Abstract
Objectives
to investigate the effects of proton pump inhibitors (PPIs) on the insulin-like-growth factor 1(IGF-1) system in the elderly.
Design
cross-sectional.
Setting
InCHIANTI study.
Participants
938 older subjects (536 women, 402 men, mean age 75.7±7.4 years).
Measurements
complete data on age, sex, BMI, liver function, medications, dietary intake, IGF-1, IGF-binding protein-1 and -3 (IGFBP-1, IGFBP-3).
Results
Participants were categorized by PPI use, identifying 903 PPI non users and 35 users. After adjusting for age, male PPI users (107.0 ± 69.6 vs 127.1 ± 55.8, p<0.001) and female PPI users (87.6 ± 29.1 vs 107.6 ± 52.3, p=0.03) had lower IGF-1 levels than non-users. IGFBP-1 levels were similar in the two groups in both sexes. In whole population, after adjustment for age and sex, PPI users had lower IGF-1 levels 81.9 [61.1–113.8] than non-users 110 [77.8–148.6], p=0.02. After further adjustment for BMI, albumin, liver function, C-reactive protein, Interleukin-6, number of medications, ACE-inhibitors use, caloric intake, protein intake, physical activity, glycemia, and IGFBP-1, the use of PPIs remained significantly and negatively associated with IGF-1 levels (β±SE=−19.60±9.83, p=0.045).
Conclusion
Use of PPIs was independently and negatively associated with IGF-1 levels.
Keywords: Proton pump inhibitors, IGF-1, older subjects
Introduction
The widespread use of proton pump inhibitors (PPIs) drugs was gradually increased in the last two decades, especially in older people (1). Recent studies suggest that PPIs may be overused or inappropriately prescribed in 25% to 81% of elderly patients taking these drugs (2). Additionally, recent studies raised concerns about the potential increased risk of fractures (3), clostridium difficile infections (4), and community-acquired pneumonia (1) in relation to long term use of PPIs. We recently found that long-term use of high dose PPIs is associated with increased risk of 1-year mortality in older patients discharged from acute hospitals (5). Among potential underlying mechanisms explaining such detrimental effects, the suppression of gastric acidity and the interference with adsorption of macronutrients (protein) (6), and micronutrients (non-heme iron, calcium, magnesium) (7) have been advocated, but other causes cannot be excluded.
Nutritional intake is an important regulator of plasma Insulin-like Growth Factor-1 (IGF-1) levels. In humans, IGF-1 plasma concentrations are reduced by few days of fasting, and their subsequent normalization depends on the adequacy of energy and protein content in the refeeding diet (8). Low IGF-1 levels are also found during obesity, metabolic syndrome and diabetes, suggesting that IGF-1 may be a sensitive marker of malnutrition rather than just a regulator of cell growth, differentiation, apoptosis and transformation. The IGF-1 bioactivity is modulated by more than six specific high affinity binding proteins (IGFBPs), which regulate and propagate IGF-1 actions in several tissues. An in-vitro study of human hepatocarcinoma HepG2 cells has shown that omeprazole can stimulate the induction of Insulin-Like Growth Factor Binding Protein-1 (IGFBP-1), through human Aryl Hydrocarbon Receptor (hAhR) activation, in an apparent non ligand-binding manner. Omeprazole, at a concentration usually used by subjects on PPI therapy, can alter IGFBP-1 expression and may influence IGFBP-1-dependent physiological processes (9). On this basis, it could be hypothesized that PPIs exert their negative effects on nutritional status by interfering with IGF-1 system.
However, the relationship between PPI use and IGF-1 system in humans has never been investigated until now. Therefore, the aim of our study was to test the relationship between use of PPIs and IGF-1 system in older people.
Methods
To address this hypothesis we used data from the InCHIANTI study. This is an epidemiological, population-based study of randomly selected older people living in 2 cities in the Chianti area, Tuscany, Italy, designed by the Laboratory of Clinical Epidemiology of the Italian Research Council of Aging (Florence) to identify risk factors for late-life disability, as previously described (10). Briefly, participants were selected from the city registries of Greve in Chianti and Bagno a Ripoli using a multistage sampling method. In 1998, 1453 persons who were randomly selected from the population agreed to participate in the project. The Italian National Research Council on Aging Ethical Committee ratified the study protocol and participants provided written consent to participate. Of the original 1453 subjects we excluded 298 participants younger than 65 years and 217 with no information on hormonal measures, or because refused physical examination or were too sick to be evaluated. The final study population included 938 older subjects (536 women and 402 men) with complete information on IGF-1, IGF-binding protein-1 (IGFBP-1), IGFBP-3 and medications. Demographic information on educational and marital status, smoking and medication use were collected using standardized questionnaires. Blood samples were collected in the morning after a 12-h fast. Aliquots of serum and plasma were stored at 80°C. IGF-1 was measured by immunoradiometric assay using commercial reagents. The Minimum Detectable Concentration (MDC) was 0.80 ng/ml. Inter and intra-assay Coefficients of Variation (CV) were <10%. IGFBP-1 and IGFBP-3 were measured by immunoradiometric assay. Serum levels of interleukin-6 (IL-6), were measured in duplicate by high sensitivity enzyme-linked immunoabsorbent assays (ELISA) using commercial kits (BIOSOURCE International, Camarillo, CA). The MDC for IL-6 was 0.1 pg/ml. The interassay CV was less than 7%. C-reactive protein (CRP) was measured in duplicate using an enzyme-linked immunosorbent assay and colorimetric competitive immunoassay with purified protein and polyclonal anti-CRP antibodies. The MDC was 0.03 mg/L and the interassay CV was 5%.
IGF-1/IGFBP-1 ratio as measure of IGF-1 bioactivity was also calculated (11).
Physical activity level in the previous year was considered as an ordinal variable and scored into seven progressive grades, from 0 (hardly any physical activity) up to 7 (intense exercise many times/week) by using a modified version of a standard questionnaire (12). For descriptive purpose, baseline characteristics of the study population were compared according to presence or absence of PPI use, using a χ2 test and ANOVA model for categorical and continuous variables, respectively. We used age and gender adjusted linear regression analysis to test the association between PPI use (predictor) and IGF-1 system (outcome). Multivariate linear regression adjusted model for age, sex, BMI, albumin, liver function, number of drugs, angiotensin-converting enzyme (ACE) inhibitor use, caloric intake, protein intake, CRP, IL-6, physical activity, fasting glycemia and IGFBP-1 was also used to estimate the association between PPI use and IGF-1.
Results
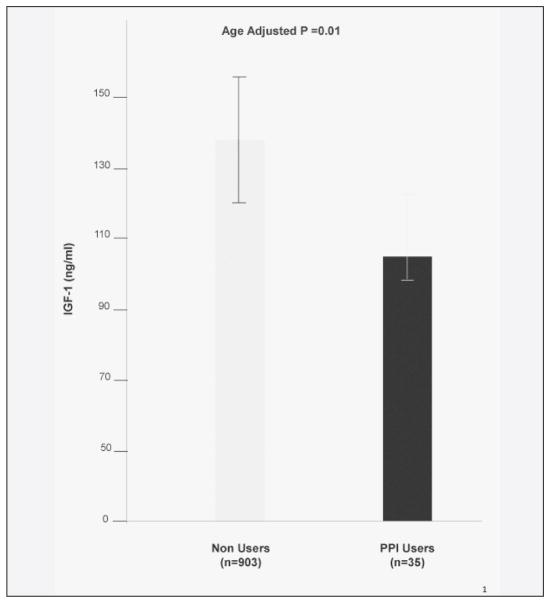
Participants were categorized according to PPI use. 35 subjects (14 men and 21 women), corresponding to 4% of the entire population, were identified as PPI users. The mean age of whole population was 75.7 ± 7.4 years ± SD. None of the participants were on GH treatment and corticosteroids. The group of PPI users had lower BMI, fasting glycemia, and were taking a greater number of medications when compared to non-users (Table 1). PPI users had lower IGF-1 levels than non-users either after adjustment for age (Figure 1) and age and sex (Table 1). PPI male users were older (77.7±7.5 vs 74.6±6.9) than male non-users, while women on PPI were younger than their non-users counterpart (74.2±4.8 vs 76.6±7.8) (data not shown).
Table 1.
General characteristics of the whole study population divided according to use of PPIs
| Baseline | ||||
|---|---|---|---|---|
| All (N=938) | Non users (N=903) | PPI users (N=35) | P* | |
| Age (years) | 75.7±7.4 | 75.7±7.5 | 75.6±6.1 | 0.88 |
| Gender, M (N,%) | 402 (42.9) | 388 (42.9) | 14 (40) | 0.72 |
| BMI (Kg/m2) | 27.4±4.05 | 27.5±4.0 | 25.8±4.1 | 0.01 |
| Albumin (g/dl) | 4.2±0.3 | 4.2±0.3 | 4.2±0.3 | 0.67 |
| MMSE | 24.3±5.0 | 24.3±5.1 | 24.0±3.8 | 0.68 |
| Caloric intake (kcal/d) | 1895.3±557.9 | 1894.5±554.3 | 1914.9±652.2 | 0.71 |
| Protein Intake (Kcal/d) | 74.10± 20.3 | 74.0 ± 20.4 | 76.2+ 18.6 | 0.52 |
| No. of drugs | 2.34±2.0 | 2.27±2.0 | 3.91±2.16 | <0.001 |
| Ace-inhibitor users (N,%) | 228 (24.3) | 218 (23.3) | 10 (28.6) | 0.54 |
| Physical Activity^ | 1.8±0.5 | 1.81±0.5 | 1.85±0.5 | 0.66 |
| ALT (UI/l) | 19.4±12.1 | 19.4±12.2 | 18.1±6.4 | 0.51 |
| AST (UI/l) | 21.1±7.9 | 21.0±8 | 21.9±6.2 | 0.52 |
| Fasting Glycemia (mg/dl) | 95.4±25.6 | 95.71± 25.9 | 86.61±11.4 | 0.04 |
| CRP (mg/dl) | 2.76[1.35–5.74] | 2.76[1.35–5.78] | 2.36[1.16–4.57] | 0.78 |
| IL-6 (pg/mL) | 1.47[0.87–2.33] | 1.47[0.87–2.33] | 1.53[0.82–2.36] | 0.91 |
| IGF BP-3 (ng/ml) | 41.4 [34.6–49.2] | 42.0 [34.7–49.5] | 39.0 [33.5–47.1] | 0.07 |
| IGF BP-1 (ng/ml) | 29.3[18.1–44.2] | 29.1[17.9–44.2] | 30.2[21.4–45.11] | 0.99 |
| IGF-1 (ng/ml) | 109.1[76.7–147.4] | 110[77.8–148.6] | 81.9[61.1–113.8] | 0.02 |
Data are presented as number of cases (percentage), mean ± SD or median and interquartile range, as appropriate. The P values are adjusted for age and sex.
intense exercise many times/week.
Figure 1.
IGF-1 serum levels in older PPI users (black column) and PPI non users (grey column) of both sexes. IGF-1 levels were lower in PPI users than non users and the difference between two groups was statistically significant after adjustment for age
After adjusting for age, male PPI users (107.0±69.6 vs 127.1±55.8 ng/ml, p<0.001) and female PPI users (87.6±29.1 vs 107.6±52.3 ng/ml, p=0.03) had lower levels of IGF-1 than non-users (data not shown). PPI users and non-users had similar IGFBP-1 levels, while IGFBP-3 levels were almost significantly lower among PPI users.
Multivariable analysis was carried out in the whole study population (Table 2). After adjusting for age, sex, BMI, albumin, liver function, CRP, IL-6, number of medications, ACE-inhibitors use, caloric intake, protein intake, physical activity, glycemia, and IGFBP-1, the use of PPIs remained significantly associated with IGF-1 (β±SE=−19.60±9.83, p=0.045). The negative association between use of PPIs and IGF-1 was attenuated when IGFBP-3 was included in the multivariable model (β±SE=−15.49±8.43, p=0.06) (data not shown). No significant association was found between PPI use and IGFBP-1 and between PPI use and IGF-1/IGFBP-1 ratio (data not shown).
Table 2.
Multivariate regression model* testing the relationship between PPI use and IGF-1
| β | SE | p | ||
|---|---|---|---|---|
| PPI use | −19.60 | ± | 9.83 | 0.045 |
| Age | −2.17 | ± | 0.31 | <.0001 |
| Sex | −15.90 | ± | 4.20 | 0.0002 |
| Ace-Inhibitor Use | 15.80 | ± | 4.50 | 0.0005 |
| CRP | −0.57 | ± | 0.28 | 0.04 |
| ALT | −0.37 | ± | 0.17 | 0.02 |
| Protein Intake | 0.26 | ± | 0.10 | 0.001 |
| Fasting glycemia | 0.61 | ± | 0.17 | 0.0004 |
| Physical Activity | −7.05 | ± | 4.40 | 0.11 |
Also adjusted for BMI, Log (IL-6), Caloric Intake, Albumin, IGFBP-1, AST, Number of Drugs.
Discussion
In this preliminary analysis performed in older male and female population of the InCHIANTI study we found a negative association between PPI use and IGF-1 levels.
This is the first human study addressing the relationship between PPI use and IGF-1 system in older population.
These results are not surprising given the specific mechanism of action of these drugs. PPIs, by inducing hypochlorhydria and reducing gastric proteolysis, decrease both adsorption and bioavailability of important nutrients and vitamins, worsening the overall nutritional status of older individuals (1).
Since IGF-1 is not only an anabolic hormone but also a sensitive nutritional marker, the reduced levels of IGF-1 in PPI users could be explained by the worse nutritional status in PPI group. In accordance with this hypothesis, PPI users had lower BMI (although in normal range) than non-users. However, the adjustment for BMI did not affect the relationship between PPI use and IGF-1.
We also tested the levels of another nutritional marker such as albumin, but we failed to detect any significant difference between the two groups. An in vitro study shows that omeprazole, a PPI very commonly used, may affect IGF bioactivity by enhancing IGFBP-1 expression (9). In our study no significant difference in IGFBP-1 levels was observed in PPI users and non users suggesting that mechanisms other than IGFBP-1 should be explored to explain the difference in IGF-1 levels in the two groups. Interestingly, the relationship between PPI use and IGF-1 levels was independent of age and sex and remained statistically significant when separate analyses in men and women were performed or when CRP, IL-6, physical activity, glycemia, protein intake, and medications known to increase IGF-1 levels such as ACE-inhibitors were accounted (13, 14).
We should acknowledge some limitations in our study.
The group on PPI treatment was composed of a small number of subjects (N=35). Information on type and duration of PPI treatment, PPI dosage was not available. The cross sectional nature of the analysis does not allow to infer any causality in the association between PPI use and IGF-1 levels. Despite the presence of these limitations, this is the first evidence of the relationship between PPI use and IGF-system in a population study. Complete information on IGF-1 and IGFBP-3 and inflammatory markers was available to test this very novel hypothesis.
In conclusion, use of PPI was independently and negatively associated with IGF-1 levels in older Italian men and women suggesting one of the potential mechanisms by which use of PPI can increase adverse outcomes in older subjects.
Acknowledgments
The InCHIANTI Study was supported as a “targeted project” (ICS 110.1/RS97.71) by the Italian Ministry of Health and in part by the US National Institute on Aging (Contracts N01-AG-916413 and N01-AG-821336), and by the Intramural Research Program of the US National Institute on Aging (Contracts 263 MD 9164 13 and 263 MD 821336) and by grant RF-2010-2312659 from the Italian Ministry of Health and Emilia Romagna Region. None of the sponsoring institutions interfered with the collection, analysis, presentation, or interpretation of the data reported here. We thank Fabrizio Ablondi, Maurizio Conca and Pietro Schianchi for their technical supports
Footnotes
Author’s roles: Marcello Maggio (MM) conceived the study, carried out the studies and data analyses and drafted the manuscript. Chiara Cattabiani (CC), Elisabetta Dall’Aglio (ED), Gian Paolo Ceda (GPC) participated in the design of the study. Fulvio Lauretani (FL) performed the statistical analysis. Francesca De Vita (FD), Valeria buttò (VB), Eleonora Sutti (ES), Giuliana Bondi (GB) helped to draft the manuscript. Bandinelli Stefania (SB), Andrea Corsonello (AC), Angela Maria Abbatecola (AA), Luigi Ferrucci (LF), Fabrizia Lattanzio (FL) participated in study design and coordination. All authors read and approved the final manuscript.
Disclosure statement: The authors have nothing to disclose.
References
- 1.McCarthy DM. Adverse effects of proton pump inhibitor drugs: clues and conclusions. Curr Opin Gastroenterol. 2010;26:624–631. doi: 10.1097/MOG.0b013e32833ea9d9. [DOI] [PubMed] [Google Scholar]
- 2.Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5:219–232. doi: 10.1177/1756283X12437358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta- analysis of 11 international studies. Am J Med. 2011;124:519–526. doi: 10.1016/j.amjmed.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linsky A, Gupta K, Lawler EV, Fonda JR, Hermos JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med. 170:772–8. doi: 10.1001/archinternmed.2010.73. Erratum in: Arch Intern Med 2010; 170:1100. [DOI] [PubMed] [Google Scholar]
- 5.Maggio M, Corsonello A, Ceda GP, Cattabiani C, Lauretani F, Buttò V, Ferrucci L, Bandinelli S, Abbatecola AM, Spazzafumo L, Lattanzio F. Proton pump inhibitors and risk of 1-year mortality and rehospitalization in older patients discharged from acute care hospitals. JAMA Intern Med. 2013;173(7):518–23. doi: 10.1001/jamainternmed.2013.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu S, Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2012;28:587–93. doi: 10.1097/MOG.0b013e328358e5cc. [DOI] [PubMed] [Google Scholar]
- 7.McColl KE. Effect of proton pump inhibitors on vitamins and iron. Am J Gastroenterol. 2009;104:S5–S9. doi: 10.1038/ajg.2009.45. [DOI] [PubMed] [Google Scholar]
- 8.Underwood LE, Clemmons DR, Maes M, D’Ercole AJ, Ketelslegers JM. Regulation of somatomedin-C/insulin-like growth factor I by nutrients. Horm Res. 1986;24:166–176. doi: 10.1159/000180556. [DOI] [PubMed] [Google Scholar]
- 9.Murray IA, Perdew GH. Omeprazole stimulates the induction of human insulin-like growth factor binding protein-1 through aryl hydrocarbon receptor activation. J Pharmacol Exp Ther. 2008;324:1102–1110. doi: 10.1124/jpet.107.132241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 11.Maggio M, Cattabiani C, Lauretani F, Bandinelli S, De Vita F, Dall’aglio E, Corsonello A, Lattanzio F, Paolisso G, Ferrucci L, Ceda GP. Insulin-Like Growth Factor-1 Bioactivity Plays a Prosurvival Role in Older Participants. J Gerontol A Biol Sci Med Sci. 2013 doi: 10.1093/gerona/glt045. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balzi D, Lauretani F, Barchielli A, Ferrucci L, Bandinelli S, Buiatti E, Milaneschi Y, Guralnik JM. Risk factors for disability in older persons over 3-year follow-up. Age Ageing. 2010;39:92–98. doi: 10.1093/ageing/afp209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maggio M, Ceda GP, Lauretani F, Pahor M, Bandinelli S, Najjar SS, Ling SM, Basaria S, Ruggiero C, Valenti G, Ferrucci L. Relation of angiotensin-converting enzyme inhibitor treatment to insulin-like growth factor-1 serum levels in subjects >65 years of age (the InCHIANTI study) Am J Cardiol. 2006;97:1525–1529. doi: 10.1016/j.amjcard.2005.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giovannini S, Cesari M, Marzetti E, Leeuwenburgh C, Maggio M, Pahor M. Effects of ACE-inhibition on IGF-1 and IGFBP-3 concentrations in older adults with high cardiovascular risk profile. J Nutr Health Aging. 2010;14:457–460. doi: 10.1007/s12603-010-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]