Abstract
The current study determined if depletion of glutathione (GSH) and inhibition of thioredoxin reductase (TR) activity could enhance radiation responses in human breast cancer stem cells by a mechanism involving thiol dependent oxidative stress. Buthionine sulfoximine (BSO), a GSH synthesis inhibitor; sulfasalazine (SSZ), an inhibitor of xc- cysteine/glutamate antiporter; auranofin (Au), a thioredoxin reductase inhibitor; or 2AAPA, a GSH-reductase inhibitor, were used to inhibit GSH- and thioredoxin (Trx)-metabolism. Clonogenic survival, Matrigel assays, flow cytometry cancer stem cell assays (CD44+CD24-ESA+ or ALDH1), and human tumor xenograft models were used to determine the antitumor activity of drug and radiation combinations. Combined inhibition of GSH and Trx-metabolism enhanced cancer cell clonogenic killing and radiation responses in human breast and pancreatic cancer cells via a mechanism that could be inhibited by N-acetylcysteine (NAC). Au, BSO, and radiation also significantly decreased breast cancer cell migration and invasion in a thiol dependent fashion that could be inhibited by NAC. In addition pre-treating cells with Au sensitized breast cancer stem cell populations to radiation in vitro as determined by CD44+CD24-ESA+ or ALDH1. Combined administration of Au and BSO, given prior to radiation significantly increased the survival of mice with human breast cancer xenografts as well as decreasing the number of ALDH1 positive cancer stem cells. These results indicate that combined inhibition of GSH- and Trx-dependent thiol metabolism using pharmacologically relevant agents can enhance responses of human breast cancer stem cells to radiation both in vitro and in vivo.
Introduction
Recurrence rates of breast cancer within 10 years of surgery followed by radiation range from 8–13%. Unfortunately, many of these patients will have metastases or locally extensive disease at the time of recurrence.(1) Pancreatic cancer has a 5 year survival rate even after surgical resection of less than 20%. Investigational therapies that use radiation in combination with resection are being pursued in an attempt to decrease metastasis.(2) It has been hypothesized that a small subset of intrinsically radioresistant early progenitor cancer stem cells are responsible for regrowth and metastasis of tumors.(3, 4) Novel therapeutic approaches for breast and pancreatic cancer that enhance radiation response of the bulk of the tumor, as well as targeting the migration and invasion of cancer stem cells, would have a high therapeutic benefit.
Glutathione (GSH) and thioredoxin (Trx) dependent metabolism are the main cellular thiol-dependent pathways that scavenge hydroperoxides, allowing for the maintenance of cell redox potential while preventing and repairing oxidative damage. GSH is a tripeptide and the thiol group on the cysteine molecule is able to donate reducing equivalents to unstable molecules in the cell. Trx is a protein that, like GSH, donates reducing equivalents to a multitude of metabolic reactions using an intermolecular cysteine thiol-disulfide exchange. Previous studies have demonstrated increased levels of GSH and Trx in breast and pancreatic tumors compared to corresponding normal tissues.(5, 6) In addition, elevated thiol metabolism has also been associated with increases in tumor grade and poorer patient outcomes.(6) It has been hypothesized that cancer cells increase GSH- and Trx-dependent metabolism to protect against hydroperoxides produced by metabolism, and that this increase in antioxidant capacity leads to resistance to radiation and chemotherapy.(7, 8) Redox status also mediates embryonic stem cell differentiation with substantial accumulation of oxidized glutathione (GSSG) by day four of differentiation.(9) Interestingly, previous studies have also found that cancer stem cell self-renewal as well as radiation sensitivity may also potentially be affected by reactive oxygen species (ROS) and thiol antioxidant capacity.(3, 4, 10)
We recently demonstrated that combined inhibition of both GSH- and Trx- dependent metabolism was necessary to consistently achieve maximal chemo-sensitization of human lung and breast cancer cells via thiol mediated oxidative stress.(7, 8) In the current study we extend this research to examine radiation responses following manipulation with other therapeutically relevant thiol modifying agents (i.e. sulfasalazine and 2AAPA) as well as combined manipulations of glutathione and thioredoxin metabolism in early progenitor breast cancer stem cells.
In order to manipulate GSH levels, an inhibitor of the rate limiting step in GSH synthesis, buthionine sulfoximine (BSO), was utilized because it is well-tolerated in humans at blood levels up to 1 mM.(11) In addition GSH levels were manipulated by limiting the availability of cysteine using sulfasalazine (SSZ), which inhibits the xc- cystine/glutamate antiporter and is well-tolerated in humans at blood levels of 100 µM after oral administration.(12) Furthermore SSZ has been shown to decrease GSH and sensitize pancreatic tumors to gemcitabine.(13) A novel inhibitor of glutathione reductase (GR), 2-acetylamino-3-[4-(2-acetylamino-2-carboxyethylsulfanylthiocarbonyamino)phenylthiocarbamoylsulfanyl] propionic acid (2-AAPA) was used to restrict GSH metabolism by inhibiting the recycling of GSSG to GSH by glutathione reductase.(14) Thioredoxin metabolism was manipulated using an excellent inhibitor of both cytosolic and mitochondrial thioredoxin reductase (TR), auranofin (Au), which is a gold phosphine used safely in humans at plasma levels of 1–2 µM.(15, 16)
In the current study GSH- and Trx-dependent metabolism was manipulated using BSO, SSZ, 2AAPA and Au, in combination with radiation to determine effects on clonogenic survival, DNA damage, migration, invasion, and breast cancer stem cell responses in vitro. Breast cancer xenograft models were used to demonstrate the consequence of pretreatment with Au+BSO on radiation-induced inhibition of tumor growth as well as effect on stem cell populations in vivo.
Materials and Methods
Cell lines and media
MDA-MB-231, MIA PaCa-2 and PANC-1 (American Type Culture Collection) SUM159 (Asterland) were maintained in the suppliers recommended media. All cells were grown at 37°C, 21% O2 except for experiments determining stem cells which were grown at the more physiologically relevant 4% O2 concentration. Oxygen percentage was measured and maintained with an O2 sensor installed in the Thermo Scientific HERAcell incubator.
Drug and radiation treatment
Cells were treated with 0.1–0.5 mM SSZ (Sigma-Aldrich) or 0.1 mM BSO (Sigma-Aldrich) for 24 hours. Au (Enzo Life Sciences) was dosed at either 250 or 500 nM for 1–3 hours. 2-AAPA prepared as previously described (12) was used at 20–100 µM final concentration for 3 hours. N-acetylcysteine (NAC) 15 mM (Cumberland Pharmaceuticals), or PEG-Catalase 100 U/mL (Sigma-Aldrich) were added simultaneously with BSO or SSZ. For the PEG control, methoxypolyethylene glycol tresylate (Sigma-Aldrich) was used at a final concentration of 0.1 mg/mL. Cells were irradiated with a dose of 2 Gy (dose rate, 0.365 Gy/min) using a 37Cs source (JL Shepherd, San Fernando, CA).
Clonogenic survival assay
Exponentially growing cells were treated as described above. Attached cells were collected using 0.25% trypsin and combined with floating cells. After resuspension in fresh media the cells were counted, plated at low densities, and allowed to grow for 11–14 days. Surviving fractions were calculated as described previously and normalized to vehicle-treated plates.(7) Cell numbers following exposure to Au were corrected to the cell number on vehicle treated dishes to account for the loss of cell sized particles caused by immediate toxicity.
Western Blot Assays
Exponentially growing MDA-MB-231 cells were treated with BSO and Au as described above. Cells were scraped one hour after IR into ice cold RIPA buffer containing Complete Mini-protease inhibitor and PhosStop phosphatase inhibitor (Roche Diagnostics). After sonication the samples were centrifuged for 10 minutes at 4°C. Protein concentrations were determined by BCA method. γH2AX Western blot analysis was carried out using polyclonal anti-phos-H2AX Ser 139 (#07-164, Upstate Biotechnology) running approximately 20 µg of protein per lane on 4 separate occasions, electrophoresed on 15% SDS-polyacrylamide gels and transferred onto PVDF membranes. Bands were detected using ECL Western Blotting Detection System (GE Healthcare) with detection by X-ray film. Band-integrated densities were determined with Image J software and averaged between four gels each normalized to its own α tubulin loading control.
Thioredoxin reductase (TR) and glutathione reductase (GR) assay
Exponentially growing cells on 100 mm dishes were treated as described above then harvested by scraping and frozen as a dry pellet until assayed. Cell pellets were lysed in 50 mM potassium phosphate buffer pH 7.8 containing 1.34 mM diethylenetriaminepentaacetic acid, centrifuged at 5000 rpm for 5 minutes and then the supernatant was assayed using a TR assay kit (Sigma-Aldrich). For GR assay 30 mM GSSG, 1% BSA and 0.8 mM NADPH and PBS was mixed, allowed to incubate 1–2 minute, the sample was added, and the rate of NADPH disappearance was measured at 340 nm. Units of GR activity were expressed by comparison to a standard curve after the blank rate was subtracted from the sample. GR and TR activity were normalized using the Lowry protein assay.
Glutathione assay
Cells were grown and processed as for the TR assay except cells were scraped into 300 µL of 5% 5-sulfosalicylic acid (Sigma-Aldrich) and stored frozen for a maximum of 72 hours. Total GSH content was determined spectrophotometrically by NADPH recycling assay as described previously.(7)
Matrigel invasion assay
MDA-MB-231 cells were plated as for the clonogenic assay. Cells were treated with 15 mM NAC and 100 µM BSO for 24 hours, 500 nM Au was added during the last hour of treatment and then culture dishes were irradiated with 2 Gy. The media was then refreshed and the plates were placed back in the incubator for 2.5 hours. Cells were then trypsinized and live cells were determined using the trypan blue assay via Countess (Invitrogen) and reseeded at 2.5 × 104 live cells in migration or invasion chambers (BD Biocoat) in serum free media. Media containing FBS was placed in the bottom chamber. After 16 hours incubation, non-invading cells were scrubbed from the top surface and the membranes stained with Giemsa violet. Cells were counted on four distinct quadrants of each chamber and averaged and the experiment was repeated at least four times.
CD44/CD24/CD326 flow cytometry
MDA-MB-231 exponentially growing on 100 mm dishes at 4% O2 37°C were treated with 500 nM Au or vehicle control followed in one hour by 2 Gy IR and put back in the incubator. 24 hours later the cells were again irradiated with 2 Gy. Approximately 1 hour after IR the cells were disassociated with trypsin, counted and 1 million cells were resuspended in 100 µL staining buffer (DPBS containing HEPES and 2% FBS). Cells were stained with PE anti-human CD24, APC anti-human CD326 (EpCAM, ESA), Brilliant Violet 421 anti-human CD44 on ice for 30 minutes (BioLegend). Cells were stained with Hoechst for live/dead determination and 100,000 cells processed through a Becton Dickinson LSR II with 405nm, 488nm, 561nm, and 639nm lasers. Compensation was calculated and gates were set using fluorescence minus one with added isotype control. The reported results are from three distinct experiments.
Tumor xenograft growth
Animals were housed in the animal care facility at the University of Iowa which has a PHS Animal Welfare Assurance, is registered with the United States Department of Agriculture and is accredited by The Association for Accreditation of Laboratory Animal Care. All animal protocols were reviewed by The Institutional Animal Care and Use Committee. 8 × 106 MDA-MB-231 cells that were previously isolated from an aggressive growing mouse xenograft, then grown in culture as before were injected subcutaneously into the right flank of 8-week-old female nu/nu mice (Harlan Laboratories). When tumor volumes measured 100 mm3 mice were divided into groups of 5 to 7 animals each and given drugs intraperitoneally (i.p.). Vehicles, BSO and Au were prepared as described previously.(6) BSO was administered at 450 mg/kg and Au was administered at 1.7 mg/kg 2 hours after BSO. All mice were anesthetized using 87.5 mg/kg ketamine and 12.5 mg/kg xylazine mixture and mice receiving IR were placed in a lead box with only the right flank exposed. 6 Gy IR was delivered using a Pantak Therapax DXT 300 X-ray machine operated at 200 kVp with added filtration of 0.35 mm Cu + 1.5 mm Al. All treatments were repeated 48 hours later. Weights and tumors volumes were measured daily [Volume = (Length × Width2)/2] and animals euthanized when tumor length exceeded 1.5 cm.
Aldefluor flow cytometric assay
2.5 × 106 SUM159 cells were grown as xenograft flank tumors. When tumors reached approximately 8 mm in diameter, mice were treated with i.p. injections of BSO 675 mg/kg followed in two hours with Au 2.7 mg/kg and four hours later with 6 Gy IR. Tumors were harvested 20 hours after IR and digested in collagenase/hyaluronidase (Stem Cell Technologies) containing media at 37°C agitating with a pipette every 10 minutes for 40 minutes followed by straining through a 70 µm nylon filter into media containing FBS on ice. Cells were labeled with anti-mouse MHC-APC (eBioscience) then the ALDEFLUOR kit (Stem Cell Technologies) was used to determine the population with a high ALDH1 enzymatic activity. Hoechst dye was added to determine viability. Samples were analyzed using a Becton Dickinson LSR II flow cytometer (Becton Dickinson System, INC.). The number of ALDH positive cells of 100,000 live cells was determined by using the sorting gate established with DEAB containing samples.
Snarf-1 flow cytometry
SUM159 xenografts were grown and harvested as above and a single cell suspension was placed in a tissue culture flask and grown at 4% O2 37°C for 24 hours. Select dishes were treated with 1 mM BSO for an additional 24 hours. 5-(and-6)-Chloromethyl SNARF-acetate (SNARF-1) (Molecular Probes) was resuspended in DMSO to make a 1 mM stock solution. 1 × 106 SUM159 cells were resuspended in ALDH buffer containing 10 µM SNARF-1 and ALDH ± DEAB for 30 minutes at 37°C.(17) After incubation cells were resuspended in ALDH buffer and analyzed as before additionally using the Argon 561 laser with 610/20 band pass filter to determine SNARF-1 mean fluorescent intensity on a minimum of 5000 ALDH positive cells.
Statistical analysis
All in vitro experiments were repeated at least three times. Statistical analysis was performed with one way ANOVA with Newman-Keul’s post-test for multiple comparisons or Student’s t test for comparison of individual groups. For tumor growth rate and survival of animal experiments the log-rank test was used to compare survival between treatment groups and a linear mixed effects regression model to estimate and compare group-specific tumor growth curves. A natural log transformation on the tumor size variable achieved the best fit for the model based on AIC. All tests were two-sided and carried out at the 5% level of significance. Analyses were performed with the SAS 9.3 software package.
Results
Au, BSO and SSZ inhibit the GSH and Trx pathway
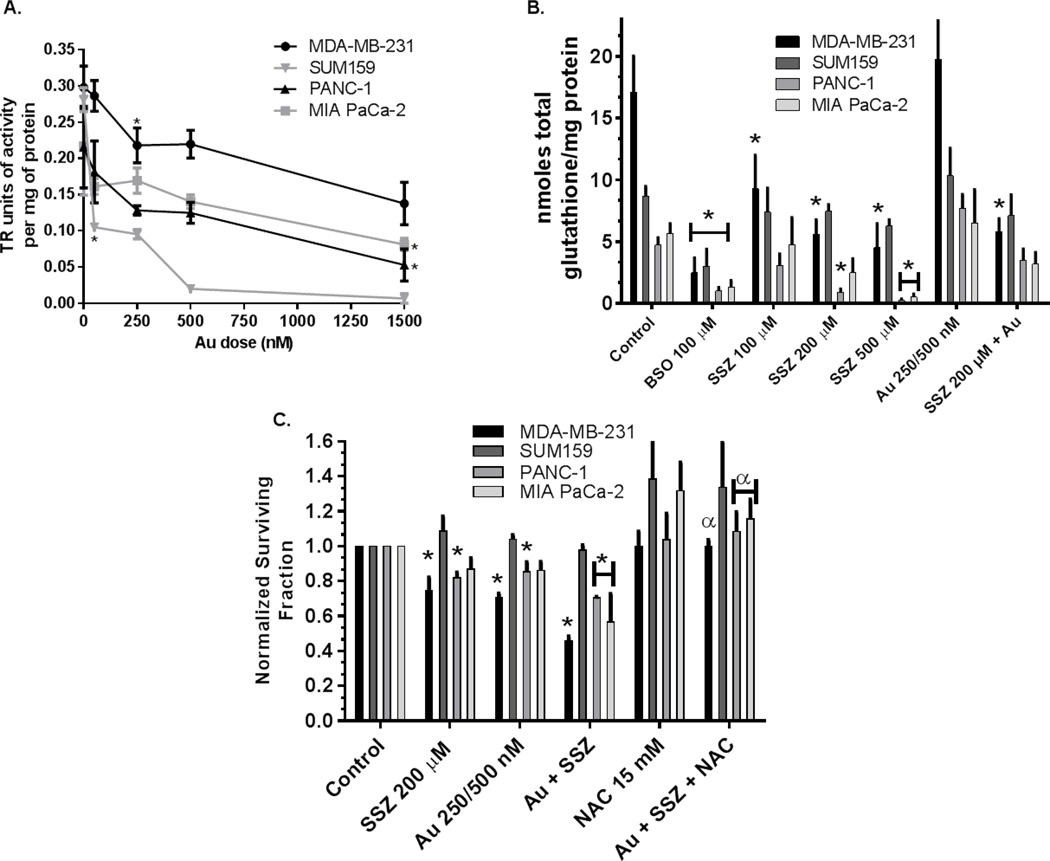
Treatment with Au+BSO has been shown to increase oxidized GSH and Trx as well as sensitize to chemotherapy agents in breast, prostate and lung cancer cells.(7, 8, 18) Figure 1A shows that Au is effective at significantly inhibiting TR activity in breast (63% decrease in SUM159 cells at 50 nM and 27% decrease in MDA-MB-231 cells at 250 nM) and pancreatic cancer cell lines (75% decrease in PANC-1 cells at 1500 nM and 63% decrease in MIA PaCa-2 cells at 1500 nM). Figure 1B shows that treatment with BSO or SSZ resulted in decreases in GSH levels in MIA PaCa-2, PANC-1 and MDA-MB-231 cell lines with higher doses of SSZ needed to get equivalent decreases as with BSO (Figure 1B). Au as a single agent did not alter GSH levels in the four cell lines tested, in addition, when Au was combined with 200 µM SSZ, GSH levels were not significantly different than 200 µM SSZ alone demonstrating that Au did not directly affect GSH levels. Clonogenic survival assays showed 200 µM SSZ resulted in significant cell killing in PANC-1 and MDA-MB-231 cells (19% and 25% respectively). Treatment with Au for 3 hours resulted in significant clonogenic killing of PANC-1 and MDA-MB-231 cells (14% and 29% respectively, Figure 1C). The combined treatment with Au+SSZ on PANC-1, MDA-MB-231 and MIA PaCa-2 cell lines resulted in significant decreases in survival (29%, 54%, and 43% respectively) that were completely reversed with NAC confirming a thiol mediated cell death mechanism (Figure 1C). In SUM159 where SSZ did not deplete GSH levels, Au+SSZ did not result in significant cell death (Figure 1B,C). These results indicate that inhibition of the Xc- transporter is effective at decreasing GSH levels and results in cell death in combination with inhibition of TR in some human cancer cell lines.
Figure 1. Au, BSO, and SSZ are effective at depleting GSH and inhibiting TR in pancreatic (PANC-1; MIA PaCa-2) and breast cancer cells (MDA-MB-231).
Cells were treated with BSO (0.1 mM), SSZ (0.1–0.5 mM), or NAC (15 mM) for 24 hours and/or with Au (250 nM for MIA PaCa-2 and SUM159 or 500 nM PANC-1 and MDA-MB-231) for 3 hours followed by analysis for TR activity * p<0.05 vs. no drug (MDA-MB-231 and SUM159) or vs. 500 nM (PANC-1 and MIA PaCa-2) (A), total GSH * p<0.05 vs. control (B) or plated for clonogenic survival assay (C). Error bars ±1 SEM of at least 3 separate experiments. * p<0.05 vs. control, α p<0.05 compared to treatments without NAC.
2-AAPA inhibits GR, TR and results in decreased clonogenic cell viability
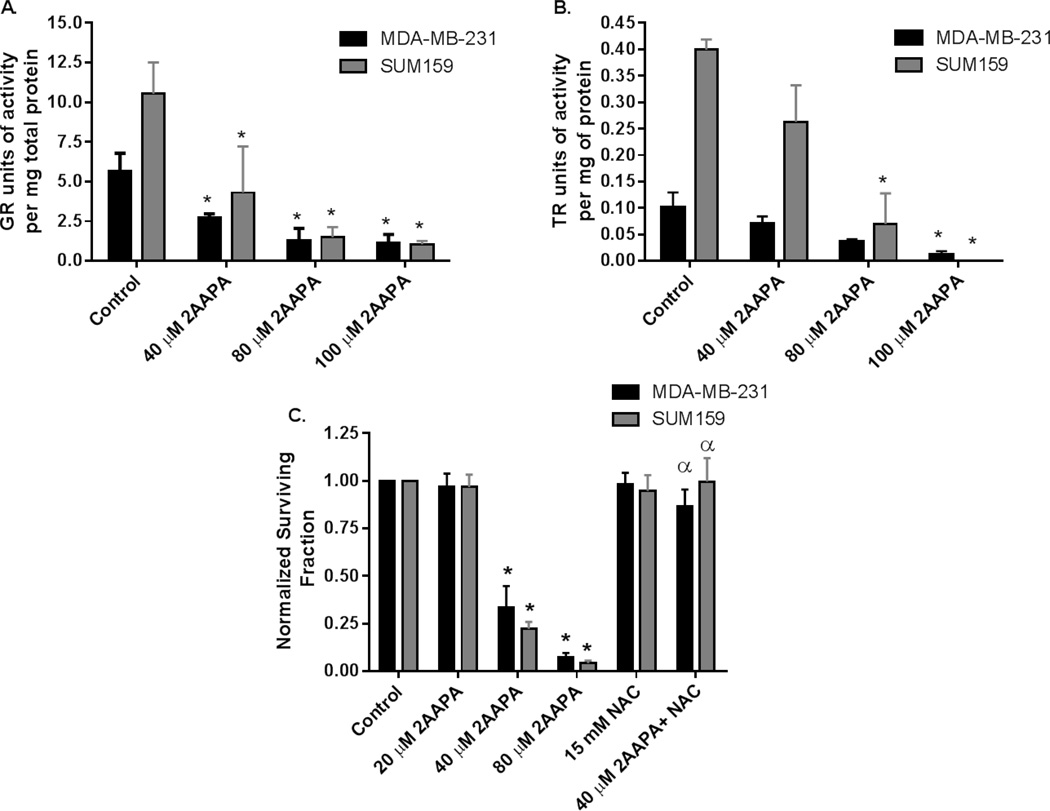
Zhao et. al. previously reported a time and dose dependent inhibition of GR in cancer cells using the novel inhibitor 2-AAPA.(14) Figure 2A, C confirmed that 2-AAPA caused a dose dependent decrease in GR activity that was accompanied by a dose dependent decrease in survival. NAC completely inhibited the cytotoxicity of 40 µM 2-AAPA in both cell lines confirming cell killing is mediated by disruption in thiol metabolism. Because inhibition of the GSH metabolism alone would not be expected to result in the level of cell killing mediated by 2-AAPA, the effect of 2-AAPA on TR activity was determined (Figure 2B). Interestingly, 2-AAPA was found to significantly inhibit TR activity in both cell lines, in a dose dependent fashion firmly establishing that 2-AAPA inhibits both Trx and GSH metabolism (Figure 2AB).
Figure 2. 2-AAPA induces cytotoxicity that is reversible with NAC as well as inhibiting both GR and TR activity.
MDA-MB-231 (black bars) and SUM159 cells (gray bars) were treated with 15 mM NAC for 24 hours and/or 20 µM – 100 µM 2-AAPA for 3 hours. Cells were harvested and then analyzed for GR activity (A), TR activity (B), or clonogenic survival (C). Errors ± 1 SEM of at least 3 separate experiments. * p<0.05 vs. control. α p<0.001 vs. treatment without NAC.
Simultaneous inhibition of the Trx and GSH metabolism enhances radiation responses
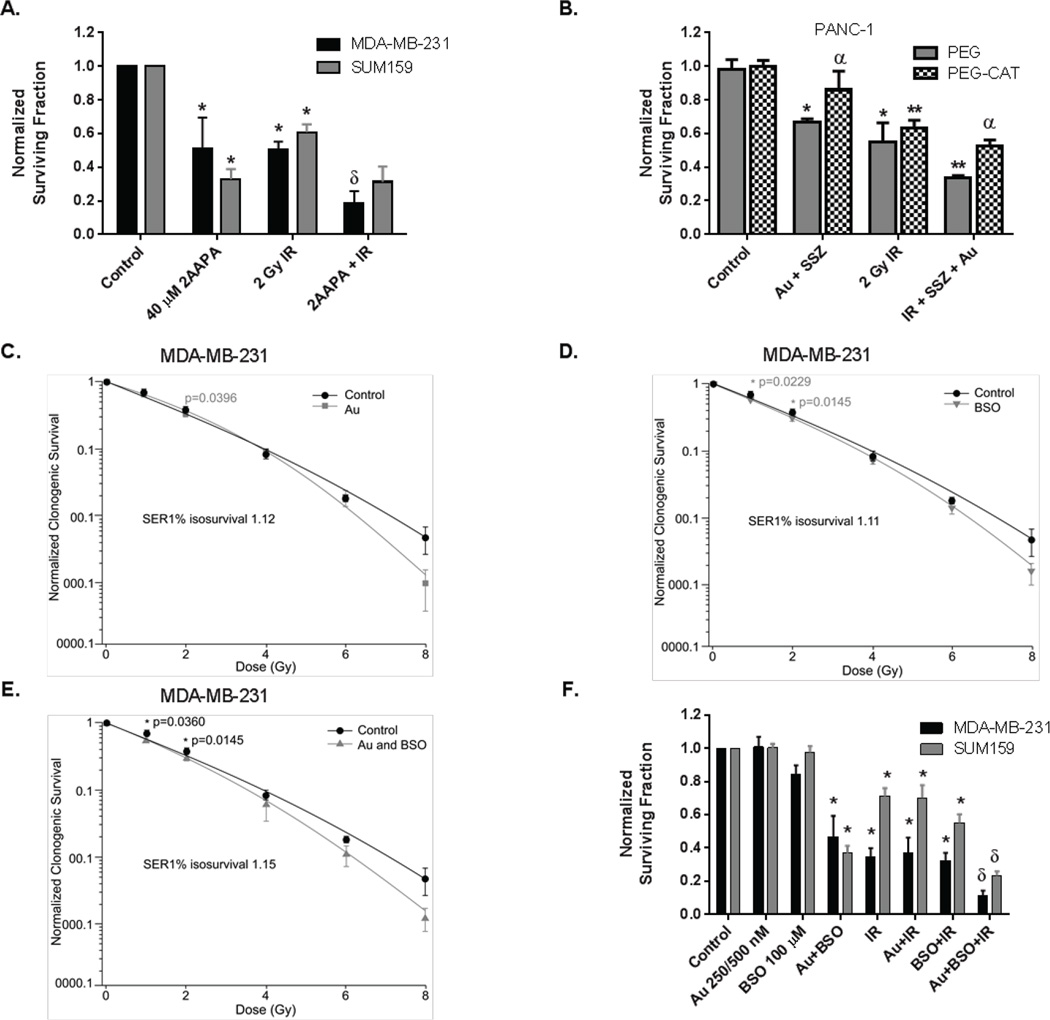
Combined treatment with 2-AAPA + 2 Gy IR caused significantly more clonogenic killing then either treatment alone in MDA-MB-231 cells but not in SUM159 breast cancer cells (Figure 3A). Treatment of breast cancer cells with BSO for 24 hours (resulting in a 65–85% decrease in GSH, see Figure 1B) or Au for the last hour as single agents (resulting in 66% and 25% decrease in TR activity, respectively, Figure 1A) did not significantly reduce clonogenic survival or enhance 2 Gy radiation (Figure 3B). In contrast, when BSO and Au were combined, significant (50–60%) increases in clonogenic cell killing were noted in MDA-MB-231 and SUM159, respectively that appeared to be at least additive when combined with exposure to 2 Gy IR (Figure 3B).
Figure 3. Simultaneous inhibition of Trx- and GSH-metabolism enhances radiation responses.
(A) Normalized clonogenic survival is shown for MDA-MB-231 (black bars) and SUM159 cells (gray bars). 2-AAPA treatment for 3 hours followed by 2 Gy IR. * p<0.05 compared to control, δ p<0.05 compared to treatment groups alone. (B) PANC-1 cells were treated 24 hours with 100 units/ml PEG-CAT or PEG alone, 200 µM SSZ and 500 nM Au for the last 3 hours of incubation followed by 2 Gy IR. Errors ± 1 SEM of at least 3 separate experiments. * p<0.05 vs control, ** p<0.05 vs treatments alone and α - p<0.05 vs treatment without PEG-CAT. (C, D, E) MDA-MB-231 cells were treated with 100 µM BSO for 24 hours and/or 500 nM Au for 1 hour followed by 1,2,4,6 and 8 Gy IR immediately followed by the clonogenic survival assay. Results were normalized to treatment alone and the resulting graph analyzed using IGOR Pro 6.36. (F) Normalized clonogenic survival is shown for MDA-MB-231 (black bars) and SUM159 cells (gray bars). 100 µM BSO for 24 hours +Au (250 nM SUM159 or 500 nM MDA-MB-231) for the last hour, followed by 2 Gy IR. * p<0.05 compared to control, δ p<0.05 compared to treatment groups alone.
To determine the sensitizer enhancement ratio (SER) of Au and BSO on breast cancer cells, MDA-MB-231 cells were treated with 100 µM BSO for 24 hours and/or Au 0.5 µM for 1 hour then IR at varying doses followed by clonogenic survival assay (Figure 3C,D,E). Au, BSO, and the combination significantly enhance radiation responses (SER 1.12, 1.11, and 1.15 respectively) at 1% iso survival when normalized to plating efficiency in the presence of drug alone.
Simultaneous inhibition of GSH- and Trx-metabolism in PANC-1 cells with Au+SSZ prior to exposure with 2 Gy IR also appeared to cause additive cell killing (Figure 3B). When PANC-1 cells were pretreated with Au+SSZ in the presence of 100 U/mL polyethylene glycol conjugated catalase (PEG-CAT) to scavenge H2O2, both the cell killing and the additive response to 2 Gy IR was inhibited. These results support the conclusion that the cell killing and enhanced response to radiation seen in PANC-1 cells treated with inhibitors of GSH-and Trx-dependent metabolism is mediated by H2O2, supporting the hypothesis that Au and SSZ are compromising H2O2 metabolism.
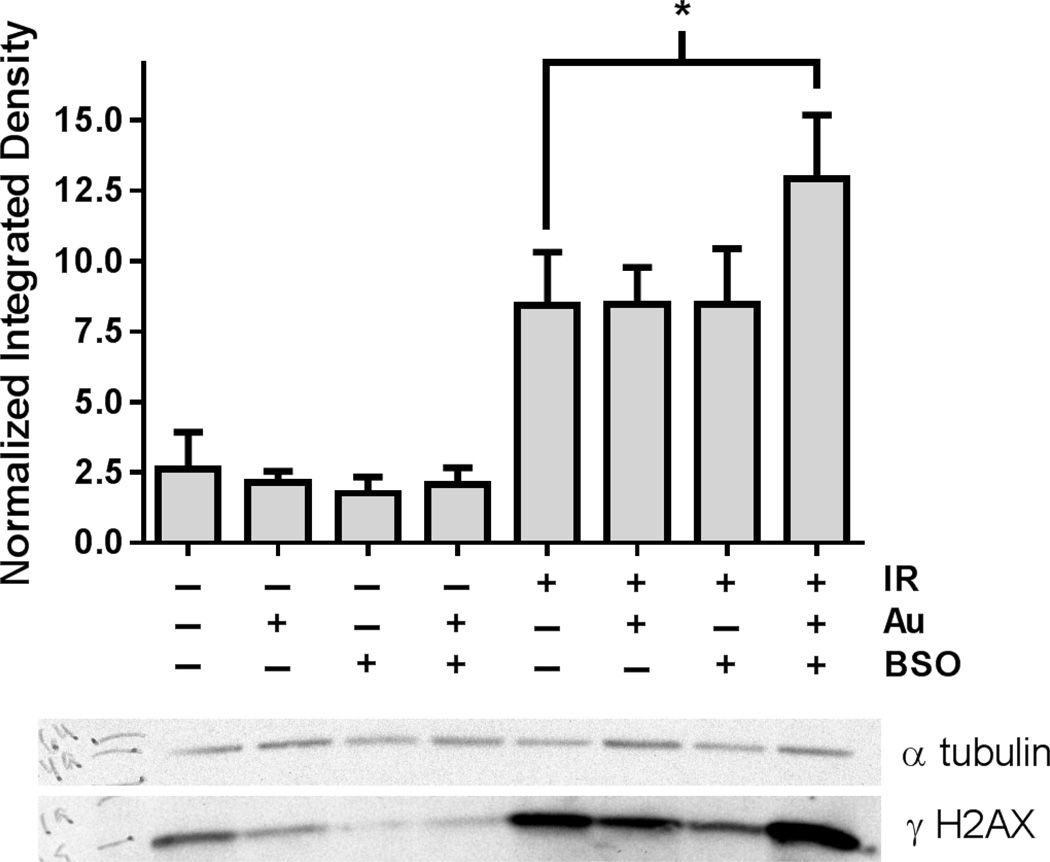
Cells were harvested for Western blots using immunoreactive phosphorylated histone H2AX (γH2AX), a marker of DNA double strand breaks, 1 hour following treatment of MDA-MB-231 cells treated with combinations of Au 0.5 µM, BSO 100 µM, and IR 2Gy. As expected γH2AX significantly increased in MDA-MB-231 cells treated with radiation (Figure 4). Neither 0.5 µM Au or 100 µM BSO alone increased γH2AX in irradiated cells. In contrast, the combination of Au+BSO significantly increased γH2AX (p=0.03), indicating an increase in steady-state level of DNA double strand breaks following radiation in the cells demonstrating enhanced radiosensitivity. These results support the conclusion that inhibition of GSH- and Trx- metabolism enhances radiation sensitivity in human breast cancer cells in vitro through a mechanism involving enhanced DNA damage.
Figure 4. Addition of Au+BSO to radiation increases DNA damage in breast cancer cells.
MDA-MB-231 cells were treated with 100 µM BSO for 24 h, 500 nM Au for 1 hour followed by 2 Gy IR. Cells were harvested and analyzed using Western blot analysis with a γ H2AX antibody and an α tubulin loading control. The gels from 4 independent experiments were quantified using Image J. * p<0.03
Disruption of redox balance inhibits cancer cell migration and invasion and sensitizes cancer stem cells to IR
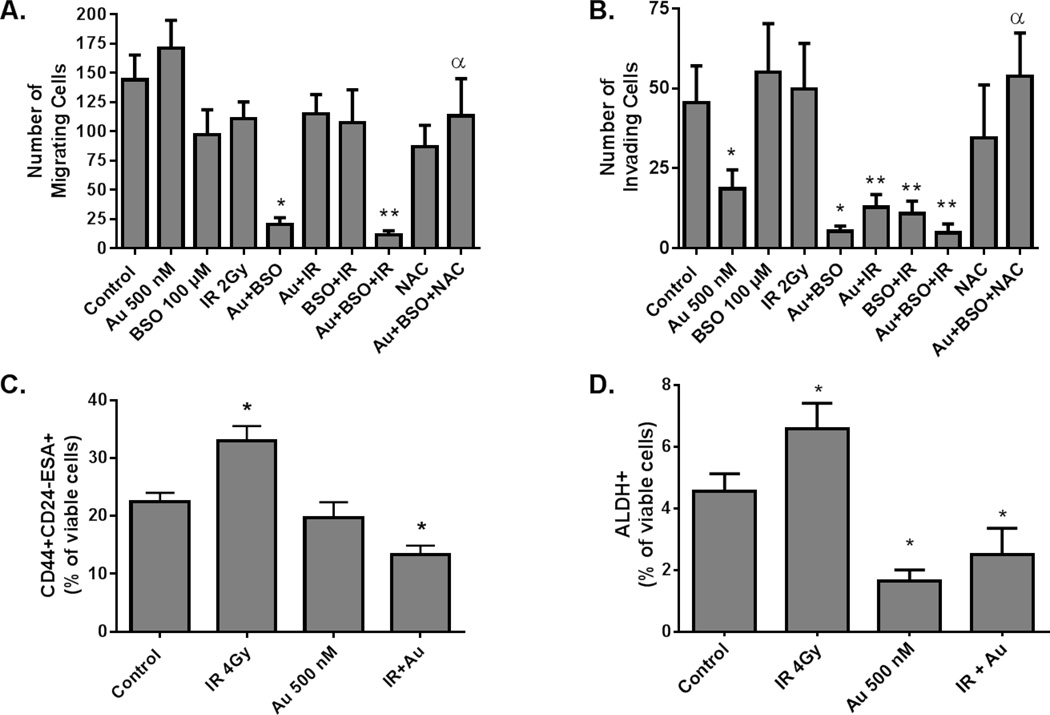
Recent evidence suggests that the cancer cell’s ability to invade surrounding tissue is dependent of epithelial-mesenchymal transition (EMT) factors that may be modulated by redox balance.(19, 20) Figure 5 shows that the combination of Au+BSO in the presence and absence of IR significantly decreased MDA-MB-231 cells’ ability to migrate through both control membranes (Figure 5A) as well as Matrigel basement membranes (Figure 5B). Furthermore, the ability of Au+BSO to inhibit invasion and migration was abrogated by treatment with NAC, supporting the conclusion that this biological effect was caused by compromised GSH- and Trx-dependent thiol metabolism (Figure 5A,B) Notably, treating with either Au or BSO before IR did not significantly affect migration through the control membrane (Figure 5A) but did significantly decrease the cells’ ability to invade the Matrigel membrane (Figure 5B) which is consistent with previous studies.(21)
Figure 5. Disruption of redox balance inhibits cancer cell migration and invasion and sensitizes cancer stem cells to IR.
MDA-MB-231 were treated with BSO, Au and IR as in Figure 3B. Immediately following IR the media was changed to fresh media, incubated 2.5 hours, trypsinized and assessed for viability. 2.5 × 104 viable cells were reseeded in BD BioCoat 8 micron migration (A) or BioCoat Matrigel coated invasion (B) chambers. After 16 hours chambers were stained and invading cells counted. Error bars represent ± 1 SEM of at least four separate experiments.* p<0.05 compared to control, ** p<0.05 compared to IR, α p<0.05 compared to Au+BSO. Exponentially growing (C) MDA-MB-231 or (D) SUM159 cells were treated with 500 nM Au followed in one hour by 2 Gy IR and an additional 2 Gy fraction 24 hours later. One hour after the second dose of radiation cells were trypsinized and then stained with CD44-BV high, CD24-PE low, ESA-APC high (MDA-MB-231 cells) or ALDFLUOR (SUM159 cells) and analyzed with flow cytometry. * p<0.05 compared to control.
Considering the results in Figure 5B and because it has been reported that cancer stem cells are resistant to radiation, we decided to investigate the effects of Au in combination with radiation treatment on the sub-population of cancer stem cells. It has been shown that a small population of MDA-MB-231 breast cancer EMT like cells exhibit self-renew capacity and grow tumors in animals when injected in small numbers (as few as 100 cells).(22) Likewise, a subpopulation of stem like cells has been identified in SUM159 cells using aldehyde dehydrogenase 1 activity (ALDH1).(23) MDA-MB-231 and SUM159 cells were treated with and without Au 500 nM followed by 4 Gy IR (in 2 Gy fractions 24 hours apart). MDA-MB-231 cells were then stained for CD44 high, CD24 low and CD326 positive (also known as EpCAM or epithelial-specific antigen) expressing cells (Figure 5C). SUM159 cells were stained for ALDH1 (Figure 5D). Interestingly treatment with IR resulted in an increase in the percent of viable (Hoechst negative) stem cells supporting that this subpopulation of breast cancer cells is resistant to radiation; however, pre-treatment with Au was able to re-sensitize this population and resulted in a decrease in the percent of viable stem cells in both cell lines (Figure 5C,D).
Au+BSO treatment before IR increases mouse survival and decreases breast cancer progenitor stem cell survival in vivo
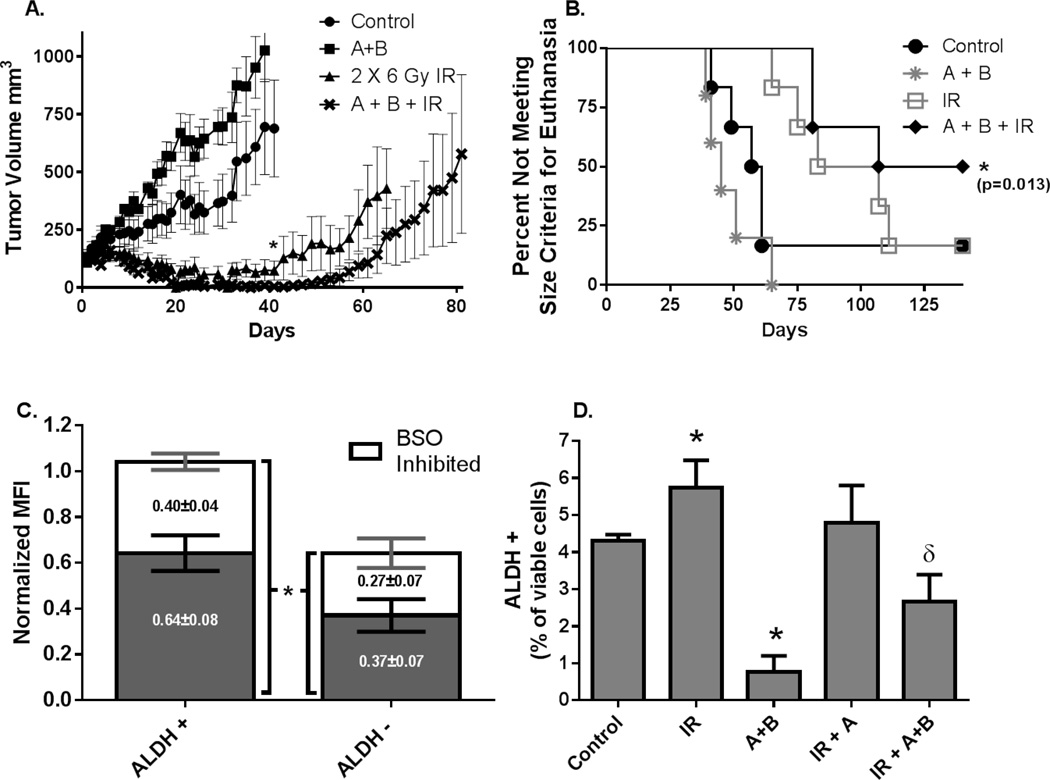
Mice bearing MDA-MB-231 xenografts were pre-treated with BSO and Au followed by IR every 48 hours for two 6 Gy doses. Mice treated with Au and BSO showed no changes in behavior or activity but lost an average of 1.2 grams body weight (~5% of body weight) during treatments. However, by one week following treatment the mice had regained weight and were identical to controls, indicating all treatments were well-tolerated. Treatment with Au+BSO appeared to result in a slight but insignificant increase in tumor growth rate over control mice, possibly due to infrequent dosing and the relatively large tumor sizes at the beginning of treatment (5 to 8 mm diameter). The mean of the tumor volumes on day 41 (day first mouse in the control group reached criteria for euthanasia) was significantly reduced in the mice pre-treated with Au+BSO before IR compared to mice treated with IR alone (p=0.02) (Figure 6A). When all the growth curves were fit using mixed linear regression analysis, the growth curve of the IR group did not reach significance (p=0.07), relative to control. In contrast the Au+BSO+IR growth curve was significantly different than control (p=0.009) (Supplemental Figure 1), but differences between the IR and IR+Au+BSO treatment groups did not reach statistical significance (p=0.24) (Supplemental Fig. 1). When time to tumor regrowth was compared using a log-rank test (Supplemental Figure 2), mice treated with A+B+IR showed a trend toward slower regrowth, relative to IR alone, that again did not reach statistical significance (p=0.08). Kaplan Meier survival curves also showed that the Au+BSO as well as the IR alone groups did not differ significantly from control (p=0.58 and p=0.15 respectively), however survival of mice treated with Au+BSO+IR was significantly different from control (p=0.013, Figure 6B).
Figure 6. Pre-treatment with Au+BSO before radiation decreases tumor growth rate and breast cancer stem cells in vivo.
MDA-MB-231 breast cancer xenografts were treated with BSO 450 mg/kg i.p. followed in 2 hours with Au 1.7 mg/kg i.p. followed in 3 hours with 2 × 6 Gy IR separated by 48 hours. (A) Tumor growth curves. Error bars are ± 1 SEM. (B) Kaplan-Meier survival plots. Criteria for euthanasia was reached when xenograft tumors measured 15 mm in one direction. (C) Three separate SUM159 xenograft tumors were grown in nude mice. Tumors were harvested, digested and plated in a tissue culture flask at 4% O2 for 48 hours. Cells were then treated with 1 mM BSO for 24 hours then harvested and subjected to flow cytometry with CM-SNARF and ALDFLUOR. At least 5,000 ALDH1+ cells were analyzed from each sample to determine the MFI of CM-SNARF (D) SUM159 xenograft tumors were grown on nude mice, and treated with 675 mg/kg BSO followed in two hours with Au 2.7 mg/kg and in four hours with 6 Gy IR. Tumors were harvested 20 hour after IR and subjected to the ALDEFLUOR flow cytometry assay. Error bars represents ± 1 SEM of at least three tumors assayed on at least two different occasions. * p<0.05 vs. control, δ p<0.05 vs. treatment groups alone.
Flow cytometric studies using chloromethyl SNARF-1 acetate (CM-SNARF, a dye that forms covalent adducts with intracellular reduced thiols) (17) along with ALDH1 activity demonstrates that breast cancer stem cells (ALHD1+) derived from SUM159 mouse xenografts, have more total reduced thiols than non-stem cells (ALDH-) (Figure 6C). Furthermore the treatments with BSO, shown to deplete >90% of total GSH (8), revealed that both the fraction of reduced thiols representing GSH (BSO sensitive) as well as the fraction of thiols that remains following GSH depletion were greater in ALDH1+ stem cells, relative to the bulk tumor cell population (Figure 6C). These results extend previous observations (4) and show that breast cancer stem cells have greater levels of both GSH and non-GSH dependent thiols.
ALDH1 was measured in SUM159 xenografts 20 hours following 6 Gy IR and consistent with the in vitro data, irradiated tumors have a significantly greater number of ALDH positive cells relative to controls (Figure 6D). Pre-treatment with Au+BSO resulted in a significant decrease in ALDH1 positive cells and inhibited the IR-induced increase in ALDH1+ cells. Interestingly, pre-treatment with Au+BSO before IR also resulted in significantly fewer ALDH positive cells compared to either IR treated or control tumors (Figure 6D). Overall the results in Figure 6 demonstrate the increased thiol antioxidant capacity of breast cancer stem cells and the effect of manipulating cellular thiols on the responses of breast cancer stem cells that could contribute to radiation resistance.
Discussion
GSH is an essential co-factor for all glutathione peroxidase and glutathione transferase enzymes that detoxify hydroperoxides and electrophiles produced as byproducts of oxidative stress.(24) Likewise, Trx acts as a cofactor in the peroxiredoxin-mediated detoxification of hydroperoxides.(6) Trx and peroxiredoxins have been shown to be overexpressed in human breast carcinomas (6) and total GSH content has been shown to be significantly increased in breast cancers as well as being correlated with poor outcomes.(25) Recently we reported that simultaneous inhibition of both Trx- and GSH-dependent metabolism was required to achieve the most effective sensitization of human lung and breast cancer cells to chemotherapeutic agents (7, 8), most likely because of redundancies between the GSH- and Trx-dependent pathways for hydroperoxide metabolism. In the current study we extended this research to examine radiation responses following manipulation with therapeutically relevant thiol modifying agents as well as combined manipulations of glutathione and thioredoxin metabolism in early progenitor breast cancer stem cells.
SSZ, 2-AAPA, and BSO were used to inhibit GSH metabolism and Au as well as 2-AAPA were used to inhibit Trx metabolism (Figures 1 and 2). When 60–80% decreases in GSH content were achieved with SSZ and combined with Au, enhancement of radiation response was seen in 3 of the 4 cell lines tested (Figure 3). Although the 200 µM concentration of SSZ that is needed to achieve these results in vitro is not achievable using current oral dosing this may be overcome with intravenous dosing.(26) Unexpectedly, the GR inhibitor, 2-AAPA, was also found to be an effective TR inhibitor suggesting a broad spectrum reactivity with thiol containing enzymes (Figure 2). From the current results comparing effective doses of SSZ and BSO for depleting GSH (Figure 1B), the most effective combination of manipulating GSH- and Trx- metabolism that was also tolerated in vivo appeared to be BSO+Au. Furthermore when pancreatic and breast cancer cells were exposed to radiation, we found that the combined inhibition of the GSH- and Trx-metabolism was also required to achieve enhancement of IR responses (Figure 3, 4, 5, and 6). The mechanism(s) responsible for these effects clearly involved disruptions in thiol and hydroperoxide metabolism as evidence by the ability of NAC and PEG-CAT to mitigate the toxic effects in cancer cells (Figures 1C, 2C, 3B, and 5A,B).
Farina et. al. recently hypothesized an important role for Trx-1 in the invasive phenotype of breast cancer cells, by a mechanism where Trx-1 overexpression stimulates matrix metalloproteinase, resulting in a more invasive phenotype.(21) The current studies clearly show the combination of Au+BSO was needed to achieve maximal inhibition of migration and invasion in vitro (Figure 5A, B). In contrast, either Au or BSO as single agents combined with IR, decreased the ability of breast cancer cells to invade Matrigel basement membranes. These effects of Au+BSO on migration and invasion were mitigated by NAC clearly supporting the importance of thiol metabolism as a critical regulator of the invasive phenotype in human breast cancer cells (Figure 5).
Cancer stem cells are capable of self-renewal and thought to represent a critical cancer cell subpopulation that determines treatment outcome.(3, 4) Intracellular redox status has been suggested to be a critical factor regulating stem cell self-renewal and altering the redox balance by inhibiting the xCT cystine transporter in breast cancer cells, impares tumor-sphere generation, delays tumor growth and decreases metastasis.(26, 27, 28) Breast cancer stem cells have been suggested to govern tumor recurrence because they are reported to produce lower steady-state levels of pro-oxidants, relative to bulk tumor cells, leading to radio-resistance.(3, 4) Although radiation is effective at shrinking tumors and is considered standard of care in many breast cancer cell populations, previous results as well as the results in the current study have demonstrated that a subpopulation of breast cancer cells appear to be resistant to radiation (Figs 5CD, 6D). In vitro radio-resistance of breast cancer stem cells has been reported to be partially abrogated by pharmacologically depleting GSH with BSO (4) and the current study clearly demonstrates inhibition of TR with Au is also effective at decreasing the % of ALDH1+ breast cancer stem cells following radiation (Figure 5C,D). In addition, the current study also clearly demonstrates (using ALDH1 as a marker for breast cancer stem cells) that percent viable breast cancer stem cells in xenografts also increase following IR and this is significantly reduced by pre-treatment with Au+BSO (Figure 6D).
Notably, the data in Figure 6A,B also support the potential for these combinations to improve tumor responses to radiation as evidenced by the fact that 3 animals showed no evidence of disease after being treated Au+BSO+IR, whereas only one animal remained disease free after IR treatment. While these data support the conclusion that radiation was the dominant treatment modality required for the antitumor effects, the tumors in this experiment were all between 5 and 8 mm in diameter at the beginning of drug and radiation treatments. This tumor size may have affected the uniformity of drug distribution to a greater extent than the uniformity of radiation dose, contributing to the fact that the radiation was the main reason for the anti-tumor effect. Most importantly, the groups that received A+B+ 2 × 6 Gy, relative to radiation alone, did respond significantly better when mean tumor volumes were compared at 41 days when the first control mouse died and did clearly show a more significant growth inhibition (supplemental Figure 1), relative to control.
Overall the results of the current study support the overarching hypothesis that intracellular thiol redox status governed by GSH- and Trx-dependent metabolism are critical regulators of the invasive/metastatic phenotype of human breast cancer cells. Furthermore these results highlight the importance of designing combined modality cancer therapies targeting both GSH- and Trx-dependent metabolism to enhance radiation responses.
Supplementary Material
Acknowledgments
The authors would like to thank The Radiation and Free Radical Research Core lab and Specifically Drs. Nukhet Aykin-Burns, Max Wicha, and Michael L. McCormick for helpful discussions and advice related to cancer stem cell experiments and enzymological analysis. The authors would like to thank Justin Fishbaugh at the University of Iowa Flow Cytometry Facility. The authors would also like to thank the Radiation and Free Radical Research Core at the University of Iowa for help in irradiations and providing instrumentation for biochemical measurements. The authors also thank Anna M. Button and the Biostatistics Core for statistical support analyzing animal data. This work was supported by NIH R01CA182804 (DRS), P30CA086862 (DRS), The Iowa Center for Research by Undergraduates (JMS, RAO, TJR), ASTRO JF2014-1 (BGA) and a Breast Cancer Research Group Pilot Grant from the Holden Comprehensive Cancer Center (MAF).
References
- 1.Recht A, Solin LJ. Breast-conserving surgery and radiotherapy in early-stage breast cancer: the importance of local control. Seminars in radiation oncology. 2011;21(1):3–9. doi: 10.1016/j.semradonc.2010.08.001. Epub 2010/12/08. PubMed PMID: 21134648. [DOI] [PubMed] [Google Scholar]
- 2.Roeder F, Timke C, Saleh-Ebrahimi L, Schneider L, Hackert T, Hartwig W, et al. Clinical phase I/II trial to investigate neoadjuvant intensity-modulated short term radiation therapy (5 × 5 Gy) and intraoperative radiation therapy (15 Gy) in patients with primarily resectable pancreatic cancer - NEOPANC. BMC cancer. 2012;12:112. doi: 10.1186/1471-2407-12-112. Epub 2012/03/27. PubMed PMID: 22443802; PubMed Central PMCID: PMC3323416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips TM, McBride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98(24):1777–1785. doi: 10.1093/jnci/djj495. PubMed PMID: 17179479. [DOI] [PubMed] [Google Scholar]
- 4.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458(7239):780–783. doi: 10.1038/nature07733. PubMed PMID: 19194462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura H, Bai J, Nishinaka Y, Ueda S, Sasada T, Ohshio G, et al. Expression of thioredoxin and glutaredoxin, redox-regulating proteins, in pancreatic cancer. Cancer Detect Prev. 2000;24(1):53–60. Epub 2000/04/11. PubMed PMID: 10757123. [PubMed] [Google Scholar]
- 6.Cha MK, Suh KH, Kim IH. Overexpression of peroxiredoxin I and thioredoxin1 in human breast carcinoma. J Exp Clin Cancer Res. 2009;28:93. doi: 10.1186/1756-9966-28-93. PubMed PMID: 19566940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fath MA, Ahmad IM, Smith CJ, Spence J, Spitz DR. Enhancement of carboplatin-mediated lung cancer cell killing by simultaneous disruption of glutathione and thioredoxin metabolism. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(19):6206–6217. doi: 10.1158/1078-0432.CCR-11-0736. Epub 2011/08/17. PubMed PMID: 21844013, PubMed Central PMCID: PMC3186854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scarbrough PM, Mapuskar KA, Mattson DM, Gius D, Watson WH, Spitz DR. Simultaneous inhibition of glutathione- and thioredoxin-dependent metabolism is necessary to potentiate 17AAG-induced cancer cell killing via oxidative stress. Free radical biology & medicine. 2012;52(2):436–443. doi: 10.1016/j.freeradbiomed.2011.10.493. Epub 2011/11/22. PubMed PMID: 22100505; PubMed Central PMCID: PMC3664944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanes O, Clark J, Wong DM, Patti GJ, Sanchez-Ruiz A, Benton HP, et al. Metabolic oxidation regulates embryonic stem cell differentiation. Nature chemical biology. 2010;6(6):411–417. doi: 10.1038/nchembio.364. PubMed PMID: 20436487; PubMed Central PMCID: PMC2873061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi X, Zhang Y, Zheng J, Pan J. Reactive oxygen species in cancer stem cells. Antioxidants & redox signaling. 2012;16(11):1215–1228. doi: 10.1089/ars.2012.4529. Epub 2012/02/10. PubMed PMID: 22316005; PubMed Central PMCID: PMC3324813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey HH. L-S,R-buthionine sulfoximine: historical development and clinical issues. Chem Biol Interact. 1998;111–112:239–254. doi: 10.1016/s0009-2797(97)00164-6. Epub 1998/07/29. PubMed PMID: 9679558. [DOI] [PubMed] [Google Scholar]
- 12.Gupta V, Jani JP, Jacobs S, Levitt M, Fields L, Awasthi S, et al. Activity of melphalan in combination with the glutathione transferase inhibitor sulfasalazine. Cancer chemotherapy and pharmacology. 1995;36(1):13–19. doi: 10.1007/BF00685726. Epub 1995/01/01. PubMed PMID: 7720170. [DOI] [PubMed] [Google Scholar]
- 13.Lo M, Ling V, Low C, Wang YZ, Gout PW. Potential use of the anti-inflammatory drug, sulfasalazine, for targeted therapy of pancreatic cancer. Current oncology. 2010;17(3):9–16. doi: 10.3747/co.v17i3.485. Epub 2010/06/23. PubMed PMID: 20567622; PubMed Central PMCID: PMC2880911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seefeldt T, Zhao Y, Chen W, Raza AS, Carlson L, Herman J, et al. Characterization of a novel dithiocarbamate glutathione reductase inhibitor and its use as a tool to modulate intracellular glutathione. J Biol Chem. 2009;284(5):2729–2737. doi: 10.1074/jbc.M802683200. PubMed PMID: 19049979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iqbal MS, Saeed M, Taqi SG. Erythrocyte membrane gold levels after treatment with auranofin and sodium aurothiomalate. Biological trace element research. 2008;126(1–3):56–64. doi: 10.1007/s12011-008-8184-x. Epub 2008/07/24. PubMed PMID: 18649049. [DOI] [PubMed] [Google Scholar]
- 16.Omata Y, Folan M, Shaw M, Messer RL, Lockwood PE, Hobbs D, et al. Sublethal concentrations of diverse gold compounds inhibit mammalian cytosolic thioredoxin reductase (TrxR1) Toxicol In Vitro. 2006;20(6):882–890. doi: 10.1016/j.tiv.2006.01.012. PubMed PMID: 16510263. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton D, Loignon M, Alaoui-Jamali MA, Batist G. Novel use of the fluorescent dye 5-(and-6)-chloromethyl SNARF-1 acetate for the measurement of intracellular glutathione in leukemic cells and primary lymphocytes. Cytometry Part A : the journal of the International Society for Analytical Cytology. 2007;71(9):709–715. doi: 10.1002/cyto.a.20433. Epub 2007/07/12. PubMed PMID: 17623874. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Fath MA, Scarbrough PM, Watson WH, Spitz DR. Combined inhibition of glycolysis, the pentose cycle, and thioredoxin metabolism selectively increases cytotoxicity and oxidative stress in human breast and prostate cancer. Redox biology. 2015;4:127–135. doi: 10.1016/j.redox.2014.12.001. PubMed PMID: 25560241; PubMed Central PMCID: PMC4309850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaiswing L, Zhong W, Liang Y, Jones DP, Oberley TD. Regulation of prostate cancer cell invasion by modulation of extra- and intracellular redox balance. Free radical biology & medicine. 2012;52(2):452–461. doi: 10.1016/j.freeradbiomed.2011.10.489. Epub 2011/11/29. PubMed PMID: 22120495; PubMed Central PMCID: PMC3253260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch S, Volkmar CM, Kolb-Bachofen V, Korth HG, Kirsch M, Horn AH, et al. A new redox-dependent mechanism of MMP-1 activity control comprising reduced low-molecular-weight thiols and oxidizing radicals. Journal of molecular medicine. 2009;87(3):261–272. doi: 10.1007/s00109-008-0420-5. Epub 2008/11/27. PubMed PMID: 19034402. [DOI] [PubMed] [Google Scholar]
- 21.Farina AR, Cappabianca L, DeSantis G, Di Ianni N, Ruggeri P, Ragone M, et al. Thioredoxin stimulates MMP-9 expression, de-regulates the MMP-9/TIMP-1 equilibrium and promotes MMP-9 dependent invasion in human MDA-MB-231 breast cancer cells. FEBS letters. 2011;585(20):3328–3336. doi: 10.1016/j.febslet.2011.09.023. Epub 2011/10/04. PubMed PMID: 21963718. [DOI] [PubMed] [Google Scholar]
- 22.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10(2):R25. doi: 10.1186/bcr1982. PubMed PMID: 18366788; PubMed Central PMCID: PMC2397524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer research. 2009;69(4):1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. Epub 2009/02/05. PubMed PMID: 19190339; PubMed Central PMCID: PMC2819227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell JB, Russo A. The role of glutathione in radiation and drug induced cytotoxicity. The British journal of cancer Supplement. 1987;8:96–104. Epub 1987/06/01. PubMed PMID: 3307879; PubMed Central PMCID: PMC2149463. [PMC free article] [PubMed] [Google Scholar]
- 25.Perquin M, Oster T, Maul A, Froment N, Untereiner M, Bagrel D. The glutathione-related detoxification system is increased in human breast cancer in correlation with clinical and histopathological features. J Cancer Res Clin Oncol. 2001;127(6):368–374. doi: 10.1007/s004320000228. PubMed PMID: 11414197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng W, Winter SM, Mayersohn M, Bishop JB, Sipes IG. Toxicokinetics of sulfasalazine (salicylazosulfapyridine) and its metabolites in B6C3F1 mice. Drug metabolism and disposition: the biological fate of chemicals. 1993;21(6):1091–1097. Epub 1993/11/01. PubMed PMID: 7905389. [PubMed] [Google Scholar]
- 27.Lanzardo S, Conti L, Rooke R, Ruiu R, Accart N, Bolli E, et al. Immunotargeting of antigen xCT attenuates stem-like cell behavior and metastatic progression in breast cancer. Cancer research. 2015 doi: 10.1158/0008-5472.CAN-15-1208. PubMed PMID: 26567138. [DOI] [PubMed] [Google Scholar]
- 28.Yae T1, Tsuchihashi K, Ishimoto T, Motohara T, Yoshikawa M, Yoshida GJ, Wada T, Masuko T, Mogushi K, Tanaka H, Osawa T, Kanki Y, Minami T, Aburatani H, Ohmura M, Kubo A, Suematsu M, Takahashi K, Saya H, Nagano O. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat Commun. 2012;3:883. doi: 10.1038/ncomms1892. PMID: 22673910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.