injection and hyperthermia | 60 days | DPPC and DSPC | NI | Cis | NI | The rats treated with CDDP liposomes + hyperthermia had the longest survival time, and the tumor CDDP level of this group was the highest when compared to the other groups. Histopathological examination showed that tumor cells were necrotized, but surrounding normal brain tissue remained undamaged. On the basis of these findings, we suggest that the combination of thermosensitive liposomes and localized hyperthermia may better focus antitumor drugs to the tumor, providing a significantly greater antitumor effect. Thermosensitive liposomes combined with localized hyperthermia provided higher antitumor activity, prolonging the median survival time of tumor-bearing rats. |
268 |
| Rats | Intravenous injection | 2 minutes | NI | NI | DiO | 2,000 | Lipid-coated microbubbles administered intravenously to rats bearing brain tumors specifically enhanced tumor visualization by ultrasound. Glioma tumors were able to internalize microbubbles. |
269 |
| Rats | Intracarotid injection | 30 minutes | PC, DPPC, and cholesterol | NI | HRP | NI | These results indicate that liposomes can penetrate the blood-brain barrier. Liposome-encapsulated Cis were able to deliver drugs across the barrier. |
270 |
| Rats | Intravenous injection | 43 days | DOTAP and DOPE | NI | Plasmid | NI | Liposomes for plasmid delivery inhibited rat glioma growth. | 271 |
| Dogs | CED administration | 14 months | DSPC, DSPE-PEG2,000, and cholesterol | NI | Irinotecan and Gd | 93-108 | This study provided a translational model system for convection-enhanced delivery in dogs. | 272 |
| Monkeys | CED administration | NI | DOPC, DSG-PEG2,000, and cholesterol | NI | Gd or RhB | 124 | Theranostic liposomes in combination with CED allowed in vivo monitoring of therapeutic distribution of liposomes into brain tumor-bearing monkeys. | 273 |
| Humans | Intracranial injection | 29 months | NI | NI | Plasmid | NI | Phase I clinical trial of IFNB gene therapy of glioma multiform via cationic liposomes demonstrated differentially expression of this and other genes of patients after gene delivery. A majority of the other genes were related to apoptosis, immune response, and angiogenesis. | 274 |
| Humans | CED administration | >25 months | Liposomal DNR | NI | DNR | NI | Progressive or recurrent high-grade gliomas are characterized by a very poor prognosis, and the relevance of second-line chemotherapy is still unassessed. Although it has been reported that liposomal anthracyclines and carboplatin show some activity in these patients, their association has never been investigated. We treated six children with recurrent high-grade glioma after surgery, radiotherapy, and chemotherapy, and one child with progressive teratoid/rhabdoid tumor with the combination of liposomal daunorubicin and carboplatin plus etoposide. Five of seven children showed a major response, and 29-month progression-free survival was 38%. The above-mentioned regimen was feasible, and children showed little and transient hematological toxicity. In our opinion, these results justify further investigation of this combination chemotherapy for recurrent or progressive malignant brain tumors in children. This study suggests the association of liposomal DNR, carboplatin, and etoposide as second-line chemotherapy in children with recurrent malignant brain tumors. |
275 |
| Humans | Intracranial injection | 6 months | NI | NI | IFNβ | NI | This study performed a pilot clinical trial by the transfer of IFNB gene therapy via cationic liposomes. The results demonstrated the feasibility and safety of this therapy for glioma treatment. | 276 |
| Humans | CED administration | 42 days | NI | NI | SFVIL-12 | 90 | A Phase I/II clinical trial of immunogenic therapy of recurrent glioblastoma multiforme with a liposome-encapsulated replication-incompetent Semliki Forest virus vector carrying the human IL12. | 277 |
| Humans | Intravenous injection | NI | Liposomal DNR | NI | DNR | NI | High concentration of DNR was detected in brain tumors after systemic administration of liposomal DNR. | 278 |
| Humans | Intravenous injection | 165 weeks | Doxil | NI | DOX | NI | Long-term stabilization of glioblastoma multiforme observed in patients after treatment with liposomal DOX. | 279 |
| Humans | Intravenous injection | 4 weeks | Doxil | NI | DOX and 99mTc-DTPA | NI | High concentration of DOX was detected in brain tumors in patients with glioblastoma multiforme and metastatic brain lesions after treatment with theranostic liposomes. | 280 |
| Humans | Intravenous administration | 48 hours | DaunoXome® (liposomal DNR) | NI | DNR | NI | Liposome-encapsulated DNR presented good cytotoxicity toward human glioma tumors. | 281 |
| Humans | Intracranial injection | 6 weeks | DPPC, DPPA and cholesterol | NI | Bleomycin | NI | Although no clinically important side effects were observed, three of six patients showed progressive deterioration in their clinical condition. | 282 |
| Humans | Intracranial injection | 24 hours | DPPC, DPPA and cholesterol | NI | Bleomycin | NI | This study demonstrated the potential of liposomes in drug delivery in patients with brain cancer. | 283 |
Abbreviations: 99mTc, technetium-99m; asOs, antisense oligonucleotides; BBB, blood–brain barrier; BSH, borocaptate sodium; CDPP, cis-diammino dichloroplatinum-II (cisplatin); CED, convection-enhanced delivery; Cer, ceramide; CHEMS, cholesteryl hemisuccinate; Cis, cisplatin; CNS, central nervous system; CTAB, Cetyltrimethylammonium bromide; CTX, chlorotoxin; DC-Chol, 3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl]-cholesterol hydrochloride; DDAB, dimethyldioctadecylammonium bromide; DiD, 4,4′-diisothiocyanatostilbene- 2,2′-disulfonic acid; DiI, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; DiO, 3,3′-dioctadecyloxacarbocyanine perchlorate; DMPC, dimyristoylphosphatidylcholine; DMPG, dimyristoylphosphatidylglycerol; DNR, daunorubicin; DODAP, 1,2-dioleoyl-3-dimethylammonium-propane; DOGS-NTA-Ni, 1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid) succinyl]; DOPC, dioleoylphosphatidylcholine; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DOPG, 1,2-dioleoyl-sn-glycero-3-phospho-(1′-rac-glycerol); DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; DOX, doxorubicin; DPPA, 1,2-dipalmitoyl-sn-glycero-3-phosphate monosodium salt; DPPC, dipalmitoylphosphatidylcholine; DQA, dequalinium; DSPC, distearoylphosphatidylcholine; DSPE, distearoylphosphatidylethanolamine; DSPG, distearoylphosphatidylglycerol; DTPA, diethylenetriaminepentaacetic acid; EPC, egg phosphatidylcholine; EPI, epirubicin; EYPC, egg-yolk phosphatidylcholine; FUS, focused ultrasound; HEPE, hydrogenated egg phosphatidylethanolamine; HRP, horseradish peroxidase; HSPC, hydrogenated soy phosphatidylcholine; IB, imipramine blue; Mal, maleimide; MAN, P-aminophenyl-α-D-mannopyranoside; MB, methylene blue; miRNA, micro-RNA; mPEG, methoxy polyethylene glycol; MR, magnetic resonance; MRI, magnetic resonance imaging; MSPC, 1-myristoyl-2-stearoyl-sn-glycero-3-phosphocholine; NI, not informed; PC, phosphatidylcholine; PEG, polyethylene glycol; PDP, pyridyldithiopropionate; PDT, photodynamic therapy; PTX, paclitaxel; QD, quantum dots; siRNA, small interfering RNA; SPC, sphingosylphosphorylcholine; TAM, tamoxifen; Tf, transferrin; TPGS, tocopheryl polyethylene glycol succinate; U1snRNA, small nuclear RNA component of U1 snRNP (small nuclear ribonucleoprotein); WGA, wheat-germ agglutinin.
Design of liposomal drug-delivery systems for glioma diagnosis
One of the most challenging problems in therapy of gliomas is their detection in the earliest stages of development. Like many tumor types, early detection correlates with successful therapy. Therefore, both new diagnostic and therapeutic approaches need to be developed for glioma-imaging oncology. For this purpose, a huge variety of contrast agents have been encapsulated into liposomes. These new nanomaterials may provide new opportunities for biomedical imaging, due to their unique magnetic, optical, and/or chemical properties, leading to the creation of better contrast-enhancement agents and increasing the sensitivity of techniques clinically available for diagnosis of brain tumors.284
Modern imaging techniques, such as magnetic resonance imaging (MRI), optical imaging, ultrasound, and single-photon-emission computed tomography (SPECT), are rapidly emerging as noninvasive modalities for detection and follow-up posttreatment of gliomas.285,286 MRI is the preferred approach for glioma imaging, since it provides high-spatial-resolution anatomic images of this tumor type.287 Optical imaging applied to glioma therapy has the potential to localize and identify intrinsic brain tumors for removal during surgery.288 Ultrasound, unlike MRI, defines tumor volume and provides intraoperative localization of tumor tissue, although its use is limited by the presence of the skull.285 SPECT yields growth rate and gives information about the heterogeneity of gliomas, but provides low-spatial-resolution images.289 Positron-emission tomography (PET) provides functional information, since this technique is highly sensitive for measurements of biological processes, such as cell proliferation, angiogenesis, and glucose consumption.290
Paramagnetic contrast agents are the most widely used agents to enhance the visibility of gliomas in MRI images. Gadolinium (Gd)-based compounds, such as Gd–diethylenetriaminepentaacetic acid, gadodiamide, and gadoteridol, are effective contrast agents, owing to their seven unpaired electrons. Although Gd-based compounds are able to cross the BBB, a key advantage of using liposomes as Gd carriers is preferential localization at the tumor site through the EPR effect. In this way, it was shown that Gd liposomes with prolonged blood-circulation time tend to accumulate in the intratumoral extravascular space after moving across the tumor’s leaky vasculature.208,254 Moreover, a recent advance was reported in the design of a pH-responsive Gd liposome that was able to release the imaging agent into a cerebral glioma rodent model, detecting with 0.2 pH precision the mildly acid tumor microenvironment.208
Methods for optical imaging of glioma are based on fluorescence. The lipid-binding fluorescent carbocyanine dyes DiD (4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid, disodium salt), DiO (3,3′-dioctadecyloxacarbocyanine perchlorate), and DiI (1,1′-dioctadecyl-3,3,3,′3,′-tetramethylinocarbocyanine perchlorate) are widely used for imaging studies. The characteristics of aqueous-insolubility ease of aggregate formation and the fact that these dyes do not readily cross the BBB suggest that it would be desirable to develop a liposome-based system. In this way, the carbocyanine dyes have been encapsulated into liposomes with the ability to demarcate tumors.60,256,269 The results of these studies suggest that those formulations, independent of the mode of administration, stained the tumor tissue and increased their bioavailability.60,256,269 However, the use of these fluorescent probes has the disadvantage of requiring low-light conditions for the visualization of tumors in vivo, which is not useful in a surgical environment. It was recently reported that Evans blue liposomally encapsulated was able to demarcate visually the margins of invasive gliomas, which may not significantly change the surgical conditions for the resection of this type of tumor.232
Also, studies reported in Table 5 suggest that the use of MRI or optical imaging alone in the imaging of gliomas is not enough for their classification and grading, optimal treatment, and follow-up after treatment,261,273 since each imaging technique is associated with individual advantages and limitations. Furthermore, it is generally observed that the presence of targeting ligands over the liposome surface improves the uptake of vesicles by target cancer cells and increases their retention time within tumors.231,233,239,247,253 In gliomas, angiogenesis seems to be the preferable target area for diagnosis of this cancer. Angiogenesis, the formation of new vessels, is a key process for glioma survival and growth.291 From the literature search, two molecules were identified for angiogenic cells: endoglin, also known as CD105, and the Ala-Pro-Arg-Pro-Gly peptide. MRI of endoglin-target liposomes was able to demonstrate tumor angiogenesis253 and delineate tumor margins, showing correlation between endoglin-associated neovasculature and tumor infiltration.231 In the same way, PET of Ala-Pro-Arg-Pro-Gly peptide-targeted liposomes was able specifically to image the different structure of glioma vessels.247 Also, liposome-targeted delivery of contrast agents containing antibodies to GFAP and the extracellular loop of Cx43 on its surface that selectively bound to brain-reactive astrocytes and faster-migrating glioma cells has been developed.239 Also developed have been PEGylated liposomes containing VEGF antibody on the surface, increasing the distribution and efficacy of the delivery of liposomes to glioma.233 However, it is still unclear if any of the strategies described could enhance detection of the earliest stage of tumors.
Design of liposomal drug-delivery systems for glioma therapy
Glioma therapy consists of surgery followed by radiotherapy, chemotherapy, or photodynamic therapy (PDT). Moreover, the stage and type of glioma often determines whether monotherapy or combined therapies are needed. Radiation therapy is a very common option for the treatment of gliomas, and has a variety of modalities, including external beam and brachytherapy.292 External beam radiotherapy, the most common approach in the clinic, uses ionizing radiation to kill cancer cells, but its application is limited by doses lower than 80 Gy due to toxicity.293 Brachytherapy or internal therapy uses a radioactive source that is delivered into or near the tumor itself, making it possible to deliver high radiation to the tumor and harming as few normal cells as possible.294 In this way, recent studies suggest that brachytherapy with liposomally encapsulated 186Re or 188Re isotopes holds significant promise for glioma therapy.229,242,245 These studies demon strated that animals treated with 186Re or 188Re liposomes had significantly prolonged survival, independent of the route of administration.229,242 Also, 188Re liposomes have been explored for diagnostic evaluation, revealing the potential of these liposomes as a future theranostic agent for brain gliomas.245
A wide variety of liposome-encapsulated anticancer drugs have also been developed for both experimental and clinical oncology. By virtue of their unique physicochemical characteristics, liposomes have mainly shown improvement in the therapeutic index of chemotherapeutic drugs by enhancing their efficacy against aggressive and chemoresistant glioma cells and/or lowering drug side effects in the body. Antitumor antibiotics include doxorubicin (DOX), daunorubicin (DNR), and bleomycin. DOX, an anthracycline antibiotic, damages DNA by intercalation, inhibiting DNA synthesis or poisoning of topoisomerase II, by alteration of membrane function, or by generation of free radicals.295,296 DNR, also an anthracycline antibiotic similar in its chemical structure to DOX, acts through intercalation into DNA, metal ion chelation, and/or by free radical formation.296 Bleomycin, a polypeptide antibiotic, exerts its action by breaking the DNA double helix.297
Furthermore, antitumor antibiotics are among the most widely used and studied chemotherapeutic drugs. They are currently available in the market as free drugs (Adriamycin®, Cerubidine®, and Blenoxane®, trade names for DOX, DNR, and bleomycin, respectively), encapsulated in PEGylated liposomes (Doxil® [PEGylated form of liposomal DOX]), and encapsulated in conventional liposomes (Myocet® and DaunoXome® [liposomal DOX and DNR, respectively]). Although the anticancer activity of free drugs has been reported to be effective against gliomas cells in vitro, they present very poor efficacy in vivo, because these antibiotics do not readily cross the BBB.298 In a rat brain-glioma model, prolonged survival of the animals was observed when PEGylated liposomes were used to deliver DOX.267 In contrast, in a cohort of patients with brain cancer, liposomal DOX, DNR, or bleomycin was found moderately effective against glioma.275,278,279,281–283
Based on the moderate efficacy of liposomal formulations against brain tumors, it is clear that more effective drug-delivery strategies are needed. One promising alternative strategy involves the combination of ultrasound and microbubbles to induce BBB opening for local and transient delivery of drugs into the brain, leading to improvement in chemotherapy treatment.226,228,243,265 Moreover, as Doxil has been already clinically approved for the treatment of some types of cancer, these results suggest that the use of ultrasonic microbubbles for glioma chemotherapy is highly clinically relevant.228,243 Other alternative strategies were found in this search. Researchers developed stimuli-responsive liposomes that were able to release DOX in a controlled manner in response to an external low-power radio frequency field209 or local temperature rise.218 Both strategies showed an improvement of DOX delivery across the BBB and prolonged survival time of animals.
In fact, over the years many liposomal formulations have been developed for the treatment of gliomas. Some formulations demonstrated their ability to improve activity of anticancer drugs in vivo, such as topotecan,220 irinotecan,214,230,235,259 arsenic trioxide,255 cisplatin,237,240,268 and oxaliplatin,237 and codelivery of synergistic two-drug combinations257,258 into brain tumor-bearing animal models. Other formulations demonstrated increased bioavailability of the bioactive compound celastrol,217 carriage of small molecules241 and large payloads,225,270 and enabled efficient gene therapy.271,274,276,277 Additionally, the design of liposomes that simultaneously carry imaging and therapeutic agents is promising for glioma therapy, since it allows the opportunity for real-time visualization of drug localization, drug delivery, and monitoring the tumor-therapy response.252,260,262,272,280 However, although passively targeting liposomes are the only ones used in clinical therapy, they suffer several limitations, such as low EPR effect within the brain, nonspecific uptake, and the crossing of both barriers. Therefore, methods for enhancing the targeting of liposomes to brain tumors were developed.
According to our search, liposomes actively targeting strategies for CNS delivery of anticancer drugs across the BBB are basically divided into adsorptive-mediated transcytosis (AMT) and receptor-mediated transcytosis (RMT). AMT is trigged by electrostatic interactions between the negatively charged surface of brain endothelial cells and the positively charged moieties of macromolecules. AMT-based drug delivery for glioma therapy was performed using the cationic cell-penetrating TAT peptide to functionalize the surface of liposomally encapsulated DOX.248 The authors demonstrated that the TAT peptide could penetrate the BBB, since DOX was efficiently delivered to brain tumor-bearing rats.248 However, this cationization strategy suffers from several limitations, such as instability of the system in the serum, nonspecific interactions, immunogenicity, and toxicity.202,299
RMT-based drug delivery has been widely explored for liposome targeting to the brain. This strategy relies on liposomal ligand interaction with the very specific receptor-mediated transport system in the BBB. In fact, there are several kinds of receptors that are expressed on the surface of endothelial cells of the barrier, such as transferrin and lactoferrin, that have been explored to facilitate the crossing of liposomes into the brain. Transferrin liposomes have been reported to be able to deliver borocaptate (BSH) and small interfering RNA (siRNA) into the CNS, which is highly significant, because these compounds do not readily cross the BBB.224,246,263 In the same way, the covalent binding of lactoferrin to the liposome surface proved to be an effective strategy for the treatment of brain tumors.244
In glioma therapy, similarly to the BBB, the BBTB also represents a challenge for glioma-targeted delivery.300 Fortunately, many kinds of receptors are highly expressed in the BBTB (tumor vessels and/or glioma cells), and these receptors have been explored for the design of actively targeting liposomes for brain delivery of anticancer drugs across the BBTB. For example, such ligands as chlorotoxin,206,215,221 TR peptide,205 RGERPPR peptides,216 folate,64,65 anti-EGFR antibody,222 and IL-13,223 have been successfully attached to the surface of liposomes. As a result, these decorated liposomes were able selectively to bind, target, and enhance uptake by glioma cells. In the same way, hemagglutinating virus of Japan liposomes have successfully delivered foreign genes into murine glioma cells, representing a good system for gene delivery.227
More recently, researchers have developed liposomes that can penetrate the BBB and targeting brain-cancer cells. This new system, known as dual-targeting liposomes, was produced to deliver DOX,211,213,238 DNR,250 epirubicin,249 topotecan,251 plasmids,236 and siRNA,212 and for codelivery of synergistic two-drug combinations.203,210 All of these dual-targeting liposomes proved to be effective in crossing the BBB and targeting glioma cells. It was also demonstrated that just angiopep-2 peptide was able to target BBB and glioma cells at the same time,219 and RGD peptide targeted both BBTB and tumor cells.204,207,209
PDT uses photosensitizing agents, such as Photofrin, for brain tumors, along with light of appropriated wavelength to kill glioma cells. The PDT cell-killing mechanism is directly related to the production of reactive oxygen species, which leads to cell apoptosis, with minimal side effects.301 Unfortunately, the efficiency of this therapy for gliomas is limited by the BBB. Just like for chemotherapy, the efficacy of PDT for the treatment of brain tumors was greatly improved when Photofrin was encapsulated into liposomes, since the photosensitizing agent was efficiently delivered within brain tumors.264,266 Finally, it is worth mentioning here that although the preferred route for delivery of liposomes seems to be intravenous injection, alternative routes of administration, such as convection-enhanced delivery and intracranial, intracarotid, and intraperitoneal injections, have been also considered.
Conclusion
The BBB is the most important obstacle to effective brain drug delivery. There has been great interest in this area, especially in the development of targeted liposomes to cross the BBB and to deliver therapeutic molecules only to the disease site within the brain. From the reported articles, we could see that liposomes can get into the brain via different mechanisms. Examples of these mechanisms are: 1) transport of liposomes via RMT, followed by their internalization by neurons or glial cells and release of therapeutic molecules within those cells; 2) adsorption of cationic liposomes in the endothelial cells, which enhanced the concentration of therapeutic molecules within the brain cells; 3) antibody- or peptide-conjugated liposomes used to transport and target encapsulated drugs into the brain via transcytotic pathways; 4) inhibition of efflux transporters, such as PgP, by coating liposomes with transporter-inhibitory substances; and 5) disruption of the BBB. In fact, liposomes have the potential to revolutionize drug development for therapy and/or diagnosis of neurological diseases. By their unique physicochemical properties, liposomes have shown great ability to compartmentalize and solubilize hydrophilic and hydrophobic drugs (Figure 4). Furthermore, liposomes are biocompatible and biodegradable systems, which make them suitable for neuromedicine.
Figure 4.
Delivery of therapeutic molecules or imaging agents to the brain by liposomes (m) is highly challenging.
Notes: Liposomes can be administered to the central nervous system via systemic delivery (a), intracarotid (b), intracranial (c), intranasal (e), and intraperitoneal (f) injections, or via convection-enhanced delivery (d/n). Liposome-based strategies consist in encapsulating the molecules of interest in liposomes (V). The ability to increase their blood-circulation time is created with the ligation of polyethylene glycol on the liposome surface (III). Liposomes can also be targeted to cross the blood–brain barrier (I), target the site of disease (IX), or both (II). Surface modification of liposomes can be achieved by covalent ligation of antibodies (IX), RNA aptamers (VI), or peptides (XII). Cationic lipids can be incorporated into the bilayer, facilitating their association with nucleic acids for gene therapy (VIII and XI). This figure also summarizes therapeutic mechanisms, such as hyperthermia (IV), temperature increase (VII), and ultrasound (X).
Also, the functionalization of liposomes surface modified with antibodies, peptides, aptamers, and other small molecules has shown promise in delivering a huge range of therapeutic molecules to targeted sites in the body (Figure 4). Liposomes usually improve the therapeutic index of new or established drugs by prolonging biological half-life and reducing their side effects. More importantly, liposomes may provide an excellent therapeutic tool for treatment or diagnosis of neurological disorders, due to their ability to cross the BBB and efficiently deliver drugs and/or contrast agents into the CNS, as discussed in this article. Additionally, theranostic liposomes have been developed, allowing real-time therapeutic efficacy. Also, high efficacy in using liposomes to deliver a drug in a spatial and temporal manner has been demonstrated (Figure 4), which we believe may be critical for the success of more effective therapy for neurological diseases.
Unfortunately, most advances and breakthroughs in liposome-based approaches have just happened for glioma therapy. The development of effective therapy for AD, PD, and stroke has been largely constrained. This might be due to our deficiency in understanding the neurological mechanisms and pathogenesis of these disorders. Increasing our comprehension about these diseases will contribute to the development of novel potential therapeutic strategies. This is essential, since we are living in a modern aging society and an effective spectrum of treatments is urgently needed.
By crossing the BBB, achieving efficient drug delivery into the brain is possible, which leads to an intensive search for alternative administration routes for liposomes. In this review, various studies used different administration routes to access the brain for the therapeutic delivery of liposomes (Figure 4). Intravenous injection of liposomes was the preferred route in the majority of the works cited here. Alternatively, intranasal injections offered a direct mode of drug delivery into the brain for AD and PD. Convection-enhanced delivery provided interesting results for efficient delivery of liposomes for drug delivery to tumor-bearing animal models (Tables 3–5). Administration of liposomal formulation via nonparenteral routes is highly desirable, since effective strategies for crossing the BBB are urgently needed.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ehrlich P. Das Sauerstoff-Bedürfnis des Organismus: Eine Farbenanalytische Studie [The oxygen demand of the organism: a color analysis study] Berlin: Hirschwald; 1885. [Google Scholar]
- 2.Goldmann EE. Vitalfarbung am Zentralnervensystem: Beitrag zur Physio-Pathologie des Plexus Chorioideus und der Hirnhäute [Vital staining of the central nervous system: contribution to the physiopathology of the plexus choroid and the brain membranes] Berlin: Königlich Akademie der Wissenschaften; 1913. German. [Google Scholar]
- 3.Lewandowsky M. Zur lehre der cerebrospinal flüssigkeit [For the teaching of cerebrospinal fluid] Z Klin Med. 1900;40:480–494. German. [Google Scholar]
- 4.Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 6.Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12:54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 8.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier – an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 10.Vorbrodt AW. Ultracytochemical characterization of anionic sites in the wall of brain capillaries. J Neurocytol. 1989;18:359–368. doi: 10.1007/BF01190839. [DOI] [PubMed] [Google Scholar]
- 11.Scherrmann JM. Drug delivery to brain via the blood-brain barrier. Vascul Pharmacol. 2002;38:349–354. doi: 10.1016/s1537-1891(02)00202-1. [DOI] [PubMed] [Google Scholar]
- 12.Poduri A, Evrony GD, Cai X, Walsh CA. Somatic mutation, genomic variation, and neurological disease. Science. 2013;341:1237758. doi: 10.1126/science.1237758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qureshi IA, Mehler MF. Epigenetic mechanisms underlying the pathogenesis of neurogenetic diseases. Neurotherapeutics. 2014;11:708–720. doi: 10.1007/s13311-014-0302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lesniak MS, Brem H. Targeted therapy for brain tumours. Nat Rev Drug Discov. 2004;3:499–508. doi: 10.1038/nrd1414. [DOI] [PubMed] [Google Scholar]
- 15.Stenehjem DD, Hartz AM, Bauer B, Anderson GW. Novel and emerging strategies in drug delivery for overcoming the blood-brain barrier. Future Med Chem. 2009;1:1623–1641. doi: 10.4155/fmc.09.137. [DOI] [PubMed] [Google Scholar]
- 16.Pavan B, Dalpiaz A, Ciliberti N, Biondi C, Manfredini S, Vertuani S. Progress in drug delivery to the central nervous system by the prodrug approach. Molecules. 2008;13:1035–1065. doi: 10.3390/molecules13051035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabathuler R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol Dis. 2010;37:48–57. doi: 10.1016/j.nbd.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 18.Pardridge WM. Drug delivery to the brain. J Cereb Blood Flow Metab. 1997;17:713–731. doi: 10.1097/00004647-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov. 2016;15:275–292. doi: 10.1038/nrd.2015.21. [DOI] [PubMed] [Google Scholar]
- 20.Fleming AB, Saltzman WM. Pharmacokinetics of the carmustine implant. Clin Pharmacokinet. 2002;41:403–419. doi: 10.2165/00003088-200241060-00002. [DOI] [PubMed] [Google Scholar]
- 21.Barua NU, Gill SS, Love S. Convection-Enhanced drug delivery to the brain: therapeutic potential and neuropathological considerations. Brain Pathol. 2014;24:117–127. doi: 10.1111/bpa.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aryal M, Arvanitis CD, Alexander PM, McDannold N. Ultrasound-mediated blood-brain barrier disruption for targeted drug delivery in the central nervous system. Adv Drug Deliv Rev. 2014;72:94–109. doi: 10.1016/j.addr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King A. Breaking through the barrier. Chem World. 2011;8:36–39. [Google Scholar]
- 24.Bertrand Y, Currie JC, Poirier J, et al. Influence of glioma tumour microenvironment on the transport of ANG1005 via low-density lipoprotein receptor-related protein 1. Br J Cancer. 2011;105:1697–1707. doi: 10.1038/bjc.2011.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spencer BJ, Verma IM. Targeted delivery of proteins across the blood-brain barrier. Proc Natl Acad Sci U S A. 2007;104:7594–7599. doi: 10.1073/pnas.0702170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ché C, Yang G, Thiot C, et al. New Angiopep-modified doxorubicin (ANG1007) and etoposide (ANG1009) chemotherapeutics with increased brain penetration. J Med Chem. 2010;53:2814–2824. doi: 10.1021/jm9016637. [DOI] [PubMed] [Google Scholar]
- 27.Vlieghe P, Khrestchatisky M. Medicinal chemistry based approaches and nanotechnology-based systems to improve CNS drug targeting and delivery. Med Res Rev. 2013;33:457–516. doi: 10.1002/med.21252. [DOI] [PubMed] [Google Scholar]
- 28.Bhaskar S, Tian F, Stoeger T, et al. Multifunctional nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: perspectives on tracking and neuroimaging. Part Fibre Toxicol. 2010;7:3. doi: 10.1186/1743-8977-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai F, Fadda AM, Sinico C. Liposomes for brain delivery. Expert Opin Drug Deliv. 2013;10:1003–1022. doi: 10.1517/17425247.2013.766714. [DOI] [PubMed] [Google Scholar]
- 30.Ramos-Cabrer P, Campos F. Liposomes and nanotechnology in drug development: focus on neurological targets. Int J Nanomedicine. 2013;8:951–960. doi: 10.2147/IJN.S30721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreuter J. Drug delivery to the central nervous system by polymeric nanoparticles: What do we know? Adv Drug Deliv Rev. 2014;71:2–14. doi: 10.1016/j.addr.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Patel T, Zhou J, Piepmeier JM, Saltzman WM. Polymeric nanoparticles for drug delivery to the central nervous system. Adv Drug Deliv Rev. 2012;64:701–705. doi: 10.1016/j.addr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren J, Shen S, Wang D, et al. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials. 2012;33:3324–3333. doi: 10.1016/j.biomaterials.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 34.Al-Jamal KT, Gherardini L, Bardi G, et al. Functional motor recovery from brain ischemic insult by carbon nanotube-mediated siRNA silencing. Proc Natl Acad Sci U S A. 2011;108:10952–10957. doi: 10.1073/pnas.1100930108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tseng Y, Kao Y, Liao J, Chen W, Liu S. Biodegradable drug-eluting poly(lactic-co-glycol acid) nanofibers for the sustainable delivery of vancomycin to brain tissue: in vitro and in vivo studies. ACS Chem Neurosci. 2013;4:1314–1321. doi: 10.1021/cn400108q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie J, Wang CH. Electrospun micro- and nanofibers for sustained delivery of paclitaxel to treat C6 Glioma in vitro. Pharm Res. 2006;23:1817–1826. doi: 10.1007/s11095-006-9036-z. [DOI] [PubMed] [Google Scholar]
- 37.Somani S, Dufès C. Applications of dendrimers for brain delivery and cancer therapy. Nanomedicine (Lond) 2014;9:2403–2414. doi: 10.2217/nnm.14.130. [DOI] [PubMed] [Google Scholar]
- 38.Pérez-Martínez FC, Ocaña AV, Pérez-Carrión MD, Ceña V. Dendrimers as vectors for genetic material delivery to the nervous system. Curr Med Chem. 2012;19:5101–5108. doi: 10.2174/0929867311209025101. [DOI] [PubMed] [Google Scholar]
- 39.Morshed RA, Cheng Y, Auffinger B, Wegscheid ML, Lesniak MS. The potential of polymeric micelles in the context of glioblastoma therapy. Front Pharmacol. 2013;4:157. doi: 10.3389/fphar.2013.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma HS, Menon PK, Lafuente JV, et al. The role of functionalized magnetic iron oxide nanoparticles in the central nervous system injury and repair: new potentials for neuroprotection with Cerebrolysin therapy. J Nanosci Nanotechnol. 2014;14:577–595. doi: 10.1166/jnn.2014.9213. [DOI] [PubMed] [Google Scholar]
- 41.Li W, Chen X. Gold nanoparticles for photoacoustic imaging. Nanomedicine (Lond) 2015;10:299–320. doi: 10.2217/nnm.14.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization . Neurological Disorders: Public Health Challenges. Geneva: WHO; 2006. [Google Scholar]
- 43.Micheli MR, Bova R, Magini A, Polidoro M, Emiliani C. Lipid-based nanocarriers for CNS-targeted drug delivery. Recent Pat CNS Drug Discov. 2012;7:71–86. doi: 10.2174/157488912798842241. [DOI] [PubMed] [Google Scholar]
- 44.Schnyder A, Huwyler J. Drug transport to brain with targeted liposomes. NeuroRX. 2005;2:99–107. doi: 10.1602/neurorx.2.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnsen KB, Moos T. Revisiting nanoparticle technology for blood-brain barrier transport: unfolding at the endothelial gate improves the fate of transferrin receptor-targeted liposomes. J Control Release. 2016;222:32–46. doi: 10.1016/j.jconrel.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 46.Noble GT, Stefanick JF, Ashley JD, Kiziltepe T, Bilgicer B. Ligand-targeted liposome design: challenges and fundamental considerations. Trends Biotechnol. 2014;32:32–45. doi: 10.1016/j.tibtech.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 48.Loyse A, Thangaraj H, Easterbrook P, et al. Cryptococcal meningitis: improving access to essential antifungal medicines in resource-poor countries. Lancet Infect Dis. 2013;13:629–637. doi: 10.1016/S1473-3099(13)70078-1. [DOI] [PubMed] [Google Scholar]
- 49.Robinson RF, Nahata MC. A comparative review of conventional and lipid formulations of amphotericin B. J Clin Pharm Ther. 1999;24:249–257. doi: 10.1046/j.1365-2710.1999.00220.x. [DOI] [PubMed] [Google Scholar]
- 50.Lippens RJ. Liposomal daunorubicin (DaunoXome) in children with recurrent or progressive brain tumors. Pediatr Hematol. 1999;16:131–139. doi: 10.1080/088800199277452. [DOI] [PubMed] [Google Scholar]
- 51.Benesch M, Urban C. Liposomal cytarabine for leukemic and lymphomatous meningitis: recent developments. Expert Opin Pharmacother. 2008;9:301–309. doi: 10.1517/14656566.9.2.301. [DOI] [PubMed] [Google Scholar]
- 52.Ananda S, Nowak AK, Cher L, et al. Phase 2 trial of temozolomide and PEGylated liposomal doxorubicin in the treatment of patients with glioblastoma multiforme following concurrent radiotherapy and chemotherapy. J Clin Neurosci. 2011;18:1444–1448. doi: 10.1016/j.jocn.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 53.Beier CP, Schmid C, Gorlia T, et al. RNOP-09: PEGylated liposomal doxorubicine and prolonged temozolomide in addition to radiotherapy in newly diagnosed glioblastoma – a phase II study. BMC Cancer. 2009;9:308. doi: 10.1186/1471-2407-9-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hau P, Fabel K, Baumgart U, et al. PEGylated liposomal doxorubicin-efficacy in patients with recurrent high-grade glioma. Cancer. 2004;100:1199–1207. doi: 10.1002/cncr.20073. [DOI] [PubMed] [Google Scholar]
- 55.Chua SL, Rosenthal MA, Wong SS, et al. Phase 2 study of temozolomide and Caelyx in patients with recurrent glioblastoma multiforme. Neuro Oncol. 2004;6:38–43. doi: 10.1215/S1152851703000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagner S, Peters O, Fels C, et al. PEGylated-liposomal doxorubicin and oral topotecan in eight children with relapsed high-grade malignant brain tumors. J Neurooncol. 2008;86:175–181. doi: 10.1007/s11060-007-9444-x. [DOI] [PubMed] [Google Scholar]
- 57.Marina NM, Cochrane D, Harney E, et al. Dose escalation and pharmacokinetics of PEGylated liposomal doxorubicin (Doxil) in children with solid tumors: a pediatric oncology group study. Clin Cancer Res. 2002;8:413–418. [PubMed] [Google Scholar]
- 58.Di Legge A, Trivellizzi IN, Moruzzi MC, Pesce A, Scambia G, Lorusso D. Phase 2 trial of nonPEGylated doxorubicin (Myocet) as second-line treatment in advanced or recurrent endometrial cancer. Int J Gynecol Cancer. 2011;21:1446–1451. doi: 10.1097/IGC.0b013e31822d754e. [DOI] [PubMed] [Google Scholar]
- 59.Vieira DB, Gamarra LF. Advances in the use of nanocarriers for cancer diagnosis and treatment. Einstein (Sao Paulo) 2016;14:99–103. doi: 10.1590/S1679-45082016RB3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joshi S, Singh-Moon RP, Ellis JA, et al. Cerebral hypoperfusion-assisted intra-arterial deposition of liposomes in normal and glioma-bearing rats. Neurosurgery. 2015;76:92–100. doi: 10.1227/NEU.0000000000000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joshi S, Singh-Moon R, Wang M, et al. Cationic surface charge enhances early regional deposition of liposomes after intracarotid injection. J Neurooncol. 2014;120:489–497. doi: 10.1007/s11060-014-1584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joshi S, Singh-Moon RP, Wang M, et al. Transient cerebral hypoperfusion assisted intraarterial cationic liposome delivery to brain tissue. J Neurooncol. 2014;118:73–82. doi: 10.1007/s11060-014-1421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huwyler J, Wu D, Pardridge WM. Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci U S A. 1996;93:14164–14169. doi: 10.1073/pnas.93.24.14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McNeeley KM, Annapragada A, Bellamkonda RV. Decreased circulation time offsets increased efficacy of PEGylated nanocarriers targeting folate receptors of glioma. Nanotechnology. 2007;18:385101. [Google Scholar]
- 65.McNeeley KM, Karathanasis E, Annapragada AV, Bellamkonda RV. Masking and triggered unmasking of targeting ligands on nanocarriers to improve drug delivery to brain tumors. Biomaterials. 2009;30:3986–3995. doi: 10.1016/j.biomaterials.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 66.Ding H, Sagar V, Agudelo M, et al. Enhanced blood-brain barrier transmigration using a novel transferrin embedded fluorescent magnetoliposome nanoformulation. Nanotechnology. 2014;25:055101. doi: 10.1088/0957-4484/25/5/055101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nair MPN, Saiyed SM, inventors. The Florida International University Board Of Trustees, assignee Magnetic nanodelivery of therapeutic agents across the blood brain barrier. 20110213193 A1. United States patent US. 2011 Sep 1;
- 68.Guo H, Chen W, Sun X, Liu YN, Li J, Wang J. Theranostic magnetoliposomes coated by carboxymethyl dextran with controlled release by low-frequency alternating magnetic field. Carbohydr Polym. 2015;118:209–217. doi: 10.1016/j.carbpol.2014.10.076. [DOI] [PubMed] [Google Scholar]
- 69.Shazeeb MS, Feula G, Bogdanov A. Liposome-encapsulated superoxide dismutase mimetic: theranostic potential of an MR detectable and neuroprotective agent. Contrast Media Mol Imaging. 2014;9:221–228. doi: 10.1002/cmmi.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karmali PP, Chaudhuri A. Cationic liposomes as non-viral carriers of gene medicines: resolved issues, open questions, and future promises. Med Res Rev. 2007;27:696–722. doi: 10.1002/med.20090. [DOI] [PubMed] [Google Scholar]
- 71.Dass CR, Choong PF. Targeting of small molecule anticancer drugs to the tumour and its vasculature using cationic liposomes: lessons from gene therapy. Cancer Cell Int. 2006;6:17. doi: 10.1186/1475-2867-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen W, Li H, Liu Z, Yuan W. Lipopolyplex for therapeutic gene delivery and its application for the treatment of Parkinson’s disease. Front Aging Neurosci. 2016;8:68. doi: 10.3389/fnagi.2016.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lajoie JM, Shusta EV. Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier. Annu Rev Pharmacol Toxicol. 2015;55:613–631. doi: 10.1146/annurev-pharmtox-010814-124852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McConnell EM, Holahan MR, DeRosa MC. Aptamers as promising molecular recognition elements for diagnostics and therapeutics in the central nervous system. Nucleic Acid Ther. 2014;24:388–404. doi: 10.1089/nat.2014.0492. [DOI] [PubMed] [Google Scholar]
- 75.Kelkar SS, Reineke TM. Theranostics: combining imaging and therapy. Bioconjug Chem. 2011;22:1879–1903. doi: 10.1021/bc200151q. [DOI] [PubMed] [Google Scholar]
- 76.Wen CJ, Zhang LW, Al-Suwayeh SA, Yen TC, Fang JY. Theranostic liposomes loaded with quantum dots and apomorphine for brain targeting and bioimaging. Int J Nanomedicine. 2012;7:1599–1611. doi: 10.2147/IJN.S29369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lerner EN, van Zanten EH, Stewart GR. Enhanced delivery of octreotide to the brain via transnasal iontophoretic administration. J Drug Target. 2004;12:273–280. doi: 10.1080/10611860400000938. [DOI] [PubMed] [Google Scholar]
- 78.Mistry A, Stolnik S, Illum L. Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm. 2009;379:146–157. doi: 10.1016/j.ijpharm.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 79.Wu H, Hu K, Jiang X. From nose to brain: understanding transport capacity and transport rate of drugs. Expert Opin Drug Deliv. 2008;5:1159–1168. doi: 10.1517/17425247.5.10.1159. [DOI] [PubMed] [Google Scholar]
- 80.Arumugam K, Subramanian GS, Mallayasamy SR, Averineni RK, Reddy MS, Udupa N. A study of rivastigmine liposomes for delivery into the brain through intranasal route. Acta Pharm. 2008;58:287–297. doi: 10.2478/v10007-008-0014-3. [DOI] [PubMed] [Google Scholar]
- 81.Migliore MM, Vyas TK, Campbell RB, Amiji MM, Waszczak BL. Brain delivery of proteins by the intranasal route of administration: a comparison of cationic liposomes versus aqueous solution formulations. J Pharm Sci. 2010;99:1745–1761. doi: 10.1002/jps.21939. [DOI] [PubMed] [Google Scholar]
- 82.Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10:S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 83.Rotman M, Welling MM, Bunschoten A, et al. Enhanced glutathione PEGylated liposomal brain delivery of an anti-amyloid single domain antibody fragment in a mouse model for Alzheimer’s disease. J Control Release. 2015;203:40–50. doi: 10.1016/j.jconrel.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 84.Balducci C, Mancini S, Minniti S, et al. Multifunctional liposomes reduce brain β-amyloid burden and ameliorate memory impairment in Alzheimer’s disease mouse models. J Neurosci. 2014;34:14022–14031. doi: 10.1523/JNEUROSCI.0284-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bana L, Minniti S, Salvati E, et al. Liposomes bi-functionalized with phosphatidic acid and an ApoE-derived peptide affect Aβ aggregation features and cross the blood-brain-barrier: implications for therapy of Alzheimer disease. Nanomedicine. 2014;10:1583–1590. doi: 10.1016/j.nano.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 86.Mutlu NB, Değim Z, Yılmaz S, Eşsiz D, Nacar A. New perspective for the treatment of Alzheimer diseases: liposomal rivastigmine formulations. Drug Dev Ind Pharm. 2011;37:775–789. doi: 10.3109/03639045.2010.541262. [DOI] [PubMed] [Google Scholar]
- 87.Tanifum EA, Dasgupta I, Srivastava M, et al. Intravenous delivery of targeted liposomes to amyloid-β pathology in APP/PSEN1 transgenic mice. PLoS One. 2012;7:e48515. doi: 10.1371/journal.pone.0048515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen ZL, Huang M, Wang XR, et al. Transferrin-modified liposome promotes α-mangostin to penetrate the blood-brain barrier. Nanomedicine. 2016;12:421–430. doi: 10.1016/j.nano.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 89.Ordóñez-Gutiérrez L, Re F, Bereczki E, et al. Repeated intraperitoneal injections of liposomes containing phosphatidic acid and cardiolipin reduce amyloid-β levels in APP/PS1 transgenic mice. Nanomedicine. 2015;11:421–430. doi: 10.1016/j.nano.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 90.Yang ZZ, Zhang YQ, Wang ZZ, Wu K, Lou JN, Qi XR. Enhanced brain distribution and pharmacodynamics of rivastigmine by liposomes following intranasal administration. Int J Pharm. 2013;452:344–354. doi: 10.1016/j.ijpharm.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 91.Ismail MF, Elmeshad AN, Salem NA. Potential therapeutic effect of nanobased formulation of rivastigmine on rat model of Alzheimer’s disease. Int J Nanomedicine. 2013;8:393–406. doi: 10.2147/IJN.S39232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li W, Zhou Y, Zhao N, Hao B, Wang X, Kong P. Pharmacokinetic behavior and efficiency of acetylcholinesterase inhibition in rat brain after intranasal administration of galanthamine hydrobromide loaded flexible liposomes. Environ Toxicol Pharmacol. 2012;34:272–279. doi: 10.1016/j.etap.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 93.Tong-Un T, Muchimapura S, Phachonpai W, Wattanathorn J. Nasal administration of quercetin liposomes modulate cognitive impairment and inhibit acetylcholinesterase activity in hippocampus. Am J Neurosci. 2010;1:21–27. [Google Scholar]
- 94.Phachonpai W, Wattanathorn J, Muchimapura S, Preechagoon D. Neuroprotective effect of quercetin encapsulated liposomes: a novel therapeutic strategy against Alzheimer’s disease. Am J Appl Sci. 2010;7:480–485. [Google Scholar]
- 95.Wattanatho J, Phachonpai W, Priprem A, Suthiparin S. Intranasal administration of quercetin liposome decreases anxiety-like behavior and increases spatial memory. Am J Agric Biol Sci. 2007;2:31–35. [Google Scholar]
- 96.Xiang Y, Wu Q, Liang L, et al. Chlorotoxin-modified stealth liposomes encapsulating levodopa for the targeting delivery against the Parkinson’s disease in the MPTP-induced mice model. J Drug Target. 2012;20:67–75. doi: 10.3109/1061186X.2011.595490. [DOI] [PubMed] [Google Scholar]
- 97.Kucherianu VG, Iurasov VV, Kryzhanovskiĭ GN, et al. The effect of liposomal form of L-Dopa on the development of parkinsonian syndrome in mice. Biull Eksp Biol Med. 1997;123:29–33. Russian. [PubMed] [Google Scholar]
- 98.Yurasov VV, Kucheryanu VG, Kudrin VS, et al. Effect of long-term parenteral administration of empty and L-Dopa-loaded liposomes on the turnover of dopamine and its metabolites in the striatum of mice with experimental Parkinson’s syndrome. Bull Exp Biol Med. 1997;123:126–129. [Google Scholar]
- 99.Yurasov VV, Podgornyi GN, Kucheryanu VG, et al. Effects of L-Dopa-carrying liposomes on striatal concentration of dopamine and its metabolites and phospholipid metabolism in experimental Parkinson’s syndrome. Bull Exp Biol Med. 1996;122:1180–1183. [Google Scholar]
- 100.Migliore MM, Ortiz R, Dye S, Campbell RB, Amiji MM, Waszczak BL. Neurotrophic and neuroprotective efficacy of intranasal GDNF in a rat model of Parkinson’s disease. Neuroscience. 2014;274:11–23. doi: 10.1016/j.neuroscience.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 101.Xia CF, Boado RJ, Zhang Y, Chu C, Pardridge WM. Intravenous glial-derived neurotrophic factor gene therapy of experimental Parkinson’s disease with Trojan horse liposomes and a tyrosine hydroxylase promoter. J Gene Med. 2008;10:306–315. doi: 10.1002/jgm.1152. [DOI] [PubMed] [Google Scholar]
- 102.Di Stefano A, Sozio P, Iannitelli A, Marianecci C, Santucci E, Carafa M. Maleic- and fumaric-diamides of (O,O-diacetyl)-L-Dopa-methylester as anti-Parkinson prodrugs in liposomal formulation. J Drug Target. 2006;14:652–661. doi: 10.1080/10611860600916636. [DOI] [PubMed] [Google Scholar]
- 103.Di Stefano A, Carafa M, Sozio P, et al. Evaluation of rat striatal L-dopa and DA concentration after intraperitoneal administration of L-dopa prodrugs in liposomal formulations. J Control Release. 2004;99:293–300. doi: 10.1016/j.jconrel.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 104.Krstic D, Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat Rev Neurol. 2012;9:25–34. doi: 10.1038/nrneurol.2012.236. [DOI] [PubMed] [Google Scholar]
- 105.Lipton SA, Gu Z, Nakamura T. Inflammatory mediators leading to protein misfolding and uncompetitive/fast off-rate drug therapy for neurodegenerative disorders. Int Rev Neurobiol. 2007;82:1–27. doi: 10.1016/S0074-7742(07)82001-0. [DOI] [PubMed] [Google Scholar]
- 106.Moreira PI, Siedlak SL, Aliev G, et al. Oxidative stress mechanisms and potential therapeutics in Alzheimer disease. J Neural Transm (Vienna) 2005;112:921–932. doi: 10.1007/s00702-004-0242-8. [DOI] [PubMed] [Google Scholar]
- 107.Oddo S, LaFerla FM. The role of nicotinic acetylcholine receptors in Alzheimer’s disease. J Physiol Paris. 2006;99:172–179. doi: 10.1016/j.jphysparis.2005.12.080. [DOI] [PubMed] [Google Scholar]
- 108.Wenk GL, Rosi S, McGann K, Hauss-Wegrzyniak B. A nitric oxide-donating flurbiprofen derivative reduces neuroinflammation without interacting with galantamine in the rat. Eur J Pharmacol. 2002;453:319–324. doi: 10.1016/s0014-2999(02)02387-7. [DOI] [PubMed] [Google Scholar]
- 109.Kan MJ, Lee JE, Wilson JG, et al. Arginine deprivation and immune suppression in a mouse model of Alzheimer’s disease. J Neurosci. 2015;35:5969–5982. doi: 10.1523/JNEUROSCI.4668-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hansen RA, Gartlehner G, Webb AP, Morgan LC, Moore CG, Jonas DE. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. Clin Interv Aging. 2008;3:211–225. [PMC free article] [PubMed] [Google Scholar]
- 111.Confaloni A, Tosto G, Tata A. Promising therapies for Alzheimer’s disease. Curr Pharm Des. 2016;22:2050–2056. doi: 10.2174/1381612822666160215154218. [DOI] [PubMed] [Google Scholar]
- 112.Zheng X, Shao X, Zhang C, et al. Intranasal H102 peptide-loaded liposomes for brain delivery to treat Alzheimer’s disease. Pharm Res. 2015;32:3837–3849. doi: 10.1007/s11095-015-1744-9. [DOI] [PubMed] [Google Scholar]
- 113.Lazar AN, Mourtas S, Youssef I, et al. Curcumin-conjugated nanoliposomes with high affinity for Aβ deposits: possible applications to Alzheimer disease. Nanomedicine. 2013;9:712–721. doi: 10.1016/j.nano.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 114.Theunis C, Crespo-Biel N, Gafner V, et al. Efficacy and safety of a liposome-based vaccine against protein tau, assessed in tau.P301L mice that model tauopathy. PLoS One. 2013;8:e72301. doi: 10.1371/journal.pone.0072301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Carrera I, Etcheverría I, Li Y, et al. Immunocytochemical characterization of Alzheimer disease hallmarks in APP/PS1 transgenic mice treated with a new anti-amyloid-β vaccine. Biomed Res Int. 2013;2013:709145. doi: 10.1155/2013/709145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nicolau C, Greferath R, Balaban TS, Lazarte JE, Hopkins RJ. A liposome-based therapeutic vaccine against β-amyloid plaques on the pancreas of transgenic NORBA mice. Proc Natl Acad Sci U S A. 2002;99:2332–2337. doi: 10.1073/pnas.022627199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee HJ, Bae EJ, Lee SJ. Extracellular α-synuclein: a novel and crucial factor in Lewy body diseases. Nat Rev Neurol. 2014;10:92–98. doi: 10.1038/nrneurol.2013.275. [DOI] [PubMed] [Google Scholar]
- 118.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 119.Nagatsu T, Sawada M. L-dopa therapy for Parkinson’s disease: past, present, and future. Parkinsonism Relat Disord. 2009;15:S3–S8. doi: 10.1016/S1353-8020(09)70004-5. [DOI] [PubMed] [Google Scholar]
- 120.Black KJ, Carl JL, Hartlein JM, Warren SL, Hershey T, Perlmutter JS. Rapid intravenous loading of levodopa for human research: clinical results. J Neurosci Methods. 2003;127:19–29. doi: 10.1016/s0165-0270(03)00096-7. [DOI] [PubMed] [Google Scholar]
- 121.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 122.Gash DM, Zhang Z, Gerhardt G. Neuroprotective and neurorestorative properties of GDNF. Ann Neurol. 1998;44:S121–S125. doi: 10.1002/ana.410440718. [DOI] [PubMed] [Google Scholar]
- 123.Grondin R, Gash DM. Glial cell line-derived neurotrophic factor (GDNF): a drug candidate for the treatment of Parkinson’s disease. J Neurol. 1998;245:P35–P42. doi: 10.1007/pl00007744. [DOI] [PubMed] [Google Scholar]
- 124.Hurelbrink CB, Barker RA. The potential of GDNF as a treatment for Parkinson’s disease. Exp Neurol. 2004;185:1–6. doi: 10.1016/j.expneurol.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 125.Wang Y, Xu H, Fu Q, Ma R, Xiang J. Protective effect of resveratrol derived from Polygonum cuspidatum and its liposomal form on nigral cells in parkinsonian rats. J Neurol Sci. 2011;304:29–34. doi: 10.1016/j.jns.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 126.Amicarelli F, Gasbarri A, Masciocco L, et al. The effect of intrastriatal injection of liposome-entrapped tyrosinase on the dopamine levels in the rat brain. Cell Mol Biol (Noisy-le-Grand) 1999;45:1093–1097. [PubMed] [Google Scholar]
- 127.Segovia J, Vergara P, Brenner M. Astrocyte-specific expression of tyrosine hydroxylase after intracerebral gene transfer induces behavioral recovery in experimental parkinsonism. Gene Ther. 1998;5:1650–1655. doi: 10.1038/sj.gt.3300776. [DOI] [PubMed] [Google Scholar]
- 128.Imaoka T, Date I, Ohmoto T, Nagatsu T. Significant behavioral recovery in Parkinson’s disease model by direct intracerebral gene transfer using continuous injection of a plasmid DNA-liposome complex. Hum Gene Ther. 1998;9:1093–1102. doi: 10.1089/hum.1998.9.7-1093. [DOI] [PubMed] [Google Scholar]
- 129.During MJ, Freese A, Deutch AY, et al. Biochemical and behavioral recovery in a rodent model of Parkinson’s disease following stereotactic implantation of dopamine-containing liposomes. Exp Neurol. 1992;115:193–199. doi: 10.1016/0014-4886(92)90053-s. [DOI] [PubMed] [Google Scholar]
- 130.Campos-Romo A, Ojeda-Flores R, Moreno-Briseño P, et al. Behavioral improvement in MPTP-treated nonhuman primates in the HALLWAY task after transfer of TH cDNA to host astrocytes. Acta Neurobiol Exp (Wars) 2012;72:166–176. doi: 10.55782/ane-2012-1889. [DOI] [PubMed] [Google Scholar]
- 131.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics – 2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 132.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 133.Yan J, Zheng M, Zhang D. Chrysophanol liposome preconditioning protects against cerebral ischemia-reperfusion injury by inhibiting oxidative stress and apoptosis in mice. Int J Pharmacol. 2014;10:55–68. [Google Scholar]
- 134.Deddens LH, van Tilborg GA, van der Toorn A, et al. MRI of ICAM-1 upregulation after stroke: the importance of choosing the appropriate target-specific particulate contrast agent. Mol Imaging Biol. 2013;15:411–422. doi: 10.1007/s11307-013-0617-z. [DOI] [PubMed] [Google Scholar]
- 135.Liu S, Zhen G, Li RC, Doré S. Acute bioenergetic intervention or pharmacological preconditioning protects neuron against ischemic injury. J Exp Stroke Transl Med. 2013;6:7–17. [PMC free article] [PubMed] [Google Scholar]
- 136.Yun X, Maximov VD, Yu J, Zhu H, Vertegel AA, Kindy MS. Nanoparticles for targeted delivery of antioxidant enzymes to the brain after cerebral ischemia and reperfusion injury. J Cereb Blood Flow Metab. 2013;33:583–592. doi: 10.1038/jcbfm.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dvoriantchikova G, Barakat DJ, Hernandez E, Shestopalov VI, Ivanov D. Liposome-delivered ATP effectively protects the retina against ischemia-reperfusion injury. Mol Vis. 2010;16:2882–2890. [PMC free article] [PubMed] [Google Scholar]
- 138.Dvoriantchikova G, Agudelo C, Hernandez E, Shestopalov VI, Ivanov D. Phosphatidylserine-containing liposomes promote maximal survival of retinal neurons after ischemic injury. J Cereb Blood Flow Metab. 2009;29:1755–1759. doi: 10.1038/jcbfm.2009.95. [DOI] [PubMed] [Google Scholar]
- 139.Chan PH. Antioxidant-dependent amelioration of brain injury: role of CuZn-superoxide dismutase. J Neurotrauma. 1992;9:S417–S423. [PubMed] [Google Scholar]
- 140.Hwang H, Jeong HS, Oh PS, et al. Improving cerebral blood flow through liposomal delivery of angiogenic peptides: potential of 18F-FDG PET imaging in ischemic stroke treatment. J Nucl Med. 2015;56:1106–1111. doi: 10.2967/jnumed.115.154443. [DOI] [PubMed] [Google Scholar]
- 141.Wang Z, Zhao Y, Jiang Y, et al. Enhanced anti-ischemic stroke of ZL006 by T7-conjugated PEGylated liposomes drug delivery system. Sci Rep. 2015;5:12651. doi: 10.1038/srep12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Agulla J, Brea D, Campos F, et al. In vivo theranostics at the peri-infarct region in cerebral ischemia. Theranostics. 2014;4:90–105. doi: 10.7150/thno.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fukuta T, Ishii T, Asai T, et al. Real-time trafficking of PEGylated liposomes in the rodent focal brain ischemia analyzed by positron emission tomography. Artif Organs. 2014;38:662–666. doi: 10.1111/aor.12350. [DOI] [PubMed] [Google Scholar]
- 144.Kaneda S, Ishizuka T, Sekiguchi A, Morimoto K, Kasukawa H. Efficacy of liposome-encapsulated hemoglobin in a rat model of cerebral ischemia. Artif Organs. 2014;38:650–655. doi: 10.1111/aor.12358. [DOI] [PubMed] [Google Scholar]
- 145.Kawaguchi AT, Endo H, Aikawa H, et al. Effects of liposome-encapsulated hemoglobin on learning ability in Tokai high-avoider rat after total brain ischemia and reperfusion. Artif Organs. 2014;38:667–674. doi: 10.1111/aor.12352. [DOI] [PubMed] [Google Scholar]
- 146.Kim H, Britton GL, Peng T, et al. Nitric oxide-loaded echogenic liposomes for treatment of vasospasm following subarachnoid hemorrhage. Int J Nanomedicine. 2013;9:155–165. doi: 10.2147/IJN.S48856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shimbo D, Abumiya T, Shichinohe H, Nakayama N, Kazumata K, Houkin K. Post-ischemic intra-arterial infusion of liposome-encapsulated hemoglobin can reduce ischemia reperfusion injury. Brain Res. 2014;1554:59–66. doi: 10.1016/j.brainres.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 148.Ishii T, Fukuta T, Agato Y, et al. Nanoparticles accumulate in ischemic core and penumbra region even when cerebral perfusion is reduced. Biochem Biophys Res Commun. 2013;430:1201–1205. doi: 10.1016/j.bbrc.2012.12.080. [DOI] [PubMed] [Google Scholar]
- 149.Ishii T, Asai T, Oyama D, et al. Treatment of cerebral ischemia-reperfusion injury with PEGylated liposomes encapsulating FK506. FASEB J. 2013;27:1362–1370. doi: 10.1096/fj.12-221325. [DOI] [PubMed] [Google Scholar]
- 150.Peng T, Britton GL, Kim H, et al. Therapeutic time window and dose dependence of xenon delivered via echogenic liposomes for neuroprotection in stroke. CNS Neurosci Ther. 2013;19:773–784. doi: 10.1111/cns.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Qu C, Ni J, Yang P, et al. Altered plasma and brain disposition of isopropylidene shikimic acid liposome in rats and the brain protection in cerebral ischemia-reperfusion. Drug Dev Ind Pharm. 2013;39:1291–1295. doi: 10.3109/03639045.2012.725056. [DOI] [PubMed] [Google Scholar]
- 152.Ishii T, Asai T, Oyama D, et al. Amelioration of cerebral ischemia-reperfusion injury based on liposomal drug delivery system with asialo-erythropoietin. J Control Release. 2012;160:81–87. doi: 10.1016/j.jconrel.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 153.Ishii T, Asai T, Fukuta T, et al. A single injection of liposomal asialo-erythropoietin improves motor function deficit caused by cerebral ischemia/reperfusion. Int J Pharm. 2012;439:269–274. doi: 10.1016/j.ijpharm.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 154.Tiebosch IA, Crielaard BJ, Bouts MJ, et al. Combined treatment with recombinant tissue plasminogen activator and dexamethasone phosphate-containing liposomes improves neurological outcome and restricts lesion progression after embolic stroke in rats. J Neurochem. 2012;123:65–74. doi: 10.1111/j.1471-4159.2012.07945.x. [DOI] [PubMed] [Google Scholar]
- 155.Ramos-Cabrer P, Agulla J, Argibay B, Pérez-Mato M, Castillo J. Serial MRI study of the enhanced therapeutic effects of liposome-encapsulated citicoline in cerebral ischemia. Int J Pharm. 2011;405:228–233. doi: 10.1016/j.ijpharm.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 156.Zhao G, Zang SY, Jiang ZH, et al. Postischemic administration of liposome-encapsulated luteolin prevents against ischemia-reperfusion injury in a rat middle cerebral artery occlusion model. J Nutr Biochem. 2011;22:929–936. doi: 10.1016/j.jnutbio.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 157.Zhao H, Bao XJ, Wang RZ, et al. Postacute ischemia vascular endothelial growth factor transfer by transferrin-targeted liposomes attenuates ischemic brain injury after experimental stroke in rats. Hum Gene Ther. 2011;22:207–215. doi: 10.1089/hum.2010.111. [DOI] [PubMed] [Google Scholar]
- 158.Britton GL, Kim H, Kee PH, et al. In vivo therapeutic gas delivery for neuroprotection with echogenic liposomes. Circulation. 2010;122:1578–1587. doi: 10.1161/CIRCULATIONAHA.109.879338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ghosh S, Das N, Mandal AK, Dungdung SR, Sarkar S. Mannosylated liposomal cytidine 5′ diphosphocholine prevent age related global moderate cerebral ischemia reperfusion induced mitochondrial cytochrome C release in aged rat brain. Neuroscience. 2010;171:1287–1299. doi: 10.1016/j.neuroscience.2010.09.049. [DOI] [PubMed] [Google Scholar]
- 160.Hamadate N, Yamaguchi T, Sugawara A, et al. Liposome-encapsulated hemoglobin ameliorates impairment of fear memory and hippocampal dysfunction after cerebral ischemia in rats. J Pharmacol Sci. 2010;114:409–419. doi: 10.1254/jphs.10207fp. [DOI] [PubMed] [Google Scholar]
- 161.Kakehata J, Yamaguchi T, Togashi H, et al. Therapeutic potentials of an artificial oxygen-carrier, liposome-encapsulated hemoglobin, for ischemia/reperfusion-induced cerebral dysfunction in rats. J Pharmacol Sci. 2010;114:189–197. doi: 10.1254/jphs.10115fp. [DOI] [PubMed] [Google Scholar]
- 162.Fukumoto D, Kawaguchi AT, Haida M, Yamano M, Ogata Y, Tsukada H. Liposome-encapsulated hemoglobin reduces the size of cerebral infarction in rats: effect of oxygen affinity. Artif Organs. 2009;33:159–163. doi: 10.1111/j.1525-1594.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- 163.Kawaguchi AT, Kurita D, Furuya H, Yamano M, Ogata Y, Haida M. Liposome-encapsulated hemoglobin alleviates brain edema after permanent occlusion of the middle cerebral artery in rats. Artif Organs. 2009;33:153–158. doi: 10.1111/j.1525-1594.2008.00700.x. [DOI] [PubMed] [Google Scholar]
- 164.Urakami T, Kawaguchi AT, Akai S, et al. In vivo distribution of liposome-encapsulated hemoglobin determined by positron emission tomography. Artif Organs. 2009;33:164–168. doi: 10.1111/j.1525-1594.2008.00702.x. [DOI] [PubMed] [Google Scholar]
- 165.Omae T, Yoshioka H, Tanaka T, et al. Antisense in vivo knockdown of synaptotagmin I by HVJ-liposome mediated gene transfer attenuates ischemic brain damage in neonatal rats. Brain Dev. 2008;30:313–320. doi: 10.1016/j.braindev.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 166.Rivera F, Costa G, Abin A, et al. Reduction of ischemic brain damage and increase of glutathione by a liposomal preparation of quercetin in permanent focal ischemia in rats. Neurotox Res. 2008;13:105–114. doi: 10.1007/BF03033562. [DOI] [PubMed] [Google Scholar]
- 167.Kawaguchi AT, Fukumoto D, Haida M, Ogata Y, Yamano M, Tsukada H. Liposome-encapsulated hemoglobin reduces the size of cerebral infarction in the rat: evaluation with photochemically induced thrombosis of the middle cerebral artery. Stroke. 2007;38:1626–1632. doi: 10.1161/STROKEAHA.106.467290. [DOI] [PubMed] [Google Scholar]
- 168.Sarkar S, Das N. Mannosylated liposomal flavonoid in combating age-related ischemia-reperfusion induced oxidative damage in rat brain. Mech Ageing Dev. 2006;127:391–397. doi: 10.1016/j.mad.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 169.Adibhatla RM, Hatcher JF, Tureyen K. CDP-choline liposomes provide significant reduction in infarction over free CDP-choline in stroke. Brain Res. 2005;1058:193–197. doi: 10.1016/j.brainres.2005.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Luk YO, Chen WY, Wong WJ, et al. Treatment of focal cerebral ischemia with liposomal nerve growth factor. Drug Deliv. 2004;11:319–324. doi: 10.1080/10717540490494104. [DOI] [PubMed] [Google Scholar]
- 171.Oda T, Kimura T, Ogata Y, Fujise Y. Hemodilution with liposome-encapsulated low-oxygen-affinity hemoglobin does not attenuate hypothermic cerebral ischemia in rats. J Artif Organs. 2005;8:263–269. doi: 10.1007/s10047-005-0309-9. [DOI] [PubMed] [Google Scholar]
- 172.Asahi M, Rammohan R, Sumii T, et al. Antiactin-targeted immunoliposomes ameliorate tissue plasminogen activator-induced hemorrhage after focal embolic stroke. J Cereb Blood Flow Metab. 2003;23:895–899. doi: 10.1097/01.WCB.0000072570.46552.DF. [DOI] [PubMed] [Google Scholar]
- 173.Omori N, Maruyama K, Jin G, et al. Targeting of post-ischemic cerebral endothelium in rat by liposomes bearing polyethylene glycol-coupled transferrin. Neurol Res. 2003;25:275–279. doi: 10.1179/016164103101201508. [DOI] [PubMed] [Google Scholar]
- 174.Cao YJ, Shibata T, Rainov N. Liposome-mediated transfer of the bcl-2 gene results in neuroprotection after in vivo transient focal cerebral ischemia in an animal model. Gene Ther. 2002;9:415–419. doi: 10.1038/sj.gt.3301676. [DOI] [PubMed] [Google Scholar]
- 175.Sinha J, Das N, Basu MK. Liposomal antioxidants in combating ischemia-reperfusion injury in rat brain. Biomed Pharmacother. 2001;55:264–271. doi: 10.1016/s0753-3322(01)00060-9. [DOI] [PubMed] [Google Scholar]
- 176.Takanashi Y, Ishida T, Kirchmeier MJ, Shuaib A, Allen TM. Neuroprotection by intrathecal application of liposome-entrapped fasudil in a rat model of ischemia. Neurol Med Chir (Tokyo) 2001;41:107–114. doi: 10.2176/nmc.41.107. [DOI] [PubMed] [Google Scholar]
- 177.Fresta M, Puglisi G. Reduction of maturation phenomenon in cerebral ischemia with CDP-choline-loaded liposomes. Pharm Res. 1999;16:1843–1849. doi: 10.1023/a:1018999225435. [DOI] [PubMed] [Google Scholar]
- 178.Yokota M, Tani E, Tsubuki S, et al. Calpain inhibitor entrapped in liposome rescues ischemic neuronal damage. Brain Res. 1999;819:8–14. doi: 10.1016/s0006-8993(98)01334-1. [DOI] [PubMed] [Google Scholar]
- 179.Fresta M, Puglisi G. Survival rate improvement in a rat ischemia model by long circulating liposomes containing cytidine-5I-diphosphate choline. Life Sci. 1997;61:1227–1235. doi: 10.1016/s0024-3205(97)00667-x. [DOI] [PubMed] [Google Scholar]
- 180.Fresta M. Biological effects of CDP-choline loaded long circulating liposomes on rat cerebral post-ischemic reperfusion. Int J Pharm. 1996;134:89–97. [Google Scholar]
- 181.Fresta M, Wehrli E, Puglisi G. Enhanced therapeutic effect of cytidine-5′-diphosphate choline when associated with GM1 containing small liposomes as demonstrated in a rat ischemia model. Pharm Res. 1995;12:1769–1774. doi: 10.1023/a:1016234226404. [DOI] [PubMed] [Google Scholar]
- 182.Fresta M, Puglisi G, Di Giacomo C, Russo A. Liposomes as in-vivo carriers for citicoline: effects on rat cerebral post-ischaemic reperfusion. J Pharm Pharmacol. 1994;46:974–981. doi: 10.1111/j.2042-7158.1994.tb03252.x. [DOI] [PubMed] [Google Scholar]
- 183.Puisieux F, Fattal E, Lahiani M, et al. Liposomes, an interesting tool to deliver a bioenergetic substrate (ATP): in vitro and in vivo studies. J Drug Target. 1994;2:443–448. doi: 10.3109/10611869408996820. [DOI] [PubMed] [Google Scholar]
- 184.Chapat S, Frey V, Claperon N, et al. Efficiency of liposomal ATP in cerebral ischemia: bioavailability features. Brain Res Bull. 1991;26:339–342. doi: 10.1016/0361-9230(91)90004-4. [DOI] [PubMed] [Google Scholar]
- 185.Phelan AM, Lange DG. Ischemia/reperfusion-induced changes in membrane fluidity characteristics of brain capillary endothelial cells and its prevention by liposomal-incorporated superoxide dismutase. Biochim Biophys Acta. 1991;1067:97–102. doi: 10.1016/0005-2736(91)90030-c. [DOI] [PubMed] [Google Scholar]
- 186.Imaizumi S, Woolworth V, Fishman RA, Chan PH. Liposome-entrapped superoxide dismutase reduces cerebral infarction in cerebral ischemia in rats. Stroke. 1990;21:1312–1317. doi: 10.1161/01.str.21.9.1312. [DOI] [PubMed] [Google Scholar]
- 187.Laham A, Claperon N, Durussel JJ, et al. Intracarotidal administration of liposomally-entrapped ATP: improved efficiency against experimental brain ischemia. Pharmacol Res Commun. 1988;20:699–705. doi: 10.1016/s0031-6989(88)80117-6. [DOI] [PubMed] [Google Scholar]
- 188.Laham A, Claperon N, Durussel JJ, et al. Liposomally entrapped adenosine triphosphate: improved efficiency against experimental brain ischaemia in the rat. J Chromatogr. 1988;440:455–458. doi: 10.1016/s0021-9673(00)94549-7. [DOI] [PubMed] [Google Scholar]
- 189.Chan PH, Fishman RA, Wesley MA, Longar S. Pathogenesis of vasogenic edema in focal cerebral ischemia: role of superoxide radicals. Adv Neurol. 1990;52:177–183. [PubMed] [Google Scholar]
- 190.Bigon E, Boarato E, Bruni A, Leon A, Toffano G. Pharmacological effects of phosphatidylserine liposomes: regulation of glycolysis and energy level in brain. Br J Pharmacol. 1979;66:167–174. doi: 10.1111/j.1476-5381.1979.tb13661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Komatsu H, Furuya T, Sato N, et al. Effect of hemoglobin vesicle, a cellular-type artificial oxygen carrier, on middle cerebral artery occlusion- and arachidonic acid-induced stroke models in rats. Neurosci Lett. 2007;421:121–125. doi: 10.1016/j.neulet.2007.04.080. [DOI] [PubMed] [Google Scholar]
- 192.Stanimirovic DB, Markovic M, Micic DV, Spatz M, Mrsulja BB. Liposome-entrapped superoxide dismutase reduces ischemia/reperfusion ‘oxidative stress’ in gerbil brain. Neurochem Res. 1994;19:1473–1478. doi: 10.1007/BF00968993. [DOI] [PubMed] [Google Scholar]
- 193.Kawaguchi AT, Haida M, Ohba H, Yamano M, Fukumoto D, Tsukada H. Liposome-encapsulated hemoglobin ameliorates ischemic stroke in nonhuman primates: longitudinal observation. Artif Organs. 2013;37:904–912. doi: 10.1111/aor.12091. [DOI] [PubMed] [Google Scholar]
- 194.Kawaguchi AT, Haida M, Yamano M, Fukumoto D, Ogata Y, Tsukada H. Liposome-encapsulated hemoglobin ameliorates ischemic stroke in nonhuman primates: an acute study. J Pharmacol Exp Ther. 2010;332:429–436. doi: 10.1124/jpet.109.160051. [DOI] [PubMed] [Google Scholar]
- 195.Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012;379:2364–2372. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lichtman JH, Krumholz HM, Wang Y, Radford MJ, Brass LM. Risk and predictors of stroke after myocardial infarction among the elderly: results from the Cooperative Cardiovascular Project. Circulation. 2002;105:1082–1087. doi: 10.1161/hc0902.104708. [DOI] [PubMed] [Google Scholar]
- 197.Overgaard K. The effects of citicoline on acute ischemic stroke: a review. J Stroke Cerebrovasc Dis. 2014;23:1764–1769. doi: 10.1016/j.jstrokecerebrovasdis.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 198.Secades J. Citicoline: pharmacological and clinical review – 2010 update. Rev Neurol. 2011;52:S1–S62. [PubMed] [Google Scholar]
- 199.Navaratna D, Guo S, Arai K, Lo EH. Mechanisms and targets for angiogenic therapy after stroke. Cell Adh Migr. 2009;3:216–223. doi: 10.4161/cam.3.2.8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2:494–503. doi: 10.1038/ncpneuro0289. [DOI] [PubMed] [Google Scholar]
- 201.Minniti G, De Sanctis V, Muni R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J Neurooncol. 2008;88:97–103. doi: 10.1007/s11060-008-9538-0. [DOI] [PubMed] [Google Scholar]
- 202.Wei X, Chen X, Ying M, Lu W. Brain tumor-targeted drug delivery strategies. Acta Pharm Sin B. 2014;4:193–201. doi: 10.1016/j.apsb.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ju RJ, Zeng F, Liu L, et al. Destruction of vasculogenic mimicry channels by targeting epirubicin plus celecoxib liposomes in treatment of brain glioma. Int J Nanomedicine. 2016;11:1131–1146. doi: 10.2147/IJN.S94467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liu Y, Mei L, Xu C, et al. Dual receptor recognizing cell penetrating peptide for selective targeting, efficient intratumoral diffusion and synthesized anti-glioma therapy. Theranostics. 2016;6:177–191. doi: 10.7150/thno.13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shi K, Long Y, Xu C, et al. Liposomes combined an integrin αvβ3-specific vector with pH-responsible cell-penetrating property for highly effective antiglioma therapy through the blood-brain barrier. ACS Appl Mater Interfaces. 2015;7:21442–21454. doi: 10.1021/acsami.5b06429. [DOI] [PubMed] [Google Scholar]
- 206.Costa PM, Cardoso AL, Custódia C, et al. MiRNA-21 silencing mediated by tumor-targeted nanoparticles combined with sunitinib: a new multimodal gene therapy approach for glioblastoma. J Control Release. 2015;207:31–39. doi: 10.1016/j.jconrel.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 207.Liu Y, Mei L, Yu Q, et al. Multifunctional tandem peptide modified paclitaxel-loaded liposomes for the treatment of vasculogenic mimicry and cancer stem cells in malignant glioma. ACS Appl Mater Interfaces. 2015;7:16792–16801. doi: 10.1021/acsami.5b04596. [DOI] [PubMed] [Google Scholar]
- 208.Pacheco-Torres J, Mukherjee N, Walko M, et al. Image guided drug release from pH-sensitive ion channel-functionalized stealth liposomes into an in vivo glioblastoma model. Nanomedicine. 2015;11:1345–1354. doi: 10.1016/j.nano.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 209.Peiris PM, Abramowski A, Mcginnity J, et al. Treatment of invasive brain tumors using a chain-like nanoparticle. Cancer Res. 2015;75:1356–1365. doi: 10.1158/0008-5472.CAN-14-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Li XT, Ju RJ, Li XY, et al. Multifunctional targeting daunorubicin plus quinacrine liposomes, modified by wheat germ agglutinin and tamoxifen, for treating brain glioma and glioma stem cells. Oncotarget. 2014;5:6497–6511. doi: 10.18632/oncotarget.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Qin L, Wang CZ, Fan HJ, et al. A dual-targeting liposome conjugated with transferrin and arginine-glycine-aspartic acid peptide for glioma-targeting therapy. Oncol Lett. 2014;8:2000–2006. doi: 10.3892/ol.2014.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yang Z, Xiang B, Dong D, Wang Z, Li J, Qi X. Dual receptor-specific peptides modified liposomes as VEGF siRNA vector for tumor-targeting therapy. Curr Gene Ther. 2014;14:289–299. doi: 10.2174/1566523214666140612151726. [DOI] [PubMed] [Google Scholar]
- 213.Zong T, Mei L, Gao H, et al. Enhanced glioma targeting and penetration by dual-targeting liposome co-modified with T7 and TAT. J Pharm Sci. 2014;103:3891–3901. doi: 10.1002/jps.24186. [DOI] [PubMed] [Google Scholar]
- 214.Chen PY, Ozawa T, Drummond DC, et al. Comparing routes of delivery for nanoliposomal irinotecan shows superior anti-tumor activity of local administration in treating intracranial glioblastoma xenografts. Neuro Oncol. 2013;15:189–197. doi: 10.1093/neuonc/nos305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Costa PM, Cardoso AL, Mendonça LS, et al. Tumor-targeted chlorotoxin-coupled nanoparticles for nucleic acid delivery to glioblastoma cells: a promising system for glioblastoma treatment. Mol Ther Nucleic Acids. 2013;2:e100. doi: 10.1038/mtna.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yang Y, Yan Z, Wei D, et al. Tumor-penetrating peptide functionalization enhances the anti-glioblastoma effect of doxorubicin liposomes. Nanotechnology. 2013;24:405101. doi: 10.1088/0957-4484/24/40/405101. [DOI] [PubMed] [Google Scholar]
- 217.Huang Y, Zhou D, Hang T, et al. Preparation, characterization, and assessment of the antiglioma effects of liposomal celastrol. Anticancer Drugs. 2012;23:515–524. doi: 10.1097/CAD.0b013e3283514b68. [DOI] [PubMed] [Google Scholar]
- 218.Gong W, Wang Z, Liu N, et al. Improving efficiency of adriamycin crossing blood brain barrier by combination of thermosensitive liposomes and hyperthermia. Biol Pharm Bull. 2011;34:1058–1064. doi: 10.1248/bpb.34.1058. [DOI] [PubMed] [Google Scholar]
- 219.Mei DY, Gao HL, Gong WH, Pang ZQ, Jiang XG, Chen J. Anti-glioma effect of doxorubicin loaded liposomes modified with angiopep-2. Afr J Pharm Pharmacol. 2011;5:409–414. [Google Scholar]
- 220.Serwer LP, Noble CO, Michaud K, et al. Investigation of intravenous delivery of nanoliposomal topotecan for activity against orthotopic glioblastoma xenografts. Neuro Oncol. 2011;13:1288–1295. doi: 10.1093/neuonc/nor139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Xiang Y, Liang L, Wang X, Wang J, Zhang X, Zhang Q. Chloride channel-mediated brain glioma targeting of chlorotoxin-modified doxorubicine-loaded liposomes. J Control Release. 2011;152:402–410. doi: 10.1016/j.jconrel.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 222.Feng B, Tomizawa K, Michiue H, et al. Delivery of sodium borocaptate to glioma cells using immunoliposome conjugated with anti-EGFR antibodies by ZZ-His. Biomaterials. 2009;30:1746–1755. doi: 10.1016/j.biomaterials.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 223.Madhankumar AB, Slagle-Webb B, Wang X, et al. Efficacy of interleukin-13 receptor-targeted liposomal doxorubicin in the intracranial brain tumor model. Mol Cancer Ther. 2009;8:648–654. doi: 10.1158/1535-7163.MCT-08-0853. [DOI] [PubMed] [Google Scholar]
- 224.Doi A, Kawabata S, Iida K, et al. Tumor-specific targeting of sodium borocaptate (BSH) to malignant glioma by transferrin-PEG liposomes: a modality for boron neutron capture therapy. J Neurooncol. 2008;87:287–294. doi: 10.1007/s11060-008-9522-8. [DOI] [PubMed] [Google Scholar]
- 225.Abounader R, Lal B, Luddy C, et al. In vivo targeting of SF/HGF and c-met expression via U1snRNA/ribozymes inhibits glioma growth and angiogenesis and promotes apoptosis. FASEB J. 2002;16:108–110. doi: 10.1096/fj.01-0421fje. [DOI] [PubMed] [Google Scholar]
- 226.Lin Q, Mao KL, Tian FR, et al. Brain tumor-targeted delivery and therapy by focused ultrasound introduced doxorubicin-loaded cationic liposomes. Cancer Chemother Pharmacol. 2016;77:269–280. doi: 10.1007/s00280-015-2926-1. [DOI] [PubMed] [Google Scholar]
- 227.Mabuchi E, Shimizu K, Miyao Y, et al. Gene delivery by HVJ-liposome in the experimental gene therapy of murine glioma. Gene Ther. 1997;4:768–772. doi: 10.1038/sj.gt.3300478. [DOI] [PubMed] [Google Scholar]
- 228.Aryal M, Park J, Vykhodtseva N, Zhang YZ, McDannold N. Enhancement in blood-tumor barrier permeability and delivery of liposomal doxorubicin using focused ultrasound and microbubbles: evaluation during tumor progression in a rat glioma model. Phys Med Biol. 2015;60:2511–2527. doi: 10.1088/0031-9155/60/6/2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Huang FY, Lee TW, Chang CH, et al. Evaluation of 188Re-labeled PEGylated nanoliposome as a radionuclide therapeutic agent in an orthotopic glioma-bearing rat model. Int J Nanomedicine. 2015;10:463–473. doi: 10.2147/IJN.S75955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mendiburu-Eliçabe M, Gil-Ranedo J. Combination therapy of intraperitoneal rapamycin and convection-enhanced delivery of nanoliposomal CPT-11 in rodent orthotopic brain tumor xenografts. Curr Cancer Drug Targets. 2015;15:352–362. doi: 10.2174/1568009615666150225123120. [DOI] [PubMed] [Google Scholar]
- 231.Qiu LH, Zhang JW, Li SP, et al. Molecular imaging of angiogenesis to delineate the tumor margins in glioma rat model with endoglin-targeted paramagnetic liposomes using 3T MRI. J Magn Reson Imaging. 2015;41:1056–1064. doi: 10.1002/jmri.24628. [DOI] [PubMed] [Google Scholar]
- 232.Roller BT, Munson JM, Brahma B, Santangelo PJ, Pai SB, Bellamkonda RV. Evans blue nanocarriers visually demarcate margins of invasive gliomas. Drug Deliv Transl Res. 2015;5:116–124. doi: 10.1007/s13346-013-0139-x. [DOI] [PubMed] [Google Scholar]
- 233.Shein SA, Nukolova NV, Korchagina AA, et al. Site-directed delivery of VEGF-targeted liposomes into intracranial C6 glioma. Bull Exp Biol Med. 2015;158:371–376. doi: 10.1007/s10517-015-2765-4. [DOI] [PubMed] [Google Scholar]
- 234.Li XY, Zhao Y, Sun MG, et al. Multifunctional liposomes loaded with paclitaxel and artemether for treatment of invasive brain glioma. Biomaterials. 2014;35:5591–5604. doi: 10.1016/j.biomaterials.2014.03.049. [DOI] [PubMed] [Google Scholar]
- 235.Noble CO, Krauze MT, Drummond DC, et al. Pharmacokinetics, tumor accumulation and antitumor activity of nanoliposomal irinotecan following systemic treatment of intracranial tumors. Nanomedicine (Lond) 2014;9:2099–2108. doi: 10.2217/nnm.13.201. [DOI] [PubMed] [Google Scholar]
- 236.Yue PJ, He L, Qiu SW, et al. OX26/CTX-conjugated PEGylated liposome as a dual-targeting gene delivery system for brain glioma. Mol Cancer. 2014;13:191. doi: 10.1186/1476-4598-13-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Charest G, Sanche L, Fortin D, Mathieu D, Paquette B. Optimization of the route of platinum drugs administration to optimize the concomitant treatment with radiotherapy for glioblastoma implanted in the Fischer rat brain. J Neurooncol. 2013;115:365–373. doi: 10.1007/s11060-013-1238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gao JQ, Lv Q, Li LM, et al. Glioma targeting and blood-brain barrier penetration by dual-targeting doxorubincin [sic] liposomes. Biomaterials. 2013;34:5628–5639. doi: 10.1016/j.biomaterials.2013.03.097. [DOI] [PubMed] [Google Scholar]
- 239.Chekhonin VP, Baklaushev VP, Yusubalieva GM, et al. Targeted delivery of liposomal nanocontainers to the peritumoral zone of glioma by means of monoclonal antibodies against GFAP and the extracellular loop of Cx43. Nanomedicine. 2012;8:63–70. doi: 10.1016/j.nano.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 240.Huo T, Barth RF, Yang W, et al. Preparation, biodistribution and neurotoxicity of liposomal cisplatin following convection enhanced delivery in normal and F98 glioma bearing rats. PLoS One. 2012;7:e48752. doi: 10.1371/journal.pone.0048752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Munson JM, Fried L, Rowson SA, et al. Anti-invasive adjuvant therapy with imipramine blue enhances chemotherapeutic efficacy against glioma. Sci Transl Med. 2012;4:127ra36. doi: 10.1126/scitranslmed.3003016. [DOI] [PubMed] [Google Scholar]
- 242.Phillips WT, Goins B, Bao A, et al. Rhenium-186 liposomes as convection-enhanced nanoparticle brachytherapy for treatment of glioblastoma. Neuro Oncol. 2012;14:416–425. doi: 10.1093/neuonc/nos060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Treat LH, McDannold N, Zhang Y, Vykhodtseva N, Hynynen K. Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med Biol. 2012;38:1716–1725. doi: 10.1016/j.ultrasmedbio.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Chen H, Qin Y, Zhang Q, et al. Lactoferrin modified doxorubicin-loaded procationic liposomes for the treatment of gliomas. Eur J Pharm Sci. 2011;44:164–173. doi: 10.1016/j.ejps.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 245.Huang FY, Lee TW, Kao CH, et al. Imaging, autoradiography, and biodistribution of 188 re-labeled PEGylated nanoliposome in orthotopic glioma bearing rat model. Cancer Biother Radiopharm. 2011;26:717–725. doi: 10.1089/cbr.2011.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Miyata S, Kawabata S, Hiramatsu R, et al. Computed tomography imaging of transferrin targeting liposomes encapsulating both boron and iodine contrast agents by convection-enhanced delivery to F98 rat glioma for boron neutron capture therapy. Neurosurgery. 2011;68:1380–1387. doi: 10.1227/NEU.0b013e31820b52aa. [DOI] [PubMed] [Google Scholar]
- 247.Oku N, Yamashita M, Katayama Y, et al. PET imaging of brain cancer with positron emitter-labeled liposomes. Int J Pharm. 2011;403:170–177. doi: 10.1016/j.ijpharm.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 248.Qin Y, Chen H, Zhang Q, et al. Liposome formulated with TAT-modified cholesterol for improving brain delivery and therapeutic efficacy on brain glioma in animals. Int J Pharm. 2011;420:304–312. doi: 10.1016/j.ijpharm.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 249.Tian W, Ying X, Du J, et al. Enhanced efficacy of functionalized epirubicin liposomes in treating brain glioma-bearing rats. Eur J Pharm Sci. 2010;41:232–243. doi: 10.1016/j.ejps.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 250.Ying X, Wen H, Lu WL, et al. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J Control Release. 2010;141:183–192. doi: 10.1016/j.jconrel.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 251.Du J, Lu WL, Ying X, et al. Dual-targeting topotecan liposomes modified with tamoxifen and wheat germ agglutinin significantly improve drug transport across the blood-brain barrier and survival of brain tumor-bearing animals. Mol Pharm. 2009;6:905–917. doi: 10.1021/mp800218q. [DOI] [PubMed] [Google Scholar]
- 252.Grahn AY, Bankiewicz KS, Dugich-Djordjevic M, et al. Non-PEGylated liposomes for convection-enhanced delivery of topotecan and gadodiamide in malignant glioma: initial experience. J Neurooncol. 2009;95:185–197. doi: 10.1007/s11060-009-9917-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zhang D, Feng XY, Henning TD, et al. MR imaging of tumor angiogenesis using sterically stabilized Gd-DTPA liposomes targeted to CD105. Eur J Radiol. 2009;70:180–189. doi: 10.1016/j.ejrad.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 254.Karathanasis E, Park J, Agarwal A, et al. MRI mediated, non-invasive tracking of intratumoral distribution of nanocarriers in rat glioma. Nanotechnology. 2008;19:315101. doi: 10.1088/0957-4484/19/31/315101. [DOI] [PubMed] [Google Scholar]
- 255.Zhao S, Zhang X, Zhang J, et al. Intravenous administration of arsenic trioxide encapsulated in liposomes inhibits the growth of C6 gliomas in rat brains. J Chemother. 2008;20:253–262. doi: 10.1179/joc.2008.20.2.253. [DOI] [PubMed] [Google Scholar]
- 256.Huynh GH, Ozawa T, Deen DF, Tihan T, Szoka FC., Jr Retro-convection enhanced delivery to increase blood to brain transfer of macromolecules. Brain Res. 2007;1128:181–190. doi: 10.1016/j.brainres.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 257.Krauze MT, Noble CO, Kawaguchi T, et al. Convection-enhanced delivery of nanoliposomal CPT-11 (irinotecan) and PEGylated liposomal doxorubicin (Doxil) in rodent intracranial brain tumor xenografts. Neuro Oncol. 2007;9:393–403. doi: 10.1215/15228517-2007-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Yamashita Y, Krauze MT, Kawaguchi T, et al. Convection-enhanced delivery of a topoisomerase I inhibitor (nanoliposomal topotecan) and a topoisomerase II inhibitor (PEGylated liposomal doxorubicin) in intracranial brain tumor xenografts. Neuro Oncol. 2006;9:20–28. doi: 10.1215/15228517-2006-016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Noble CO, Krauze MT, Drummond DC, et al. Novel nanoliposomal CPT-11 infused by convection-enhanced delivery in intracranial tumors: pharmacology and efficacy. Cancer Res. 2006;66:2801–2806. doi: 10.1158/0008-5472.CAN-05-3535. [DOI] [PubMed] [Google Scholar]
- 260.Saito R. Convection-enhanced delivery of Ls-TPT enables an effective, continuous, low-dose chemotherapy against malignant glioma xenograft model. Neuro Oncol. 2006;8:205–214. doi: 10.1215/15228517-2006-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Mamot C, Nguyen JB, Pourdehnad M, et al. Extensive distribution of liposomes in rodent brains and brain tumors following convection-enhanced delivery. J Neurooncol. 2004;68:1–9. doi: 10.1023/b:neon.0000024743.56415.4b. [DOI] [PubMed] [Google Scholar]
- 262.Saito R, Bringas JR, McKnight TR, et al. Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res. 2004;64:2572–2579. doi: 10.1158/0008-5472.can-03-3631. [DOI] [PubMed] [Google Scholar]
- 263.Zhang Y, Boado RJ, Pardridge WM. In vivo knockdown of gene expression in brain cancer with intravenous RNAi in adult rats. J Gene Med. 2003;5:1039–1045. doi: 10.1002/jgm.449. [DOI] [PubMed] [Google Scholar]
- 264.Jiang F, Lilge L, Grenier J, Li Y, Wilson MD, Chopp M. Photodynamic therapy of U87 human glioma in nude rat using liposome-delivered photofrin. Lasers Surg Med. 1998;22:74–80. doi: 10.1002/(sici)1096-9101(1998)22:2<74::aid-lsm2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 265.Ho SY, Barbarese E, D’Arrigo JS, Smith-Slatas C, Simon RH. Evaluation of lipid-coated microbubbles as a delivery vehicle for Taxol in brain tumor therapy. Neurosurgery. 1997;40:1260–1268. doi: 10.1097/00006123-199706000-00028. [DOI] [PubMed] [Google Scholar]
- 266.Jiang F, Lilge L, Logie B, Li Y, Chopp M. Photodynamic therapy of 9L gliosarcoma with liposome-delivered photofrin. Photochem Photobiol. 1997;65:701–706. doi: 10.1111/j.1751-1097.1997.tb01913.x. [DOI] [PubMed] [Google Scholar]
- 267.Sharma US, Sharma A, Chau RI, Straubinger RM. Liposome-mediated therapy of intracranial brain tumors in a rat model. Pharm Res. 1997;14:992–998. doi: 10.1023/a:1012136925030. [DOI] [PubMed] [Google Scholar]
- 268.Kakinuma K, Tanaka R, Takahashi H, Watanabe M, Nakagawa T, Kuroki M. Targeting chemotherapy for malignant brain tumor using thermosensitive liposome and localized hyperthermia. J Neurosurg. 1996;84:180–184. doi: 10.3171/jns.1996.84.2.0180. [DOI] [PubMed] [Google Scholar]
- 269.Barbarese E, Ho SY, D’Arrigo JS, Simon RH. Internalization of microbubbles by tumor cells in vivo and in vitro. J Neurooncol. 1995;26:25–34. doi: 10.1007/BF01054766. [DOI] [PubMed] [Google Scholar]
- 270.Shibata S, Ochi A, Mori K. Liposomes as carriers of cisplatin into the central nervous system: experiments with 9L gliomas in rats. Neurol Med Chir (Tokyo) 1990;30:242–245. doi: 10.2176/nmc.30.242. [DOI] [PubMed] [Google Scholar]
- 271.Zhang H, Wen Y, Mao B, Gong Q, Qian Z, Wei Y. Plasmid encoding matrix protein of vesicular stomatitis viruses as an antitumor agent inhibiting rat glioma growth in situ. Exp Oncol. 2007;29:85–93. [PubMed] [Google Scholar]
- 272.Dickinson PJ, LeCouteur RA, Higgins RJ, et al. Canine spontaneous glioma: a translational model system for convection-enhanced delivery. Neuro Oncol. 2010;12:928–940. doi: 10.1093/neuonc/noq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Krauze MT, Mcknight TR, Yamashita Y, et al. Real-time visualization and characterization of liposomal delivery into the monkey brain by magnetic resonance imaging. Brain Res Brain Res Protoc. 2005;16:20–26. doi: 10.1016/j.brainresprot.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 274.Wakabayashi T, Natsume A, Hashizume Y, Fujii M, Mizuno M, Yoshida J. A phase I clinical trial of interferon-beta gene therapy for high-grade glioma: novel findings from gene expression profiling and autopsy. J Gene Med. 2008;10:329–339. doi: 10.1002/jgm.1160. [DOI] [PubMed] [Google Scholar]
- 275.Fiorillo A, Maggi G, Greco N, et al. Second-line chemotherapy with the association of liposomal daunorubicin, carboplatin and etoposide in children with recurrent malignant brain tumors. J Neurooncol. 2004;66:179–185. doi: 10.1023/b:neon.0000013471.53015.52. [DOI] [PubMed] [Google Scholar]
- 276.Yoshida J, Mizuno M, Fujii M, et al. Human gene therapy for malignant gliomas (glioblastoma multiforme and anaplastic astrocytoma) by in vivo transduction with human interferon beta gene using cationic liposomes. Hum Gene Ther. 2004;15:77–86. doi: 10.1089/10430340460732472. [DOI] [PubMed] [Google Scholar]
- 277.Ren H, Boulikas T, Lundstrom K, Söling A, Warnke PC, Rainov NG. Immunogene therapy of recurrent glioblastoma multiforme with a liposomally encapsulated replication-incompetent Semliki forest virus vector carrying the human interleukin-12 gene: a phase I/II clinical protocol. J Neurooncol. 2003;64:147–154. doi: 10.1007/BF02700029. [DOI] [PubMed] [Google Scholar]
- 278.Albrecht KW, de Witt Hamer PC, Leenstra S, et al. High concentration of daunorubicin and daunorubicinol in human malignant astrocytomas after systemic administration of liposomal daunorubicin. J Neurooncol. 2001;53:267–271. doi: 10.1023/a:1012287212388. [DOI] [PubMed] [Google Scholar]
- 279.Fabel K, Dietrich J, Hau P, et al. Long-term stabilization in patients with malignant glioma after treatment with liposomal doxorubicin. Cancer. 2001;92:1936–1942. doi: 10.1002/1097-0142(20011001)92:7<1936::aid-cncr1712>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 280.Koukourakis MI, Koukouraki S, Fezoulidis I, et al. High intratumoural accumulation of stealth liposomal doxorubicin (Caelyx) in glioblastomas and in metastatic brain tumours. Br J Cancer. 2000;83:1281–1286. doi: 10.1054/bjoc.2000.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Zucchetti M, Boiardi A, Silvani A, Parisi I, Piccolrovazzi S, D’Incalci M. Distribution of daunorubicin and daunorubicinol in human glioma tumors after administration of liposomal daunorubicin. Cancer Chemother Pharmacol. 1999;44:173–176. doi: 10.1007/s002800050964. [DOI] [PubMed] [Google Scholar]
- 282.Firth GB, Firth M, McKeran RO, et al. Application of radioimmunoassay to monitor treatment of human cerebral gliomas with bleomycin entrapped within liposomes. J Clin Pathol. 1988;41:38–43. doi: 10.1136/jcp.41.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.McKeran RO, Firth G, Oliver S, Uttley D, O’Laoire S. A potential application for the intracerebral injection of drugs entrapped within liposomes in the treatment of human cerebral gliomas. J Neurol Neurosurg Psychiatry. 1985;48:1213–1219. doi: 10.1136/jnnp.48.12.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Chapman S, Dobrovolskaia M, Farahani K, et al. Nanoparticles for cancer imaging: the good, the bad, and the promise. Nano Today. 2013;8:454–460. doi: 10.1016/j.nantod.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Keunen O, Taxt T, Grüner R, et al. Multimodal imaging of gliomas in the context of evolving cellular and molecular therapies. Adv Drug Deliv Rev. 2014;76:98–115. doi: 10.1016/j.addr.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 286.Hernández-Pedro NY, Rangel-López E, Magaña-Maldonado R, et al. Application of nanoparticles on diagnosis and therapy in gliomas. Biomed Res Int. 2013;2013:351031. doi: 10.1155/2013/351031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Blasiak B, van Veggel FC, Tomanek B. Applications of nanoparticles for MRI cancer diagnosis and therapy. J Nanomater. 2013;2013:148578. [Google Scholar]
- 288.Haglund MM, Berger MS, Hochman DW. Enhanced optical imaging of human gliomas and tumor margins. Neurosurgery. 1996;38:308–317. doi: 10.1097/00006123-199602000-00015. [DOI] [PubMed] [Google Scholar]
- 289.Minn H. PET and SPECT in low-grade glioma. Eur J Radiol. 2005;56:171–178. doi: 10.1016/j.ejrad.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 290.la Fougère C, Suchorska B, Bartenstein P, Kreth FW, Tonn JC. Molecular imaging of gliomas with PET: opportunities and limitations. Neuro Oncol. 2011;13:806–819. doi: 10.1093/neuonc/nor054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Anderson JC, McFarland BC, Gladson CL. New molecular targets in angiogenic vessels of glioblastoma tumours. Expert Rev Mol Med. 2008;10:e23. doi: 10.1017/S1462399408000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9:193–199. doi: 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Werner-Wasik M, Scott CB, Nelson DF, et al. Final report of a phase I/II trial of hyperfractionated and accelerated hyperfractionated radiation therapy with carmustine for adults with supratentorial malignant gliomas. Cancer. 1996;77:1535–1543. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1535::AID-CNCR17>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 294.Schwarz SB, Thon N, Nikolajek K, et al. Iodine-125 brachytherapy for brain tumours: a review. Radiat Oncol. 2012;7:30. doi: 10.1186/1748-717X-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Thorn CF, Oshiro C, Marsh S, et al. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics. 2011;21:440–446. doi: 10.1097/FPC.0b013e32833ffb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Yaqub F. Mechanism of action of anthracycline drugs. Lancet Oncol. 2013;14:e296. [Google Scholar]
- 297.Della Latta V, Cecchettini A, Del Ry S, Morales MA. Bleomycin in the setting of lung fibrosis induction: from biological mechanisms to counteractions. Pharmacol Res. 2015;97:122–130. doi: 10.1016/j.phrs.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 298.von Holst H, Knochenhauer E, Blomgren H, et al. Uptake of adriamycin in tumour and surrounding brain tissue in patients with malignant gliomas. Acta Neurochir (Wien) 1990;104:13–16. doi: 10.1007/BF01842886. [DOI] [PubMed] [Google Scholar]
- 299.Hervé F, Ghinea N, Scherrmann JM. CNS delivery via adsorptive transcytosis. AAPS J. 2008;10:455–472. doi: 10.1208/s12248-008-9055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Zhan C, Lu W. The blood-brain/tumor barriers: challenges and chances for malignant gliomas targeted drug delivery. Curr Pharm Biotechnol. 2012;13:2380–2387. doi: 10.2174/138920112803341798. [DOI] [PubMed] [Google Scholar]
- 301.Koo YE, Reddy GR, Bhojani M, et al. Brain cancer diagnosis and therapy with nanoplatforms. Adv Drug Deliv Rev. 2006;58:1556–1577. doi: 10.1016/j.addr.2006.09.012. [DOI] [PubMed] [Google Scholar]