Abstract
Background
A whole-body vibration technique delivered in a horizontal position through a massage mattress was introduced in 2007. The present study analyzed the effects of different exposure periods to these vibrations on microcirculation of mice.
Material/Methods
Different periods of vibrations (30Hz) were locally delivered in a horizontal position on the external abdominal skin in 3 randomized groups of mice (N=42). The 3 groups receiving vibrations were compared to an untreated control group (N=14). The 3 experimental groups received 3, 6, and 10 min of vibrations. The in vivo measurement of the arterial and venous diameters was done before and after each vibration period.
Results
Average venous diameters (μm) after 6 to 10 min of vibrations were significantly increased (7% and 12%, p values 0.026 and 0.013, respectively), but 3 min did not significantly change average venous diameters. Arterial diameters (μm) did not significantly vary after 3, 6, and 10 min. In the control group, variations of arterial and venous diameters during 10 min were not significant.
Conclusions
This study shows a vasodilatory effect of low-frequency vibrations. The hypothesis of local cutaneous blood flow increase is retained. A phenomenon of shear stress of the endothelium induced by skin massage generates this local venous vasodilation and blood flow increase.
MeSH Keywords: Massage, Mice, Microcirculation, Vasodilation, Vibration
Background
Whole-body vibrations delivered in a vertical position are frequently used by athletes, in wellness centers, and for medical purposes [1]. Their effects on blood circulation and cutaneous microcirculation in humans are thoroughly established. In 2007, a new technique of whole-body vibrations was introduced for medical purposes. Two biophysical treatment modalities (multidirectional stochastic vibrations and infra-red light) are delivered in a horizontal position through a massage mattress. The future therapeutic possibilities of this new method are very promising for skin blood microcirculation and lymphatic vessels stimulation, especially in the field of prevention and treatment of blood and lymphatic system diseases from which many people suffer. Mechanical skin massage induced in this position by the vibrations seems to have a positive effect on the superficial lymphatic system. In fact, a first study on laboratory animals about the effects of this type of vibration upon the lymphatic system showed an increased accumulation in the lymphatic nodes of a subcutaneously injected tracer after vibrations [2]. There has been no study published about the effects of multidirectional stochastic vibrations delivered in a horizontal position on skin blood microcirculation. We propose to investigate it in skin microcirculation laboratory animal models.
The aim of this study was to analyze the immediate effects of different periods of exposure to multidirectional vibrations delivered in a horizontal position (andullation) on arterial and venous cutaneous microcirculation of the abdomen in mice.
Material and Methods
Animals
Experiments were performed on white female mice of N.M.R.I. origin (Naval Medical Research Institute, Bethesda, Maryland, USA), aged between 6 and 8 weeks and weighing around 28–30 grams.
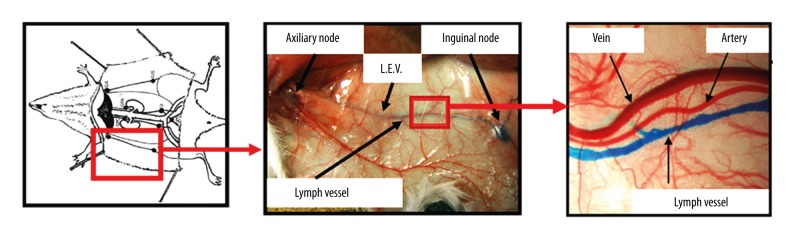
The epigastric lateral superficial vein and its artery, both situated between the lymphatic axillary and inguinal nodes, were used as a subcutaneous vascular model during the experiments (Figure 1).
Figure 1.
Lateral epigrastic vein (LEV) (and artery) of the right hemi-abdomen of the mouse after dissection.
The distribution of the animals into 3 groups was randomized (N=42). Each experimental group, as well as the control group, consisted of 14 mice.
Investigations were approved by the Ethics Committee for Animal Experimentation of Brussels Free University (Vrije Universiteit Brussel, V.U.B.). Animal care and housing in the facility followed the recommendations of the Federation of European Laboratory Animal Science Associations (http://www.felasa.eu). Upon arrival, animals were placed in cages in a special room (5 animals per cage). Mice were given an adaptation period of 1 week to acclimatize to the conditions in the facility.
Before anesthesia, mice were given a period of 20 min of adaptation to acclimatize to the ambient laboratory temperature (20–22°C). Before interventions, the animal welfare was evaluated with the control of the absence of general signs of pain or suffering (http://www.felasa.eu). All animals were anaesthetized with urethane (2 mg/g i.p.).
During this intervention, clinical signs of the depth of the anesthesia were used to control the pain and suffering and humane end-points were selected for determining when animal experiments should be discontinued (http://www.felasa.eu).
At the end of the experiments, the animals are euthanized by cervical dislocation.
Visualization of cutaneous microcirculation and vibrating platform
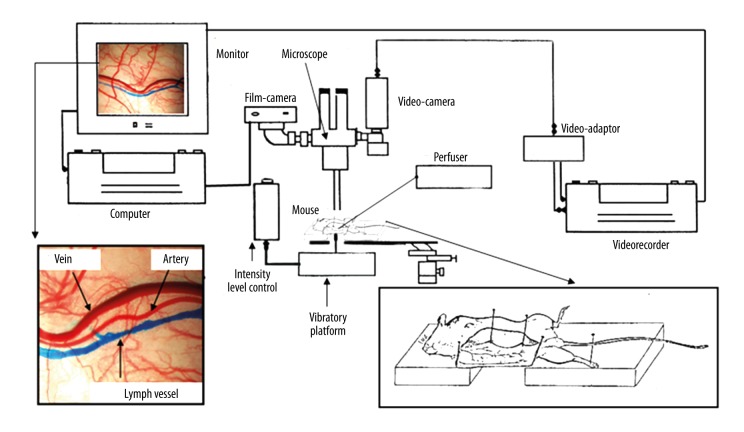
The in vivo visualization technique under a microscope (Figure 2) was used to investigate subcutaneous microcirculation [3]. After anesthesia and hair removal using small scissors, the right hemi-abdomen skin of the animals was retracted to uncover the subcutaneous epigastric vessels. Then, each dissected animal was placed in an identical position (right lateral decubitus) on a mobile platform (Figure 2) to obtain a perfect contact (without stretching) between the external skin side and a support able to generate vibrations (Figure 3). Microcirculation was observed under a microscope (ZEISS 50, West Germany, OpMi-1 89183). Photographs and a video recording were made during experiments with a digital photo camera (Nikon D80) and a video camera (Sony DXC-101P) (Figure 2).
Figure 2.
Experimental material and assembly used to visualize in vivo cutaneous microcirculation of the hemi-abdomen of the mouse.
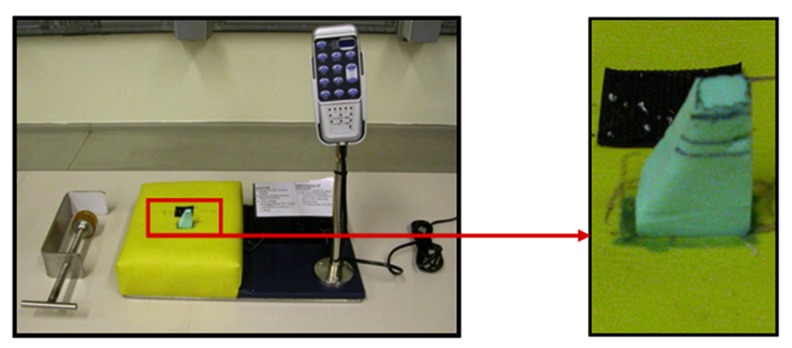
Figure 3.
Support used during experiments to generate vibrations through a built-in motor with associated control. The right image shows the external part (made of foam) delivering the vibrations on the external side of the retracted skin directly from the internal motor.
Measuring the diameter of the blood vessels
We measured vessel diameters under a microscope. This type of measuring in vivo allowed us to obtain direct data on the influence of multidirectional vibrations (delivered through andullation technology) on blood microcirculation of the skin, especially on vasomotricity of subcutaneous arterial and venous vessels. The diameters were measured by means of image J software on photographs taken during the experiments.
Photographs were taken and opened at the same size (1936×1296 pixels, RGB, 9,6 MB). Before starting the experiments, several enlargement scales were calculated for each enlargement possibility of the microscope. Therefore, a squared plate (1mmx1mm) was photographed at each enlargement of the microscope in order to mathematically determine the enlargement relationship between the real vessel diameters and the distances measured on the photographs with the software.
Experimental protocol
After the animals were placed in an experimental position, a rest period of 10 min was allowed for each animal. At the beginning of this period, the internal side of the retracted skin was moistened with 3 drops of physiological solution (Na Cl, 0.9%, kept at ambient temperature) to avoid skin dehydration during the experiments.
After this rest period, the mice of the 3 experimental groups received either 3, 6 or 10 min of continuous local vibrations (30 Hz), while the mice in the control group were submitted to the same experimental conditions without receiving any vibrations. Photographs of the epigastric vessels situated in the selected cutaneous zone were taken at the end of the rest period (T1) and at the end of the treatment period or the placebo period (T2). A summary of this experimental protocol is given in Figure 4.
Figure 4.
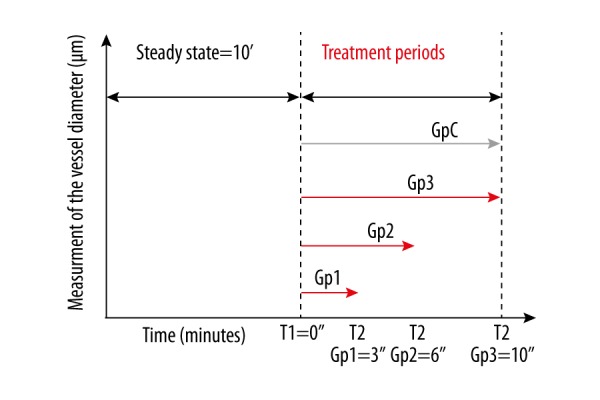
Graphical synthesis of the experimental protocol used for the 4 groups of mice. (Gp=group, Gp C=control group, T1 and T2 are the moments at which the photographs of the vessels were taken.)
The ambient room temperatures near the microscope, the rectal temperatures (temperature probe TM-100, recorder iWorx HK-214S), and the temperatures of the internal skin sides at the concerned spots (infra-red thermometer, TFI 220 Ebro), were also taken at the T1 and T2 moments. These temperature measurements were done with the purpose of controlling the possible influence of the evolution of these 3 temperatures upon the vasomotricity of the vessels. All the experiments were done by the same experimenter.
Statistical analysis
Statistical analysis was performed using SPSS software (Statistical Package for the Social Sciences) version 22.
Descriptive statistics for the continuous outcome variables are presented by mean ± standard deviation. To compare vessel diameters at T1 before vibration and T2 after vibration for groups 1, 2, and 3, a paired t test was used. The same test was performed to compare vessel diameters at T1 before vibration and T3 after vibration (or placebo period) for the 3 experimental groups and the control group. Variations of local cutaneous temperatures measured for the 3 groups and the control group were tested with an unpaired t test. All tests were performed with α=0.05.
To determine the possible correlation between arterial and venous diameters and temperature variations, the Pearson test was used.
Results
Is there a local vasomotor effect upon the arterial and venous vessels of cutaneous microcirculation, when the skin is subjected for different periods of exposure to multidirectional vibrations generated by andullation technology?
Arterial diameters
After exposure periods of 3, 6, and 10 min, the mean arterial diameters at T1 (μm, groups 1, 2, 3: 65, 33±14.11; 78.30±14.04; 83.20±12.59, respectively) do not undergo any significant vasomotor effect (T2 values, groups 1, 2, 3: 67.33±16.23; 80±15.86; 85, 30±10.95 and respective p values: 0.158; 0.2149; 0.0638, respectively). The same was true for the control group: 10 min of exposure to this situation in the same experimental conditions did not significantly modify the mean diameter of the arteries (T1: 90.58±16.57 μm, T2: 92.67±20.11, p value=0.4094) (Figure 5).
Figure 5.
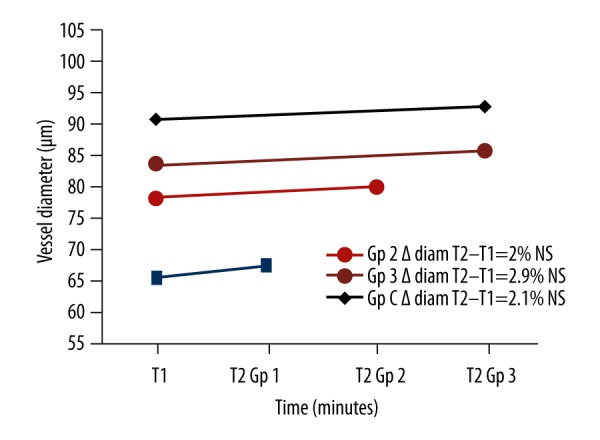
Arterial diameters for the 4 groups.
Venous diameters (Figure 6)
Figure 6.
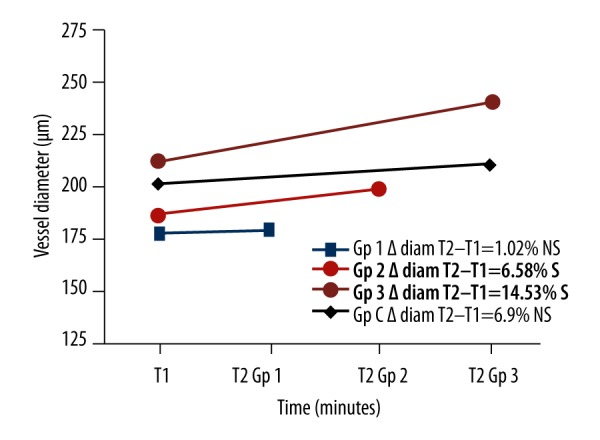
Venous diameters for the 4 groups.
After exposure periods of 3 min (group 1), the mean venous diameters (T1: 177.2±61.62 μm) did not undergo any significant vasomotor effect (T2: 178.8±61.61, p values=0.616). The same was true for the control group: 10 min of exposure to this situation in the same experimental conditions did not significantly modify the average diameter of the veins (T1: 200.5±44.66 μm, T2: 210.3±33.62, p=0.192). In contrast, 6 and 10 min of local vibrations (T1: 185.9±37.6; 210.8±32.53 μm) significantly increased the average venous diameters by 6.58% and 14.53% (T2: 198.6±45.78; 239.5±39.8 μm, respective p values: 0.026; 0.013) (Figure 7).
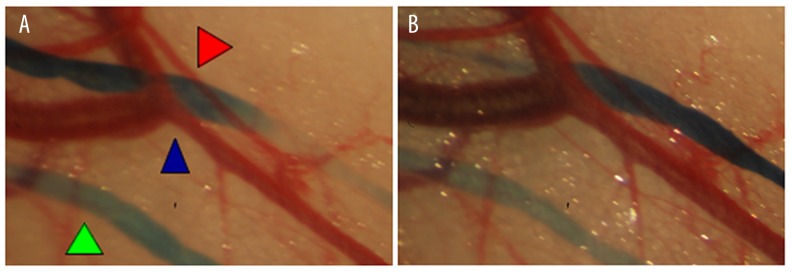
Figure 7.
Example of a local venous dilatation after 6-min vibrations. Microscopic view of the vascular vessels of the mouse abdominal skin internal face. Green arrow – lymphatic vessels after blue dye injection, red arrow – artery, blue arrow – vein. (A) Before vibrations, (B) after 6-min vibration.
Influence of experimental temperature conditions on vasomotricity of the blood vessels
During experiments in all groups, mean room temperature variations near the experimental side were 0.03±0.12°C during the measurements on mice of which arteries could be measured, and 0.04±0.14°C during measurements on mice of which veins could be measured. We consider there very small ambient temperature variations to be insignificant, as well as their effects upon the vasomotricity of the analyzed vessels.
Mean temperature variations of the internal cutaneous sides at times T1 and T2 of arterial diameters were −0.4553±0.5574°C, while mean variations measuring venous diameters were 0.4542±0.5473°C.
There was no statistically significant difference between the variations of local cutaneous temperatures measured for the 3 groups and the control group (skin temperature variations T1 before vibrations and T2 after vibrations) concerning the measures of the venous diameters (p value=0.5277). The same can was true for the variations of local cutaneous temperatures concerning the arterial diameters (p value=0.379).
Statistical analysis did not reveal any direct correlation between temperature decreases measured at the internal skin sides (or rectal temperature reductions) and the variations of arterial and venous diameters (Table 1). The only proven relationships were the correlations between rectal temperature reductions and temperature reductions measured at the retracted internal cutaneous sides (Table 1) during the 2 types of arterial and venous measurement.
Table 1.
Correlation between the temperature differences measured at the internal skin side in function of arterial diameter variations (above) and venous diameter variations (below).
| Δ Arterial diameter T2–T1 | Δ rectal temp T2–T1 | ||
|---|---|---|---|
| Δ skin temp T2–T1 | Pearson Correlation | 0.104 | 0.527** |
| Sig. (2-tailed) | 0.535 | 0.001 | |
| N | 38 | ||
|
| |||
| Δ Venous diameter T2-T1 | Δ rectal temp T2-T1 | ||
|
| |||
| Δ skin temp T2–T1 | Pearson Correlation | −0.050 | 0.496** |
| Sig. (2-tailed) | 0.742 | 0.000 | |
| N | 46 | 46 | |
Significant correlation: 0.01 level (2-tailed).
Discussion
Results
In the above-mentioned experimental conditions, the obtained results show that periods of exposure exceeding or equal to 6 min to multidirectional vibrations delivered through direct skin contact generate a significant venous vasodilation.
These exposure times do not seem to have any significant vasomotor effect upon arteries. Nevertheless, it is important to emphasize that after an exposure time of 10 min to horizontal vibrations, a non-significant vasodilation of the arteries was nevertheless observed, with a p value of 0.0638, showing a possible trend towards artery diameter increase after a 10-min vibration period. This situation can be explained by the small number of arterial diameters measured at the end.
A first possible hypothesis to explain the obtained results is that multidirectional vibrations delivered in a horizontal position have a warming-up effect upon the skin, which can influence vasomotricity. In our experimental conditions (humidification and open-air exposure of cutaneous microcirculation), 10 min of localized vibrations did not bring about any increase of local temperature through the ‘mechanical friction effect’. Indeed, there was no significant difference between the variations of local cutaneous temperatures measured for the 3 groups and the control group in venous and arterial diameters.
A second possible hypothesis is that multidirectional vibrations delivered in a horizontal position have a vasodilatory effect through increasing cutaneous blood flow. Lohman et al. [4] showed in 2007 that in healthy men, 3 min of continuous exposure to vibrations of 30 Hz delivered in a horizontal position (group 3, where the calves of the subjects were resting horizontally on a vibrating platform) very significantly increase the cutaneous blood flow as measured by Doppler laser. In their study, skin contact with the vibrating element was quite considerable, whereas in our study the contact zone was very small (5×5 mm). This can also explain the more ambiguous results obtained for arterial diameters.
In 2008, Maloney-Hinds et al. [5] showed that in healthy men, periods of exposure of 4, 6, and 9 min to vibrations of 30 Hz delivered in a horizontal position (the forearms of the subjects were resting in a horizontal position on a vibrating platform) very significantly increased cutaneous blood flow measured by Doppler laser. The vibrations in their study were delivered in a discontinuous way in order to allow measuring with a Doppler laser. This type of protocol with discontinuous vibrations, as well as the difference of vibrated cutaneous surfaces, can explain the different results of our study.
The mechanism hypothesized to support our second hypothesis is the “massage effect” generated by the vibrations. This local massaging of the skin, which is done under a certain frequency (30 Hz), logically gives rise to cell friction in the vibrated tissues. As for the vascular aspect, the most plausible explanation, also proposed by Lohman et al. [4], is that vasodilation is induced by the liberation of nitric oxide (NO) as a result of “shear stress” of the vascular wall.
In vivo diameter measuring technique
The first major difficulty with in vivo measuring of vascular diameters under a microscope is the possibility of having luminous artefacts while photographing. These artefacts obscure certain zones of the microscopic field, even if all precautions were taken. The reason of this phenomenon is the presence of a layer of indispensable physiological serum, which sometimes can reflect the light shining on the microscopic field. The second difficulty lies in the fact that sometimes it is impossible to delimitate in a precise way on photographs the walls of the smaller vessels, due to the thickness of subcutaneous adipose tissue in which vessels occur at unequal depth. For these 2 reasons, the final numbers of exploitable vessels of each group were not equivalent, especially for the arteries. This technique also necessitates the selection of a microscopic field in which the vein and the artery are visible and clearly distinguishable. This necessity forces the experimenter to choose a specific observation spot that can be situated at anyplace on the entire visible length of the vessels. The diameter of the vessels depends upon the localization of the chosen observation spot. This explains most of the non-homogeneity of vascular diameters in the 4 groups of mice.
The Doppler laser used to obtain a quantitative measurement of the cutaneous blood flow did not allow us to dissociate a possible vasomotor effect from an action through shear stress upon each type of small blood vessels. Usually, in clinical investigation, this shear stress effect is analyzed by echography on vessels having a larger diameter, measuring the flow- mediated dilatation (FMD), for example, on the brachial artery [6]. In microcirculation, animal experimentation with direct in vivo measurement of the vessel diameters allows this type of investigation (measuring of the FMD) on each vessel type, which is one of the advantages of animal experimentation to understand the action mechanisms of certain physical techniques on the vascular endothelium.
Possible influences of experimental conditions (cutaneous temperature falls due to dissection, humidification and exposure time) upon vasomotricity of local cutaneous microcirculation
One of our hypotheses was that subcutaneous arterial and venous vasodilation can appear in order to remedy a cutaneous temperature fall due to experimental factors, such as exposure to open air of the internal skin side of the hemi-abdomen. However, this cannot be proven. There was no significant variation of the arterial and venous diameters in the control group during at least 20 min of exposure to open air of the internal side of the skin of the hemi-abdomen, in spite of the local cutaneous temperature fall and results regarding arterial and venous diameters.
The same conclusions have to be reached concerning the possible effects of humification of the internal side of the skin and the 20 min exposure time to open air of the examined cutaneous microcirculation.
Control group
The choice of a single control group with a placebo treatment period of 10 min can be justified by the first R (Reduce the number of used animals) of the 3 R’s rule (Refine methodology, Replace animal models), applying to animal experimentation [7]. For this reason, we chose a single control group with a placebo treatment time at least equal to that of group 3 (the longest vibration treatment).
Protocol
A 10-min rest time before vibrations or placebo period was selected in order to cover the time necessary to normalize cutaneous microcirculation after dissection, retraction, and humidification of the skin. We did not choose to measure the vessel diameters over a prolonged period after vibration, as was done in the study by Maloney-Hinds et al. [5]. Indeed, an excessive period of exposure of the cutaneous vessels to open air and dehydration would not allow making the measurements, as the measurements would probably be distorted over this period, during which too many external factors would intervene.
Upcoming clinical research
It has recently been shown that low-frequency vibrations (about 30 Hz) are beneficial for microcirculation [4,5] and, conversely, that high-frequency whole-body vibrations of 80–100 Hz harm microcirculation and in certain cases produce Raynaud phenomena [8]. The first results of this study show a vasodilatory effect of low-frequency vibrations (30 Hz) in a horizontal position through andullation technology upon veins, as well as a vasodilatory tendency of arteries. These very encouraging results encourage us to continue research on humans, especially on patients with microcirculation disorders [9]. External skin massages provided by medical devices have more medical importance in the treatment of blood microcirculations dysfunction due to vascular diseases, such as intermittent compression therapy for the treatment of postoperative leg swelling in patients with chronic leg ischemia [10] Low-frequency and multidirectional stochastic vibrations delivered in horizontal position are a very promising additional physical treatment method in vascular medicine. Maloney-Hinds et al. [5] found that the cutaneous blood flow is more abundant in cutaneous zones in direct contact with the vibrating element, as well as at the opposite side of this contact zone. Based on this observation, it seems logical from a medical point of view that better blood supply to the vibrated cutaneous tissue can be obtained with whole-body vibrations in a horizontal position (patient in dorsal or ventral decubitus) and with the largest surface of the body in direct contact with the vibrating elements. This is precisely the concept of andullation technology, which allows use of low-frequency whole-body vibrations through a mattress (containing 21 vibration generating sources/motors, ANDUMEDIC®3 Professional, Home Health Products, Oudenaarde, Belgium) on which patients are lying down.
Limitations of the study
The small number of animals is the first limitation, but can also be justified by the first R (Reduce the number of used animals) of the 3 R’s rule (Refine methodology, Replace animal models) applying to animal experimentation [7]. The selected animal model and the direct in vivo method to assess vessel diameter did not allows us to extend time of experiments as described above.
Finally, the measurement of vessel diameter does not provide direct quantitative data on blood flow.
Conclusions
This preliminary study on mice shows a vasodilatory effect of low-frequency multidirectional vibrations (30 Hz) delivered in a horizontal position upon veins, as well as a vasodilatory effect on the arteries of cutaneous microcirculation. To explain our results, we retain the hypothesis proposed by Lohmann et al. [4] concerning the increase of the local cutaneous blood flow generated by the vibrations. Indeed, it is very likely that a phenomenon of shear stress of the vascular endothelium induced by skin massage brings about this venous vasodilation and this increase of local blood stream. These very encouraging results encourage us to continue our research on the therapeutic possibilities of low-frequency vibrations delivered in a horizontal position on humans with microcirculation disorders.
Acknowledgements
We thank the International Association for Andullation Technologies (I.A.A.T.) for the material and scientific support.
Footnotes
Source of support: Departmental sources
References
- 1.Prisby RD, Lafage-Proust MH, Malaval L, et al. Effects of whole body vibration on the skeleton and other organ systems in man and animal models: What we know and what we need to know. Ageing Res Rev. 2008;7(4):319–29. doi: 10.1016/j.arr.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Pastouret F, Cardozo L, Ruault J-P, et al. Spectrophotometric determination of lymph nodes dye accumulation after short exposure to multidirectional vibrations (andullation) or manual massage, in mice. Lymphology, Progress in Lymphology XXIV. 2014;47(Suppl):61–67. [Google Scholar]
- 3.Pastouret F, Lievens P, Leduc O, et al. Short time effects of radiotherapy on lymphatic vessels and restorative lymphatic pathways: Experimental approaches ina mouse model. Lymphology. 2014;47(2):92–100. [PubMed] [Google Scholar]
- 4.Lohman EB, 3rd, Petrofsky JS, Maloney-Hinds C, et al. The effect of whole body vibration on lower extremity skin blood flow in normal subjects. Med Sci Monit. 2007;13(2):CR71–76. [PubMed] [Google Scholar]
- 5.Maloney-Hinds C, Petrofsky JS, Zimmerman G. The effect of 30 Hz vs. 50 Hz passive vibration and duration of vibration on skin blood flow in the arm. Med Sci Monit. 2008;14(3):CR112–16. [PubMed] [Google Scholar]
- 6.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 7.Richmond J. The 3Rs-Past, present and future. Scand J Lab Anim Sci. 2000;27:84–92. [Google Scholar]
- 8.Stoyneva Z, Lyapina M, Tzvetkov D, Vodenicharov E. Current pathophysiological views on vibration-induced Raynaud’s phenomenon. Cardiovas Res. 2003;57(3):615–24. doi: 10.1016/s0008-6363(02)00728-9. [DOI] [PubMed] [Google Scholar]
- 9.Maloney-Hinds C, Petrofsky JS, Zimmerman G, Hessinger DA. The role of nitric oxide in skin blood flow increases due to vibration in healthy adults and adults with type 2 diabetes. Diabetes Technol Ther. 2009;11(1):39–43. doi: 10.1089/dia.2008.0011. [DOI] [PubMed] [Google Scholar]
- 10.Pawlaczyk K, Gabriel M, Urbanek T, et al. Effects of intermittent pneumatic compression on reduction of postoperative lower extremity edema and normalization of foot microcirculation flow in patients undergoing arterial revascularization. Med Sci Monit. 2015;21:3986–92. doi: 10.12659/MSM.895229. [DOI] [PMC free article] [PubMed] [Google Scholar]