Abstract
Objectives
In participants with peripheral arterial disease (PAD), we determined whether more sedentary behavior and slower outdoor walking speed were associated with faster functional decline and more adverse changes in calf muscle characteristics over time.
Background
Modifiable behaviors associated with faster functional decline in lower-extremity PAD are understudied.
Methods
Participants were 384 men and women with an ankle brachial index <0.90 followed for a median of 47 months. At baseline, participants reported the number of hours they spent sitting per day and their walking speeds outside their homes. Participants underwent baseline and annual measures of objective functional performance. Calf muscle characteristics were measured with computed tomography at baseline and every 2 years subsequently. Analyses were adjusted for age, sex, race, comorbidities, ankle brachial index, and other confounders.
Results
Slower walking speed outside the home was associated with faster annual decline in calf muscle density (brisk/ striding pace −0.32 g/cm3, average pace −0.46 g/cm3, casual strolling −1.03 g/cm3, no walking at all −1.43 g/cm3, p trend <0.001). Greater hours sitting per day were associated with faster decline in 6-min walk (<4 h: −35.8 feet/year; 4 to <7 h: −41.1 feet/year; 8 to <11 h: −68.7 feet; ≥12 h: −78.0 feet; p trend = 0.008). Similar associations were observed for greater hours sitting per day and faster declines in fast-paced (p trend = 0.018) and usual-paced (p trend < 0.001) 4-m walking velocity.
Conclusions
Greater sedentary hours per day and slower outdoor walking speed are modifiable behaviors that are associated with faster functional decline and greater decline in calf muscle density, respectively, in patients with PAD.
Keywords: intermittent claudication, modifiable behaviors, peripheral arterial disease, physical activity, physical functioning
Men and women with lower-extremity peripheral arterial disease (PAD) have greater functional impairment and faster functional decline compared with those without PAD (1–3). Few therapies have been identified that improve functional performance in men and women with PAD. Although supervised treadmill exercise improves treadmill walking endurance in patients with PAD (4), medical insurance does not typically cover supervised treadmill exercise and few patients with PAD participate (5).
Few data are available regarding associations of modifiable lifestyle behaviors with functional decline and other important outcomes in people with PAD. Therefore, in a prospective, observational study, we assessed associations of the number of sedentary hours per day with rates of functional decline and changes in calf muscle characteristics among men and women with PAD. We also studied associations of outdoor walking speed with functional decline and changes in calf muscle characteristics among participants with PAD. We hypothesized that more sedentary hours per day and slower walking speed outside the home would be associated with faster functional decline and more adverse calf muscle changes over time among men and women with PAD.
Methods
Study overview
The institutional review boards of Northwestern University and Catholic Health Partners Hospital approved the protocol. Participants gave written informed consent. Participants were enrolled in the WALCS (Walking and Leg Circulation Study) II cohort, a prospective, observational study designed to identify mechanisms of functional decline in PAD (6,7). Participants underwent baseline measures and returned annually for up to 4 follow-up visits.
Participant identification
Recruitment, participation rates, and exclusion criteria for the WALCS II cohort have been described (6,7) and are summarized briefly here. Participants were age 59 years and older and were identified from among consecutive patients diagnosed with PAD in Chicago-area noninvasive vascular laboratories (5,6). A small number of participants with PAD were identified from among consecutive patients in a large general internal medicine practice at Northwestern University. PAD was defined as ankle brachial index (ABI) <0.90 (6,7). Patients with dementia were excluded because of their inability to answer questions accurately. Nursing home residents, wheelchair-bound patients, and patients with foot or leg amputations were excluded because they had severely impaired functioning at baseline. Non–English-speaking patients were excluded because investigators were not fluent in non-English languages. Patients with recent major surgery were excluded.
ABI measurement
A handheld Doppler probe (Nicolet Vascular Pocket Dop II, Nicolet Biomedical Inc, Golden, Colorado) was used to measure systolic pressures in the right and left brachial, dorsalis pedis, and posterior tibial arteries (6–8). Each pressure was measured twice. For each leg, the ABI was calculated by dividing the mean of the dorsalis pedis and posterior tibial pressures by the mean of the 4 brachial pressures (8). Zero values for the dorsalis pedis and posterior tibial pulses were set to missing for the ABI calculation. Average brachial pressures in the arm with the highest pressure were used when 1 brachial pressure was higher than the opposite brachial pressure in both measurement sets and the 2 brachial pressures differed by 10 mm Hg or more in at least 1 measurement set. In these cases, subclavian stenosis was possible (9). The leg with the lowest ABI was used in analyses.
Measures of sedentary hours per day and participant-reported walking speed outside the home
At baseline, participants were asked, “In a typical day, how many hours do you spend sitting? Be sure to include time spent sitting at a desk, riding in a car, eating, and sitting up watching television” (10,11). Participants were also asked, “In a typical day, how many hours do you spend lying down? Include time spent sleeping, lying down resting, napping, and trying to get to sleep” (10). Based on responses to these questions, we calculated: 1) the number of hours spent sitting per day; and 2) the total number of sedentary hours per day (including hours spent sitting and lying down). At baseline, participants were asked to describe their typical walking speed when walking outside their house. Specifically, participants were asked, “When you walk outside your house, what is your usual walking speed?” (10). Participants selected from one of the following responses: 1) no walking at all; 2) casual strolling (0 to 2 miles/h); 3) average or normal (2 to 3 miles/h); and 4) brisk or striding (>3 miles/h).
Functional outcomes
Functional outcomes were assessed at baseline and annually and consisted of average annual declines in 6-min walk performance, usual-paced 4-m walking velocity, and fastest-paced 4-m walking velocity.
6-MIN WALK
The 6-min walk test is a validated and reliable measure of walking performance that predicts mortality, mobility loss, and correlates significantly with physical activity levels during daily life (12–15). Following a standardized protocol (12–15), participants walk up and down a 100-foot hallway for 6 min after instructions to cover as much distance as possible.
4-M WALKING VELOCITY
Walking velocity was measured with a 4-m walk performed at “usual” and “fastest” paces, based on a previous study (14–16). Each walk was performed twice. The faster walk in each pair was used in analyses (14–16).
Calf muscle characteristics
Calf muscle measurements were assessed with computed tomography (CT) at baseline and at the 2- and 4-year follow-up visits using a CT scanner (LightSpeed, General Electric Medical Systems, Waukesha, Wisconsin) (6,7). Cross-sectional (2.5 mm) images of the calves were obtained at 66.7% of the distance from the distal to the proximal tibia (6,7). Cross-sectional images were analyzed using BonAlyse (BonAlyse Oy, Jyvaskyla, Finland), a software for processing CT images that identifies muscle tissue, fat, and bone (6,7,17). The muscle outline was traced manually, excluding subcutaneous fat and bone. When quantifying muscle area, the BonAlyse software quantifies voxels within a range corresponding to muscle density (9 to 271 mg/cm3) and excludes voxels corresponding to fat density (−270 to 8 mg/cm3) (6,7). Intramuscular fat is quantified by summing voxels corresponding to fat within muscle tissue. Muscle density measures the quantity of muscle per volume within the voxel range corresponding to muscle (9 to 271 mg/cm3) and is a measure of muscle quality (6,7). Cadaver studies demonstrate that these methods provide an estimate of muscle cross-sectional area that is highly correlated with direct anatomic measures (18). Because larger individuals require greater muscle mass to support their frames, muscle area was adjusted for the square of individual tibia length (6,7).
Comorbidities
Comorbidities assessed at baseline were diabetes, angina, myocardial infarction, heart failure, cancer, chronic lung disease, lower-extremity arthritis, spinal stenosis, spinal disc disease, and stroke (19). Disease-specific algorithms that combine data from patient report, medical record review, medications, laboratory values, and a questionnaire completed by the participant’s primary care physician were used to verify and document baseline comorbidities other than knee and hip arthritis, based on criteria previously developed (20). American College of Rheumatology criteria were used to document presence of knee and hip osteoarthritis (21,22).
Physical activity measurement using the Caltrac vertical accelerometer
We used a vertical accelerometer (Caltrac, Muscle Dynamics Fitness Network, Inc., Torrence, California) to measure physical activity continuously over 7 days using previously described methods (23,24). Vertical accelerometers do not measure activities performed standing or sitting that do not involve vertical motion. Test-retest reliability of the Caltrac vertical accelerometer is excellent among participants with PAD (25). We programmed the accelerometer identically for all participants, allowing us to compare physical activity levels among participants, irrespective of individual variation in age, weight, height, and sex (23,24). Programmed in this way, the accelerometers measured “activity units.”
Other measures
Height and weight were measured at baseline. Body mass index (BMI) was calculated as: weight (kg)/(height [m])2. Cigarette smoking history was determined with patient report. At each follow-up visit, we used patient report, a primary care physician questionnaire, and medical record review to identify lower-extremity revascularizations since the previous visit.
Statistical analyses
Before data analyses, categories of the number of hours spent sitting per day were defined as follows, based on the distribution of baseline data: 1) <4 h/day; 2) 4 to <7 h/day; 3) 8 to <11 h/day; and 4) ≥12 h/day. Before data analyses, categories of the total number of sedentary hours per day (including hours spent either sitting or lying down) were defined as follows: 1) <10 h/day; 2) 10 to <14 h/day; 3) 14 to <18 h/day; and 4) ≥18 h/day. Analysis of covariance (ANCOVA) was used to compare continuous baseline clinical characteristics across categories of number of sedentary hours per day and across categories of participant-reported walking intensity, with adjustments for age. Logistic regression was used to compare categorical baseline characteristics across categories of sedentary hours, with adjustments for age. Spearman correlation coefficients were calculated between the Caltrac vertical accelerometer value and measures of total hours spent sitting per day, total hours spent sedentary per day, and walking speed outside the home.
Changes in functional performance (6-min walk distance and 4-m walking velocity) and calf muscle characteristics (calf muscle density, calf muscle area, and calf muscle percent fat) were compared across categories of 1) hours spent sitting per day; 2) total sedentary hours (both sitting and lying down) per day; and 3) participant-reported walking speed outside the home using a longitudinal or repeated-measure ANCOVA and a mixed-model approach. PROC MIXED with the command “repeat” was used to perform these analyses. Participants who underwent lower-extremity revascularization were excluded from analyses after the date of their lower-extremity revascularization. The within-subject correlations were modeled with a subject-specific random effect. The primary dependent variables for each analysis were the successive annual differences in each outcome of interest (i.e., the difference in 6-min walk distance between baseline and the first follow-up visit, between the first and second follow-up visits, between the second and third follow-up visits, and so on). Differences in decline for each outcome were compared using ANCOVA, adjusting for baseline age, sex, race, BMI, smoking, ABI, physical activity, comorbidities, leg symptoms, and the corresponding prior year performance or muscle characteristic. A statistical test for trend was performed to determine the degree to which the associations of each independent variable of interest with each dependent variable were linear. Pairwise statistical comparisons were made between a reference category (least sedentary hours and fastest outdoor walking speed, respectively) and each remaining category of sedentary hours and outdoor walking speed. Analyses of changes in muscle area included adjustment for tibia length. Analyses were performed using SAS statistical software (version 9.2, SAS Institute Inc., Cary, North Carolina).
Results
Among 463 WALCS II participants with PAD, 384 were eligible for the current analyses. Among the 79 participants with PAD not included, 2 participants did not complete baseline questions regarding their walking speed outside the home or number of hours spent sitting and lying down per day. Ten participants had missing data relevant to our fully adjusted models, and 67 participants were not eligible for analyses because they did not return for at least 1 follow-up visit or they underwent lower-extremity revascularization before their first follow-up visit. Median follow-up was 47 months (interquartile range: 25 to 50 months).
Spearman correlation coefficients of vertical accelerometer–measured physical activity with sitting hours per day and total sedentary hours per day were −0.077 (p = 0.239) and −0.155 (p = 0.017), respectively. The Spearman correlation coefficient of vertical accelerometer–measured physical activity and participant-reported walking speed outside the home was 0.281 (p < 0.001). The Spearman correlation coefficient between the number of hours sitting per day and total sedentary hours per day was 0.892 (p < 0.001). The Spearman correlation coefficients between participant-reported walking speed outside the home and total hours sitting per day as well as total hours spent lying down per day were −0.106 (p = 0.039) and −0.161 (p = 0.002), respectively.
Average ages across categories of total hours spent sitting per day were <4 h: 73.0 ± 7.8 years; 4 to 7 h: 75.5 ± 7.9 years; 8 to 11 h: 74.0 ± 8.6 years; and >12 h: 74.5 ± 6.8 years (p trend = 0.832). Average ages across categories of total hours spent sedentary per day (combining time spent sitting and lying down) were <10 h: 71.9 ± 8.5 years; 10 to 14 h: 75.6 ± 7.8 years; 14 to <18 h: 73.3 ± 8.5 years; and ≥ 18 h: 75.2 ± 7.6 years (p trend = 0.518). Table 1 shows age-adjusted baseline characteristics of study participants according to the number of hours spent sitting per day and the total sedentary hours (sitting and lying down) per day. Greater hours sitting per day and greater total sedentary hours per day were associated with higher prevalence of pulmonary disease and angina. Greater hours sitting per day were associated with higher BMI. Greater hours sitting and greater total sedentary hours per day were each associated with poorer 6-min walk performance at baseline (Table 1).
Table 1.
Age-Adjusted Associations of Baseline Characteristics With the Number of Hours Spent Seated and Total Sedentary Hours per Day Among Participants With Peripheral Arterial Disease
| Total Hours Sitting per Day | <4 h | 4–7 h | 8–11 h | ≥12 h | p Trend |
|---|---|---|---|---|---|
| No. of patients | 60 | 163 | 111 | 50 | |
| Body mass index, kg/m2 | 27.0 ± 0.65 | 28.0 ± 0.39 | 28.3 ± 0.48 | 29.8 ± 0.71 | 0.006 |
| Ankle brachial index | 0.66 ± 0.02 | 0.61 ± 0.01 | 0.64 ± 0.01 | 0.64 ± 0.02 | 0.833 |
| Male, % | 54.0 | 47.9 | 62.9 | 58.1 | 0.136 |
| African American, % | 17.2 | 20.4 | 12.0 | 15.7 | 0.310 |
| Current smoker, % | 17.4 | 13.8 | 13.6 | 15.2 | 0.728 |
| Diabetes, % | 26.2 | 35.5 | 26.6 | 45.9 | 0.226 |
| Pulmonary disease, % | 24.3 | 46.5 | 42.9 | 50.0 | 0.030 |
| Cancer, % | 21.9 | 20.7 | 14.5 | 22.0 | 0.552 |
| Hip arthritis, % | 0.00 | 5.35 | 3.59 | 7.94 | 0.146 |
| Knee arthritis, % | 10.2 | 14.6 | 10.7 | 13.8 | 0.965 |
| Disc disease, % | 39.8 | 43.7 | 31.5 | 50.0 | 0.993 |
| Spinal stenosis, % | 17.1 | 12.1 | 10.6 | 25.7 | 0.339 |
| Angina, % | 33.1 | 29.6 | 31.5 | 58.0 | 0.012 |
| Myocardial infarction, % | 18.6 | 28.0 | 26.2 | 38.0 | 0.063 |
| Stroke, % | 21.1 | 19.8 | 14.2 | 36.0 | 0.262 |
| Heart failure, % | 28.5 | 27.5 | 26.2 | 38.0 | 0.401 |
| Calf muscle area, mm2 | 5,526 ± 181 | 5,439 ± 108 | 5,606 ± 132 | 5,597 ± 201 | 0.497 |
| Calf muscle fat, % | 10.2 ± 1.71 | 11.5 ± 1.03 | 10.9 ± 1.26 | 12.1 ± 1.90 | 0.629 |
| Calf muscle density, g/cm3 | 32.4 ± 0.54 | 32.8 ± 0.32 | 33.1 ± 0.39 | 31.9 ± 0.60 | 0.819 |
| 6-min walk distance, feet | 1,196 ± 50.1 | 1,164 ± 30.0 | 1,132 ± 37.0 | 912 ± 53.9 | <0.001 |
| 4-m usual-paced walking velocity, m/s | 0.88 ± 0.03 | 0.87 ± 0.02 | 0.89 ± 0.02 | 0.77 ± 0.03 | 0.054 |
| 4-m fastest-paced walking velocity, m/s | 1.16 ± 0.04 | 1.17 ± 0.02 | 1.18 ± 0.03 | 1.09 ± 0.04 | 0.314 |
|
| |||||
| Total Sedentary Hours per Day (Sitting and Lying Down) | <10 h | 10 to <14 h | 14 to <18 h | ≥18 h | p Trend |
|
| |||||
| No. of patients | 37 | 148 | 103 | 96 | |
| Body mass index, kg/m2 | 27.4 ± 0.83 | 28.0 ± 0.42 | 28.4 ± 0.50 | 28.5 ± 0.52 | 0.252 |
| Ankle brachial index | 0.66 ± 0.03 | 0.62 ± 0.01 | 0.62 ± 0.02 | 0.65 ± 0.02 | 0.531 |
| Male, % | 49.5 | 47.4 | 64.4 | 56.8 | 0.079 |
| African American, % | 22.3 | 19.9 | 11.8 | 15.6 | 0.163 |
| Current smoker, % | 23.0 | 11.4 | 15.3 | 15.3 | 0.843 |
| Diabetes, % | 18.3 | 38.1 | 23.9 | 40.0 | 0.252 |
| Pulmonary disease, % | 36.6 | 39.6 | 36.3 | 55.6 | 0.025 |
| Cancer, % | 19.4 | 17.3 | 25.5 | 15.5 | 0.909 |
| Hip arthritis, % | 2.82 | 4.56 | 2.95 | 6.09 | 0.519 |
| Knee arthritis, % | 11.4 | 15.4 | 10.8 | 11.0 | 0.429 |
| Disc disease, % | 56.7 | 37.2 | 38.8 | 40.6 | 0.442 |
| Angina, % | 26.6 | 30.6 | 32.8 | 44.9 | 0.016 |
| Myocardial infarction, % | 19.3 | 26.1 | 23.5 | 36.3 | 0.052 |
| Stroke, % | 20.7 | 17.8 | 16.1 | 29.3 | 0.108 |
| Heart failure, % | 35.7 | 24.1 | 29.3 | 32.1 | 0.557 |
| Calf muscle area, mm2 | 5,459 ± 231 | 5,492 ± 114 | 5,616 ± 137 | 5,485 ± 144 | 0.831 |
| Calf muscle fat, % | 10.3 ± 2.19 | 12.4 ± 1.08 | 10.7 ± 1.30 | 10.3 ± 1.36 | 0.415 |
| Baseline muscle density, g/cm3 | 31.7 ± 0.69 | 32.8 ± 0.34 | 32.8 ± 0.41 | 32.9 ± 0.43 | 0.317 |
| Baseline 6-min walk, feet | 1,167 ± 64.4 | 1,152 ± 31.5 | 1,197 ± 38.5 | 992 ± 40.0 | 0.008 |
| Baseline 4-m usual pace, m/s | 0.85 ± 0.03 | 0.88 ± 0.02 | 0.89 ± 0.02 | 0.82 ± 0.02 | 0.197 |
| Baseline 4-m fast pace, m/s | 1.16 ± 0.05 | 1.17 ± 0.02 | 1.20 ± 0.03 | 1.11 ± 0.03 | 0.294 |
Data are presented as n, mean ± SD, or %. N = 384.
Average ages across categories of walking speed outside the home were no walking at all: 76.0 ± 8.1 years; casual strolling: 75.6 ± 7.6 years; average or normal: 73.5 ± 8.2 years; and brisk or striding: 70.4 ± 7.9 years (p trend <0.001). After adjustment for age, slower walking speed outside the home was associated with higher BMI and lower ABI values and higher prevalence of disc disease, spinal stenosis, hip arthritis, angina, myocardial infarction, and heart failure (Table 2). Slower walking speed outside the home was associated with more adverse baseline calf muscle characteristics and poorer performance on each measure of baseline walking performance (Table 2).
Table 2.
Age-Adjusted Associations of Baseline Characteristics With Reported Walking Speed Outside the Home Among Participants With Peripheral Arterial Disease
| No Walking Outside the Home (n =16) | Casual Strolling (0–2 miles/h) (n =197) | Average or Normal (2–3 miles/h) (n =142) | Briskly or Striding (>3 miles/h) (n =26) | p Trend | |
|---|---|---|---|---|---|
| Body mass index, kg/m2 | 29.3 ± 1.26 | 28.6 ± 0.36 | 27.5 ± 0.42 | 27.4 ± 1.00 | 0.032 |
| Ankle brachial index | 0.55 ± 0.04 | 0.62 ± 0.01 | 0.64 ± 0.01 | 0.73 ± 0.03 | 0.001 |
| Male, % | 44.6 | 52.5 | 54.4 | 75.2 | 0.076 |
| African American, % | 44.9 | 15.4 | 17.4 | 9.85 | 0.131 |
| Current smoker, % | 19.0 | 15.0 | 15.3 | 5.25 | 0.267 |
| Diabetes, % | 32.3 | 35.3 | 31.3 | 15.4 | 0.094 |
| Pulmonary disease, % | 44.4 | 47.2 | 34.7 | 44.2 | 0.141 |
| Cancer, % | 18.4 | 17.5 | 20.6 | 27.9 | 0.255 |
| Hip arthritis, % | 12.1 | 6.93 | 0.0 | 0.0 | 0.001 |
| Knee arthritis, % | 11.6 | 14.4 | 10.6 | 8.56 | 0.317 |
| Disc disease, % | 62.9 | 42.9 | 37.1 | 26.1 | 0.018 |
| Spinal stenosis, % | 42.7 | 16.2 | 9.21 | 8.36 | 0.002 |
| Angina, % | 37.9 | 40.4 | 24.4 | 37.4 | 0.051 |
| Myocardial infarction, % | 31.1 | 32.3 | 21.9 | 11.8 | 0.010 |
| Stroke, % | 12.7 | 23.6 | 19.3 | 7.04 | 0.169 |
| Heart failure, % | 25.0 | 34.5 | 22.5 | 15.4 | 0.019 |
| Baseline muscle area, mm2 | 4,700 ± 333 | 5,341 ± 97.6 | 5,710 ± 114 | 6,197 ± 268 | <0.001 |
| Baseline fat percentage, % | 19.4 ± 3.18 | 12.6 ± 0.93 | 8.88 ± 1.09 | 8.73 ± 2.56 | 0.001 |
| Baseline muscle density gm/cm3 | 31.1 ± 0.99 | 32.1 ± 0.29 | 33.4 ± 0.34 | 34.6 ± 0.80 | <0.001 |
| Baseline 6-min walk, feet | 680 ± 96 | 990 ± 24 | 1,281 ± 28 | 1,579 ± 67.0 | <0.001 |
| Baseline 4-m usual pace, m/s | 0.65 ± 0.05 | 0.81 ± 0.01 | 0.93 ± 0.02 | 1.04 ± 0.04 | <0.001 |
| Baseline 4-m fast pace, m/s | 0.89 ± 0.07 | 1.09 ± 0.02 | 1.25 ± 0.02 | 1.38 ± 0.05 | <0.001 |
Data are presented as mean ± SD or %. N = 381.
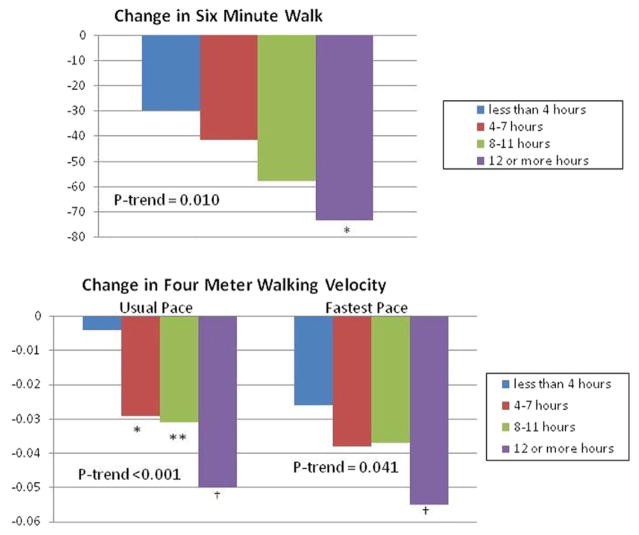
Figure 1 shows associations of the number of hours sitting per day with average annual decline in functional performance. Greater hours sitting per day at baseline was associated with greater average annual decline in 6-min walk performance (p trend = 0.008), usual-paced 4-m walking velocity (p trend <0.001), and fast-paced 4-m walking velocity (p trend = 0.018), with adjustments for age, sex, race, BMI, comorbidities, smoking, study cohort, leg symptoms, ABI, and prior year performance. Figure 2 shows associations of the total sedentary hours per day (both sitting and lying down) with average annual decline in functional performance. Greater total sedentary hours/day at baseline were associated with greater average annual decline in 6-min walk performance (p trend = 0.010), usual-paced 4-m walking velocity (p trend <0.001), and fast-paced 4-m walking velocity (p trend = 0.041), with adjustments for age, sex, race, BMI, comorbid diseases, smoking, study cohort, leg symptoms, ABI, and prior year performance. There were no significant associations of walking speed outside the home with average annual decline in 6-min walk performance, usual-paced 4-m walking velocity, or fastest-paced 4-m walking velocity (data not shown).
Figure 1. Adjusted Associations of Hours Spent Sitting per Day With Decline in Functional Performance Among Participants With Peripheral Arterial Disease.
Analyses adjusted for age, sex, race, body mass index, comorbidities, ankle brachial index, smoking, study cohort, leg symptoms, and prior year performance (N = 384). The reference group for pairwise comparisons was less than 4 h of sitting per day. Top: *Pairwise p = 0.052. **Pairwise p = 0.054. Bottom: *Pairwise p = 0.02. †Pairwise p = 0.054. **Pairwise p = 0.019.
Figure 2. Adjusted Associations of Total Sedentary Hours per Day With Functional Decline Among Participants With Peripheral Arterial Disease.
Analyses adjusted for age, sex, race, body mass index, comorbidities, ankle brachial index, smoking, study cohort, leg symptoms, and prior year performance (N = 384). The reference group for pairwise comparisons was <4 sedentary h/day. Top: *Pairwise p = 0.034. Bottom: *Pairwise p = 0.034. †Pairwise p < 0.001. ‡Pairwise p = 0.037. **Pairwise p = 0.008.
We observed no significant associations of hours spent sitting per day or total sedentary hours per day with average annual change in calf muscle characteristics (Table 3). However, slower walking speed outside the home was associated with greater average annual decline in calf muscle density (p trend <0.001), with adjustments for age, sex, race, comorbidities, BMI, smoking, study cohort, leg symptoms, prior year calf muscle density, and ABI. Participants with PAD who walked at a casual strolling pace outside the home had significantly greater decline in calf muscle density, compared with participants with a brisk or striding pace (p = 0.025) (Table 3). In fully adjusted analyses, the association of slower walking speed outside the home with greater calf muscle density decline remained statistically significant even after participants who reported no outdoor walking (casual strolling: −1.05 g/cm3; average or normal: −0.46 g/cm3; brisk or striding: −0.331 g/cm3; p = 0.001) were excluded from the analyses. In these latter analyses, participants with PAD who reported a walking speed of “casual strolling” outside the home had significantly greater decline in calf muscle density than those who reported a walking speed of brisk or striding (p = 0.024).
Table 3.
Adjusted Associations of Total Sitting Hours per Day, Total Sedentary Hours per Day, and Walking Speed Outside the Home With Change in Calf Muscle Characteristics Among Participants With Peripheral Arterial Disease
| Average Annual Changes in Calf Muscle Characteristics | No Walking Outside the Home (n =12) | Casual Strolling (0–2 miles/h) (n =148) | Average or Normal (2–3 miles/h) (n =114) | Briskly or Striding (>3 miles/h) (n =23) | p Trend |
|---|---|---|---|---|---|
| Walking speed outside the home | |||||
| Calf muscle area, mm2/yr | −381 | − 291 | −286 | −334 | 0.965 |
| Calf muscle percent fat, %/yr | +1.25 | +1.39 | +1.33 | +1.33 | 0.932 |
| Calf muscle density, g/cm3/yr | −1.43* | −1.03† | −0.46 | −0.32 | 0.0007 |
|
| |||||
| Hours spent sitting per day | |||||
| Average annual declines | <4 h (n = 49) | 4–7 h (n = 128) | 8–11 h (n = 82) | ≥12 h (n = 40) | |
| Calf muscle area, mm2/yr | −285 | − 309 | −277 | −314 | 0.983 |
| Calf muscle percent fat, %/yr | +0.74 | +1.44 | +1.57 | +1.47 | 0.220 |
| Calf muscle density, g/cm3/yr | −0.92 | −0.57 | −0.74 | −1.06 | 0.554 |
|
| |||||
| Total sedentary hours (sitting and lying down) per day | |||||
| Average annual declines | <10 h (n = 31) | 10 to < 14 h (n = 112) | 14 to <18 h (n = 81) | ≥18 h (n = 75) | |
| Calf muscle area, mm2/yr | −284 | − 323 | −296 | −262 | 0.437 |
| Calf muscle percent fat, %/yr | +0.99 | +1.30 | +1.73 | +1.22 | 0.641 |
| Calf muscle density, g/cm3/yr | −0.69 | −0.61 | −0.73 | −0.95 | 0.196 |
Analyses adjusted for age, sex, race, tibia length (muscle area only), prior year muscle measurement, body mass index, smoking, ankle brachial index, study cohort, leg symptoms, and comorbidities. N = 437.
p = 0.060 for the pairwise comparison between no walking outside the home and a walking speed of brisk or striding.
p = 0.025 for the pairwise comparison between casual strolling and a walking speed of brisk or striding.
Discussion
Among 384 participants with PAD, greater hours spent sitting per day and greater total sedentary hours per day (both sitting and lying down) were associated with greater average annual declines in 6-min walk performance, usual-paced 4-m walking velocity, and fastest-paced walking velocity. These associations were independent of potential confounders including age, sex, race, comorbidities, BMI, and ABI.
Patients with PAD experience significantly faster rates of functional decline and mobility loss compared with those without PAD (3,14). However, few treatments have been identified that improve functional performance in PAD. Supervised treadmill exercise therapy significantly increases treadmill walking performance in patients with PAD (4,26), but few patients participate, in part because medical insurance typically does not pay for this therapy (5). Home-based exercise interventions do not have demonstrated efficacy in patients with PAD (27). Identifying modifiable lifestyle behaviors associated with slower functional decline in patients with PAD provides opportunities to test interventions that modify behavior in an effort to improve functional performance and/or reduce functional decline in PAD. Patients with PAD can potentially reduce the number of hours they spend sitting or being sedentary per day, even without participating in exercise programs. Interventions that reduce the number of hours spent seated or sedentary per day may be more acceptable to patients with PAD than interventions attempting to increase exercise behavior (26).
Our results also showed that among participants with PAD, slower walking speed outside the home was associated with greater decline in calf muscle density at 4-year follow-up, compared with faster walking speed outside the home. This association was independent of age, sex, race, BMI, comorbidities, and ABI. Lower calf muscle density has been previously associated with higher rates of mobility loss in patients with PAD (6). Thus, calf muscle density is a meaningful outcome in patients with PAD (6).
Walking speed outside the home is also a modifiable behavior but may be more difficult for patients with PAD to change than the number of sedentary hours per day. This is because walking speed outside the home is determined in part by functional ability. Furthermore, increasing walking speed outside the home may induce ischemic pain in patients with PAD. Nonetheless, our data suggest that encouraging patients with PAD to increase their walking speed outside the home may prevent decline in calf muscle density. Further study is needed to identify interventions that increase walking speed outside the home and to determine whether these interventions prevent declines in calf muscle density and other outcomes in patients with PAD.
Previous studies in people without PAD have demonstrated that greater sitting hours per day are associated with weight gain and incident diabetes mellitus (10,11,28). However, to our knowledge, associations of sedentary hours per day with clinically important outcomes have not been evaluated previously in patients with PAD. Because patients with PAD have lower physical activity levels, greater functional impairment, and faster functional decline than people without PAD (1–3), prior studies linking greater sedentary behavior to more adverse outcomes may not be generalizable to patients with PAD.
Our findings are consistent with previous studies demonstrating that lower physical activity levels during daily life, measured with a vertical accelerometer over 7 days, are associated with higher rates of faster functional decline in people with PAD (23,24). However, vertical accelerometers measure both quantity and intensity of vertical motion. Vertical accelerometers do not measure activities performed standing or sitting that do not include vertical movement. We observed no correlation between the numbers of hours spent sitting per day with vertical accelerometer–measured physical activity. We observed only a modest correlation of total sedentary hours per day with vertical accelerometer–measured physical activity levels. Because nonsedentary hours include standing and light activity, it is possible to reduce the number of sedentary hours per day without substantially altering physical activity.
Our finding that greater sedentary hours per day were associated with faster decline in functional performance but not change in calf muscle characteristics suggests that the causal pathway for the association of greater sedentary hours with faster functional decline is not likely to be mediated by adverse calf muscle characteristics. Instead, the association of greater sedentary hours per day with faster functional decline may be mediated by worsening cardiovascular fitness, greater inflammation, worsening calf muscle mitochondrial function, or greater progression of lower-extremity atherosclerosis among participants with PAD who were more sedentary. Similarly, our finding that slower walking speed outside the home was associated with greater declines in calf muscle density but no change in functional performance measures suggests that declines in calf muscle density associated with slower walking speed outside the home are insufficient to cause changes in functional performance over the 4-year follow-up. However, at baseline, slower walking speeds outside the home were associated with markedly lower levels of functional performance. It may be that associations of slower walking speed outside the home with poorer functional performance develop over many years and that additional declines in functional performance among those with slowest walking speeds were not observable because of a floor effect related to the association with baseline walking speed outside the home and poor baseline functional performance. Longer follow-up periods may be needed to demonstrate significant associations of slower walking speed with average annual functional decline among patients with PAD.
Study limitations
First, data were observational. Significant associations cannot be construed as causal. Second, participants were identified from an academic medical center. Findings may not be generalizable to participants with PAD outside of this setting. Third, the mechanisms of associations reported here were not discernable from data collected in this study.
Conclusions
Among people with PAD, greater hours spent sitting per day and greater total sedentary hours per day are associated with faster functional decline. Slower walking speed outside the home is associated with greater declines in calf muscle density. Reducing the number of sedentary hours per day and increasing walking speed outside the home are modifiable behaviors that may be more readily adopted by patients with PAD than interventions designed to promote exercise. Further study is needed to determine whether reducing sedentary hours per day can slow decline in objective measures of functional performance in PAD. Further study is also needed to determine whether interventions that increase walking speed outside the home can prevent declines in calf muscle density in people with PAD.
Acknowledgments
This work was supported by grants R01-HL58099, R01-HL64739, R01-HL071223, and R01-HL076298 from the National Heart, Lung and Blood Institute, by grant RR-00048 from the National Center for Research Resources, National Institutes of Health, and in part by the Intramural Research Program, National Institute on Aging.
Abbreviations and Acronyms
- ABI
ankle brachial index
- BMI
body mass index
- CT
computed tomography
- PAD
peripheral arterial disease
Footnotes
The authors have reported that they have no relationships to disclose.
References
- 1.McDermott MM, Greenland P, Liu K, et al. Leg symptoms commonly reported by men and women with lower extremity peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 2.McDermott MM, Greenland P, Liu K, et al. The ankle brachial index as a measure of leg functioning and physical activity in peripheral arterial disease: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–83. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 3.McDermott MM, Guralnik JM, Tian L, et al. Associations of borderline and low normal ankle brachial index values with functional decline at 5-year follow-up: the Walking and Leg Circulation Study. J Am Coll Cardiol. 2009;53:1056–62. doi: 10.1016/j.jacc.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled clinical trial. JAMA. 2009;301:165–74. doi: 10.1001/jama.2008.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regensteiner JG. Exercise rehabilitation for the patient with intermittent claudication: a highly effective yet underutilized treatment. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:233–9. doi: 10.2174/1568006043336195. [DOI] [PubMed] [Google Scholar]
- 6.McDermott MM, Ferrucci L, Guralnik J, et al. Pathophysiological changes in calf muscle predict mobility loss at 2-year follow-up in men and women with peripheral arterial disease. Circulation. 2009;120:1048–55. doi: 10.1161/CIRCULATIONAHA.108.842328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDermott MM, Hoff F, Ferrucci L, et al. Lower extremity ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2007;55:400–6. doi: 10.1111/j.1532-5415.2007.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDermott MM, Criqui MH, Liu K, et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–71. doi: 10.1067/mva.2000.108640. [DOI] [PubMed] [Google Scholar]
- 9.Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–23. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 10.Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–25. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 11.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289:1785–91. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 12.McDermott MM, Ades PA, Dyer A, Gurlanik JM, Kibbe M, Criqui MH. Corridor-based functional performance measures correlate better with physical activity during daily life than treadmill measures in persons with peripheral arterial disease. J Vasc Surg. 2008;48:1231–7. doi: 10.1016/j.jvs.2008.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc. 1998;46:706–11. doi: 10.1111/j.1532-5415.1998.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 14.McDermott MM, Guralnik JM, Tian L, et al. Baseline functional performance predicts the rate of mobility loss in persons with peripheral arterial disease. J Am Coll Cardiol. 2007;50:974–82. doi: 10.1016/j.jacc.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott MM, Tian L, Liu K, et al. Prognostic value of functional performance for mortality in patients with peripheral artery disease. J Am Coll Cardiol. 2008;51:1482–9. doi: 10.1016/j.jacc.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guralnik JM, Ferrucci L, Simonsick E, Salive ME, Wallace RB. Lower extremity function in persons over 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermott MM, Guralnik JM, Albay M, Bandinelli S, Miniati B, Ferrucci L. Impairments of muscles and nerves associated with peripheral arterial disease and their relationship with lower extremity functioning: the inCHIANTI study. J Am Geriatr Soc. 2004;52:405–10. doi: 10.1111/j.1532-5415.2004.52113.x. [DOI] [PubMed] [Google Scholar]
- 18.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1999;86:1097–8. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 19.Boult C, Kane RL, Louis TA, Boult L, McCaffrey D. Chronic conditions that lead to functional limitation in the elderly. J Gerontol. 1994;49:M28–36. doi: 10.1093/geronj/49.1.m28. [DOI] [PubMed] [Google Scholar]
- 20.Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME. The Women’s Health and Aging Study: health and social characteristics of older women with disability. Bethesda, MD: National Institute on Aging; 1995. Appendix E. NIH publication 95-4009. [Google Scholar]
- 21.Altman R, Alarcon G, Appelrouth D. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505–14. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 22.Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K The American College of Rheumatology. Development of criteria for the classification and reporting of osteoarthritis. Arthritis Rheum. 1986;29:1039–49. [Google Scholar]
- 23.Garg PK, Tian L, Criqui MH, et al. Physical activity during daily life and mortality in patients with peripheral arterial disease. Circulation. 2006;114:242–8. doi: 10.1161/CIRCULATIONAHA.105.605246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garg P, Liu K, Guralnik JM, et al. Physical activity during daily life and functional decline in peripheral arterial disease. Circulation. 2009;119:251–60. doi: 10.1161/CIRCULATIONAHA.108.791491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sieminski DJ, Cowell LL, Montgomery PS, Pillai SB, Gardner AW. Physical activity monitoring in patients with peripheral arterial occlusive disease. J Cardiopulm Rehabil. 1997;17:43–7. doi: 10.1097/00008483-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Watson L, Ellis B, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2008;(4):CD00990. doi: 10.1002/14651858.CD000990.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Regensteiner JG, Meyer TJ, Krupski WC, Cranford LS, Hiatt WR. Hospital vs. home-based exercise rehabilitation for patients with peripheral arterial occlusive disease. Angiology. 1997;48:291–300. doi: 10.1177/000331979704800402. [DOI] [PubMed] [Google Scholar]
- 28.Dunstan DW, Barr EL, Healy GN, et al. Television viewing time and mortality: the Australian Diabetes, Obesity, and Lifestyle Study. Circulation. 2010;121:384–91. doi: 10.1161/CIRCULATIONAHA.109.894824. [DOI] [PubMed] [Google Scholar]