Abstract
The B7 family of immune costimulatory ligands is a group of cell surface proteins that bind to the surface receptors of lymphocytes to fine-tune immune responses. The aberrant expression of these proteins plays a key role in tumor immune evasion. Immunotherapy targeting certain B7 family members, including programmed death ligand 1, has proven quite effective in suppressing tumor growth. However, why such therapy works in only a subgroup of tumors is unclear. We hypothesized that other B7 family members, either alone or in concert with programmed death ligand 1, play a crucial role in tumor pathogenesis and progression. We therefore examined the expression of a newly discovered B7 family member, B7-H4, in 306 cases of ovarian serous carcinoma by immunohistochemistry. We found that 91% (267/293) of the high-grade ovarian serous carcinomas and 69% (9/13) of the low-grade ovarian serous carcinomas expressed B7-H4. The difference between B7-H4 expression in high-grade and low-grade ovarian serous carcinoma was statistically significant (P = 0.002). Moreover, B7-H4 protein expression in high-grade serous carcinoma was associated with tumor stage (P < 0.01) but not overall survival or disease-free survival. In conclusion, B7-H4 is frequently expressed in ovarian serous carcinomas, especially high-grade serous carcinomas, and may represent a novel immunotherapeutic target in this cancer.
Keywords: Ovarian cancer, fallopian tube, B7-H4, immunohistochemistry, immunotherapy
1. Introduction
The B7 family of immune costimulatory ligands is a group of cell surface proteins that bind to the surface receptors of lymphocytes to fine-tune immune responses. The aberrant expression of these proteins plays a key role in tumor immune evasion [1-4]. Immunotherapy targeting certain B7 family members, including programmed death ligand 1 (PD-L1), has proven quite effective in suppressing tumor growth. However, why such therapy works in only a subgroup of tumors is unclear. We hypothesized that other B7 family members, either alone or in concert with programmed death ligand 1, play a crucial role in tumor pathogenesis and progression.
One recently discovered member of the B7 family is B7-H4. The human B7-H4 gene is located on chromosome 1, and a possible B7-H4 pseudogene is located on chromosome 20. The B7-H4 gene has six exons and five introns, and the first two exons encode a signal peptide. Alternative splicing of the B7-H4 gene produces two different transcripts [5]. Although many normal human tissues (e.g., lung, liver, kidney, ovary, testis, placenta) express B7-H4 messenger RNA (mRNA), normal tissues have no or little B7-H4 protein expression, which suggests that B7-H4 expression is regulated post-transcriptionally [5]. B7-H4 has been shown to inhibit T-lymphocyte proliferation and cytokine production and thus may serve as a means by which some ovarian serous carcinomas circumvent anti–PD-L1 immunotherapy [5]. Although some small studies have reported B7-H4 protein expression in ovarian cancers [5-9], the patterns, rates, and levels of B7-H4 expression in ovarian serous carcinoma by histological grade or disease stage have not been assessed definitively in a large-scale study.
In the present study, to determine the potential of B7-H4 as an immunotherapeutic target, we assessed its expression in a large number of ovarian serous carcinoma samples. Our study's findings may provide the basis for expanding the scope of immunotherapeutic drugs used against this disease.
2. Materials and Methods
2.1. Patients and samples
With approval from the Institutional Review Board, we identified 306 patients who underwent surgery for ovarian serous carcinoma at our institution between 1990 and 2009. The patients’ relevant clinical data, including demographic information, pathologic diagnosis, laboratory findings, radiologic findings, and follow-up information, were obtained from electronic medical records. The diagnosis of ovarian serous carcinoma was based on 2014 World Health Organization criteria [10]. Ovarian serous carcinomas were graded using a 2-tier system (low-grade versus high-grade) [11] and staged using the International Federation of Gynecology and Obstetrics (FIGO) system [12]. In addition, we assessed the expression of B7-H4 in normal ovary (n = 24) and fallopian tube (n = 20). Tissue microarrays were constructed as described previously [13-17].
2.2. Immunohistochemical staining for B7-H4
Immunohistochemical staining for B7-H4 was performed with an anti–B7-H4 rabbit monoclonal antibody (D1M8I, 1:200; Cell Signaling, Danvers, MA) as per the manufacturer's recommendations. The patients’ formalin-fixed, paraffin-embedded tissue specimens (3-μm-thick sections) were deparaffinized, exposed to Peroxidazed 1 (PX968; Biocare Medical, Concord, CA, USA) for 5 minutes to decrease background staining, and then immersed in a universal decloaker (UD1000M; Biocare Medical) and placed in a pressure cooker at 121°C for 5 minutes. Next, the sections were blocked using blocking reagent (BS966M; Biocare Medical) for 30 minutes, incubated with primary antibody overnight at 4°C, incubated with the biotinylated secondary antibody (GU600H; Biocare Medical) for 10 minutes, and incubated with Streptavidin HRP Label (HP604; Biocare Medical) for 10 minutes. Finally, the sections were stained with 3,3′-diaminobenzidine chromogen (DB801L; Biocare Medical) and then counterstained with hematoxylin.
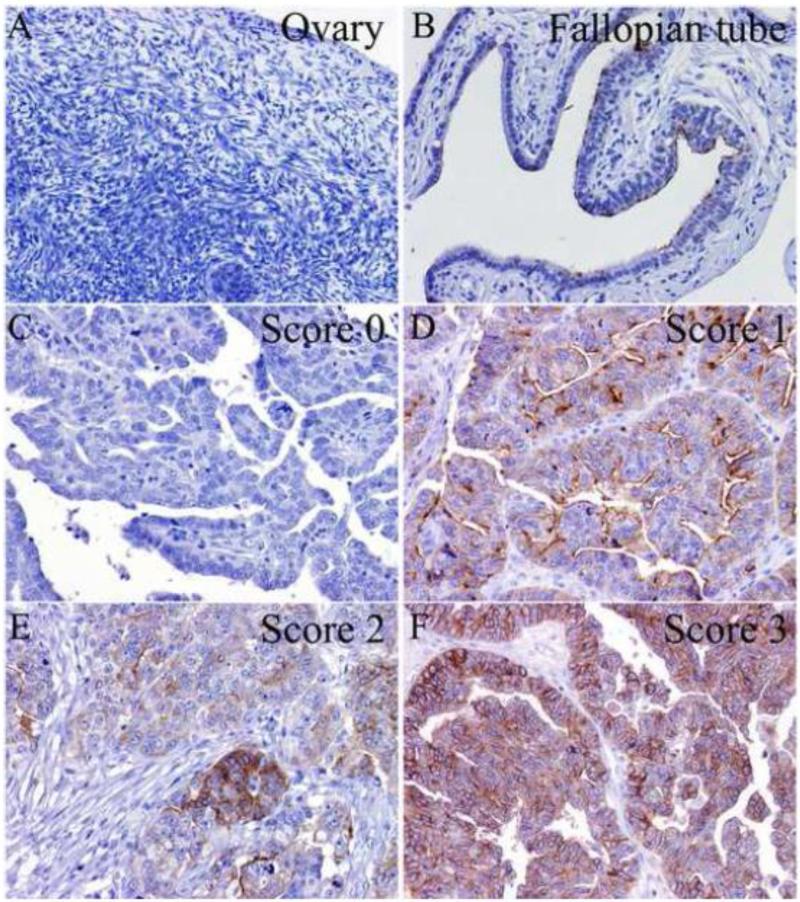
The pattern of B7-H4 immunohistochemical staining was recorded using a 4-score grading system (Figure 1): Score 0, no staining/negative; Score 1, apical pattern; Score 2, mixed apical and circumferential membranous staining with circumferential membranous staining in <10% of tumor cells; and Score 3, circumferential membranous staining in ≥10% of tumor cells.
Fig. 1.
B7-H4 protein expression in normal ovary (A), fallopian tube (B), and high-grade ovarian serous carcinoma (C-F). B7-H4 immunohistochemical staining in ovarian serous carcinoma was semi-quantified using a four-score grading system: Score 0 (C), negative; Score 1 (D), apical pattern; Score 2 (E), mixed apical and circumferential membranous staining with circumferential membranous staining in <10% of tumor cells; Score 3 (F), circumferential membranous staining in ≥10% of tumor cells (immunohistochemical stain; original magnification ×200).
2.3. Statistical analysis
Summary statistics including mean, median, and range were provided for the continuous variable age. Categorical variables such as histologic types and FIGO stage were summarized in count and frequency. The Fisher's exact test was used to compare the expression of B7-H4 between high-grade and low-grade ovarian serous carcinomas. To assess the effects of the B7-H4 immunohistochemical score on the tumor stage, we performed logistic regression analysis, in which patients with the stage I or II disease were combined in an early-stage disease group and patients with stage III or IV disease were combined in an advanced-stage disease group. P-values < 0.05 were considered statistically significant.
Disease-free survival (DFS) was measured from the date of original diagnosis to the date of tumor relapse (i.e., the appearance of recurrent lesions or a doubling of serum CA125 levels from the upper limit of normal) or last follow-up. Overall survival (OS) was measured from the date of original diagnosis to the date of death or last follow-up [13, 17]. OS and DFS curves were estimated using the Kaplan-Meier method and compared using log-rank tests. Cox proportional hazards regression models were used to assess the effect of the B7-H4 immunohistochemical score on OS and DFS. All statistical analyses were performed with SAS (version 9.3, SAS Institute, Cary, NC).
3. Results
3.1 B7-H4 expression in normal ovary and fallopian tube
B7-H4 was not expressed in normal ovary (n = 24; Figure 1a). However, some epithelial cells in normal fallopian tube showed an apical pattern of B7-H4 expression (n = 20; Figure 1b).
3.2 B7-H4 expression in high-grade and low-grade ovarian serous carcinoma
The patients’ clinical characteristics are summarized in Table 1. The expression of B7-H4 in high-grade and low-grade ovarian serous carcinomas is summarized in Table 2. We found that 91% (267/293) of the high-grade ovarian serous carcinomas and 69% (9/13) of the low-grade ovarian serous carcinomas expressed B7-H4. The difference between B7-H4 expression in high-grade and low-grade ovarian serous carcinoma was statistically significant (P = 0.002).
Table 1.
Clinicopathologic data of 306 patients with ovarian serous carcinoma.
| Histologic types | |
| Low-grade serous carcinoma | 13 |
| High-grade serous carcinoma | 293 |
| Age at diagnosis (years) | |
| Mean | 60 |
| Median | 61 |
| Range | 22-87 |
| FIGO Stage | |
| I | 6 |
| II | 9 |
| III | 211 |
| IV | 68 |
| Unknown | 12 |
Table 2.
B7-H4 immunohistochemical scores in high-grade versus low-grade ovarian serous carcinoma.
| B7-H4 Immunohistochemical Score |
|||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Total | |
| High-grade serous carcinoma | |||||
| Stage I&II | 1 | 5 | 4 | 4 | 14 |
| Stage III | 18 | 49 | 43 | 90 | 200 |
| Stage IV | 7 | 6 | 19 | 36 | 68 |
| Stage | 0 | 3 | 4 | 4 | 11 |
| Unknown | |||||
| Total (%) | 26 (8.9%) | 63 (21.5%) | 70 (23.9%) | 134 (45.7%) | 293 (100%) |
| Low-grade serous carcinoma | |||||
| Stage I&II | 0 | 1 | 0 | 0 | 1 |
| Stage III | 4 | 5 | 1 | 1 | 11 |
| Stage IV | 0 | 0 | 0 | 0 | 0 |
| Stage | 0 | 0 | 1 | 0 | 1 |
| Unknown | |||||
| Total (%) | 4 (30.8%) | 6 (46.2%) | 2 (15.4%) | 1 (7.7%) | 13 (100%) |
3.3 B7-H4 expression in high-grade ovarian serous carcinoma is associated with tumor stage but not OS or DFS
The results of the logistic regression models for high-grade serous carcinoma are given in Table 3. The association between the B7-H4 immunohistochemical score and tumor stage was statistically significant in high-grade serous carcinoma (P < 0.01). Patients with a higher B7-H4 immunohistochemical score had a higher risk of having an advanced tumor stage.
Table 3.
Ordered logistic regression for high-grade ovarian serous carcinoma with tumor stage as dependent variable.
| Characteristic | Coefficient (95% CI) | P-value | |
|---|---|---|---|
| B7-H4 IHC score | <0.01 | ||
| 1 | −1.04 (−2.09,0.01) | 0.05 | |
| 2 | 0.01 (−0.97,0.99) | 0.99 | |
| 3 | 0.06 (−0.86,0.97) | 0.90 | |
| 0 | |||
| Tumor stage (Intercept) | |||
| I&II | −3.25 (−4.23,−2.26) | <0.01 | |
| III&IV | 1.00 (0.17,1.84) | 0.02 |
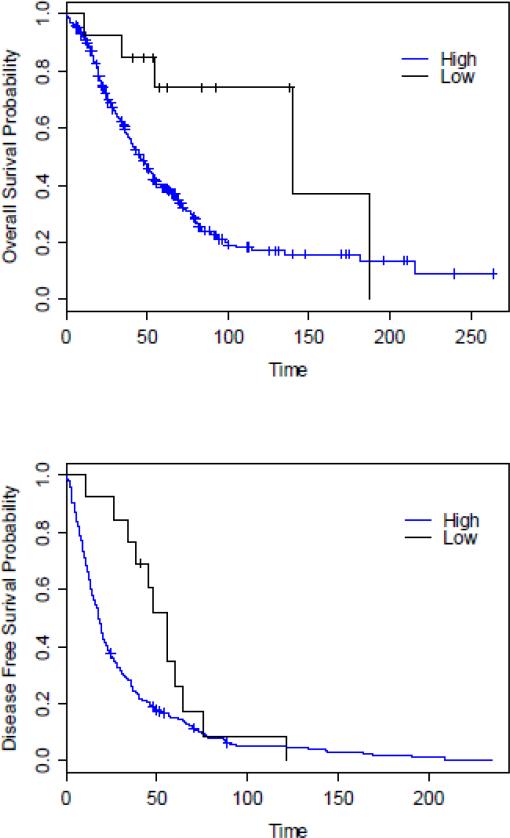
The Kaplan-Meier estimates of the OS and DFS rates of patients with high-grade or low-grade serous carcinoma are shown in Figure 2. The OS and DFS rates of patients with low-grade serous carcinoma were significantly longer than those of patients with high-grade serous carcinoma (P = 0.014 and 0.041, respectively). We found no significant association between B7-H4 immunohistochemical score and OS or DFS (Table 4).
Fig. 2.
Kaplan-Meier estimates of the overall survival (OS) and disease-free survival (DFS) rates of patients with high-grade ovarian serous carcinoma versus those with low-grade ovarian serous carcinoma (P = 0.017 for OS and P = 0.041 for DFS).
Table 4.
Cox regression models for overall survival and disease-free survival for patients with high-grade ovarian serous carcinoma.
| Characteristic | HR (95% CI) | Cox Model P-value | ||
|---|---|---|---|---|
| Overall survival | B7-H4 IHC score | 0.816 | ||
| 1 | 0.84 (0.48,1.48) | 0.551 | ||
| 2 | 0.78 (0.45,1.37) | 0.392 | ||
| 3 | 0.79 (0.47,1.32) | 0.365 | ||
| 0 | ||||
| Disease-free survival | B7-H4 IHC score | 0.657 | ||
| 1 | 0.77 (0.47,1.25) | 0.282 | ||
| 2 | 0.76 (0.47,1.24) | 0.268 | ||
| 3 | 0.84 (0.54,1.32) | 0.457 | ||
| 0 |
HR, Hazard Ratio; CI, Confidence Interval.
4. Discussion
We found that the B7-H4 protein was frequently expressed in ovarian serous carcinoma, especially in high-grade serous carcinoma. The tumor specimens had different B7-H4 staining patterns, including apical, circumferential membranous, and mixed apical and circumferential membranous patterns. The immunohistochemistry score of B7-H4 protein expression in high-grade serous carcinoma was associated with tumor stage. These findings suggest that B7-H4 is a promising immunotherapeutic target for ovarian serous carcinoma.
Our results are largely in keeping with those of earlier studies with smaller sample sizes [5-9]. Choi and colleagues [5], using the monoclonal antibodies hH4.3 and hH4.2 and defining positive staining as B7-H4 positivity in >10% of tumor cells regardless of the immunostaining pattern, found that 85% (22/26) of ovarian tumors expressed B7-H4 [5]. Tringeler and colleagues [7], using the monoclonal antibody A57.1 and a three-tier system identifying negative, apical, and circumferential membranous and strong cytoplasmic staining patterns, found that 100% (32/32) of ovarian serous carcinomas, 78% (18/23) of serous borderline tumors, and 77% (20/26) of serous cystadenomas expressed B7-H4. Moreover, the authors found that a circumferential membranous and strong cytoplasmic pattern of B7-H4 staining occurred mainly in invasive ovarian carcinomas. Dangaj and colleagues [8], using flow cytometry, demonstrated cell surface expression of B7-H4 in 100% (15/15) of ovarian cancer samples. In our study, since a subset of the cases showed a mixed apical and circumferential membranous pattern of B7-H4 staining, we used a four-tier system to interpret immunohistochemistry results.
In addition, earlier studies showed that whereas B7-H4 messenger RNA is expressed in many normal tissues, B7-H4 protein is expressed in only antigen-presenting cells and cancer cells, likely because the protein is regulated post-transcriptionally [5]. Therefore, reverse-transcriptase polymerase chain reaction, mRNA sequencing, and RNA in-situ hybridization are not useful in studying B7-H4 expression. In the present study, B7-H4 was not expressed in normal ovary. Although B7-H4 was expressed in some epithelial cells in normal fallopian tube, it demonstrated an apical (incomplete membranous) pattern of expression instead of the circumferential membranous pattern seen in the majority of high-grade ovarian serous carcinomas. The mechanism of this phenomenon is unclear and needs be studied further. Previous studies have used the enzyme-linked immunosorbent assay to detect the B7-H4 protein in serum and ovarian cancer tissue lysates [18-20]. Serum B7-H4 level is a potential diagnostic marker of ovarian cancer. Our results also suggest that for high-grade ovarian serous carcinoma, B7-H4 expression is associated with tumor stage but not DFS or OS. However, Kryczek and colleagues [9] reported that B7-H4 expression in tumor-associated macrophages was associated with worse prognosis in ovarian cancer patients.
Currently, immunotherapeutic drugs targeting B7-H4 is under development. Dangaj and colleagues [8, 21, 22] found that the intraperitoneal injection of anti–B7-H4 single-chain variable fragments slowed the growth of OCVAR-5–derived ovarian cancer xenografts. In the future, anti–B7-H4 therapy may be tested in patient-derived xenograft models and/or clinical trials. Our results, together with those of other authors [23], suggest that B7-H4 is a promising immunotherapeutic target in ovarian cancer.
In conclusion, our large cohort study showed that ovarian serous carcinomas frequently express B7-H4, which raises the possibility that the immunomodulatory molecule is a promising immunotherapeutic target in ovarian cancers. Moreover, the assessment of other B7 family members (i.e., B7.1, B7.2, inducible costimulator ligand, B7-H3, B7-H5, B7-H6, B7-H7) in ovarian and other types of cancers may also expand our knowledge of tumor immune escape and provide new immunotherapeutic targets.
Research highlights.
➢ B7-H4 is a member of the B7 family of immune costimulatory ligands.
➢ B7-H4 protein is frequently expressed in ovarian serous carcinoma, especially in high-grade serous carcinoma.
➢ B7-H4 may represent a novel immunotherapeutic target in ovarian cancer.
Acknowledgements
We thank Joe Munch in MD Anderson's Department of Scientific Publications for editing the manuscript.
funding disclosures: This work was supported in part by grant from the Cancer Prevention and Research Institute of Texas, The MD Anderson Cancer Center SPORE in Ovarian Cancer (National Institutes of Health Grant), a Sister Institution Grant from MD Anderson Cancer Center Global Academic Programs. Dr. Li Liang is supported by a training grant (T32CA163185) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors have no conflicts of interest to declare.
References
- 1.Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. 2013;34:556–563. doi: 10.1016/j.it.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins M, Ling V, Carreno BM. The B7 family of immune-regulatory ligands. Genome Biol. 2005;6:223. doi: 10.1186/gb-2005-6-6-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carreno BM, Carter LL, Collins M. Therapeutic opportunities in the B7/CD28 family of ligands and receptors. Curr Opin Pharmacol. 2005;5:424–430. doi: 10.1016/j.coph.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Leung J, Suh WK. The CD28-B7 family in anti-tumor immunity: emerging concepts in cancer immunotherapy. Immune Netw. 2014;14:265–276. doi: 10.4110/in.2014.14.6.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi IH, Zhu G, Sica GL, et al. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J Immunol. 2003;171:4650–4654. doi: 10.4049/jimmunol.171.9.4650. [DOI] [PubMed] [Google Scholar]
- 6.Salceda S, Tang T, Kmet M, et al. The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp Cell Res. 2005;306:128–141. doi: 10.1016/j.yexcr.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Tringler B, Liu W, Corral L, et al. B7-H4 overexpression in ovarian tumors. Gynecol Oncol. 2006;100:44–52. doi: 10.1016/j.ygyno.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 8.Dangaj D, Lanitis E, Zhao A, et al. Novel recombinant human b7-h4 antibodies overcome tumoral immune escape to potentiate T-cell antitumor responses. Cancer Res. 2013;73:4820–4829. doi: 10.1158/0008-5472.CAN-12-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kryczek I, Wei S, Zhu G, et al. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67:8900–8905. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 10.Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO Classification of Tumours of Female Reproductive Organs. 4th ed. IARC Press; Lyon: 2014. [Google Scholar]
- 11.Malpica A, Deavers MT, Lu K, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28:496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Prat J, FIGO Committee on Gynecologic Oncology Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124:1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Guo X, Chang DY, Rosen DG, Mercado-Uribe I, Liu J. CD133 expression associated with poor prognosis in ovarian cancer. Mod Pathol. 2012;25:456–464. doi: 10.1038/modpathol.2011.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Chang DY, Mercado-Uribe I, Liu J. Sex-determining region Y-box 2 expression predicts poor prognosis in human ovarian carcinoma. Hum Pathol. 2012;43:1405–1412. doi: 10.1016/j.humpath.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon RA, di Sant'Agnese PA, Huang LS, et al. CD44 expression is a feature of prostatic small cell carcinoma and distinguishes it from its mimickers. Hum Pathol. 2009;40:252–258. doi: 10.1016/j.humpath.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Rosen DG, Huang X, Deavers MT, Malpica A, Silva EG, Liu J. Validation of tissue microarray technology in ovarian carcinoma. Mod Pathol. 2004;17:790–797. doi: 10.1038/modpathol.3800120. [DOI] [PubMed] [Google Scholar]
- 17.Chang B, Liu G, Xue F, et al. ALDH1 expression correlates with favorable prognosis in ovarian cancers. Mod Pathol. 2009;22:817–823. doi: 10.1038/modpathol.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon I, Zhuo S, Corral L, et al. B7-h4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res. 2006;66:1570–1575. doi: 10.1158/0008-5472.CAN-04-3550. [DOI] [PubMed] [Google Scholar]
- 19.Simon I, Liu Y, Krall KL, et al. Evaluation of the novel serum markers B7-H4, Spondin 2, and DcR3 for diagnosis and early detection of ovarian cancer. Gynecol Oncol. 2007;106:112–118. doi: 10.1016/j.ygyno.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Oikonomopoulou K, Li L, Zheng Y, et al. Prediction of ovarian cancer prognosis and response to chemotherapy by a serum-based multiparametric biomarker panel. Br J Cancer. 2008;99:1103–1113. doi: 10.1038/sj.bjc.6604630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dangaj D, Scholler N. Blocking the B7-H4 pathway with novel recombinant antibodies enhances T cell-mediated antitumor responses. Oncoimmunology. 2013;2:e25913. doi: 10.4161/onci.25913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dangaj D, Scholler N. Isolation and validation of anti-B7-H4 scFvs from an ovarian cancer scFv yeast-display library. Methods Mol Biol. 2015;1319:37–49. doi: 10.1007/978-1-4939-2748-7_2. [DOI] [PubMed] [Google Scholar]
- 23.Smith JB, Stashwick C, Powell DJ., Jr B7-H4 as a potential target for immunotherapy for gynecologic cancers: a closer look. Gynecol Oncol. 2014;134:181–189. doi: 10.1016/j.ygyno.2014.03.553. [DOI] [PMC free article] [PubMed] [Google Scholar]