Abstract
Introduction
Cardiac implantable electronic device (CIED) leads frequently develop echogenic masses. However, the nature of these masses is not well understood. In patients in whom atrial fibrillation (AF) catheter ablation is planned, there is concern that transseptal puncture may result in cerebrovascular embolism of these masses. The optimal therapeutic strategy in this setting remains undefined.
Methods
We describe six patients identified over a 6-year period (2008–2014) with device lead-based masses prior to or at the time of AF ablation. We examined the anticoagulation strategy and periprocedural management based on mass identification.
Results
In all six patients (age 39–73; four males), the device lead mass was found in the right atrium. The average mass size was 11 ± 1.3 mm. The majority of patients were already on anticoagulation (5/6; 83 %), and an intensified anticoagulation regimen was initiated (INR goal 3.0). In all six patients, the size of the device lead mass decreased on repeat imaging. In two sixths (33 %) patients, the lead-based mass completely resolved within 2 months. The remaining four patients had persistent lead-based masses (average follow-up of 10.9 ± 9.6 months).
Discussion
We describe a series of patients with CIED lead-based masses found at the time of ablation. These cases illustrate that lead-based masses can disappear while patients are on high-intensity anticoagulation, most compatible with a thrombotic origin. These early data will need to be assessed in larger cohorts for further validation and evaluation of safety.
Keywords: Catheter ablation, Lead-based masses, Lead thrombus, Anticoagulation, Cardiac implantable electronic device
1 Introduction
Catheter ablation for atrial fibrillation (AF) is a recommended treatment for symptomatic AF, especially in patients intolerant or refractory to trials of anti-arrhythmic agents [1]. Patients with AF who undergo catheter ablation are at increased periprocedural risk for thromboembolic events [2–7]. It is currently recommended that transesophageal echocardiogram (TEE) be performed prior to procedure in those patients to identify thrombus within the left atrium at risk for embolization [1, 8]. In spite of this screening, risk of thromboembolism remains high in the periprocedural period [1]. Therefore, continued effort to identify patients at highest risk for thromboembolism is of the utmost importance. Recently, the presence of an echogenic mass identified on a lead of a cardiac implantable electronic device (CIED) prior to procedure has become a focus of concern. At the time of autopsy, it has been shown that 48 % of patients with a CIED have lead vegetations [9]. Although the incidence of clinically significant pulmonary embolism has been shown to be small with lead-based masses [10], the risk for spontaneous embolism does exist [11, 12]. Intracardiac manipulation during ablation likely increases the risk for embolism. The potential consequences of embolism are increased by the transseptal puncture performed during ablation procedure allowing potential paradoxical embolization and cerebrovascular events [3, 5, 13].
Currently there are no recommendations for CIED lead-based mass management in the periprocedural setting. This is in part due to a lack of knowledge regarding the nature of these lead-based masses which have been variously referred to in the literature as thrombus, vegetations, and fibrin deposits [14, 15]. If thrombotic, the echogenic masses would be expected to respond to continued anticoagulation allowing for natural thrombus dissolution. Sterile fibrin deposits would not be expected to dissolve with antithrombotic management strategies and may require more invasive treatment. We hypothesize that many of these masses do in fact represent thrombus and present herein a series of cases representing a potential management strategy of intensified anticoagulation in these patients prior to AF ablation.
2 Methods
We identified six patients who were scheduled for AF ablation at the Mayo Clinic Rochester from 2008 to 2014 who were found to have a CIED lead-based mass prior to or at the time of the procedure. A detailed chart review was performed to evaluate how the echogenic mass was identified, subsequent course, complications, management, and outcomes.
3 Results
Among our six patients, four were male with ages ranging from 39 to 73. Echogenic masses were exclusively located on the right atrial aspect of a device lead in all patients (three on right ventricular (RV) lead, three on right atrial (RA) lead). All patients had dual-chamber devices in place, with five devices being permanent pacemakers (PPM) and one being an ICD. The size of the mass on initial evaluation ranged from 9 to 12 mm (mean 11 ± 1.3 mm). In one patient, anticoagulation was initiated with warfarin to an INR goal of 2.5, and the lead-based mass disappeared on follow-up. In the remaining five patients, anticoagulation was intensified to warfarin with a goal INR of 3.0. On follow-up imaging studies, the lead-based mass either decreased in size or disappeared completely in all patients. In two patients, after resolution of the mass, ablation was performed successfully. In one patient, ablation was performed without complication despite persistent echogenic mass. Table 1 illustrates a summary of the cases and outcomes as described below.
Table 1.
Cases with lead-based mass characteristics and outcomes
| Case no. | Age | Previous anticoagulation | Size of mass (mm) | Durationa (months) | Size on recheck (mm) | Outcome |
|---|---|---|---|---|---|---|
| 1 | 66 | No | 12 | 2 | 0 | Ablation completed, no complications |
| 2 | 69 | Yes | 12 and unclear | 23 | 7 and 6 | Ablation aborted |
| 3 | 71 | Yes | 11 | 1 | 0 | Ablation completed, no complications |
| 4 | 44 | Yes | 10 | 1.5 | 8 | Ablation completed, no complications |
| 5 | 73 | Yes | 9 | 14 | 4 | Ablation aborted |
| 6 | 39 | Yes | 12 | 5 | 10 | Ablation aborted |
Duration on intensified warfarin regimen prior to vegetation size recheck
3.1 Case 1
A 66-year-old female with a dual-chamber PPM for tachy-brady syndrome presented for ablation due to recurrent symptomatic paroxysmal atrial fibrillation not on anticoagulation. Pre-procedural transthoracic echocardiogram (TTE) revealed a 12-mm mass on the atrial aspect of the screw fixated RV pacemaker lead. The planned procedure was aborted and the patient was initiated on anticoagulation with warfarin with a goal INR of 2–2.5. Approximately 2 months later, a repeat transesophageal echocardiogram (TEE) revealed complete resolution of the mass, and ablation was performed without complication.
3.2 Case 2
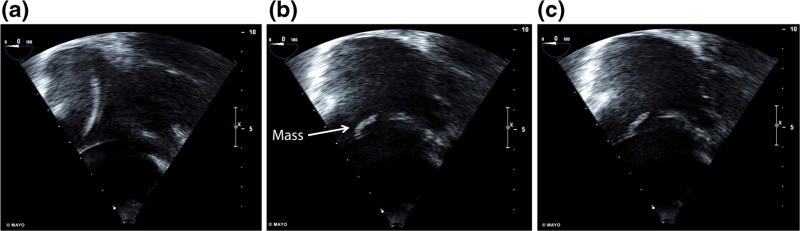
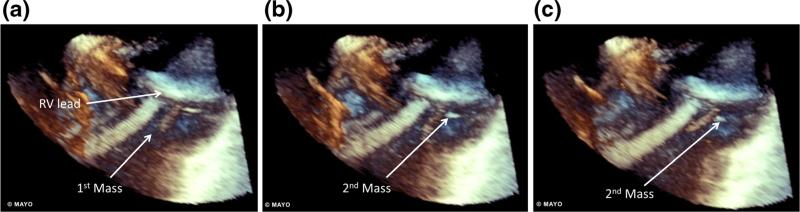
A 69-year-old female with a dual-chamber PPM for bradycardia presented for ablation of symptomatic AF. Pre-procedural standard TEE (TEE) revealed a 12-mm mass on the atrial aspect of the passively fixated tined RV lead as seen in Fig. 1 and Supplemental Video 1. However, with the addition of three-dimensional imaging to the TEE (3D-TEE), a second size-indeterminate mass was seen within the RA on the RV lead Fig. 2 and Supplemental Video 2. INR at the time of initial TEE and 3D-TEE was 2.0 with a goal of 2.5. Her ablation was postponed and goal INR was raised to 3.0. After 7 months with a higher goal INR repeat TEE revealed a decrease in the size of the 12-mm mass to 5 mm and the second mass was measured at 10 mm. Anticoagulation was therefore continued for an additional 16 months. Repeat imaging revealed slight changes in the sizes of masses to 7 and 6 mm, respectively. The ablation was canceled due to persistent echogenic mass, and the patient remains symptomatic on medical therapy for her AF.
Fig. 1.
Right ventricular inflow view on transesophageal echocardiogram focused on right atrium demonstrating a intra-atrial cardiac implantable electronic device leads, b a mobile 12-mm mass within the atrium, and c apparent attachment of the mass to the right ventricular lead
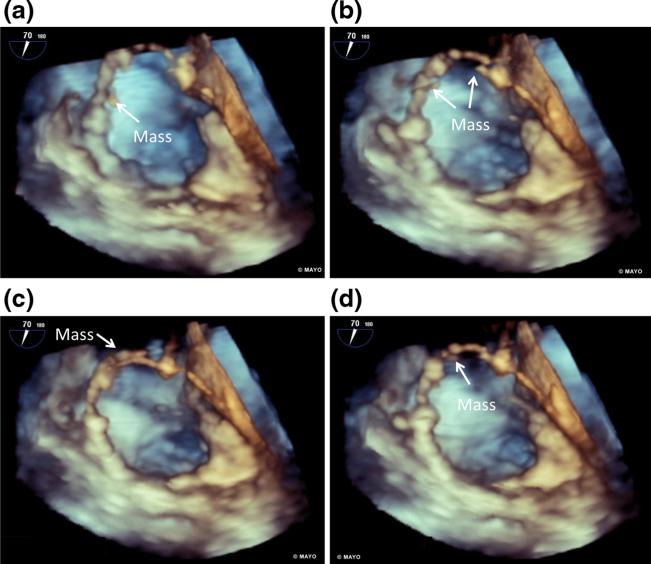
Fig. 2.
Consecutive frames from a 3D-transesophageal echocardiogram view of the right atrium demonstrating a mobility of a 12-mm mass within the atrium and attachment of the mass to the right ventricular lead. b, c A second mass not seen with 2D-imaging can be seen moving independently of the first mass
3.3 Case 3
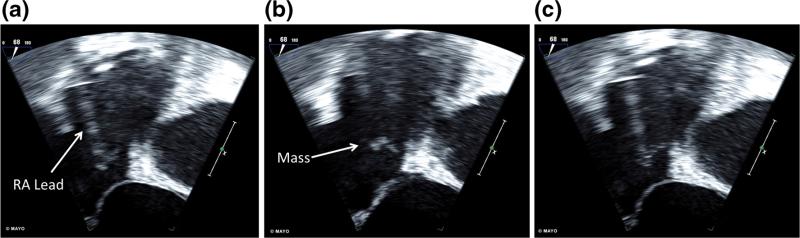
A 71-year-old male with a dual-chamber PPM for sinus node dysfunction presented for AF ablation. Pre-procedural TEE revealed an 11-mm mass on the screw fixated RA lead as seen in Fig. 3 and Supplemental Video 3. His INR at the time of imaging was 1.6 with a goal of 2.5. The procedure was delayed and the INR goal was intensified to 3.0. Repeat TEE 1.5 months later revealed complete resolution of mass, and AF ablation was performed successfully.
Fig. 3.
Transesophageal echocardiogram four-chamber view with focus on right atrial and right ventricular views shows the a RA lead of the permanent pacemaker, b an 11-mm mass within the RA, and c the attachment of the mass to the RA lead
3.4 Case 4
A 44-year-old male with a dual-chamber PPM for sinus node dysfunction presented for AF ablation. Pre-procedural TEE did not reveal any intracardiac mass. However, during the procedure, intracardiac echocardiography (ICE) revealed a 10-mm mass on the screw fixated right atrial lead. The procedure was aborted and the INR goal was intensified to a goal of 3.0. INR at the time of mass identification was 1.0. Warfarin had been held prior to the procedure. Repeat TEE performed 6 weeks later showed only a small reduction in size to 8 mm. The decision was made to continue with the ablation despite persistent echogenic mass due to severity of symptoms. Ablation was completed without complications.
3.5 Case 5
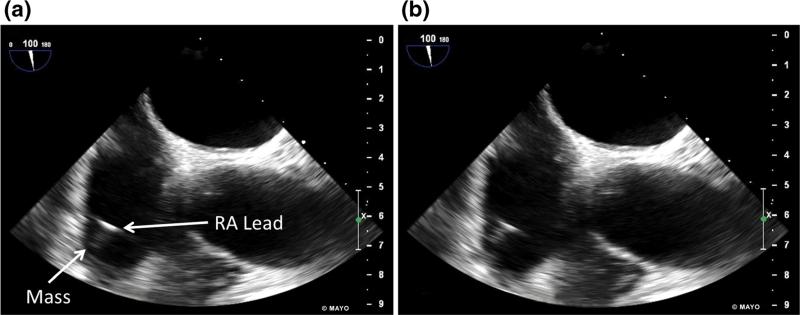
A 73-year-old male with third degree heart block and dual-chamber PPM presented for AF ablation. A small echogenic mass, thought to be a sleeve thrombus, was noted on pre-procedural TEE on the screw fixated RV lead (Fig. 4 and Supplemental Video 4). 3D-TEE revealed multiple mobile components to the mass (Fig. 5 and Supplemental Video 5). The patient was on anticoagulation with dabigatran at the time. The procedure was initiated as scheduled due to small size of visualized mobile masses on TEE. ICE performed during the procedure revealed that a mass was larger than previously thought at 9 mm (Supplemental Video 6). The procedure was aborted and the patient was initiated on warfarin with a goal INR of 3.0–3.5. Repeat TEE was performed twice within the next 5 months with persistent lead-based mass at 9 mm. Fourteen months after the initial ablation procedure, TEE revealed a persistent 4-mm mass. The patient was continued on warfarin and clopidogrel was initiated. The patient has not yet followed up.
Fig. 4.
Transesophageal echocardiogram biplane right atrial view shows a, b mobile mass of unclear size attached to the right atrial lead
Fig. 5.
3D-transesophageal echocardiogram view focusing on the right atrium demonstrates a–d appearance and disappearance of multiple RA lead-based masses indicating mobility of masses associated with stationary sleeve thrombus identified on traditional TEE
3.6 Case 6
A 39-year-old male with prior cardiac arrest and dual-chamber ICD presented for ablation for symptomatic AF. A small mass was seen attached to the screw fixated RA lead on pre-procedural TEE. The procedure was initiated. However, the mass was found on ICE to be larger than expected at 12 mm. The procedure was aborted and the patient was transitioned from dabigatran to warfarin therapy with a goal INR of 3.0–3.5. Repeat TEE 5 months later revealed a decrease in size of the mass to 10 mm. Ablation was not performed due to lack of follow-up after repeat TEE.
4 Discussion
We describe a series of six patients who presented for AF ablation and had echogenic masses identified on CIED leads prior to ablation. The procedure was delayed in each case and an intensified anticoagulation regimen was initiated. Our principle findings in these patients include the following: (1) Lead-based masses can disappear during follow-up while on high-intensity anticoagulation, most compatible with a thrombotic origin (2) pre-procedural TEE can miss masses on device leads which can be potentially identified by 3D-TEE or ICE.
4.1 High-intensity anticoagulation as treatment for CIED lead-based masses
There are no guidelines as to how to proceed when CIED lead-based masses are identified prior to ablation procedures. In our series, we demonstrate that intensification of warfarin therapy is associated with a decrease in mass size. In each case, there was a decrease in mass size after either intensification of anticoagulation therapy to an INR goal of 3.0 or initiation of anticoagulation to an INR goal of 2.5. However, the magnitude of change in size is small in cases 4 and 6, and it is possible that the documented change in size could be secondary to interobserver variability and inherent limitations of our imaging modalities. In two patients, we observed complete resolution of the echogenic mass. This is consistent with a recently reported case series by Rahbar et al. in which eight out of nine patients had some response to intensification of warfarin therapy and seven out of nine had complete resolution of the echogenic masses [16]. In their case series, none of the patients who had resolution of the echogenic mass had a therapeutic INR prior to intensification of warfarin therapy. This may account for their better rate of mass resolution as compared to our series. In our series, both patients that had resolution of a lead-based mass were subtherapeutic at the time of initial discovery of the mass. Three of our other four patients were therapeutically anticoagulated at the time of mass identification. Further prospective studies are needed to identify those patients likely to have resolution of masses and further delineate association with intensified anticoagulation. Of note, there were no recorded bleeding incidents despite the intense anticoagulation regimen utilized.
4.2 Composition of a CIED lead-based mass
The improvement noted in mass size with anticoagulation provides some insight into the nature of these lead-based masses. If these vegetations were representative of fibrin deposits or other non-thrombotic material, it would be unlikely that they would respond to anticoagulation. Only if these masses represent thrombi would they be expected to respond as has been shown with previous intracardiac thrombus [17]. It should be noted that it is possible that resolution of the lead-based masses may in fact represent embolization rather than dissolution.
The timing of resolution of the clot was variable. In one previous report of left atrial thrombus, successful resolution of thrombus occurred within 47 days in 80 % of patients [17]. This time frame is consistent with our cases in which resolution occurred within 2 months. The lack of complete response in four patients may suggest a composition of lead-based masses intermediate between thrombus and sterile fibrin deposits. Thrombotic components of the mass may dissolve quickly while fibrotic deposits remain. Alternatively, longstanding thrombus may become endothelialized [18] shielding the thrombus from the patient's natural mechanism for thrombus dissolution. Duration of thrombus presence could not be determined in our study given the retrospective nature limited by timing of imaging evaluation. Further research into this area will need to be performed to better identify the nature of these echogenic masses and likelihood of response to anticoagulation.
4.3 Embolic events in patients with CIED lead-based masses
CIED lead-based masses have the potential to cause catastrophic outcomes via embolization to the cerebral vasculature. In our study, we did not identify any clinical adverse events associated with these lead-based masses. However, previous studies have revealed that silent pulmonary embolism may occur in as many as 15 % of patients following CIED placement [5]. No clinically significant emboli were identified but that does not rule out silent thromboembolism in this retrospective study. It has previously been shown that passively fixated tined RA leads are associated with a higher risk of TIA [19]. In our study, there were no masses identified on passively fixated tined RA leads, and there was only one mass located on a passively fixated tined RV lead. Further research will need to be performed to better delineate those at highest risk for lead-based mass formation and embolism.
4.4 Identification of CIED lead-based mass prior to proceeding with ablation
Given the risk of thromboembolism with CIED lead-based masses in patients scheduled to undergo ablation for atrial fibrillation, identification is ideal prior to procedures. It is often felt that TEE is sufficient for visualization of lead-based masses [20]. We demonstrate that this is not always true. In case 4, a pre-procedural TEE failed to identify a CIED lead-based mass. Furthermore, in two other cases (5 and 6) the size of the mass was significantly underestimated on TEE as compared to ICE. This propensity for TEE and TTE to miss significant lead-based masses has been previously demonstrated [12, 21]. Cases 2 and 5, demonstrate that 3D imaging can be utilized to clarify the extent of echogenic masses and find masses not seen with traditional TEE. Given the higher sensitivity of ICE, the question is raised as to whether ICE, 3D imaging, or both should become standard of care during and before ablation procedures. Currently only 50 % of expert consensus statement committee members routinely utilize ICE in their practice [8].
5 Conclusion
We demonstrate that in follow-up resolution and shrinking of lead-based masses can occur while patients are on higher intensity anticoagulation, most consistent with a thrombotic etiology. Larger studies will need to be performed to better define the nature of CIED echogenic masses, risk factors for formation, treatment, outcomes, and management in patients planning to undergo AF ablation. Given the potential for adverse outcomes in patients with lead-based masses at the time of ablation, it is important that we define how best to identify and manage these patients. Our case series serves as an impetus for further research in this area.
Supplementary Material
Acknowledgments
Financial support CVD is supported by NIH T32 Training grant no. HL007111.
Abbreviations
- AF
Atrial fibrillation
- CIED
Cardiac implantable electronic device
- ICE
Intracardiac echocardiography
- PPM
Permanent pacemakers
- RA
Right atrial
- RV
Right ventricular
- TEE
Transesophageal echocardiogram
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10840-016-0110-0) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
References
- 1.January CT, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology. 2014;64(21):e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Cappato R, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. Arrhythmia and Electrophysiology. 2010;3(1):32–38. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 3.Korkeila P, et al. Clinical and laboratory risk factors of thrombotic complications after pacemaker implantation: a prospective study. Europace. 2010;12(6):817–824. doi: 10.1093/europace/euq075. [DOI] [PubMed] [Google Scholar]
- 4.Scherr D, et al. Incidence and predictors of periprocedural cerebrovascular accident in patients undergoing catheter ablation of atrial fibrillation. Journal of Cardiovascular Electrophysiology. 2009;20(12):1357–1363. doi: 10.1111/j.1540-8167.2009.01540.x. [DOI] [PubMed] [Google Scholar]
- 5.Seeger W, Scherer K. Asymptomatic pulmonary embolism following pacemaker implantation. Pacing and Clinical Electrophysiology : PACE. 1986;9(2):196–199. doi: 10.1111/j.1540-8159.1986.tb05392.x. [DOI] [PubMed] [Google Scholar]
- 6.Vazquez SR, Johnson SA, Rondina MT. Periprocedural anticoagulation in patients undergoing ablation for atrial fibrillation. Thrombosis Research. 2010;126(2):e69–e77. doi: 10.1016/j.thromres.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruce CJ, et al. Early heparinization decreases the incidence of left atrial thrombi detected by intracardiac echocardiography during radiofrequency ablation for atrial fibrillation. Journal of Interventional Cardiac Electrophysiology. 2008;22(3):211–219. doi: 10.1007/s10840-008-9270-x. [DOI] [PubMed] [Google Scholar]
- 8.Calkins H, et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14(4):528–606. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 9.Novak M, et al. Autopsy and clinical context in deceased patients with implanted pacemakers and defibrillators: intracardiac findings near their leads and electrodes. Europace. 2009;11(11):1510–1516. doi: 10.1093/europace/eup216. [DOI] [PubMed] [Google Scholar]
- 10.Noheria A, et al. Pulmonary embolism in patients with transvenous cardiac implantable electronic device leads. Europace. 2015 doi: 10.1093/europace/euv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman DB, et al. Pacemaker lead thrombosis treated with atrial thrombectomy and biventricular pacemaker and defibrillator insertion. The Annals of Thoracic Surgery. 2004;78(5):e83–e84. doi: 10.1016/j.athoracsur.2003.09.115. [DOI] [PubMed] [Google Scholar]
- 12.Supple GE, et al. Mobile thrombus on device leads in patients undergoing ablation: identification, incidence, location, and association with increased pulmonary artery systolic pressure. Circulation. 2011;124(7):772–778. doi: 10.1161/CIRCULATIONAHA.111.028647. [DOI] [PubMed] [Google Scholar]
- 13.DeSimone CV, et al. Stroke or transient ischemic attack in patients with transvenous pacemaker or defibrillator and echocardiographically detected patent foramen ovale. Circulation. 2013;128(13):1433–1441. doi: 10.1161/CIRCULATIONAHA.113.003540. [DOI] [PubMed] [Google Scholar]
- 14.Baddour LM, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121(3):458–477. doi: 10.1161/CIRCULATIONAHA.109.192665. [DOI] [PubMed] [Google Scholar]
- 15.Dumont E, et al. Suspected pacemaker or defibrillator transvenous lead infection. Prospective assessment of a TEE-guided therapeutic strategy. European Heart Journal. 2003;24(19):1779–1787. doi: 10.1016/j.ehj.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Rahbar AS, et al. Risk factors and prognosis for clot formation on cardiac device leads. Pacing and Clinical Electrophysiology. 2013;36(10):1294–1300. doi: 10.1111/pace.12210. [DOI] [PubMed] [Google Scholar]
- 17.Jaber WA, et al. Efficacy of anticoagulation in resolving left atrial and left atrial appendage thrombi: a transesophageal echocardiographic study. American Heart Journal. 2000;140(1):150–156. doi: 10.1067/mhj.2000.106648. [DOI] [PubMed] [Google Scholar]
- 18.Almekhlafi MA, et al. Calcification and endothelialization of thrombi in acute stroke. Annals of Neurology. 2008;64(3):344–347. doi: 10.1002/ana.21404. [DOI] [PubMed] [Google Scholar]
- 19.Vaidya V, et al. Implanted endocardial lead characteristics and risk of stroke or transient ischemic attack. Journal of Interventional Cardiac Electrophysiology. 2014;41(1):31–38. doi: 10.1007/s10840-014-9900-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korkeila PJ, et al. Transesophageal echocardiography in the diagnosis of thrombosis associated with permanent transvenous pacemaker electrodes. Pacing and Clinical Electrophysiology : PACE. 2006;29(11):1245–1250. doi: 10.1111/j.1540-8159.2006.00519.x. [DOI] [PubMed] [Google Scholar]
- 21.Ren J-F, Marchlinski FE, Callans DJ. Left atrial thrombus associated with ablation for atrial fibrillation: identification with intracardiac echocardiography. Journal of the American College of Cardiology. 2004;43(10):1861–1867. doi: 10.1016/j.jacc.2004.01.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.