Abstract
Brain-derived neurotrophic factor (BDNF) is an active neurotrophin abundantly expressed throughout the nervous system. It plays an important role in synaptic transmission, plasticity, neuronal proliferation, differentiation, survival, and death. The Bdnf gene in rodents has eight non-coding exons and only a single coding exon (IX). Despite its recognized regulation by neuronal activity, relatively little is known about its transcriptional regulation, and even less about the transcription factor candidates that may play such a role. The goal of the present study was to probe for such a candidate that may regulate exon IX in the rat Bdnf gene. Our in silico analysis revealed tandem binding sites for nuclear respiratory factor 2 (NRF-2) on the promoter of exon IX. NRF-2 is of special significance because it co-regulates the expressions of mediators of energy metabolism (cytochrome c oxidase) and mediators of neuronal activity (glutamatergic receptors). To test our hypothesis that NRF-2 also regulates the Bdnf gene, we performed electrophoretic mobility shift assay (EMSA), chromatin immunoprecipitation (ChIP), promoter cloning, and site-directed mutagenesis, real-time quantitative PCR (RT-qPCR), and Western blotting analysis. Results indicate that NRF-2 functionally regulates exon IX of the rat Bdnf gene. The binding sites of NRF-2 are conserved between rats and mice. Overexpressing NRF-2 up-regulated the expression of Bdnf exon IX, whereas knocking down NRF-2 down-regulated such expression. These findings are consistent with our hypothesis that NRF-2, in addition to regulating the coupling between neuronal activity and energy metabolism, also regulates the expression of BDNF, which is intimately associated with energy-demanding neuronal activity.
Keywords: brain-derived neurotrophic factor (BDNF), chromatin immunoprecipitation (ChiP), short hairpin RNA (shRNA), transcription factor, transcription regulation
Introduction
Brain-derived neurotrophic factor (BDNF)2 is regarded as the most widely expressed, active, and well characterized neurotrophin in developing and adult mammalian central nervous system (1–3). BDNF is synthesized as a precursor protein known as pro-BDNF and secreted as a mixture of pro-BDNF and mature BDNF (4). BDNF supports the survival and differentiation of embryonic neurons and controls various neural processes in development and adulthood, including growth, differentiation, and survival, synaptic formation, function, and plasticity (reviewed in Refs. 5 and 6), as well as memory and learning (7). Alterations in BDNF gene expression contribute to disorders and pathologies such as depression, epilepsy, cancer, drug abuse, and neurodegenerative diseases, such as Alzheimer's, Huntington's, and Parkinson's diseases (8–15). Thus, uncovering the mechanisms of BDNF regulation is of significant clinical importance.
BDNF expression is tissue-specific, as it is differentially expressed in different brain regions as well as in peripheral tissues (16, 17). In rodents, the Bdnf gene consists of nine exons, including eight non-coding exons and a single coding one (exon IX). These yield a single, identical, mature BDNF protein (reviewed in Ref. 18). Although the regulation of BDNF expression by neuronal activity has received a great deal of attention, relatively little is known about the transcriptional regulation of the coding exon of the Bdnf gene, nor about transcription factor candidates that may play a significant role.
Thus far, exon IV (previously known as exon III) has received the most attention. Its promoter reportedly contains Ca2+-response elements and bind to cAMP responsive element-binding protein and calcium-responsive factor (CaRF) to mediate transcription (19, 20). Virtually nothing is known about transcription factor candidates for the coding exon IX. The goal of the present study was to conduct an in-depth study of such a candidate in the rat Bdnf gene.
Our in silico analysis of the Bdnf exon IX promoter revealed tandem repeats of “GGAA” and “TTCC” characteristic of the binding motif for nuclear respiratory factor 2 (NRF-2) or GA-binding protein (GABP) (21). NRF-2 is a member of E26 transformation-specific (ETS) family that binds the GGAA core DNA sequence (reviewed in Refs. 22 and 23). It is composed of two distinct proteins: the α subunit contains the ETS DNA binding domain that binds to the GGAA or TTCC motif, and the β subunit contains the transcriptional activation domain (21). NRF-2/GABP is involved in cell cycle progression, protein synthesis, and mitochondrial biogenesis (reviewed in Refs. 23 and 24). Previously, we have found that it transcriptionally couples energy metabolism and neuronal activity by co-regulating the expression of all 13 subunits of an important energy-generating enzyme, cytochrome c oxidase (COX) (25), as well as glutamatergic receptor subunit genes (26, 27).
By means of a combination of bioinformatics, biochemical, and molecular biological approaches, including in silico analysis, electrophoretic mobility shift and supershift assays, real-time quantitative PCR (RT-qPCR), chromatin immunoprecipitation, promoter mutational analysis, overexpression, shRNA, functional assays, and Western blot analysis, we have documented that NRF-2 functionally regulates the transcription of exon IX of the Bdnf gene in rat cortical neurons.
Results
Bdnf Exons' Expression in Visual Cortex
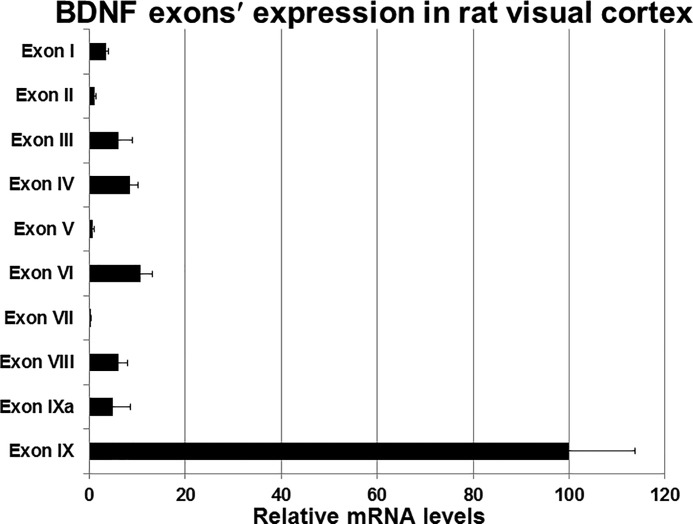
Using PCR primers specific to each of exons I to IX (Table 1) and RT-qPCR, we detected transcripts for each Bdnf exon in the rat visual cortex. Fig. 1 illustrates that, in comparison to exon IX (expressed as 100%), all of the other exons, including exon IXA that contributes to the pro-BDNF, were expressed in much lower quantities. The almost non-detectable ones were exons II, V, and VII. Clearly, the expression of exon IX, being at least 10-fold higher than any of the other exons, dominated over all of the other exons. As this is the only coding exon and very little was known about the cis elements in its promoter region that may regulate its expression, we chose to focus on the promoter of exon IX.
TABLE 1.
Real-time qPCR primers
| Gene | Primer sequence |
|---|---|
| Bdnf exon I | F: 5′-CAGGGCAGTTGGACAGTCAT-3′ |
| R: 5′-TACGCAAACGCCCTCATTCT-3′ | |
| Bdnf exon II | F: 5′-TTCGGCTCACACTGAGATCG-3′ |
| R: 5′-CAGTATACCAACCCGGAGCTT-3′ | |
| Bdnf exon III | F: 5′-TTGGAGGGCTCCTGCTTTCT-3′ |
| R: 5′-CTGGGCTCAATGAAGCATCCAG-3′ | |
| Bdnf exon IV | F: 5′-ACTGAAGGCGTGCGAGTATT-3′ |
| R: 5′-TGGTGGCCGATATGTACTCC-3′ | |
| Bdnf exon V | F: 5′-AAACCATAACCCCGCACACT-3′ |
| R: 5′-CTTCCCGCACCACAGAGCTA-3′ | |
| Bdnf exon VI | F: 5′-GATGAGACCGGGTTCCCTCA-3′ |
| R: 5′-TTGTTGTCACGCTCCTGGTC-3′ | |
| Bdnf exon VII | F: 5′-ACTGTCACCTGCTTTCTAGGG-3′ |
| R: 5′-GAGTTCCGCAGACCCTTTCA-3′ | |
| Bdnf exon VIII | F: 5′-GTGCTCAGGCTAATCCTCGTT-3′ |
| R: 5′-CTTTCTCCTGGGATGCACAGT-3′ | |
| Bdnf exon IXA | F: 5′-ACGGCGTGAACAGAGATCAT-3′ |
| R: 5′-ACGGTTTCTAAGCAAGTGACG-3′ | |
| Bdnf exon IX | F: 5′-GAGAAGAGTGATGACCATCCT-3′ |
| R: 5′-TCACGTGCTCAAAAGTGTCAG-3′ | |
| NRF2-α | F: 5′-CTCCCGCTACACCGACTAC-3′ |
| R: 5′-TCTGACCATTGTTTCCTGTTCTG-3′ | |
| NRF2-β | F: 5′-ACCAACCAGTGGGATGGGTCAG-3′ |
| R: 5′-GCACATTCCACCCGGCTCTCAAT-3′ | |
| COX7c | F: 5′-ATGTTGGGCCAGAGTATCCG-3′ |
| R: 5′-ACCCAGATCCAAAGTACACGG-3′ | |
| Gapdh | F: 5′-AGGTCGGTGTGAACGGATTTG-3′ |
| R: 5′-GGGGTCGTTGATGGCAACA-3′ |
FIGURE 1.
Analysis of all Bdnf exons' mRNA levels in the rat visual cortex. Values given are mean ± S.E. The expression level of exon IX is represented as 100%, and those of all other exons are depicted as percentages in comparison to exon IX (n for each exon = 6).
In Silico Analysis of the Promoter Region of Bdnf Exon IX
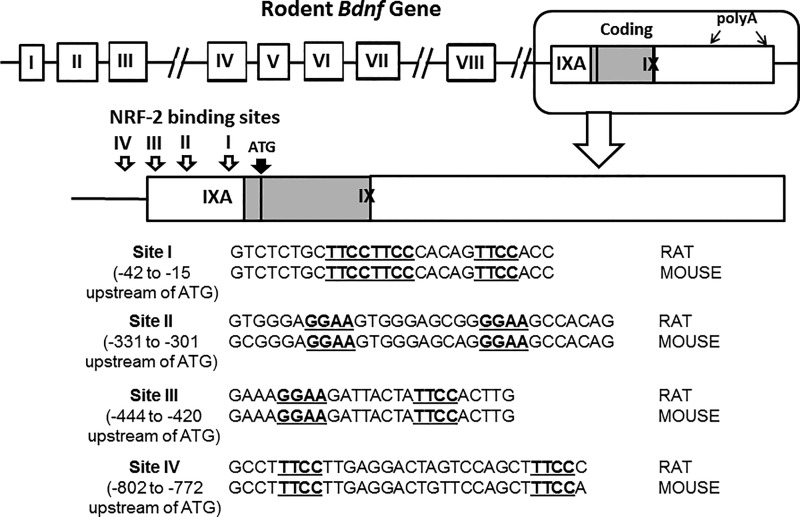
Fig. 2 shows a schematic diagram of the rat Bdnf gene, with eight non-coding exons and a single coding exon, exon IX (modified from 28). Proximal promoters of mouse and rat Bdnf exon IX with DNA sequence 1 kb 5′ upstream and 1 kb 3′ beyond the transcription start point (TSP) were analyzed in silico. Megalign analysis was used to find tandem repeats of GGAA or TTCC typical of NRF-2 binding motifs in the promoter region upstream of the coding portion of exon IX. We chose four putative binding sites that showed high homology between rats and mice, and partial homology with humans. The positions of these four sites upstream of the ATG codon (translation start site) in exon IX are: site I (−42 to −15), site II (−331 to −301), site III (−444 to −420), and site IV (−802 to −772). Sequences for sites I to IV are shown in Fig. 2.
FIGURE 2.
Schematic representation of the rat Bdnf gene showing its eight non-coding exons and the single, coding exon IX, with IXA providing the precursor portion of the pro-BDNF (modified from Ref. 28, not drawn to scale). The enclosed portion at the top right for exons IXA and IX is enlarged to indicate the four putative NRF-2 binding sites tested. Each binding site contains a tandem repeat of GGAA or TTCC. The translation start site (ATG) of exon IX is located 21 nucleotides downstream of the transcription start point of exon IX. The sequences for sites I and II used for EMSA are indicated below, with the NRF-2 binding motifs underlined. There is high homology between rat and mouse sequences at these two sites. Nucleotide sequences for sites III and IV are also given.
In Vitro Binding of NRF-2 to the Promoter of Bdnf Exon IX
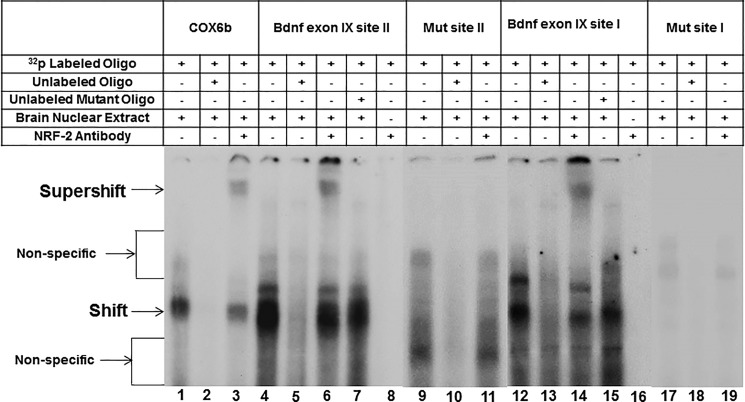
To determine the binding capacity of NRF-2 to its candidate sites, we performed EMSA. In agreement with our previous findings (26), the promoter of cytochrome c oxidase subunit 6b (COX6b) formed a specific DNA/NRF-2 shift and a DNA/NRF-2/NRF-2 antibody supershift complex when incubated with visual cortical nuclear extract (Fig. 3, lanes 1 and 3, respectively). The shift band was competed out with the addition of an excess of unlabeled COX6b (Fig. 3, lane 2).
FIGURE 3.
In vitro EMSA and supershift assays to show the binding of NRF-2 to putative sites II and I on the promoter of exon IX in the Bdnf gene. All lanes contain 32P-labeled oligonucleotides containing either site II or site I, and they are indicated by a ”+“ or a ”−“ sign depending on whether they also contain rat brain nuclear extract, excess unlabeled oligos, unlabeled mutant oligos, or NRF-2 antibody. NRF-2 shift, supershift, and nonspecific complexes are indicated by arrows. The positive control for NRF-2 binding is COX6b. Specific NRF-2 shift bands are revealed upon incubation with cortical nuclear extract (lanes 1 and 3). Excess unlabeled competitor competed out the shift band (lane 2). The addition of NRF-2 antibody yielded a strong supershift band (lane 3). Incubation of cortical nuclear extract with the Bdnf probes yielded a specific NRF-2 shift band (lanes 4 and 6 for site II, lanes 12 and 14 for site I) that was competed out with the addition of excess cold probe (lanes 5 and 13). The addition of NRF-2 antibody yielded specific supershift band (lanes 6 and 14). The addition of excess unlabeled mutant Bdnf probes did not compete out the shift reaction (lanes 7 and 15). Labeled Bdnf probe with NRF-2 antibody without any nuclear extract was used as a control to check for any antibody-oligo interaction, and no shift or supershift bands are evident (lanes 8 and 16). Labeled Bdnf probes with mutant NRF-2 sites did not yield prominent specific NRF-2 shift or supershift bands (lanes 9–11 and 17–19).
When 32P-labeled site II of the promoter region of Bdnf exon IX was subjected to EMSA, it showed a shift complex with the rat visual cortical nuclear extract (Fig. 3, lane 4). The complex was competed out with the addition of unlabeled competitors (Fig. 3, lane 5). A supershift band was evident when NRF-2 antibody was added to the Bdnf sequence (Fig. 3, lane 6). The addition of unlabeled probes with mutated NRF-2 binding sites was not able to compete out the shift band (Fig. 3, lane 7). Labeled Bdnf oligo with NRF-2 antibody in the absence of any brain extract did not show any shift or supershift band, indicating that there was no direct antibody-oligo interaction (Fig. 3, lanes 8). Shift, competitor, and supershift reactions with the mutated NRF-2 site on the 32P-labeled Bdnf oligo also did not show any distinct binding to the NRF-2 site (Fig. 3, lanes 9–11). Site I was also tested with EMSA, and it yielded a specific shift and a supershift complex for NRF-2 that was competed out with an excess of cold competitor (Fig. 3, lanes 12, 14, and 13, respectively), but not with an excess of unlabeled probe with mutated NRF-2 sites (Fig. 3, lane 15). Again, there was no antibody-oligo interaction without the brain extract (Fig. 3, lane 16). Mutating the binding motif for NRF-2 in site I also did not produce any shift or supershift bands (Fig. 3, lanes 17–19). We have also tested sites III and IV with EMSA, but neither of them gave a shift or a supershift band (data not shown).
In Vivo Interaction via Chromatin Immunoprecipitation Assay (ChIP)
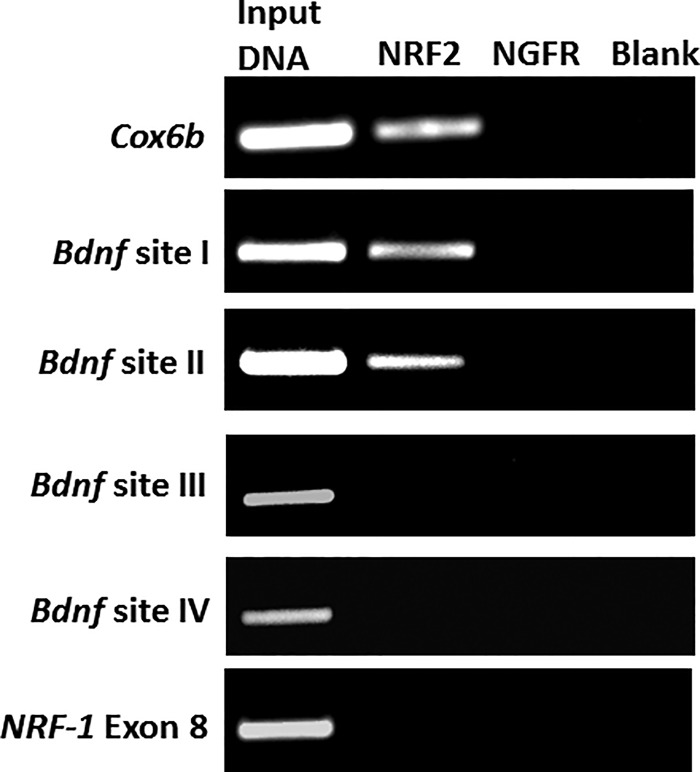
To provide further corroboration of the physical association of NRF-2 with its binding sites on the promoter of Bdnf exon IX gene, we performed a ChIP assay using rat visual cortical tissue (Fig. 4). A 0.5% dilution of input chromatin was used as a standard to indicate the efficiency of the PCR. As with EMSA, COX6b was used as a positive control. Exon 8 of NRF-1, which does not contain a NRF-2 binding motif, served as a negative control.
FIGURE 4.
In vivo chromatin immunoprecipitation assay using rat visual cortical nuclear extract shows that NRF-2 interacts with the promoter of Bdnf exon at both sites I and II, but not at sites III and IV. Immunoprecipitation was carried out with anti-NRF-2α antibody (NRF-2 lane), anti-nerve growth factor receptor p75 antibody (negative control, NGFR lane), or no antibody (negative control, blank lane). 0.5% of input chromatin (input DNA lane) served as the control for PCR. The positive control for NRF-2 binding was COX6b, whereas exon 8 of NRF-1 was the negative control.
As shown in Fig. 4, the anti-NRF-2 antibody specifically immunoprecipitated the Bdnf exon IX promoter fragments. The appearance of a distinct band with NRF-2 antibody verified an in vivo interaction of the Bdnf gene with NRF-2. This was true for both sites I and II of the promoter region for exon IX (Fig. 4). Nerve growth factor receptor (NGFR) antibody was used as a control for the immunoprecipitation reaction, and it did not yield any PCR product. An additional “no antibody” control was used to rule out the possibility of a bead-DNA interaction. Indeed, it also did not yield any PCR product. Sites III and IV were also tested with ChIP on visual cortical tissue immunoprecipitated with NRF-2 antibodies, but both sites did not reveal any interactions (Fig. 4).
Effect of Mutating NRF-2 Binding Sites on the Promoter Activity of Exon IX
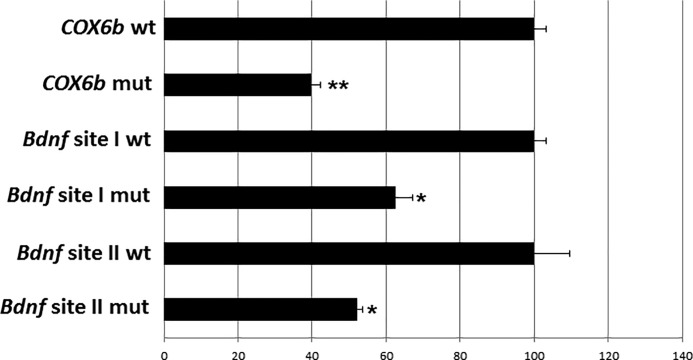
Luciferase reporter assays were done to verify the significance of NRF-2 binding. Site-directed mutations of the putative NRF-2 binding sites on the promoter of exon IX were constructed. Wild type controls or mutants were transfected into cultured N2a cells. COX6b wild type and mutated NRF-2 binding sites served as controls. Fig. 5 shows a significant reduction in COX6b promoter activity in mutants to 40% of controls, and a significant fall to 62.44 and 52% in the Bdnf site I and II mutants, respectively, as compared with controls (*, p < 0.05, **, p < 0.01 when compared with respective controls).
FIGURE 5.
Promoter mutational analysis shows relative luciferase activity of wild type (wt) and mutant (mut) promoters of Bdnf exon IX. COX6b served as the positive control. Mutating the NRF-2 binding site in COX6b, and sites I and II in Bdnf resulted in a significant decrease in luciferase activity as compared with the wild type controls (down to 40% for COX6b mutant and to 62.44 and 52% for Bdnf sites I and II mutants, respectively). n = 3 for each construct. **, p < 0.01; *, p < 0.05 as compared with the respective wild type controls.
Knockdown of NRF-2α Decreased mRNA Levels of Bdnf Exon IX and the Effect of KCl Depolarization in Primary Neurons
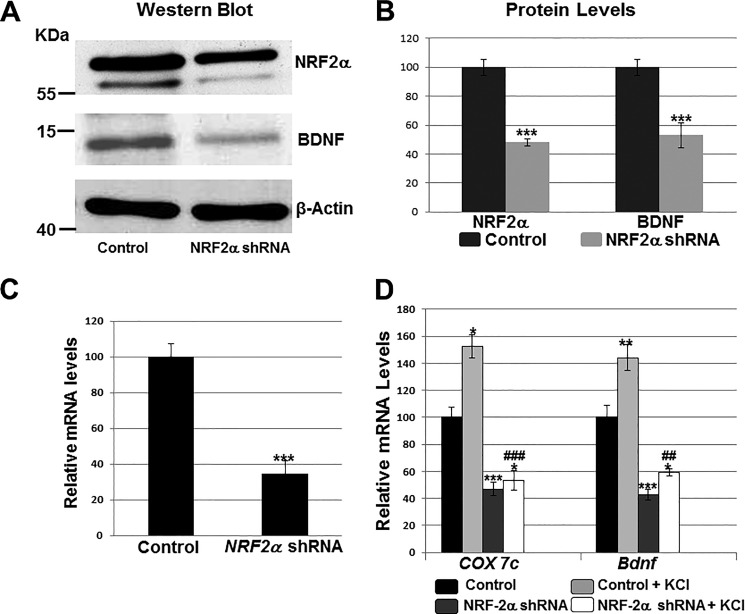
A knockdown of NRF-2α was achieved in cultured primary neurons with a NRF-2α shRNA construct. For transfection controls, neurons received scrambled shRNA, which failed to bind to any known mRNA. In NRF-2α-silenced neurons, NRF-2α protein was significantly reduced to 48%, whereas BDNF protein fell to 53% of control values (p < 0.001 for both) (Fig. 6, A and B). Silencing of NRF-2α also resulted in a significant decrease in NRF-2α mRNA levels to 34.65% of controls (Fig. 6C, p < 0.001). Likewise, the transcripts for COX7c (positive control) was reduced to 47% and those for Bdnf exon IX to 43% when compared with controls (p < 0.001 for both, Fig. 6D).
FIGURE 6.
Effect of silencing NRF-2α with shRNA and KCl depolarizing stimulation. Scrambled shRNA vectors served as the control. A and B, Western blots revealed a down-regulation of NRF-2α and BDNF protein levels in shRNA-transfected primary visual cortical neurons. β-Actin served as a loading control (n = 4 for each). The molecular masses of NRF-2α, BDNF, and β-actin are ∼56, ∼14, and ∼42 kDa, respectively. ***, p < 0.001 when compared with controls. C and D, RT-qPCR revealed a down-regulation of NRF-2α, COX7c, and Bdnf transcripts in neurons transfected with NRF-2α shRNA (n = 4 for each) as compared with those transfected with scrambled shRNA controls. ***, p < 0.001 when compared with controls. Primary neurons exposed to KCl up-regulated their mRNA levels for COX7c and Bdnf (n = 4 for each). *, p < 0.05; **, p < 0.01 when compared with unstimulated controls. In the presence of NRF-2α shRNA, KCl could no longer up-regulate COX7c and Bdnf transcripts to control + KCl levels (n = 4 for each). ##, p < 0.01; ###, p < 0.001 when compared with control + KCl. There were no statistically significant differences in transcript levels of Bdnf and COX7c in neurons treated with NRF-2α shRNA with or without KCl.
We have shown previously that KCl depolarization up-regulated COX and specific excitatory glutamatergic neurotransmitter receptor subunits, and that this involved NRF-2 (25, 26, 29). Indeed, 20 mm KCl for 5 h induced an up-regulation of COX7c (to 152%) as well as Bdnf exon IX (to 142%) in cultured visual cortical neurons (p < 0.05 and p < 0.01, respectively) (Fig. 6D). However, if neurons were transfected with NRF-2α shRNA, then the same KCl regimen could no longer increase transcripts levels of COX7c and Bdnf to those of control-plus KCl (p < 0.001 and p < 0.01, respectively, when compared with control-plus KCl).
Overexpressing NRF-2 Increased mRNA Levels of Bdnf Exon IX and the Effect of TTX Blockade in Primary Neurons
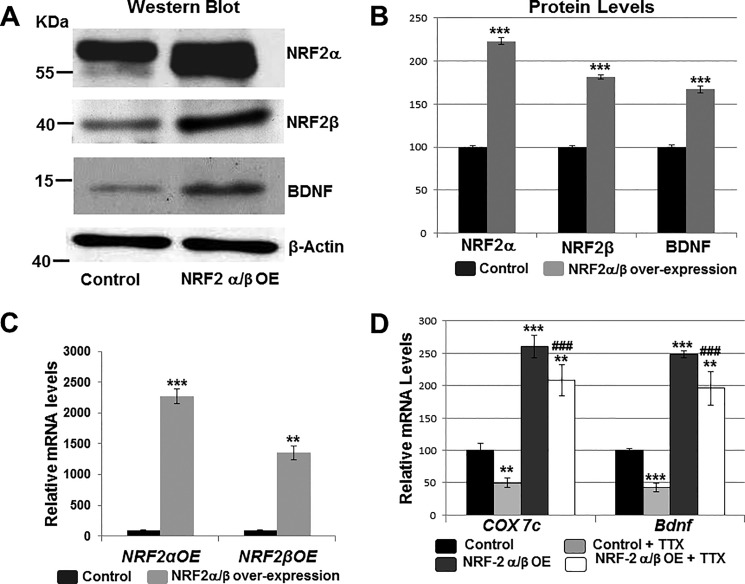
NRF-2α and NRF-2β overexpression plasmids were transfected into cultured primary visual cortical neurons. β-Actin was the internal control. Both subunits were used to form the functional NRF-2αβ complex. NRF-2 overexpression increased the protein levels of NRF-2α to 223%, NRF-2β to 181.67%, and those of BDNF to 167% (p < 0.001 for all, Fig. 7, A and B). Overexpressing NRF-2α/β also significantly increased the mRNA levels of NRF-2α by 20–22-fold and that of NRF-2β by 13-fold (Fig. 7C). Transcript levels of COX7c and Bdnf were likewise increased to 260.68 and 248.51%, respectively, when compared with empty vector controls (p ≤ 0.001 for both, Fig. 7C).
FIGURE 7.
Effect of overexpressing NRF-2 on BDNF levels and TTX impulse blockade. Empty vectors served as the control. A and B, Western blots showed that primary neurons transfected with NRF-2α and NRF-2β overexpression vectors up-regulated their NRF-2α, NRF-2β, and BDNF protein levels (n = 4 for each). ***, p < 0.001 when compared with empty vector controls. C and D, overexpressing NRF-2α and -β in primary neurons increased mRNA levels of NRF-2α, NRF-2β, COX7c, and Bdnf in primary neurons (n = 4 for each). **, p < 0.01; ***, p < 0.001 when compared with empty vector controls. Transcript levels of COX7c and Bdnf were reduced with 3 days of 0.4 μm TTX treatment (n = 4 for each). **, p < 0.01; ***, p < 0.001 when compared with no-TTX controls. NRF-2α/β overexpression rescued the TTX-mediated down-regulation of COX7c and Bdnf transcripts (n = 4). **, p < 0.01 when compared with controls. ###, p < 0.001 when compared with control + TTX. There were no statistically significant differences in transcript levels of Bdnf and COX7c in neurons treated with NRF-2 overexpression vectors with or without TTX.
Previous studies in our laboratory showed that TTX-induced impulse blockade decreased the transcript levels of COX subunits as well as those of NMDA, AMPA, and GABAA receptor subunit genes (25–27, 30). To determine whether NRF-2 overexpression could rescue Bdnf transcripts from being down-regulated by TTX, primary cultured neurons that were transfected with empty vector controls or with NRF-2α/β overexpression vectors were subjected to 0.4 μm TTX treatments for 3 days. Control neurons showed a significant down-regulation of COX7c and Bdnf transcripts to 50 and 42%, respectively (p < 0.01 and p < 0.001, respectively; Fig. 7D). However, neurons that were transfected with NRF-2 overexpression vectors did not exhibit significant down-regulation of these transcripts with TTX. Rather, the level of COX7c was 208.28% and that of Bdnf was 195.99% when compared with controls (p < 0.01 for both; and p < 0.001 when compared with control + TTX; Fig. 7D). There was no statistically significant difference between NRF-2 overexpression and NRF-2 overexpression + TTX for Cox7c (p = 0.061) or Bdnf (p = 0.063).
Homology
The functional NRF-2 binding sites (sites I and site II) are conserved between rats and mice (Fig. 2) and only partially with humans (data not shown). Although homology exists for sites III and IV, they were not functional for NRF-2.
Discussion
Using multiple techniques, including bioinformatics, biochemical, and molecular biological approaches, the present study documented the functional role of NRF-2 in the transcriptional regulation of the single coding exon, exon IX, of the Bdnf gene in rat primary visual cortical neurons. The expression of Bdnf exon IX transcripts was at least 10-fold higher than all of the other exons. Knocking down NRF-2 significantly down-regulated BDNF protein and mRNA levels, whereas overexpressing NRF-2 markedly up-regulated these levels. Moreover, activity-dependent Bdnf expression was significantly altered by the prevalence of NRF-2. NRF-2 tandem binding sites on the promoter of exon IX are conserved between mice and rats.
BDNF is the second neurotrophin discovered (31) almost three decades after the discovery of nerve growth factor (32). It plays an important role in both developing and the adult central nervous system, including neurogenesis, survival, differentiation, synaptic transmission, synaptic plasticity, and neural degeneration (33–40). Decreased BDNF levels in humans have been associated with bipolar disorders, severe depression, schizophrenia, and neurodegenerative diseases, such as Alzheimer's and Huntington's diseases (15, 41, 42). Given the critical role of BDNF in neuronal development, plasticity, and regeneration, the transcriptional regulation of the Bdnf gene should be of significant importance (43).
The Bdnf gene is unique in that it contains eight non-coding exons (I to VIII), each reportedly has its own promoter and, with tissue- and age-specific expression, may be spliced upstream of a common coding exon, exon IX (15, 16). Being the only coding exon, exon IX obviously commands a special dominance in this structural arrangement. However, very little was known about the transcriptional regulation of this exon.
Much of the attention has been paid to the regulation of exon IV (previously known as exon III). The cAMP signaling system phosphorylates and activates the transcription factor, cAMP responsive element-binding protein, and stimulates the transcription of exon IV (15, 45, 46). Other transcription factors, such as basic HLHB2 and NFκB, have also been implicated in the expression of exon IV (47, 48). Little attention has been paid to the regulation of exon IX, except for a reported epigenetic regulation by Gadd45b, a neural immediate early gene, which promotes activity-dependent demethylation of the exon IX promoter (49). Virtually nothing is known about transcription factor candidates for exon IX, let alone their characterization.
Previous reports on the expression levels of various exons have noted the presence of exons I, IV, and IXA in the rat brain (28). However, they did not analyze the expression of exon IX. Our quantitative analysis of transcript levels of all exons in the rat visual cortex indicates that exon IX, by far, has the highest expression, and all others, including exon IXA, are expressed at a much lower level (Fig. 1). This is consistent with findings by Barde's group (50) that the mature BDNF is 10-fold more abundant than the pro-BDNF, and that the pro-BDNF is a transient biosynthetic intermediate that is rapidly converted to mature BDNF intracellularly in neurons. The pro-BDNF is also an apoptotic ligand that binds to the nonspecific p75NTR (51), whereas the mature BDNF is known to bind to the high-affinity TrkB receptors and is pro-survival (52). The greater prevalence of exon IX transcript underscores its functional significance, and yet so little was known about its transcriptional regulation.
Our in silico analysis of the promoter region of exon IX indicated multiple GGAA motifs for the binding of NRF-2. Based on our previous experience, we focused only on tandem repeats of GGAA or TTCC. We concentrated on four such repeat sites in the more proximal promoter region of exon IX, and found that only two of these sites are functional. Site I is located in the region between −15 to −42, whereas site II is located in the region between −301 to −331, both upstream of the translation start site of exon IX (Fig. 2). The other two sites are much more upstream to the first two sites, and they did not exhibit any functional binding. Thus, sites I and II may be considered as part of the basal promoter region of exon IX, and they are both located in exon IXA. Both sites I and II bound to NRF-2 in vitro (EMSA) and functionally in vivo (ChIP). Promoter mutational analysis also confirmed their functionality.
NRF-2 is an ETS-related transcription factor, and is a human homologue of the murine GA-binding protein, GABP (21). NRF-2 has two distinct subunits, NRF-2α and NRF-2β. The α subunit contains the DNA binding domain, whereas the β subunit has the transactivation domain (reviewed in Ref. 53). Functional NRF-2 is a tetramer of α2β2. NRF-2 regulates the expression of many genes in various tissues, including genes for mitochondrial biogenesis (21, 22, 25), lineage-restricted myeloid and lymphoid genes required for innate immunity (23), and genes for cell cycle control in fibroblasts (24).
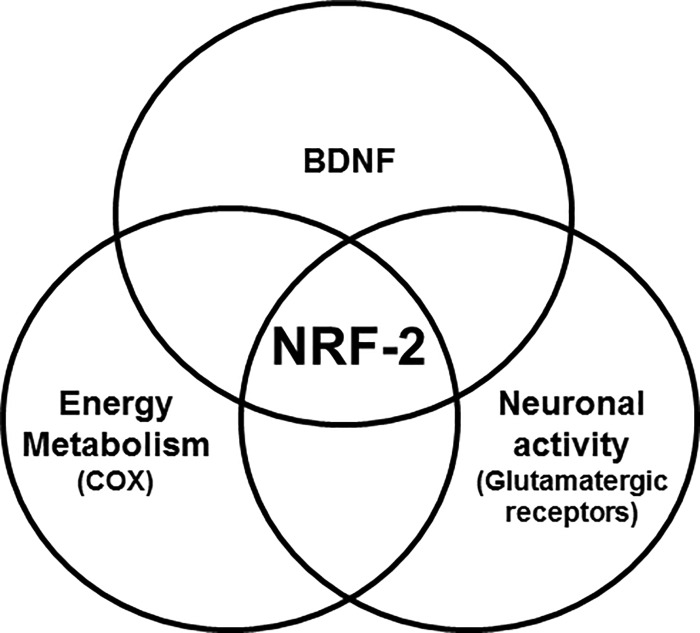
The brain is a highly metabolic organ that needs a constant supply of energy to perform critical steps of neuronal activity. Disruptions of metabolic homeostasis can have dramatic consequences for information processing and cognitive function (54). Thus, the effective control of mitochondrial biogenesis and turnover is critical for the maintenance of energy homeostasis in the brain. Our laboratory has shown that NRF-2 regulates all 10 nuclear-encoded and all three mitochondrial-encoded subunit genes of cytochrome c oxidase, an important energy generating enzyme in the mitochondria (25). Moreover, we found that NRF-2 links energy metabolism and neuronal activity at the transcriptional level by also regulating specific subunits of glutamatergic receptors (26, 27). The present study adds a third component to this coupling, i.e. BDNF (Fig. 8). BDNF functions at the interface of metabolism and synaptic plasticity. On the one hand, it interacts with metabolic molecules (55), influences mitochondrial energy management efficiency (56, 57), and alters glucose utilization in neurons (58). On the other hand, it enhances glutamate release and increases the abundance of NMDA and AMPA receptors and their delivery to the plasma membrane, thereby up-regulating receptor activity in hippocampal and cortical neurons (59–61). Previously, we have found that NRF-2 functionally regulates key NMDA and AMPA receptor subunit genes (26, 27). With our current discovery of direct regulation of BDNF by NRF-2 in visual cortical neurons, it is highly possible that the actions of BDNF on energy metabolism and glutamatergic synapses result from upstream activation by NRF-2.
FIGURE 8.
Venn diagram shows how NRF-2 transcriptionally regulates the coupling of energy metabolism (represented by COX), neuronal activity (represented by glutamatergic receptor subunit genes), and BDNF, which is intimately associated with both energy metabolism and neuronal activity.
It has been reported that BDNF-TrkB signaling plays a pivotal role on mitochondrial bioenergetics through metabolic activators like PGC-1α (peroxisome proliferator-activated receptor-γ co-activator 1α) (54), a master regulator of mitochondrial biogenesis (62). PGC-1α is known to powerfully induce NRF-2 (63). We have also found that PGC-1α responds to changes in neuronal activity earlier than that of NRF-2 (64). Thus, the coupling of neuronal activity, energy metabolism, and BDNF at the transcriptional level by NRF-2, with upstream induction likely by PGC-1α, is an efficient mechanism adopted by neurons for proper functioning, at least in the rat visual cortex.
Experimental Procedures
Experiments involving animals were approved by and conducted in accordance with the Institutional Animal Care and Use Committee (IACUC) of the Medical College of Wisconsin (Milwaukee, WI). All efforts were made to reduce the number of animals used and their suffering.
RNA Isolation and RT-qPCR of Visual Cortical Tissue
Visual cortices dissected from P1 rat pup brains were collected in microcentrifuge tubes kept in dry ice, homogenized immediately, and followed by the extraction of total RNA by the TRIzol method according to the manufacturer's instructions (Life Technologies). Any residual genomic DNA was digested with DNase I on total RNA, and cDNA synthesis was carried out using the iScript cDNA synthesis kit (170-8891, Bio-Rad).
Quantification of Gene Expression by RT-qPCR
mRNA levels of Bdnf exons I to IX were determined in a Bio-Rad iCycler using IQ SYBR Green Supermix and/or a Cepheid Smart Cycler Detection system (Cepheid, Sunnyvale, CA) following the manufacturer's protocols. Primers for real-time are shown in Table 1. PCR runs were: hot start 2 min at 95 °C, denaturation 10 s at 95 °C, annealing 15 s according to the Tm of each primer, and extension 10 s at 72 °C for 15–30 cycles. Melt curve analyses verified the formation of a single desired PCR product. Mouse/rat compatible Actb (β-actin) and Gapdh were used as internal controls and the 2−ΔΔCT method (65) was performed for the relative amount of transcripts.
Primary Neuronal Cultures
Primary visual cortical neuronal cultures were done as described previously (66). Briefly, P0 to P1 rat pups were euthanized by decapitation. After clearing the meningeal layers from the brain, visual cortices were dissected out and chopped into small pieces. The tissues were treated with trypsin and suspended by pipetting. Neurons were then dissociated by trituration, and cells were seeded in a six-well plate (35 mm; pre-coated with poly-l-lysine) at a density of 1 × 106 cells/well. Cells were grown in Neurobasal-A media containing l-glutamine and B27 supplement (Life Technologies) and maintained in a humidified incubator with 5% CO2 at 37 °C. The addition of cytosine arabinoside (Ara-C) (Sigma) to the culture media helped to suppress the proliferation of glial/non-neuronal cells.
In Silico Analysis of Bdnf Exon IX
In silico analysis of Bdnf exon IX promoter was done using sequence builder and Genequest software (DNAStar Lasergene 8 Suite). Sequences including 1 kb upstream and 1 kb downstream of the TSPs of mouse, rat, and human BDNF were derived from the genome database in GenBankTM. Alignment was done to find the conserved sites among mice, rats, and humans using Megalign. Computer-assisted search for the typical NRF-2 tandem repeats GGAA and TTCC was conducted to reveal putative NRF-2 binding sites.
Preparation of Nuclear Extracts and Electrophoretic Mobility Shift and Supershift Assays
Nuclear extraction protocol was as reported previously (67). In brief, the frozen brain tissue was rinsed in the PBS buffer, spun, and transferred to a Dounce tissue homogenizer. Buffer A was added at 2.5 times/g of tissue weight with a minimum volume of 2 ml. Five strokes of a pestle were used to homogenize the tissue to a liquid mass. After induction with Nonidet P-40 (0.5%), five additional strokes were applied and the tissue was incubated for 10 min to lyse the cells with the detergent. The homogenate was transferred equally into fresh tubes and centrifuged at 13,000 rpm for 30 s. The supernatant containing the cytoplasmic constituents was removed, and buffer C was added to the nuclear pellet. After thorough mixing of the content, the tubes were placed on a rotary shaker for 15 min, then centrifuged. The supernatant containing the protein from the nuclear extract was carefully removed and transferred to a fresh tube. The protein was measured, aliquoted, and stored at −80 °C until use.
The protocols for performing EMSA and supershift assays were as described previously (25). Based on the in silico analysis, oligonucleotide probes with NRF-2 tandem binding sites on each promoter were synthesized (Table 2), annealed, and labeled by a fill-in reaction with Klenow fragment and [32P]dATP (50 μCi/200 ng; PerkinElmer Life Sciences). Each labeled probe was incubated with 2 g of calf thymus DNA and 5 g of brain nuclear extract. Supershift assays were conducted using GABPα (NRF-2α) polyclonal antibodies (GABPα, H-180, SC-22810, Santa Cruz Biotechnology). The reactions were subjected to an incubation of 20 min with the antibody at room temperature. For competition, a 100-fold excess of unlabeled oligonucleotides were incubated with brain nuclear extract before adding labeled or nonspecific oligonucleotides. Shift reactions were loaded onto 4% polyacrylamide gel and run at 200 V for 3 h in 0.25× TBE buffer. Gels were dried and exposed for 4–24 h at −80 °C. COX6b with a known NRF-2 binding site was designed as previously described (25) and was used as a positive control. NRF-2 mutants with mutated sequences were used as negative controls.
TABLE 2.
EMSA probes
NRF-2 tandem binding sites are underlined.
| Gene Promoter | EMSA Sequence |
|---|---|
| COX6b | F: 5′-TTTTTCCTCTTGCAGCTTCCGGCCAGTC-3′ |
| R: 5′-TTTTGACTGGCCGGAAGCTGCAAGAGGA-3′ | |
| Bdnf exon IX site I | F: 5′-TTTTGTCTCTGCTTCCTTCCCACAGTTCCACC-3′ |
| R: 5′-TTTTGGTGGAACTGTGGGAAGGAAGCAGAGAC-3′ | |
| Bdnf exon IX site II | F: 5′-TTTTGTGGGAGGAAGTGGGAGCGGGGAAGCCACAG-3′ |
| R: 5′-TTTTCTGTGGCTTCCCCGCTCCCACTTCCTCCCAC-3′ | |
| Bdnf exon IX site III | F: 5′-TTTTGACTTGAAAGGAAGATTACTATTCCACTTGCA-3′ |
| R: 5′-TTTTTGCAAGTGGAATAGTAATCTTCCTTTCAAGTC-3′ | |
| Bdnf exon IX site IV | F: 5′-TTTTTGCCTTTCCTTGAGGACTAGTCCAGCTTTCCCC-3′ |
| R: 5′-TTTTGGGGAAAGCTGGACTAGTCCTCAAGGAAAGGCA-3′ | |
| Mutant Bdnf binding site I | F: 5′-TTTTGTCTCTGCTTACATACCACAGTACCAC-3′ |
| R: 5′-TTTTGGTGGTACTGTGGTATGTAAGCAGAGAC-3′ | |
| Mutant Bdnf binding site II | F: 5′-TTTTGTGGGATTAAGTGGGAGCGGGACAGCCACAG-3′ |
| R: 5′-TTTTCTGTGGCTGTCCCGCTCCCACTTAATCCCAC-3′ |
ChIP Assays
The protocol was as described previously (26) with minor modifications. The procedure was done with visual cortical tissues kept on ice at all times to avoid protein degradation. Tissue was cut in a Petri dish resting on a block of dry ice and then chopped into tiny pieces, fixed in 1% formaldehyde, and resuspended in the swelling buffer (85 mm KCl, 5 mm PIPES, pH 8.0, 1% Nonidet P-40, and protease inhibitors). The tissue was homogenized using a Dounce homogenizer and centrifuged to isolate the nuclei. The nuclei were resuspended and sonicated in SDS lysis buffer (1% SDS, 50 mm Tris-HCl, pH 8.1, 10 mm EDTA). Immunoprecipitation was performed with 2 μg of anti-GABPα (NRF-2α) polyclonal antibodies. Two μg of anti-NGFR antibodies (sc-6188, Santa Cruz Biotechnology) or no antibody blanks were used as negative controls. Based on our in silico analysis, putative NRF-2 binding sites were identified and semi-quantitative PCR was performed by utilizing primers surrounding the NRF-2 binding sites. Table 3 shows the PCR primers used for sites I–IV (see Fig. 2) of Bdnf exon IX promoter, as well as those for the positive control COX6b and the negative control exon 8 of NRF-1. PCR additives (betaine) and cycling parameters were used to improve the reproducibility and quality of ChIP. PCR products were run on 2% agarose gels to visualize the efficiency.
TABLE 3.
Primers used for ChIP analyses
| Gene Promoter | Sequence |
|---|---|
| COX6b | F: 5′-AAAGTGCGCAGGCGCTGGAG-3′ |
| R: 5′-CCGAGACGCTGACAGCACCG-3′ | |
| Bdnf exon IX site I | F: 5′-TGATTTGTGTCCCCTGCAGC-3′ |
| R: 5′-CAGCCTTCATGCAACCGAAG-3′ | |
| Bdnf exon IX site II | F: 5′-GTGACGGCGTGAACAGAGAT-3′ |
| R: 5′-GCTGCAGGGGACACAAATCA-3′ | |
| Bdnf exon IX site III | F: 5′-AGAGATGTCCGCACGTGAAA-3′ |
| R: 5′-TTCACGCCGTCACCAGAAAC-3′ | |
| Bdnf exon IX site IV | F: 5′-AGAGATGTCCGCACGTGAAA-3′ |
| R: 5′-TTCACGCCGTCACCAGAAAC-3′ | |
| NRF-1 exon 8 | F: 5′-GTGGAACAAAATTGGGCCAC-3′ |
| R: 5′-CTGTTAAGGGCCATGGTGA-3′ |
Luciferase Reporter Assay
Luciferase reporter constructs of the promoter of Bdnf exon IX were made by PCR cloning the proximal promoter sequences, using genomic DNA prepared from N2a cells as template. Digestion with restriction enzymes MluI and BglII was performed, followed by ligation of the product directionally into pGL3 basic vector (Promega, Madison, WI). Sequences of primers used for PCR cloning are provided (Tables 4). Site-directed mutations of NRF-2 tandem binding sites in the promoter of IX were generated using QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). Wild type and mutant COX6b were used as positive controls. Primers used for mutagenesis are listed in Table 5. All constructs were verified by sequencing.
TABLE 4.
Primers used for cloning analyses
| Gene promoter | Primer sequence |
|---|---|
| COX6b | F: 5′-TTGGTACCACTCTGCAGACAGCCTCAC-3′ |
| R: 5′-TTAAGCTTCGGAGCAGCGTTACTTCAAT-3′ | |
| Bdnf exon IX | F: 5′-CAGACGCGTGACGGCGTGAACAGAGATCA-3′ |
| R: 5′-CAGAGATCTTCCGTGGACGTTTGCTTCTT-3′ |
TABLE 5.
Primers used for site-directed mutagenesis analyses
Mutated NRF-2 binding sites are underlined.
| Gene promoter | Primer sequence |
|---|---|
| Mutant NRF-2 of COX6b | F: 5′-TCTCCTCTTGCAGCTAGAGGCCAGTCGGAATTCCG-3′ |
| R: 5′-CGGAATTCCGACTGGCCTCTAGCTGCAAGAGGAGA-3′ | |
| Mutant NRF-2 of Bdnf exon IX site I | F: 5′-GTGTCTGTCTCTGCTGACATACCACAGTACCACCAGGTGAG-3′ |
| R: 5′-CTCACCTGGTGGTACTGTGGTATGTCAGCAGAGACAGACAC-3′ | |
| Mutant NRF-2 of Bdnf exon IX site II | F: 5′-GTGGGATTAAGTGGGAGCGGGACAGCCACAGTGCCG-3′ |
| R: 5′-CGGCACTGTGGCTGTCCCGCTCCCACTTAATCCCAC-3′ |
Neuroblastoma cells were plated into 24-well plates before transfection. Each promoter construct was transfected into N2a cells using Lipofectamine 2000 (Invitrogen). Each well received 0.6 μg of reporter construct and 0.06 μg of pRL-TK Renilla luciferase vector (68). Firefly luciferase and Renilla luciferase activities were measured sequentially using the Dual Luciferase Reporter Assay System (Promega). The firefly luciferase activity was normalized according to Renilla and expressed as relative luciferase units to reflect the promoter activity.
Plasmid Construction for NRF-2α Knockdown, Transfection, and KCl Treatment
To knockdown NRF-2α expression, two target sequences of a vector-based shRNA (5′-ATTGCCCAGCCAGTCACG-3′ and 5′-AGAAGACAGAAGTTCACCG-3′) were cloned into the pBS/U6 parent vector. The PLKO.1-puro-CMV-TurboGFP positive control vector (SHC003, Sigma) containing TurboGFP and puromycin resistance was used to visualize transfection efficiency and selection. The pLK0.1 non-mammalian shRNA vector, which has a scrambled shRNA sequence with no known mammalian gene targets, was used as the negative control (SHC002, Sigma).
Transfection of primary neurons was carried out 4 days post-plating with both NRF-2α shRNA constructs (2 μg of each construct) or the pLKO.1 non-mammalian control (2 μg), using Neurofect transfection reagent per 6-well plate according to the manufacturer's instructions (Genlantis). TurboGFP (0.5 μg) vector was added to each well for transfection visualization and selection efficiency. Transfection efficiency was around 50–60% before selection. Puromycin selection, however, effectively yielded 100% transfected cells. Transfection efficiency was observed using green fluorescence. Primary neurons transfected with shRNA against NRF-2α were further stimulated with KCl. A final concentration of 20 mm KCl was added to the culture medium for 5 h according to our published method (68). After this period, cells were harvested for RNA and protein isolation.
NRF-2 Overexpression and TTX Treatment
NRF-2α and NRF-2β subunit expressing vectors were constructed by PCR cloning the human NRF-2α and NRF-2β from HeLa cell cDNA and human skeletal muscle cDNA library, respectively, as described earlier (69) (Table 6). The primer pairs used to amplify NRF-2α and NRF-2β had added HindIII and KpnI and NotI/BamHI restriction sites to their products, respectively. Amplification was done with Taq polymerase and products were cloned into pGEMT-EZ using TA cloning kit (Promega).
TABLE 6.
NRF-2α and NRF2-β cloning primers
| Gene | Primer sequence |
|---|---|
| NRF-2α | F: 5′-AAGCTTACTCCAGCCATGACTAAAAG-3′ |
| R: 5′-GGTACCAGCTATACTTGCTCTAAACAT-3′ | |
| NRF2-β | F: 5′-TTGCGGCCGCGATGTCCCTGGTAGATTTG-3′ |
| R: 5′-AAGGATCCTTAAACAGCTTCTTTATTAGTC-3′ |
As mentioned above, the transfection protocol for overexpressing NRF-2 in primary cultures was similar to that described for shRNA. Either 2 μg of NRF-2 overexpression vector, or 1.5 μg of the pcDNA3.1 empty vector and 0.5 μg of TurboGFP vector for green fluorescence were used for primary neuronal cultures. Transfected primary neurons were blocked for 3 days with TTX at a final concentration of 0.4 μmand starting on the day after transfection as previously described (68). Two to 4 days after transfection, neurons were harvested for RNA and protein isolation.
Transfected neurons were washed with PBS, followed by the extraction of total RNA by the TRIzol method according to the manufacturer's instructions and as mentioned above. Any residual genomic DNA was digested with DNase I on total RNA, and cDNA synthesis was carried out using an iScript cDNA synthesis kit. mRNA levels of various genes were determined in a Bio-Rad iCycler using IQ SYBR Green Supermix and/or a Cepheid Smart Cycler Detection system as described above. Primers for real-time are shown in Table 1.
Western Blots
Control, NRF-2 shRNA, and NRF-2 overexpression protein samples were harvested using the sample buffer (12.5 mm EDTA, 50 mm Tris-HCl, pH 6.8, 1% SDS, 10% glycerol). Equal protein amounts of each sample were electrophoresed in SDS gels and transferred onto polyvinylidene difluoride membranes. Membranes were blocked with 5% nonfat dry milk in TBS-T (150 mm NaCl, 50 mm Tris-HCl, pH 8.0, and 0.05% Tween 20) at room temperature for 1 h, then incubated with primary antibodies against NRF-2α (H-180, 1:1000; Santa Cruz Biotechnology), NRF-2β (gift of Dr. Scarpulla, Northwestern University), and BDNF (N-20, 1:500, Santa Cruz Biotechnology). The loading control was β-actin (1:5000; Sigma) followed by an incubation with horseradish peroxidase-conjugated secondary antibodies from Vector Laboratories (Burlingame, CA). ECL reagent was used to visualize protein position and intensity on blots, which were exposed to autoradiographic films (RPI, Mount Prospect, IL). The Gel Doc system (Bio-Rad) was used to perform quantitative analyses of relative changes.
Statistical Analysis
Data were analyzed using analysis of variance. Significance between two groups was analyzed by Student's t test. p values of 0.05 or less were considered significant.
Author Contributions
B. N. conducted all of the experiments, analyzed the results, and wrote most of the paper. M. W. R. conceived the idea for the project, oversaw the experiments, helped with the analysis of the data, and wrote the paper with B. N.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 EY018441. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- BDNF
- brain-derived neurotrophic factor
- COX
- cytochrome c oxidase
- GABP
- GA-binding protein
- NRF-2
- nuclear respiratory factor 2
- PGC-1α
- peroxisome proliferator-activated receptor-γ co-activator 1α
- TTX
- tetrodotoxin
- ETS
- E26 transformation-specific
- NGFR
- nerve growth factor receptor
- TSP
- transcription start point
- qPCR
- quantitative PCR.
References
- 1. Ernfors P., Wetmore C., Olson L., and Persson H. (1990) Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron 5, 511–526 [DOI] [PubMed] [Google Scholar]
- 2. Conner J. M., Lauterborn J. C., Yan Q., Gall C. M., and Varon S. (1997) Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J. Neurosci. 17, 2295–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Katoh-Semba R., Takeuchi I. K., Semba R., and Kato K. (1997) Distribution of brain-derived neurotrophic factor in rats and its changes with development in the brain. J. Neurochem. 69, 34–42 [DOI] [PubMed] [Google Scholar]
- 4. McCarthy D. M., Brown A. N., and Bhide P. G. (2012) Regulation of BDNF expression by cocaine. Yale J. Biol. Med. 85, 437–446 [PMC free article] [PubMed] [Google Scholar]
- 5. Yoshii A., and Constantine-Paton M. (2010) Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev. Neurobiol. 70, 304–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park H., and Poo M. M. (2013) Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 14, 7–23 [DOI] [PubMed] [Google Scholar]
- 7. Bekinschtein P., Cammarota M., Izquierdo I., and Medina J. H. (2008) BDNF and memory formation and storage. Neuroscientist 14, 147–156 [DOI] [PubMed] [Google Scholar]
- 8. Bibel M., and Barde Y. A. (2000) Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 14, 2919–2937 [DOI] [PubMed] [Google Scholar]
- 9. Murer M. G., Yan Q., and Raisman-Vozari R. (2001) Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog. Neurobiol. 63, 71–124 [DOI] [PubMed] [Google Scholar]
- 10. Binder D. K., and Scharfman H. E. (2004) Brain-derived neurotrophic factor. Growth Factors 22, 123–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bolaños C. A., and Nestler E. J. (2004) Neurotrophic mechanisms in drug addiction. Neuromolecular Med. 5, 69–83 [DOI] [PubMed] [Google Scholar]
- 12. Castrén E. (2004) Neurotrophins as mediators of drug effects on mood, addiction, and neuroprotection. Mol. Neurobiol. 29, 289–302 [DOI] [PubMed] [Google Scholar]
- 13. Cattaneo E., Zuccato C., and Tartari M. (2005) Normal huntingtin function: an alternative approach to Huntington's disease. Nat. Rev. Neurosci. 6, 919–930 [DOI] [PubMed] [Google Scholar]
- 14. Martinowich K., Manji H., and Lu B. (2007) New insights into BDNF function in depression and anxiety. Nat. Neurosci. 10, 1089–1093 [DOI] [PubMed] [Google Scholar]
- 15. Adachi N., Numakawa T., Richards M., Nakajima S., and Kunugi H. (2014) New sight in expression, transport, and secretion of brain-derived neurotrophic factor: implications in brain-related diseases. World J. Biol. Chem. 5, 409–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Timmusk T., Palm K., Metsis M., Reintam T., Paalme V., Saarma M., and Persson H. (1993) Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron 10, 475–489 [DOI] [PubMed] [Google Scholar]
- 17. Bishop J. F., Mueller G. P., and Mouradian M. M. (1994) Alternate 5′ exons in the rat brain-derived neurotrophic factor gene: differential patterns of expression across brain regions. Brain Res. Mol. Brain Res. 26, 225–232 [DOI] [PubMed] [Google Scholar]
- 18. Noble E. E., Billington C. J., Kotz C. M., and Wang C. (2011) The lighter side of BDNF. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R1053–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tao X., West A. E., Chen W. G., Corfas G., and Greenberg M. E. (2002) A calcium-responsive transcription factor, CaRF, that regulates neuronal activity dependent expression of BDNF. Neuron 33, 383–395 [DOI] [PubMed] [Google Scholar]
- 20. Zheng F., Zhou X., Moon C., and Wang H. (2012) Regulation of brain-derived neurotrophic factor expression in neurons. Int. J. Physiol. Pathophysiol. Pharmacol. 4, 188–200 [PMC free article] [PubMed] [Google Scholar]
- 21. Virbasius J. V., and Scarpulla R. C. (1991) Transcriptional activation through ETS domain binding sites in the cytochrome c oxidase subunit IV gene. Mol. Cell. Biol. 11, 5631–5638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelly D. P., and Scarpulla R. C. (2004) Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 18, 357–368 [DOI] [PubMed] [Google Scholar]
- 23. Rosmarin A. G., Resendes K. K., Yang Z., McMillan J. N., and Fleming S. L. (2004) GA-binding protein transcription factor: a review of GABP as an integrator of intracellular signaling and protein-protein interactions. Blood Cells Mol. Dis. 32, 143–154 [DOI] [PubMed] [Google Scholar]
- 24. Yang Z. F., Mott S., and Rosmarin A. G. (2007) The Ets transcription factor GABP is required for cell-cycle progression. Nat. Cell Biol. 9, 339–346 [DOI] [PubMed] [Google Scholar]
- 25. Ongwijitwat S., and Wong-Riley M. T. (2005) Is nuclear respiratory factor 2 a master transcriptional coordinator for all ten nuclear-encoded cytochrome c oxidase subunits in neurons? Gene 360, 65–77 [DOI] [PubMed] [Google Scholar]
- 26. Priya A., Johar K., and Wong-Riley M. T. (2013) Nuclear respiratory factor 2 regulates the expression of the same NMDA receptor subunit genes as NRF-1: both factors act by a concurrent and parallel mechanism to couple energy metabolism and synaptic transmission. Biochim. Biophys. Acta 1833, 48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Priya A., Johar K., Nair B., and Wong-Riley M. T. (2014) Nuclear respiratory factor 2 regulates the transcription of AMPA receptor subunit GluA2 (Gria2). Biochim. Biophys. Acta 1843, 3018–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aid T., Kazantseva A., Piirsoo M., Palm K., and Timmusk T. (2007) Mouse and rat BDNF gene structure and expression revisited. J. Neurosci. Res. 85, 525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang C., and Wong-Riley M. (1999) Expression and regulation of NMDA receptor subunit R1 and neuronal nitric-oxide synthase in cortical neuronal cultures: correlation with cytochrome oxidase. J. Neurocytol. 28, 525–539 [DOI] [PubMed] [Google Scholar]
- 30. Nair B., Johar K., Priya A., and Wong-Riley M. T. (2016) Specificity protein 4 (Sp4) transcriptionally regulates inhibitory GABAergic receptors in neurons. Biochim. Biophys. Acta 1863, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barde Y. A., Edgar D., and Thoenen H. (1982) Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1, 549–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cohen S., Levi-Montalcini R., and Hamburger V. (1954) A nerve growth-stmulating factor isolated from sarcomas 37 and 180. Proc. Natl. Acad. Sci., U.S.A. 40, 1014–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McAllister A. K., Lo D. C., and Katz L. C. (1995) Neurotrophins regulate dendritic growth in developing visual cortex. Neuron 15, 791–803 [DOI] [PubMed] [Google Scholar]
- 34. Ventimiglia R., Mather P. E., Jones B. E., and Lindsay R. M. (1995) The neurotrophins BDNF, NT-3 and NT-4/5 promote survival and morphological and biochemical differentiation of striatal neurons in vitro. Eur. J. Neurosci. 7, 213–222 [DOI] [PubMed] [Google Scholar]
- 35. Patterson S. L., Abel T., Deuel T. A., Martin K. C., Rose J. C., and Kandel E. R. (1996) Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron 16, 1137–1145 [DOI] [PubMed] [Google Scholar]
- 36. Takei N., Sasaoka K., Inoue K., Takahashi M., Endo Y., and Hatanaka H. (1997) Brain-derived neurotrophic factor increases the stimulation evoked release of glutamate and the levels of exocytosis-associated proteins in cultured cortical neurons from embryonic rats. J. Neurochem. 68, 370–375 [DOI] [PubMed] [Google Scholar]
- 37. Lessmann V. (1998) Neurotrophin-dependent modulation of glutamatergic synaptic transmission in the mammalian CNS. Gen. Pharmacol. 31, 667–674 [DOI] [PubMed] [Google Scholar]
- 38. Poo M. M. (2001) Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2, 24–32 [DOI] [PubMed] [Google Scholar]
- 39. Nestler E. J., Barrot M., DiLeone R. J., Eisch A. J., Gold S. J., and Monteggia L. M. (2002) Neurobiology of depression. Neuron 34, 13–25 [DOI] [PubMed] [Google Scholar]
- 40. Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., Weisstaub N., Lee J., Duman R., Arancio O., Belzung C., and Hen R. (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809 [DOI] [PubMed] [Google Scholar]
- 41. Cunha A. B., Frey B. N., Andreazza A. C., Goi J. D., Rosa A. R., Gonçalves C. A., Santin A., and Kapczinski F. (2006) Serum brain-derived neurotrophic factor is decreased in bipolar disorder during depressive and manic episodes. Neurosci. Lett. 398, 215–219 [DOI] [PubMed] [Google Scholar]
- 42. Palomino A., Vallejo-Illarramendi A., Gonáalez-Pinto A., Aldama A., González-Gómez C., Mosquera F., González-García G., and Matute C. (2006) Decreased levels of plasma BDNF in first-episode schizophrenia and bipolar disorder patients. Schizophr. Res. 86, 321–322 [DOI] [PubMed] [Google Scholar]
- 43. Pruunsild P., Sepp M., Orav E., Koppel I., and Timmusk T. (2011) Identification of cis-elements and transcription factors regulating neuronal activity-dependent transcription of human BDNF gene. J. Neurosci. 31, 3295–3308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Deleted in proof
- 45. Tao X., Finkbeiner S., Arnold D. B., Shaywitz A. J., and Greenberg M. E. (1998) Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20, 709–726 [DOI] [PubMed] [Google Scholar]
- 46. Zheng F., Zhou X., Luo Y., Xiao H., Wayman G., and Wang H. (2011) Regulation of brain-derived neurotrophic factor exon IV transcription through calcium response elements in cortical neurons. PLoS ONE 6, e28441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jiang X., Tian F., Du Y., Copeland N. G., Jenkins N. A., Tessarollo L., Wu X., Pan H., Hu X. Z., Xu K., Kenney H., Egan S. E., Turley H., Harris A. L., Marini A. M., and Lipsky R. H. (2008) BHLHB2 controls Bdnf promoter 4 activity and neuronal excitability. J. Neurosci. 28, 1118–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kairisalo M., Korhonen L., Sepp M., Pruunsild P., Kukkonen J. P., Kivinen J., Timmusk T., Blomgren K., and Lindholm D. (2009) NF-κB-dependent regulation of brain-derived neurotrophic factor in hippocampal neurons by X-linked inhibitor of apoptosis protein. Eur. J. Neurosci. 30, 958–966 [DOI] [PubMed] [Google Scholar]
- 49. Ma D. K., Jang M. H., Guo J. U., Kitabatake Y., Chang M. L., Pow-Anpongkul N., Flavell R. A., Lu B., Ming G. L., and Song H. (2009) Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 323, 1074–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Matsumoto T., Rauskolb S., Polack M., Klose J., Kolbeck R., Korte M., and Barde Y.-A. (2008) Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat. Neurosci. 11, 131–133 [DOI] [PubMed] [Google Scholar]
- 51. Teng H. K., Teng K. K., Lee R., Wright S., Tevar S., Almeida R. D., Kermani P., Torkin R., Chen Z.-Y., Lee F. S., Kraemer R. T., Nykjaer A., and Hempstead B. L. (2005) ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J. Neurosci. 25, 5455–5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Klein R., Nanduri V., Jing S. A., Lamballe F., Tapley P., Bryant S., Cordon-Cardo C., Jones K. R., Reichardt L. F., and Barbacid M. (1991) The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell 66, 395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Scarpulla R. C. (1997) Nuclear control of respiratory chain expression in mammalian cells. J. Bioenerg. Biomembr. 29, 109–119 [DOI] [PubMed] [Google Scholar]
- 54. Agrawal R., Tyagi E., Vergnes L., Reue K., and Gomez-Pinilla F. (2014) Coupling energy homeostasis with a mechanism to support plasticity in brain trauma. Biochim. Biophys. Acta 1842, 535–546 [DOI] [PubMed] [Google Scholar]
- 55. Gomez-Pinilla F., Vaynman S., and Ying Z. (2008) Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur. J. Neurosci. 28, 2278–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Markham A., Cameron I., Franklin P., and Spedding M. (2004) BDNF increases rat brain mitochondrial respiratory coupling at complex I, but not complex II. Eur. J. Neurosci. 20, 1189–1196 [DOI] [PubMed] [Google Scholar]
- 57. Markham A., Bains R., Franklin P., and Spedding M. (2014) Changes in mitochondrial function are pivotal in neurodegenerative and psychiatric disorders: how important is BDNF? Br. J. Pharmacol. 171, 2206–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Burkhalter J., Fiumelli H., Allaman I., Chatton J. Y., and Martin J. L. (2003) Brain-derived neurotrophic factor stimulates energy metabolism in developing cortical neurons. J. Neurosci. 23, 8212–8220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Caldeira M. V., Melo C. V., Pereira D. B., Carvalho R., Correia S. S., Backos D. S., Carvalho A. L., Esteban J. A., and Duarte C. B. (2007) Brain-derived neurotrophic factor regulates the expression and synaptic delivery of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J. Biol. Chem. 282, 12619–12628 [DOI] [PubMed] [Google Scholar]
- 60. Caldeira M. V., Melo C. V., Pereira D. B., Carvalho R. F., Carvalho A. L., and Duarte C. B. (2007) BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol. Cell. Neurosci. 35, 208–219 [DOI] [PubMed] [Google Scholar]
- 61. Martin J. L., and Finsterwald C. (2011) Cooperation between BDNF and gluatamate in the regulation of synaptic transmission and neuronal development. Commun. Integr. Biol. 4, 14–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Puigserver P., Wu Z., Park C. W., Graves R., Wright M., and Spiegelman B. M. (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 [DOI] [PubMed] [Google Scholar]
- 63. Wu Z., Puigserver P., Andersson U., Zhang C., Adelmant G., Mootha V., Troy A., Cinti S., Lowell B., Scarpulla R. C., and Spiegelman B. M. (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124 [DOI] [PubMed] [Google Scholar]
- 64. Liang H. L., Dhar S. S., and Wong-Riley M. T. (2010) p38 mitogen-activated protein kinase and calcium channels mediate signaling in depolarization-induced activation of peroxisome proliferator-activated receptor gamma coactivator-1α in neurons. J. Neurosci. Res. 88, 640–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Livak K. J., and Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−[/Delta][/Delta] CT) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 66. Ongwijitwat S., and Wong-Riley M. T. (2004) Functional analysis of the rat cytochrome c oxidase subunit 6A1 promoter in primary neurons. Gene 337, 163–171 [DOI] [PubMed] [Google Scholar]
- 67. Lahiri D. K., and Ge Y. (2000) Electrophoretic mobility shift assay for the detection of specific DNA-protein complex in nuclear extracts from the cultured cells and frozen autopsy human brain tissue. Brain Res. Brain Res. Protoc. 5, 257–265 [DOI] [PubMed] [Google Scholar]
- 68. Dhar S. S., and Wong-Riley M. T. (2009) Coupling of energy metabolism and synaptic transmission at the transcriptional level: role of nuclear respiratory factor 1 in regulating both cytochrome c oxidase and NMDA glutamate receptor subunit genes. J. Neurosci. 29, 483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ongwijitwat S., Liang H. L., Graboyes E. M., and Wong-Riley M. T. (2006) Nuclear respiratory factor 2 senses changing cellular energy demands and its silencing down-regulates cytochrome oxidase and other target gene mRNAs. Gene 374, 39–49 [DOI] [PubMed] [Google Scholar]