PSORIASIS UPDATE
Patient-reported Outcomes: Association with Clinical Response to Psoriasis Therapies
Patient-reported outcomes (PROs) of psoriasis control do not always align with objective clinical observations of the disease. PROs are inherently subjective, but these self-reported assessments can complement objective observations, provide important insights into how patients perceive their own disease and its treatment, and may help advance care by incorporating this point of view into how clinicians treat psoriasis.
In a retrospective study of patients with moderate-to-severe psoriasis treated with biologics (adalimumab and etanercept), methotrexate, or placebo drawn from several recent clinical trials (n=11,015 patient-visits), a total of 534 had a Physician’s Global Assessment (PGA) score of “clear” and 1,725 of “minimal.”1 A subset of these 2,259 patient-visits had a Patient’s Global Assessment (PtGA) of “uncontrolled” (n=60) or “limited disease control” (n=450). In those patients with a PGA of “clear” or “minimal,” the factors most associated with increased odds of patient-assessed limited disease control or uncontrolled disease were: pain, pruritus, and current joint inflammation. Pain (defined as 10mm on a visual analog scale [VAS]) was associated with a 12-percent increase (odds ratio [OR] 1.12, 95% confidence interval [CI], 1.01–1.23, p=0.027), pruritus with a 49-percent increase (1.49, 95% CI, 1.38–1.60, p<0.001), and current joint inflammation with a 67-percent increase (1.67 OR, 95% CI, 1.19–2.34, −p=0.003). Thus, persistent symptoms are associated with a patient perception of uncontrolled or limited control of psoriasis even if the assessing practitioner views the disease as clear or almost clear.
The metrics differ in terms of how clinicians evaluate psoriasis versus patient-reported outcomes. Clinicians use the Psoriasis Area and Severity Index (PASI),2 the Physician’s or Investigator’s Global Assessment (PGA or IGA),3,4 and body surface area (BSA)5 evaluations. On the other hand, patient-reported outcomes are generally expressed using the Dermatology Life Quality Index (DLQI)6 or psoriasis-specific tools, such as the Psoriasis Symptom Assessment (PSA) Scale,7 the Psoriasis Symptom Diary,8 or the Psoriasis Symptom Inventory (PSI).9 Obviously, these tools capture different aspects of psoriasis (Table 1).
TABLE 1.
| INSTRUMENT | DISEASE FACTORS ASSESSED | RECALL PERIOD | COMMENTS |
|---|---|---|---|
| DLQI | Symptoms Psychosocial impact Physical impairment Treatment | 1 week | Does not assess
|
| PSA scale | Symptom frequency and burden | 2 weeks | Does not assess
|
| PSI | Symptom frequency and severity | 1 day or 1 week | Does not assess
|
| Psoriasis symptom diary | Symptom frequency, burden, and severity Psychosocial impact Physical impairment | 1 day | Has been used in clinical trials |
DLQI=Dermatology Life Quality Index; PSA=Psoriasis Symptom Assessment; PSI=Psoriasis Symptom Inventory
PROs related to psoriasis should meet four criteria. First, they should assess patient experiences most relevant to psoriasis. Second, they must frame questions in such a way that the patient can easily understand what is being asked and reply appropriately. Third, PRO tools should not place an unreasonable burden on the patient’s ability to recall past events or sequences of events. Finally, any PRO that will be well-accepted by patients and clinicians must be easy to complete in about five minutes or less.8
The DLQI tool lists 10 items that are not specific to psoriasis, such as symptoms (itchiness, pain, stinging sensations), feelings (embarrassment or self-consciousness because of skin), and how the condition might affect choice of clothing, leisure activities, work or study, relationships, and sex. The DLQI even allows patients to evaluate how much time and messiness is associated with their treatment.6 The DLQI in patients with chronic moderate-to-severe plaque psoriasis being currently treated with biologic agents correlates with the mean percent reduction in PASI score (R2=0.8055).10 A reduction in PASI of at least 75 percent can result in significant improvements in quality of life for patients.10 When the Psoriasis Symptom Diary (PSD) was correlated with data from two double-blind, placebo-controlled, Phase 3 studies in patients with moderate-to-severe plaque psoriasis treated with secukinumab (n=820), items on the PSD achieved high intraclass coefficients (>0.90) and were considered moderate-to-strong in magnitude (0.41–0.73) by Week 12 of the study. This suggests that the PSD is a valid tool that can help in reaching appropriate treatment choices in these patients.11
Sex-specific differences in quality of life of patients with moderate-to-severe plaque psoriasis were evaluated using data from the Phase 3 REVEAL study12 of adalimumab.13 Women had significantly worse mean scores than men for DLQI, mental component score (MCS), and physical component score (PCS) than men (MCS and PCS are part of the SF-36 metric). Women had significantly higher scores in presenteeism (20.5% vs. 16.6%, p=0.0229), in total work productivity impairment (21.8% vs. 17.0%, p=0.0079, and total activity impairment (32.3% vs. 24.0%, p<0.0001). Absenteeism was similar in women and men (4.9% vs. 3.1%, p=0.0980). This suggests that female patients with moderate-to-severe psoriasis suffered more in their health-related quality of life and work productivity compared to male patients.13
DLQI scores are associated with the body region(s) affected by psoriasis. There was a clear impact on patients with head/neck PASI 100 response compared to similar patients with PASI <100 in terms of the percentage of patients who had a DLQI score of 0 or 1 (this corresponds with a lack of or very little effect of psoriasis on the patient’s health-related quality of life). For other areas of the body PASI >75 versus PASI <75 also impacted those patients who scored DLQI 0 or 1. This suggests that head/neck clearance might disproportionately impact patient-reported satisfaction with treatment and quality of life (Navarini AA, et al. EADV 2012—Annual Congress of the European Academy of Dermatology and Venereology, Prague, Czech Republic poster 949). These findings were supported by a study of REVEAL patients (n=15,280 assessments in 4,988 patients), which found that more severe skin lesions on the head or upper extremity had a disproportionately large impact on the DLQI compared to BSA, particularly for younger patients (both male and female).14 Thus, the location of skin symptoms can exert a significant impact on the health-related quality of life of psoriasis patients.
In conclusion, the use of psoriasis-specific PROs may reflect patient status better than the DLQI or other objective instruments. PROs are designed to capture symptoms associated with psoriasis that are important to the patient and are also valuable in measuring treatment efficacy. The Psoriasis Symptom Inventory (PSI) is an eight-item instrument that allows patients to self-report symptom severity; the PSI has demonstrated good reliability and validity in testing.9,15 The PSI assesses symptoms in the last 24 hours. Patients rate each symptom on a five-point verbal scale ranging from “not at all” to “very severe.” Patients are asked about itchiness, redness, scaling, burning, stinging, cracking, flaking, and pain associated with psoriasis. Responses are converted to numbers (0=not at all, 4=very severe) and the total score evaluated (best possible score is 0, worst possible score is 32).
When the PSI was contrasted with the static Physician’s Global Assessment (sPGA), which rates psoriasis in terms of clearance (0=clear of disease and 5=very severe disease), it helped to elucidate the patients’ perception in terms of how an sPGA score of 0 (clear) and 1 (almost clear) might differ. From the vantage point of the clinical observer, sPGA 0 and 1 are very similar. Among patients with PSI of 0, 60.8 percent were sPGA 0 and only 5.3 percent were sPGA 1. This was a significant difference (p<0.001). Among patients who could be classified as a “PSI responder,” defined as those with a PSI score ≤8 and no single item scoring ≤1 point), 94.9 percent were sPGA 0 and 54.3 percent sPGA 1 (p<0.001). This indicates that significantly more patients with complete skin clearance (sPGA 0) report no psoriasis symptoms severity compared to patients who are “almost clear” (sPGA).16
In conclusion, PROs both complement and qualify objective (“observer only”) assessments of psoriasis. In terms of understanding the patient’s perspective on psoriasis, not all body areas are equal. Psoriasis in visible and socially important areas of the body (face and neck) is more distressing than psoriasis on the legs or the arms, which can be effectively hidden from most onlookers. Sex and age play an important role in terms of how patients respond to psoriasis. In terms of clearance, the more complete the clearance the better. While clinicians might be tempted to view “clear” and “almost clear” as similar outcomes, patients see them as significantly different endpoints. A variety of important new psoriasis-specific PROs have been and are being developed. These are important new tools that may help improve treatment of patients.
Systemic Treatment of Psoriasis: What’s New?
This overview of the newest systemic treatments and advances in small molecules is presented by small molecules and then by drug targets.
Small molecules. Apremilast. The LIBERATE study (Evaluation in a Placebo-Controlled Study of Oral Apremilast and Etanercept in Plaque Psoriasis) is a double-blind, double-dummy study that evaluated the safety and efficacy of apremilast or etanercept versus placebo in 250 biologic-naive patients with moderate-to-severe psoriasis over a 16-week period. At Week 16, significantly more patients achieved PASI 75 with apremilast 30mg twice daily (39.8%) or etanercept 50mg once a week (48.2%) than placebo (11.9%), p<0.0001 for both active treatments. Although the study was not powered to compare apremilast to etanercept, a post-hoc analysis found results between these two active treatments were not significantly different. After 16 weeks, all patients were switched to apremilast 30mg twice daily, and efficacy in former etanercept patients could be maintained. No unexpected adverse events were observed.17 A post-treatment observational follow-up phase to 104 weeks has not yet been reported.
Weight is associated with psoriasis disease severity and may affect treatment outcomes. In a post-hoc analysis of the ESTEEM 1 study, it was found that in patients who took apremilast 30mg, the percentage change in body weight over baseline was not correlated with the percentage change in PASI score at Week 16 or Week 52.18
Tofacitinib. Tofacitinib (CP-690,550) is a janus-kinase (JAK) inhibitor that primarily inhibits the JAK 1 and 3 pathways. Tofacitinib is approved therapy for treating rheumatoid arthritis and is known to decrease inflammatory cytokines, chemokines, and several types of inflammatory cells. Tofacitinib is also effective in treating psoriasis and is relatively well-tolerated.19 PASI 75 results over time demonstrate tofacitinib is more effective than placebo and PASI 75 rates increase in dose-dependent fashion.
A total of 1,106 adults with chronic plaque psoriasis were randomized in a 12-week, Phase 3 clinical study into four treatment groups: tofacitinib 5mg twice daily, tofacitinib 10mg twice daily, etanercept 50mg twice weekly, and placebo. This study found tofacitinib was non-inferior to etanercept with rates of adverse events similar across groups.20
Tofacitinib is also used off-label in dermatology practices; among these currently reported off-label uses are the treatment of atopic nails,21 severe atopic dermatitis,22 and vitiligo.23
Certolizumab-pegol. Certolizumab-pegol is a PEG-ylated anti-tumor necrosis factor alpha (TNFα) biologic approved for treatment of rheumatoid arthritis and Crohn’s disease. In 2007, results from a Phase 2 clinical trial of certolizumab explored the role of this small molecule in the treatment of psoriasis. This study found PASI 75 results obtained by 75 and 83 percent of patients (based on dose) compared to seven percent placebo with PGA scores of clear or almost clear obtained by 53 and 72 percent (p<0.001 for both treatment doses versus placebo) with no unexpected adverse events.24 A Phase 3 trial is currently underway evaluating two doses (400mg at Weeks 0, 2, 4 and then 200mg every two weeks starting at Week 6 for 48 weeks; 400mg every two weeks over 47 weeks) versus placebo. The primary endpoint of this study is PASI 75 at 16 weeks. Inclusion criteria for this study are PASI ≥12, BSA affected ≥10 percent, and PGA ≥3.
Drug targets and new treatments. Cytokines and cytokine inhibitors occur across a spectrum ranging from very pro-inflammatory (TNFα, for example) to very anti-inflammatory (interleukin [IL]-4, IL-10, and others, for example). Drug targets may be IL-12 (ustekinumab), IL-23 (ustekinumab, guselkumab, tildrakizumab, BI-655066), and IL-17 (secukinumab, ixekizumab, brodalumab). Since IL-17 and IL-22 occur downstream in the cascade from IL-23, guselkumab, tildrakizumab, and BI-655066 are thought to have an indirect effect on those cytokines. IL-17a is expressed by memory natural killer (NK) and T-cells and occurs in increased amounts in psoriatic skin. IL-17a is known to increase inflammation and enhance angiogenesis. IL-22 is expressed in high levels by Th17 and occurs abundantly in psoriasis (both skin and plasma) with levels increasing with severity of the disease. IL-22 is known to induce keratinocyte hyperproliferation both in vitro and in vivo.25
Guselkumab. When compared to adalimumab in a randomized, placebo-controlled, Phase 2 clinical trial of plaque psoriasis patients, at 16 weeks, significantly more guselkumab patients achieved PASI 75 response than placebo patients (43.9% 5mg every 12 weeks; 75.6% 15mg every 8 weeks; 81.0% 50mg every 12 weeks; 78.6% 100mg every 8 weeks; 81.0% 200mg every 12 weeks compared to 4.8% placebo and 69.8% adalimumab patients, p<0.001 all comparisons to placebo).26 Three serious infections, one malignancy, and three major adverse cardiac events (MACEs) were reported, but no anaphylaxis was reported.
Tildrakizumab. Tildrakizumab is a novel anti-IL-23p19 monoclonal antibody, but unlike ustekinumab (which blocks IL-12), tildrakizumab blocks only IL-23. In a phase 2 study of psoriasis patients, at 16 weeks, 76.2 percent of patients achieved PASI 75 and 51.2 percent PASI 90 with all doses (5, 25, 100, and 200mg) statistically significant versus placebo. Tildrakizumab was reported to be generally safe and well-tolerated by investigators.27
BI-655066. BI-655066 is a novel anti-IL23p19 monoclonal antibody that has been termed an “immunologic disrupter.” In a Phase 2 proof-of-concept study, patients with a history of psoriasis achieved PASI 75 and PASI 90 at rates of 87 and 58 percent with a single intravenous or subcutaneous dose. Further, 33 percent of responders were able to maintain results over 66 weeks with that single dose.28
Secukinumab. Secukinumab is a human IL-17A antagonist indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. The recommended dose regimen is 300mg subcutaneous injection at Weeks 0, 1, 2, 3, and 4, followed by 300mg subcutaneous injection every four weeks. Note that in some patients, a dose of 150mg may be acceptable. Efficacy of secukinumab in placebo-controlled studies and extension periods has been demonstrated with response in a Phase 3 trial occurring as early as three or four weeks.29 The adverse events associated with secukinumab are of special interest. Mild-to-moderate candidiasis occurred in 4.7 percent (300mg) and 2.3 percent (150mg) of secukinumab patients versus less than one percent in etanercept patients and in no placebo patients. Grade 3 neutropenia was reported in one percent of secukinumab patients, 0.3 percent of etanercept patients, and in no placebo patients.29 According to the package insert, secukinumab may exacerbate Crohn’s disease.30
Ixekizumab. Ixekizumab is a humanized monoclonal antibody that blocks IL-17A. This new agent was evaluated in two large Phase 3 randomized clinical trials (UNCOVER-2 and UNCOVER-3), which found ixekizumab was more effective than placebo and etanercept at 12 weeks in treating moderate-to-severe psoriasis.31 The rate of any infection in these studies was 26 percent (for both doses of ixekizumab), 22 percent for etanercept, and 21 percent for placebo. No deaths were reported for any treatments. The rate of serious non-fatal adverse events was two percent for all groups, including placebo.
Brodalumab. The novel agent brodalumab faces an unclear future. Although effective in treatment of psoriasis, its manufacturer, Amgen, halted development on the drug in the middle of 2015 although partners are continuing efforts to advance it. It has been accepted for review by the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Of particular concern are certain psychological symptoms associated with the drug, ranging from depression to suicidal ideation and suicide. However, the mechanism that might explain a relationship between IL-17 inhibition and suicidal ideation remains unclear.32
New choices in highly effective drugs. With multiple potential agents on the market and different treatment strategies available to clinicians, it can be difficult to determine the most effective course of therapy. An important and readily grasped concept in terms of choosing effective drugs is the number needed to treat (NNT). The NNT may be defined as the number of patients who need to be treated in order to achieve one good outcome, that is, how many patients need to be treated for one to benefit compared with a control is a clinical trial.33 Thus, NNT might be seen as the inverse of the absolute risk reduction. Based on information from package inserts, the NNT values for certain psoriasis drugs appears in Table 2, 3, and 4.
TABLE 2.
Number needed to treat (NNT) of main drugs used to treat psoriasis (the NNT values are the number needed to treat in order to achieve a PASI 75 score as a primary endpoint of a clinical trial)
| DRUG | THERAPY (%) | PLACEBO (%) | THERAPEUTIC EFFECT | PRIMARY ENDPOINT (WEEKS) | NNT | COMMENT |
|---|---|---|---|---|---|---|
| Alefacept | 21 | 5 | 16 | 14 | 6.3 | 15mg IM |
| Methotrexate | 35.5 | 18.9 | 16.6 | 12 | 6.0 | CHAMPION |
| Apremilast | 33.1 | 5.3 | 27.8 | 16 | 3.6 | ESTEEM 1 |
| Efalizumab | 39 | 2.4 | 36.6 | 12 | 2.7 | 1 mg/kg/wk |
| Etanercept | 49 | 4 | 45 | 12 | 2.2 | 50mg biw |
| Adalimumab | 71 | 6.5 | 74.5 | 16 | 1.6 | 40mg EOW |
| Ustekinumab | 66 | 3 | 63 | 12 | 1.6 | 90mg |
| Infliximab | 75.5 | 1.9 | 73.6 | 10 | 1.4 | 5mg/kg IV |
| Secukinumab | 81.6 | 4.5 | 77.1 | 12 | 1.3 | 300mg |
| Ixekizumab | 89.1 | 3.9 | 85.2 | 12 | 1.2 | 80mg |
TABLE 3.
Main drugs used to treat psoriasis, number needed to treat (NNT) required to achieve PASI 90 at primary endpoint
| DRUG | THERAPY (%) | PLACEBO (%) | THERAPEUTIC EFFECT | PRIMARY ENDPOINT (WEEKS) | NNT | COMMENT |
|---|---|---|---|---|---|---|
| Methotrexate | 13.6 | 11.3 | 2.3 | 12 | 43.5 | CHAMPION |
| Etanercept | 22 | 1 | 21 | 12 | 4.8 | 50mg biw |
| Adalimumab | 45 | 2 | 43 | 16 | 2.3 | 40mg EOW |
| Ustekinumab | 36.7 | 2 | 34.7 | 12 | 2.9 | 90mg |
| Infliximab | 45.2 | 0.5 | 44.7 | 10 | 2.2 | 5mg/kg IV |
| Secukinumab | 59.2 | 1.2 | 58 | 12 | 1.7 | 300mg |
| Ixekizumab | 70.9 | 0.5 | 70.4 | 12 | 1.4 | 80mg |
TABLE 4.
Main drugs used to treat psoriasis, number needed to treat (NNT) required to achieve PASI 100 at primary endpoint
| DRUG | THERAPY (%) | PLACEBO (%) | THERAPEUTIC EFFECT | PRIMARY ENDPOINT (WEEKS) | NNT | COMMENT |
|---|---|---|---|---|---|---|
| Etanercept | 4.3 | 0 | 4.3 | 12 | 23.3 | 50mg biw (Fixture) |
| Adalimumab | 29 | 1 | 18 | 16 | 5.3 | 40mg EOW |
| Ustekinumab | 10.9 | 0 | 10.9 | 12 | 9.2 | 90mg |
| Infliximab | 25.6 | 1 | 24.6 | 10 | 4.1 | 5mg/kg IV |
| Secukinumab | 28.6 | 0.8 | 27.8 | 12 | 3.6 | 300mg |
| Ixekizumab | 35.3 | 0 | 35.3 | 12 | 2.8 | 80mg Q2W |
Dermatologists at one time tried to manage patient expectations about psoriasis clearance, advising patients that complete clearance was an unrealistic therapeutic goal. Today, many forces are driving us toward agents that will allow complete or nearly complete clearance, as our increased understanding of IL17 and IL23 antagonism creates ever-improved biologics. The old treatment paradigm for psoriasis involved a stepwise progression of over-the-counter remedies to prescription topical agents, then phototherapy, and finally systemic therapy. It was only when a patient failed to achieve results with one step that he or she moved to the next more aggressive step. Today, psoriasis treatment does not follow that old sequential pattern. If a patient failed topical therapy, the patient should advance to either traditional systemic agents (such as apremilast, methotrexate, and others) or biologics (such as adalimumab, infliximab, and others), or phototherapy. The choice should be based on the individual patient’s characteristics and objectives. In other words, a patient need not “fail” phototherapy before receiving a biologic agent.
Topical Treatments for Psoriasis
Even in the age of breakthrough systemic agents to treat psoriasis, topical therapy still plays an important role in that many patients have localized disease or localized flares. There is considerable evidence in the literature in support of class I, III/IV, V/VI/VII corticosteroids, vitamin D analogs, tazarotene, and combination therapy (corticosteroid plus vitamin D analog or tazarotene).34 Topical steroids are available in a range of potency, from low (Class VII), such as hydrocortisone 1% cream or ointment, to super-high-potency products (Class I), such as augmented betamethasone dipropionate 0.05% ointment or gel or also fluocinonide 0.1%.
The greatest shift in our thinking about topical agents of late has been the role of the vehicle or carrier of the active agent. According to the old paradigm, an occlusive agent (ointment) was needed to provide the best penetration and thus efficacy, but new types of vehicles are changing the landscape of topical products. These so-called “designer vehicles” allow for greater skin penetration while modulating permeation into receptor fluid (blood). In that way, a vehicle may improve efficacy of the active agent and it may minimize adverse events. Vehicles can optimize penetration of the agent, which, in turn, can enhance product efficacy.
The efficacy of calcipotriene is vehicle-dependent. In a study of calcipotriene, the ointment formulation resulted in 70-percent marked improvement at eight weeks with 11 percent of patients clear and skin irritation occurring at a rate of 10 to 15 percent. Calcipotriene in cream formulation resulted in 50-percent marked improvement, four percent clear, and irritation at 10 to 15 percent over the same time period. Solution reduced irritation to 1 to 5 percent, but with a 31-percent marked improvement and 14 percent clearance. Foam calcipotriene resulted in an irritation rate of two percent, 41 percent clear or almost clear on the scalp, and 14 to 27 percent clear or almost clear on the body.
Combination or sequential therapy enhances efficacy, minimizes side effects, and may offer a steroid-sparing treatment for managing psoriasis. In a three-arm trial evaluating the efficacy of combination therapy (calcipotriene ointment 0.005% applied mornings and halobetasol propionate 0.05% ointment evenings) versus calcipotriene ointment 0.005% only applied twice daily versus halobetasol propionate ointment 0.05% applied twice daily, the study (n=127) found that after two weeks, combination therapy was more effective than the monotherapies (71% of combination patients were clear or almost clear at 14 days versus 57% of halobetasol only patients and 30% of calcipotriene only patients). Fewer cutaneous adverse effects were observed in the combination group than the monotherapies.35 The rationale behind combination therapy in this study was that the patient could obtain the efficacy conferred by steroids while mitigating associated risks.
In another study evaluating fixed combination of betamethasone dipropionate/calcipotriene ointment, 1,603 patients with chronic plaque psoriasis were randomized to one of four groups: betamethasone dipropionate ointment plus calcipotriene daily, betamethasone dipropionate only daily, calcipotriene only daily, or vehicle only (placebo).36 The combination product (betamethasone dipropionate plus calcipotriene) was significantly more effective than either monotherapy or placebo at Week 1 and Week 4 (39.2% mean change in PASI score at one week and 71.3% at four weeks compared to 33.3% and 57.2% [betamethasone dipropionate only], 23.4% and 46.1% [calcipotriene only] and 18.1% and 22.7% [placebo]). Of particular clinical relevance in this study was the rapid response, with noticeable improvement at one week in combination therapy.
Maintenance therapy plays a crucial role in efficacy. In a study of calcipotriene combined with betamethasone dipropionate in 885 patients with scalp psoriasis, patients were treated daily until they were clear or almost clear; they were then put on a maintenance program and followed for 12 weeks.37 One group applied the product twice weekly regardless of whether they felt it was needed or not, while the other group was advised to use the product only as needed. This study found that the twice-weekly mandatory application was more effective and associated with a lower rate of relapse than on-demand application only (41.7% vs. 19.5%, p<0.001).
Our emerging understanding of the role of the vehicle in topical products and designer vehicles leads to an important question for real-world clinical practice. Can a cosmetically acceptable vehicle be found that enhances penetration? For instance, it is known that calcipotriene plus betamethasone dipropionate is more effective as an ointment than a suspension, but the ointment formulation is not cosmetically elegant. A new foam formulation was designed to enhance efficacy in a cosmetically acceptable vehicle. Data show that the aerosol foam combination product (calcipotriene plus betamethasone dipropionate) allowed for better penetration of both active agents (betamethasone dipropionate and calcipotriene) into the skin compared to the ointment in an in vitro porcine skin assay. In a Phase 3 randomized, placebo-controlled study of this same fixed-combination foam product in 426 adult mild-to-severe plaque psoriasis patients (the PSO-FAST study), significantly more patients achieved “treatment success” based on PGA score at four weeks with the foam product than placebo at four weeks (53.3% vs. 4.8%, p<0.001). Adverse drug reactions were reported in 3.1 percent of foam and 1.9 percent of placebo patients.38 No significant changes in albumin-corrected serum calcium levels or the ratio of urinary calcium to creatinine were observed.
In a randomized investigator-blinded trial of 376 plaque psoriasis patients, treatment arms compared two fixed combination products of calcipotriene plus betamethasone dipropionate in aerosol foam versus ointment. Patients were randomized into one of four groups for four-weeks of once daily treatment: calcipotriene plus betamethasone dipropionate aerosol foam, foam vehicle, calcipotriene betamethasone dipropionate ointment, and ointment vehicle.39 Treatment success, defined as being clear or almost clear with at least a two-step improvement, occurred among significantly more aerosol foam combination product patients than ointment patients (54.6% vs. 43.0%, p=0.025). Rapid and continuous relief of itching was reported with both active treatments.
The MUSE Phase 2 trial evaluated product safety in 35 patients with extensive psoriasis (defined as body surface area of 15–30%) treated for four weeks with calciprotiol and betamethasone dipropionate foam; no evidence of effect on calcium metabolism was found, no serious adverse events were reported, and the product was described as generally well-tolerated. None of the patients exhibited adrenal suppression, as indicated by a 30-minute, post-stimulation cortisol level of ≤18ug/dL at Week 4.40
Tazarotene is a familiar topical retinoid product commercially available on 0.1% and 0.05% cream and gel formulations. It is a pregnancy Category X drug and causes adverse events in about 10 to 30 percent of patients, including pruritus, burning, stinging, erythema, exacerbation of psoriasis, irritation, and skin pain. Nevertheless, when tazarotene 0.1% is combined with topical corticosteroids, tazarotene’s efficacy is enhanced while side effects are minimized.41 A placebo-controlled clinical trial of a tazarotene 0.045% product combined with a lotion formulation of halobetasol propionate 0.01% (product is currently named IDP-118) is currently underway in adult patients with moderate-to-severe psoriasis.
Other important ongoing clinical trials include a trial of a product called GSK-2894512, a small molecule being tested in 0.5% and 1.0% concentrations in two different application frequencies (once versus twice daily) in a randomized, vehicle-controlled, six-arm, parallel-group, dose-finding, 12-week study of patients with moderate-to-severe plaque psoriasis (excluding psoriasis on the scalp).
There is still a significant role for topical therapy in the treatment of psoriasis and new combination approaches and dosing regimens may further enhance their efficacy and tolerability. Novel small molecules are also in the pipeline for topical applications: there are studies of a phosphodiesterase 4 (PDE4) inhibitor ointment, an integrin inhibitor cream, a JAK-1/JAK-2 inhibitor cream (ruxolitinib), a tyrosine kinase inhibitor cream and ointment formulation, and a dihydrofolate reductase inhibitor (methotrexate) proprietary vehicle.42
Update on Psoriasis Comorbidities
The new millennium has seen a tremendous upsurge in our awareness of psoriasis as a disease, the critical importance of the Th17 pathway, biologic treatments targeting T-cells, TNF, IL 12/23, and IL-17, the genetic component of psoriasis, and its many comorbid conditions. There has been a marked increase in literature on the important topic of psoriasis comorbidities lately. The old paradigm that psoriasis is “just a skin disease” has given way to our understanding of psoriasis as a systemic disease. On December 30, 2015, results from a search on the Scopus database with the keywords “psoriasis and cardiovascular disease” indicate the marked increase in the quantity of publications on this topic from 1965 to 2015.
The phenotypically diverse conditions of diabetes mellitus type II, coronary artery disease, myocardial infarction, and obesity are all comorbid with psoriasis and share similar pathologic changes, such as chronic inflammation, angiogenesis, oxidative stress, and certain genetic factors. Indeed, the comorbidities paradigm for psoriasis would include genes and loci (PSORS2/3/4, CDKAL1, ApoE4, and TNFAIP32, environmental factors (such as smoking and obesity), and mediating factors such as inflammatory response, endothelial dysfunction, and epidermal proliferation.43
The list of established comorbidities with psoriasis is lengthy.44 It includes myocardial infarction,45 stroke,46 cardiovascular mortality,47–49 metabolic syndrome (obesity, insulin resistance, cholesterol abnormalities, hypertension, diabetes mellitus),50, 51 psoriatic arthritis,52 mood disorders (notably anxiety, depression, and suicide),53–55 Crohn’s disease,56–58 multiple sclerosis,59 and T-cell lymphoma,60 among other conditions.
One of the most important comorbidities associated with psoriasis is the risk of cardiometabolic disorders. In patients with more severe psoriasis, the risk of cardiovascular disease is roughly the same as the risk conferred by diabetes.45,46 Patients treated for severe psoriasis are 30 times more likely to experience a MACE attributable to psoriasis than to develop a melanoma.47,61 Severe psoriasis is associated with approximately five years life lost compared to patients without psoriasis, mainly owing to cardiovascular disease, but also other disorders including infection and kidney disorders.62 The risk appears to vary with psoriasis disease severity (Table 5).
TABLE 5.
| OUTCOME | RISK RATIO | |
|---|---|---|
| MILD PSORIASIS | SEVERE PSORIASIS | |
| Myocardial infarction | 1.05 | 1.5 |
| Stroke | 1.06 | 1.4 |
| Cardiovascular death | * | 1.6 |
| Major adverse cardiac event | * | 1.5 |
| Diabetes | 1.11 | 1.5 |
These evaluations are not available.
Using a retrospective review of medical records from 9,035 psoriasis patients (aged 25–64 years) and 90,350 age- and practice-matched patients without psoriasis, the psoriasis patients were grouped into those with mild (<3% of BSA affected), moderate (3–10% of BSA affected), and severe (>10% of BSA affected).44 These three groups accounted for 51.8, 35.8, and 12.4 percent of the psoriasis patients, respectively. Using the Charlson Comorbidity Index (CCI), it was found that the CCI score was higher in patients with psoriasis than without (p<0.05), and trend analysis demonstrated that the severity of psoriasis was associated with an increasing number of serious comorbidities (p<0.05).
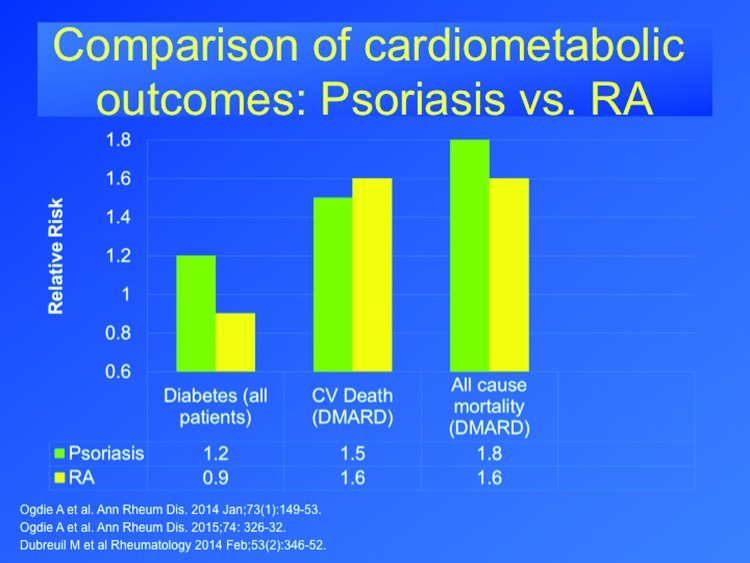
Rheumatoid arthritis (RA) is widely recognized to be associated with cardiovascular morbidity, and is a chronic inflammatory disease that responds to similar treatments for psoriasis, such a methotrexate and TNF inhibitors. Therefore, it makes a useful comparison in contextualizing psoriasis comorbidities. In a longitudinal cohort study in the United Kingdom, patients with psoriatic arthritis (n=8,706), RA (n=41,752), and psoriasis (n=138,424) were compared to control patients (n=82,258) for a total of 1,442,357 person years. Psoriasis and RA were associated with an increased risk of mortality, but psoriatic arthritis was not associated with greater mortality.64 In a population-based study (n=4,196 subjects with psoriatic arthritis, n=59,281 subjects with psoriasis, and n=11,158 subjects with RA), the incidence of diabetes was greater in patients with psoriasis, psoriatic arthritis, but not RA, compared to the general population.65 The findings suggest that more severe psoriasis has similar rates of excess MACE events and mortality compared to RA treated with similar systemic drugs, but that psoriasis is more specifically associated with diabetes, whereas RA is not (Figure 164–66).
Figure 1.
The risk for diabetes, cardiovascular death, or all-cause mortality in patients with psoriasis or rheumatoid arthritis taking a disease-modifying antirheumatic drug (DMARD) is shown. The use of a DMARD is thought to indicate patients with a more severe form of the disease.64–66
New pediatric data suggest that metabolic problems associated with psoriasis emerge early. Among pediatric patients, metabolic syndrome occurs in 30 percent of psoriasis patients versus 7.4 percent of controls (p<0.05).67 Pediatric psoriasis patients are more likely to be obese than similar control patients68 and their lipoprotein profile is more atherogenic.69
Metabolic and cardiovascular gene expression have been associated with inflammatory disease categories in lesional and nonlesional psoriasis biopsies, providing new mechanistic insights into psoriasis comorbid with cardiometabolic disorder. In a murine model, KC-Tie2 psoriasis-specific skin inflammation was associated with the development of aortic inflammation and thrombosis.70,71 This raises the intriguing question as to whether or not the aggressive treatment of psoriasis might lower the patient’s risk of cardiovascular disease. Observational data suggest that psoriasis patients treated with methotrexate and TNF inhibitors (similar data are not yet available for phototherapy, apremilast, ustekinumab, or secukinumab) have a lower rate of cardiovascular events.72–76 This aligns with a meta-analysis in patients with RA, psoriatic arthritis (PsA), or psoriasis treated with TNF inhibitors or methotrexate, which found a relative risk for all cardiovascular adverse events was 0.70 for TNF inhibitors and 0.72 for methotrexate, indicating a cardioprotective effect.77 In a five-year study of 6,902 patients with severe psoriasis, methotrexate and TNF inhibitors were associated with significantly lower rates of cardiovascular events (hazard ratio [HR] 0.52 and 0.46, respectively) than other anti-psoriasis treatments (HR 0.58, 1.06, 1.80, and 1.52 for biologics, cyclosporine, retinoids, and ustekinumab, respectively).78 Two ongoing randomized clinical trials are further studying the potential impact of psoriasis treatment on cardiovascular risk. The Vascular Inflammation in Psoriasis Trial (VIP) and VIP-Ustekinumab are studying whether the treatment of moderate-to-severe psoriasis with adalimumab or phototherapy will lower vascular inflammation and improve lipid metabolism. The Cardiovascular Inflammation Reduction Trial (CIRT) NCT01594333, is testing the hypothesis that methotrexate will lower the risk of major vascular events in patients with a history of myocardial infarction (MI) or metabolic syndrome.
In clinical practice, dermatologists should consider that severe psoriasis implies an elevated cardiovascular risk. While patients with severe psoriasis should be screened for hypertension, diabetes, and standard cardiovascular risk, less than half of U.S. dermatologists surveyed report performing such screenings.79 The prevalence of uncontrolled hypertension in the non-psoriasis population is 51.6 versus 53.9 percent of psoriasis patients overall; in patients with severe psoriasis, uncontrolled hypertension prevalence is 59.5 percent.80,81 A study of 693 Danish patients with severe psoriasis found cardiovascular risk factors under-treated and that these patients were less likely than matched control patients to receive pharmacotherapy for cardiovascular risk factors.82
Emerging comorbidity associations with psoriasis include conditions such as sleep apnea and other sleep disorders, nonalcoholic steatohepatitis (NASH), chronic obstructive pulmonary disease (COPD), adverse infectious disease outcomes, chronic and end-stage renal disease, peptic ulcer disease, and sexual dysfunction.44,83–87 Moderate-to-severe psoriasis has been implicated as a risk factor in chronic kidney disease (CKD). Patients with moderate-to-severe psoriasis have a two-fold risk of chronic kidney disease compared to control patients and have a greater than four-fold risk of needing dialysis.88 These risks are independent of other known risk factors, such as diabetes, hypertension, and potentially nephrotoxic pharmacotherapy.88,89 Moreover, moderate-to-severe patients have a two-fold increased risk of death from kidney disease versus controls.90 The prevalence of CKD increases with the severity of psoriasis.
The association between psoriasis and cancer is an ongoing subject for research. A recent study of 937,716 control patients matched with 198,366 psoriasis patients (186,076 had mild and 12,290 had moderate-to-severe psoriasis) found an association between psoriasis and incident lymphoma and lung cancer, but not for breast, colon, or prostate cancer or leukemia.91 Dermatologists should advise their psoriasis patients to keep up to date on appropriate cancer screenings particularly when considering immune modulating treatments, which may theoretically impact the risk of cancer as described in FDA prescribing information.
It has long been known that psoriasis is associated with depression, anxiety, and other mood disorders. There is some evidence suggesting that cognitive-behavioral therapies and meditation may enhance the patient’s response to psoriasis treatment.92,93 Finally, dermatologists should monitor psoriasis patients closely for signs and symptoms of psoriatic arthritis. These symptoms include morning joint stiffness, joint pain that improves upon activity, swollen or tender joints, dactylitis, and enthesitis.
When treating psoriasis, prescribing decisions should consider potential comorbidities. For example, TNF inhibitors may be effective in treating psoriasis, but are contraindicated in patients with multiple sclerosis. IL-17 should generally not be prescribed to patients with active Crohn’s disease. Other important prescribing considerations should include obesity (weight-based dosing), rapid onset of action, and long-term persistence.
Important Psoriasis Papers Published in 2015
A literature search for “psoriasis” work published in 2015 turns up a wealth of material, including articles published in high-impact international medical journals, such as the Journal of the American Medical Association, The New England Journal of Medicine, and Lancet, among others. Many of these articles focus on our emerging understanding of the IL-23/Th17 immunologic pathway, which is critical in psoriasis pathogenesis. With this wealth of important new findings, a few stand out in terms of clinical trial results, new work in comorbidities, immunology highlights, and recent important clinical reviews.
Clinical trials. The results of eight important psoriasis trials were published in 2015 (Table 6). Tildrakizumab is a monoclonal antibody that targets the IL-23 p19 subunit and was the subject this year of a major Phase 1 and Phase 2 trial. The novel agent, BI-655066, or risankizumab, is a fully human immunoglobulin G1(IgG1) mAb specific molecule for the IL-23 p19 subunit. Results from this Phase 1 study were particularly noteworthy and generated national news interest in that many patients achieved excellent results that endured over a year following one single intravenous injection of the agent.28 Another important Phase 2 study by Gordon et al, published in The New England Journal of Medicine, evaluated several dosing schemes for guselkumab (CNTO-1959), an anti-IL-23 monoclonal antibody.26 Phase 3 studies of note in 2015 evaluated the anti-IL-17A/17RA blockers, ixekizumab (UNCOVER-2 and UNCOVER-3 studies) and brodalumab.31,94 Ixekizumab is a humanized monoclonal antibody that selectively blocks IL-17A.
TABLE 6.
Eight selected clinical trials published in 2015 with important findings for psoriasis*
| STUDY | AGENT/DOSE | EFFICACY | SAFETY | OF NOTE |
|---|---|---|---|---|
| Kopp95 Phase 1 rising multiple-dose, three-part study | Tildrakizumab 3 or 10mg/kg | PASI 75 achieved by 100% in parts 1 and 3 of this study by Day 196 | Well tolerated | This study shows IL-23 is crucial in the pathogenesis of psoriasis |
| Krueger28 Phase 1, single-rising dose | Single IV dose of BI-655066 or same agent subcutaneously | PASI 75, 90, and 100 were achieved by 87%, 58%, and 16% of active treatment patients at 12 weeks vs. none in placebo | AEs with BI-655066 similar to placebo | Results durable for 66 wk after single IV dose |
| Papp96 Phase 2a, three part | Subcutaneous tildrakizumab, 5, 25, 100, or 200mg or placebo on Weeks 0 and 4 (part 1), then every 12 weeks thereafter until Week 52 (part 2), at which point the drug was discontinued and patients observed through Week 72 (part 3) | PASI 75 was achieved by 33.3% (5mg), 64.4% (25mg), 66.3% (100mg), and 74.4% (200mg) and 4.4% (placebo) at 16 weeks (p<0.001 for all groups vs. placebo) | Generally well tolerated but possible drug-related SAEs were observed (bacterial arthritis, lymphedema, melanoma, stroke, epiglottis and knee infection) | Most PASI 75 patients could maintain this response through Week 52; only 8/222 participants who reached 72 wk relapsed |
| Gordon26 Phase 2 dose-ranging active-comparator study (adalimumab) | Guselkumab in 4 dose groups: (1) 5mg at Weeks 0 and 4, then every 12 wk thereafter; (2) 50mg at Weeks 0 and 4 and every 12 wk thereafter; (3) 100mg every 8 wk; or (4) 200mg at Weeks 0 and 4 and every 12 wk thereafter. Adalimumab group doses at standard therapeutic levels, also placebo group | PGA scores of 0–1 were achieved by significantly more guselkumab patients than placebo (34% for 5mg, 61% for 15mg, 79% for 50mg, 86% for 100mg, 83% for 200mg) vs. 7% placebo (p<0.05 for all comparisons). More guselkumab patients achieved PASI 75 scores or better at 16 wk (p<0.001 for all comparisons) and at Week 40, significantly more patients had PGA 0–1 in the 50, 100, and 200mg guselkumab groups than in the adalimumab groups (71%, 77%, 81%, respectively, vs. 49%, p<0.05 for all comparisons) | From Weeks 0 to 16, infections occurred at a rate of 20% in all guselkumab groups, 12% in the adalimumab group, and 14% in the placebo patients | |
| Griffiths31 Results reported from two Phase 3 double-blind studies (UNCOVER-2 and -3) | Subcutaneous placebo, etanercept (50mg twice weekly) or one injection of 80mg ixekizumab every 2 wk or every 4 wk following a 160mg starting dose | Both ixekizumab dose regimens were more effective than placebo and etanercept in 2 studies for proportion of patients achieving PASI 75 | SAEs were reported in about 2% of all patients (all groups, ixekizumab, etanercept, placebo) | |
| Lebwohl94 Results from two Phase 3 studies (AMAGINE-2 and -3) | Brodalumab (210 or 140mg every 2 wk) or ustekinumab (45mg or 90mg, based on weight), or placebo for 12 wk; at Week 12 brodalumab patients were randomly assigned to 210 or 150mg maintenance doses every 2, 4, or 8 wk; placebo patients were changed to 210mg brodalumab every 2 wk | PASI 75 response rates were higher with brodalumab 210 (86%-85%) and 140 (67%-69%) mg doses (AMAGINE 2- and 3 results, respectively) compared to placebo (6%), p<0.001. PASI 100 response rates were significantly higher with 210mg brodalumab than ustekinumab (44% vs. 22% or 37% vs. 19%) | Mild or moderate candida infections occurred more often with brodalumab than ustekinumab or placebo, serious infection rates through 52 wk were around 1% with brodalumab |
Note that studies are named after the first author. Griffiths and Lebwohl each report on two studies in their papers. All studies were randomized, placebo-controlled trials. AE=adverse event; IV=intravenous; kg=kilogram; mg=milligram; PGA=Physician’s Global Assessment; SAE=serious adverse event; wk=week
Comorbidities. The subject of psoriasis comorbidities continues to be an important area of ongoing exploration with significant publications appearing in 2015 with respect to cardiovascular comorbidities, psoriatic arthritis, and infections.
In a murine study, it was found that mice with long-term psoriasis-like disease were at greater risk of developing thromboses than mice with short-term psoriasis disease, suggesting that it is chronic rather than acute skin-specific inflammation that promotes thrombosis in mice.97 Cardiovascular comorbidities were further explored in a population-based cross-section study from the United Kingdom that found that uncontrolled hypertension (defined as systolic pressure of 140mmHg or greater and diastolic pressure of90mmHg) was more likely in patients with moderate-to-severe psoriasis than those with milder forms of psoriasis.80 The severity of psoriasis also associated with aortic vascular inflammation beyond cardiovascular risk factors in an observational study of 50 psoriasis patients, mean age 47 years, low cardiovascular risk factors.98
A 500,000 person-year study found that the risk of herpes zoster infection increased with combination systemic psoriasis therapy with a biologic, but did not increase when psoriasis was treated with phototherapy, methotrexate, cyclosporine, or a biologic alone. By the same token, systemic psoriasis treatment with acitretin decreased the risk of herpes zoster infection.99 The risk of serious infection with biologic and systemic treatment of psoriasis was published with results from the PSOLAR registry.100
Immunology. The association of genetic variants in the IL-23- and NF-KB-mediated inflammatory pathways appears to be different in mild versus severe psoriasis skin disease, such that the IL-23 genes (p19, p40, IL-23R) and TNF genes (NFKB1, TNIP1) are associated with the more severe forms of psoriasis.101 This suggests the value of phenotyping patients for long-term genetic studies. In a study of transgenic mice engineered to produce continuous low levels of IL-17A, the mice eventually developed psoriasis-like skin lesions in a process that could be accelerated with skin trauma.102 An intriguing murine study was published in 2015 that is based on the observation that conventional αβ T-cells can form long-lasting resident memory T-cells (TRM), related to a concept called “innate memory,” where nonadaptive branches of the immune system can deliver rapid, intensified immune response upon re-infection or re-challenge.103 This study identified a subset of γδ cells in mice capable of establishing long-lived memory in the skin. This finding may explain psoriasis recurrences in the same areas, namely areas where there are long-lived IL-17 producing cells.
Small-molecule-mediated inhibition of the retinoic receptor-related orphan nuclear receptor g (RORg) t-dependent gene expression and autoimmune disease pathology was evaluated in vivo. RORgt is a transcription factor that regulates both IL-17A and IL-17F production. An RORgt inhibitor selectively blocked IL-17A/F production and improved psoriasis-like disease in mice,104 making it a potentially relevant target for future drug development. This novel drug is in early phase development for potential application in psoriasis treatment. In another in vitro (human and murine cells) and in vivo study, cells were genetically engineered to detect high levels of pro-psoriatic cytokines, TNF and IL-22, and respond to them by turning on the production of anti-psoriatic cytokines, IL-4 and IL-10. These designer cells prevented the onset of psoriatic flares, stopped acute episodes of psoriasis, and improved psoriatic skin lesions in mice.105 Protein therapeutics may one day be able to provide personalized gene-based and/or cell-based treatments for psoriasis and other disorders.
Reviews. In a rapidly changing, dynamic field like psoriasis research, clinical reviews help digest and organize information from the vast amount of research. A number of relevant reviews for psoriasis appeared in 2015 (Table 7106–109).
TABLE 7.
A selection of some of the most important reviews from 2015 relating to psoriasis and its treatment
| REVIEW | FINDINGS | OF NOTE |
|---|---|---|
| European League Against Rheumatism, Gossec et al106 | New treatment guidelines issued for psoriatic arthritis: (1) NSAIDs, (2) methotrexate, (3) TNF blockers, (4) ustekinumab/secukinumab/apremilast | Updated pharmacological recommendations |
| Effects of TNF inhibitors, methotrexate, NSAIDs, and corticosteroids on CV events in RA, psoriasis, and psoriatic arthritis, Roubille et al77 | In RA patients, TNF blockers and methotrexate are associated with decreased MACE risk, but not in psoriasis or psoriatic arthritis patients | Emerging information in CV comorbidities; systematic review and meta-analysis |
| Rationale for using biologics in moderate-to-severe psoriasis107 | The role of biologics as “gold standard” in moderate-to-severe psoriasis | Addressed to non-biologic users |
| IL-23/IL-17A dysfunction phenotypes108 | These phenotypes may inform possible clinical effects of IL-17A therapies | Offers insight into the biologic basis of possible side effects of anti-IL-17 therapy |
| Ixekizumab109 | Comprehensive review of the newest biologic for treating psoriasis | FDA approval imminent at time of publication |
Psoriatic Arthritis Update
Psoriatic arthritis (PsA), which occurs among approximately 30 percent of patients with skin psoriasis, has a substantial impact on affected patients. There has been tremendous recent progress in delineating the immunopathophysiological basis of PsA. In a recent study, gene expression in PsA synovial samples was compared to that from synovial samples from other inflammatory arthritides as well as affected skin samples with active psoriasis using principal component analysis. Interestingly, gene expression in PsA synovia was more closely related to gene expression in psoriatic skin lesions than to gene expression in synovia in patients with other forms of arthritis, including osteoarthritis (OA), RA, and systemic lupus erythematosus (SLE). In that same paper, microarray results quantifying mRNA expression of biologically significant genes by real-time polymerase chain reaction, normalizing expression values to the housekeeping gene hARP, found greater elevation of mRNA for IL-17A and IL-17F in psoriatic lesional skin compared to inflamed psoriatic synovium, while TNF expression was similar for skin and synovium.110 These intriguing results seem to align with results from recent clinical trials using various targeted therapies, and may help inform future studies.
Because PsA is a progressive disorder that can result in severe joint damage early in the disease course, and because the vast majority of patients develop skin involvement prior to joint involvement, early detection of articular manifestations among psoriasis patients would be valuable. A variety of screening questionnaires to detect PsA have been developed (e.g., PASE, EARP, PEST, TOPAS, etc). In a cross-sectional study of adult primary care patients with psoriasis, patients completed the four questionnaires and then were evaluated by a trained research nurse.111 The clinical assessments included a PASI score, LEI/MASES, and 66/68-joint count and evaluation for the presence of nail psoriasis. Patients who reported a painful enthesis on LEI/MASES then underwent an ultrasound examination of the entheses. A PsA case fulfilled the CASPAR criteria. Sensitivity and specificity for PEST and EARP cutoff ≥3 and PASE cutoff ≥44 and ≥47 were obtained. A total of 473 psoriasis patients (mean age 55.7±13.9 years, 50.9% men) were examined. Median PASI score was 2.3 (IQR 1–4) and 15 percent exhibited nail abnormalities consistent with psoriasis. In the study, 3.6 percent of patients could be diagnosed with PsA (as confirmed by a rheumatologist) and 36 new cases of enthesitis were found, confirmed by ultrasound examination. For enthesitis, EARP had a sensitivity and specificity of 87 and 33 percent, respectively; PEST was 68 and 71 percent, respectively; and PASE was 66 and 55 percent for cutoff ≥44 and 59 and 64 percent for cutoff ≥47. Of note, a number of patients refused further evaluation for their musculoskeletal symptoms. This may partly explain both the relatively low sensitivity and specificity that has been seen for all of these questionnaires, especially in studies subsequent to their initial development.
The PEST screening tool for primary care involves showing psoriasis patients a simplified drawing of a person with various joints called out (shoulder, hip, wrist, ankle, knee, and so on) and asking them to indicate by tic marks if any of those body areas had ever been swollen, stiff, or painful.112,113 They would then be asked five questions and scores of three or more positive answers warranted referral to a rheumatologist. The questions included the following: Have you ever had a swollen or painful joint (as indicated in the drawing)? Have you ever been told by a doctor that you had arthritis? Do your fingernails or toenails have pits or holes in them? Do you have heel pain? Have you ever experienced a finger or toe that swelled up completely and was painful for no apparent reason?
Because not all psoriasis patients with musculoskeletal signs and symptoms desire further evaluation and/or treatment for their PsA, Dr. Arthur Kavanaugh suggests perhaps a single question could be used instead of the questionnaires noted: “Do you have pain or any other symptoms in or around your joints that you want to do something about?” Patients answering affirmatively may wish to discuss tenderness, swelling, pain with motion, loss of function, loss of range, or other symptoms that may relate to PsA, and try to achieve improved outcomes. All rheumatologists would be happy to see such patients in consultation.
There has been notable development in newer therapies for PsA. However, questions also remain concerning the efficacy of older agents, for example methotrexate. While it is frequently used in PsA, there have been relatively few studies of methotrexate in PsA. In a subanalysis of the TICOPA (treat-to-target study in early, drug-naive PsA patients), 188 patients were randomized to receive oral methotrexate monotherapy for 12 weeks versus standard care, which was left to physician discretion but was often methotrexate.114 At 12 weeks, 22.4 percent of patients had minimal disease activity (MDA) and patients treated with methotrexate had less dactylitis (63% decrease) and less enthesitis (26% drop). There was a trend for more PsA patients receiving methotrexate doses of greater than 15mg/wk to achieve ACR 20, ACRO 50, and PASI 75 scores. Thus, in PsA patients, the efficacy of methotrexate showed a slightly greater ACR-20 response in the TICOA trial versus the MIPA trial (41% vs. 34%), which suggests that methotrexate can have some efficacy for some PsA patients, although the extent of benefit was relatively low for most. In addition, there appears to be a dose reponse, with weekly doses of 15mg or higher being more effective.
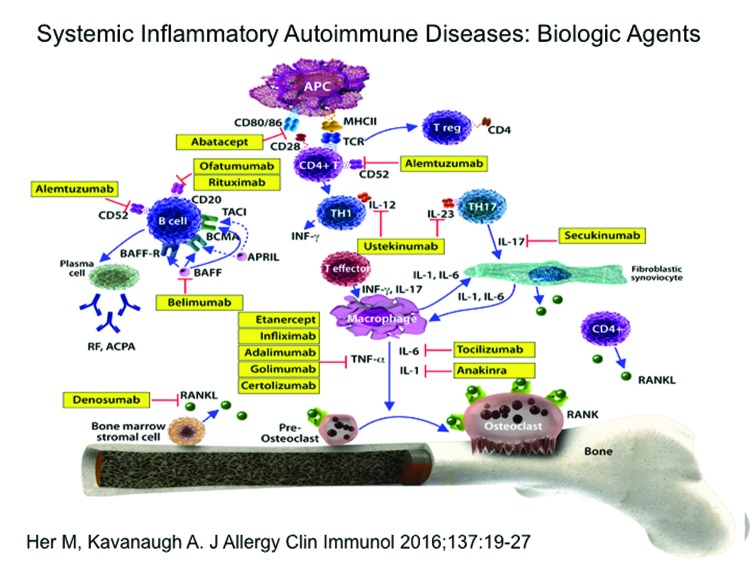
The availability of additional biologic agents has expanded therapeutic options in PsA care by adding a wealth of new weapons to the armamentarium (Figure 2).115 At present, there are three main classes of biological agents proven effective in treating PsA: TNF-α inhibitors (etanercept, infliximab, adalimumab, golimumab, and certolizumab pegol), agents that target IL-12 and/or IL-23 (ustekinumab, with others in development), and agents that target IL-17A (secukinumab, with others in development).116
Figure 2.
Biologic agents and their targets in systemic inflammatory autoimmune diseases.
Reprinted with permission: Her M, Kavanaugh A. Alterations in immune function with biologic therapies for autoimmune disease. J Allergy Clin Immunol. 2016;137(1 ):19–27.
A meta-analysis of biologic agents for the treatment of PsA (assessing data from 12 randomized clinical trials) suggested that that patients treated with TNF inhibitors may have achieved higher levels of articular response than those treated with newer biologic agents, whereas the newer biologic agents, such as ustekinumab and secukinumab, may achieve higher levels of dermatologic response. However, there are issues that make comparisons of therapies not assessed head-to-head potentially tenuous. Also, in studies average responses for groups of patients are reported, whereas individual patients can have distinct results.
Although TNF inhibitors have been available for the treatment for several years now, we continue to learn more about these agents. The recently published GO-REVEAL trial, a Phase 3, randomized, double-blind trial with placebo control through 24 weeks evaluated MDA outcomes in PsA patients treated with golimumab 50/100mg.118 After 24 weeks, patients were followed by open-label extension up to five years. Golimumab treatment was associated with significantly higher rates of MDA responses versus placebo at Week 14 (23.5% vs. 1.09%, p<0.0001), Week 24 (28.1% vs. 7.7%, p<0.0001), and Week 52 (42.4% vs. 30.2%, p=0.037). About half of all patients in the golimumab group achieved MDA at least once during the study. Further, golimumab was associated with greater functional improvement, higher patient global assessments, and better radiographic outcomes than placebo.
The relationship between trough serum concentrations of adalimumab and clinical response was explored in a 28-week study of 103 PsA patients.119 Patients were treated with 40mg of subcutaneous adalimumab every two weeks with serum trough levels of the agent and clinical response (change over baseline) measured at 28 weeks. At the end of the study, the mean serum trough concentration was 7.2mg/L (range 0.018.8), which falls within the presumed optimal range (5–8mg/L) based on work done in RA patients. About 35 percent of patients in the study had serum trough concentrations <5mg/L and 58 percent of these patients had antidrug antibodies (ADAb). About 47 percent had serum trough concentrations >8mg/L and some had low titer or intermittent ADAb. Thus, it appears that concentrations of 1.0mg/L and higher showed efficacy, whereas concentrations beyond 8mg/L did not confer additional benefits.
A topic of increasing interest across many immunologic diseases treated with biologic agents is whether patients who achieve appropriate goals of therapy might be able to taper or even discontinue treatment. Factors that spur such interest include cost, consideration of potential adverse effects, and patient preferences.120 There is a paucity of evidence from the literature to guide tapering strategies, so clinicians should be aware of the patient’s history, have clear goals and how to measure them, reach an agreement with the patient about how to define failure (for example, return of disease activity), and determine how long the patient will be followed once the drug is tapered or discontinued.
There is great interest in newer agents for PsA, including those that target IL-17. In a Phase 2 placebo-controlled study of 606 PsA patients randomized to receive IV secukinumab (10mg/kg) at Weeks 0, 2, and 4 followed by subcutaneous secukinumab (doses of either 75 or 150mg) every four weeks versus placebo.121 At 24 weeks, secukinumab patients were significantly more likely to achieve an ACR20 rating (50.0% of 150mg and 50.5% of 75mg) versus placebo (17.3%, p<0.001 for both comparisons to placebo). Results were durable to 52 weeks although infections, including candida, were more frequent in secukinumab patients than placebo patients. These results confirm the efficacy of drugs targeting IL-17A as a potential target in the treatment of PsA.
The FUTURE-2 study was a Phase 3, double-blind,placebo-controlled, multicenter study of 397 PsA patients randomized to be treated with subcutaneous placebo or secukinumab (300, 150, or 75mg) weekly from baseline to Week 4, then once every four weeks thereafter.122 At 24 weeks, significantly more secukinumab patients achieved an ACR20 score versus placebo patients (54%, 51%, 29%, and 15% for secukinumab 300, 150, and 75mg for placebo, respectively). Serious adverse events were reported by 5, 1, and 4 percent of secukinumab patients (300, 150, 75mg, respectively) compared to two percent in the placebo group.
In a Phase 3 clinical trial, 417 biologic disease-modifying antirheumatic drug (bDMARD)-naive PsA patients were randomized to receive placebo; adalimumab 40mg once every two weeks (active control); or another anti-IL-17A monoclonal antibody, ixekizumab, at a dose of 80mg every two or every four weeks after an initial 160mg dose at Week 0. At 12 and 24 weeks, significantly more ixekizumab patients achieved ACR20, ACR50, ACR70 scores or PASI 75, PASI 90, and PASI 100 responses than placebo (p<0.01 all comparisons to placebo).123 Ixekizumab patients in both groups also experienced significantly greater reductions in dactylitis at Weeks 12 and 24 than placebo patients, but not for enthesitis. Efficacy for adalimumab was also observed. Treatment-emergent adverse events occurred significantly more often (p<0.05) in both adalimumab and ixekizumab groups compared to placebo. Both active treatments resulted in inhibition of radiographic progression of structural joint damage compared to placebo (p<0.025).124
Apremilast, an oral phosphodiesterase-4 inhibitor, has been approved for the treatment of PsA based on data including that from the PALACE-1 trial. A total of 504 patients with active PsA and prior DMARD and/or biologic therapy were randomized to be treated with placebo or apremilast 20mg twice daily or apremilast 30mg twice daily.125 At 16 weeks, significantly more apremilast 20mg (31%) and 30mg (40%) patients achieved ACR20 compared to placebo (19%), p<0.001. Data were recently presented on two-year follow-up of these patients indicating durable ACR20 results to 104 weeks (65.3% and 60.9% for 30 and 20mg, twice daily, respectively).
The clinical treatment of PsA patients requires a careful and individualized assessment of the patient. Clinicians must take a detailed patient history to know what other treatments the patient has taken, patient preferences, prognostic factors, disease involvement, comorbidities, and the patient’s activity levels and lifestyle considerations. The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) recently published treatment recommendations for PsA based on the following six key domains or principles: arthritis, spondylitis, enthesitis, dactylitis, skin disease, nail disease, and comorbidities.126
The clinical importance of addressing comorbidities in PsA is increasingly recognized. The main comorbidities associated with PsA recently recognized by the Canadian Dermatology-Rheumatology Comorbidity Initiative include the risk of cardiovascular disease, smoking, obesity, malignancies (either new cancer or recurrence), infections, osteoporosis, and depression.127 For brevity, their comorbidity list did not include some other conditions, such as asthma, chronic kidney disease, hypertension, or manifestations associated with systemic disease, for example, uveitis. The Canadian recommendations were comprehensive and multidisciplinary and represent a new, approach of this important topic. Limitations of these recommendations are that clinical trial evidence is sometimes weak and it is unclear how these recommendations should translate into changes in real-world clinical practice.
Further study of PsA is warranted, and there are important considerations for those designing clinical trials in order to result in highly relevant data to guide modern clinical practice. Clinical trials must select the specific domain(s) to be assessed and, if multiple domains are addressed, how they combine.128 Outcome metrics must be selected that are clinically relevant and understandable, as well as the optimal imaging techniques. Most studies evaluate patients with active PsA and there should be more effort to evaluate early PsA. Study outcomes may describe low disease activity or remission, but careful and objective definitions should be clarified. When patients reach their therapeutic goal, studies should help clinicians understand how to taper or discontinue therapy appropriate to maintain results. Finally, the role of biomarkers should be explored to better understand individual patient risks and needs.
CUTANEOUS ONCOLOGY
Paradigm Shifts in Cutaneous Oncology
There are currently several topical treatments for field actinic keratosis (AK) approved by the FDA for use in the United States. These are 5-fluorouracil (available in 0.5%, 1%, and 5% formulations), imiquimod (2.5%, 2.75%, and 5% formulations), diclofenac, photodynamic therapy (PDT) with 5-aminolevulinic acid (ALA) or methyl aminolevulinate (MAL), and ingenol mebutate (0.015% and 0.05% formulations). Among the recent breakthroughs in dermatology, the paradigm shift in how field AK is treated is an important change. While most field AK is treated with cryotherapy and sometimes adjunctive topical treatments, topical treatments are infrequently used as monotherapy. This is changing with the advent of new treatment options that overcome some of the drawbacks of conventional AK field therapy. Typically, AK field therapy took weeks or months to complete, during which time patients often felt their appearance was cosmetically unacceptable. While compliance is a major challenge in all of clinical practice, it can be particularly difficult for field AK therapy when patients are required to undergo prolonged “downtime.” Furthermore, field AK therapy requires a great deal of patient education, reassurance, and motivation, and many busy clinics simply cannot handle this aspect of field AK therapy well.
The paradigm shift in field AK therapy is that treatments that typically required weeks (diclofenac, imiquimod, even 5-fluorouracil) are being replaced by treatments that can be conducted in days or weeks, such as ingenol mebutate (IM) 0.15% and PDT-ALA. These newer options not only do not compromise efficacy, they may enhance it (Table 8).
TABLE 8.
A short summary of topical treatment strategies for field AK therapy
| TREATMENT | DOSAGE | DOSAGE TOTAL CLEARANCE | MEDIAN CLEARANCE | ONE-YEAR CLEARANCE | COMMENTS |
|---|---|---|---|---|---|
| 5-FU | BID x 2–6 wk | 47.5–84% | 75–88% | 33–40% | Painful Poor appearance |
| Diclofenac | BID x 90 d | 12–48% | 83% | 14–30% | Reduces inflammation Long course |
| Imiquimod 5% | 2x/wk for 16 wk | 20–45.1% | 83.3% | 40–73% | Compliance issues Poor appearance Flu-like symptoms |
| Imiquimod 3.75% | 6 wk cycle Rx | 35.6% | 81.5% | 40.5% | Poor cosmetic appearance |
| IM 0.015% | Daily x 3 (face/scalp) | 50% | 85% | 46.1% | Shortest course |
| IM 0.05% | Daily x 2 (torso/extremities) | 27.8% | 69.1% | 44% | Effective on chest, other areas less so |
| PDT-ALA | RX x 1 to 2 | 70–90% | ≥80% | ≥50% | Painful |
BID=twice a day; d=day; FU=fluorouracil; IM=ingenol mebutate; Rx=prescription; wk=week; x=times
After field AK treatment, the AK burden can further be reduced by treating the patient once per quarter with liquid nitrogen, possibly doing more field therapy, adding a topical tretinoin, and patient education, especially regarding the consistent use of sunscreen.
Imiquimod 3.75% is an effective topical treatment for AK field therapy, but it can result in prolonged irritation and downtime. A novel protocol for its use requires the patient to apply 3.75% imiquimod daily for seven days, then take a two-week “vacation” with no use of the agent, followed by an application of imiquimod 3.75% once a week. The concept behind continuous once weekly use of 3.75% imiquimod after one week of daily therapy is that because AKs are a chronic disease with recurrences after field therapy, chronic treatment should be required beyond what is traditionally recommended. Preliminary investigator-initiated “proof of concept” studies (George Martin, MD) have shown dramatic AK clearances beyond two years. Patients should be educated about the advantages (less irritation, less “downtime,” convenience, ongoing AK targeted therapy) and drawbacks (must continue weekly applications indefinitely) to this course.
PDT is a well-established treatment for field AK and is carried out with ALA or MAL, which is no longer marketed in the United States, but used extensively in the European Union. In the United States, the approved light sources are red light (MAL) or blue light (ALA). FDA-approved treatment protocols require a multi-hour incubation period (for example, 14 hours) followed by light exposure. Results of PDT-ALA on head/scalp and torso/extremities demonstrate good and durable results after one or two treatments (Figure 3A and Figure 3B). Using fluorokinetic analysis, it is possible to predict maximum accumulation during PDT-ALA using fluorescence detection (Figure4A and Figure 4B).
Figures 3A and 3B.
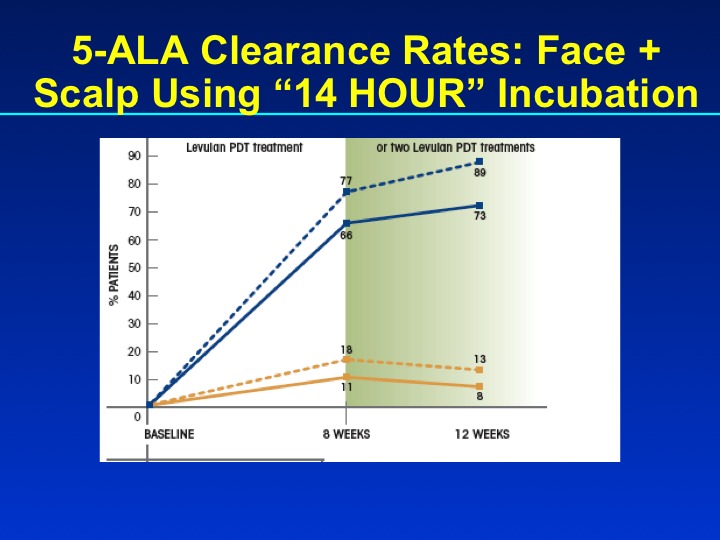
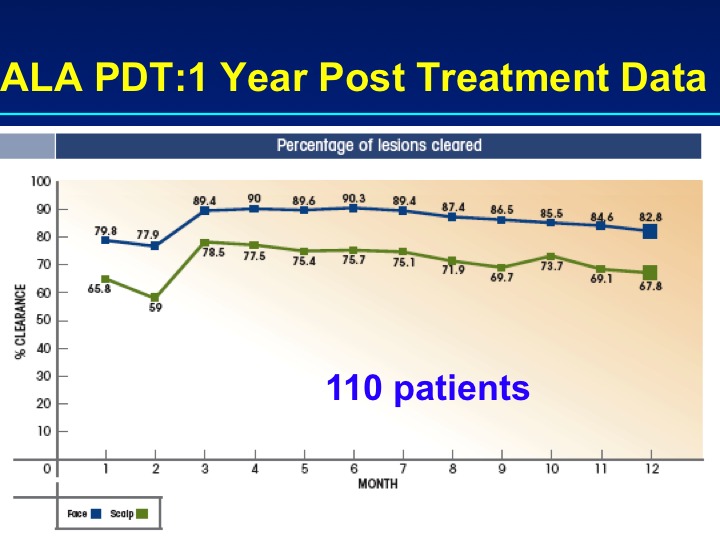
Clearance rates for PDT-ALA using a 14-hour incubation protocol (n=110) and results at one year
Figure 4.
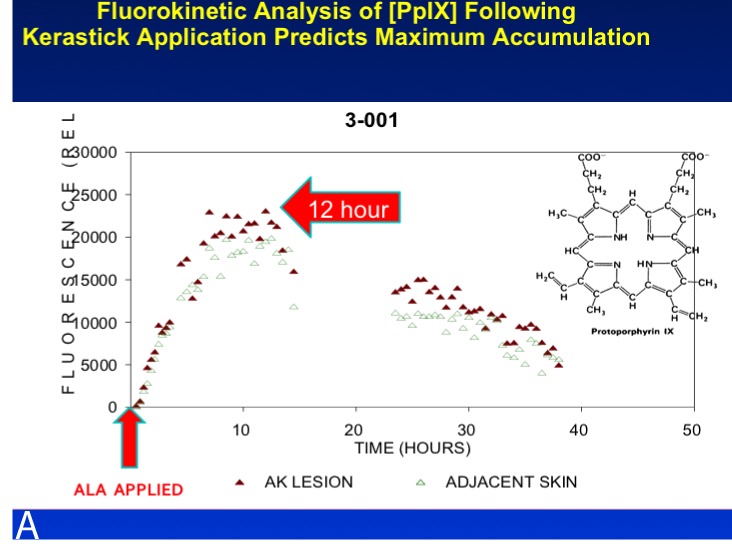
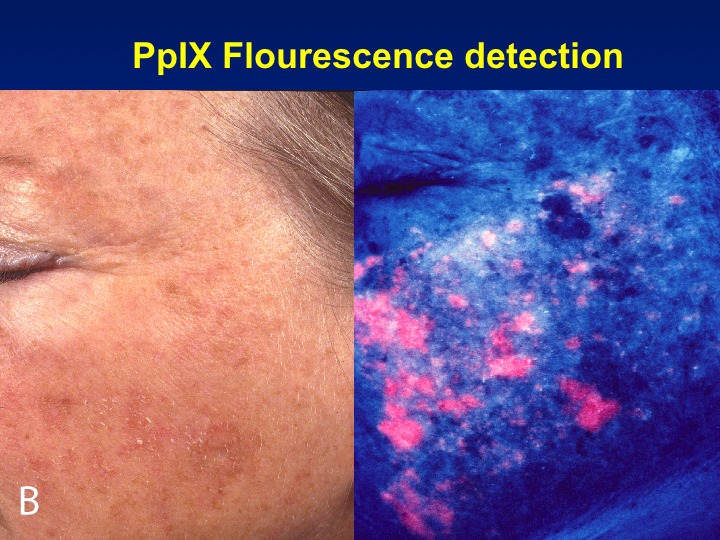
A fluorokinetic analysis carried out after ALA application predicts ALA accumulation patterns
While PDT is an effective field AK treatment, it is associated with pain that can be treatment limiting. A “kinder, gentler” version of PDT was proposed in which patients were incubated with MAL and exposed to daylight for 1.5 or 2.5 hours.129 The protocol in this study required MAL to be applied topically to the skin (affected areas on face and/or scalp) by the patient; this was left in place for 30 minutes. After that, the patient applied sunscreen and was asked to walk outside for 1.5 or 2.5 hours (two treatment groups). Daylight exposure was monitored using a wristwatch-type dosimeter calibrated to 412nm. A total of 120 patients with 1,572 thin AKs on the face and/or scalp participated in the study. The mean lesion response rate at three months was about 75 percent for both treatment groups with grade 1 AKs. The mean overall effective light dose was 9.4J/cm2 (range 0.2–28.3), and the response rate was not associated with the effective daylight dose, exposure duration (1.5 or 2.5 hours), time of day, or time of year. Patients were asked to rate their pain on an 11-point numeric scale with 0 representing no pain at all and 10 being the worst pain imaginable. Ninety-two percent of patients rated their pain 0 to 3, meaning they experienced little to no pain with treatment. Moderate pain was reported by 7.5 percent of patients (pain score 4–7), and only one patient (0.8%) reported severe pain (range 8–10). Intriguingly, in this study there was no correlation between pain and efficacy.
While “daylight mediated” PDT could be a game-changing therapeutic option for field AK therapy, there are some limitations to its use. Since it relies on daylight, weather represents a confounding and uncontrollable variable and there is no standardized way to dose light exposure. There may be times of year or locations in which this treatment is simply not possible. Furthermore, the therapy requires the patient to spend some time outdoors in an environment that is not clinically supervised or controlled. Perhaps most restricting is the fact that this new painless PDT protocol is not yet covered by insurance; reimbursement for PDT-ALA requires exposure to blue light in a clinical setting.
A novel iteration to painless PDT evolved in the form of “in-office painless PDT.” The protocol requires that the patient apply ALA to the affected areas of the face or scalp and incubate it for 15 minutes. After the 15-minute incubation period expires, the patient is exposed to blue light for 60 minutes. The standard in-clinic PDT-ALA treatment is generally performed using 1 to 3 hours of ALA incubation followed by 16 minutes and 45 seconds of blue light exposure. These two in-clinic PDT protocols were tested in three patients using the “split face” approach. Half of the face was treated using the 15 minute ALA/60 minute blue light treatment and the other half was treated with 75 minute ALA/16 minute and 45 seconds blue light. Study results are reported in Table 9. Painless PDT can be reproduced in the clinic.
TABLE 9.
Split-face study (n=3) for field actinic keratosis (AK) on the face and neck using two “in-clinic” painless photodynamic therapy (PDT) protocols. In this study, pain was rated from 0 (no pain at all) to 5 (worst pain imaginable)
| PROTOCOL | DESCRIPTION | AK COUNT | PERCENTAGE REDUCTION IN AKS | AVERAGE PAIN SCORE | |
|---|---|---|---|---|---|
| AT BASELINE | POST-TREATMENT | ||||
| Painless PDT | 15 min aminolevulinic acid incubation then 60 minutes blue light | 27 | 13 | 52% | 0/5 (range 0–0) |
| Standard PDT | 75 min aminolevulinic acid incubation then 16 minutes 45 seconds blue light | 32 | 18 | 44% | 3.5/5 (range 3–4) |
Update on the Safety of Ingenol Mebutate
Ingenol mebutate (Picato®, Leo Pharma, Inc.) was the subject of an FDA Drug Safety Communication on August 21, 2015. This type of statement from the FDA announces future changes to product labeling and is not a “black box warning.”135 The FDA warned about the possibility of severe adverse events associated with application of ingenol mebutate (IM), specifically severe allergic reactions, herpes zoster, and severe eye injuries. These events are very rare. Based on one million patients, severe allergic reactions were reported in about five, and both herpes zoster and eye injuries in fewer than 20 each.
Safety Communications are published relatively frequently with the FDA issuing about 400 to 500 of them annually. It should be noted that the other agents that treat field AK have previously been the subject of such communications.136 Safety Communications improve drug safety by providing clinicians with relevant advice for their practice.
Delving Deeper: Clinically Evident and Subclinical Lesions
Skin cancer is one of the most frequently occurring forms of malignancy around the world. Risk factors for skin cancer are well known. Human papillomaviruses (HPV) can infect the cutaneous and mucosal epithelia, resulting in lesions.137 About 15 percent of all cancerous tumors are caused by viruses, with nonmelanoma skin cancer (NMSC) correlating with HPV infection.138 Ultraviolet (UV) radiation from the sun can damage cells and is another risk factor for NMSC, which includes basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). When a noninfected cutaneous cell is exposed to UV radiation, cell damage is either repaired (resulting in a normal phenotype) or if the damage cannot be repaired, the cell undergoes apoptosis (killing the abnormal phenotype). If the UV radiation affects an HPV-positive cell, no repair or programmed cell death can be initiated, with the result that cells with the abnormal phenotype proliferate and accumulate, resulting in skin cancer. It is believed that homeodomain-interacting protein kinase 2 (HIPK2) can suppress tumorigenesis in the skin and regulates apoptosis through interactions with tumor-suppressor p53, in that it mediates p53 phosphorylation at Ser46 and, in that way, promotes the expression of pro-apoptotic genes.139
In the case of field cancerization, at any given point in time, the cascade is at different stages at multiple sites over the region of sun-exposed skin. This means that the clinician must treat early AK, AK, and SCC, sometimes in close proximity to each other. AK affects about 58 million Americans and has a prevalence of 11 to 25 percent in the Northern Hemisphere.140 While NMSC is not a public health threat in terms of mortality, it is associated with high costs and a substantial burden to the healthcare system. NMSC prevalence increases with age, so the burden of NMSC is likely to increase with the “graying” of industrialized populations.141 In fact, hospitalizations for skin melanomas generally exceed those for NMSC up to around age 50, when hospitalizations for NMSC increase sharply and by age 80 far exceed those for melanoma.141
AKs are predictors of SCC, which is a prominent type of cancer in Caucasians. The disease continuum may be described as sun damage to the skin, early-stage AK, full AK, and then SCC.142 The classical pathway involves AK I transforming to AK II, then AK III, and finally to invasive SCC. However, a new differentiated pathway is now being described in which an AK I transforms directly into an invasive SCC without the intervening AK II and AK III forms.
The European Prevention Initiative for Dermatological Malignancies (EPIDERM) was founded to raise awareness about cutaneous oncology and to capture data important to its diagnosis and treatment. There are currently EPIDERM groups active in Scotland, Spain, the Netherlands, Germany, Italy, Malta, Poland, Finland, and Greece. In an evaluation of how dermatologists in these countries treat skin cancer, the majority in all nations favored surgical over nonsurgical approaches.143 The relative differences were highest in Germany (88% surgical vs. 22% nonsurgical) and lowest in Greece (65% surgical vs. 35% nonsurgical).
In order to better understand the different subgroups of AK and differentiate treatment strategies, the International League of Dermatological Societies (ILDS) issued their international guidelines for the treatment of AK.144 Its diagnosis paradigm requires the clinician to evaluate both patients with actual AK and those at high risk for AK development, such as immunosuppressed patients. High-risk patients should be specifically managed to monitor for the onset of AK. Those with active AK should be grouped into those who have solitary lesions or those with multiple lesions, defined as a high number of lesions concentrated in a compact area or patients with a history of multiple lesions. In this latter group, lesion-and field-directed treatment is recommended, either with imiquimod 3.75% or 5%, diclofenac 3% gel, ingenol mebutate, resiquimod, PDT-ALA, or 5-fluorouracil. For patients with solitary lesions, a lesion-directed treatment should be commenced using a topical agent on the lesion and surrounding field; treatment choices include diclofenac 3% gel, imiquimod, PDT-ALA, or 5-fluorouracil. In some patients, cryotherapy may be appropriate to remove a specific lesion and should be followed by topical treatment. Regardless of the type of AK and the treatment selected, all patients with AK should be educated about the importance of sun protection.144
Field therapy for AK is improving, in that efficacy is better, recurrence rates are low, there are fewer side effects, cosmesis has been improved, application times are shorter, and it has a good safety profile. AK field therapy can be considered cost effective, an increasingly important consideration for today’s cost-conscious healthcare system.
Since AK can be a precursor of more serious forms of potentially lethal cancers, it is important to not only recognize and treat AK appropriately, but to understand its natural history. The disease process begins with a cell with a genetic mutation, leading to hyperplasia or the build-up of abnormal cells. Hyperplasia then becomes dysplasia and finally in situ cancer, which tends to be invasive and may be aggressive. Gene expression patterns support the notion that AK is a precursor lesion to SCC as the two types of lesions are closely related genetically.145
In a survey of 427 physicians, 70 percent of respondents believed that AK should be treated with a topical field treatment.146 Compliance can be a major challenge in AK field treatment, and AK therapy should be considered from the patient’s vantage point, namely that lengthy courses of treatment can be burdensome and inconvenient and local skin reactions can limit treatment. Thus, physicians should find topical AK field therapy that is effective, but of short duration and results in rapid clearance with good tolerability. More than 90 percent of physicians surveyed believed that shorter treatment durations for topical AK therapy would improve adherence and persistence.146
Treating AK means deciding on a strategy based on whether the treatment should address single lesions or the field. Cryotherapy is a good option for treating single, well-defined, hyperkeratotic lesions. Field therapy with a topical agent is an appropriate treatment option for subclinical lesions not treated by cryotherapy.147 Indeed, an important consideration for topical AK field treatment is the fact that it treats not only visible lesions, but also the expanded patches at the subclinical and cellular level.
Although rare, disseminated actinic porokeratosis (DSAP) is also the subject of a paradigm shift in terms of treatment. Previously, these lesions were not well understood or diagnosed by physicians and even dermatologists did not know the optimal course of treatment. DSAP lesions are characterized by an irregular fiber-like ring (coronoid lamella) around them which produces a visible, palpable ridge. The interior of the lesion may be smooth or rough and is often discolored. Many patients find the lesions itchy and irritating, but at times they produce no symptoms. Lesions are typically small, but may grow to a diameter of 10mm.
DSAP may develop in the presence of certain immune deficiencies (HIV infection, diabetes mellitus, cirrhosis, acute pancreatitis, Crohn’s disease, and others) or it may be an inherited condition. It is more common in women than men, although it occurs in both sexes. Focal clonal expansion of atypical keratinocytes gives rise to the unusual shape of the cornoid lamella. Phenotypic variants indicate a simultaneous expression of closely linked genes, the loci of which can be mapped to chromosomes 12q, 15q, and 18q with various mutations identified in the SSH1 gene on chromosome 12. Further, mutations were found in the mevalonate kinase (MVK) gene, which is important in lipid metabolism.
The literature reports a case study of effective DSAP treatment using three treatments with a Q-switched ruby laser.130 Other treatment options are associated with various drawbacks. Topical treatments have a low clearance rate and systemic retinoids are associated with side effects and do not produce long-term remission. PDT-ALA shows no benefit. Excision, cryotherapy, and ablative laser treatments appear to be the most effective and safe options although likely to produce scarring.131
A therapeutic strategy that is evolving now for DSAP includes low-dose acitretin (10mg if possible) or, alternately, topical tazarotene 0.1% gel every night. Treatment with imiquimod 3.7% cream applied every other day (if tolerated) or every third day may be effective. Patients might also benefit from the use of 5-fluorouracil 5% on weekends. Although PDT was reported to be ineffective in treating DSAP, the following new protocol has been proposed: every two months, the patient undergoes ALA incubation under occlusion, followed by 16 minutes and 40 seconds of blue light performed with cryotherapy performed two weeks later to ablate what might still remain.
It is important for dermatologists to raise awareness about DSAP and to offer patients therapeutic options. DSAP is a distressing skin condition particularly when lesions cannot be readily concealed by clothing. In some areas, support groups have emerged to help DSAP patients cope with their skin condition.
Unusual Tumors
Of sebaceous lesions, sebaceous gland carcinoma (SGC) is considered a particularly aggressive albeit uncommon neoplasm. It arises from the sebaceous glands in the skin, and in 75 percent of cases, treatment is made difficult by the periocular location of the lesions. The risk of local recurrence is high, but the metastatic risk is controversial and may be substantial. Although SGC tumors make up less than one percent of eyelid tumors, they comprise 1 to 5 percent of all eyelid malignancies. In such cases, a malignancy on the upper eyelid is two to three times more common than one on the lower lid and it typically afflicts patients in their sixties and seventies. Women are more often affected than men and it occurs more in Asians than other groups.
Ocular SGC occurs when tumors arise in the periocular sebaceous glands. In most cases (50–70%), these tumors arise in the meibomian glands in the tarsal plates, but they may also arise in the glands of Zeis (eyelash cilia) in about 10 percent of cases. In <10 percent of cases, the tumors occur in the glands of caruncle. About 12 to 15 percent of ocular SGC can be described as multicentric or involving multiple periocular locations.
The natural history of an ocular SGC begins subtly with a slowly enlarging, but painless, nodule that may exhibit yellowish or pinkish/red coloration. Since it grows slowly and is often asymptomatic and because it mimics other benign eye conditions (chalazion, keratoconjunctivitis, blepharoconjunctivitis, ocular pemphigoid), diagnosis of SGC can be delayed as long as three years. It may also resemble a BCC or SCC, although these latter two tumors occur more often on the lower than upper eyelid. Dermatologists presented with this condition should look for nonresolving “chalazion,” a loss of cilia in the area, yellow streaks in the patient’s conjunctiva, increased vascularity, and chronic but unilateral inflammation. Such signs should make the clinician suspect SGC.
A chalazion is an inflammation of the Meibomian glands or the glands of Zeis, resulting in a painless, granulomatous inflammation. SGC is often misdiagnosed as a chalazion. Ocular SCG can appear on the pagetoid or intraepithelial area and spread onto the conjunctiva, resulting in keratoconjunctivitis or blepharo-conjunctivitis, which can be misdiagnosed as blepharitis.
About a quarter of all SGC are extraocular, typically appearing on the parotid gland, head, or neck. They emerge as a nodule or papule and may be pink, tan, or yellow in color. Although unusual, there have been cases of SGC that occurred in the genitalia, ear canal, breast, nasal vestibule, and axilla. Although there is some sense that extraocular SGC are not as aggressive as ocular SVG, a retrospective study of 91 cases found that extraocular SGC have a recurrence rate of 29 percent and a metastasis rate of 21 percent.
SCG are associated with Muir-Torre syndrome, although most SGC are of unknown etiology. Muir-Torre is a rare autosomal dominant genodermatosis, which may be a phenotypic variant of Lynch syndrome (hereditary nonpolyposis colorectal cancer syndrome). It is caused by a mutation in one of the mismatch repair genes, that is, MLH1, MSH2, or MSH6. Muir-Torre is characterized by the simultaneous or sequential development of a sebaceous neoplasm along with an internal malignancy. The internal malignancies are often visceral, low-grade tumors that occur most frequently in the colorectal area or genitourinary tracts. Not all patients with Muir-Torre will have a sebaceous carcinoma, but approximately 25 percent do. If it is suspected that a patient might have Muir-Torre, the sebaceous lesions should be tested for microsatellite instability. Immunohistochemical staining should be carried out to look for mismatch repair genes.
Merkel cell carcinoma (MCC) is an unusual malignant tumor arising from the neuroendocrine cells with features of epithelial differentiation and in 1972 was described as a “trabecular carcinoma of the skin.” Originally thought to be a sweat gland tumor, it is actually a cytoplasmic neurosecretory granule. Although rare, MCC is biologically aggressive and its incidence has tripled in the past two decades. It affects primarily the elderly Caucasian population and about 70 percent of cases are thought to be caused by the polyoma virus. The MCC presents as a non-tender, solitary, dome-shaped nodule, which may be blue or red in color. Occasionally, it may appear as a plaque-like or subcutaneous mass. It is typically less than 2cm in length, but may be as large as 20cm. It mainly appears on the head and neck area (40% of MCC).
On histopathology, MCC is a dermal tumor nodule with small blue cells revealing round or oval hyperchromatic nuclei. It invades the vasculature and there may be evidence of tumor necrosis, perineural invasion, and a high mitotic rate. Immunostains should look for CK-20 and thyroid transcription factor.
About 70 percent of patients who present with MCC have localized disease (and about one third of those with clinically negative nodes will actually have microscopic nodal disease). Since MCC is a rare form of cancer, there are few data to guide practice or to verify the validity of prognostic features. In an analysis of 5,823 MCC patients, it was found that five-year survival was 40 percent and that when lesions were <2cm in size, there was a more favorable prognosis. Furthermore, sentinel lymph node biopsy could be an important predictor in that a negative biopsy was associated with a more favorable prognosis.132 The treatment of MCC can be a subject of some controversy. Mohs surgery is a tissue-sparing procedure to excise the tumor and a modified Mohs procedure with an additional final margin may be appropriate (1 to 2cm margin to fascia with complete circumferential and peripheral deep-margin assessment) to achieve the surgical goal of complete tumor extirpation at initial resection. Unfortunately, there are no controlled clinical trials comparing various surgical margins. Following surgery, a sentinel lymph node biopsy is recommended and postoperative radiation and/or chemotherapy may be appropriate.
The prognostic value of a sentinel lymph node biopsy was evaluated in a meta-analysis of 10 studies with MCC. In this study, 67 percent of patients had biopsy-negative and 33 percent of patients had biopsy-positive sentinel lymph nodes and the recurrence rates of cancer were three percent (negative patients, mean follow-up 7.3 months) and 33 percent (positive patients, mean follow-up 12 months).133 This clearly shows that a biopsy of the sentinel lymph node has predictive value in MCC cancer recurrence. However, of the 10 biopsy-negative patients in this study (n=23 sentinel node biopsies), immunostaining was positive in 22 percent of biopsies in 40 percent of the patients. The greatest sensitivity and specificity for diagnosis of micrometastatic MCC in sentinel lymph nodes is staining for the anti-CK-20 antibody.134 Thus, following surgery for MCC, a sentinel lymph node biopsy should be performed. For biopsy-positive patients, therapeutic node dissection with or without adjuvant radiation and chemotherapy should be considered. For biopsy-negative patients, immunostaining for the anti-CK-20 antibody should be carried out and then treatment options considered.
CONCLUSION
The MauiDerm 2016 conference provided an excellent overview of the achievements and challenges of 2015 for dermatologists. Psoriasis continues to be a difficult condition for patients, causing extreme distress in some, but new treatment modalities are providing better avenues for patients to achieve complete or nearly complete skin clearance. The many comorbid conditions associated with psoriasis continues to be explored as medicine stops treating psoriasis as “just a skin disease,” but rather as a serious systemic condition with wide-ranging effects. Skin cancer continues to be a global public health threat, and our understanding of actinic keratosis as a precursor to squamous cell cancer is increasing the urgency with which we address actinic keratosis. Further, the link between the human papillo-mavirus and skin cancer continues to be better elucidated. As dermatologists, we are on the forefront of medical breakthroughs on several fronts that can translate into safer, more effective, and more tolerable treatments for our patients. These in turn will bring our patients improved outcomes, better health, and enhanced quality of life.
REFERENCES
- 1.Kimball AB, Sundaram M, Samuelson T, Signorovitch JE. Characteristics of psoriasis patients with self-assessed limited disease control despite physician-assessed clear or minimal skin disease. Presented at the 71st Annual Meeting of the American Academy of Dermatology; Miami, FL; March 1–5, 2013. [Google Scholar]
- 2.Fredriksson T, Pettersson U. Severe psoriasis—oral therapy with a new retinoid. Dermatologica. 1978;157(4):238–244. doi: 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- 3.Langley RG, Ellis CN. Evaluating psoriasis with Psoriasis Area and Severity Index, Psoriasis Global Assessment, and Lattice System Physician’s Global Assessment. J Am Acad Dermatol. 2004;51(4):563–569. doi: 10.1016/j.jaad.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168(2):402–411. doi: 10.1111/bjd.12112. [DOI] [PubMed] [Google Scholar]
- 5.Puzenat E, Bronsard V, Prey S, et al. What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. J Eur Acad Dermatol Venereol. 2010;24(Suppl 2):10–16. doi: 10.1111/j.1468-3083.2009.03562.x. [DOI] [PubMed] [Google Scholar]
- 6.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 7.Shikiar R, Bresnahan BW, Stone SP, et al. Validity and reliability of patient reported outcomes used in psoriasis: results from two randomized clinical trials. Health Qual Life Outcomes. 2003;1:53. doi: 10.1186/1477-7525-1-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebwohl M, Swensen AR, Nyirady J, et al. The Psoriasis Symptom Diary: development and content validity of a novel patient-reported outcome instrument. Int J Dermatol. 2014;53(6):714–722. doi: 10.1111/j.1365-4632.2012.05798.x. [DOI] [PubMed] [Google Scholar]
- 9.Bushnell DM, Martin ML, McCarrier K, et al. Validation of the Psoriasis Symptom Inventory (PSI), a patient-reported outcome measure to assess psoriasis symptom severity. J Dermatolog Treat. 2013;24(5):356–360. doi: 10.3109/09546634.2012.742950. [DOI] [PubMed] [Google Scholar]
- 10.Mattei PL, Corey KC, Kimball AB. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI): the correlation between disease severity and psychological burden in patients treated with biological therapies. J Eur Acad Dermatol Venereol. 2014;28(3):333–337. doi: 10.1111/jdv.12106. [DOI] [PubMed] [Google Scholar]
- 11.Strober B, Zhao Y, Tran MH, et al. Psychometric validation of the Psoriasis Symptom Diary using Phase III study data from patients with chronic plaque psoriasis. Int J Dermatol. 2016;55(3):e147–e155. doi: 10.1111/ijd.13117. [DOI] [PubMed] [Google Scholar]
- 12.Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58(1):106–115. doi: 10.1016/j.jaad.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Cather J, Horn E, Sundaram M, Bao Y. Sex-specific differences in health-related quality of life in patients with moderate to severe psoriasis. American Academy of Dermatology. 2012 [Google Scholar]
- 14.Kimball A, Sundaram M, Mulani P, Bao Y. Quality of life effects on psoriasis skin symptoms affecting different body regions. American Academy of Dermatology; 2012. Poster 4795.
- 15.Martin ML, McCarrier KP, Chiou CF, et al. Early development and qualitative evidence of content validity for the Psoriasis Symptom Inventory (PSI), a patient-reported outcome measure of psoriasis symptom severity. J Dermatolog Treat. Aug 2013;24(4):255–260. doi: 10.3109/09546634.2012.759639. [DOI] [PubMed] [Google Scholar]
- 16.Feldman S, Bushnell DM, Kircik L, et al. Differences in patient reported psoriasis symptom severity between patients rated as “clear” versus “almost clear” based on Physician Global Assessment. American Academy of Dermatology; 2015. [Google Scholar]
- 17.Soung J, Chapman S, Langley RG, et al. Psoriasis efficacy of apremilast or etanercept compared with placebo in patients with moderate to severe psoriasis: Results from the LIBERATE study. EADV 20152015.
- 18.Reich K, Thaci D, Ghislain P-D, et al. American Academy of Dermatology; 2015. Analysis of psoriasis area and severity index and weight change during long term treatment with apremilast in patients with moderate to severe plaque psoriasis (ESTEEM 1). [Google Scholar]
- 19.Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a Phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167(3):668–677. doi: 10.1111/j.1365-2133.2012.11168.x. [DOI] [PubMed] [Google Scholar]
- 20.Bachelez H, van de Kerkhof PC, Strohal R, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386(9993):552–561. doi: 10.1016/S0140-6736(14)62113-9. [DOI] [PubMed] [Google Scholar]
- 21.Dhayalan A, King BA. Tofacitinib citrate for the treatment of nail dystrophy associated with alopecia universalis. JAMA Dermatol. 2016;152(4):492–493. doi: 10.1001/jamadermatol.2015.3772. [DOI] [PubMed] [Google Scholar]
- 22.Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73(3):395–399. doi: 10.1016/j.jaad.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 23.Craiglow BG, King BA. Tofacitinib citrate for the treatment of vitiligo: a pathogenesis-directed therapy. JAMA Dermatol. 2015;151(10):1110–1112. doi: 10.1001/jamadermatol.2015.1520. [DOI] [PubMed] [Google Scholar]
- 24.Ortonne JP, Tasset C, Reich K, Sterry W. Safety and efcacy of subcutaneous certolizumab pegol, a new anti-TNFα monoclonal antibody, in patients with moderate-to-severe chronic plaque psoriasis: preliminary results from a double-blind, placebo-controlled trial. J Am Acad Dermatol. 2007;56(Suppl 2):AB6. [Google Scholar]
- 25.Fitch E, Harper E, Skorcheva I, Kurtz SE, Blauvelt A. Pathophysiology of psoriasis: recent advances on IL-23 and Th17 cytokines. Curr Rheumatol Rep. 2007;9(6):461–467. doi: 10.1007/s11926-007-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon KB, Duffin KC, Bissonnette R, et al. A Phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. New Eng J Med. 2015;373(2):136–144. doi: 10.1056/NEJMoa1501646. [DOI] [PubMed] [Google Scholar]
- 27.Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a Phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173(4):930–939. doi: 10.1111/bjd.13932. [DOI] [PubMed] [Google Scholar]
- 28.Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebocontrolled trial. J Allergy Clin Immunol. 2015;136(1):116–124; e117. doi: 10.1016/j.jaci.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. New Eng J Med. 2014;371(4):326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 30.Oussova T. Cosentyx (secukinumab) for injection, for subcutaneous use for the treatment of moderate to severe plaque psoriasis. Dermatologic and Ophthalmic Drugs Advisory Committee Meeting 2014. [May 25, 2015]. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DermatologicandOphthalmicDrugsAdvisoryCommittee/UCM420461.pdf
- 31.Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–551. doi: 10.1016/S0140-6736(15)60125-8. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt CG. Suicidal thoughts end Amgen’s blockbuster aspirations for psoriasis drug. Nat Biotechnol. 2015;33(9):894–895. doi: 10.1038/nbt0915-894b. [DOI] [PubMed] [Google Scholar]
- 33.Citrome L, Ketter TA. When does a difference make a difference? Interpretation of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Int J Clin Pract. 2013;67(5):407–411. doi: 10.1111/ijcp.12142. [DOI] [PubMed] [Google Scholar]
- 34.Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60(4):643–659. doi: 10.1016/j.jaad.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 35.Lebwohl M, Siskin SB, Epinette W, et al. A multicenter trial of calcipotriene ointment and halobetasol ointment compared with either agent alone for the treatment of psoriasis. J Am Acad Dermatol. 1996;35(2 Pt 1):268–269. doi: 10.1016/s0190-9622(96)90349-7. [DOI] [PubMed] [Google Scholar]
- 36.Kaufmann R, Bibby AJ, Bissonnette R, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology (Basel, Switzerland) 2002;205(4):389–393. doi: 10.1159/000066440. [DOI] [PubMed] [Google Scholar]
- 37.Saraceno R, Camplone G, D’Agostino M, et al. Efficacy and maintenance strategies of two-compound formulation calcipotriol and betamethasone dipropionate gel (Xamiol® gel) in the treatment of scalp psoriasis: results from a study in 885 patients. J Dermatolog Treat. 2014;25(1):30–33. doi: 10.3109/09546634.2013.800182. [DOI] [PubMed] [Google Scholar]
- 38.Leonardi C, Bagel J, Yamauchi P, et al. Efficacy and safety of calcipotriene plus betamethasone dipropionate aerosol foam in patients with psoriasis vulgaris—a randomized Phase III study (PSO-FAST) J Drugs Dermatol. 2015;14(12):1468–1477. [PubMed] [Google Scholar]
- 39.Koo J, Tyring S, Werschler WP, et al. Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris—a randomized phase II study. J Dermatolog Treat. 2016;27(2):120–127. doi: 10.3109/09546634.2015.1083935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taraska V, Tuppal R, Olesen M, et al. A novel aerosol foam formulation of calcipotriol and betamethasone has no impact on HPA axis and calcium homeostasis in patients with extensive psoriasis vulgaris. J Cutan Med Surg. 2016;20(1):44–51. doi: 10.1177/1203475415597094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lebwohl MG, Breneman DL, Goffe BS, et al. Tazarotene 0.1% gel plus corticosteroid cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;39(4 Pt 1):590–596. doi: 10.1016/s0190-9622(98)70008-8. [DOI] [PubMed] [Google Scholar]
- 42.Feely MA, Smith BL, Weinberg JM. Novel psoriasis therapies and patient outcomes, part 1: topical medications. Cutis. 2015;95(3):164–168;170. [PubMed] [Google Scholar]
- 43.Azfar RS, Gelfand JM. Psoriasis and metabolic disease: epidemiology and pathophysiology. Curr Opin Rheumatol. 2008;20(4):416–22. doi: 10.1097/BOR.0b013e3283031c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149(10):1173–1179. doi: 10.1001/jamadermatol.2013.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 46.Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129(10):2411–2418. doi: 10.1038/jid.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31(8):1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neimann AL, Shin DB, Wang X, et al. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55(5):829–835. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 49.Horreau C, Pouplard C, Brenaut E, et al. Cardiovascular morbidity and mortality in psoriasis and psoriatic arthritis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27(Suppl 3):12–29. doi: 10.1111/jdv.12163. [DOI] [PubMed] [Google Scholar]
- 50.Gelfand JM, Yeung H. Metabolic syndrome in patients with psoriatic disease. J Rheumatol. 2012;89(Suppl):24–28. doi: 10.3899/jrheum.120237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 Pt 1):556–562. doi: 10.1038/jid.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gelfand JM, Gladman DD, Mease PJ, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol. 2005;53(4):573. doi: 10.1016/j.jaad.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 53.Egeberg A, Khalid U, Gislason GH, et al. Association between depression and risk of atrial fibrillation and stroke in patients with psoriasis: a Danish nationwide cohort study. Br J Dermatol. 2015;173(2):471–479. doi: 10.1111/bjd.13778. [DOI] [PubMed] [Google Scholar]
- 54.Lakshmy S, Balasundaram S, Sarkar S, et al. A cross-sectional study of prevalence and implications of depression and anxiety in psoriasis. Indian J Psychol Med. 2015;37(4):434–440. doi: 10.4103/0253-7176.168587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135(4):984–991. doi: 10.1038/jid.2014.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fiorino G, Omodei PD. Psoriasis and Inflammatory Bowel Disease: Two sides of the same coin? J Crohns Colitis. 2015;9(9):697–698. doi: 10.1093/ecco-jcc/jjv110. [DOI] [PubMed] [Google Scholar]
- 57.Skroza N, Proietti I, Pampena R, et al. Correlations between psoriasis and inflammatory bowel diseases. Biomed Res Int. 2013;2013:983902. doi: 10.1155/2013/983902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Najarian DJ, Gottlieb AB. Connections between psoriasis and Crohn’s disease. J Am Acad Dermatol. 2003;48(6):805–821. doi: 10.1067/mjd.2003.540. quiz 822–804. [DOI] [PubMed] [Google Scholar]
- 59.Egeberg A. Psoriasis and comorbidities. Epidemiological studies. Danish Medical Journal. 2016;63(2) [PubMed] [Google Scholar]
- 60.Donigan JM, Snowden C, Carter JB, Kimball AB. The temporal association between cutaneous T-cell lymphoma and psoriasis: implications for common biologic processes. J Eur Acad Dermatol Venereol. doi: 10.1111/jdv.13281. 2015 September 3. [DOI] [PubMed] [Google Scholar]
- 61.Mehta NN, Yu Y, Pinnelas R, et al. Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med. 2011;124(8):775;e771–776. doi: 10.1016/j.amjmed.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abuabara K, Azfar RS, Shin DB, Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K. Br J Dermatol. 2010;163(3):586–592. doi: 10.1111/j.1365-2133.2010.09941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Azfar RS, Seminara NM, Shin DB, et al. Increased risk of diabetes mellitus and likelihood of receiving diabetes mellitus treatment in patients with psoriasis. Arch Dermatol. 2012;148(9):995–1000. doi: 10.1001/archdermatol.2012.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ogdie A, Haynes K, Troxel AB, et al. Risk of mortality in patients with psoriatic arthritis, rheumatoid arthritis and psoriasis: a longitudinal cohort study. Ann Rheum Dis. 2014;73(1):149–153. doi: 10.1136/annrheumdis-2012-202424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dubreuil M, Rho YH, Man A, et al. Rheumatology. 2. Vol. 53. Oxford; England: 2014. Diabetes incidence in psoriatic arthritis, psoriasis and rheumatoid arthritis: a UK population-based cohort study; pp. 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74(2):326–332. doi: 10.1136/annrheumdis-2014-205675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Au SC, Goldminz AM, Loo DS, et al. Association between pediatric psoriasis and the metabolic syndrome. J Am Acad Dermatol. 2012;66(6):1012–1013. doi: 10.1016/j.jaad.2011.11.935. [DOI] [PubMed] [Google Scholar]
- 68.Paller AS, Mercy K, Kwasny MJ, et al. Association of pediatric psoriasis severity with excess and central adiposity: an international cross-sectional study. JAMA Dermatol. 2013;149(2):166–176. doi: 10.1001/jamadermatol.2013.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tom WL, Playford MP, Admani S, et al. Characterization of lipoprotein composition and function in pediatric psoriasis reveals a more atherogenic profile. J Invest Dermatol. 2016;136(1):67–73. doi: 10.1038/JID.2015.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Gao H, Loyd CM, et al. Chronic skin-specific inflammation promotes vascular inflammation and thrombosis. J Invest Dermatol. 2012;132(8):2067–2075. doi: 10.1038/jid.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suarez-Farinas M, Li K, Fuentes-Duculan J, Hayden K, Brodmerkel C, Krueger JG. Expanding the psoriasis disease profile: interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J Invest Dermatol. 2012;132(11):2552–2564. doi: 10.1038/jid.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Michna E, Blonsky ER, Schulman S, et al. Subcutaneous methylnaltrexone for treatment of opioid-induced constipation in patients with chronic, nonmalignant pain: a randomized controlled study. J Pain. 2011;12(5):554–562. doi: 10.1016/j.jpain.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 73.Prodanovich S, Ma F, Taylor JR, et al. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52(2):262–267. doi: 10.1016/j.jaad.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 74.Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273(2):197–204. doi: 10.1111/j.1365-2796.2012.02593.x. [DOI] [PubMed] [Google Scholar]
- 75.Barnabe C, Martin BJ, Ghali WA. Systematic review and meta-analysis: anti-tumor necrosis factor alpha therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2011;63(4):522–529. doi: 10.1002/acr.20371. [DOI] [PubMed] [Google Scholar]
- 76.Wu JJ, Poon KY, Channual JC, Shen AY. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148(11):1244–1250. doi: 10.1001/archdermatol.2012.2502. [DOI] [PubMed] [Google Scholar]
- 77.Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, nonsteroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(3):480–489. doi: 10.1136/annrheumdis-2014-206624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. J Eur Acad Dermatol Venereol. 2015;29(6):1128–1134. doi: 10.1111/jdv.12768. [DOI] [PubMed] [Google Scholar]
- 79.Manalo IF, Gilbert KE, Wu JJ. Survey of trends and gaps in dermatologists’ cardiovascular screening practices in psoriasis patients: Areas still in need of improvement. J Am Acad Dermatol. 2015;73(5):872–874; e874. doi: 10.1016/j.jaad.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 80.Takeshita J, Wang S, Shin DB, et al. Effect of psoriasis severity on hypertension control: a population-based study in the United Kingdom. JAMA Dermatol. 2015;151(2):161–169. doi: 10.1001/jamadermatol.2014.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alamdari HS, Gustafson CJ, Davis SA, et al. Psoriasis and cardiovascular screening rates in the United States. J Drugs Dermatol. 2013;12(1):e14–e19. [PubMed] [Google Scholar]
- 82.Ahlehoff O, Skov L, Gislason G, et al. Pharmacological undertreatment of coronary risk factors in patients with psoriasis: observational study of the Danish nationwide registries. PloS One. 2012;7(4):e36342. doi: 10.1371/journal.pone.0036342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Callis Duffin K, Wong B, Horn EJ, Krueger GG. Psoriatic arthritis is a strong predictor of sleep interference in patients with psoriasis. J Am Acad Dermatol. 2009;60(4):604–608. doi: 10.1016/j.jaad.2008.10.059. [DOI] [PubMed] [Google Scholar]
- 84.Cohen JM, Jackson CL, Li TY, et al. Sleep disordered breathing and the risk of psoriasis among US women. Arch Dermatol Res. 2015;307(5):433–438. doi: 10.1007/s00403-015-1536-4. [DOI] [PubMed] [Google Scholar]
- 85.van der Voort EA, Koehler EM, Dowlatshahi EA, et al. Psoriasis is independently associated with nonalcoholic fatty liver disease in patients 55 years old or older: results from a population-based study. J Am Acad Dermatol. 2014;70(3):517–524. doi: 10.1016/j.jaad.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 86.Wakkee M, de Vries E, van den Haak P, Nijsten T. Increased risk of infectious disease requiring hospitalization among patients with psoriasis: a population-based cohort. J Am Acad Dermatol. 2011;65(6):1135–1144. doi: 10.1016/j.jaad.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 87.Yang YW, Keller JJ, Lin HC. Medical comorbidity associated with psoriasis in adults: a population-based study. Brit J Dermatol. 2011;165(5):1037–1043. doi: 10.1111/j.1365-2133.2011.10494.x. [DOI] [PubMed] [Google Scholar]
- 88.Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. doi: 10.1136/bmj.f5961. (Clinical research ed.). 2013 October 15;347:f5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chi CC, Wang J, Chen YF, et al. Risk of incident chronic kidney disease and end-stage renal disease in patients with psoriasis: a nationwide population-based cohort study. J Dermatolog Sci. 2015;78(3):232–238. doi: 10.1016/j.jdermsci.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 90.Svedbom A, Dalen J, Mamolo C, et al. Increased cause-specific mortality in patients with mild and severe psoriasis: a population-based Swedish register study. Acta Derm Venereol. 2015;95(7):809–815. doi: 10.2340/00015555-2095. [DOI] [PubMed] [Google Scholar]
- 91.Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, Gelfand JM. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152(3):282–290. doi: 10.1001/jamadermatol.2015.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zachariae R, Oster H, Bjerring P, Kragballe K. Effects of psychologic intervention on psoriasis: a preliminary report. J Am Acad Dermatol. 1996;34(6):1008–1015. doi: 10.1016/s0190-9622(96)90280-7. [DOI] [PubMed] [Google Scholar]
- 93.Kabat-Zinn J, Wheeler E, Light T, et al. Influence of a mindfulness meditation-based stress reduction intervention on rates of skin clearing in patients with moderate to severe psoriasis undergoing phototherapy (UVB) and photochemotherapy (PUVA) Psychosomatic Medicine. 1998;60(5):625–632. doi: 10.1097/00006842-199809000-00020. [DOI] [PubMed] [Google Scholar]
- 94.Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. New Eng J Med. 2015;373(14):1318–1328. doi: 10.1056/NEJMoa1503824. [DOI] [PubMed] [Google Scholar]
- 95.Kopp T, Riedl E, Bangert C, et al. Clinical improvement in psoriasis with specific targeting of interleukin-23. Nature. 2015;521(7551):222–226. doi: 10.1038/nature14175. [DOI] [PubMed] [Google Scholar]
- 96.Papp K, Pariser D, Catlin M, et al. Phase 2a randomised, double-blind, placebo-controlled, sequential doseescalation study to evaluate the efficacy and safety of ASP015K, a novel Janus kinase (JAK) inhibitor, in patients with moderate to severe psoriasis. Br J Dermatol. 2015;173(3):767–776. doi: 10.1111/bjd.13745. [DOI] [PubMed] [Google Scholar]
- 97.Golden JB, Wang Y, Fritz Y, et al. Chronic, not acute, skin-specific inflammation promotes thrombosis in psoriasis murine models. J Transl Med. 2015;13:382. doi: 10.1186/s12967-015-0738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Naik HB, Natarajan B, Stansky E, et al. Severity of psoriasis associates with aortic vascular inflammation detected by FDG PET/CT and neutrophil activation in a prospective observational study. Arterioscler Thromb Vasc Biol. 2015;35(12):2667–2676. doi: 10.1161/ATVBAHA.115.306460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shalom G, Zisman D, Bitterman H, et al. Systemic therapy for psoriasis and the risk of herpes zoster: a 500,000 person-year study. JAMA Dermatol. 2015;151(5):533–538. doi: 10.1001/jamadermatol.2014.4956. [DOI] [PubMed] [Google Scholar]
- 100.Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the psoriasis longitudinal assessment and registry (PSOLAR) JAMA Dermatol. 2015;151(9):961–969. doi: 10.1001/jamadermatol.2015.0718. [DOI] [PubMed] [Google Scholar]
- 101.Nikamo P, Lysell J, Stahle M. Association with genetic variants in the IL-23 and NF-kappaB pathways discriminates between mild and severe psoriasis skin disease. J Invest Dermatol. 2015;135(8):1969–1976. doi: 10.1038/jid.2015.103. [DOI] [PubMed] [Google Scholar]
- 102.Wohn C, Brand A, van Ettinger K, et al. Gradual development of psoriatic skin lesions by constitutive low-level expression of IL-17A. Cell Immunol. doi: 10.1016/j.cellimm.2015.11.006. 2015 Nov 26. [DOI] [PubMed] [Google Scholar]
- 103.Hartwig T, Pantelyushin S, Croxford AL, Kulig P, Becher B. Dermal IL-17-producing gammadelta T cells establish long-lived memory in the skin. Eur J Immunol. 2015;45(11):3022–3033. doi: 10.1002/eji.201545883. [DOI] [PubMed] [Google Scholar]
- 104.Banerjee D, Zhao L, Wu L, et al. Small molecule mediated inhibition of RORgamma-dependent gene expression and autoimmune disease pathology. in vivo. Immunology. 2016;147(4):399–413. doi: 10.1111/imm.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schukur L, Geering B, Charpin-El Hamri G, Fussenegger M. Implantable synthetic cytokine converter cells with AND-gate logic treat experimental psoriasis. Science Translational Medicine. 2015;7(318):318ra201. doi: 10.1126/scitranslmed.aac4964. [DOI] [PubMed] [Google Scholar]
- 106.Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75(3):499–510. doi: 10.1136/annrheumdis-2015-208337. [DOI] [PubMed] [Google Scholar]
- 107.Blauvelt A, Armstrong AW, Krueger GG. Essential truths for the care and management of moderate-to-severe psoriasis. J Drugs Dermatol. 2015;14(8):805–812. [PubMed] [Google Scholar]
- 108.Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135(8):1946–1953. doi: 10.1038/jid.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blauvelt A. Ixekizumab: a new anti-IL-17A monoclonal antibody therapy for moderate-to-severe plaque psoriasis. Expert Opin Biol Ther. 2016;16(2):255–263. doi: 10.1517/14712598.2016.1132695. [DOI] [PubMed] [Google Scholar]
- 110.Belasco J, Louie JS, Gulati N, et al. Comparative genomic profiling of synovium versus skin lesions in psoriatic arthritis. Arthritis & Rheumatology (Hoboken, NJ). 2015;67(4):934–944. doi: 10.1002/art.38995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karreman M, Weel A, van der Ven M, et al. Screening for PSA in primary care psoriasis patients with muscoloskeletal complaints with PEST, PASE, and EARP. Rome: EULAR 2015;2015. [Google Scholar]
- 112.Helliwell PS. Psoriasis Epidemiology Screening Tool (PEST): a report from the GRAPPA 2009 annual meeting. J Rheumatol. 2011;38(3):551–552. doi: 10.3899/jrheum.101119. [DOI] [PubMed] [Google Scholar]
- 113.Ibrahim GH, Buch MH, Lawson C, et al. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol. 2009;27(3):469–474. [PubMed] [Google Scholar]
- 114.Coates LC, Helliwell PS. Methotrexate efficacy in the tight control in psoriatic arthritis study. J Rheumatol. 2016;43(2):356–361. doi: 10.3899/jrheum.150614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Her M, Kavanaugh A. Alterations in immune function with biologic therapies for autoimmune disease. J Allergy Clin Immunol. 2016;137(1):19–27. doi: 10.1016/j.jaci.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 116.Boyd T, Kavanaugh A. Novel approaches to biological therapy for psoriatic arthritis. Expert Opin Biol Ther. 2016;16(2):173–186. doi: 10.1517/14712598.2016.1118045. [DOI] [PubMed] [Google Scholar]
- 117.Ungprasert P, Thongprayoon C, Davis JM. 3rd. Indirect comparisons of the efficacy of biological agents in patients with psoriatic arthritis with an inadequate response to traditional disease-modifying anti-rheumatic drugs or to nonsteroidal anti-inflammatory drugs: a meta-analysis. Semin Arthritis Rheum. 2016;45(4):428–438. doi: 10.1016/j.semarthrit.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 118.Kavanaugh A, van der Heijde D, Beutler A, et al. Radiographic progression of patients with psoriatic arthritis who achieve minimal disease activity in response to golimumab tTherapy: results through 5 years of a randomized, placebo-controlled study. Arthritis Care Res (Hoboken). 2016;68(2):267–274. doi: 10.1002/acr.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vogelzang EH, Kneepkens EL, Nurmohamed MT, et al. Anti-adalimumab antibodies and adalimumab concentrations in psoriatic arthritis; an association with disease activity at 28 and 52 weeks of follow-up. Ann Rheum Dis. 2014;73(12):2178–2182. doi: 10.1136/annrheumdis-2014-205554. [DOI] [PubMed] [Google Scholar]
- 120.Kavanaugh A, Smolen JS. The when and how of biologic agent withdrawal in rheumatoid arthritis: learning from large randomised controlled trials. Clin Exp Rheumatol. 2013;31(4 Suppl 78):S19–S21. [PubMed] [Google Scholar]
- 121.Mease PJ, McInnes IB, Kirkham B, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. New Engl J Med. 2015;373(14):1329–1339. doi: 10.1056/NEJMoa1412679. [DOI] [PubMed] [Google Scholar]
- 122.McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386(9999):1137–1146. doi: 10.1016/S0140-6736(15)61134-5. [DOI] [PubMed] [Google Scholar]
- 123.Mease PJ, van der Heijde D, Ritchlin CT, et al. A randomized, double-blind, active- and placebo-controlled phase 3 study of efficacy and safety of ixekizumab, adalimumab, and placebo therapy in patients naive to biologic disease modifying anti-rheumatic drugs with active psoriatic arthritis [abstract] [August 22, 2016];Arthritis Rheumatol. 2015 67(Suppl 10) http//acrabstracts.org/abstract/arandomized-double-blind-active-and-placebo-controlled-phase-3-study-of-efficacy-and-safety-of-ixekizumab-adalimumab-and-placebo-therapy-in-patients-naive-to-biologic-disease-modifying-anti/ [Google Scholar]
- 124.Gottlieb AB, Mease PJ, Cuchacovich RS, et al. Ixekizumab improves physical function, quality of life, and work productivity in biologic disease-modifying antirheumatic drug-naive patients with active psoriatic arthritis [abstract] [August 22, 2016];Arthritis Rheumatol. 2015 67(suppl 10) http://acrabstracts.org/abstract/ixekizumab-improves-physical-function-quality-of-life-and-work-productivity-in-biologic-disease-modifying-antirheumatic-drug-naive-patients-with-active-psoriatic-arthritis/ [Google Scholar]
- 125.Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73(6):1020–1026. doi: 10.1136/annrheumdis-2013-205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Coates LC, Kavanaugh A, Mease PJ, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol (Hoboken, NJ). 2016;68(5):1060–1071. doi: 10.1002/art.39573. [DOI] [PubMed] [Google Scholar]
- 127.Roubille C, Richer V, Starnino T, et al. Evidence-based recommendations for the management of comorbidities in rheumatoid arthritis, psoriasis, and psoriatic arthritis: expert opinion of the Canadian Dermatology-Rheumatology Comorbidity Initiative. J Rheumatol. 2015;42(10):1767–1780. doi: 10.3899/jrheum.141112. [DOI] [PubMed] [Google Scholar]
- 128.Kavanaugh A, Boyd T. Trial design in psoriatic arthritis: what could be changed? Clin Exp Rheum. 2015;33(4 Suppl 92):S15–S18. [PubMed] [Google Scholar]
- 129.Wiegell SR, Fabricius S, Stender IM, et al. A randomized, multicentre study of directed daylight exposure times of 1(1/2) vs. 2(1/2) h in daylight-mediated photodynamic therapy with methyl aminolaevulinate in patients with multiple thin actinic keratoses of the face and scalp. Br J Dermatol. 2011;164(5):1083–1090. doi: 10.1111/j.1365-2133.2011.10209.x. [DOI] [PubMed] [Google Scholar]
- 130.Lolis MS, Marmur ES. Treatment of disseminated superficial actinic porokeratosis (DSAP) with the Q-switched ruby laser. J Cosmet Laser Ther. 2008;10(2):124–127. doi: 10.1080/14764170802068971. [DOI] [PubMed] [Google Scholar]
- 131.Bakardzhiev I, Kavaklieva S, Pehlivanov G. Successful treatment of disseminated superficial actinic porokeratosis with calcipotriol. Int J Dermatol. 2012;51(9):1139–1142. doi: 10.1111/j.1365-4632.2010.04704.x. [DOI] [PubMed] [Google Scholar]
- 132.Lemos BD, Storer BE, Iyer JG, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010;63(5):751–761. doi: 10.1016/j.jaad.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mehrany K, Otley CC, Weenig RH, et al. A meta-analysis of the prognostic significance of sentinel lymph node status in Merkel cell carcinoma. Dermatol Surg. 2002;28(2):113–117; discussion 117. doi: 10.1046/j.1524-4725.2002.02901.x. [DOI] [PubMed] [Google Scholar]
- 134.Su LD, Lowe L, Bradford CR, et al. Immunostaining for cytokeratin 20 improves detection of micrometastatic Merkel cell carcinoma in sentinel lymph nodes. J Am Acad Dermatol. 2002;46(5):661–666. doi: 10.1067/mjd.2002.119563. [DOI] [PubMed] [Google Scholar]
- 135. [April 23, 2016]. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm459311.htm FDA. Picato (ingenol mebutate) Gel: Drug Safety Communication-FDA Warns of Severe Adverse Events, Requires Label Changes. Safety Alerts for Human Medical Products 2015.
- 136.Kircik L, Sung JC, Stein-Gold L, Goldenberg G. United States Food and Drug Administration Product Label Changes. J Clin Aesthet Dermatol. 2016;9(1):39–48. [PMC free article] [PubMed] [Google Scholar]
- 137.Nindl I, Gottschling M, Stockfleth E. Human papillomaviruses and nonmelanoma skin cancer: basic virology and clinical manifestations. Disease Markers. 2007;23(4):247–259. doi: 10.1155/2007/942650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nindl I, Rosl F. Molecular concepts of virus infections causing skin cancer in organ transplant recipients. Am J Transplant. 2008;8(11):2199–2204. doi: 10.1111/j.1600-6143.2008.02392.x. [DOI] [PubMed] [Google Scholar]
- 139.Muschik D, Braspenning-Wesch I, Stockfleth E, et al. Cutaneous HPV23 E6 prevents p53 phosphorylation through interaction with HIPK2. PloS One. 2011;6(11):e27655. doi: 10.1371/journal.pone.0027655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1 Pt 2):4–7. doi: 10.1067/mjd.2000.103342. [DOI] [PubMed] [Google Scholar]
- 141.Stang A, Stausberg J, Boedeker W, et al. Nationwide hospitalization costs of skin melanoma and non-melanoma skin cancer in Germany. J Eur Acad Dermatol Venereol. 2008;22(1):65–72. doi: 10.1111/j.1468-3083.2007.02334.x. [DOI] [PubMed] [Google Scholar]
- 142.Fernandez-Figueras MT, Carrato C, Saenz X, et al. Actinic keratosis with atypical basal cells (AK I) is the most common lesion associated with invasive squamous cell carcinoma of the skin. J Eur Acad Dermatol Venereol. 2015;29(5):991–997. doi: 10.1111/jdv.12848. [DOI] [PubMed] [Google Scholar]
- 143.Ferrandiz L, Ruiz-de-Casas A, Trakatelli M, et al. Assessing physicians’ preferences on skin cancer treatment in Europe. Br J Dermatol. 2012;167(Suppl 2):29–35. doi: 10.1111/j.1365-2133.2012.11084.x. [DOI] [PubMed] [Google Scholar]
- 144.Werner RN, Stockfleth E, Connolly SM, et al. Evidence- and consensus-based (S3) guidelines for the treatment of actinic keratosis—International League of Dermatological Societies in cooperation with the European Dermatology Forum—short version. J Eur Acad Dermatol Venereol. 2015; 29(11):2069–2079. doi: 10.1111/jdv.13180. [DOI] [PubMed] [Google Scholar]
- 145.Padilla RS, Sebastian S, Jiang Z, et al. Gene expression patterns of normal human skin, actinic keratosis, and squamous cell carcinoma: a spectrum of disease progression. Arch Dermatol. 2010;146(3):288–293. doi: 10.1001/archdermatol.2009.378. [DOI] [PubMed] [Google Scholar]
- 146.Stockfleth E. From a new vision of actinic keratosis to imiquimod 3.75%, the new treatment standard. Introduction. J Eur Acad Dermatol Venereol. 2015;29(Suppl 1):1–2. doi: 10.1111/jdv.12827. [DOI] [PubMed] [Google Scholar]
- 147.Poulin Y, Lynde CW, Barber K, et al. Nonmelanoma skin cancer in Canada Chapter 3: Management of actinic keratoses. J Cutan Med Surg. 2015;19(3):227–238. doi: 10.1177/1203475415583414. [DOI] [PubMed] [Google Scholar]