Abstract
Purpose
Local radiation therapy (RT) in combination with systemic anti-CTLA-4 immunotherapy may enhance induction of systemic anti-melanoma immune responses. The primary objective of this trial was to assess the safety and efficacy of combining ipilimumab with RT in patients with stage IV melanoma. Secondary objectives included laboratory assessment of induction of anti-melanoma immune responses.
Materials/Methods
In our prospective clinical trial, 22 patients with stage IV melanoma were treated with palliative RT and ipilimumab for 4 cycles. RT to 1-2 disease sites was initiated within 5 days after starting ipilimumab. Patients had ≥1 nonirradiated metastasis measuring 7≥1.5 cm for response assessment. Tumor imaging studies were obtained at baseline, 2-4 weeks following cycle 4 of ipilimumab, and every 3 months until progression. Laboratory immune response parameters were measured before and during treatment.
Results
Combination therapy was well-tolerated without unexpected toxicities. Eleven patients (50.0%) had clinical benefit from therapy, including complete and partial responses (CR, PR) and stable disease (SD) at median follow-up of 55 weeks. Three (27.3%) achieved an ongoing systemic CR at median follow-up of 55 weeks (range 32-65), and 3 (27.3%) had initial PR for a median of 40 weeks. Analysis of immune response data suggests a relationship between elevated CD8-activated T-cells and response.
Conclusion
This is the second prospective clinical trial of treatment of metastatic melanoma with the combination of RT and systemic immunotherapy and the first using this sequence of therapy. Results from this trial demonstrate that a subset of patients can benefit from combination therapy, arguing for continued clinical investigation into the use of radiation therapy in combination with immunotherapy including PD-1 inhibitors, which may have the potential to be even more effective in combination with radiation.
INTRODUCTION
Melanoma is a relatively immunogenic malignancy with well-defined tumor antigens [1] [2], and infiltration of melanoma lesions by T-lymphocytes has been associated with a better clinical prognosis [3]. Recent studies of immunotherapy in the treatment of patients with metastatic melanoma have shown promise, with improved outcomes as compared with previous systemic approaches [4] [5] [6]. There is currently great interest in strategies aimed at modulation of the immune response in order to achieve an anti-tumor immune response. One early success in this area has been in the area of anti-cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) therapy. Ipilimumab is a monoclonal antibody which targets CTLA-4 and was the first immune checkpoint inhibitor to show improved overall survival in patients with advanced melanoma [4], although most patients do not respond, and responses are often incomplete. As such, efforts have been made to try to enhance treatment efficacy, including through the incorporation of targeted radiation therapy with systemic therapy as an in situ tumor vaccine strategy.
Several case reports describe abscopal responses in distant metastatic sites outside of the radiation therapy field when radiation is given in combination with immunotherapy [2] [7] [8]. A recent review of a single retrospective clinical study of 21 patients treated with sequential ipilimumab and radiation and 23 case reports describing a variety of abscopal responses, and 13 pre-clinical papers suggested synergy between radiotherapy and immune treatments [9]. The only prospective clinical trial reported to date is a recent phase I clinical trial performed at the University of Pennsylvania, which enrolled 22 patients with metastatic melanoma who were treated with hypofractionated radiation to a single metastatic lesion, followed by four cycles of ipilimumab. In this study, 18% of patients had a partial response as the best clinical response, 18% had stable disease, and 64% had progressive disease [10]. Pre- and post-treatment sera were examined in a subset of trial patients, with results suggesting that markers of T-cell reinvigoration may correlate with treatment response, but it remains unclear how to predict which patients are likely to respond to combination therapy, and how to detect early responders.
We performed a prospective clinical trial investigating the safety and efficacy of combining local radiation therapy (RT) with systemic anti-CTLA-4 immunotherapy in patients with metastatic melanoma, with the goal of enhancing the induction of systemic anti-melanoma immune responses. In our trial design, treatment was sequenced with delivery of immunotherapy prior to the start of radiation therapy, in order to have checkpoint blockade in effect at the time of irradiation, and to maximize the potential effect of combination therapy. The primary objective of this trial was to assess the safety and efficacy of combining ipilimumab with RT in patients with stage IV melanoma. Secondary objectives included assessment of induction of anti-melanoma immune responses using laboratory correlative studies.
METHODS
Eligibility Criteria
Patient and Treatment Characteristics
Follow Up
Patients were clinically evaluated every 3 weeks at the time of administration of ipilimumab. Follow-up diagnostic imaging occurred 2-4 weeks after the last dose of ipilimumab. Evaluation of treatment response was assessed by clinical exam and radiographic studies. Imaging modality, including computed tomography (CT), positron emission tomography (PET) and magnetic resonance (MR) imaging, was performed at the discretion of the treating physician at the time points specified by the protocol, with imaging every 12 weeks until taken off study for progression of disease or serious adverse event. Both Response Evaluation Criteria in Solid Tumors (RECIST) and Immune Response Criteria (IRC) were used to define response to treatment [11] [12]. By IRC criteria, new lesions do not necessarily define progressive disease but are incorporated into overall tumor burden. Furthermore, IRC criteria require multiple assessments at least 4 weeks apart in order to account for different kinetics of response to immunotherapy. For evaluation by RECIST, irradiated tumors were not included as part of measurable disease; for evaluation by IRC, irradiated tumors were included as part of measurable disease.
Laboratory Assays and Statistics
All assays were performed in the Human Immune Monitoring Center at XXXXX.
RESULTS
Clinical Outcomes
Of 22 evaluable patients, 11 (50.0%) achieved clinical benefit from therapy, including complete and partial responses (CR, PR) as well as stable disease (SD) at median follow-up of 55 weeks (Table 1). Three patients (27.3%, 95% CI 9.7-56.9%) achieved an ongoing systemic CR to the combination therapy with no evidence of disease at a median follow-up of 55 weeks (range 32-65 weeks). Three patients (27.3%, 95% CI 9.7-56.9%) had an initial PR following treatment without progression for a median of 40 weeks (range 29-53 weeks) and 5 additional patients (45.4%, 95% CI 20.2-65.6%) initially had SD following treatment without progression by RECIST and IRC for a median of 39 weeks (range 26-76 weeks). Median time to response among patients with CR/PR was 19 weeks (range 12-52 weeks). Nine had progressive disease by RECIST, and eight by IRC on the first post-treatment scan (Table 2). Two patients were taken off study early for adverse events and were not evaluable. Median progression-free survival for all 20 evaluable patients was 26 weeks (range 2-65 weeks, 95% CI 16.3-35.7 weeks). Median overall survival for all 22 patients was 55 weeks (range 8-141 weeks, 95% CI 39.2-70.8 weeks).
Table 1A.
Patient and Treatment Characteristics
| Patient Number |
Ge nd er |
A g e |
Sites of Metastatic Disease |
Prior Treatments |
Baseline Sum of Products of Diameters (SPD) of Unirradiated Lesions (cm2) |
Site Radiated |
RT Dose, Fractionation (BED10), and Technique |
Time Elapsed During RT (days) |
Local Respo nse (Irradia ted Lesion s) |
Symptom Palliation |
Cycl es of Ipili mu mab |
Best System ic Respon se by RECIST |
Tim e to Prog ress ion by REC IST |
Overall Survival |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 62 | Lungs, liver, intraventricular septum |
Nasal endoscopic resection, RT, interferon, PD-1 |
21.3 | Lung (RLL) |
45 Gy/15 fx (48.8 Gy) IMRT |
19 | PR | N/A | 2 | Interim PD |
6 wee ks |
9 weeks |
| 2 | M | 73 | Left parotid, duodenum, small bowel |
None | 12.1 | Left parotid |
37.5 Gy/15 fx (39.0 Gy) 3D |
23 | CR | Y | 4 | SD | 76 wee ks |
121 weeks |
| 3 | M | 57 | Right cervical lymph nodes, liver, lumbar spine, subcutaneous nodules |
Resection, IL-2 |
2.5 | Right neck |
30 Gy/10 fx (32.5 Gy) IMRT |
12 | PR | Y | 4 | Interim PD |
5 wee ks |
21 weeks |
| 4 | F | 56 | Left cervical lymph nodes, lungs, left upper quadrant mesentery |
Left parotidecto my and left MRND, RT, wedge resection, IL-2 |
12.7 | Lung (LUL) |
25 Gy/1 fx (72.9 Gy) SBRT |
1 | PR | N/A | 4 | SD | 36 wee ks |
141 weeks+ |
| 5 | M | 47 | Left cervical lymph nodes, lungs, liver, brain |
None | 6.9 | Liver (R lobe) |
30 Gy/5 fx (40.0 Gy) SBRT |
7 | CR | N/A | 4 | SD | 50 wee ks |
54 weeks |
| 6 | M | 70 | Liver, lungs, mediastinal and hilar lymph nodes |
Mohs surgery, left radical neck dissection, RT, interferon |
15.0 | Liver (R lobe) |
30 Gy/5 fx (40.0 Gy) SBRT |
7 | PR | N/A | 4 | SD | 29 wee ks |
51 weeks |
| 7 | M | 57 | Liver, mediastinum, supraclavicular, paraaortic, pelvic and inguinal lymph nodes |
Multiple resections, IL-2 |
34.1 | R iliac/ingui nal lymph nodes |
36 Gy/12 fx (39.0 Gy) 3D |
21 | SD | Y | 4 | PD | 12 wee ks |
85 weeks |
| 8 | M | 33 | Cervical, abdominal, pelvic, and inguinal lymph nodes; jejunum; gluteus medius; right breast |
Multiple resections, SRS |
70.0 | Gluteus medius; pelvic and inguinal lymph nodes |
37.5 Gy/15 fx (39.0 Gy) 3D |
22 | SD | Y | 4 | SD | 26 wee ks |
64 weeks |
| 9 | F | 78 | L1; L5; liver; adrenal nodules; retroperitoneal implants; left inguinal and right supraclavicular lymph nodes |
Multiple resections, intra- tumoral ipilimumab |
15.2 | L1; L5 | 30 Gy/10 fx (32.5 Gy) 3D |
14 | SD | N/A | 2 | Interim PD |
7 wee ks |
13 weeks |
| 10 | M | 41 | Right lung, soft tissue in RLQ, pancreas; bilateral adrenals; paratracheal and peri-rectal lymph nodes |
Surgical excision, pleurodesis |
15.8 | R lung and paratrach eal node |
39 Gy/13 fx (42.3 Gy) IMRT |
17 | PR | Y | 4 | PD | 14 wee ks |
84 weeks+ |
| 11 | F | 69 | Right gracilis; posterior popliteal; cervical and axillary lymph nodes; left breast and chest wall; hepatogastric; left adrenal, right kidney. |
Resection, ipilimumab |
37.2 | R gracilis and popliteal |
24 Gy/3 fx (36.0 Gy) SBRT |
7 | PR | Y | 2 | Interim PD |
2 wee ks |
16 weeks |
| 12 | F | 69 | Left lung, liver, left breast, left supraclavicular nodes |
Resection | 40.4 | L lung (LUL) |
45 Gy/15 fx (48.8 Gy) IMRT |
23 | CR | N/A | 4 | PR | 52 wee ks |
103 weeks+ |
| 13 | M | 18 | Right clavicle, left lung; bilateral flanks; right adrenal; brain |
Resection, MRND, interferon, IL-2 |
10.3 | R clavicle | 20 Gy/5 fx (23.3 Gy) 3D |
7 | SD | N | 3 | Interim PD |
9 wee ks |
55 weeks |
| 14 | M | 53 | Jejunum; right hilum; omentum; axillary and mesenteric lymph nodes; C7. T3. T9. L3-5 |
Resection, SRS, RT |
8.3 | Jejunum | 33 Gy/11 fx (35.8 Gy) 3D |
15 | PR | Y | 2 | NE - SAE |
8 weeks | |
| 15 | M | 83 | Left upper lobe of lung, left lingula, right middle lobe, occipital calvarium |
Resection, RT |
8 | L lung (LUL) |
50 Gy/4 fx (93.8 Gy) SBRT |
4 | CR | N/A | 4 | CR | 55 wee ks+ |
55 weeks+ |
| 16 | M | 19 | Brain, calvarium, bilateral lungs, axillary lymph nodes, premaxillary soft tissue |
Resection, IL-2 |
6.1 | Brain | 18 Gy/1 fx (42.0 Gy) SBRT |
1 | SD | Y | 4 | PD | 16 wee ks |
65 weeks |
| 17 | F | 68 | Left upper lobe of lung, left lower lobe, right occipital lobe, right parietal, left frontal |
Resection; brain metastases treated with SRS prior to study enrollment |
4.3 | L lung (LUL) |
24 Gy/3 fx (36.0 Gy) SBRT |
3 | CR | N/A | 4 | CR | 65 wee ks+ | 65 weeks+ |
| 18 | M | 46 | Pancreas, supraclavicular nodes, subcutaneous nodules in right shoulder, chest wall; left adrenal, gallbladder, paracolic gutter. |
Resection, debullking |
27.3 | Pancreas | 24 Gy/3 fx (36.0 Gy) SBRT |
3 | CR | N/A | 4 | PR | 38 wee ks |
56 weeks+ |
| 19 | M | 73 | T1 paraspinous mass; right pterygoid, paratracheal lymph nodes; left upper lobe; left adrenal; right kidney |
Resection, SRS |
74.6 | T1 paraspino us mass |
20 Gy/5 fx (23.3 Gy) 3D |
7 | PR | N | 4 | PR | 26 wee ks |
45 weeks+ |
| 20 | M | 66 | Right posterior occipital scalp, inferior right scalp, superior right scalp, neck, distant skin |
Resection, RND, interferon, IL-12 |
22.8 | R posterior occipital scalp R neck |
40 Gy/10 fx (46.7 Gy) IMRT 40 Gy/10 fx |
15 | CR | Y | 4 | CR | 32 wee ks+ |
32 weeks+ |
| 21 | F | 89 | Right buttock; precarinal nodes; left thigh; body of pancreas; duodenum; abdominal wall musculature |
Resection, imiquimod, RT |
6.6 | Right buttock |
40 Gy/10 fx (46.7 Gy) 3D |
15 | SD | Y | 2 | N.E. (SAE) |
16 weeks+ | |
| 22 | M | 70 | Right breast subcutaneous nodule; bilateral lungs |
Resection, interferon |
2.9 | Right breast nodule |
30 Gy/5 fx (40.0 Gy) 3D |
7 | PR | Y | 4 | PD by RECIST , SD by IRC |
12 wee ks |
12 weeks+ |
Abbreviations: RT: radiation therapy, BED: biologically effective dose, N/A: not applicable, CR: complete response, PR: partial response, SD: stable disease, PD: progressive disease, SRS: stereotactic radiosurgery
Table 1B.
Patient responses by RECIST and IRC
| Patient Number | Initial Response (RECIST) |
Best Response (RECIST) |
Best Response (IRC) |
|---|---|---|---|
| 1 | Interim PD | Interim PD | NE |
| 2 | SD | SD | SD |
| 3 | Interim PD | Interim PD | PD |
| 4 | SD | SD | SD |
| 5 | SD | SD | SD |
| 6 | SD | SD | SD |
| 7 | PD | PD | PD |
| 8 | SD | SD | SD |
| 9 | Interim PD | Interim PD | NE |
| 10 | PD | PD | PD |
| 11 | Interim PD | Interim PD | PD |
| 12 | PR | PR | PR |
| 13 | Interim PD | Interim PD | PD |
| 14 | NE-SAE | NE - SAE | NE |
| 15 | PR | CR | CR |
| 16 | PD | PD | PD |
| 17 | PR | CR | CR |
| 18 | PR | PR | PR |
| 19 | PR | PR | PR |
| 20 | PR | CR | CR |
| 21 | NE | NE (SAE) | NE |
| 22 | PD | PD | SD |
Completion of Planned Treatment/Adverse Events
Sixteen of 22 patients completed all 4 cycles of ipilimumab as scheduled. Patients were taken off study prior to completion of treatment due to either adverse events thought to be likely related to treatment (3 patients), or clinical symptoms prompting a scan that showed unequivocal progression of disease necessitating change in treatment (3 patients). Overall, there were no unexpected toxicities, and no apparent exacerbation of either radiation or ipilimumab-associated toxicities. Adverse events (AEs) included colitis, hypophysitis, rash, and anemia (Table 3). There was a 14% rate of grade 3-4 toxicity. We did not find immune-related adverse effects when radiation was delivered near the affected organ and did not observe a correlation between dose/fractionation and AEs.
Patients with Complete Response
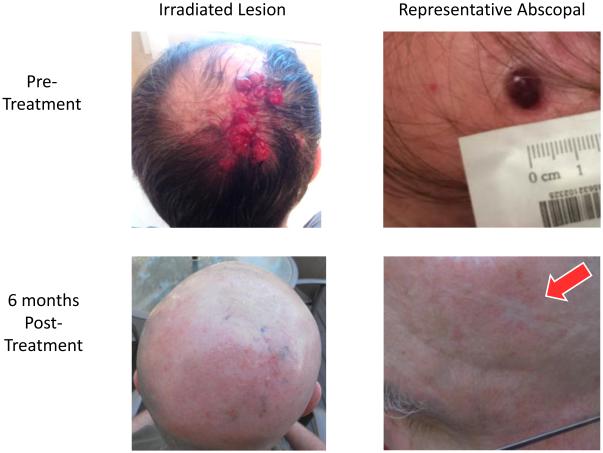
Three patients achieved an ongoing complete response to therapy (Table 1). These included a patient treated to a left upper lobe lesion to 50 Gy in 4 fractions, with unirradiated lesions in the left lingula, two lesions in the right middle lobe, and posterior occiput, who has an ongoing CR at 55 weeks after treatment. A second patient was treated to a left upper lobe lesion to 24 Gy in 3 fractions, with an unirradiated lesion in the left lower lobe, and has an ongoing CR at 65 weeks after treatment. The third patient was treated to a right posterior occipital scalp lesion and neck to 40 Gy in 10 fractions, with unirradiated lesions in the inferior right occipital, superior right occipital, mid-right occipital, and left temporal regions, and has an ongoing CR at 32 weeks (Figure 2). Median SPD among patients with a CR was 8.0 cm, versus 15.0 cm in patients without a CR. We did not find an association between total time elapsed during radiation therapy and clinical benefit.
Figure 2.
Abscopal response in patient with complete response to combination therapy. This patient had extensive dermal disease outside the radiation field and achieved a complete response to therapy; depicted here is one example lesion.
Immune Response Data
PBMC from 9 patients (3 with PD and 6 with either CR or PR) were used for immunological analyses. Samples from the baseline visit and two follow-up visits samples were analyzed to evaluate the immune response in patients with PD or CR/PR. We used a variety of assays to identify potential predictive biomarkers by comparing immune cell phenotype and function between patients with progressive disease and CR/PR.
Serum cytokine responses
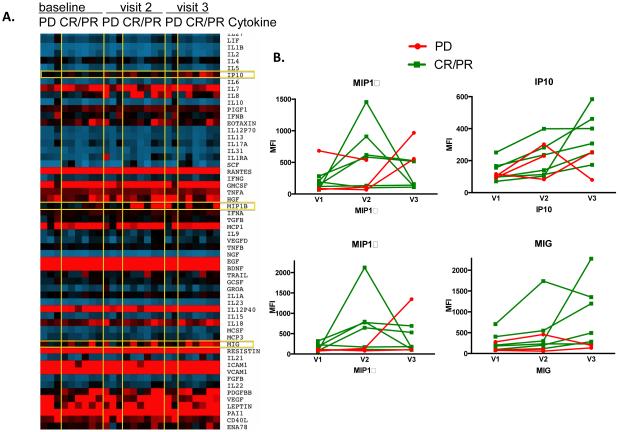
We used a human 63-plex Luminex assay to determine the cytokines present in patient serum at baseline and throughout treatment (Figure 3A). After analyzing the levels of 63 different cytokines in patient serum, we found that patients with CR/PR tended to have an increase in MIP1α and MIP-1β after the second dose of ipilimumab, as compared to patients with PD (Figure 3B). However, MIP1α and MIP-1β levels dropped by the 4th dose of ipilimumab suggesting that these cytokines may be important in the early response induced by ipilimumab, but are not maintained throughout continued treatment. IP-10 and MIG tended to increase through the treatment course in patients with CR/PR as compared to PD (Figure 3B). Our data suggest that MIP1α, MIP-1β, IP-10 and MIG may be more likely to increase from pre-treatment baseline in the serum of patients with CR/PR compared to PD. However, these differences did not reach statistical significance in this small sample set. There was no correlation between lymphocyte counts, cytokine production, and chemokine levels.
Figure 3.
Serum cytokines in melanoma patients treated with radiation and ipilimumab analyzed by Luminex (A) Heat map showing the expression of 63 cytokines in patients with progressive disease (PD) or complete response/partial response (CR/PR) following treatment, at baseline and 2nd and 4th doses of ipilimumab (visit 2 and 3, resp.). (B) Serum levels of (clockwise) MIP1α, IP-10, MIG and MIP1β. PD=Progressive Disease, CR/PR=Complete Response/Partial Response.
T cell responses following radiation plus immunotherapy
DISCUSSION
The primary objectives of this prospective clinical trial were to evaluate the safety and efficacy of combination therapy with anti-CTLA-4 therapy and local radiation therapy, as well as to identify potential correlates of immune response. This study was initiated, in part, because of our patient prospectively treated with this combination therapy prior to the opening of the trial, who achieved a CR to therapy and has had no disease recurrence for over 4 years since completion of combination radiotherapy and ipliumumab [7].
Three groups emerged from our study: those with a complete or partial response as best response to treatment, those with stable disease, and those with progressive disease. Among our patients who had a complete response to therapy, we sought to determine whether there was anything unique about their response predictors or characteristics that correlated with their excellent outcomes. Patients who achieved a CR tended to have a smaller volume of disease at baseline than those who did not. The three patients with a CR had baseline unirradiated sums of the product of diameters (SPD) of 4.3 cm, 8.0 cm, and 22.8 cm, as compared to a median value of 15.0 cm in those patients who did not experience a CR. We did not find a correlation between location of treated disease and outcome. All 3 patients who achieved a CR to therapy were treated with doses of at least 4 Gy per fraction, with a range of 4 Gy – 12.5 Gy per fraction. We did not find an association between SPD or location of treated disease and overall response rate or clinical benefit rate. Interestingly, we did found that all 3 patients who achieved a CR to therapy also experienced grade 2-3 hypophysitis, which was not seen in patients who did not achieve a CR, perhaps reflecting the robustness of the immune response in these patients. Others have also reported a higher disease control rate among patients who experience more significant immune-related adverse events [13] . Nine patients had received previous radiation therapy, including both patients who experienced a serious adverse event and 5 patients who had a PR or CR to therapy.
Treatment was tolerated without excess toxicity above what has been reported with ipilimumab alone; however three patients did not complete the four cycles of ipilimumab due to toxicity attributable to ipilimumab. Three other patients also did not complete the four cycles of ipilimumab due to clinical evidence of extensive progressive disease and were classified as such. In comparing toxicities between our study of combination treatment, and the rates of toxicity with ipilimumab alone, we found similar types, rates, and grade of toxicity, and no increase in adverse events with the combination treatment. Postow et al. report a 24% rate of grade 3-4 toxicity in patients who received ipilimumab monotherapy, and a 4.4 month progression-free survival in patients with previously untreated metastatic melanoma treated with ipilimumab alone [14]. No patients treated with ipilimumab alone achieved a CR to treatment [14]. Other studies have shown grade 3-4 toxicity rates related to ipilimumab of 7-19.9%, with rare CRs [4, 13] [15] [16] Our results show a benefit to the combination therapy in overall response rate, and also importantly demonstrate that a subset of patients achieved a durable complete response for 32-55+ weeks. Importantly, the majority of the patients treated on this study were heavily pretreated, including two patients who had progressed on ipilimumab monotherapy and one patient who had progressed on anti-PD-1 therapy, and may have been expected to have had a lower response to therapy in this setting.
The only other prospective clinical trial treating patients with metastatic melanoma with the combination of radiation with ipilimumab to date was reported earlier this year by Twyman-Saint Victor et al.[10] In this phase I study, 22 patients were treated with hypofractionated radiation to a single index lesion in 2 to 3 fractions, followed by 4 cycles of ipilimumab. By RECIST criteria, 18% of patients had a partial response in unirradiated lesions as best response, 18% had stable disease as best response, and 64% had progressive disease. Median PFS and OS in this cohort were 3.8 months and 10.7 months, respectively [10]. This trial study differs from ours in several ways: in our patients, radiation was delivered within 5 days of the first dose of ipilimumab being given, but not prior to ipilimumab, and our radiation dose/fractionation regimen was at the discretion of the treating physician rather than being standardized. This study used overall lower doses of radiation than was received by most patients in our study, with two dose levels in two strata tested (8 Gy × 2 or 3 for lung/bone lesions, and 6 Gy × 2 or 3 for liver and subcutaneous lesions). They report no grade 4 toxicities, with 1 grade 3 colitis, 1 grade 3 pneumothorax, 1 grade 3 anaphylaxis, and 4 grade 3 anemias.
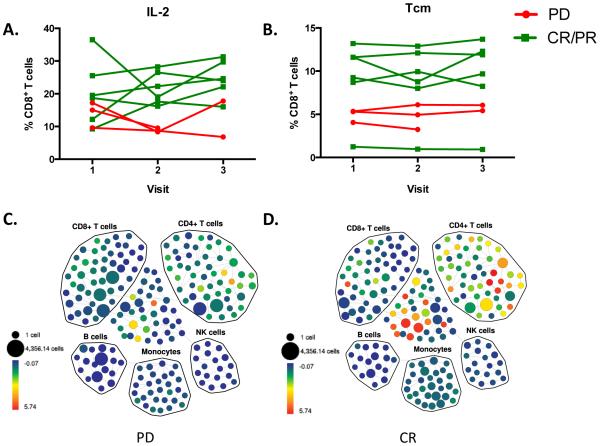
In an effort to assess whether serum markers could differentiate responders versus non-responders to therapy, we analyzed markers of immune response from blood drawn prior to ipilimumab, at the administration of the second cycle of ipilimumab, and at the administration of the fourth cycle of ipilimumab. We found that patients with CR/PR showed increased serum levels of MIP-1α, and MIP-β at the second time point 3 weeks after the first cycle compared to patients with PD, indicating that these patients appear to have generated a stronger cytokine response detectable in the serum following treatment with ipilimumab, though this did not reach statistical significance. There was also a trend toward increase in IP-10 and MIG in responders, which may indicate regulatory mechanisms being triggered in CR/PR patients in response to their strong initial cytokine response, though not statistically significant. Patients who had a complete or partial response also had higher levels of IL-2-producing CD8+ T cells and central memory CD8+ T cells compared to patients with progressive disease, which again points to an anti-tumor responses mounted by CD8+ T cells in patients who respond to ipilimumab treatment. These early data in a subset of patients suggest that it may be possible to identify biomarkers that are indicative of a therapeutic response, and suggest that CD8+ T cell immunity may be an important mechanism of this anti-tumor response. In this study there was an increased frequency of Tcm cells in responders, but no functional differences between responders and progressors. While this data suggests that patients with higher Tcm frequencies are more likely to respond to this therapy, perhaps the larger pool of Tcm cells allowed these patients to more effectively mount effector CD8+ T cell responses following the blockade of negative signaling through CTLA-4. In order to draw any definitive conclusions, more data from a larger number of patients is needed. Interestingly, we did not find a correlation between lymphocyte counts, cytokine production, and chemokine levels, which may have been related to the small sample size. We did not find an association between changes in MDSCs and outcomes, though others have reported that decreases in MDSCs may be a mechanism through which combined radiation and immunotherapy efficacy occurs [2]. The sample size in this study is too small to draw definitive conclusions, and the correlation may depend upon the definition of specific MDSC subsets.
To our knowledge, this is the second prospective clinical trial assessing treatment of metastatic melanoma with the combination of RT and systemic immune checkpoint inhibitors, and the first to report immune response data with potential biomarkers of response. Limitations of our study include small sample size and lack of statistical power for robust efficacy analyses, variable radiation doses and treatment sites limiting interpretation of safety analyses, and immune response data only in a subset of patients. We also note that as irradiated tumors are not assessable for response by RECIST, fewer sites of disease are assessable for response in patients treated with radiation plus immunotherapy as compared to immunotherapy alone, thereby potentially introducing bias toward more favorable response rates. Still, our results demonstrate that a subset of patients may achieve significant clinical benefit from combination therapy, and that this in situ tumor vaccine strategy is a promising area for continued clinical investigation. As there is currently no clinical data providing strong evidence that a certain dose/fractionation scheme is superior in promoting an abscopal response, the dose/fractionation for each patient was determined by the treating physician based on clinical indications in each case. Combination therapy is promising even if the dose/fractionation utilized was not standardized. Our data suggests that a variety of dose/fractionation regimens may be effective in this setting, though in preclinical models, hypofractionated regimens have generally shown more benefit in combination with immunotherapy. Furthermore, our findings demonstrate that the combination of ipilimumab with radiation therapy did not induce supra-additive toxicity and resulted in excellent responses in a subset of patients, although the optimal dose/fractionation of radiation to use in combination with immunotherapy still needs to be determined. As anti-PD-1/anti-PD-L1 therapies are more effective and have less toxicity than ipilimumab, they are ideal to study in combination with RT, as is planned in an upcoming multi-center randomized trial. The preliminary biomarker results reported here suggest that immune response biomarkers may be useful for early assessment of response to therapy. These results may have applicability to ongoing and future trials of immunotherapy combined with radiation, and highlight the importance of further work in this area.
Supplementary Material
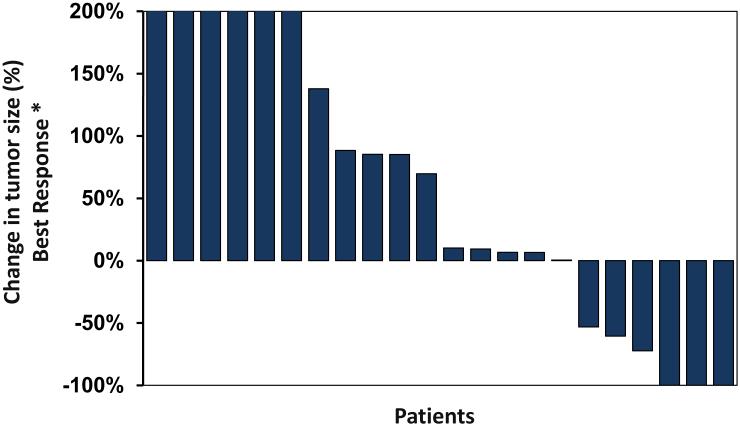
Figure 1. Waterfall plot of best response of non-irradiated lesions.
* non-irradiated lesions only
Figure 4.
Intracellular cytokine staining and mass cytometric analyses of ex vivo stimulated PBMC from melanoma patients treated with radiation and ipilimumab (A) PBMC from melanoma patients receiving ipilimumab were collected and cryopreserved at baseline pre-treatment (visit 1), 2nd ipilimumab dose (visit 2) and 4th ipilimumab dose (visit 3). PBMC samples were rested and stimulated with PMA and Ionomycin for 4 hours, and then stained with the antibody panel shown in Table 1, and analyzed by CyTOF. IL-2 expression in CD8+ T cells is shown in A. PD and CR/PR groups were significantly different (p<0.01) (B) Mass cytometric analysis of samples from A revealed differences in CCR7+CD45RA− CD8+ T cells (central memory, Tcm). PD and CR/PR groups were found to be significantly different (p<0.05) (C-D) SPADE analysis of baseline IL-2 CyTOF data from one representative patient each for progressive disease and complete response. PD=Progressive Disease, CR/PR=Complete Response/Partial Response. *p<0.05.
SUMMARY.
We report the promising results of a prospective nonrandomized clinical trial, in which patients with metastatic melanoma were treated with systemic immunotherapy plus radiation therapy to 1-2 sites of disease with the goal of achieving an abscopal response. Our results demonstrate a higher response rate than reported with ipilimumab alone, as well as durable complete responses in a subset of patients. These findings provide further insight into our understanding of combining immunotherapy with radiation therapy.
ACKNOWLEDGEMENTS
This work has been supported by the NIH (S10RR017582), Stanford Cancer Center Grant 5P30CA124435, Stanford Cancer Institute Translational Research Award – Developmental Cancer Research Award, and Biomed Valley Discoveries.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST NOTIFICATION:
The authors have no actual or potential conflicts of interest relating to this work.
Presented in part at the 2015 ASTRO Annual Meeting, San Antonio, TX, October 2015.
REFERENCES
- 1.Barrow C, et al. Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res. 2006;12(3):764–71. doi: 10.1158/1078-0432.CCR-05-1544. Pt 1. [DOI] [PubMed] [Google Scholar]
- 2.Postow MA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clemente CG, et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77(7):1303–10. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamid O, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiniker SM, et al. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol. 2012;5(6):404–7. doi: 10.1593/tlo.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golden EB, et al. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1(6):365–72. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynders K, et al. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41(6):503–10. doi: 10.1016/j.ctrv.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Twyman-Saint Victor C, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–7. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Wolchok JD, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 13.Weber J, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15(17):5591–8. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 14.Postow MA, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robert C, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 16.Wolchok JD, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–64. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.