Abstract
Rationale
Idiopathic subglottic stenosis (iSGS) is a rare and devastating extrathoracic obstruction involving the lower laryngeal and upper tracheal airway. It arises without known antecedent injury or associated disease process. Persistent mucosal inflammation and a localized fibrotic response are hallmarks of the disease. Despite the initial clinical description of iSGS more than 40 year ago, there have been no substantive investigations into the pathogenesis of this enigmatic and progressive airway obstruction.
Objectives
In these studies, we present the initial characterization of the molecular pathogenesis underlying the fibrosing phenotype of iSGS.
Methods
Utilizing 20 human iSGS and healthy control specimens we applied histologic, immunohistochemical, molecular and immunologic techniques.
Main Results
We demonstrate significant activation of the canonical IL-23/IL-17A pathway in the tracheal mucosa of iSGS patients, as well as identify γδ T cells as the primary cellular source of IL-17A.
Conclusions
Our results suggest that aberrant mucosal immune activation is a component in of the pathogenesis of iSGS. Most critically, our work offers new targets for future therapeutic intervention.
Level of Evidence
NA
Keywords: IL-17A, IL-17, Idiopathic Subglottis Stenosis, Tracheal Stenosis, Laryngotracheal Stenosis, iSGS, ISS
Introduction
Laryngotracheal stenosis is a devastating, fixed extrathoracic obstruction that can occur without known antecedent injury (idiopathic subglottic stenosis; iSGS). It also can accompany collagen vascular disease e.g. Wegeners Granulomatosis) or follow iatrogenic injury (e.g., endotracheal intubation; iLTS). iSGS is clinically rare and histopathologically distinct and manifests as persistent mucosal inflammation with a localized fibrotic tissue response. Despite the initial clinical description of iSGS more than 40 years ago1, there have been no substantive investigations into the pathogenesis of this enigmatic and progressive airway obstruction. A recent international multicenter study (NoAAC RP-01)2 demonstrated that the iSGS population is strikingly homogeneous (female, Caucasian, and peri-menopausal). Classically, iSGS patients present with unexplained prodromal dyspnea (often extending several month or years prior to presentation). Physical examination demonstrates a short (1-3cm), concentric stenosis centered at the cricoid cartilage (below the level of the vocal cords). Patients require urgent treatment to reestablish airway patency. Unfortunately, surgical interventions are rarely definitive and many iSGS patients require several treatments3. High disease recidivism is common and associated with disabling side effects in breathing and voicing that significantly affect the patient's quality of life4.
Histologically, the majority of iSGS cases show extensive fibrosis with relatively normal cartilage5. Diverse diseases in divergent organ systems are associated with fibrosis, suggesting common pathogenic pathways6. The process is defined by the excessive accumulation of fibrous connective tissue components of the extracellular matrix (ECM, i.e. collagen and fibronectin) in inflamed tissue7. Despite an increasing understanding of the pathogenic mechanisms of fibrosis in alternate organ systems, there have been no studies in iSGS delineating the the biologic process driving the characteristic tissue remodeling in the subglottis.
We utilized specialized histologic staining to anatomically define the aberrant tissue remodeling in iSGS. We then employed electron microscopy to probe these changes in greater structural detail. Real-time polymerase chain reaction (RT-PCR) was preformed to verify the molecular underpinnings to the observed ultrastructural fibrotic changes in iSGS. Since persistent inflammation is linked to fibrosis6, and inflammation in the tracheal scar is a cardinal feature of iSGS, we then investigated central immunologic mechanisms involved in inflammation that we hypothesize promote the fibrosing phenotype of this disease.
Experimental procedures
This study was performed in accordance with the Declaration of Helsinki, Good Clinical Practice, and was approved by the Institutional Review Board at Vanderbilt University Medical Center.
Patients
20 iSGS, 20 iLTS, and 23 normal controls were utilized for experiments. Each iSGS & iLTS diagnosis was confirmed using previously described clinical and serologic criteria8. Subglottic scar was the source of all specimens from the iSGS, and iLTS patients. The control population consisted of patients without known subglottic pathology or systemic infection.
Transmission Electron Microscopy
Human tissue samples from cases and controls were harvested in the operating theater and immediately fixed with chilled buffer (50 mM sodium cacodylate [pH 7.4]) containing 2.5% glutaraldehyde and 2.0% paraformaldehyde and placed in 4°C overnight. The samples were then prepared as previsouly described9. Samples analyzed with an FEI T-12 transmission electron microscope equipped with a side-mounted digital camera.
Immunohistochemistry
Staining
3 μm paraffin embedded sections of iSGS or iLTS airway stenosis derived from patients that underwent open tracheal or cricotracheal resection were obtained from the Vanderbilt pathology archive. Normal subglottis from healthy controls was obtained from US Biomax Inc. (product# RS321). Specimins were deparaffinized, and a heat induced antigen retrieval was performed for 20 minutes. Slides were placed in DakoCytomation Biotin Blocking System (Ref#x0590, DAKO) for 15 minutes in each solution. Slides then were placed in a Protein Block (Ref# x0909, DAKO) for 10 minutes. Slides were incubated with anti-IL-17A (Cat. AF-317-NA, R&D Systems) for one hour at a 1:200 dilution and followed by a biotinylated anti-goat (Cat. BA-5000, Vector Laboratories, Inc.) for 30 minutes at a 1:200 dilution. The Bond Polymer Refine detection system was used for visualization. Slides were then dehydrated, cleared and coverslipped.
Quantification
Immunostained slides were imaged on a Leica SCN400 Slide Scanner (Leica Biosystems). Slides were imaged at 20X magnification to a resolution of 0.5 μm/pixel. Cells were identified utilizing standard Ariol® analysis scripts. (Leica) Upper and lower thresholds for color, saturation, intensity, size, roundness, and axis length were set for both blue Hematoxylin staining of nuclei and for brown DAB reaction products. Thus, brown (DAB) positive cells can be distinguished from blue (Hematoxylin only) negative cells.
qPCR profiling of respiratory epithelial immune activation
Following mechanical digestion of surgical biopsy specimins, mRNA was extracted with the RNAeasy extraction kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Healthy control tracheal mRNA was obtained from 23 pooled donors (clontech cat# 636127)10. The RNA concentration and quality were confirmed using the Bioanalyzer 2100 system (Agilent, CA, USA). cDNA was transcribed using established reagents according to manufacturer's instructions. The human innate and adaptive immune response qPCR array (Qiagen) was utilized as per manufacture's instructions in a StepOnePlus™ instrument (Applied Biosystems®, USA). Expression analysis was preformed using PCR analysis software employing the 2-ΔΔCt method as previously described11 (Qiagen; https://www.qiagen.com).
Flow Cytometry
Surgical specimens of tracheal scar were digested and single cell suspensions were rested overnight in RPMI 1640 medium supplemented with 10% FCS. 5×105 cells were activated with PMA and ionomycin at 37 °C under 5 % CO2 for 30 min before addition of BD GolgiStop (BD Biosciences). After 4h of stimulation, the cells were harvested and washed for surface staining to CD3, TCR γδ, (BD Biosciences). Cells were then washed, fixed and permeabilized, using a Fix & Perm Kit according to the manufacturer's instructions (BD Biosciences). Intracellular antibodies to IL-17A were added at 4 °C for 45 min. Cells were washed and analyzed via flow cytometry. For the gating strategy, lymphocytes were gated by FSC/SSC, then doublets were gated out, then dead cells, then cells were analyzed for CD3 and SSC. CD3+ cells were gated and subsequently analyzed for γδ TCR and IL-17A expression. All flow cytometry experiments were acquired with a LSR-II flow cytometer (BD Biosciences). Analysis was performed using FlowJo software (Tree Star). A minimum of 200,000 events were acquired for each sample.
Statistical Analysis
Statistical significance was set at p value less than 0.05 and a mean difference equal to or greater than 2-fold change in expression levels. Normal distribution of the variables was tested using the Shapiro-Wilk test. Differences between x and y groups were determined using the Kruskal Wallis and Mann Whitney tests for normal and non-normal distributions, respectively. Data were expressed as median ± SD for non-normal distributed variables. All statistical analyses were performed with Prism version 6.0 software.
Results
iSGS Anatomic, Physiologic and Histologic characteristics
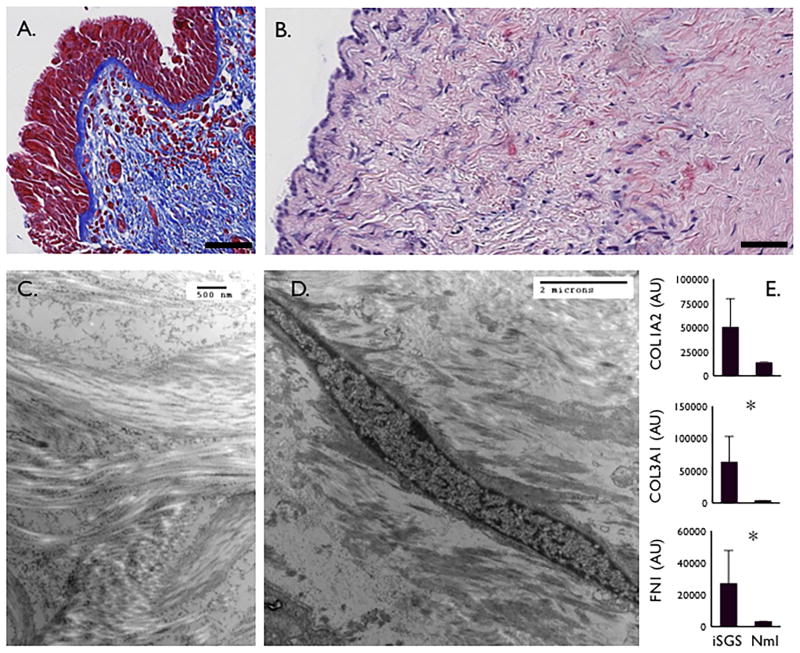
iSGS is defined by classic anatomic, physiologic and histologic characteristics. All iSGS patients develop airway stenosis below the vocal cords in the region where the larynx transitions to the trachea (Fig. S1A., S1B.). This luminal scar produces impairment in airflow manifesting as a flow-volume loop finding of fixed extrathoracic obstruction on pulmonary function testing (PFT), (Fig. S1C.). Histologically, the airway mucosa at the site of scar is frequently characterized by an intact basement membrane (Fig. 1A.) with pronounced subepithelial fibrosis (Fig. 1B.). Transmission electron microscopy demonstrates the disordered arrangement of collagen bundles (Fig. 1C.) driven by abundant tissue fibroblasts (Fig. 1D.). Molecular analysis of areas of subglottic fibrotic remodeling shows increased mRNA expression of established extracellular matrix constituents type I collagen (COL1A2), type III collagen (COL3A1), and Fibronectin (FN1) (Fig. 1E.). These results were confirmed at the protein level with histologic analysis (data not shown).
Figure 1. iSGS Anatomic, Physiologic and Histologic characteristics.
Trichome blue staining of iSGS subglottic scar demonstrating extensive blue collagen staining and an intact basement membrane (Magnification 20x, scale bar = 100μM) (A.). Sirius red staining highlights subepithelial collagen deposition in red (Magnification 40x, scale bar = 100μM) (B.). Transmission electron microscopy shows irregular, disordered subepithelial collagen bundles (C.), as well as abundant fibroblasts (D.). qPCR from 10 iSGS patients for extracellular matrix constituents (Types I & III collagen, Fibronectin) from iSGS scar compared with 23 healthy controls (E.), asterisk indicates statistical significance (Two-tailed, Mann Whitney test; p<0.05).
Host Inflammatory Response
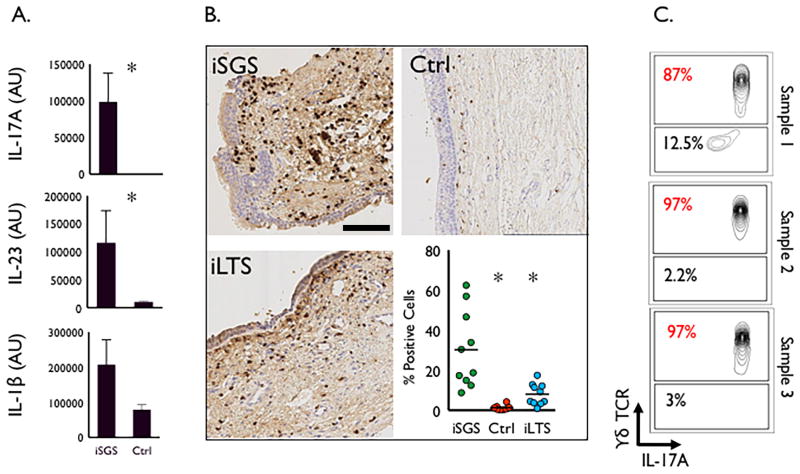
Affected tracheal mucosa in iSGS patients consistently demonstrates acute and chronic inflammation (Fig. S2A), with an infiltration of CD3+ T lymphocytes (Fig. S2B). To delineate the host mucosal immune response in iSGS, we utilized a qPCR array consisting of 84 unique inflammatory genes (Qiagen Inc, Vallencia, CA) to compare gene expression in the tracheal mucosa of 10 iSGS patients with 23 healthy controls (Fig. S3). mRNA expression analysis showed the IL-17A to be the most significantly unregulated gene in iSGS mucosa compared to healthy control trachea (p<0.005) (Fig. 2A). The upstream driver of the IL-17A pathway, IL-23 demonstrated significant up-regulation in iSGS and IL-1β showed a trend towards increased expression, suggesting a potential role for the IL-17A/IL-23 pathway in the pathogenesis of inflammation and fibrosis seen in iSGS.
Figure 2. Host Inflammatory Response.
qPCR results for panel IL-17A/IL-23 pathway gene products from 10 iSGS patients demonstrating significant upregulation in IL-17A and IL-23 when compared to control healthy trachea, asterisk denotes significance (Two-tailed, Mann Whitney test; p<0.005). IL-1β tended to be higher in iSGS patients but did not reach statistical significance (p=0.06) (A.). Representative images from IHC of IL-17A in surgical specimens from iSGS (n=10), iLTS (n=10), and control (n=10) patients (Magnification 20x, scale bar = 100μM). Accompanying graph summarizing digitally quantified IL-17A levels, depicting significantly more IL-17A expression in iSGS patients, compared with either iLTS, or controls, asterisk denotes significance (Kruskal-Wallis test; p<0.0001) (B.). Flow cytometry from tracheal mucosa in 3 surgical specimens from different iSGS patients, demonstrating that the majority of IL-17A positive cells were γδ TCR positive (C.).
Increased IL-17A expression within the mucosa of iSGS scar was confirmed at the protein level utilizing immunohistochemistry. As seen in the representative images (Fig. 2B) and quantified digitally by computerized algorithm, the mean number of IL-17A positive cells in iSGS tracheal mucosal biopsy specimens was 30.3% compared to 8.1% in iLTS (who developed their tracheal injury following prolonged endotracheal intubation in an ICU for medical illness) and 1.3% in normal healthy tracheal mucosa; p<0.001].
In order to localize the cellular source of mucosal IL-17A, we utilized flow cytometry with intracellular cytokine staining on cells obtained from surgical specimens containing subglottic mucosal scar tissue from 3 iSGS patients. After digestion and nonspecific stimulation, Gr1+neutrophils, CD3+CD4+, and CD3+ γδ TCR+ positive cells were assessed for intracellular IL-17A levels. In iSGS, 87-97% of IL-17A expressing cells were identified as γδ TCR+(Fig. 2C).
Discussion
Although the clinical description of iSGS is more than 40 years old1, our study represents the first molecular characterization of the pathogenic tissue remodeling in this rare disorder. The in-depth histologic, electron microscopic, and molecular techniques utilized in this study confirm an abundance of ECM components in the subglottic scar in iSGS patients. This evidence strongly supports a central role for the pathogenic process of fibrosis in the tissue remodeling seen in iSGS. Furthermore, our studies highlight the inflammatory nature of the airway scar in iSGS, and begin to characterize the immunologic mechanisms driving the observed inflammation.
While acute and chronic inflammation are characteristic clinical findings in the subglottic scar of iSGS patients, our study begins to dissect the molecular circuits involved. Our data reveals a significant up-regulation of the IL-17A/IL23 inflammatory axis in iSGS airway scar. Although crucial in protecting the host from invasion by many types of pathogens, dysregulated IL-17A production can drive chronic inflammation12-14 with subsequent tissue damage and fibrinogenesis15,16. IL-17A is also specifically implicated in many inflammatory lung diseases, including asthma12,17, cystic fibrosis13 and chronic obstructive pulmonary disease14. Classically, the expression of IL-17A is derived from immune cells located in non-lymphoid tissues where they are poised to respond immediately to tissue injury or pathogenic insults. Stimulation of these cells by IL-23 induces IL-17A expression and subsequent local tissue inflammation. Consistent with established mechanistic work, our investigations reveal an increase in IL-23 accompanying upregulated IL-17A expression within an infiltrating immune cell subset in the subglottis.
Several studies in the last 5 years have linked IL-17A and the development of fibrotic lung disease. Human studies offer strong support for a critical role of IL-17A in the pathogenesis of obliterative bronchiolitis16 seen after pulmonary transplant. However, the mechanisms governing IL-17A-mediated fibrosis in pulmonary fibroinflamatory disease remain poorly understood. The elevated inflammatory IL-17A expression we observed in the subglottis may directly influence fibroblasts in iSGS leading to pathologic tissue fibrosis18, although the precise molecular mechanism linking IL-17A to fibrogenesis merits future study.
Given the rarity of iSGS, our results will require confirmation in larger cohorts pooled from multiple institutions. Furthermore, while we provide molecular characterization of the fibroinflamatory phenotype as mechanistic support for the hypothesis that a conserved and consistent biologic process drives a singular disease, the explanation for why involvement is nearly universally restricted to adult Caucasian women remains opaque. The role of genetics and the influence of estrogen on host inflammation and fibrosis are novel questions that demand future studies.
The inciting event behind the dramatic increase in IL-17A is also unclear. However, given the established role of γδ T cell IL-17A in host defense against pathogens at mucosal barriers19, as well as an emerging understanding of disrupted microbiota in other chronic inflammatory mucosal disease20-26, our results raise important questions about a potential role for pathogens in the etiopathogenesis of iSGS. Given the advent of robust culture-independent techniques27,28 future studies may be able to define a role for bacteria, fungus, or virus in disease initiation, progression, or recurrence.
Alternatively, a role for IL-17A has been increasingly recognized in autoimmune collagen vascular disorders such as rheumatoid arthritis (RA)18, systemic lupus erythematosus (SLE)29, and granulomatosis with polyangitis (GPA, i.e. Wegener's granulomatosis)30,31. In these diseases, increased circulating IL-17A+ cells have been closely associated with tissue inflammation and overall disease activity. Our findings may offer support for the phenotypic similarity between iSGS and GPA observed clinically. However, future study will be important to full clarify the molecular overlap31 between the diseases.
Our results advance the understanding of iSGS by developing the concept that this disease results from aberrant mucosal immune activation in the large airway rather than simply idiopathic extracellular matrix deposition. Most critically, demonstration of IL-23/IL-17A pathway activation offers a novel approach for therapeutic intervention in iSGS patients. Several established drugs limit IL-17A production32, or neutralize its effects33,34. Future insight into the molecular mechanism linking IL-17A to canonical pathways of tissue fibrosis may reveal additional pathways amenable to directed targeting. Thus, the implications this work may extend beyond the confines of iSGS to impact other fibrotic diseases driven by pathogenic inflammation.
Supplementary Material
Figure S1. Normal glottic and subglottic anatomy (A.) contrasted with the classic endolunimal subglottic scar arising below the vocal cords in iSGS patients (B.). This stenosis results in airflow restriction manifesting as a flow-volume loop finding of fixed extrathoracic obstruction (C.).
Figure S2. H&E stained surgical specimen from iSGS patient showing acute and chronic inflammation (A.). IHC for CD3+ cells (B.).
Figure S3. Innate and adaptive immunologic gene profiling. mRNA from 10 iSGS patients and healthy controls were characterized with a human innate & adaptive immunity qPCR array (Qiagen, Valencia, CA). Scatter plots of the relative expression levels measured for iSGS samples (y axis) vs. controls (x axis). The dashed black lines indicate the ± 2-fold changes. Relative to normal tissue, the gene encoding IL-17A was the most up-regulated gene in iSGS among the subset examined.
Acknowledgments
This was a North American Airway Collaborative (NoAAC) Study. Funding: Research in NoAAC is made possible by infrastructure supported by the Patient-Centered Outcomes Research Institute under award number 1409-22214. We would also like to acknowledge the Vanderbilt the genomics core laboratory VANTAGE (Vanderbilt Technologies for Advanced Genomics) supported by an ARRA funded NIH award as well as the Translational Pathology Shared Resource supported by NCI/NIH Cancer Center Support Grant 2P30 CA068485-14. The content is solely the responsibility of the authors.
Financial Disclosure: Research in NoAAC is made possible by infrastructure supported by the Patient-Centered Outcomes Research Institute under award number 1409-22214. We would also like to acknowledge the Vanderbilt the genomics core laboratory VANTAGE (Vanderbilt Technologies for Advanced Genomics) supported by an ARRA funded NIH award as well as the Translational Pathology Shared Resource supported by NCI/NIH Cancer Center Support Grant 2P30 CA068485 14.
Footnotes
Competing Interests: None of the others has any competing interest to disclose.
Author Contribution: AG designed & performed experiments, analyzed data, & wrote the paper, NK analyzed data, MM designed & performed experiments, DN designed & performed experiments, BR aided in experimental design, JD, EE, DE, JK, AH analyzed data, preformed critical scientific review, GG aided in experimental design, LY analyzed data, JR conducted experiments, JN aided in experimental design, CW aided in experimental design, DF aided in experimental design, statistical analysis, CS conducted experiments, KJ conducted experiments, TM aided in experimental design, data analysis, review of manuscript, TB aided in experimental design, experiments, data analysis, review of manuscript, JG conducted experiments, WD aided in experimental design, experiments, data analysis, review of manuscript. Competing Interests: None of the others has any competing interest to disclose.
References
- 1.Brandenburg JH. Idiopathic subglottic stenosis. Transactions - American Academy of Ophthalmology and Otolaryngology American Academy of Ophthalmology and Otolaryngology. 1972;76(5):1402–6. [PubMed] [Google Scholar]
- 2.Gelbard A, Donovan DT, Ongkasuwan J, et al. Disease homogeneity and treatment heterogeneity in idiopathic subglottic stenosis. Laryngoscope. 2015 doi: 10.1002/lary.25708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gelbard A, Francis DO, Sandulache VC, et al. Causes and consequences of adult laryngotracheal stenosis. Laryngoscope. 2014 doi: 10.1002/lary.24956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryans L, Palmer AD, Schindler JS, et al. Subjective and objective parameters of the adult female voice after cricotracheal resection and dilation. The Annals of otology, rhinology, and laryngology. 2013;122(11):707–16. doi: 10.1177/000348941312201108. [DOI] [PubMed] [Google Scholar]
- 5.Mark EJ, Meng F, Kradin RL, et al. Idiopathic tracheal stenosis: a clinicopathologic study of 63 cases and comparison of the pathology with chondromalacia. Am J Surg Pathol. 2008;32(8):1138–43. doi: 10.1097/PAS.0b013e3181648d4a. [DOI] [PubMed] [Google Scholar]
- 6.Rockey DC, Bell PD, Hill JA. Fibrosis--a common pathway to organ injury and failure. N Engl J Med. 2015;372(12):1138–49. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- 7.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nature medicine. 2012;18(7):1028–40. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nouraei SA, Sandhu GS. Outcome of a multimodality approach to the management of idiopathic subglottic stenosis. Laryngoscope. 2013;123(10):2474–84. doi: 10.1002/lary.23949. [DOI] [PubMed] [Google Scholar]
- 9.Parekh VV, Wu L, Boyd KL, et al. Impaired autophagy, defective T cell homeostasis, and a wasting syndrome in mice with a T cell-specific deletion of Vps34. J Immunol. 2013;190(10):5086–101. doi: 10.4049/jimmunol.1202071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dezso Z, Nikolsky Y, Sviridov E, et al. A comprehensive functional analysis of tissue specificity of human gene expression. BMC Biol. 2008;6:49. doi: 10.1186/1741-7007-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Wang YH, Voo KS, Liu B, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207(11):2479–91. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan HL, Regamey N, Brown S, et al. The Th17 pathway in cystic fibrosis lung disease. Am J Respir Crit Care Med. 2011;184(2):252–8. doi: 10.1164/rccm.201102-0236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Stefano A, Caramori G, Gnemmi I, et al. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol. 2009;157(2):316–24. doi: 10.1111/j.1365-2249.2009.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters M, Kohler-Bachmann S, Lenz-Habijan T, et al. Influence of an Allergen-specific Th17 Response on Remodeling of the Airways. Am J Respir Cell Mol Biol. 2015 doi: 10.1165/rcmb.2014-0429OC. [DOI] [PubMed] [Google Scholar]
- 16.Vanaudenaerde BM, De Vleeschauwer SI, Vos R, et al. The role of the IL23/IL17 axis in bronchiolitis obliterans syndrome after lung transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(9):1911–20. doi: 10.1111/j.1600-6143.2008.02321.x. [DOI] [PubMed] [Google Scholar]
- 17.Chakir J, Shannon J, Molet S, et al. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol. 2003;111(6):1293–8. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 18.Saxena A, Raychaudhuri SK, Raychaudhuri SP. Interleukin-17-induced proliferation of fibroblast-like synovial cells is mTOR dependent. Arthritis Rheum. 2011;63(5):1465–6. doi: 10.1002/art.30278. [DOI] [PubMed] [Google Scholar]
- 19.Sutton CE, Mielke LA, Mills KH. IL-17-producing gammadelta T cells and innate lymphoid cells. Eur J Immunol. 2012;42(9):2221–31. doi: 10.1002/eji.201242569. [DOI] [PubMed] [Google Scholar]
- 20.Abreu NA, Nagalingam NA, Song Y, et al. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci Transl Med. 2012;4(151):151ra24. doi: 10.1126/scitranslmed.3003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erb-Downward JR, Thompson DL, Han MK, et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PloS one. 2011;6(2):e16384. doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blainey PC, Milla CE, Cornfield DN, et al. Quantitative analysis of the human airway microbial ecology reveals a pervasive signature for cystic fibrosis. Sci Transl Med. 2012;4(153):153ra30. doi: 10.1126/scitranslmed.3004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drake WP, Newman LS. Mycobacterial antigens may be important in sarcoidosis pathogenesis. Curr Opin Pulm Med. 2006;12(5):359–63. doi: 10.1097/01.mcp.0000239554.01068.94. [DOI] [PubMed] [Google Scholar]
- 24.Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(34):13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauth JC, Goldenberg RL, Andrews WW, et al. Reduced incidence of preterm delivery with metronidazole and erythromycin in women with bacterial vaginosis. N Engl J Med. 1995;333(26):1732–6. doi: 10.1056/NEJM199512283332603. [DOI] [PubMed] [Google Scholar]
- 26.Gupta K, Stapleton AE, Hooton TM, et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli colonization in women with recurrent urinary tract infections. J Infect Dis. 1998;178(2):446–50. doi: 10.1086/515635. [DOI] [PubMed] [Google Scholar]
- 27.Relman DA, Loutit JS, Schmidt TM, et al. The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N Engl J Med. 1990;323(23):1573–80. doi: 10.1056/NEJM199012063232301. [DOI] [PubMed] [Google Scholar]
- 28.Relman DA, Schmidt TM, MacDermott RP, et al. Identification of the uncultured bacillus of Whipple's disease. N Engl J Med. 1992;327(5):293–301. doi: 10.1056/NEJM199207303270501. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Chu Y, Yang X, et al. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 2009;60(5):1472–83. doi: 10.1002/art.24499. [DOI] [PubMed] [Google Scholar]
- 30.Wilde B, Thewissen M, Damoiseaux J, et al. Th17 expansion in granulomatosis with polyangiitis (Wegener's): the role of disease activity, immune regulation and therapy. Arthritis Res Ther. 2012;14(5):R227. doi: 10.1186/ar4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenbaum JT, Choi D, Wilson DJ, et al. Orbital pseudotumor can be a localized form of granulomatosis with polyangiitis as revealed by gene expression profiling. Exp Mol Pathol. 2015;99(2):271–8. doi: 10.1016/j.yexmp.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva JC, Mariz HA, Rocha LF, Jr, et al. Hydroxychloroquine decreases Th17-related cytokines in systemic lupus erythematosus and rheumatoid arthritis patients. Clinics (Sao Paulo) 2013;68(6):766–71. doi: 10.6061/clinics/2013(06)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–51. doi: 10.1016/S0140-6736(15)60125-8. [DOI] [PubMed] [Google Scholar]
- 34.Lebwohl M, Strober B, Menter A, et al. Phase 3 Studies Comparing Brodalumab with Ustekinumab in Psoriasis. N Engl J Med. 2015;373(14):1318–28. doi: 10.1056/NEJMoa1503824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Normal glottic and subglottic anatomy (A.) contrasted with the classic endolunimal subglottic scar arising below the vocal cords in iSGS patients (B.). This stenosis results in airflow restriction manifesting as a flow-volume loop finding of fixed extrathoracic obstruction (C.).
Figure S2. H&E stained surgical specimen from iSGS patient showing acute and chronic inflammation (A.). IHC for CD3+ cells (B.).
Figure S3. Innate and adaptive immunologic gene profiling. mRNA from 10 iSGS patients and healthy controls were characterized with a human innate & adaptive immunity qPCR array (Qiagen, Valencia, CA). Scatter plots of the relative expression levels measured for iSGS samples (y axis) vs. controls (x axis). The dashed black lines indicate the ± 2-fold changes. Relative to normal tissue, the gene encoding IL-17A was the most up-regulated gene in iSGS among the subset examined.