Abstract
Background
This study assessed functional performance, calf muscle characteristics, peripheral nerve function, and quality of life in asymptomatic persons with peripheral arterial disease (PAD).
Methods and Results
PAD participants (n=465) had an ankle brachial index <0.90. Non-PAD participants (n=292) had an ankle brachial index of 0.90 to 1.30. PAD participants were categorized into leg symptom groups including intermittent claudication (n=215) and always asymptomatic (participants who never experienced exertional leg pain, even during the 6-minute walk; n=72). Calf muscle was measured with computed tomography. Analyses were adjusted for age, sex, race, ankle brachial index, comorbidities, and other confounders. Compared with participants with intermittent claudication, always asymptomatic PAD participants had smaller calf muscle area (4935 versus 5592 mm2; P<0.001), higher calf muscle percent fat (16.10% versus 9.45%; P<0.001), poorer 6-minute walk performance (966 versus 1129 ft; P=0.0002), slower usual-paced walking speed (P=0.0019), slower fast-paced walking speed (P<0.001), and a poorer Short-Form 36 Physical Functioning score (P=0.016). Compared with an age-matched, sedentary, non-PAD cohort, always asymptomatic PAD participants had smaller calf muscle area (5061 versus 5895 mm2; P=0.009), poorer 6-minute walk performance (1126 versus 1452 ft; P<0.001), and poorer Walking Impairment Questionnaire speed scores (40.87 versus 57.78; P=0.001).
Conclusions
Persons with PAD who never experience exertional leg symptoms have poorer functional performance, poorer quality of life, and more adverse calf muscle characteristics compared with persons with intermittent claudication and a sedentary, asymptomatic, age-matched group of non-PAD persons.
Keywords: claudication, epidemiology, inflammation, peripheral vascular disease
Eight million men and women in the United States have peripheral arterial disease (PAD).1,2 Intermittent claudication (IC) is the classic manifestation of PAD.3 However, when noninvasive testing with the ankle brachial index (ABI) is used to diagnose PAD, ≈20% to 50% of people with PAD are “asymptomatic” (ie, they report no exertional leg symptoms).4–6 However, the clinical significance of asymptomatic PAD compared with intermittent claudication is poorly understood.
Approximately one third of persons with PAD who report no exertional leg symptoms develop leg symptoms during a 6-minute walk test.5 These individuals with asymptomatic PAD who develop leg symptoms during a 6-minute walk test presumably have restricted their physical activity to avoid exertional leg symptoms during daily life. The clinical significance of asymptomatic PAD among those who do not develop leg symptoms during the 6-minute walk test is unclear. These individuals may have such a mild form of PAD that they never experience leg symptoms. Alternatively, these “always asymptomatic” individuals with PAD may have slowed their walking speed to avoid symptoms, even during the 6-minute walk test.
In the present study, participants with PAD who reported no exertional leg symptoms during daily life were further categorized according to whether they developed leg symptoms during a 6-minute walk test (sometimes asymptomatic) or never developed leg symptoms during the 6-minute walk test (always asymptomatic). To understand the clinical significance of always asymptomatic PAD, we compared lower extremity functional performance, calf muscle characteristics, peripheral nerve function, and quality-of-life measures between individuals with always asymptomatic PAD and those with PAD and IC. We also compared characteristics of individuals with always asymptomatic PAD to those of an asymptomatic, sedentary, age-matched, non-PAD cohort.
Methods
Subjects
The protocol was approved by the Institutional Review boards of Northwestern University Feinberg School of Medicine and Catholic Health Partners Hospitals. Participants gave informed consent. Participants included 227 individuals with PAD attending their fourth annual follow-up visit in the Walking and Leg Circulation Study (WALCS)5,7 and 238 individuals with newly identified PAD.8,9 Together, these individuals made up the PAD participants in WALCS II. Non-PAD participants in WALCS II included 130 attending their fourth WALCS annual follow-up visit and 162 newly identified non-PAD participants. Participants were ≥59 years of age. Most PAD participants were identified from among consecutive patients diagnosed with PAD by 3 Chicago-area noninvasive vascular laboratories. Remaining PAD participants were identified from consecutive patients in a general medicine practice at Northwestern with an ABI <0.90. Non-PAD participants were identified from among consecutive patients with normal lower extremity arterial tests in the 3 noninvasive vascular laboratories and from consecutive patients in the general medicine practice with a normal ABI.8,9
Participation rates and exclusion criteria for our WALCS cohort have been described.5,7 The 238 newly identified PAD participants and 162 newly identified non-PAD participants were recruited from among 1804 contacted for participation. Of these, 176 (9.8%) met exclusion criteria, 131 (7.3%) refused participation, 1021 (57%) did not respond to letters inviting their participation, 74 (4.1%) did not show up for their study appointment, and 2 did not have leg symptom data, leaving 238 newly identified PAD and 162 newly identified non-PAD participants.
Exclusion Criteria
PAD was defined as ABI <0.90 at the baseline WALCS II visit. Absence of PAD was defined as an ABI of 0.90 to 1.30. The following exclusion criteria were applied at the time of original study enrollment to participants in the WALCS cohort and those newly identified for WALCS II. Patients with dementia were excluded because of their inability to answer questions accurately. Nursing home residents, wheelchair-bound patients, and patients with foot or leg amputations were excluded because of their severely impaired functioning. Non–English-speaking patients were excluded because investigators were not fluent in non-English languages. Patients with recent major surgery were excluded. Participants with an ABI of 0.90 to 1.30 with prior lower extremity revascularization were excluded.
ABI Measurement
After participants rested supine for 5 minutes, a hand-held Doppler probe (Nicolet Vascular Pocket Dop II, Golden, Colo) was used to measure systolic pressures in this order: right brachial, dorsalis pedis, and posterior tibial arteries and left dorsalis pedis, posterior tibial, and brachial arteries. Pressures were repeated in reverse order. The ABI was calculated in each leg by dividing average pressures in each leg by the average of the 4 brachial pressures.5,7–9 Average brachial pressures in the arm with highest pressure were used when 1 brachial pressure was higher than the opposite brachial pressure in both measurement sets and the 2 brachial pressures differed by ≥10 mm Hg in at least 1 measurement set because in such cases subclavian stenosis was possible.10 Zero values for the dorsalis pedis and posterior tibial vessels were excluded. Lowest leg ABI was used in the analyses.7
Calf Skeletal Muscle
We selected calf muscle for study because the superficial femoral artery is the most common site of lower extremity atherosclerosis11,12 and calf muscle receives blood from the superficial femoral artery. Using a computed tomography scanner (LightSpeed, General Electric Medical Systems, Waukesha, Wis), we obtained 2.5-mm cross-sectional images of the calves at 66.7% of the distance from the distal to the proximal tibia.13 Cross-sectional images were analyzed with BonAlyse (BonAlyse Oy, Jyvaskyla, Finland), a software for processing computed tomography images that identifies muscle tissue, fat, and bone.13 When quantifying muscle area, the BonAlyse software quantifies voxels within a range corresponding to muscle density and excludes voxels corresponding to fat density.14 Intramuscular fat is quantified by summing voxels corresponding to fat within muscle tissue. Muscle density represents the average amount of muscle tissue within a defined volume.
Peripheral Nerve Function
The electrodiagnostic supervisor at Northwestern Memorial Hospital used electroneurography to measure nerve function. Methods have been reported previously.9
Peroneal Nerve Conduction Velocity (Lower Extremity Motor Nerve Testing)
Two stimulating electrodes were placed over the peroneal nerve, over the anterior ankle and behind the knee. A recording electrode was placed over the belly of the extensor digitorum brevis. Time for electrical impulses to travel from the ankle to the recording electrode (t1) and from the electrode at the fibular head to the recording electrode (t2) were measured, along with the distance between the 2 pairs of electrodes (distance) and the amplitude of the sinusoids (a1 and a2). The nerve conduction velocity (NCV) was calculated as follows: (distance)/(t2–t1). Peroneal amplitude was measured from baseline to the negative peak.
Sural Nerve Testing (Lower Extremity Sensory Nerve)
A recording electrode was placed behind the lateral malleolus. The sural nerve was stimulated 14 cm proximal to the recording electrode. Sural amplitude and latency were recorded, and NCV was calculated.
Ulnar NCV (Upper Extremity Motor Nerve)
The active electrode was placed over the abductor digiti minimi muscle. The reference was at the base of the fifth digit. A ground electrode was placed over the dorsum of the hand. The ulnar nerve was stimulated at the wrist and above the elbow. The distance between the 2 stimulation points and the travel time for electric stimulation between these points were used to calculate ulnar motor NCV.
Functional Data
Performance on each objective measure of leg functioning described below predicts the incidence of mobility loss at the 4-year follow-up among persons with PAD.15 Six-minute walk performance and usual and fastest 4-m walking speed predict mortality in persons with PAD.16
Six-Minute Walk
Participants walk up and down a 100-ft hallway for 6 minutes after instructions to cover as much distance as possible.7,17 At the end of this test, all participants were asked whether they were experiencing leg discomfort.
Repeated Chair Rises
Participants sit in a straight-backed chair with arms folded across their chests and stand 5 times consecutively as quickly as possible. Time to complete 5 chair rises is measured.18,19
Standing Balance
Participants were asked to hold 3 increasingly difficult standing positions for 10 seconds each: standing with feet together side by side and parallel (side-by-side stand), standing with feet parallel with the toes of 1 foot adjacent to and touching the heel of the opposite foot (semitandem stand), and standing with 1 foot directly in front of the other (tandem stand).18,19
Four-Meter Walking Velocity
Walking velocity was measured with a 4-m walk performed at “usual” and “fastest” pace based on previous study.15,16 Each walk was performed twice. The faster walk in each pair was used in the analyses.18,19
Short Physical Performance Battery
The Short Physical Performance Battery combines data from the usual-paced 4-m walking velocity, time to rise from a seated position 5 times, and standing balance. Individuals receive a 0 score for each task they are unable to complete. One to 4 scores are assigned for remaining tasks based on quartiles of performance for >5000 participants in the Established Populations for the Epidemiologic Study of the Elderly.18,19 Scores are summed to obtain the Short Physical Performance Battery, ranging from 0 to 12.
Leg Symptom Groups
Leg symptoms for each participant were characterized into 5 mutually exclusive groups using the San Diego claudication questionnaire.20 These symptom groups, previously defined for participants with exertional leg symptoms, are as follows: (1) IC (exertional leg pain that does not begin at rest and causes the participant to stop walking), (2) pain on exertion and rest (exertional leg symptoms that sometimes begin at rest), and (3) exertional leg pain/carry on (exertional leg symptoms that do not begin at rest and do not stop the individual from walking). We previously defined an “atypical exertional leg pain” symptom category for individuals who met criteria for IC except that their exertional leg symptoms did not involve the calf or did not resolve within 10 minutes of rest. Because outcomes for this group were similar to those of participants with IC, the 2 groups were combined into IC.
Participants reporting no exertional leg symptoms on the San Diego Claudication Questionnaire were considered asymptomatic. One group was designated always asymptomatic because they did not develop exertional leg symptoms during the 6-minute walk. The second group was designated sometimes asymptomatic because they developed leg symptoms during the 6-minute walk.
Comorbidities
Comorbidities were selected because they are known or likely to influence lower extremity functional performance.21,22 Algorithms developed for the Women's Health and Aging Study and the Cardiovascular Health Study were used to document comorbidities.23 These algorithms combine data from patient report, physical examination, medical record review, medications, laboratory values, and a primary care physician questionnaire. Comorbidities assessed were angina pectoris, diabetes mellitus, myocardial infarction, stroke, heart failure, pulmonary disease, cancer, spinal stenosis, and disk disease. Criteria from the American College of Rheumatology were used to diagnose knee and hip osteoarthritis.24,25
Quality-of-Life Measures
The Walking Impairment Questionnaire (WIQ) measures self-reported walking limitations within 3 domains: walking speed, walking distance, and stair climbing.26 In the distance component, participants rank the degree of difficulty walking distances ranging from within their home (50 ft) to 5 blocks (1500 ft) on a Likert scale of 0 to 4 (0=unable, 4=no difficulty). Likert scale responses are multiplied by the corresponding number of feet for each question. These products are summed and divided by the maximum possible score to achieve a percent score ranging from 0 to 100, where 0 represents the inability to walk any of the distances and 100 represents no difficulty walking any of the distances. Similar percent scores are used for walking speed and stair climbing. Walking speed is assessed by asking participants to indicate the degree of difficulty walking a block “slowly,” “average speed,” or “quickly” or “running/jogging.” Responses on the Likert scale of 0 to 4 are multiplied by the approximate miles per hour for each speed.26 For stair climbing, participants rank difficulty climbing 1 to 3 flights of stairs on a Likert scale of 0 to 4. We used the Medical Outcomes Study Short-Form 36 (SF-36) to assess functional status.27 We used the Physical Functioning domain because it includes activities that are likely to be impaired in PAD, including walking, carrying groceries, or climbing stairs.27
Other Measures
Height and weight were measured at the study visit. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Cigarette smoking history was measured with self-report. Lower extremity revascularization was based on patient report and required confirmation with either medical record review or the primary care physician questionnaire. Physical activity was measured using patient-reported number of blocks walked during the past week. Additionally, 60% of WALCS II participants wore a vertical accelerometer continuously over 7 days (Caltrac, Muscle Dynamics Fitness Network, Inc, Torrence, Calif) to measure physical activity using defined methods.28,29 We programmed the accelerometer identically for all participants, allowing us to compare physical activity levels between participants regardless of individual variations in age, weight, height, and sex. Programmed in this way, the accelerometers measured “activity units.”
Statistical Analyses
Differences in continuous and dichotomous variables were compared across categories of leg symptoms by use of ANOVA and χ2 tests, respectively.
Calf muscle characteristics, lower extremity performance, quality-of-life measures, and nerve conduction parameters were compared across leg symptom categories by use of ANCOVA with adjustment for age, race, sex, smoking, BMI, ABI, recruitment cohort (original WALCS versus newly identified), lower extremity revascularization, blocks walked during the past week, and comorbidities. Tibia length was a covariate for calf muscle area comparisons because greater tibia length is associated with greater calf muscle area.30 Height and alcohol use were additional covariates for the nerve analyses. Zero values for NCV indicate the absence of any measurable nerve function. Analyses for NCV were performed with and without inclusion of “0” values, enabling us to compare nerve function across leg symptom groups in the entire cohort and in the subset with any measurable nerve function.
For each outcome, pairwise comparisons were made between each symptom group other than IC with the IC group. IC was the reference because IC is the most classic manifestation of PAD. Analyses were adjusted for multiple comparisons with the Bonferroni method. A value of P<0.0125 was considered statistically significant.
Among the 292 non-PAD WALCS II participants, 142 were always asymptomatic. Among the always asymptomatic non-PAD participants, we identified a subset matched by age and physical activity (number of blocks walked in the last week) to the always asymptomatic PAD participants. Fifty-seven always asymptomatic PAD participants had ≥1 always asymptomatic non-PAD participants who were matched by physical activity and age. When >1 matched non-PAD participant was available, random selection of the non-PAD participant was performed to ensure equal distributions of age and physical activity between the PAD and non-PAD participants. Calf muscle characteristics, functional performance, and quality of life were compared between the always asymptomatic PAD and matched always asymptomatic non-PAD participants by use of ANCOVA with adjustment for age, sex, race, BMI, comorbidities, smoking, recruitment cohort, and blocks walked during the past week.
Because associations studied here were not among the primary aims of WALCS II, sample size estimates were not calculated for the study questions addressed here. Analyses were performed with SAS Statistical Software version 9.0 (SAS Institute Inc, Cary, NC).
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Among the 465 PAD participants, the average age was 75.03±8.26 years and mean ABI was 0.63±0.16. Forty-seven percent were women, 16.99% were black, and 32.69% had diabetes mellitus. Table 1 shows characteristics of PAD participants according to leg symptom categories. Significant differences across all leg symptom groups were observed for age, gender, black race, ABI, and prevalences of angina pectoris, stroke, pulmonary disease, knee arthritis, and lumbar disk disease (Table 1).
Table 1.
Clinical Characteristics Among Participants With PAD According to Leg Symptom Category
| Always Asymptomatic (n=72) | Sometimes Asymptomatic (n=32) | Pain on Exertion and Rest (n=102) | Pain/Carry on (n=44) | IC (n=215) | P | |
|---|---|---|---|---|---|---|
| Age, y | 77.4 (9.1) | 75.4 (7.2) | 74.8 (8.3) | 72.2 (8.6) | 74.9 (7.9) | 0.020 |
| Male, % | 63.89 | 56.25 | 34.31 | 61.36 | 56.74 | <0.001 |
| Black, % | 18.06 | 15.63 | 29.41 | 11.36 | 12.09 | 0.003 |
| ABI | 0.67 (0.16) | 0.68 (0.12) | 0.63 (0.15) | 0.66 (0.15) | 0.60 (0.16) | 0.001 |
| BMI, kg/m2 | 26.72 (4.25) | 28.02 (4.59) | 28.89 (5.63) | 27.80 (4.82) | 27.67 (5.13) | 0.093 |
| Diabetes mellitus, % | 26.39 | 31.25 | 36.27 | 27.27 | 34.42 | 0.591 |
| Angina pectoris, % | 25.35 | 21.88 | 49.00 | 32.56 | 33.96 | 0.007 |
| Myocardial infarction, % | 26.39 | 18.75 | 33.33 | 13.64 | 26.64 | 0.126 |
| Stroke, % | 18.06 | 6.25 | 29.41 | 13.64 | 22.79 | 0.033 |
| Heart failure, % | 27.78 | 37.50 | 32.35 | 22.73 | 31.16 | 0.653 |
| Pulmonary disease, % | 36.11 | 56.25 | 54.90 | 27.27 | 43.26 | 0.008 |
| Knee arthritis, % | 8.33 | 18.75 | 22.55 | 4.55 | 11.63 | 0.010 |
| Lumbar disk disease, % | 30.56 | 37.50 | 58.82 | 27.27 | 36.28 | <0.001 |
| Current cigarette smoker | 9.72 | 15.63 | 18.63 | 9.09 | 17.21 | 0.347 |
| History of lower extremity revascularization, % | 29.17 | 21.88 | 37.25 | 22.73 | 35.35 | 0.219 |
Values shown are mean (SD) when appropriate. n=465.
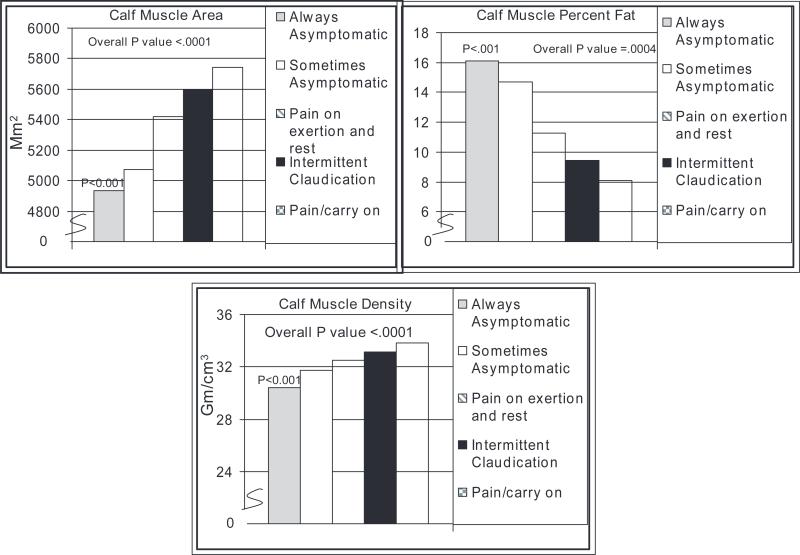
Figure 1 shows associations of leg symptom categories with calf muscle characteristics with adjustment for age, sex, race, BMI, ABI, comorbidities, recruitment cohort, lower extremity revascularization, physical activity, and cigarette smoking. Compared with individuals with PAD and IC, those who were always asymptomatic had lower calf muscle area (P<0.001), higher calf muscle percent fat (P<0.001), and lower calf muscle density (P<0.001) (Figure 1). No other leg symptom group had significantly different muscle characteristics compared with IC after adjustment for multiple comparisons.
Figure 1.
Adjusted associations of leg symptom categories with calf muscle characteristics in persons with PAD. Pairwise P values shown are in reference to participants with IC symptoms. Results are adjusted for age, sex, race, BMI, comorbidities, ABI, smoking, lower extremity revascularization, recruitment cohort, and blocks walked during the past week. Analyses of calf muscle area also are adjusted for tibia length.
Table 2 shows associations of leg symptom categories with peripheral nerve function with adjustment for age, sex, race, BMI, ABI, comorbidities, recruitment cohort, smoking, height, and alcohol use. PAD participants who were always asymptomatic had poorer peroneal nerve amplitude, peroneal NCV, sural nerve amplitude, and sural nerve latency than those with IC. When analyses for NCV were repeated after exclusion of participants with 0 values (ie, after exclusion of participants with no evidence of any nerve activity), peroneal NCV was no longer significantly different between the always asymptomatic and IC groups. No significant differences in upper extremity nerve function were found between the always asymptomatic and IC groups, suggesting that differences in peripheral nerve function were specific to the lower extremities.
Table 2.
Adjusted Associations of Leg Symptom Categories With Peripheral Nerve Function in Persons With PAD*
| Always Asymptomatic (n=67) | Sometimes Asymptomatic (n=29) | Pain on Exertion and Rest (n=95) | Pain/Carry on (n=42) | IC (n=194) | |
|---|---|---|---|---|---|
| Peroneal NCV m/s | 33.07† | 41.17 | 37.08 | 41.15 | 40.32 |
| Peroneal nerve amplitude, mV | 1.92† | 3.44 | 2.68 | 2.78 | 3.10 |
| Peroneal latency, ms | 5.00 | 4.97 | 5.02 | 4.84 | 4.93 |
| Sural NCV, m/s | 40.67 | 40.13 | 41.80 | 42.18 | 43.08 |
| Sural amplitude, mV | 3.29‡ | 4.28 | 4.59 | 6.76 | 5.52 |
| Sural latency, ms | 5.49§ | 3.56 | 3.66 | 3.18 | 3.33 |
| Ulnar motor NCV, m/s | 49.84 | 50.35 | 50.28 | 51.77 | 50.62 |
| Ulnar motor nerve amplitude, mV | 8.42 | 8.71 | 8.86 | 9.14 | 8.92 |
| Ulnar motor nerve latency, ms | 2.89 | 2.82 | 2.82 | 2.74 | 2.80 |
Analyses include participants with 0 values for NCV. When analyses were repeated excluding participants with 0 values for NCV differences in peroneal NCV between participants with IC and those who were always asymptomatic were no longer statistically significant.
Analyses adjust for age, sex, race, BMI, ABI, comorbidities, recruitment cohort, cigarette smoking, height, lower extremity revascularization, and alcohol use.
P= 0.0002
P= 0.0051
P= 0.0056 for pairwise comparisons of always asymptomatic PAD participants vs IC.
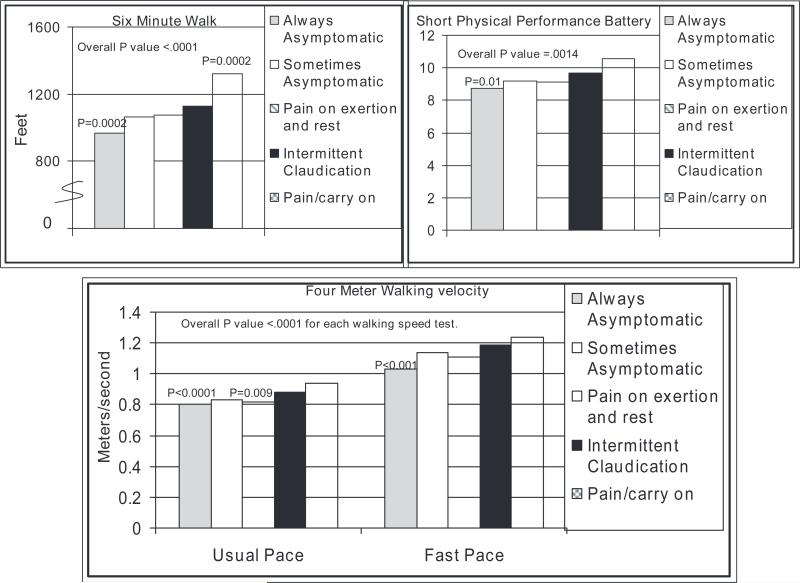
Figure 2 shows associations of leg symptom categories with lower extremity performance in PAD persons with adjustment for age, sex, race, comorbidities, BMI, smoking, ABI, physical activity, and recruitment cohort. Always asymptomatic PAD participants had significantly poorer performance on the 6-minute walk, usual- and fast-paced 4-m walks, and the Short Physical Performance Battery compared with those with IC (Figure 2). PAD participants with exertional leg pain/carry on had significantly better 6-minute walk performance compared with participants with IC. PAD participants with pain on exertion and rest had significantly slower usual-paced walking speed compared with IC.
Figure 2.
Adjusted associations of leg symptom categories with lower extremity performance in persons with PAD. P values represent pairwise relationships relative to IC (reference group). Results are adjusted for age, sex, race, BMI, comorbidities, ABI, smoking, lower extremity revascularization, patient-reported blocks walked during the past week, and recruitment cohort.
Within the subset of 282 PAD participants with Caltrac vertical accelerometer data, no significant difference was noted in physical activity between the always asymptomatic and IC groups (582 versus 691 activity units; P=0.155) after adjustment for age, sex, race, BMI, comorbidities, smoking, ABI, recruitment cohort, and history of lower extremity revascularization. The pain/carry on group had higher physical activity than the IC group (1086 versus 691 activity units; P<0.001).
After adjustment for age, sex, race, comorbidities, BMI, smoking, physical activity, lower extremity revascularization, recruitment cohort, and ABI, always asymptomatic PAD participants had lower Short Form-36 Physical Functioning scores (43.19 versus 50.49; P=0.0157) and higher WIQ distance scores (47.58 versus 34.16; P=0.004) than PAD persons with IC. Findings for the Short Form-36 Physical Functioning score were not significant after adjustment for multiple comparisons. No significant differences were found in the WIQ speed and stair climbing scores between PAD participants with IC versus those always asymptomatic (data not shown).
Table 3 compares outcomes between the always asymptomatic PAD participants and the age- and physical activity–matched always asymptomatic non-PAD participants. After adjustment for confounders, the always asymptomatic non-PAD participants had significantly poorer performance on each functional outcome, more adverse findings for each calf skeletal muscle characteristic, lower objectively measured physical activity, and lower scores for each WIQ measure (Table 3).
Table 3.
Adjusted Associations of Calf Muscle Characteristics, Functional Performance, and Quality of Life Between Always Asymptomatic Participants With PAD and Sedentary Always Asymptomatic Participants Without PAD*
| Always Asymptomatic PAD Participants† (n=57) | Always Asymptomatic Non-PAD Participants† (n=57) | P | |
|---|---|---|---|
| Calf muscle area, mm2 | 5061 | 5895 | 0.0095 |
| Calf muscle fat, % | 15.61 | 9.07 | 0.022 |
| Calf muscle density, g/cm3 | 31.08 | 33.95 | <0.001 |
| Six-minute walk, ft | 1126 | 1452 | <0.001 |
| Usual-paced 4-m walking speed, m/s | 0.84 | 0.94 | 0.010 |
| Fastest-paced 4-m walking speed, m/s | 1.08 | 1.29 | <0.001 |
| Caltrac-measured physical activity over 7 d, activity units | 655 | 1034 | 0.004 |
| Short-form 36 physical functioning score (0–100 scale) | 53.38 | 63.90 | 0.079 |
| WQ distance score (0–100 scale) | 59.40 | 75.10 | 0.019 |
| WQ speed score (0–100 scale) | 40.87 | 57.78 | 0.0011 |
| WQ stair climbing score (0–100 scale) | 45.82 | 68.22 | 0.0002 |
Always asymptomatic PAD participants and sedentary always asymptomatic non-PAD participants were matched for physical activity by patient-reported blocks walked during the previous week. Analyses were adjusted for age, sex, race, comorbidities, smoking, recruitment cohort, and blocks walked during the past week (except for the Caltrac outcome).
Always asymptomatic non-PAD participants are matched by age and physical activity (blocks walked in the past week) to the always asymptomatic PAD participants. Random selection was used to ensure matched distributions of age and activity. Matches were identified for 57 of the always asymptomatic PAD participants
Discussion
Fully 20% to 50% of persons with PAD are asymptomatic.4–6 However, the clinical significance and pathophysiological correlates of asymptomatic PAD are poorly understood. Among 429 men and women with PAD identified by the noninvasive vascular laboratory, we found that 104 (24%) were asymptomatic. Most of the asymptomatic PAD participants (70%) were designated always asymptomatic because they did not develop leg symptoms even during a 6-minute walk test. We found that individuals with PAD who are always asymptomatic have significantly smaller calf muscle area, higher calf muscle percent fat, lower calf muscle density, poorer lower extremity peripheral nerve function, and poorer lower extremity functional performance compared with PAD participants with classic symptoms of IC.
To the best of our knowledge, no prior studies have compared calf muscle characteristics or peripheral nerve function between specific leg symptom categories among persons with PAD. Findings reported here are significant for several reasons. First, previous research demonstrates that asymptomatic PAD is common and that asymptomatic PAD is more likely to be underdiagnosed than symptomatic PAD.4,6,31 Second, our results indicate that clinicians should not equate lack of exertional leg symptoms with a “benign” form of PAD. Third, findings reported here challenge the current paradigm of using leg symptoms as an eligibility criterion for therapeutic clinical trials designed to improve walking performance in persons with PAD. Finally, our results underscore the importance of identifying therapies to improve walking performance in asymptomatic individuals with PAD.
A potential explanation for our findings is that always asymptomatic PAD participants may slow their walking speed to avoid ischemic leg symptoms. Over time, reduced walking speed may result in adverse pathophysiological findings such as the smaller calf muscle area, greater calf muscle fat, and lower calf muscle density observed here. These calf muscle changes may further contribute to impaired lower extremity functioning in always asymptomatic individuals with PAD. However, it also is possible that these PAD participants have limited their activity or slowed their walking speeds for reasons unrelated to leg symptoms. Alternatively, always asymptomatic participants may have experienced a primary insult on their lower extremity nerves, perhaps related to lower extremity ischemia, which may induce denervation atrophy of calf skeletal muscle and reduce perception of lower extremity ischemic symptoms. However, this study is not able to determine the mechanism of asymptomatic PAD.
Without exertional leg symptoms, quality of life in always asymptomatic PAD participants could be superior to that of PAD persons with IC and similar to that of age- and physical activity–matched always asymptomatic non-PAD persons. However, our results show that always asymptomatic PAD participants did not have better quality of life than PAD persons with IC or always asymptomatic non-PAD participants. Our finding that always asymptomatic PAD participants had higher WIQ distance scores than IC participants indicates that always asymptomatic PAD participants perceive greater ease of walking long distances than persons with IC, perhaps consistent with the lack of exertional leg symptoms in the former group. However, all WIQ scores were significantly lower in always asymptomatic PAD participants compared with age- and activity-matched always asymptomatic non-PAD participants.
This study has limitations. First, data are cross-sectional. Associations reported here cannot be construed as causal. Longitudinal study is needed to determine whether always asymptomatic PAD is associated with higher rates of limb loss or critical limb ischemia. Second, most PAD participants were identified by noninvasive vascular laboratories. Third, a large proportion of the potential participants in this study either did not respond to the invitation to participate or refused participation. Our findings may not be generalizable to asymptomatic persons with unrecognized PAD, to individuals with PAD from community settings, or to individuals who did not respond to our mailed invitation or refused to participate.
Our results demonstrate that asymptomatic PAD is not a benign condition. Individuals with PAD who never develop leg symptoms have greater functional impairment and more adverse pathophysiological findings in calf muscle and lower extremity nerves than PAD patients with IC. Further study is needed to identify the natural history of always asymptomatic PAD over time and to develop interventions to improve functional performance in persons with asymptomatic PAD.
CLINICAL PERSPECTIVE.
Eight million men and women in the United States have lower extremity peripheral arterial disease (PAD). Fully 20% to 50% of persons with PAD have no exertional leg symptoms (ie, are asymptomatic). Asymptomatic individuals with PAD who develop leg symptoms during a 6-minute walk test presumably have restricted their physical activity to avoid exertional leg symptoms during daily life. The clinical significance of asymptomatic PAD among those who do not develop leg symptoms during the 6-minute walk test is unclear. In this study, asymptomatic PAD participants who did not develop leg symptoms during the 6-minute walk test were classified as always asymptomatic. Among 429 men and women with PAD identified by the noninvasive vascular laboratory, we found that persons with PAD who are always asymptomatic have significantly smaller calf muscle area, higher calf muscle percent fat, lower calf muscle density, poorer lower extremity peripheral nerve function, and poorer lower extremity functional performance compared with PAD participants with classic symptoms of intermittent claudication. Our results indicate that clinicians should not equate lack of exertional leg symptoms with a “benign” form of PAD. Our results also underscore the importance of ankle brachial index screening to identify persons with PAD who are asymptomatic. Further study is needed to identify therapies that improve walking performance in asymptomatic individuals with PAD.
Acknowledgments
Sources of Funding
This work was supported by grants R01-HL58099, R01-HL64739, R01-HL71223, and R01-HL076298 from the National Heart, Lung, and Blood Institute and by grant RR-00048 from the National Center for Research Resources, National Institutes of Health. This work also was supported by the Intramural Research Program, National Institute on Aging, National Institutes of Health.
Footnotes
Disclosures
None.
References
- 1.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, Criqui MH. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–333. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thorn T, Wasserthiel-Smoller S, Hong Y, for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics: 2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–el71. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 3.Rose GA. The diagnosis of ischemic heart pain and intermittent claudication in field surveys. Bull World Health Org. 1962;27:645–658. [PMC free article] [PubMed] [Google Scholar]
- 4.McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: the Women's Health and Aging Study. Circulation. 2000;101:1007–1012. doi: 10.1161/01.cir.101.9.1007. [DOI] [PubMed] [Google Scholar]
- 5.McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, Chan C, Celic L, Pearce WH, Schneider JR, Sharma L, Clark E, Gibson D, Martin GJ. Leg symptoms in peripheral arterial disease: assodated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, ffiatt WR. The PARTNERS program: A national survey of peripheral arterial disease detection, awareness, and treatment. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 7.McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, Chan C, Martin GJ, Schneider J, Pearce WH, Taylor LM, Clark E. The ankle brachial index as a measure of leg functioning and physical activity in peripheral arterial disease: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 8.McDermott MM, Hoff F, Ferrucci L, Pearce WH, Guralnik JM, Tian L, Liu K, Schneider JR, Sharma L, Tan J, Criqui MH. Lower extremity ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2007;55:400–406. doi: 10.1111/j.1532-5415.2007.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDermott MM, Sufit R, Nishida T, Guralnik JM, Ferrucci L, Tian L, Liu K, Tan J, Pearce WH, Schneider JR, Sharma L, Criqui MH. Lower extremity nerve function in patients with lower extremity ischemia. Arch Intern Med. 2006;166:1986–1992. doi: 10.1001/archinte.166.18.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shadman R, Criqui MH, Bundens WP, Fronek A, Denenberg JO, Gamst AC, McDermott MM. Subclavian artery stenosis: Prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–623. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 11.Lindbom A. Arteriosclerosis and arterial thrombosis in the lower limb: a roentgenological study. Acta Radiol Suppl. 1950;80:1–80. [PubMed] [Google Scholar]
- 12.Hyvarinen S. Arteriographic findings of claudication patients. Ann Clin Res. 1984;16:1–45. [PubMed] [Google Scholar]
- 13.McDermott MM, Guralnik JM, Albay M, Bandinelli S, Miniati B, Ferrucci L. Impairments of muscles and nerves associated with peripheral arterial disease and their relationship with lower extremity functioning: The InCHIANTI Study. J Am Geriatr Soc. 2004;52:405–410. doi: 10.1111/j.1532-5415.2004.52113.x. [DOI] [PubMed] [Google Scholar]
- 14.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1999;86:1097–1098. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 15.McDermott MM, Guralnik JM, Tian L, Ferrucci L, Liu K, Liao Y, Criqui MH. Baseline functional performance predicts the rate of mobility loss in persons with peripheral arterial disease. J Am Coll Cardiol. 2007;50:974–982. doi: 10.1016/j.jacc.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDermott MM, Tian L, Liu K, Guralnik JM, Ferrucci L, Tan J, Pearce WH, Schneider JR, Criqui MH. Prognostic value of functional performance for mortality in persons with peripheral arterial disease. J Am Coll Cardiol. 2008;51:1482–1489. doi: 10.1016/j.jacc.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc. 1998;46:706–711. doi: 10.1111/j.1532-5415.1998.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 18.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol Med Sci. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 19.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. Lower extremity function in persons over 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vase Med. 1996;1:65–71. doi: 10.1177/1358863X9600100112. [DOI] [PubMed] [Google Scholar]
- 21.Boult C, Kane RL, Louis TA, Boult L, McCaffrey D. Chronic conditions that lead to functional limitation in the elderly. J Gerontol Med Sci. 1994;49:M28–M36. doi: 10.1093/geronj/49.1.m28. [DOI] [PubMed] [Google Scholar]
- 22.Fried LP, Bandeen-Roche K, Kasper JD, Guralnik JM. Association of comorbidity with disability in older women: the Women's Health and Aging Study. J Clin Epidemiol. 1999;52:27–37. doi: 10.1016/s0895-4356(98)00124-3. [DOI] [PubMed] [Google Scholar]
- 23.Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME. The Women's Health and Aging Study: Health and Social Characteristics of Older Women With Disability. National Institute on Aging; Bethesda, Md: 1995. NJH publication No. 95-4009, appendix E. [Google Scholar]
- 24.Airman R, Alarcon G, Appelrouth D. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505–514. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 25.Airman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, for the American College of Rheumatology Development of criteria for the classification and reporting of osteoarthritis. Arthritis Rheum. 1986;29:1039–1049. [Google Scholar]
- 26.Regensteiner JG, Steiner JF, Panzer RJ, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vase Med Biol. 1990;2:142–152. [Google Scholar]
- 27.Ware JE, Snow KK, Kosisnki M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. The Health Institute, New England Medical Center; Boston, Mass: 1993. [Google Scholar]
- 28.McDermott MM, OMmiller SM, Liu K, Guralnik JM, Martin GJ, Pearce WH, Greenland P. Gait alterations associated with walking impairment in people with peripheral arterial disease with and without intermittent claudication. J Am Geriatr Soc. 2001;49:747–754. doi: 10.1046/j.1532-5415.2001.49151.x. [DOI] [PubMed] [Google Scholar]
- 29.Garg PK, Tian L, Criqui MH, Liu K, Ferrucci L, Guralnik JM, Tan J, McDermott MM. Physical activity during daily life and mortality in patients with peripheral arterial disease. Circulation. 2006;114:242–248. doi: 10.1161/CIRCULATIONAHA.105.605246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruff C. Allometry between length and cross-sectional dimensions of the femur and tibia in Homo sapiens. Am J Phys Anthropol. 1984;65:347–358. doi: 10.1002/ajpa.1330650403. [DOI] [PubMed] [Google Scholar]
- 31.McDermott MM, Kerwin DR, Liu K, Martin GJ, O'Brien E, Kaplan H, Greenland P. Prevalence and significance of unrecognized lower extremity peripheral arterial disease in general medicine practice. J Gen Intern Med. 2001;16:384–390. doi: 10.1046/j.1525-1497.2001.016006384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]