Abstract
Placental malaria (PM) due to Plasmodium falciparum is a major cause of maternal, fetal and infant mortality, but the mechanisms of pathogenesis and protective immunity are relatively well-understood for this condition, providing a path for vaccine development. P. falciparum parasites bind to chondroitin sulfate A (CSA) to sequester in the placenta, and women become resistant over 1–2 pregnancies as they acquire antibodies that block adhesion to CSA. The protein VAR2CSA, a member of the PfEMP1 variant surface antigen family, mediates parasite adhesion to CSA, and is the leading target for a vaccine to prevent PM. Obstacles to PM vaccine development include the large size (~350 kD), high cysteine content, and sequence variation of VAR2CSA. A number of approaches have been taken to identify the combination of VAR2CSA domains and alleles that can induce broadly active antibodies that block adhesion of heterologous parasite isolates to CSA. This review summarizes these approaches, which have examined VAR2CSA fragments for binding activity, antigenicity with naturally acquired antibodies, and immunogenicity in animals for inducing anti-adhesion or surface-reactive antibodies. Two products are expected to enter human clinical studies in the near future based on N-terminal VAR2CSA fragments that have high binding affinity for CSA, and additional proteins preferentially expressed by placental parasites are also being examined for their potential contribution to a PM vaccine.
Keywords: Plasmodium falciparum, Placental malaria, Vaccine
1. Introduction
Placental malaria (PM) leads to poor outcomes for pregnant women and their babies, and is caused by Plasmodium falciparum sequestration in the intervillous spaces of placenta and the ensuing inflammation. Severe maternal anemia, low birth weight delivery and fetal loss are common sequelae, resulting in 10,000 maternal deaths and 200,000 infant deaths annually in Africa by some estimates, and causing a third of perinatal mortality in the absence of preventive measures [1]. The poor outcomes of PM have been associated with the inflammatory infiltrates and levels of inflammatory cytokines observed in the placenta [2–6]. Adult residents of malaria endemic areas enjoy immunity that protects them from severe disease; women become susceptible to infection and disease again during first gestation, then regain immunity over successive pregnancies. P. falciparum infected erythrocytes (IE) bind chondroitin sulfate A (CSA) on the syncytiotrophoblast surface and in intervillous spaces; unlike placental IE, IE in non-pregnant individuals bind receptors like CD36 and ICAM-1 but not CSA to sequester in other vascular beds [7].
With increasing parity, women acquire specific antibody against CSA-binding parasites, including that which inhibits IE adhesion, and this antibody is associated with heavier babies and higher maternal hemoglobin levels [8–10]. These naturally acquired antibodies are broadly active to placental IE collected around the world, indicating that the target epitopes are conserved [11]. This model of pathogenesis and immunity provides a framework to develop PM vaccines, and also predicts that vaccine-induced immunity should be naturally boosted when pregnant women are exposed to malaria. Other malaria parasite species such as Plasmodium vivax infect pregnant women, but disease sequelae are less severe [12], and these species do not sequester in placenta [13] hence the path to a vaccine against these forms of pregnancy malaria is not clear.
P. falciparum IE adhesion to CSA is mediated by the large (~350 kD) protein called VAR2CSA (Fig. 1), a member of the PfEMP1 variant surface antigen family. PfEMP1 proteins including VAR2CSA are encoded in the genome of P. falciparum but not that of other human malaria parasites. VAR2CSA has extracellular, transmembrane, and intracytoplasmic regions, and its extracellular region is uniquely structured among PfEMP1 family members. The extracellular region of VAR2CSA includes an N-terminal sequence, 6 cysteine-rich Duffy binding like (DBL) domains, and inter-domain (ID) regions that increasingly appear to play a key role in adhesion and immunogenicity of recombinant VAR2CSA protein fragments. VAR2CSA is preferentially expressed by placental parasites and isolates selected to bind CSA [14,15], and is currently the leading candidate for a vaccine to prevent malaria during pregnancy. The high molecular weight, multiple extracellular domains, and extensive sequence variation of VAR2CSA pose unique challenges in designing a vaccine that will mimic the broadly neutralizing activity of naturally acquired immunity. The most challenging step is to define the domain or domain combination and boundaries that can elicit potent pan-reactive antibody. Currently, the first 2 candidate VAR2CSA-based products derived from the protein N-terminus region are entering clinical trials evaluation.
Fig. 1. Domain architecture of VAR2CSA.
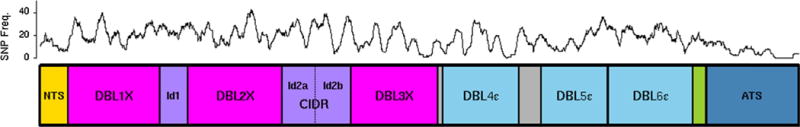
Schematic organization of VAR2CSA protein, showing its N-terminal sequence, 6 cysteine-rich Duffy binding like (DBL) domains, inter-domain (ID) regions, and intracytoplasmic tail (acidic terminal sequence, ATS). Prevalence of sequence variability along the extent of the protein is graphically displayed as the number of single nucleotide polymorphisms (SNPs) per 100 nucleotides, measured as a moving average along the sequence. Analysis does not include insertion/deletion differences. SNP data obtained from PlasmoDB. Figure and sequence analysis prepared by Robert Morrison.
We review here the approaches to design VAR2CSA immunogens that can be used in a vaccine to prevent PM, either by defining CSA-binding fragments of VAR2CSA, or by defining the domains and epitopes that induce broadly active antibodies in animals or that react to such antibodies from immune women. We conclude with a discussion of the antigens other than VAR2CSA that are preferentially expressed by placental parasites, as these may also contribute to a protective vaccine.
2. CSA-binding domains of VAR2CSA
PM vaccine development is currently based on mimicking naturally acquired functional antibodies that block parasite adhesion to CSA. Because these antibodies are likely to target epitopes within the binding domains of VAR2CSA, several approaches have been applied to identify CSA binding regions of the protein. Initial in vitro studies examined binding to CSA of 6 VAR2CSA recombinant DBL domains of 3D7 allele and the DBL1, DBL2 and DBL3 of the A4 allele: DBL2 and DBL6 domains of 3D7 and DBL2 and DBL3 of A4 bound immobilized CSA [16,17]. In another study, DBL3 of both 3D7 and A4 alleles bound CSA, but the 3D7 form demonstrated stronger binding than the A4 form [18]. Following these observations, two crystallography studies using recombinant DBL3 protein of the A4 allele confirmed binding to CSA [19,20].
In another approach to identify CSA binding motifs, a phage library of var2csa gene fragments was panned on CSA-expressing cells or soluble CSA, and identified CSA-binding peptides derived from the first five domains (DBL1 through DBL5) and not DBL6 [21]. Based on these results, peptides representing the sequences of the phage clones were synthesized. One of the peptides from DBL2 domain bound to CSA and anti-peptide antibodies recognized native VAR2CSA [21].
In vitro binding studies have shown that DBL domains bind specifically to CSA but not to the closely related glycosaminoglycan chondroitin sulfate C. However, similar results have been obtained with DBL from other PfEMP1s expressed by IE that do not bind CSA [22]. This raises questions about data interpretation and complicates identification of specific binding domains and vaccine targets. After the full-length extracellular region of VAR2CSA was expressed as recombinant protein, individual DBL domains or domain combinations were re-evaluated using the full-length protein as a bench mark for strength of binding. Full length VAR2CSA binds CSA at a nanomolar affinity [23,24]. Kinetic analysis showed that single domains DBL2, DBL3 and DBL6 bind CSA with affinity in the micromolar range [24,25]. In comparison, recombinant multi-domain DBL1–ID1–DBL2 fragment bound CSA with similar kinetics as the full length protein in one study [25], but did not bind CSA in another study [26]. Instead, the latter study found high affinity binding with DBL1–ID1–DBL2–ID2a domain combination. ID2a and ID2b comprise the so-called cysteine-rich inter-domain region (CIDR) of VAR2CSA, and the evidence suggested that ID2a or a portion of it is required for specific binding to CSA and not to other sulfated glycans [26]. For example, recombinant DBL1–ID1–DBL2–CIDR (ID2a–ID2b) binds to CSA and to heparan sulfate equally, while a recombinant protein truncated after the first 90–100 amino acids of ID2a regained specificity [26]. Interestingly, the binding of DBL1–ID1–DBL2 to decorin was stronger than to CSA [25], a difference that was not seen in binding of intact IE (unpublished data) and that may reflect differences between the native epitopes and the binding epitopes of the recombinant protein. In summary, it appears that the highest binding affinity to CSA was obtained with domain combinations from the N-terminal portion of VAR2CSA, but the specificity of binding is influenced by inclusion or exclusion of different CIDR (ID2a–ID2b) fragments.
3. Naturally acquired antibodies to VAR2CSA domains
Over successive pregnancies women develop specific immunity to the parasites that sequester in the placenta. Antibodies that block parasite adhesion to CSA have been associated with a reduced infection risk and increased newborn birthweight [8,10,11]. Low antibody reactivity with the IE surface was associated with lower hemoglobin level and reduced birthweight [9]. Antibodies that react to the IE surface by flow, or that opsonize or agglutinate CSA-binding IE, also develop in naturally exposed women [9,27–38], although the evidence base is smaller that these antibodies might be associated with clinical outcomes.
Because VAR2CSA is the leading vaccine target, several studies have compared VAR2CSA domains for reactivity to sera of primigravid and multigravid women in order to support vaccine design (Table 1). Tuikue Ndam et al. [39] reported that antibodies to domains DBL5 and DBL6 but not DBL1 measured during the 2nd trimester or at delivery correlated with parity in an area of Senegal with seasonal malaria transmission. In a recent study from Benin, Tuikue Ndam et al. [10] reported that antibody levels to DBL1–ID1–DBL2 and to DBL3 were higher among multigravid than primigravid women both during pregnancy and at the time of delivery, while antibody levels to DBL4 or DBL5 were only higher in multigravid women at delivery or during pregnancy, respectively. Babakhanyan et al. [40] reported that antibody levels to ID1–DBL2–ID2a did not differ between Cameroonian pregnant women and men. Similarly, Gnidehou et al. [41] reported that in Colombia, children, men and pregnant women exposed to malaria have similar antibody levels to ID1–DBL2–ID2a, DBL3 and DBL5 domains. Oleinikov et al. [42] reported significantly higher levels of antibodies to DBL1, DBL3 and DBL6 (but not DBL4 or DBL5) in multigravidae compared to primigravidae living in a malaria holoendemic area of Tanzania. Brolin et al. [33] found that antibodies to DBL5 were higher among multigravid compared to primigravid women, while antibody levels to DBL3 and DBL6 did not differ by gravidity. Several other studies reported parity-dependent serum reactivity to DBL5 domain [14,43,44]. In summary, parity-dependent acquisition of VAR2CSA-specific antibody appears to be a robust correlate of protective immunity, but in general it does not appear to discriminate well between different candidate VAR2CSA immunogens. Further, studies differ as to whether a particular domain specificity is elevated in multigravid women, possibly due to differences in recombinant antigens, in assay format such as ELISA versus Luminex platform, or in study populations.
Table 1.
Studies of acquired antibody to VAR2CSA DBL domains in African women, indicating the relationship between antibody levels and maternal parity. Timing of sample collection during pregnancy or delivery is indicated.
| DBL domain | Significant increase with parity | No difference with parity |
|---|---|---|
| DBL1 | Oleinikov [42] (Delivery) | Tuikue Ndam [39] (Enrollmenta & Delivery) |
| DBL1–DBL2 | Tuikue Ndam [10] (Enrollmenta & Delivery) |
|
| ID1–ID2a | Babakhanyanb [40] | |
| DBL3 | Oleinikov [42] (Delivery) Tuikue Ndam [10] (Enrollmenta & Delivery) |
Brolin [33] (3rd trimester) |
| DBL4 | Tuikue Ndam [10] (Delivery) | Oleinikov [42] (Delivery) Tuikue Ndam [10] (Enrollmenta) |
| DBL5 | Brolin [33] (3rd trimester) Gangnard [44] (During pregnancy)c Gnidehou [43] (2nd and 3rd trimester) Salanti [14] (Delivery) Tuikue Ndam [39] (Enrollmenta & Delivery) Tuikue Ndam [10] (Enrollmenta) |
Oleinikov [42] (Delivery) Tuikue Ndam [10] (Delivery) |
| DBL6 | Oleinikov [42] (Delivery) Tuikue Ndam [39] (Enrollmenta & Delivery) |
Brolin [33] (3rd trimester) Tuikue Ndam [10] (Enrollmenta & Delivery) |
Samples collected at enrollment at any time during the first 6 months of gestation.
Samples collected during the 1st, 2nd and 3rd trimester.
Samples collected during pregnancy, but timing is not specified.
More data are emerging on the relationships between antibodies to DBL domains and birthweight or maternal anemia. Tutterrow et al. [45] examined antibody levels to DBL1, DBL1–ID1–DBL2, DBL3, DBL4, DBL5, and DBL6 of 3 alleles over the course of pregnancy in Cameroon, and concluded that the number of domains and allelic types recognized by naturally acquired antibodies during second trimester predicted a reduction in placental infection status at delivery. Among Kenyan women with acute or chronic malaria infection, higher antibody levels to DBL5 were associated with reduced risk of low birthweight delivery and with increased birthweight [14]. In Mozambique, antibody levels to VAR2CSA domains, to merozoite antigens (EBA-175 and MSP1), and to IE surface were lower among uninfected pregnant women compared to pregnant women with acute, chronic or past infection. Among women with at least one malaria infection during their pregnancy, high antibody levels to VAR2CSA domains and other proteins not unique to placental parasites like AMA-1, positively correlated with birthweight and gestational age [46]. In a recent study in Benin, high antibody levels to the full recombinant VAR2CSA and DBL3 domain at enrollment (before gestational week 24) were associated with a reduction in the risk of infection in the period between enrollment and delivery; reduced placental malaria risk was associated with higher antibody levels to DBL3, although a similar trend was also observed with an unrelated VSA [10], and high antibody levels to DBL1–ID1–DBL2 were associated with a reduction in low birthweight deliveries [10].
4. VAR2CSA immunization of animals to elicit anti-adhesion antibodies
VAR2CSA is a large molecular weight protein that cannot be commercially manufactured as a full-length recombinant molecule with existing technologies. Identifying smaller fragments that elicit functional antibodies similar to those induced by natural infection has probably been the greatest challenge for VAR2CSA vaccine development. Multiple studies have examined functional activity of antibodies raised in animals to DBL domains of VAR2CSA, as an approach to identify the optimal VAR2CSA-based immunogen(s) for inducing cross-reactive and/or functional antibodies. An important caveat for these studies is that different groups use different assay formats to measure anti-adhesion activity of antibody, and therefore it is not possible to compare activity measurements between studies.
Multi-domain fragments NTS–DBL1–ID1–DBL2, DBL3–DBL4 and DBL5–DBL6 of FCR3 allele induced antibodies that reacted with the surface of homologous parasites at higher levels than did antibodies induced by the six single DBL domains of FCR3 allele [47]. However, antibodies to the single DBL4 domain almost completely inhibited the binding of homologous and 2 heterologous IE to CSA [47,48] while antibodies to other single or multi-domain immunogens only reduced homologous parasite binding to CSA by 50–60% [47]. Anti-adhesion antibodies induced by immunization with DBL4 domain of FCR3 allele were species-dependent: anti-adhesion activity was observed in samples collected from immunized rats but not from rabbits [48]. Antibodies raised to all DBL domains of 3D7 allele type reduced the adhesion of heterologous FCR3 IE by 50–65% compared to control, with the exception of antibodies to DBL1 that almost completely inhibited FCR3 binding to CSA [49]. Antibodies to DBL4 of 3D7 allele as well as that of a placental parasite sample reduced the binding of heterologous maternal parasites by more than 50% [50]. Of the DBL domains of HB3 allele, only DBL3 induced anti-adhesion antibodies that almost completely inhibited the binding of FCR3 IE to CSA [49]. Other studies reported that recombinant DBL5 domain and the multi-domain DBL5–DBL6 of several allelic forms inhibited the binding of FCR3 IE by more than 60% [51] and the binding of culture-adapted isolates from pregnant women [50]. The N-terminal region of VAR2CSA, NTS–DBL1–ID1–DBL2, elicited antibodies that almost completely inhibited the binding of homologous FCR3 and heterologous HB3 IE [52], consistent with an earlier study reporting anti-adhesion activity by antibodies raised against multidomain immunogens including NTS–DBL1–ID1–DBL2 [47]. Smaller fragments of the N-terminal region of VAR2CSA like ID1–DBL1–ID2a and ID1–DBL2–ID2a–ID2b also elicited strong homologous anti-adhesion antibodies [26].
Antibody anti-adhesion activity against IE collected from pregnant women has also been induced by the FCR3 allelic forms of DBL4 and of N-terminal multi-domain fragments of VAR2CSA. DBL4 domain of FCR3 inhibited 5/13 isolates from binding to CSA by >50% [53]. An extended form of DBL4 with an additional C-terminal 40 amino acids had broader anti-adhesion activity, inhibiting 10/13 maternal isolates by >50% [53]. In separate studies, antibodies raised in mice and rats to DBL4 failed to inhibit the binding of maternal isolates [52,54]. A recent analysis of the cross reactivity of this form showed minimal activity against heterologous isolates selected for CSA binding [55]. DNA vaccination with NTS–DBL1–ID1–DBL2 elicited antibodies that blocked the binding of the majority of the isolates to CSA by >50% (FCR3 allele: 12/15; 3D7 allele: 13/18; FCR3 allele: 11/18) [52,56]. The smaller fragment ID1–DBL2 reduced the binding to CSA of 6/8 maternal isolates, of which the reduction was significant for 2 isolates [57].
Notably antibodies to the full length VAR2CSA inhibited the binding of homologous parasite isolate [23,58] but not heterologous parasites [56,58]. Previously we showed that plasma from multigravid women inhibited the binding of maternal isolates in a strain independent manner [11]. Thus, it is not clear whether immunization with multiple allelic types is required to induce broadly reactive antibodies, or whether animal immunization with recombinant protein induces a response to different and possibly more variant epitopes than does natural infection, resulting in a strain-dependent response.
5. Epitope mapping
Immune multigravid women develop strain-independent functional antibodies against placental parasites, and these antibodies are associated with clinical protection. Identifying epitopes targeted by protective antibodies can facilitate development of a broadly reactive vaccine to prevent pregnancy malaria. Several approaches have been applied to identify protective epitopes, including screening of peptide libraries, producing nanobody, and developing monoclonal antibodies from B cells of immune women.
For screening peptide libraries, Pepscan analysis was performed on a peptide array corresponding to the extracellular portion of VAR2CSA [59]. Pooled plasma from immune women recognized multiple peptides across all VAR2CSA domains, except for a small reduction in reactivity with peptides corresponding to DBL2 domain; most reactivity was not reduced after depleting the plasma on native VAR2CSA protein [59].
Antibodies to the DBL4 domain raised in rats had adhesion blocking activity against homologous and heterologous parasites [47,53]. Using a DBL4 peptide array, the reactivity of anti-DBL4 antisera to 3 regions within DBL4 was associated with adhesion blocking activity [60]. This region was also recognized by sera from 4/6 immune women, with reactivity directed to a conserved sequence in DBL4 [60]. However, antibodies raised to these peptides neither reacted with VAR2CSA expressed on the IE surface nor inhibited parasite adhesion to CSA. One possible explanation is that the linear epitopes did not mimic the conformational epitopes required to induce functional antibodies [60]. Recombinant DBL4 mutated in the region corresponding to the peptides recognized by native sera induced antibodies with lower adhesion blocking activity than the wild type form. The loss of potency may have resulted from a change in a specific B-cell epitope, or alternatively from a change in the overall protein folding resulting in a less immunogenic protein [60].
Nanobody technology has been used as another approach to identify vaccine targets in VAR2CSA. This technology is based on camelid heavy-chain only antibody in which the variable heavy chain (VHH) alone comprises the antigen binding site. Recombinant forms of the VHH are called nanobodies. Using this approach, nanobodies that reacted with the recombinant DBL domains ID1–DBL1–ID2a, DBL4, DBL5, and DBL6 [61] and DBL1, DBL4, DBL5, and DBL6 [62] were produced. Several nanobodies to DBL1 and ID1–ID2a reduced parasite adhesion to CSA while nanobodies to DBL4, DBL5 and DBL6 had no inhibitory activity [61,62]. In one study, the majority of nanobodies reacted to DBL1 domain [62], while the other study did not identify any nanobodies to this domain [61], making it difficult to determine the utility of this information for designing a vaccine.
Finally, B cells from immune African women were immortalized as a source of specific reagents, and using this approach 8 monoclonal antibodies that reacted with the surface of CSA-selected isolates were identified [63]. Of those, 3 monoclonal antibodies reacted with recombinant DBL3 domain and 4 with DBL5 domain of VAR2CSA; the 8th monoclonal antibody named PAM1.4 did not react with recombinant DBL domains and reacted poorly with full length VAR2CSA [63,64]. Monoclonal antibody PAM1.4 recognized the surface of placental parasites and inhibited their adhesion to CSA, while it only moderately inhibited the adhesion to CSA of the laboratory isolate FCR3-CSA [64]. These results raised an important issue that maternal parasites may express additional proteins mediating adhesion to CSA that are targeted by natural immunity and are not expressed in model laboratory isolates selected for CSA binding phenotype.
Taken together, these different approaches to epitope mapping have provided insights into domains or epitopes that react to functional antibody. However, these studies have not definitively identified a single VAR2CSA fragment that is the ideal target for a vaccine.
6. Invariant proteins expressed by placental parasites
In addition to VAR2CSA, several conserved proteins expressed by placental parasites have been identified in transcriptomic and proteomics analysis of placental parasites. While gene knockout studies have demonstrated that VAR2CSA is required to maintain the CSA-binding phenotype [65], other genes and proteins are upregulated in placental parasites and therefore might contribute to the binding phenotype or to placental malaria pathogenesis as well. Comparative transcriptome analyses identified 11 genes that were upregulated in placental parasites compared to children’s parasites [66] and 38 genes in placental parasites compared to the reference parasite line 3D7 [67]. Two comparative proteomics analyses of placental parasites identified 17 and 53 conserved proteins respectively that were expressed exclusively or at higher levels in placental parasites than in parasites collected from children [68,69]. Because these novel genes and proteins are upregulated in VAR2CSA-expressing parasites, studies should be undertake to determine whether deletion of VAR2CSA may influence their expression or localization.
Two conserved proteins PFB0115w and PFI1785w were common between three (PFB0115w) or four (PFI1785w) comparative studies. PFB0115w is expressed at higher levels in placental parasites compared to children’s isolates, while PFI1785w is exclusively expressed by placental parasite. PFI1785w is a member of the Plasmodium helical interspersed subtelomeric family, and is annotated as an exported protein. Antibodies to a recombinant form of PFI1785w reacted with membrane protein extracted from placental parasites but not with membrane proteins extracted from children’s isolates or FCR3 parasites selected to bind CSA [68]. Antibody levels to recombinant PFI1785w are significantly higher in plasma from pregnant women than men [67]. Antibody to recombinant PFI1785w expressed in Escherichia coli failed to react with the surface of live IE, suggesting that the antibody recognizes non-conformational epitopes [68]. Further studies with protein expressed in a eukaryotic system that better reflects native structure could be valuable for studies of localization and adhesion to CSA.
Of 5 conserved genes that were upregulated in transcriptomic studies of placental parasites [66], one of the proteins named PfCSAL binds to CSA and co-localizes with VAR2CSA on the surface of maternal IE (Keitany, Duffy, et al., unpublished data). This protein is currently being evaluated as an additional placental malaria vaccine candidate.
7. Summary
The rational design of a vaccine to prevent malaria during pregnancy is based on the observations that a distinct form of the parasite that infects the placenta is associated with disease and death in mothers and newborns, and that women become resistant to pregnancy malaria as they develop antibodies against proteins expressed on the surface of this parasite form. The identification of a single human monoclonal antibody (PAM1.4) from immune multigravid women that recapitulates anti-adhesion activity in plasma from multigravidae [64], together with the observation that plasma from multigravidae have strain-transcending functional activity [11], strongly suggest that a limited number of epitopes can be targeted by an effective PM vaccine.
Currently, VAR2CSA is the leading pregnancy malaria vaccine candidate. Different research approaches have been taken to identify the targets that should be included in a VAR2CSA-based vaccine, including mapping the CSA-binding sites, mapping antibody epitopes, defining DBL domains preferentially recognized by immune sera, and assessing functional activity by antibodies raised against recombinant DBL domains. While multiple VAR2CSA domains bind CSA in in vitro assays, an N-terminal fragment (ID1–DBL2–ID2a–ID2b) binds CSA with similar kinetics as the full length protein [26]. Antibody levels to N-terminal fragments of VAR2CSA are similar between men, primigravid and multigravid women in Africa [40]. However, antibodies induced in animals to the NTS–DBL1–ID1–DBL2–ID2a multi-domain have heterologous functional activity, and the serum activity induced by this recombinant form has been broader than that induced by the smaller ID1–DBL2 fragment [52,56].
In addition to VAR2CSA, several conserved proteins expressed by placental parasites have been identified [66–69]. These conserved proteins may play an independent role in IE adhesion, or may interact with VAR2CSA to form conformational epitopes targeted by naturally acquired immunity. One example is the invariant protein PfCSAL that interacts with VAR2CSA (Keitany, Duffy, et al., unpublished data). Further studies of PFI1785w, an exported protein exclusively expressed by placental parasites, are required to determine whether it also has a role in inducing protective immunity.
The development of a placental malaria vaccine is based on naturally occurring immunity. Based on this model, the vaccine is predicted to induce antibodies in nulligravidae that will be boosted by malaria exposures during subsequent pregnancies, and similarly should boost functional antibodies that have been acquired by multigravidae in endemic areas. The first field trials will soon begin to assess whether individual VAR2CSA immunogens may be sufficient for inducing functional antibodies that have been associated with protection from placental malaria, or whether additional components may be required for an effective vaccine that protects pregnant women.
Acknowledgments
The authors are supported by the Intramural Research Program, NIAID, NIH.
Footnotes
Open Access provided for this article by the PATH Malaria Vaccine Initiative and ExxonMobil Foundation.
References
- 1.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 2.Fried M, Muga RO, Misore AO, Duffy PE. Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J Immunol. 1998;160:2523–30. [PubMed] [Google Scholar]
- 3.Rogerson SJ, Brown HC, Pollina E, Abrams ET, Tadesse E, Lema VM, et al. Placental tumor necrosis factor alpha but not gamma interferon is associated with placental malaria and low birth weight in Malawian women. Infect Immun. 2003;71:267–70. doi: 10.1128/IAI.71.1.267-270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kabyemela ER, Fried M, Kurtis JD, Mutabingwa TK, Duffy PE. Fetal responses during placental malaria modify the risk of low birth weight. Infect Immun. 2008;76:1527–34. doi: 10.1128/IAI.00964-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ordi J, Ismail MR, Ventura PJ, Kahigwa E, Hirt R, Cardesa A, et al. Massive chronic intervillositis of the placenta associated with malaria infection. Am J Surg Pathol. 1998;22:1006–11. doi: 10.1097/00000478-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Dong S, Kurtis JD, Pond-Tor S, Kabyemela E, Duffy PE, Fried M. CXC ligand 9 response to malaria during pregnancy is associated with low-birth-weight deliveries. Infect Immun. 2012;80:3034–8. doi: 10.1128/IAI.00220-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–4. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 8.Duffy PE, Fried M. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect Immun. 2003;71:6620–3. doi: 10.1128/IAI.71.11.6620-6623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staalsoe T, Shulman CE, Bulmer JN, Kawuondo K, Marsh K, Hviid L. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet. 2004;363:283–9. doi: 10.1016/S0140-6736(03)15386-X. [DOI] [PubMed] [Google Scholar]
- 10.Tuikue Ndam N, Denoeud-Ndam L, Doritchamou J, Viwami F, Salanti A, Nielsen MA, et al. Protective antibodies against placental malaria and poor outcomes during pregnancy, Benin. Emerg Infect Dis. 2015;21:813–23. doi: 10.3201/eid2105.141626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. Maternal antibodies block malaria. Nature. 1998;395:851–2. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- 12.Nosten F, McGready R, Simpson JA, Thwai KL, Balkan S, Cho T, et al. Effects of Plasmodium vivax malaria in pregnancy. Lancet. 1999;354:546–9. doi: 10.1016/s0140-6736(98)09247-2. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed R, Singh N, ter Kuile FO, Bharti PK, Singh PP, Desai M, et al. Placental infections with histologically confirmed Plasmodium falciparum are associated with adverse birth outcomes in India: a cross-sectional study. Malar J. 2014;13:232. doi: 10.1186/1475-2875-13-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salanti A, Dahlback M, Turner L, Nielsen MA, Barfod L, Magistrado P, et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J Exp Med. 2004;200:1197–203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuikue Ndam NG, Salanti A, Bertin G, Dahlback M, Fievet N, Turner L, et al. High level of var2csa transcription by Plasmodium falciparum isolated from the placenta. J Infect Dis. 2005;192:331–5. doi: 10.1086/430933. [DOI] [PubMed] [Google Scholar]
- 16.Gamain B, Trimnell AR, Scheidig C, Scherf A, Miller LH, Smith JD. Identification of multiple chondroitin sulfate A (CSA)-binding domains in the var2CSA gene transcribed in CSA-binding parasites. J Infect Dis. 2005;191:1010–3. doi: 10.1086/428137. [DOI] [PubMed] [Google Scholar]
- 17.Avril M, Gamain B, Lepolard C, Viaud N, Scherf A, Gysin J. Characterization of anti-var2CSA-PfEMP1 cytoadhesion inhibitory mouse monoclonal antibodies. Microbes Infect. 2006;8:2863–71. doi: 10.1016/j.micinf.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Dahlback M, Rask TS, Andersen PH, Nielsen MA, Ndam NT, Resende M, et al. Epitope mapping and topographic analysis of VAR2CSA DBL3X involved in P. falciparum placental sequestration. PLoS Pathog. 2006;2:e124. doi: 10.1371/journal.ppat.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh K, Gittis AG, Nguyen P, Gowda DC, Miller LH, Garboczi DN. Structure of the DBL3x domain of pregnancy-associated malaria protein VAR2CSA complexed with chondroitin sulfate A. Nat Struct Mol Biol. 2008;15:932–8. doi: 10.1038/nsmb.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins MK. Overproduction, purification and crystallization of a chondroitin sulfate A-binding DBL domain from a Plasmodium falciparum var2csa-encoded PfEMP1 protein. Acta Crystallogr Sect F: Struct Biol Cryst Commun. 2008;64:221–3. doi: 10.1107/S1744309108004211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Resende M, Nielsen MA, Dahlback M, Ditlev SB, Andersen P, Sander AF, et al. Identification of glycosaminoglycan binding regions in the Plasmodium falciparum encoded placental sequestration ligand, VAR2CSA. Malar J. 2008;7:104. doi: 10.1186/1475-2875-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Resende M, Ditlev SB, Nielsen MA, Bodevin S, Bruun S, Pinto VV, et al. Chondroitin sulphate A (CSA)-binding of single recombinant Duffy-binding-like domains is not restricted to Plasmodium falciparum Erythrocyte Membrane Protein 1 expressed by CSA-binding parasites. Int J Parasitol. 2009;39:1195–204. doi: 10.1016/j.ijpara.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Khunrae P, Dahlback M, Nielsen MA, Andersen G, Ditlev SB, Resende M, et al. Full-length recombinant Plasmodium falciparum VAR2CSA binds specifically to CSPG and induces potent parasite adhesion-blocking antibodies. J Mol Biol. 2010;397:826–34. doi: 10.1016/j.jmb.2010.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srivastava A, Gangnard S, Round A, Dechavanne S, Juillerat A, Raynal B, et al. Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA. Proc Natl Acad Sci USA. 2010;107:4884–9. doi: 10.1073/pnas.1000951107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srivastava A, Gangnard S, Dechavanne S, Amirat F, Lewit Bentley A, Bentley GA, et al. Var2CSA minimal CSA binding region is located within the N-terminal region. PLoS ONE. 2011;6:e20270. doi: 10.1371/journal.pone.0020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clausen TM, Christoffersen S, Dahlback M, Langkilde AE, Jensen KE, Resende M, et al. Structural and functional insight into how the Plasmodium falciparum VAR2CSA protein mediates binding to chondroitin sulfate A in placental malaria. J Biol Chem. 2012;287:23332–45. doi: 10.1074/jbc.M112.348839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ricke CH, Staalsoe T, Koram K, Akanmori BD, Riley EM, Theander TG, et al. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J Immunol. 2000;165:3309–16. doi: 10.4049/jimmunol.165.6.3309. [DOI] [PubMed] [Google Scholar]
- 28.Staalsoe T, Megnekou R, Fievet N, Ricke CH, Zornig HD, Leke R, et al. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum-infected erythrocytes that protect against placental parasitemia. J Infect Dis. 2001;184:618–26. doi: 10.1086/322809. [DOI] [PubMed] [Google Scholar]
- 29.Megnekou R, Staalsoe T, Taylor DW, Leke R, Hviid L. Effects of pregnancy and intensity of Plasmodium falciparum transmission on immunoglobulin G subclass responses to variant surface antigens. Infect Immun. 2005;73:4112–8. doi: 10.1128/IAI.73.7.4112-4118.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fievet N, Le Hesran JY, Cottrell G, Doucoure S, Diouf I, Ndiaye JL, et al. Acquisition of antibodies to variant antigens on the surface of Plasmodium falciparum-infected erythrocytes during pregnancy. Infect Genet Evol. 2006;6:459–63. doi: 10.1016/j.meegid.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Feng G, Aitken E, Yosaatmadja F, Kalilani L, Meshnick SR, Jaworowski A, et al. Antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes are associated with protection from treatment failure and the development of anemia in pregnancy. J Infect Dis. 2009;200:299–306. doi: 10.1086/599841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aitken EH, Mbewe B, Luntamo M, Maleta K, Kulmala T, Friso MJ, et al. Antibodies to chondroitin sulfate A-binding infected erythrocytes: dynamics and protection during pregnancy in women receiving intermittent preventive treatment. J Infect Dis. 2010;201:1316–25. doi: 10.1086/651578. [DOI] [PubMed] [Google Scholar]
- 33.Brolin KJ, Persson KE, Wahlgren M, Rogerson SJ, Chen Q. Differential recognition of P. falciparum VAR2CSA domains by naturally acquired antibodies in pregnant women from a malaria endemic area. PLoS ONE. 2010;5:e9230. doi: 10.1371/journal.pone.0009230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayor A, Rovira-Vallbona E, Machevo S, Bassat Q, Aguilar R, Quinto L, et al. Parity and placental infection affect antibody responses against Plasmodium falciparum during pregnancy. Infect Immun. 2011;79:1654–9. doi: 10.1128/IAI.01000-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaworowski A, Fernandes LA, Yosaatmadja F, Feng G, Mwapasa V, Molyneux ME, et al. Relationship between human immunodeficiency virus type 1 coinfection, anemia, and levels and function of antibodies to variant surface antigens in pregnancy-associated malaria. Clin Vaccine Immunol. 2009;16:312–9. doi: 10.1128/CVI.00356-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beeson JG, Brown GV, Molyneux ME, Mhango C, Dzinjalamala F, Rogerson SJ. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. J Infect Dis. 1999;180:464–72. doi: 10.1086/314899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maubert B, Fievet N, Tami G, Cot M, Boudin C, Deloron P. Development of antibodies against chondroitin sulfate A-adherent Plasmodium falciparum in pregnant women. Infect Immun. 1999;67:5367–71. doi: 10.1128/iai.67.10.5367-5371.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keen J, Serghides L, Ayi K, Patel SN, Ayisi J, van Eijk A, et al. HIV impairs opsonic phagocytic clearance of pregnancy-associated malaria parasites. PLoS Med. 2007;4:e181. doi: 10.1371/journal.pmed.0040181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuikue Ndam NG, Salanti A, Le-Hesran JY, Cottrell G, Fievet N, Turner L, et al. Dynamics of anti-VAR2CSA immunoglobulin G response in a cohort of senegalese pregnant women. J Infect Dis. 2006;193:713–20. doi: 10.1086/500146. [DOI] [PubMed] [Google Scholar]
- 40.Babakhanyan A, Leke RG, Salanti A, Bobbili N, Gwanmesia P, Leke RJ, et al. The antibody response of pregnant Cameroonian women to VAR2CSA ID1–ID2a, a small recombinant protein containing the CSA-binding site. PLoS ONE. 2014;9:e88173. doi: 10.1371/journal.pone.0088173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gnidehou S, Doritchamou J, Arango EM, Cabrera A, Arroyo MI, Kain KC, et al. Functional antibodies against VAR2CSA in nonpregnant populations from colombia exposed to Plasmodium falciparum and Plasmodium vivax. Infect Immun. 2014;82:2565–73. doi: 10.1128/IAI.01594-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oleinikov AV, Rossnagle E, Francis S, Mutabingwa TK, Fried M, Duffy PE. Effects of sex, parity, and sequence variation on seroreactivity to candidate pregnancy malaria vaccine antigens. J Infect Dis. 2007;196:155–64. doi: 10.1086/518513. [DOI] [PubMed] [Google Scholar]
- 43.Gnidehou S, Jessen L, Gangnard S, Ermont C, Triqui C, Quiviger M, et al. Insight into antigenic diversity of VAR2CSA-DBL5epsilon domain from multiple Plasmodium falciparum placental isolates. PLoS ONE. 2010;5:e13105. doi: 10.1371/journal.pone.0013105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gangnard S, Tuikue Ndam NG, Gnidehou S, Quiviger M, Juillerat A, Faure G, et al. Functional and immunological characterization of the var2CSA-DBL5epsilon domain of a placental Plasmodium falciparum isolate. Mol Biochem Parasitol. 2010;173:115–22. doi: 10.1016/j.molbiopara.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Tutterrow YL, Avril M, Singh K, Long CA, Leke RJ, Sama G, et al. High levels of antibodies to multiple domains and strains of VAR2CSA correlate with the absence of placental malaria in Cameroonian women living in an area of high Plasmodium falciparum transmission. Infect Immun. 2012;80:1479–90. doi: 10.1128/IAI.00071-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayor A, Kumar U, Bardaji A, Gupta P, Jimenez A, Hamad A, et al. Improved pregnancy outcomes in women exposed to malaria with high antibody levels against Plasmodium falciparum. J Infect Dis. 2013;207:1664–74. doi: 10.1093/infdis/jit083. [DOI] [PubMed] [Google Scholar]
- 47.Nielsen MA, Pinto VV, Resende M, Dahlback M, Ditlev SB, Theander TG, et al. Induction of adhesion-inhibitory antibodies against placental Plasmodium falciparum parasites by using single domains of VAR2CSA. Infect Immun. 2009;77:2482–7. doi: 10.1128/IAI.00159-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinto VV, Ditlev SB, Jensen KE, Resende M, Dahlback M, Andersen G, et al. Differential induction of functional IgG using the Plasmodium falciparum placental malaria vaccine candidate VAR2CSA. PLoS ONE. 2011;6:e17942. doi: 10.1371/journal.pone.0017942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salanti A, Resende M, Ditlev SB, Pinto VV, Dahlback M, Andersen G, et al. Several domains from VAR2CSA can induce Plasmodium falciparum adhesion-blocking antibodies. Malar J. 2010;9:11. doi: 10.1186/1475-2875-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saveria T, Oleinikov AV, Wiliamson K, Chaturvedi R, Lograsso J, Keitany GJ, et al. Antibodies to Escherichia coli-expressed C-terminal domains of Plasmodium falciparum variant surface antigen 2-chondroitin sulfate A (VAR2CSA) inhibit binding of CSA-adherent parasites to placental tissue. Infect Immun. 2013;81:1031–9. doi: 10.1128/IAI.00978-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fernandez P, Petres S, Mecheri S, Gysin J, Scherf A. Strain-transcendent immune response to recombinant Var2CSA DBL5-epsilon domain block P. falciparum adhesion to placenta-derived BeWo cells under flow conditions. PLoS ONE. 2010;5:e12558. doi: 10.1371/journal.pone.0012558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bigey P, Gnidehou S, Doritchamou J, Quiviger M, Viwami F, Couturier A, et al. The NTS-DBL2X region of VAR2CSA induces cross-reactive antibodies that inhibit adhesion of several Plasmodium falciparum isolates to chondroitin sulfate A. J Infect Dis. 2011;204:1125–33. doi: 10.1093/infdis/jir499. [DOI] [PubMed] [Google Scholar]
- 53.Magistrado PA, Minja D, Doritchamou J, Ndam NT, John D, Schmiegelow C, et al. High efficacy of anti DBL4varepsilon-VAR2CSA antibodies in inhibition of CSA-binding Plasmodium falciparum-infected erythrocytes from pregnant women. Vaccine. 2011;29:437–43. doi: 10.1016/j.vaccine.2010.10.080. [DOI] [PubMed] [Google Scholar]
- 54.Fried M, Avril M, Chaturvedi R, Fernandez P, Lograsso J, Narum D, et al. Multilaboratory approach to preclinical evaluation of vaccine immunogens for placental malaria. Infect Immun. 2013;81:487–95. doi: 10.1128/IAI.01106-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nielsen MA, Resende M, de Jongh WA, Ditlev SB, Mordmuller B, Houard S, et al. The influence of sub-unit composition and expression system on the functional antibody response in the development of a VAR2CSA based Plasmodium falciparum placental malaria vaccine. PLoS ONE. 2015;10:e0135406. doi: 10.1371/journal.pone.0135406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doritchamou J, Bigey P, Nielsen MA, Gnidehou S, Ezinmegnon S, Burgain A, et al. Differential adhesion-inhibitory patterns of antibodies raised against two major variants of the NTS-DBL2X region of VAR2CSA. Vaccine. 2013;31:4516–22. doi: 10.1016/j.vaccine.2013.07.072. [DOI] [PubMed] [Google Scholar]
- 57.Bordbar B, Tuikue Ndam N, Bigey P, Doritchamou J, Scherman D, Deloron P. Identification of Id1-DBL2X of VAR2CSA as a key domain inducing highly inhibitory and cross-reactive antibodies. Vaccine. 2012;30:1343–8. doi: 10.1016/j.vaccine.2011.12.065. [DOI] [PubMed] [Google Scholar]
- 58.Avril M, Hathaway MJ, Srivastava A, Dechavanne S, Hommel M, Beeson JG, et al. Antibodies to a full-length VAR2CSA immunogen are broadly strain-transcendent but do not cross-inhibit different placental-type parasite isolates. PLoS ONE. 2011;6:e16622. doi: 10.1371/journal.pone.0016622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andersen P, Nielsen MA, Resende M, Rask TS, Dahlback M, Theander T, et al. Structural insight into epitopes in the pregnancy-associated malaria protein VAR2CSA. PLoS Pathog. 2008;4:e42. doi: 10.1371/journal.ppat.0040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ditlev SB, Nielsen MA, Resende M, Agerbaek MO, Pinto VV, Andersen PH, et al. Identification and characterization of B-cell epitopes in the DBL4epsilon domain of VAR2CSA. PLoS ONE. 2012;7:e43663. doi: 10.1371/journal.pone.0043663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ditlev SB, Florea R, Nielsen MA, Theander TG, Magez S, Boeuf P, et al. Utilizing nanobody technology to target non-immunodominant domains of VAR2CSA. PLoS ONE. 2014;9:e84981. doi: 10.1371/journal.pone.0084981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nunes-Silva S, Gangnard S, Vidal M, Vuchelen A, Dechavanne S, Chan S, et al. Llama immunization with full-length VAR2CSA generates cross-reactive and inhibitory single-domain antibodies against the DBL1X domain. Sci Rep. 2014;4:7373. doi: 10.1038/srep07373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barfod L, Bernasconi NL, Dahlback M, Jarrossay D, Andersen PH, Salanti A, et al. Human pregnancy-associated malaria-specific B cells target polymorphic, conformational epitopes in VAR2CSA. Mol Microbiol. 2007;63:335–47. doi: 10.1111/j.1365-2958.2006.05503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barfod L, Dobrilovic T, Magistrado P, Khunrae P, Viwami F, Bruun J, et al. Chondroitin sulfate A-adhering Plasmodium falciparum-infected erythrocytes express functionally important antibody epitopes shared by multiple variants. J Immunol. 2010;185:7553–61. doi: 10.4049/jimmunol.1002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Viebig NK, Gamain B, Scheidig C, Lepolard C, Przyborski J, Lanzer M, et al. A single member of the Plasmodium falciparum var multigene family determines cytoadhesion to the placental receptor chondroitin sulphate A. EMBO Rep. 2005;6:775–81. doi: 10.1038/sj.embor.7400466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Francis SE, Malkov VA, Oleinikov AV, Rossnagle E, Wendler JP, Mutabingwa TK, et al. Six genes are preferentially transcribed by the circulating and sequestered forms of Plasmodium falciparum parasites that infect pregnant women. Infect Immun. 2007;75:4838–50. doi: 10.1128/IAI.00635-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tuikue Ndam N, Bischoff E, Proux C, Lavstsen T, Salanti A, Guitard J, et al. Plasmodium falciparum transcriptome analysis reveals pregnancy malaria associated gene expression. PLoS ONE. 2008;3:e1855. doi: 10.1371/journal.pone.0001855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fried M, Hixson KK, Anderson L, Ogata Y, Mutabingwa TK, Duffy PE. The distinct proteome of placental malaria parasites. Mol Biochem Parasitol. 2007;155:57–65. doi: 10.1016/j.molbiopara.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 69.Bertin GI, Sabbagh A, Guillonneau F, Jafari-Guemouri S, Ezinmegnon S, Federici C, et al. Differential protein expression profiles between Plasmodium falciparum parasites isolated from subjects presenting with pregnancy-associated malaria and uncomplicated malaria in Benin. J Infect Dis. 2013;208:1987–97. doi: 10.1093/infdis/jit377. [DOI] [PubMed] [Google Scholar]


