TO THE EDITOR
Gamma-glutamyl carboxylase (GGCX) is an integral membrane protein that catalyzes the post-translational modification of certain glutamates to gamma-carboxyglutamates in vitamin K-dependent (VKD) proteins. Carboxylation is required for the biological activity of numerous VKD proteins involved in a broad range of physiological functions. Mutations in GGCX have been mainly associated with bleeding disorders because these mutations cause undercarboxylation of VKD coagulation factors and of the anticoagulant proteins (Napolitano et al., 2010). GGCX mutations have also been linked to pseudoxanthoma elasticum (PXE)-like syndrome, a non-bleeding disorder caused by functional defects in the matrix Gla protein (MGP) (Vanakker et al., 2007). Patients with PXE-like syndrome have been reported to have comorbid bleeding disorder of vitamin K-dependent coagulation factors deficiency (VKCFD), which is characterized by the simultaneous functional defects of multiple VKD coagulation factors (Li et al., 2009; Rongioletti et al., 1989; Vanakker et al., 2007).
Recently, Kariminejad et al. (2014) reported thirteen patients with phenotypes typical of PXE-like syndrome, but with no coagulation abnormalities (Kariminejad et al., 2014). Genetic analysis of these patients’ ATP-binding cassette subfamily C member 6 (ABCC6) gene (the causative gene for the classical PXE syndrome) excluded the ABCC6 mutations. However, all affected members were found to be homozygous for a splice-site mutation (c.373+3G>T) in the GGCX gene, which causes the deletion of exon 3 in the GGCX mRNA (GGCX-Δex3). It has been suggested that the phenotypes displayed by the affected patients were associated with the GGCX-Δex3 mutation (Kariminejad et al., 2014). However, the reason for the absence of bleeding diathesis in these patients remained unclear, and no functional study on the GGCX-Δex3 mutation was available.
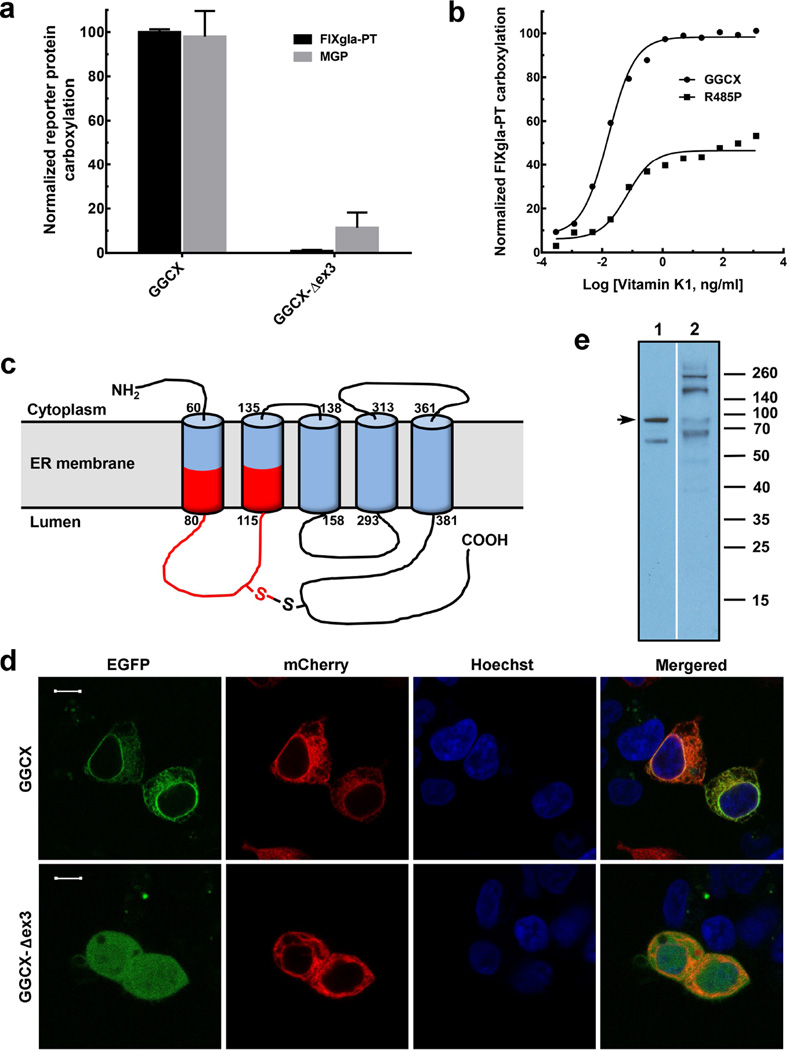
To examine the functional consequences of the GGCX-Δex3 mutation, we determined the carboxylation activity of this mutant using our recently established GGCX-deficient cell-based assay with two reporter proteins (a chimeric coagulation factor, FIXgla-PT and MGP) (Supplementary Methods). This approach, unlike the traditional in vitro GGCX activity assay, allows us to assess the functionality of GGCX using its natural protein substrates in a cellular milieu that requires the enzyme to interact with its physiologic components for VKD carboxylation (Tie et al., 2016). Our result shows that the GGCX-Δex3 mutant abolished carboxylation activity for both reporter proteins (Figure 1a). These results agree with the findings of Kariminejad et al. (2014), who observed uncarboxylated MGP in the affected patients. However, the inability of the GGCX-Δex3 mutant to carboxylate the coagulation factor reporter-protein disagrees with their observation that all the affected patients had normal coagulation factor activities.
Figure 1. Characterization of GGCX mutations identified in PXE-like and VKCFD patients.
(a) Cell-based activity assay of GGCX and its mutant identified from PXE-like patients. Wild-type GGCX and the GGCX-Δex3 mutant were transiently expressed in GGCX-deficient HEK293 reporter cells. Transfected cells were cultured in complete medium containing 5 µg/ml vitamin K. The concentrations of the carboxylated reporter proteins (FIXgla-PT, black bars and MGP, gray bars) in the cell culture medium were measured by ELISA. Wild-type GGCX activity was normalized to 100%. Data are presented as mean±SD (n=3). (b) Carboxylation activity of GGCX and the Arg485Pro mutant in response to increasing concentrations of vitamin K. Wild-type GGCX (filled circles) and the Arg485Pro mutant (filled squares) were transiently expressed in GGCX-deficient HEK293 reporter cells. The enzymatic activity for FIXgla-PT carboxylation was determined as described above. (c) Schematic representation of the proposed membrane topology of GGCX. The exon 3 encoded region is shown in red. (d) Subcellular localization of GGCX and the GGCX-Δex3 mutant in HEK293 cells. EGFP-tagged GGCX or GGCX-Δex3 was transiently co-expressed with the ER marker mCherry-ER-3 in HEK293 cells. Forty-eight hours post-transfection, cell nucleus was stained by Hoechst 33342 and directly used for fluorescence confocal image collection. GGCX fusions were visualized by the green fluorescence of EGFP, ER marker was visualized by the red fluorescence of mCherry, and cell nuclei were visualized by the blue fluorescence of Hoechst. Scale bar = 10 µm. (e) Immunoblotting analysis of GGCX (lane 1) and the GGCX-Δex3 mutant (lane 2) proteins. Full-length GGCX band is indicated by arrow.
To further clarify the effect of GGCX-Δex3 mutation on the carboxylation of coagulation factors, we characterized GGCX mutants of a VKCFD patient with compound heterozygous mutations of GGCX-Δex3 and Arg485Pro (Rost et al., 2004). If the GGCX-Δex3 mutation located on one allele expresses an inactive enzyme, as we observed (Figure 1a), the Arg485Pro mutation on the other allele should play a major role in the clinical phenotype of this VKCFD patient, whose coagulation disorder has been partially corrected by vitamin K administration (Rost et al., 2004). To test this hypothesis, we titrated the carboxylation activity of the Arg485Pro mutant in response to increasing concentrations of vitamin K. Compared with the wild-type enzyme, the Arg485Pro mutant requires a ~5-fold higher vitamin K concentration to reach half-maximal carboxylation of FIXgla-PT (Figure 1b). At the optimum vitamin K concentration, the Arg485Pro mutant has ameliorated coagulation factor carboxylation up to ~50%. This agrees with the patient’s clinical result, in which vitamin K administration partially restored coagulation factor activities to ~60% (Rost et al., 2004). These results support our hypothesis that, when the GGCX-Δex3 mutation on one allele encodes an inactive enzyme, the Arg485Pro mutation on the other allele plays a key role in the clinical phenotype of this VKCFD patient.
Deletion of exon 3 results in an in-frame deletion of 53 amino acid residues (between Phe73 to Gly125) in the GGCX molecule, which includes part of the first and second transmembrane domains (TMDs) and the lumenal loop between TMD1 and TMD2 (Figure 1c) (Tie et al., 2000). Therefore, exon 3 deletion could disrupt the proper integration of TMD1 and TMD2 into the endoplasmic reticulum (ER) membrane, which would result in the misfolding of the GGCX protein. Exon 3 also encodes residue Cys99, which forms the only disulfide bond with Cys450 in the GGCX molecule that is essential for GGCX folding and maturation (Tie et al., 2003). To test the effect of exon 3 deletion on GGCX maturation, we fused an enhanced green fluorescence protein (EGFP) at the C-terminus of the wild-type GGCX and the GGCX-Δex3 mutant, co-expressed these fusion proteins with the ER marker protein mCherry-ER-3 in HEK293 cells, and examined their expression and localization by fluorescence confocal microscopy and immunoblotting analysis (Supplementary Methods). Our results show that, while the wild-type GGCX appears to be localized to the ER (Figure 1d), the GGCX-Δex3 mutant shows a ubiquitous diffusion throughout the entire cell, which suggests the mis-localization and probable misfolding of the mutant protein. The immunoblotting result is consistent with this interpretation, revealing that the GGCX-Δex3 mutant shows more protein bands than the wild-type GGCX, suggesting both degradation (the protein bands are smaller than the wild-type GGCX) and aggregation (the protein bands are larger than the wild-type GGCX) of the mutant protein (Figure 1e). Together, these results suggest that the GGCX-Δex3 mutant protein is misfolded and mis-localized in the cell, and is therefore unable to carry out its biological function in the ER.
Our results suggest that exon 3 deletion in the GGCX gene results in an inactive enzyme. This result cannot explain the normal coagulation activity of the patients who had a homozygous splice-site mutation of c.373+3G>T, causing exon 3 to be skipped in the GGCX mRNA (Kariminejad et al., 2014). One possible explanation for this discrepancy is that our result is based on cell-based characterization of the GGCX-Δex3 mutant. However the patients’ splice-site mutation (c.373+3G>T) may not completely abolish normal processing of the GGCX pre-mRNA, and this could produce a portion of correctly processed mRNA for translating the functional protein, as has been reported in other diseases (Mikkola et al., 1997; Schernthaner-Reiter et al., 2016). It is also possible that this splice-site mutation could result in several variant mRNA transcripts of GGCX, as has previously described for other genes (Gallinaro et al., 2006; Kallabi et al., 2015). The affected patients reported by Kariminejad et al. (2014) have normal coagulation factor carboxylation, but defects in their MGP carboxylation; it is therefore possible that some of the mRNA transcript may produce a GGCX mutant protein that causes MGP-carboxylation defects but has a smaller effect on coagulation factor carboxylation, as we have previously observed in a VKCFD patient (Tie et al., 2016).
Splice-site mutations make a significant contribution to human genetic disease, but the effects of these mutations on pre-mRNA splicing remain to be elucidated (Krawczak et al., 2007). Mutations in exon-intron junctions could lead to mis-splicing and could result in exon skipping, activation of a cryptic splice-site, or intron retention. Importantly, a single splice-site mutation can result in multiple mRNA transcripts and can affect the protein function (Gallinaro et al., 2006; Kallabi et al., 2015; Mikkola et al., 1997; Schernthaner-Reiter et al., 2016). Therefore, it is important to clarify the effect of the GGCX splicing-site mutation (c.373+3G>T) on the splicing mechanism and the possible GGCX mRNA transcripts in these affected patients, especially when mutations in the causative gene for the classical PXE syndrome have been excluded. It would also be beneficial to identify possible variations on the vitamin K epoxide reductase (VKOR) gene in the affected patients, as VKOR is the essential enzyme that provides the reduced form of vitamin K needed for GGCX to function.
In conclusion, we have characterized the exon 3 deletion mutation of GGCX which was identified from patients with a homozygous splice-site mutation (c.373+3G>T) in the GGCX gene. Our results suggest that clarifying the correlation between the GGCX genotypes and their clinical phenotypes will remain challenging, especially when these mutations are located in splice junctions that could affect pre-mRNA processing.
Supplementary Material
Acknowledgments
We thank Dr. Tony Perdue for the help on confocal fluorescence microscopy. This work was supported by grant HL077740 from the National Institutes of Health (to D.W.S. and J.K.T.).
Abbreviations
- ABCC6
ATP-binding cassette subfamily C member 6
- ER
endoplasmic reticulum
- FIXgla-PT
prothrombin with its Gla domain replaced by that of FIX
- EGFP
enhanced green fluorescence protein
- GGCX
gamma-glutamyl carboxylase
- MGP
matrix Gla protein
- PXE
pseudoxanthoma elasticum
- TMD
transmembrane domain
- VKCFD
vitamin K-dependent coagulation factors deficiency
- VKD
vitamin K-dependent
- VKOR
vitamin K epoxide reductase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Dr. Vermeer received salary from VitaK. The other authors state no conflict of interest.
References
- Gallinaro L, Sartorello F, Pontara E, Cattini MG, Bertomoro A, Bartoloni L, et al. Combined partial exon skipping and cryptic splice site activation as a new molecular mechanism for recessive type 1 von Willebrand disease. Thromb Haemost. 2006;96:711–716. [PubMed] [Google Scholar]
- Kallabi F, Hadj Salem I, Ben Chehida A, Ben Salah G, Ben Turkia H, Tebib N, et al. Splicing defects in ABCD1 gene leading to both exon skipping and partial intron retention in X-linked adrenoleukodystrophy Tunisian patient. Neurosci Res. 2015;97:7–12. doi: 10.1016/j.neures.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Kariminejad A, Bozorgmehr B, Najafi A, Khoshaeen A, Ghalandari M, Najmabadi H, et al. Retinitis pigmentosa, cutis laxa, and pseudoxanthoma elasticum-like skin manifestations associated with GGCX mutations. J Invest Dermatol. 2014;134:2331–2338. doi: 10.1038/jid.2014.191. [DOI] [PubMed] [Google Scholar]
- Krawczak M, Thomas NS, Hundrieser B, Mort M, Wittig M, Hampe J, et al. Single base-pair substitutions in exon-intron junctions of human genes: nature, distribution, and consequences for mRNA splicing. Hum Mutat. 2007;28:150–158. doi: 10.1002/humu.20400. [DOI] [PubMed] [Google Scholar]
- Li Q, Schurgers LJ, Smith AC, Tsokos M, Uitto J, Cowen EW. Co-existent pseudoxanthoma elasticum and vitamin K-dependent coagulation factor deficiency: compound heterozygosity for mutations in the GGCX gene. Am J Pathol. 2009;174:534–540. doi: 10.2353/ajpath.2009.080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola H, Muszbek L, Laiho E, Syrjala M, Hamalainen E, Haramura G, et al. Molecular mechanism of a mild phenotype in coagulation factor XIII (FXIII) deficiency: a splicing mutation permitting partial correct splicing of FXIII A-subunit mRNA. Blood. 1997;89:1279–1287. [PubMed] [Google Scholar]
- Napolitano M, Mariani G, Lapecorella M. Hereditary combined deficiency of the vitamin K-dependent clotting factors. Orphanet journal of rare diseases. 2010;5:21. doi: 10.1186/1750-1172-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongioletti F, Bertamino R, Rebora A. Generalized pseudoxanthoma elasticum with deficiency of vitamin K-dependent clotting factors. J Am Acad Dermatol. 1989;21:1150–1152. doi: 10.1016/s0190-9622(89)70320-0. [DOI] [PubMed] [Google Scholar]
- Rost S, Fregin A, Koch D, Compes M, Muller CR, Oldenburg J. Compound heterozygous mutations in the gamma-glutamyl carboxylase gene cause combined deficiency of all vitamin K-dependent blood coagulation factors. Br J Haematol. 2004;126:546–549. doi: 10.1111/j.1365-2141.2004.05071.x. [DOI] [PubMed] [Google Scholar]
- Schernthaner-Reiter MH, Adams D, Trivellin G, Ramnitz MS, Raygada M, Golas G, et al. A novel AVPR2 splice site mutation leads to partial X-linked nephrogenic diabetes insipidus in two brothers. Eur J Pediatr. 2016 doi: 10.1007/s00431-015-2684-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie J, Wu SM, Jin D, Nicchitta CV, Stafford DW. A topological study of the human gamma-glutamyl carboxylase. Blood. 2000;96:973–978. [PubMed] [Google Scholar]
- Tie JK, Carneiro JD, Jin DY, Martinhago CD, Vermeer C, Stafford DW. Characterization of vitamin K-dependent carboxylase mutations that cause bleeding and non-bleeding disorders. Blood. 2016 doi: 10.1182/blood-2015-10-677633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie JK, Mutucumarana VP, Straight DL, Carrick KL, Pope RM, Stafford DW. Determination of disulfide bond assignment of human vitamin K-dependent gamma-glutamyl carboxylase by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Biol Chem. 2003;278:45468–45475. doi: 10.1074/jbc.M309164200. [DOI] [PubMed] [Google Scholar]
- Vanakker OM, Martin L, Gheduzzi D, Leroy BP, Loeys BL, Guerci VI, et al. Pseudoxanthoma elasticum-like phenotype with cutis laxa and multiple coagulation factor deficiency represents a separate genetic entity. J Invest Dermatol. 2007;127:581–587. doi: 10.1038/sj.jid.5700610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.