Abstract
Objective
Electrical stimulation of the pudendal nerve (PN) is a potential therapy for bladder dysfunction, but voiding efficiency (VE) produced by PN stimulation appears limited to 60–70%. We conducted experiments in rats and cats to investigate the hypothesis that introduction of artificial phasic bursting activity of the external urethral sphincter (EUS) would enhance VE under conditions where such activity was absent.
Materials and Methods
Cystometry experiments were conducted in 17 urethane anesthetized female Sprague-Dawley rats and 4 α-chloralose anesthetized male cats. The effects of phasic stimulation of the pudendal motor branch on VE were quantified in intact conditions, following bilateral transection of the motor branch of the PN, and following subsequent bilateral transection of the sensory branch of the PN.
Results
Artificial phasic bursting activity in the EUS generated by electrical stimulation of the motor branch of the PN increased VE in both rats and cats. Subsequent transection of the sensory branch of the PN abolished the increased VE elicited by phasic stimulation in both rats and cats.
Conclusions
Artificial phasic EUS bursting restored efficient voiding in rats. Introduction of artificial phasic bursting in cats, which normally exhibit EUS relaxation while voiding, was also effective in promoting efficient voiding. In both species phasic EUS activity increased voiding efficiency via activation of pudendal sensory pathways. These results provide further insight into the function of phasic EUS activity in efficient voiding and highlight a novel approach to increase VE generated by pudendal afferent nerve stimulation.
Keywords: bladder, micturition, electrical stimulation, deep perineal nerve, augmentation reflex, pudendal-pudendal reflex
Introduction
Electrical stimulation of the pudendal nerve is under investigation for restoration of bladder emptying in persons with spinal cord injury (Kennelly et al., 2011; Yoo et al., 2009, 2011). Selective electrical stimulation of individual branches of the pudendal nerve evokes sustained bladder contractions and increases voiding efficiency (VE) beyond that of distention-evoked reflex voiding in cats (Woock et al., 2008; Yoo et al., 2008a). However, while pudendal nerve stimulation is a promising approach, VE is limited to 60–70% in anesthetized cats (Boggs et al., 2006; Woock et al., 2008; Yoo et al., 2008a), and larger VE is desirable for clinical translation. The objective of the present study was to test the hypothesis that artificial phasic activation of the external urethral sphincter (EUS), as is observed during voiding in rats and dogs, would enhance VE in cats.
Phasic bursting activity in the EUS, which occurs during voiding in rats (Chen et al., 2011; Chen et al., 2012; Kakizaki et al., 1997; Kruse et al., 1990; Peng et al., 2008; Vera and Nadelhaft, 2001) and dogs (Nishizawa et al., 1984a; Nishizawa et al., 1984b), is observed in the electromyogram (EMG) of the striated EUS and as high frequency oscillations (HFOs) in intravesical pressure. While the origin and function of this activity remain unclear, elimination of phasic EUS activity either by transection of the pudendal nerve innervating the EUS in the rat (Cruz and Downie, 2005; Peng et al., 2006) and dog (Nishizawa et al., 1984a) or by neuromuscular blockade in the rat (Conte et al., 1991; Kruse et al., 1993; Maggi et al., 1986; Peng et al., 2006; Vera and Nadelhaft, 2001; Yoshiyama et al., 2000) causes a significant reduction in VE.
We conducted a series of experiments in rats and cats to investigate the hypothesis that introduction of artificial EUS bursting activity by electrical stimulation could increase VE under conditions where such activity was absent. We first conducted a series of experiments in rats to verify the role of phasic EUS bursting in efficient voiding and to test the hypothesis that reintroduction of phasic EUS activity following motor nerve transection could restore efficient voiding. Subsequently, we quantified the effects of introducing phasic EUS bursting activity on bladder contractions and VE in the cat, which, unlike the rat, normally exhibits relaxation of the EUS while voiding (Fedirchuk and Shefchyk, 1993). Finally, in both rats and cats, transection of the sensory branches of the pudendal nerves revealed that phasic EUS activity increased voiding efficiency through a sensory pathway, presumably by amplifying the sensory input to the augmenting reflex (Barrington, 1941). Collectively, these results provide further insight into the function of phasic EUS activity in efficient voiding and highlight a novel approach to increase VE generated by pudendal afferent nerve stimulation.
Materials and Methods
All animal care and experimental procedures were reviewed and approved by the Duke University Institutional Animal Care and Use Committee.
Rat surgical preparation and procedures
Female Sprague-Dawley rats (n=17) weighing between 256 and 343 g were anesthetized with urethane (1.2 g/kg s.c., and supplemented as necessary). Body temperature was monitored using an esophageal temperature probe and maintained at 36°–38° C with a recirculating water blanket. Heart rate and arterial blood oxygen saturation levels (Sp02) were monitored using a pulse oximeter (Nonin Medical Inc., 2500A VET).
For cystometrogram (CMG) measurements, the bladder was exposed via a midline abdominal incision. A PE-50 polyethylene catheter, the tip of which was heated to create a collar, was inserted into the bladder lumen through a small incision in the apex of the bladder dome, and a 6–0 silk suture was tied around the collar. The abdominal wall was closed with 4–0 silk suture. The bladder catheter was connected via a 3-way stopcock to an infusion pump (Braintree Scientific Inc., BS-8000) and to a pressure transducer (ArgoTrans, Argon Medical Devices Inc., Plano, TX) connected to a bridge amplifier and filter (13-6615-50, Gould Instruments, Valley View, Ohio) for measuring intravesical pressure (IVP).
Two PFA-coated platinum-iridium wires (0.0055 inch-diameter, A-M Systems, Sequim, WA) were bilaterally inserted percutaneously into the EUS to record EUS EMG. EUS EMG leads were connected through a preamplifier (HIP5, Grass Products, Warwick, RI) to an amplifier (P511, Grass Products). IVP and EUS EMG signals were amplified, filtered, and sampled at 1,000 Hz (IVP) or 4,000 Hz (EUS EMG) using a PowerLab/16SP acquisition unit (AD Instruments, Colorado Springs, CO) and displayed for off-line analysis using LabChart 7 Pro (v7.3.7, AD Instruments).
After placement of the bladder catheter, abdominal closure, and introduction of the percutaneous EUS electrodes, the rat was flipped to a prone position. The motor and sensory branches of the pudendal nerves were then exposed bilaterally via the ischiorectal fossa (Damaser et al., 2007; McKenna and Nadelhaft, 1986; Pacheco et al., 1989). Using a posterior approach, the gluteus muscles were incised to expose the rostral portion of the iliac crests, the ilium and sacrum were separated, and the pudendal nerves were carefully dissected. At approximately 2.4 cm distal to the S1 plexus at the anastomosis juncture, a pair of bipolar electrodes (AS 631, Cooner Wire Company, Chatsworth, CA) were glued onto each motor branch (left and right sides) with Kwik-Cast sealant (World Precision Instruments, Inc., Sarasota, FL) for electrical stimulation. For the duration of the experiment, the rats remained in the prone position and were not suspended or secured to a frame.
The bladder was continuously filled with physiological saline at room temperature (4–8 ml/hr) using an infusion pump with an open urethra for at least 45 minutes to allow post-surgical recovery. The bladder was subsequently emptied, and CMGs recorded. For each CMG, the bladder was filled until a micturition event was observed, at which time the infusion pump was turned off. Approximately one minute after the bladder pressure returned to baseline, the bladder was emptied. Voided and residual volumes were recorded and used to calculate bladder capacity and VE. At least three repeated CMG trials were recorded for each experimental condition, and parameters from multiple trials within an animal were averaged.
After control CMGs, the motor branches were transected bilaterally, approximately 2 mm central to the stimulation electrodes. The distal pudendal motor stump was stimulated with regulated current, 0.1 ms per phase, biphasic pulses delivered at a stimulus amplitude which produced a maximal EUS EMG response. During micturition, pudendal efferent nerves in rats have a median burst frequency of 40 Hz with 3 to 4 action potentials per burst (D’Amico and Collins, 2012). Therefore, a pattern of stimulation that produced 3 EUS EMG evoked potentials per burst at a frequency of 40 Hz with 160 ms inter-burst frequency (≈6 Hz) was used to mimic this bursting activity (Fig. 1G). For the stimulation trials, stimulation was initiated when the infused volume reached 92 % ± 0.11 % (mean ± SD) of the infused volume necessary to evoke a micturition contraction in trials following bilateral pudendal motor branch transection. The stimulation was turned off when the bladder pressure returned to baseline (Fig. 1E). To determine the impact of surgical placement of the pudendal electrode on the motor branch, pre-surgical control CMGs were recorded prior to pudendal surgery and implantation of the electrodes in n=5 of 13 experiments. Additionally, we investigated the effect of unilateral stimulation of both the left and right motor branches (stimulated independently) on the CMGs in n=7 of 13 experiments.
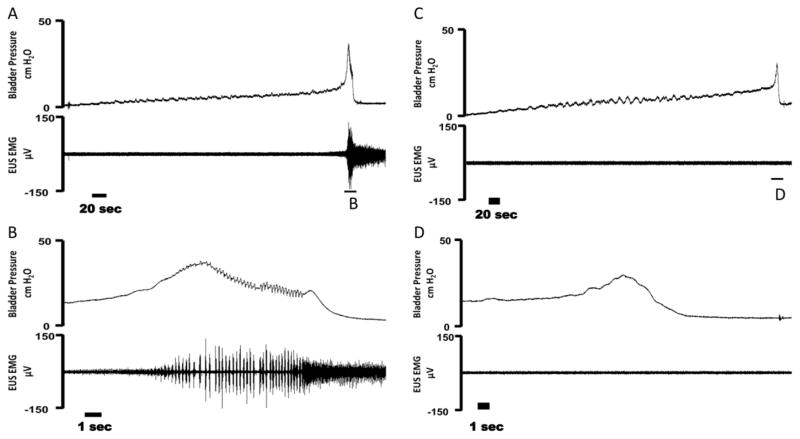
Figure 1.
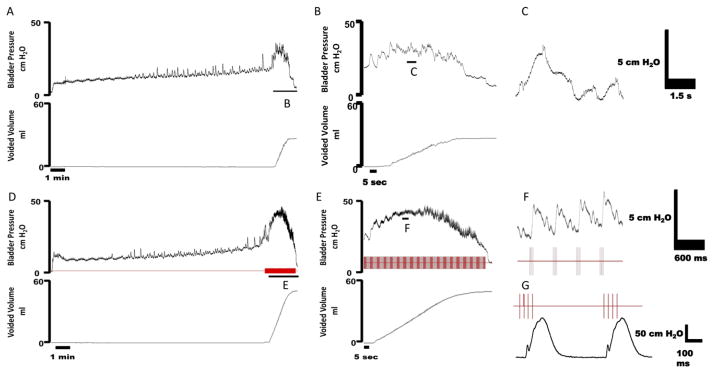
Example cystometrogram (CMG) trials in female rats during intact (A–B), bilateral pudendal motor transection (C–D), and bilateral phasic stimulation of the distal pudendal motor stump (E–H). (A) Bladder pressure (top) and external urethral sphincter (EUS) EMG activity (bottom) during a distention evoked trial. (B) An expanded trace from (A) showing bladder pressure (top) and EUS EMG activity confirming the presence of high frequency oscillations (HFOs) during a void event. (C) Bladder pressure (top) and EUS EMG activity (bottom) during a bilateral pudendal motor transection trial. (D) An expanded trace from (C) showing bladder pressure (top) and EUS EMG activity confirming the absence HFOs during a void event due to motor transection. (E) Bladder pressure (top) and EUS EMG activity (bottom) during bilateral phasic stimulation of the distal pudendal motor stump. Stimulation is turned on during a voiding event and is subsequently turned off after the end of the void. (F) An expanded trace from (E) showing bladder pressure (top) and EUS EMG (bottom) during the bladder contraction. Phasic stimulation of the distal pudendal motor stump elicited HFO’s during stimulation. (G) An expanded trace from (F) of bladder pressure (top) and EUS EMG (bottom) during phasic stimulation. Oscillations in bladder pressure follow each stimulation burst. (H) An expanded trace from (G) of EUS EMG activity during a single phasic burst. Arrow indicates the stimulation artifact and * denotes an evoked motor response.
To determine the mechanisms underlying the effects of EUS burst activity on VE, following the initial experimental protocol, the pudendal sensory branches were bilaterally transected approximately 2.4 cm distal to the S1 plexus at the anastomosis juncture in n=6 of 13 experiments, and subsequent CMGs were performed with and without bilateral motor branch stimulation. For the stimulation trials, stimulation was initiated when the infused volume reached 94 % ± 0.04% (mean ± SD) of the infused volume necessary to evoke a micturition contraction in the preceding trials following both motor and sensory pudendal nerve transection.
In n=4 experiments, α-bungarotoxin was administered to block muscle activity as an alternative to surgical transection of the pudendal motor branch, and CMGs and EUS EMG activity were recorded prior to and after drug administration. The right jugular vein was cannulated with a PE-50 polyethylene catheter for administration of 0.4 mg/kg α-bungarotoxin (R&D Systems, Inc., Minneapolis, MN), and the trachea was cannulated for artificial ventilation using the PhysioSuite Monitor. End tidal CO2 was measured using a ML206 Gas Analyzer (AD Instruments) and maintained between 3–4%.
Data analysis
Voided volume and residual volume were measured after each trial to calculate the VE:
The area under the curve (AUC) of the bladder contraction as a function of time (the pressure-time integral) was calculated between the start of micturition contraction to the end of the void. The bladder contraction amplitude was defined as the peak bladder pressure during a micturition event. For pre-surgical control vs. electrode placement comparisons of the EUS EMG bursting phase, the smoothed (0.08 s window moving average) rectified EUS EMG activity was used. The area under the EUS EMG bursting phase as a function of time (the voltage-time integral), maximum burst amplitude, burst phase frequency, and burst duration were calculated.
All parameters were analyzed using GraphPad Prism version 6.05 for Windows (GraphPad Software, La Jolla California USA). Comparisons of electrode placement vs. pre-surgical control, left vs. right unilateral stimulation, unilateral vs. bilateral stimulation, and α-bungarotoxin were analyzed using a paired t-test. The effect of pudendal motor transection followed by bilateral motor stimulation as well as, in a subset of animals, pudendal sensory nerve transection and subsequent bilateral motor stimulation were analyzed using repeated measures one-way analysis of variance (RM-ANOVA) followed by post-hoc Tukey’s multiple comparison test. P<0.05 was considered statistically significant for all analyses.
Cat surgical preparation and procedures
Acute experiments were conducted in four adult, neurologically intact male cats (3.3 – 3.5 kg). Anesthesia was induced with isoflurane (3%) and maintained with α-chloralose (initial 65 mg/kg, supplemented at 5 mg/kg/hr, i.v.). Artificial respiration (ADS 1000, Engler Engineering Corporation, Hialeah, FL) maintained end-tidal CO2 between 3–4 % (Capnogaurd, Novametrix Medical Systems Inc., Wallingford, CT). Body temperature was measured using an esophageal temperature probe connected to a TCAT-2AC controller (Physitemp, Clifton, New Jersey) and maintained at 38° C with a recirculating water blanket. Arterial blood pressure was monitored via a catheter in the carotid artery (Tektronix 413A Neonatal Monitor), and fluids (0.9 % physiological saline with 5 % dextrose and 8.4 g/L NaHCO3) were administered continuously (15 ml/kg/hr, i.v.). Following a midline abdominal incision, the bladder was cannulated through the dome with a modified 14 g BD Angiocath catheter connected to PE 90 tubing introduced with a hypodermic needle, secured with a purse string suture, and connected to a solid-state pressure transducer (Deltran, Utah Medical, UT) to measure bladder pressure. Wire electrodes were inserted into the external anal sphincter (EAS) to measure EMG activity. EAS EMG signals were amplified (x1000) and filtered (30 Hz to 10 kHz). All signals were sampled at 20 kHz and recorded (PowerLab, ADInstruments Inc.). The cat was then placed in the prone position prior to pudendal nerve isolation. The deep perineal branch (DPeriN) of the pudendal nerve, the motor branch innervating the EUS (Yoo et al., 2008b), was exposed bilaterally via the ischiorectal fossa. A bipolar cuff electrode (AirRay research Micro Cuff Tunnel 300μM, CorTec GmbH, Freiburg Germany) was placed on the left side for stimulation of the DPeriN approximately 0.5 cm distal to the division of the compound pudendal nerve into the motor and sensory branches. An analog stimulus isolator (model 2200, A-M Systems, WA) was used to deliver electrical stimulation. The cat remained in the prone position for the duration of the experiment and was not suspended or secured to a frame.
A catheter tip pressure transducer (CT/S2, Medical Measurements, Inc.) was placed intraurethrally to measure the urethral pressure response to DPeriN stimulation. Trains of biphasic constant current pulses (0.1 ms per phase, 0.25 – 0.9 mA, 1 Hz) were delivered unilaterally to the DPeriN. The DPeriN stimulation amplitude required to evoke the largest urethral pressure response was determined from an intensity response curve. To mimic phasic EUS bursting, the DPeriN was stimulated at 40 Hz for a duration 100 ms delivered at 2 Hz (Fig. 4F). Although phasic bursting activity in the rat also appears in the EUS EMG before voiding, phasic DPeriN stimulation began at the initiation of a void and was terminated when voiding ended.
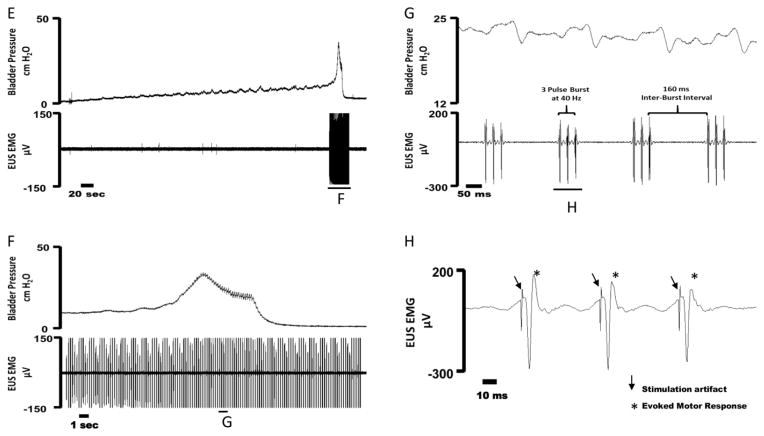
Figure 4.
Example cystometrogram trials in cats during distention evoked voiding (A–C) and voiding with unilateral DPeriN phasic stimulation (D–F). (A) Bladder pressure (top) and voided volume (bottom) during a distention evoked trial. (B) An expanded trace from (A) showing bladder pressure (top) and voided volume during the bladder contraction. (C) An expanded view of the bladder pressure demonstrating the absence of high frequency oscillations (HFOs) during a void event. (D) Bladder pressure (top) and voided volume (bottom) during DPeriN phasic stimulation (red trace). DPeriN stimulation is turned on during a voiding event and is subsequently turned off after the end of the void. (E) An expanded trace from (D) showing bladder pressure (top) and voided volume during the bladder contraction. DPeriN phasic stimulation (red trace) elicited HFO’s during stimulation. (F) An expanded view of the bladder pressure during phasic stimulation. Oscillations in bladder pressure follow each stimulation burst. G) Example of urethral pressure in response to 2 Hz bursts of DPeriN stimulation. Urethral pressure recorded during bladder empty conditions. The inter-pulse frequency was 40 Hz, and each burst was 100 ms in duration.
The bladder was continuously filled with physiological saline at room temperature (0.9–2 ml/min) using an infusion pump with an open urethra for 45 minutes to allow post-surgical recovery. The bladder was subsequently emptied and CMGs recorded. For each CMG, the bladder was filled until micturition occurred, at which time the infusion pump was turned off. Approximately one minute after the bladder pressure returned to baseline, the bladder was emptied. For the DPeriN stimulation trials, stimulation was initiated at the beginning of a void. Two repeated CMG trials were recorded for control (distention evoked contraction), DPeriN stimulation in the intact condition (in continuity), DPeriN stimulation of the distal stump following bilateral pudendal motor branch transection, and DPeriN stimulation of the distal stump following subsequent bilateral transection of the sensory branches of the pudendal nerve, and parameters from multiple trials within an animal were averaged. The motor branch on the left side was transected approximately 0.2 cm central to the stimulation cuff, and the contralateral side was transected approximately 0.5 cm distal to the division of the compound pudendal nerve into the motor and sensory branches. The pudendal sensory branches on both sides were transected approximately 0.5 cm distal to the division of the compound pudendal nerve into the motor and sensory branches.
Data analysis
Voided volume, residual volume, voiding efficiency, and bladder contraction AUC were calculated as described for the rat. Mean bladder contraction amplitude during a micturition event was also calculated. All parameters were averaged and analyzed using GraphPad Prism version 6.05 for Windows (GraphPad Software, La Jolla California USA). The effects of DPeriN burst stimulation on VE, bladder capacity, and bladder contraction AUC in the intact condition, following bilateral motor transection, and following complete transection were analyzed using repeated measures two-way analysis of variance (RM-two-way ANOVA) followed by post hoc Sidak’s multiple comparison test. P<0.05 was considered statistically significant for all analyses. All experimental values are expressed as mean ± standard error, unless specified otherwise.
Results
Artificial phasic bursting activity of the EUS increased voiding efficiency in both rats and cats, and selective nerve transections revealed that this effect was mediated by sensory feedback via pudendal afferents.
Effects of Phasic Activation of the EUS on Voiding Efficiency in the Rat
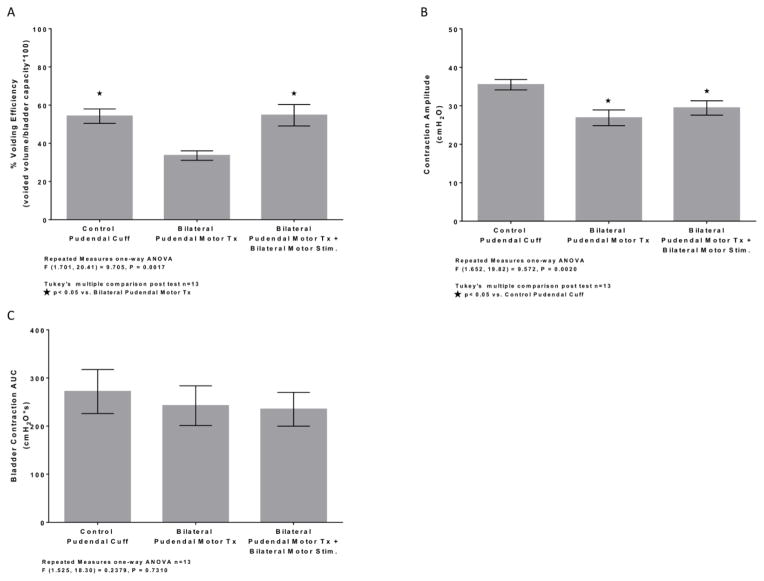
The bladder pressure and EUS EMG activity during CMGs were recorded in urethane – anesthetized female rats before (Figure 1A–B) and after (Figure 1C–D) bilateral transection of the motor branches of the pudendal nerves. Bilateral transection of the pudendal motor branches resulted in complete loss of EUS EMG activity (Figure 1C–D), and VE and contraction amplitude decreased significantly compared to pudendal cuff implanted controls (Fig. 2A–B). Bladder contraction AUC was not significantly decreased post transection (Fig. 2C). Similarly, both VE (49 ± 7.7 % vs. 82 ± 5.4 %, mean ± SE, n=4, P = 0.0033, two-tailed paired t-test) and contraction amplitude (23 ± 2.2 cm H2O vs. 30 ± 2.7 cm H2O, n=4, P = 0.0032, two-tailed paired t-test) decreased following administration of α-bungarotoxin, while bladder contraction AUC remained unchanged (140 ± 24 vs. 170 ± 30 cm H2O*s, mean ± SE, n=4, P = 0.058, two-tailed paired t-test). Bilateral implantation of stimulating electrodes on the motor branches did not affect voiding, bladder contraction, or EUS EMG parameters compared to pre-implantation controls (Table 1).Bilateral stimulation of the distal transected motor branches to produce phasic activation of the EUS restored VE to the levels measured prior to motor branch transection. Stimulation of the transected pudendal motor branch resulted in direct activation of the EUS EMG (Fig. 1H) with an average latency response of 1.7 ± 0.27 ms (mean ± SD, n=13). Stimulus amplitude which produced a maximal EUS EMG response ranged from 80–800 μA (240 ± 140 μA, mean ± SD). Bilateral motor branch stimulation significantly increased VE compared to motor transection (Fig. 2A). No significant differences in bladder contraction amplitude (Fig. 2B) or AUC (Fig. 2C) were observed during bilateral motor branch stimulation compared to pudendal motor transection, suggesting that smooth muscle contractions were not affected by phasic stimulation. Additionally, when comparing left and right unilateral motor branch stimulation or the averaged unilateral stimulation responses to bilateral stimulation, no significant differences were observed in VE, bladder contraction amplitude, or bladder contraction AUC (Table 2).
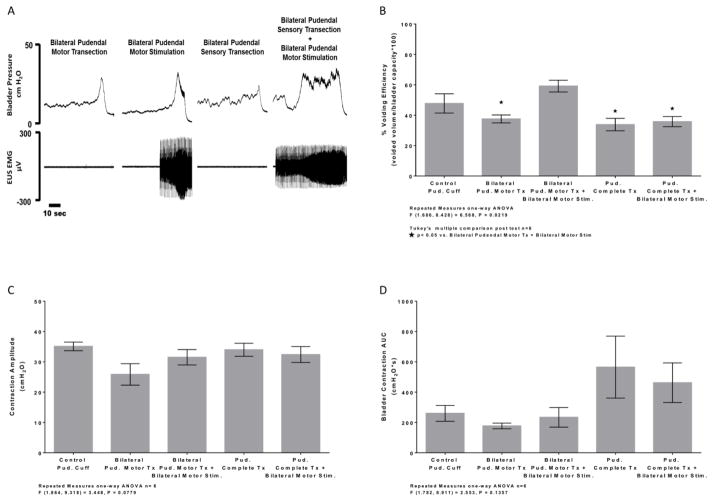
Figure 2.
Average % voiding efficiency (A), bladder contraction amplitude (B), and bladder contraction AUC (C) during voiding trials between distention evoked control, bilateral motor transection, and bilateral phasic stimulation of the distal motor stump post pudendal motor transection conditions. (A) Voiding efficiency was significantly decreased after pudendal motor transection compared to control. Artificial phasic stimulation following pudendal motor transection significantly increased % voiding efficiency back to control level compared to pudendal motor transection. (B) Bladder contraction amplitude was significantly decreased after pudendal motor transection compared to control. Artificial phasic stimulation following pudendal motor transection was also significantly decreased compared to control. No difference was noted between pudendal motor transection and phasic stimulation post pudendal motor transection. (C) No significant change in bladder contraction AUC was observed after pudendal motor transection and phasic stimulation post pudendal motor transection compared to control.
Table 1.
EUS EMG and bladder parameters pre and post pudendal cuff implantation.
| Parameter | Pre-Pudendal Surgery (n=6) | Control Pudendal Cuff (n=6) | Two-tailed Paired t test |
|---|---|---|---|
| EUS EMG Bursting Phase Rectified Smoothed AUC (mV*s) | 0.276 ± 0.06 | 0.178 ± 0.03 | M = −0.098 SD = 0.12 P = 0.10 |
| EUS EMG Bursting Phase Rectified Smoothed Maximum Amplitude (mV) | 0.249 ± 0.09 | 0.212 ± 0.12 | M = −0.036 SD = 0.11 P = 0.46 |
| EUS EMG Bursting Phase Duration (s) | 3.79 ± 0.45 | 4.19 ± 0.45 | M = 0.40 SD = 0.98 P = 0.36 |
| EUS EMG Bursting Phase Frequency (Hz) | 5.85 ± 0.23 | 6.36 ± 0.39 | M = 0.50 SD = 0.58 P = 0.09 |
| Bladder Contraction AUC (cmH2O*s) | 245.23 ± 38.21 | 281.80 ± 89.98 | M = 36 SD = 210 P = 0.69 |
| Bladder Contraction Amplitude (cmH2O) | 37.42 ± 1.55 | 36.08 ± 2.70 | M = −1.3 SD = 5.0 P = 0.54 |
| % Voiding Efficiency (voided volume/bladder capacity*100) | 66.17 ± 6.81 | 58.14 ± 3.88 | M = −8.0 SD = 12 P = 0.16 |
All values represent mean ± SE, M=mean difference
SD= standard deviation
Table 2.
Subset of experiments comparing left vs. right unilateral stimulation and unilateral (averaged response of left and right) vs. bilateral stimulation on % voiding efficiency, bladder contraction amplitude, and bladder contraction AUC.
| Parameter | Left Pudendal Motor Stimulation (n=7) | Right Pudendal Motor Stimulation (n=7) | Two- tailed Paired t test | Unilateral Pudendal Motor Stimulation (n=7) | Bilateral Pudendal Motor Stimulation (n=7) | Two- tailed Paired t test |
|---|---|---|---|---|---|---|
| % Voiding Efficiency (voided volume/bladder capacity*100) | 56.75 ± 8.64 | 54.69 ± 9.68 | M = −2.0 SD = 13 P = 0.70 |
55.72 ± 8.81 | 50.87 ± 10.03 | M = −4.8 SD = 12 P = 0.34 |
| Bladder Contraction Amplitude (cmH2O) | 26.36 ± 2.05 | 27.30 ± 2.92 | M = 0.93 SD = 4.0 P = 0.55 |
26.83 ± 2.41 | 27.69 ± 2.73 | M = 0.86 SD = 1.7 P = 0.23 |
| Bladder Contraction AUC (cmH2O*s) | 257.97 ± 41.81 | 228.60 ± 28.21 | M = −29 SD = 77 P = 0.35 |
243.28 ± 32.52 | 236.05 ± 40.07 | M = −7.2 SD = 55 P = 0.74 |
All values represent mean ± SE
M=mean difference, SD= standard deviation
Subsequent bilateral transection of the sensory branches of the pudendal nerve eliminated the increase in VE generated by distal stimulation of the transected motor branches (Fig. 3B). No significant differences were observed in either bladder contraction amplitude (Fig. 3C) or AUC (Fig. 3D) during bilateral motor branch stimulation following bilateral sensory branch transection.
Figure 3.
(A) Example bladder pressure (top) and EUS EMG activity (bottom) during the voiding contraction phase of a CMG trial after motor transection, phasic stimulation post pudendal motor transection, complete pudendal transection, and phasic stimulation post complete pudendal transection conditions. (B) Artificial phasic stimulation post motor transection significantly increased % voiding efficiency compared to distention evoked trials after pudendal motor transection. Phasic stimulation evoked increase in % voiding efficiency was abolished after complete bilateral pudendal transection (motor and sensory). No changes in Bladder contraction amplitude (C) or bladder contraction AUC (D) were observed.
Effects of Phasic Activation of the EUS on Voiding Efficiency in the Cat
Although the EUS is largely silent during voiding in the cat, we hypothesized that the introduction of exogenous EUS burst activity by electrical stimulation would enhance VE in the cat, as we observed in the rat. Electrical stimulation of the DPeriN resulted in activation of the EUS and increases in urethral pressure. The stimulation amplitude that produced maximum urethral pressure response ranged from 0.60–0.9 mA (0.72 ± 0.12 mA, mean ± SD). Burst-patterned DPeriN stimulation resulted in transient contraction of the EUS reflected in transient increases in urethral pressure. Between bursts, the pressure declined to baseline (Fig. 4G) thus providing an opportunity for urine flow.
Example CMGs during distention evoked and DPeriN stimulation in an α-chloralose anesthetized male cat are shown in Figure 4. 2 Hz burst DPeriN stimulation produced high frequency oscillations in bladder pressure (Fig. 4E–F) that were absent during distention evoked contractions without stimulation (Fig. 4B–C).
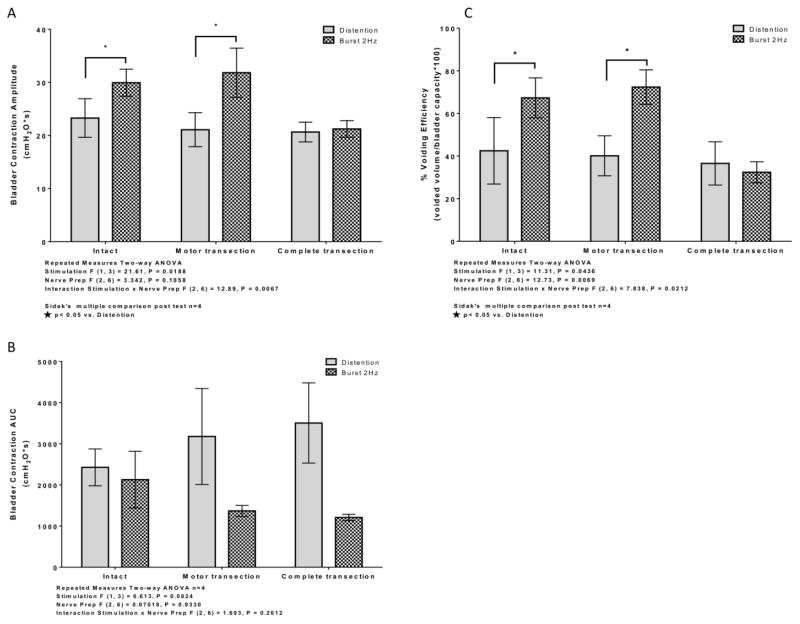
A two-way ANOVA of variance showed a significant effect on both VE (Fig. 5C) and mean bladder contraction amplitude (Fig. 5A) for the stimulation factor. No significant effect on either VE or mean bladder contraction amplitude was observed for the nerve preparation factor. However, the interaction between stimulation and nerve preparation was significant for both VE and mean bladder contraction amplitude. For both intact and pudendal motor transection preparations, unilateral phasic burst stimulation of the DPeriN resulted in significantly increased VE (Fig. 5C) and mean bladder contraction amplitude (Fig. 5A) compared to distention evoked voiding. However, for the complete pudendal nerve transection condition, VE and mean bladder contraction amplitude were not different between distention evoked voiding and unilateral phasic burst stimulation.
Figure 5.
Average bladder contraction amplitude (A), bladder contraction AUC (B), and voiding efficiency (C) in cat during voiding trials between distention evoked control and DPeriN 2 Hz burst stimulation during intact, motor transection, and complete (sensory and motor) transection conditions. (A) Bladder contraction amplitude evoked by artificial phasic stimulation was significantly larger than distention controls in both the intact and bilateral pudendal motor transection conditions. Phasic stimulation evoked increase in bladder contraction amplitude was abolished after complete bilateral pudendal transection (motor and sensory). (B) Artificial phasic stimulation did not significantly decrease bladder contraction AUC compared to distention controls in intact, bilateral pudendal motor transection, and complete bilateral pudendal transection (motor and sensory) conditions. (C) Artificial phasic stimulation significantly increased % voiding efficiency compared to distention controls in both the intact and bilateral pudendal motor transection conditions. Phasic stimulation evoked increase in % voiding efficiency was abolished after complete bilateral pudendal transection (motor and sensory).
No significant differences in bladder contraction AUC were observed during unilateral phasic burst stimulation of the DPeriN compared to distention evoked voiding in either the intact condition, following bilateral motor branch transection, or following subsequent bilateral sensory branch transection (Fig. 5B).
Discussion
Artificial phasic burst activation of the external urethral sphincter (EUS) evoked by electrical stimulation of the pudendal motor branch increased voiding efficiency in both rats and cats. Our initial experiments verified the importance of phasic EUS bursting for efficient voiding in rats. Phasic bursting of the EUS was present during micturition in control conditions (see Fig 1A–B) (Chen et al., 2011; Chen et al., 2012; D’Amico and Collins, 2012; Kakizaki et al., 1997; Kruse et al., 1990; Peng et al., 2008; Streng et al., 2004; Vera and Nadelhaft, 2001), and disruption of the EUS by pudendal motor transection resulted in significantly decreased VE compared to control conditions. Block of EUS activity by α-bungarotoxin also significantly reduced VE, consistent with the effects of motor nerve transection. Furthermore, the magnitude of change after α-bungarotoxin or bilateral pudendal motor transection were similar for both VE (40 and 38%) and contraction amplitude (21 and 22 %), suggesting that these changes were indeed the result of the (common) loss of EUS bursting activity. Our results are in agreement with other studies reporting reduced VE after elimination of EUS burst activity by either pharmacological blockade (Conte et al., 1991; Kruse et al., 1993; Maggi et al., 1986; Peng et al., 2008; Vera and Nadelhaft, 2001; Yoshiyama et al., 2000) or transection of the pudendal motor nerve (Peng et al., 2008). The elimination of phasic bursting also significantly decreased mean bladder contraction amplitude. The role of phasic bursting in efficient voiding in rats motivated the hypothesis that artificial introduction of phasic EUS activity in conditions when it was not present could increase voiding efficiency.
Artificial Phasic Bursting in Rats and Cats
Bilateral distal stimulation of the transected motor branches produced phasic EUS activation (see Fig. 1E–H), and, during micturition, high frequency oscillations in bladder pressure as observed during naïve voiding in the rat. Re-introduction of phasic bursting following motor nerve transection significantly increased VE, returning it back to levels measured prior to motor branch transection. Moreover, introduction of phasic EUS bursting activity in the cat (via DPeriN stimulation), which normally exhibits EUS relaxation during voiding (Fedirchuk and Shefchyk, 1993), also elicited high frequency oscillations in bladder pressure during micturition (Fig. 4). Unilateral stimulation of the DPeriN significantly increased VE and bladder contraction amplitude compared to distention evoked reflex voiding with intact pudendal nerves and after bilateral motor transection. In cats, pudendal motor stimulation to effect voiding function has been previously reported. Continuous or intermittent low frequency stimulation of the rectal perineal branch of the pudendal nerve (the somatic motor branch that contains both the DPeriN, innervating the EUS and the caudal rectal branch innervating the EAS) evoked sustained bladder contractions under isovolumetric conditions and increased voiding efficiency (Yoo et al., 2008a). Additionally, Boggs et al. (2006) reported that intermittent stimulation of the compound pudendal nerve produced voiding that was more efficient than distention evoked voiding. Stimulation amplitude during these trials were sufficient to evoke both a direct motor response as well as a pudendal-EUS reflex response. While both Yoo et al. (2008a) and Boggs et al. (2006) described the potential impact of pudendal motor stimulation, the present results are the first investigation of pudendal motor burst stimulation patterned similarly to what has been observed in rats as an approach to increase voiding efficiency.
Mechanism of Enhanced Voiding Efficiency by Phasic Bursting of the EUS
In both rats and cats, phasic burst stimulation of the pudendal motor branch increased VE, which raised the question of how phasic contraction of the EUS was influencing micturition. Bilateral transection of the pudendal sensory branches completely eliminated the increases in VE produced by phasic stimulation in both rats and cats. This observation indicated that, in both species, phasic bursting of the EUS increased voiding efficiency through activation of pudendal afferents. The EUS contraction evoked by pudendal motor stimulation produced constriction of the urethra which in turn activated pudendal afferents innervating the urethra (Danziger and Grill, 2015; Snellings et al., 2012), as well as potentially the perineal skin (Lagunes-Cordoba et al., 2010). Thus, phasic activation of the pudendal motor nerve generates activation of pudendal afferents via this motor – sensory coupling mechanism; and, in turn, these afferents activated or amplified the augmenting reflex (Barrington, 1941). In cats, phasic bursting also increased bladder contraction amplitude, which was abolished after bilateral pudendal sensory transection, providing further evidence for the augmenting reflex mechanism.
In contrast to cats, no change in bladder contraction amplitude was observed during phasic motor branch stimulation in rats, and mechanisms other than or in addition to the augmenting reflex may have contributed to the increased VE in rats. Le Feber and Van Asselt (1999) reported that stimulation of the compound pudendal nerve in rats elicited urethral striated muscle contraction as well as smooth muscle relaxation, even after bilateral pelvic and hypogastric nerve transections. Together with our results, these data suggest that phasic activation of the pudendal nerve may cause urethral relaxation via a pudendal-pudendal reflex, and that the subsequent reduction in outlet resistance contributed to the increased VE generated by phasic pudendal nerve stimulation.
We investigated the effect of phasic bursting in female urethane anesthetized rats and α-chloralose male cats. While phasic bursting of the EUS in both species increased voiding efficiency through activation of pudendal sensory pathways, gender and anesthesia may have contributed to differences in mechanism between rats and cats.
Conclusions
Phasic bursting activity of the EUS is critical for efficient voiding in rats, and artificial restoration of phasic bursting lost following nerve transection could restore efficient voiding in rats. Imposition of artificial phasic bursting in cats, which normally exhibits EUS relaxation while voiding, was also effective in promoting efficient voiding. In both species phasic EUS activity increased voiding efficiency through activation of pudendal sensory pathways. Phasic bursting is presumably affecting micturition by activating/amplifying the augmenting reflex in cats while decreased outlet resistance resulting from a pudendal-pudendal reflex may also contribute in rats. Collectively, these results provide further insight into the function of phasic EUS activity in efficient voiding and highlight a novel approach to increase voiding efficiency generated by pudendal afferent nerve stimulation.
Acknowledgments
The authors thank Gilda Mills for her assistance during these experiments. This work was supported by a grant from the National Institutes of Health (R01 NS050514).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barrington FJF. The component reflexes of micturition in the cat Part III. Brain. 1941;64:239–243. [Google Scholar]
- Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Bladder emptying by intermittent electrical stimulation of the pudendal nerve. Journal of neural engineering. 2006;3:43–51. doi: 10.1088/1741-2560/3/1/005. [DOI] [PubMed] [Google Scholar]
- Chen SC, Cheng CL, Fan WJ, Chen JJ, Lai CH, Peng CW. Effect of a 5-HT1A receptor agonist (8-OH-DPAT) on external urethral sphincter activity in a rat model of pudendal nerve injury. American journal of physiology Regulatory, integrative and comparative physiology. 2011;301:R225–235. doi: 10.1152/ajpregu.00260.2010. [DOI] [PubMed] [Google Scholar]
- Chen SC, Fan WJ, Lai CH, Jason Chen JJ, Peng CW. Effect of a 5-HT(1A) receptor agonist (8-OH-DPAT) on the external urethral sphincter activity in the rat. Journal of the Formosan Medical Association = Taiwan yi zhi. 2012;111:67–76. doi: 10.1016/j.jfma.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Conte B, Maggi CA, Parlani M, Lopez G, Manzini S, Giachetti A. Simultaneous recording of vesical and urethral pressure in urethane-anesthetized rats: effect of neuromuscular blocking agents on the activity of the external urethral sphincter. Journal of pharmacological methods. 1991;26:161–171. doi: 10.1016/0160-5402(91)90041-3. [DOI] [PubMed] [Google Scholar]
- Cruz Y, Downie JW. Sexually dimorphic micturition in rats: relationship of perineal muscle activity to voiding pattern. American journal of physiology Regulatory, integrative and comparative physiology. 2005;289:R1307–1318. doi: 10.1152/ajpregu.00088.2005. [DOI] [PubMed] [Google Scholar]
- D’Amico SC, Collins WF., 3rd External urethral sphincter motor unit recruitment patterns during micturition in the spinally intact and transected adult rat. Journal of neurophysiology. 2012;108:2554–2567. doi: 10.1152/jn.00927.2011. [DOI] [PubMed] [Google Scholar]
- Damaser MS, Samplaski MK, Parikh M, Lin DL, Rao S, Kerns JM. Time course of neuroanatomical and functional recovery after bilateral pudendal nerve injury in female rats. American journal of physiology Renal physiology. 2007;293:F1614–1621. doi: 10.1152/ajprenal.00176.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danziger ZC, Grill WM. Sensory and circuit mechanisms mediating lower urinary tract reflexes. Auton Neurosci. 2015 doi: 10.1016/j.autneu.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedirchuk B, Shefchyk SJ. Membrane potential changes in sphincter motoneurons during micturition in the decerebrate cat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13:3090–3094. doi: 10.1523/JNEUROSCI.13-07-03090.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizaki H, Fraser MO, De Groat WC. Reflex pathways controlling urethral striated and smooth muscle function in the male rat. The American journal of physiology. 1997;272:R1647–1656. doi: 10.1152/ajpregu.1997.272.5.R1647. [DOI] [PubMed] [Google Scholar]
- Kennelly MJ, Bennett ME, Grill WM, Grill JH, Boggs JW. Electrical stimulation of the urethra evokes bladder contractions and emptying in spinal cord injury men: case studies. The journal of spinal cord medicine. 2011;34:315–321. doi: 10.1179/2045772311Y.0000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse MN, Belton AL, de Groat WC. Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. The American journal of physiology. 1993;264:R1157–1163. doi: 10.1152/ajpregu.1993.264.6.R1157. [DOI] [PubMed] [Google Scholar]
- Kruse MN, Noto H, Roppolo JR, de Groat WC. Pontine control of the urinary bladder and external urethral sphincter in the rat. Brain research. 1990;532:182–190. doi: 10.1016/0006-8993(90)91758-9. [DOI] [PubMed] [Google Scholar]
- Lagunes-Cordoba R, Hernandez PR, Raya JG, Munoz-Martinez EJ. Functional Coupling Between Motor and Sensory Nerves Through Contraction of Sphincters in the Pudendal Area of the Female Cat. Journal of neurophysiology. 2010;103:74–82. doi: 10.1152/jn.00712.2009. [DOI] [PubMed] [Google Scholar]
- Le Feber J, Van Asselt E. Pudendal nerve stimulation induces urethral contraction and relaxation. Am J Physiol-Reg I. 1999;277:R1368–R1375. doi: 10.1152/ajpregu.1999.277.5.R1368. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Giuliani S, Santicioli P, Meli A. Analysis of factors involved in determining urinary bladder voiding cycle in urethan-anesthetized rats. The American journal of physiology. 1986;251:R250–257. doi: 10.1152/ajpregu.1986.251.2.R250. [DOI] [PubMed] [Google Scholar]
- McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. The Journal of comparative neurology. 1986;248:532–549. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- Nishizawa O, Satoh S, Harada T, Nakamura H, Fukuda T, Tsukada T, Tsuchida S. Role of the pudendal nerves on the dynamics of micturition in the dog evaluated by pressure flow EMG and pressure flow plot studies. The Journal of urology. 1984a;132:1036–1039. doi: 10.1016/s0022-5347(17)49994-0. [DOI] [PubMed] [Google Scholar]
- Nishizawa O, Satoh S, Tsukada T, Fukuda T, Moriya I, Tsuchida S. Role of the striated urethral sphincter in the voiding cycle of the decerebrated dog. Nihon Heikatsukin Gakkai Zasshi. 1984b;20:413–417. doi: 10.1540/jsmr1965.20.413. [DOI] [PubMed] [Google Scholar]
- Pacheco P, Martinez-Gomez M, Whipple B, Beyer C, Komisaruk BR. Somato-motor components of the pelvic and pudendal nerves of the female rat. Brain research. 1989;490:85–94. doi: 10.1016/0006-8993(89)90433-2. [DOI] [PubMed] [Google Scholar]
- Peng CW, Chen JJ, Chang HY, de Groat WC, Cheng CL. External urethral sphincter activity in a rat model of pudendal nerve injury. Neurourology and urodynamics. 2006;25:388–396. doi: 10.1002/nau.20229. [DOI] [PubMed] [Google Scholar]
- Peng CW, Chen JJ, Cheng CL, Grill WM. Role of pudendal afferents in voiding efficiency in the rat. American journal of physiology Regulatory, integrative and comparative physiology. 2008;294:R660–672. doi: 10.1152/ajpregu.00270.2007. [DOI] [PubMed] [Google Scholar]
- Snellings AE, Yoo PB, Grill WM. Urethral flow-responsive afferents in the cat sacral dorsal root ganglia. Neuroscience letters. 2012;516:34–38. doi: 10.1016/j.neulet.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streng T, Santti R, Andersson KE, Talo A. The role of the rhabdosphincter in female rat voiding. BJU international. 2004;94:138–142. doi: 10.1111/j.1464-4096.2004.04875.x. [DOI] [PubMed] [Google Scholar]
- Vera PL, Nadelhaft I. Clozapine inhibits micturition parameters and the external urethral sphincter during cystometry in anesthetized rats. Brain research. 2001;901:219–229. doi: 10.1016/s0006-8993(01)02352-6. [DOI] [PubMed] [Google Scholar]
- Woock JP, Yoo PB, Grill WM. Activation and inhibition of the micturition reflex by penile afferents in the cat. American journal of physiology Regulatory, integrative and comparative physiology. 2008;294:R1880–1889. doi: 10.1152/ajpregu.00029.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo PB, Horvath EE, Amundsen CL, Webster GD, Grill WM. Intraurethral activation of excitatory bladder reflexes in persons with spinal cord injury. Conference proceedings :... Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society Conference; 2009; 2009. pp. 6781–6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo PB, Horvath EE, Amundsen CL, Webster GD, Grill WM. Multiple pudendal sensory pathways reflexly modulate bladder and urethral activity in patients with spinal cord injury. The Journal of urology. 2011;185:737–743. doi: 10.1016/j.juro.2010.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo PB, Woock JP, Grill WM. Bladder activation by selective stimulation of pudendal nerve afferents in the cat. Experimental neurology. 2008a;212:218–225. doi: 10.1016/j.expneurol.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo PB, Woock JP, Grill WM. Somatic innervation of the feline lower urinary tract. Brain research. 2008b;1246:80–87. doi: 10.1016/j.brainres.2008.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama M, deGroat WC, Fraser MO. Influences of external urethral sphincter relaxation induced by alpha-bungarotoxin, a neuromuscular junction blocking agent, on voiding dysfunction in the rat with spinal cord injury. Urology. 2000;55:956–960. doi: 10.1016/s0090-4295(00)00474-x. [DOI] [PubMed] [Google Scholar]