Abstract
Background
Lack of consensus in management guidelines for women with minor cervical lesions, coupled with novel screening approaches, such as human papillomavirus (HPV) genotyping, necessitate revisiting prevention policies. We evaluated the cost-effectiveness and resource trade-offs of alternative triage strategies to inform cervical cancer prevention in Norway.
Methods
We used a decision-analytic model to compare the lifetime health and economic consequences associated with ten novel candidate approaches to triage women with minor cervical lesions. Candidate strategies varied by: 1) the triage test(s): HPV testing in combination with cytology, HPV testing alone with or without genotyping for HPV-16 and-18, and immediate colposcopy, and 2) the length of time between index and triage testing (i.e., 6, 12 or 18 months). Model outcomes included quality-adjusted life-years (QALYs), lifetime societal costs, and resource use (e.g., colposcopy referrals).
Results
The current Norwegian guidelines were less effective and more costly than candidate strategies. Given a commonly-cited willingness-to-pay threshold in Norway of $100,000 per QALY gained, the preferred strategy involved HPV genotyping with immediate colposcopy referral for HPV-16 or -18 positive and repeat HPV testing at 12 months for non-HPV-16 or -18 positive ($78,010 per QALY gained). Differences in health benefits among candidate strategies were small, while resource use varied substantially. More effective strategies required a moderate increase in colposcopy referrals (e.g., a 9% increase for the preferred strategy) compared with current levels.
Conclusion
New applications of HPV testing may improve management for women with minor cervical lesions, yet are accompanied by a trade-off of increased follow-up procedures.
Keywords: mass screening, cost-effectiveness, cervical intraepithelial neoplasia, human papillomavirus, mathematical model
Introduction
A better understanding of cervical carcinogenesis has led to the development of several prevention approaches that target high-risk human papillomavirus (HPV), the causative agent of cervical cancer and one of the most common sexually transmitted infections [1]. The majority of infections clear within 1-2 years; however, the risk of developing cervical precancer and cancer increases with HPV persistence [2, 3]. The relationship between HPV and cervical cancer led to the development of HPV vaccines, which target the two most oncogenic HPV genotypes (i.e., HPV -16 and -18) that contribute to ∼70% of all cervical cancers [4]. Vaccination of adolescent girls against HPV infections has been adopted by nearly all developed countries; yet cervical cancer screening remains an essential preventive measure for those individuals not offered the HPV vaccine or who are past the age of vaccination.
HPV DNA testing for high-risk infections is more sensitive in detecting cervical precancer and cancer than cytology and represents an opportunity to improve screening effectiveness [5]. HPV testing has been recommended to triage women with cytology results indicating minor cervical lesions (i.e., atypical squamous cells of undetermined significance (ASC-US) and/or low-grade squamous intraepithelial lesion (LSIL)) since the beginning of the 2000s; recent applications involve replacing cytology as the primary screening test [6, 7]. In Norway, a randomized implementation study was initiated in 2015 to evaluate switching women from primary cytology-based screening to HPV-based screening at age 34 years [8]; however, national scale-up is not scheduled for several years. In the interim, revisiting the application of HPV testing within the current cytology-based screening may help improve screening effectiveness and efficiency.
Women with cytology results of ASC-US and LSIL have a higher risk of progressing to a more severe lesion within the next screening round than those with normal cytology [9, 10], but the elevated risk may not warrant direct referral to diagnostic colposcopy with biopsy. Management guidelines for these women differ among developed countries, and determining the optimal follow-up approach as well as the threshold to prompt colposcopy referral remains a challenge. For example, decision-makers in Norway updated the screening guidelines for women with either ASC-US or LSIL in July 2014 to include re-testing a woman's initial cytology sample for the presence of high-risk HPV (i.e., reflex HPV testing). Women testing positive for high-risk HPV are recommended to return 6 to 12 months later for repeat testing to identify persistent high-risk HPV infections or cytologic abnormalities. In other European countries and the United States, reflex HPV testing is reserved for women with ASC-US [11, 12], while women with LSIL are referred directly to colposcopy due to the high prevalence of HPV in these women [13]. A recently published cohort study from the U.S. demonstrates the importance of risk-stratifying women with ASC-US according to HPV genotype, prompting the authors to call for cost-effectiveness analyses that assess the value of HPV genotype testing to triage women with minor cervical cytological lesions [14]. Revisiting cytology-based algorithms will be important not only for women of all ages prior to the national scale-up of primary HPV testing, but also for younger women unlikely to be recommended primary HPV testing due to the high prevalence of transient HPV infections [13].
Decision-analytic modelling has been previously applied to assess the cost-effectiveness of cervical cancer screening in Norway [15-17] and elsewhere [18], as well as management of ASC-US in the U.S. [19]. To our knowledge, there are no recent studies that evaluate alternative triage applications of HPV testing, such as HPV genotyping and delayed repeat testing, on the long-term health and economic consequences. Our objective was to identify the optimal triage management approach for women with cytology results of ASC-US and LSIL within the context of the Norwegian Cervical Cancer Screening Program.
Materials and Methods
Analytic approach
We adapted a previously developed microsimulation model [20, 21] to reflect the natural history of HPV and cervical cancer in Norway. We projected the long-term health and economic consequences associated with ten alternative management strategies for women aged 25 to 69 years with either ASC-US or LSIL on their index cytology and who were positive for high-risk HPV on their reflex test (Figure 1). The alternative triage strategies varied with respect to 1) the triage test(s): HPV with cytology in combination (i.e., co-testing), and HPV testing alone with or without genotyping for HPV-16 and -18, and 2) the length of time in between index and triage testing (i.e., at 6, 12 or 18 months following the index test result). We also considered one strategy that allowed direct referral to colposcopy with biopsy for all women who had ASC-US or LSIL and were positive for high-risk HPV on their index screen (Figure 1). Our primary health outcomes included life expectancy, quality-adjusted life years (QALYs), and the lifetime risk of developing cervical cancer. Economic outcomes included the total lifetime cost per screened woman, expressed in 2014 USD ($USD = NOK6.30) [22], as well as resource use in terms of number of cytology and HPV tests, colposcopy referrals, and precancer treatments. We adopted a societal perspective, accounting for patient time and transportation costs (Table 1), and discounted monetary costs and health benefits by 4% per year, consistent with Norwegian guidelines for economic evaluation [23].
Figure 1. Alternative strategies to triage women with ASC-US or LSIL, and high-risk HPV-positive on index screen.
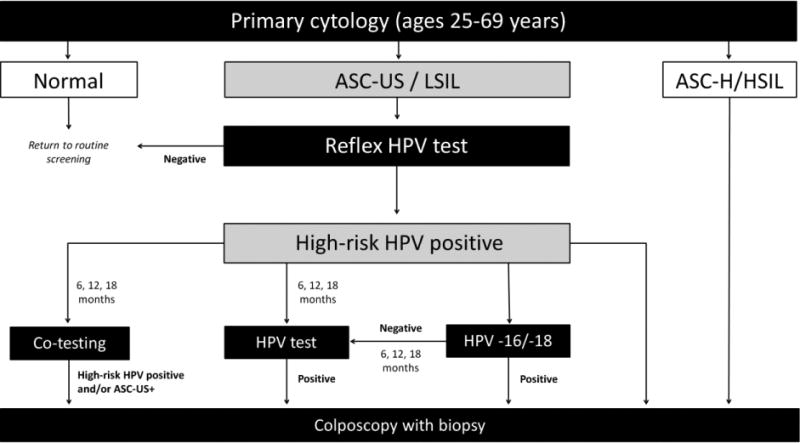
ASC-US+: atypical squamous cells of undetermined significance or worse, ASC-H: atypical squamous cells, cannot rule out high-grade squamous intraepithelial lesions, HPV: human papillomavirus, HSIL: high-grade intraepithelial lesion.
Flow diagram representing alternative screening strategies. This analysis focused on the follow-up of women with ASC-US/LSIL on their primary cytology screen, with a positive HPV result using reflex HPV DNA testing. We compared four main alternative strategies for screening triage; co-testing (i.e., HPV DNA testing and cytology in combination), HPV testing (i.e., HPV DNA testing to detect high-risk HPV), HPV -16/-18 genotyping (i.e., only referring HPV-16/-18 positives to colposcopy and requiring a persistent HPV positive result at 6, 12, or 18 months for women positive for other high-risk HPV types), or direct colposcopy for all HPV positive women. We varied the wait-time between index result and triage procedure by 6, 12 and 18 months for strategies other than direct colposcopy. Women negative for high-risk HPV could return to a routine screening schedule.
Table 1. Selected model inputs.
| Variable | Base-case value (%) | Range (%) |
|---|---|---|
| Screening test characteristicsa | ||
|
| ||
| Probability of abnormal cytology (ASC-US+) given high-grade precancer or worse | 70 | 40 |
| Probability of positive high-risk HPV DNA test given high-risk HPV | 100 | 90 |
| Probability of abnormal biopsy (CIN2+) given high-grade precancer or worse | 86 | |
|
| ||
| Unit costs | Baseline ($) | Range ($) |
|
| ||
| Physician consultationb | ||
| Screening (cytology and/or HPV test) | 122 | 61 - 244 |
| Verification of high-grade lesion (colposcopy w/biopsy) | 258 | 129 - 516 |
| Analyzing test sample at pathology laboratoryc | ||
| Cytology | 45 | 22 - 89 |
| HPV DNA test | 39 | 20 - 79 |
| Cervical biopsy | 124 | 62 - 247 |
| Patient time and travel costsd | ||
| Screening consultation | 120 | 0 |
| Colposcopy examination | 150 | 0 |
| Precancer and cancer treatmente | ||
| High-grade precancers | 1,682 | 841 - 3,364 |
| Local cancer | 26,941 | 13,471 - 53,882 |
| Regional cancer | 56,601 | 28,301 - 113,202 |
| Distant cancer | 41,367 | 20,684 - 82,735 |
ASC-US+: Atypical squamous cells of undetermined significance or worse, CIN2+: ce rvical intraepithelial neoplasia of grade 2 or worse, HPV: Human papillomavirus.
Based on published Norwegian fee schedules.
Based on actual resource use in Norwegian pathology laboratories (details available in the Supplementary Appendix).
Includes round-trip travel costs to attend physician consultation, as well as productivity losses associated with travel-, wait-, and consultation-time.
Based on published Norwegian fee schedules and includes patient time and travel costs. All costs are expressed in 2014 US dollars (US$ = NOK6.30).
We identified cost-efficient strategies by calculating the incremental cost-effectiveness ratio (ICER), defined as the additional cost per QALY gained, of a strategy compared to the next most costly strategy. Strategies that were more effective and less costly, or had a lower cost per QALY gained than other less costly strategies were considered cost-efficient. In Norway, there is no consensus for a single threshold value below which an intervention is considered cost-effective; therefore, we used a commonly-cited threshold value of 500,000 Norwegian Kroner (∼$80,000 in 2005-values [24]) per QALY gained, and adjusted to 2014-values using changes in real income wage in Norway during 2005-2014 [25]. Consequently, we considered the strategy that provided the most health benefits with an ICER below $100,000 to be cost-effective.
Simulation model
The individual-based model simulates a hypothetical cohort of women through the natural history of HPV-induced squamous cell cervical carcinoma.[20, 21] Individuals girls enter the model at age 9 with no HPV infections or cervical abnormalities and face monthly transitions between health states until death. Health states reflect HPV infection status (stratified by HPV -16, -18, -31, -33, -45, -52 and -58, pooled other high-risk HPV types, and pooled low-risk HPV types), grade of precancer (stratified by cervical intraepithelial neoplasia grade 2 (CIN2) and grade 3 (CIN3)) and invasive cancer (stratified by local, regional and distant stages). Monthly transitions can depend on HPV genotype, duration of infection or lesion, history of prior HPV infection, and age. For each individual woman, the model tracks clinical events such as screening and treatment histories, as well as the resource use and expenditures. We assumed that the underlying natural history of cervical cancer is similar across countries, but geographical variations in risk factors (e.g., sexual behavior) influence country-specific epidemiology; therefore, we allowed baseline transition parameters to vary across a plausible range of values. We used a likelihood-based calibration approach to identify 50 unique parameter sets that simultaneously achieve good fit to Norwegian epidemiologic data including type-specific HPV prevalence and HPV type distribution in cervical intraepithelial neoplasia grade 3 (CIN3) and cervical cancer (see Technical Appendix available at the author's website [26]). We calculated the base-case health and economic outcomes as the average value across all 50 parameter sets, and used the minimum and maximum values to reflect uncertainty bounds.
Costs
We included the direct medical and non-medical costs associated with screening, diagnosis, and treatment of precancer and cancer, which were updated from previous analyses [15, 17]. Briefly, relevant cost components were valued using Norwegian fee schedules and micro-costing of Norwegian pathology laboratories (Table 1 and Technical Appendix available at the author's website [26]), based on Norwegian guidelines for economic evaluation [23]. In sensitivity analysis, we explored uncertainty around cost estimates assuming 50% and 200% of base-case values (Table 1). In addition, we explored the impact of restricting the scope of the analysis to include only direct medical costs or broadening the scope of the analysis to include productivity losses associated with sick leave after precancer and cancer treatments.
Screening strategies and scenarios
The Norwegian Cervical Cancer Screening program invites women aged 25 to 69 years to cytology-based screening every three years. The screening program is managed by the Cancer Registry of Norway, which mails information letters about the screening program to all women aged 25 years (the age at which they are eligible to initiate screening), as well as reminder letters to women who have not attended routine screening or guidelines-based follow-up procedures. Women with a normal cytology result (i.e., no intraepithelial lesion or malignancy (NILM)) return to a routine screening schedule, while women with a high-grade result (i.e., atypical squamous cells, cannot rule out high-grade squamous intraepithelial lesions (ASC-H), or high-grade intraepithelial lesion (HSIL)) are referred directly to diagnostic colposcopy with biopsy. For women with cytology results indicating minor cervical lesions (i.e., ASC-US or LSIL) the current Norwegian guidelines recommend reflex HPV testing, allowing HPV negative women to return for routine screening in three years (Figure 1). Women testing positive for high-risk HPV are recommended to return 6 to 12 months later for repeat cytology and HPV co-testing, and are referred to colposcopy if results indicate the presence of a persistent high-risk HPV infection and/or cytologic abnormalities of LSIL or worse. For this analysis, we assumed the current Norwegian algorithm involved delayed co-testing at 12 months, but included 6 and 18 month delayed co-testing to reflect the variation in screening guidelines.
We compared the current triage algorithm in Norway with seven alternative strategies to triage women with ASC-US or LSIL on their index cytology and who were positive for high-risk HPV on their reflex test (Figure 1). Candidate strategies involved three management approaches: (1) HPV testing with genotyping for HPV-16 and -18, (2) HPV testing without genotyping for HPV-16 and -18, and (3) immediate colposcopy referral. The HPV genotyping strategy involves referring women who test positive for the two most oncogenic HPV genotypes (i.e., HPV-16 and -18) on their index reflex test directly to diagnostic colposcopy. Women positive for the other pooled high-risk HPV types are required to return for repeat HPV testing. Similar to the co-testing strategy, we varied the length of time in between index and repeat test(s) by 6, 12 or 18 months following their index results. Surveillance following a negative biopsy was constant across all strategies and reflected current practice in Norway (i.e., delayed co-testing at 12 months).
The alternative screening strategies were outlined in collaboration with key decision-makers in Norway for a previous analysis [16]. To reflect the policy decision currently on the table in Norway, we did not consider differential management for women diagnosed with ASC-US and LSIL (e.g., immediate colposcopy for all women with LSIL and reflex HPV testing for women with ASC-US) in our primary analysis; however, we included this strategy in a secondary analysis. We also expanded the secondary analysis to identify whether the optimal triage strategy may differ for younger women (i.e., < age 34), accounting for the likely switch to primary HPV testing starting at either age 31 or 34 (every 5 years) [8]. For all analyses, we assumed perfect adherence to screening guidelines, but varied this assumption in sensitivity analysis using data on observed screening and follow-up compliance from the Cancer Registry of Norway (see Technical Appendix available at the author's website [26]) [27, 28]. For example, we assumed that 72.3% of women with cytology results indicating ASC-US or LSIL attended recommended triage testing [27]. Screening test characteristics for cytology, HPV testing, and diagnostic colposcopy with biopsy were based on primary data and published literature (Table 1) [29-33], and are conditioned on a woman's underlying health state.
Results
Primary analysis: Management of women within current cytology-based program
For women with a cytology result of ASC-US or LSIL and who are positive for high-risk HPV on reflex testing, the current Norwegian guidelines involving co-testing at 12 months was projected to reduce the lifetime risk of cervical cancer by 85.9% compared with no screening (Table 2). For the alternative triage strategies, the reductions in lifetime risk of cervical cancer ranged from 85.4% to 87.0%. Despite the modest differences in effectiveness, resource use varied considerably among the candidate strategies (Figure 2). For example, compared with current guidelines-based management, the most effective strategy (i.e., immediate colposcopy for all women with ASC-US or LSIL on index cytology and high-risk HPV) was expected to increase colposcopy referrals and precancer treatments by 21.4% and 14.9%, respectively. In comparison, the strategy involving genotyping with colposcopy for women positive for HPV -16 or -18 (with repeat HPV testing in 12 months for non HPV -16 or -18 positive women) increased colposcopy referrals and precancer treatment rates by 8.7% and 6.8 %, respectively. The duration of time in between index and triage testing was an important resource-driver. For example, within the same management approach, delaying repeat testing from 6 to 18 months decreased colposcopies by as much as 17% and precancer treatments by as much as 12% with only nominal impacts on health benefits.
Table 2. Costs, health benefits, and cost-effectiveness of alternative triage strategies for women diagnosed with ASC-US or LSIL, and high-risk HPV-positive.
| Management of women with ASC-US/LSIL and high-risk HPVa | Costs | Health outcomes | Cost-effectiveness | |||
|---|---|---|---|---|---|---|
|
|
|
|
|
|||
| Screening procedure | Follow-up wait (months) b | Discounte d lifetime costs ($) per woman | Cancer incidence reduction (%)e | Discounted QALYsf | Discounted life-years | ICERg ($ per QALY) |
|
|
|
|
|
|||
| No screening | - | 180 | - | 21.46887 | 23.97505 | - |
| HPV testing | 18 | 1,249 | 85.40 % | 21.50136 | 24.00466 | 32,914 |
| Co-testingc | 18 | 1,253 | 85.46 % | 21.50139 | 24.00468 | - |
| HPV testing | 12 | 1,264 | 85.95 % | 21.50164 | 24.00489 | - |
| Genotypingd | 18 | 1,268 | 86.31 % | 21.50171 | 24.00488 | 52,552 |
| Co-testingc (current) | 12 | 1,268 | 85.92 % | 21.50164 | 24.00490 | - |
| Genotypingd | 12 | 1,276 | 86.51 % | 21.50182 | 24.00499 | 78,012 |
| HPV testing | 6 | 1,285 | 86.52 % | 21.50179 | 24.00494 | - |
| Genotypingd | 6 | 1,288 | 86.82 % | 21.50188 | 24.00500 | - |
| Co-testingc | 6 | 1,289 | 86.49 % | 21.50178 | 24.00493 | - |
| Colposcopy | - | 1,293 | 87.01 % | 21.50198 | 24.00508 | 104,402 |
ASC-US: Atypical squamous cells of undetermined significance, HPV: high-risk human papillomavirus, ICER: incremental cost-effectiveness ratio, LSIL: low-grade squamous intraepithelial lesion, Lys: life-years, QALYs: quality-adjusted life-years.
Results at the primary screen, assuming cytology screening every three years for women aged 25-69 years. Women detected with ASC-US/LSIL receive a reflex HPV DNA test, and return to a routine screening schedule if negative for high-risk HPV.
Number of months between primary screen and triage screening procedure.
Repeat HPV DNA testing and cytology in combination.
Genotyping indicates stratified management for women with HPV-16 or -18 versus other pooled high-risk HPV types, involving direct colposcopy for HPV-16/-18 positives and HPV DNA testing for other high-risk HPV positives.
Compared to no screening.
The incremental cost-effectiveness ratio (ICER) represents the incremental costs per QALY gained of a strategy compared with the next most costly strategy. Rows highlighted in bold reflect strategies on the efficiency frontier (i.e., strategies providing health benefits in terms of QALYs at lower costs, or lower ICER, than alternative strategies). Health benefits and costs are discounted by 4% per year. All costs are expressed in 2014 US dollars (US$ = NOK6.30).
Figure 2. Resource trade-offs associated with candidate triage algorithms compared with current guidelines.
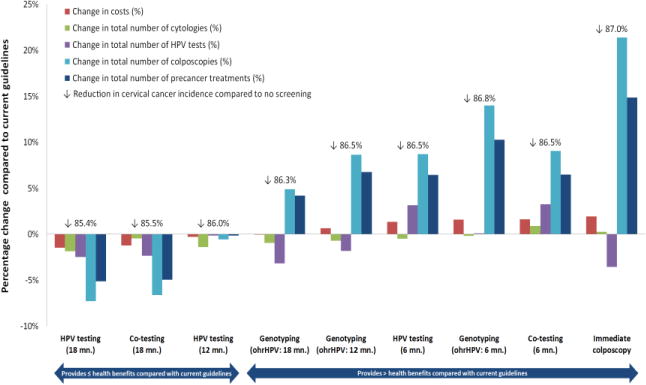
ASC-US = Atypical squamous cells of undetermined significance; HPV, human papillomavirus; LSIL = Low-grade intraepithelial lesion; ohrHPV, positive for non HPV-16/-18 high-risk genotypes.
Colored bars denote percentage change in total costs per woman, total number of cytologies, total number of HPV tests, total number of colposcopies, and total number of treatments, of each alternative strategy compared with current guidelines in Norway (i.e., co-testing at 12 months). The strategies are sorted by increasing change in costs.
When we translated health benefits and resource use into a single composite measure of cost per QALY gained to identify cost-efficient strategies, we found that all but four triage management strategies were inefficient, including all strategies that involved co-testing (Table 2, Figure 3). The remaining efficient strategies involved repeat HPV testing at 18 months without genotyping, HPV genotyping with immediate colposcopy for HPV -16 or -18 positive (and repeat testing at 12 or 18 months for women positive for non-HPV-16 or -18 high-risk HPV types), and immediate colposcopy for all women with ASC-US or LSIL on index cytology and high-risk HPV. The latter three strategies were projected to improve both the effectiveness and the efficiency of the Norwegian Cervical Cancer Screening Program. In Norway, for a willingness-to-pay threshold of $100,000 per QALY gained, the preferred (i.e., most cost-effective) strategy involved genotyping with a 12-month delayed repeat HPV test for non-HPV-16 or -18 high-risk genotypes (i.e., $78,010 per QALY gained). Immediate colposcopy for any high-risk HPV positive result had a cost per QALY that only slight exceeded the willingness-to-pay threshold (i.e., $104,400 per QALY gained).
Figure 3. Efficiency frontier showing the trade-off of projected health benefits and costs of alternative triage algorithms for women with ASC-US or LSIL and high-risk HPV-positive results.
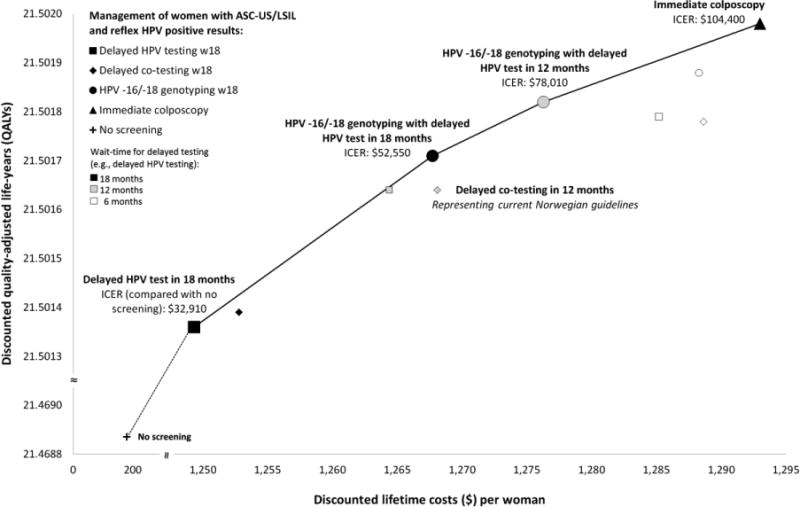
ASC-US: atypical squamous cells of undetermined significance, HPV: high-risk human papillomavirus, ICER: incremental cost-effectiveness ratio, QALYs: quality-adjusted life-years.
Discounted QALYs and lifetime costs ($) per screened woman (discount rate: 4% per year). Strategies connected by the solid line represent the efficiency frontier (i.e., strategies providing health benefits in terms of QALYs at lower costs, or lower ICER, than alternative strategies). All costs are expressed in 2014 US dollars (US$ = NOK6.30).
Secondary analysis: Additional screening strategies
When we included a strategy that allowed differential management of women with ASC-US or LSIL (i.e., reflex HPV testing for women with ASC-US and immediate colposcopy for women with LSIL), we found that this strategy was efficient but not cost-effective as the cost per QALY gained was exceedingly high (see Supplementary Appendix Table S4). For example, when holding all other assumptions constant, this strategy yielded an ICER of >$9 million per QALY gained compared to the next most costly strategy. The HPV genotyping strategy remained the preferred strategy for both primary HPV-based screening start ages (i.e., age 31 and 34) (Supplementary Appendix Table S5 and S6).
Sensitivity analysis
Results were most sensitive to assumptions around screening and follow-up compliance, HPV test characteristics, and when we expanded the analysis to include productivity losses associated with sick leave after precancer and cancer treatments. For example, when we assumed compliance reflected empirical data from the Cancer Registry of Norway, only three strategies remained cost-efficient, including repeat HPV testing at 18 months without genotyping, genotyping (with HPV testing at 18 months for women positive for non-HPV-16 or -18 high-risk HPV types), and immediate colposcopy for all women positive for high-risk HPV (Appendix Table S3). Given current willingness-to-pay recommendations in Norway, the preferred strategy involved immediate colposcopy for all women positive for high-risk HPV. This strategy was also preferred when we reduced the sensitivity of the HPV test, in which case the strategies involving repeat HPV testing at 18 months and HPV genotyping (with HPV testing at 12 months for non-HPV-16 or -18 positive) were no longer cost-efficient, and were replaced by co-testing at 18 months on the efficiency frontier. Results were moderately influenced by a 50% reduction in the cost associated with analyzing a cytology or biopsy, a colposcopy office visit, and precancer treatment, when we doubled the cost of local cancer treatment, or when we only included direct medical costs (Appendix Table 4), in which case immediate colposcopy for all high-risk HPV positive was the preferred strategy. Of note, the current Norwegian guidelines remained unattractive under all sensitivity analysis assumptions. Across the 50 simulated parameter sets, and given a willingness-to-pay threshold of $100,000 per QALY gained, the HPV genotyping strategy (requiring non-HPV-16/-18 to return 12 or 18 months later) was the preferred strategy in 52% of the simulations, while immediate colposcopy for all HPV-positive women was the preferred strategy in 48% of the simulations.
Discussion
Our better understanding of the carcinogenic potential of persistent HPV infection and the advent of new HPV diagnostics necessitates revisiting management of women with minor cervical lesions. Our study indicates that improvements in effectiveness and efficiency can be made to the current Norwegian guidelines for management of women with ASC-US or LSIL. Given current benchmarks for what constitutes ‘good value for money’ in Norway, the preferred strategy involves HPV genotyping to expedite management for women positive for HPV-16 or -18 infections (requiring non-HPV-16/-18 to return 12 months later), while immediate colposcopy for all high-risk HPV positive would be preferred for a small increase in the willingness-to-pay threshold. Due to the proximity of these two strategies to a willingness-to-pay threshold of $100,000 per QALY gained, there is decision uncertainty around which of these two strategies is preferred. However, immediate colposcopy for all high-risk HPV positive accompanies a considerable increase in the number of colposcopy referrals and precancer treatments compared to current levels, both of which may be subject to short-term capacity constraints in Norway. In contrast, the HPV genotyping strategies require only a moderate increase in resource use, with nominal compromises in health gains.
To our knowledge, this is the first analysis to investigate the impact of using novel applications (e.g., HPV genotyping) to triage women with ASC-US or LSIL on long-term health benefits and resource use (both monetary and non-monetary). Previous studies evaluated the cost-effective management of women with minor cervical lesions in Norway, but only considered surrogate health (i.e., detected precancers) and short-term economic outcomes associated with alternative triage strategies [16, 17]. Despite different time horizons and outcomes, the current Norwegian guidelines were identified as more costly and less effective in all analyses. Our results were similar to another study performed within the Italian context that compared the short-term cost-effectiveness of three alternative triage strategies for women with ASC-US or LSIL, including immediate colposcopy and reflex HPV DNA testing [34]. Although the authors did not consider differential management of ASC-US and LSIL or HPV genotyping, their results suggest that reflex HPV DNA testing would reduce colposcopy referrals by more than 50% without considerably reducing the number of CIN2+ detected compared to referring all women with minor cervical lesions to immediate colposcopy. Consistent with a U.S.-based study published in 2002 that compared alternative triage algorithms for women with ASC-US using a lifetime perspective [19], we found that the health benefits (e.g., reductions in cervical cancer risk) associated with varying the management of women with ASC-US or LSIL results are small. In contrast, both studies found that resource requirements vary substantially.
Our study has several implications for resource utilization. First, the model used in this analysis is one of the only natural history models that explicitly accounts for the role of HPV persistence in progression to precancer and cancer. Interestingly, by allowing time for HPV infections to clear, the strategies involving 12- and 18-month delays were more efficient than strategies involving a 6-month delay. The current Norwegian guidelines recommend repeat testing as early as 6 months; our results suggest that delaying repeat testing to ≥12 months impacts the specificity of a triage algorithm and can help reduce the costs and resource use of screening triage with little compromise in health gains. Second, in several European countries and the U.S., reflex HPV testing is restricted to women with ASC-US while women with LSIL are advised immediate colposcopy. We found that referring women diagnosed with LSIL directly to colposcopy would require an additional cost per QALY gained that far exceeded current willingness-to-pay threshold recommendations in Norway. In Norway, and other countries with similar epidemiologic characteristics and relative costs, the cost savings associated with reducing colposcopy referrals for HPV-negative LSILs may outweigh the incremental benefit achieved by referring these women to colposcopy.
Our analysis also highlights the value of using HPV genotype testing, a novel screening technology not yet commonly used in triage algorithms. In the U.S., HPV -16 or - 18 genotyping is currently only recommended to triage women who are HPV-positive and cytology-negative [12], yet a recent study suggests that HPV genotype testing may also benefit management guidelines for women with minor cervical cytological lesions [14]. We found that extending genotyping to triage ASC-US and LSIL is projected to increase the efficiency of screening algorithms, and continues to be the preferred strategy for young adult women (i.e., <34 years) unlikely to be recommended primary HPV-based screening.
Our analysis has several limitations. First, data availability for other candidate biomarkers such as HPV mRNA testing is limited; consequently, we restricted the scope of this analysis to variations of HPV DNA testing. Analyses can be reevaluated as data accumulate. Second, we did not consider the optimal triage strategy for women who are vaccinated against HPV. Decision-makers in Norway have yet to reach consensus on the primary screening algorithm for women vaccinated against HPV during adolescence, therefore future analyses will need to evaluate the optimal primary and triage screening algorithm as vaccinated women enter screening target age. The capacity limits for Norwegian laboratories and hospitals are unknown; therefore, in the short-term, we cannot state whether or not a strategy identified as cost-effective is also feasible. Quantifying non-monetary resource requirements may help inform implementation decisions. Similarly, strategies that increase the number of colposcopy referrals and precancer treatments place a higher burden on women attending screening. Although women's preferences for the trade-offs between reducing the risk of developing cervical cancer and additional diagnostic tests is unknown, quantifying expected changes in screening procedures may aid decision-makers in designing screening policies that provide an acceptable balance between benefits (e.g., reduced cancer risk) and harms (e.g., unnecessary colposcopy referrals) [16]. No single willingness-to-pay threshold value in Norway exists; therefore, other strategies on the efficiency frontier may be preferred. Lastly, although our model is based on the best available evidence and analyses were performed using multiple parameter sets, uncertainty in the natural history and structure of the model remains. Model validation, utilizing external Norwegian data not used in the calibration process, has been performed in accordance with good modeling practice (see Technical Appendix available at the author's website [26]) [35].
Prior to implementing a new screening policy, and following European guidelines for quality assurance in cervical cancer screening [13], decision-makers should recommend screening algorithms that maximize the benefits and minimize the harms of screening, while simultaneously ensuring the feasibility and cost-effectiveness of the recommendations. We have identified four strategies that provide efficient use of resources, and three strategies with a potential to improve both the effectiveness and efficiency of the Norwegian Cervical Cancer Screening Program. However, more effective strategies also require more colposcopy referrals and precancer treatments than current levels. The optimal prevention policy will ultimately depend on a compendium of factors that decision-makers must consider, including investments of monetary and non-monetary resources and the availability of these resources.
Supplementary Material
Highlights.
-
➢
New screening approaches, such as human papillomavirus (HPV) genotyping, necessitate revisiting cervical cancer prevention policies.
-
➢
This study evaluates the cost-effectiveness and resource trade-offs associated with novel triage approaches for women with minor cervical lesions.
-
➢
HPV-16/-18 genotyping is the preferred strategy given current willingness-to-pay recommendations in Norway, but requires a moderate increase in colposcopy referrals.
Acknowledgments
We appreciate helpful comments from Dr. Peder Halvorsen, Dr. Andreas Pahle, Dr. Daniel Sørli, Dr. Torbjørn Wisløff, Dr. Sveinung W. Sørbye, Dr. Christian Widnes, Dr. June Hansen, Dr. Nils Natvig and Dr. Margrethe Renå, regarding Norwegian cost data. We are grateful for the contributions of the Center for Health Decision Science (Harvard T. H. Chan School of Public Health) and the Department of Health Management and Health Economics (University of Oslo) throughout analysis. The study has used data from the Cancer Registry of Norway. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Cancer Registry of Norway is intended nor should be inferred.
Funding/Support: This study was funded by the University of Oslo and the Norwegian Research Council (grant number 238042). JJK is partially supported by the U.S. National Cancer Institute of the National Institutes of Health (R01CA160744).
Role of the Sponspor: The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384(2):260–5. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 2.Plummer M, Schiffman M, Castle PE, et al. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007;195(11):1582–9. doi: 10.1086/516784. [DOI] [PubMed] [Google Scholar]
- 3.Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet (London, England) 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. IARC Monographs on th eEvaluation of Carcinogenic Risks to Human. Lyon, France: International Agency for Research on Cancer; 2012. Available at: http://monographs.iarc.fra/ENG/Monographs/vol100B/mono100B.pdf. [Google Scholar]
- 5.Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(Suppl 5):F88–99. doi: 10.1016/j.vaccine.2012.06.095. [DOI] [PubMed] [Google Scholar]
- 6.NHS Cervical Screening Programme. [16.06.2015];HPV Primary Screening Pilot Announced. 2012 URL: www.cancerscreening.nhs.uk/cervical/nes/021.html.
- 7.Vink MA, Bogaards JA, Meijer CJ, et al. Primary human papillomavirus DNA screening for cervical cancer prevention: Can the screening interval be safely extended? International journal of cancer Journal international du cancer. 2015;137(2):420–7. doi: 10.1002/ijc.29381. [DOI] [PubMed] [Google Scholar]
- 8.Nygard M, Andreassen T, Berland J, et al. The Norwegian Directorate of Health. Oslo: 2013. HPV-test i primærscreening mot livmorhalskreft. Kontrollert implementering og evaluering av forbedret helsetjeneste. [Google Scholar]
- 9.Nygard M, Roysland K, Campbell S, et al. Comparative effectiveness study on human papillomavirus detection methods used in the cervical cancer screening programme. BMJ Open. 2014;4(1):e003460. doi: 10.1136/bmjopen-2013-003460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castle PE, Sideri M, Jeronimo J, et al. Risk assessment to guide the prevention of cervical cancer. American journal of obstetrics and gynecology. 2007;197(4):356.e1–6. doi: 10.1016/j.ajog.2007.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordan J, Arbyn M, Martin-Hirsch P, et al. European guidelines for quality assurance in cervical cancer screening: recommendations for clinical management of abnormal cervical cytology, part 1. Cytopathology : official journal of the British Society for Clinical Cytology. 2008;19(6):342–54. doi: 10.1111/j.1365-2303.2008.00623.x. [DOI] [PubMed] [Google Scholar]
- 12.Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Journal of lower genital tract disease. 2013;17(5 Suppl 1):S1–s27. doi: 10.1097/LGT.0b013e318287d329. [DOI] [PubMed] [Google Scholar]
- 13.Arbyn M, Anttila A, Jordan J, et al. European Guidelines for Quality Assurance in Cervical Cancer Screening. Second edition--summary document. Ann Oncol. 2010;21(3):448–58. doi: 10.1093/annonc/mdp471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiffman M, Burk RD, Boyle S, et al. A study of genotyping for management of human papillomavirus- positive, cytology-negative cervical screening results. Journal of clinical microbiology. 2015;53(1):52–9. doi: 10.1128/JCM.02116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burger EA, Ortendahl JD, Sy S, et al. Cost-effectiveness of cervical cancer screening with primary human papillomavirus testing in Norway. British journal of cancer. 2012;106(9):1571–8. doi: 10.1038/bjc.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedersen K, Sørbye SW, Burger EA, et al. Using Decision-Analytic Modeling to Isolate Interventions That Are Feasible, Efficient and Optimal: An Application from the Norwegian Cervical Cancer Screening Program. Value in Health. 2015;18(8):1088–97. doi: 10.1016/j.jval.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen K, Sørbye SW, Kristiansen IS, et al. Using novel biomarkers to triage young adult women with minor cervical lesions: A cost-effectiveness analysis. BJOG: An International Journal of Obstetrics and Gynaecology. 2016 doi: 10.1111/1471-0528.14135. Accepted March 24, 2016. [DOI] [PubMed] [Google Scholar]
- 18.Mendes D, Bains I, Vanni T, et al. Systematic review of model-based cervical screening evaluations. BMC cancer. 2015;15:334. doi: 10.1186/s12885-015-1332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JJ, Wright TC, Goldie SJ. Cost-effectiveness of alternative triage strategies for atypical squamous cells of undetermined significance. Jama. 2002;287(18):2382–90. doi: 10.1001/jama.287.18.2382. [DOI] [PubMed] [Google Scholar]
- 20.Campos NG, Burger EA, Sy S, et al. An updated natural history model of cervical cancer: derivation of model parameters. American journal of epidemiology. 2014;180(5):545–55. doi: 10.1093/aje/kwu159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JJ, Kuntz KM, Stout NK, et al. Multiparameter calibration of a natural history model of cervical cancer. American journal of epidemiology. 2007;166(2):137–50. doi: 10.1093/aje/kwm086. [DOI] [PubMed] [Google Scholar]
- 22.Federal Reserve. [November 22, 2015];Average rates of exchange 2014. 2015 Available at: http://www.federalreserve.gov/releases/G5A/current/
- 23.Norwegian Directorate of Health. [15.09.2014];Økonomisk evaluering av helsetiltak - en veileder. 2012 2012 URL: http://helsedirektoratet.no/publikasjoner/okonomisk-evaluering-av-helsetiltak--en-veileder/Publikasjoner/IS-1985.pdf.
- 24.Norwegian Directorate of Health Health effects of socio-economic analyses. Oslo: Norwegian Directorate of Health; 2007. [Google Scholar]
- 25.Statistics Norway. [December 11, 2015];2015 URL: https://www.ssb.no/nasjonalregnskap-og-konjunkturer/artikler-og-publikasjoner/norsk-okonomi-i-moderat-fart--180967?tabell=180881.
- 26.Technical Appendix. [July 28, 2016];Harvard Cervical Cancer Natural History Model Calibration and Costing Approach for Norway. Available at: http://www.med.uio.no/helsam/english/research/projects/preventive-strategies-hpv/harvardmodel-norway-technicalappendix.pdf.
- 27.Cancer Registry of Norway. Annual Report of the Norwegian Cervical Cancer Screening Program, 2013-14. Oslo, Norway: 2015. [Google Scholar]
- 28.Pedersen K, Burger EA, Campbell S, et al. Risk of Cervical Cancer by Screening Intensity: A Registry- based Analysis. International Papillomavirus Conference; September 17-21; Lisbon, Portugal. 2015. [Google Scholar]
- 29.Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. The New England journal of medicine. 2007;357(16):1579–88. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 30.Ronco G, Dillner J, Elfstrom KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet (London, England) 2014;383(9916):524–32. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- 31.Nanda K, McCrory DC, Myers ER, et al. Accuracy of the Papanicolaou test in screening for and follow- up of cervical cytologic abnormalities: a systematic review. Annals of internal medicine. 2000;132(10):810–9. doi: 10.7326/0003-4819-132-10-200005160-00009. [DOI] [PubMed] [Google Scholar]
- 32.Arbyn M, Bergeron C, Klinkhamer P, et al. Liquid compared with conventional cervical cytology: a systematic review and meta-analysis. Obstetrics and gynecology. 2008;111(1):167–77. doi: 10.1097/01.AOG.0000296488.85807.b3. [DOI] [PubMed] [Google Scholar]
- 33.Stoler MH, Ronnett BM, Joste NE, et al. The Interpretive Variability of Cervical Biopsies and Its Relationship to HPV Status. The American journal of surgical pathology. 2015;39(6):729–36. doi: 10.1097/PAS.0000000000000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zappacosta R, Gatta DM, Marinucci P, et al. Role of E6/E7 mRNA test in the diagnostic algorithm of HPV-positive patients showing ASCUS and LSIL: clinical and economic implications in a publicly financed healthcare system. Expert Rev Mol Diagn. 2015;15(1):137–50. doi: 10.1586/14737159.2015.961915. [DOI] [PubMed] [Google Scholar]
- 35.Caro JJ, Briggs AH, Siebert U, et al. Modeling good research practices--overview: a report of the ISPOR- SMDM Modeling Good Research Practices Task Force--1. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2012;15(6):796–803. doi: 10.1016/j.jval.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 36.Burger EA, Sy S, Nygard M, et al. Prevention of HPV-related cancers in Norway: cost-effectiveness of expanding the HPV vaccination program to include pre-adolescent boys. PloS one. 2014;9(3):e89974. doi: 10.1371/journal.pone.0089974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myers E, GS IL. Patient preferences for health states related to HPV infection: visual analygue scales versus time trade-off eliciation [abstract]. Poceeding of the 21st International Papillomavirus Conference; Mexico City, Mexico. 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


